Abstract
In recent years, the interest in biological treatment of knee lesions has increased, especially the application of platelet-rich plasma is of particular note. The number of articles evaluating platelet-rich plasma (PRP) efficacy in the recovery of knee disorders and during knee surgery has exponentially increased over the last decade. A systematic review with meta-analyses was performed by assessing selected studies of local PRP injections to the knee joint. The study was completed in accordance with 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. A multistep search of PubMed, Embase, Cochrane Database of Systematic Reviews, and Clinicaltrials.gov was performed to identify studies on knee surgery and knee lesion treatment with PRP. Of the 4004 articles initially identified, 357 articles focusing on knee lesions were selected and, consequently, only 83 clinical trials were analyzed using the revised Cochrane risk-of-bias tool to evaluate risk. In total, seven areas of meta-analysis reported a positive effect of PRP. Among them, 10 sub-analyses demonstrated significant differences in favor of PRP when compared to the control groups (p < 0.05). This study showed the positive effects of PRP, both on the recovery of knee disorders and during knee surgery; however further prospective and randomized studies with a higher number of subjects and with lower biases are needed.
Keywords: PRP, platelet-rich plasma, meniscus, anterior cruciate ligament (ACL), osteoarthritis, tendinopathy, arthroscopy, knee lesion, total knee arthroplasty, osteoarthritis (OA), meniscal repair
1. Introduction
Knee disorders are among the most frequent disorders treated by orthopedic surgeons. Traumatic knee injuries, as well as knee degeneration, require special attention and appropriate treatment. The first line of treatment is usually conservative and includes physical therapy, rehabilitation, braces or non-steroid inflammatory drugs. Recently, orthobiologics—naturally occurring substances in the body—were introduced to clinics [1,2]. One type of orthobiologic substance, platelet-rich plasma (PRP) shows promising results for minimally invasive treatment of knee lesions through enhanced healing potential of damaged cartilage, tendons, and ligaments [1]. PRP, also known as platelet-rich fibrin (PRF), platelet concentrate or platelet-rich growth factors (PRGFs) is a concentration of platelets derived from the patient’s whole blood, which has to be centrifuged to obtain a ready-to-use product [2,3]. The mechanism of action relies on releasing cytokines and growth factors from alpha granules such as interleukin 1β, interleukin 8, tumor necrosis factor (TNF-α), platelet derived growth factor (PDGF), platelet derived endothelial growth factor (PDEGF), transforming growth factor β1 (TGF-β1), insulin-like growth factor 1 (IGF-1), fibroblast growth factor 2 (FGF-2), hepatocyte growth factor (HGF), and vascular endothelial growth factor A (VEGF-A). These enhance healing by stimulating cell proliferation, migration, and differentiation, alongside interaction with the immune system, inflammation, and angiogenesis [1,2,3,4]. Possible indications for PRP application in knee disorders and knee surgery are cartilage degeneration in osteoarthritis and soft tissue injuries in sports medicine. Well documented clinical trials are related to patients with degenerative meniscus lesions, patellar tendinopathy, graft remodeling in anterior cruciate ligament (ACL) reconstruction, hamstring tendinopathy, and medial collateral ligament (MCL) injuries [1,5]. There is also some evidence for pain reduction after total knee arthroplasty (TKA) and bone remodeling after osteotomies. Several systematic reviews and meta-analyses have been published, although with contradictory results; therefore, we aimed to elucidate these controversial issues and performed a systematic review and meta-analysis on the efficacy of PRP use in disorders around the knee.
2. Results
2.1. Literature Search
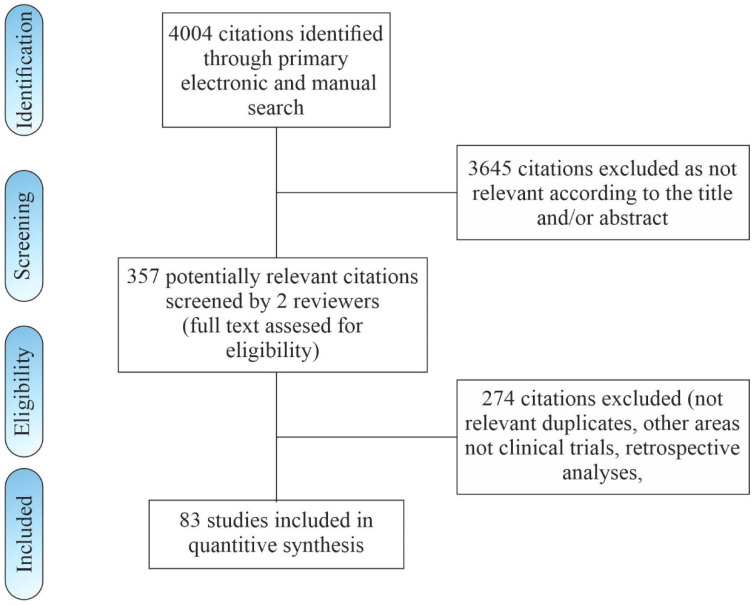
A literature search through electronic databases identified a total of 4002 records according to the selected search algorithm and two additional studies were included through reference list evaluation. A total of 3645 citations was excluded as irrelevant according to title and/or abstract. The abstracts of 357 remaining articles were assessed for eligibility. From these, 274 were excluded. The remaining 83 clinical studies published between 2005 and 2020 with 5323 patients were included in this review. The literature search flowchart is shown in Figure 1.
Figure 1.
Flow chart of study inclusion.
2.2. Study Characteristics
A total of 83 randomized controlled trials (RCTs) and seven non-RCTs was included in our study. The characteristics of the selected articles are summarized in Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8 and Table 9. All of the selected studies were included into a systematic review. Mean follow-up period was 12 months (ranging from 10 days to 3 years) and the mean number of patients included was 62 (ranging from 20 to 315).
Table 1.
Platelet-rich plasma (PRP) compared with control intervention for patellar tendinopathy.
| LOE | Type of Study | Exp | Cont | Follow-up | Control | Preparation Kit | LR/LP | Platelet Conc. | Number of Inj. | PROM | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abate et al. | III | 3 arms | PRP 18(18) HVIGI + PRP 18(18) |
18(18) | 6 months | HVIGI saline | Regen Lab A-PRP Kit (Regenlab) | LP | 1.6× NPC (Native Platelet Concentration) | 2 | VISA VAS |
[9] |
| Dragoo et al. | I | 2 arms | 10(8) | 12(9) | 6 months | Dry needling | GPS III (Biomet) | LR | N/R | 1 | VISA Tegner Lysholm VAS SF-12 |
[7] |
| Scott et al. | I | 3 arms | LR 19(19) LP 19(19) |
19(19) | 12 months | Saline | ACs (Arthrex) | LR/LP | LR 3.8 × 230,000 (51,000)/µL LP 3.0 × 227,000 (43,000)/µL |
1 | VISA NPRS GROC |
[6] |
| Vetrano et al. | I | 2 arms | 23(23) | 23(23) | 12 months | ESWT | Recover ps kit (Kaylight) | N/R | 0.89–1.1 × 109 µL | 2 | VISA VAS Blazina |
[8] |
LOE—level of evidence; exp.—no. of patients receiving treatment in experimental group (no. of patients analyzed at final follow-up); cont.—no. of patients receiving treatment in control group (no. of patients analyzed at final follow-up); ESWT—extracorporeal shock wave therapy; HVIGI—high volume image guided injection; LR—leukocyte rich; LP—leukocyte poor; PROM—patient related outcome measures; VAS—visual analog scale; NPRS—Numeric Pain Rating Scale; VISA—Victorian Institute of Sport Assessment; GROC—Global Rating of Change Scales; SF-12—Short Form Survey.
Table 2.
Platelet-rich plasma (PRP) compared with control intervention for the knee adjacent muscle injuries.
| LOE | Type of Study | Exp. | Cont. | Follow-up | Control | Preparation Kit | LR/LP | Platelet Concentration | Number of Injections | PROM | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hamid et al. | II | 2 arms | 14(12) | 14(12) | 39 weeks | No injection | GPS III (Biomet) | 38.3 × 103/µL | 1297 × 103µL | 1 | RTS BPI-SF |
[11] |
| Hamilton et al. | I | 3 arms | PRP 30(26) PPP 30(28) |
30(29) | 6 months | No injection | GPS III (Biomet) | 26.1(13.7) × 103/µL | 765.8(23.6) × 109/L | 1 | RTS Re-injury |
[12] |
| Reurink et al. | I | 2 arms | 41(37) | 39(36) | 1 year | Saline | ACP (Athrex) | 1.9(2.1) × 103/µL | 433(128) × 103/µL | 2 | RTS Re-injury |
[13] |
| Rossi et al. | I | 2 arms | 35(34) | 40(38) | 2 years | No injection | N/R | N/R | N/R | 1 | RTS VAS Re-injury |
[10] |
LOE—level of evidence; exp.—no. of patients receiving treatment in experimental group (no. of patients analyzed at final follow-up); cont.—no. of patients receiving treatment in control group (no. of patients analyzed at final follow-up); LR—leukocyte rich; LP—leukocyte poor; PROM—patient related outcome measures; VAS—visual analog scale; RTS—time for return to sports; BPI-SF—Brief Pain Inventory-Short Form.
Table 3.
Platelet-rich plasma (PRP) compared with control intervention for high tibial osteotomy.
| LOE | Type of Study | Exp. | Cont. | Follow-up | Control | Preparation Kit | LR/LP | Platelet Concentration | Number of Injections | PROM | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dallari et al. | I | 3 arms | PG 1(9) PG + BM 12(10) |
10(9) | 1 year | Lyophilized bone chips |
N/R | N/R | 1 × 106/µL | 1 | KSS ROM Osteointegration histomorphometric |
[15] |
| Koh et al. | II | 2 arms | 26(23) | PRP + MSC 26(21) | 2 years | PRP + MSC | N/R | N/R | 1303.27 (375.2) × 103/µL | 1 | Lysholm VAS KOOS |
[14] |
LOE—level of evidence; exp.—no. of patients receiving treatment in experimental group (no. of patients analyzed at final follow-up); cont.—no. of patients receiving treatment in control group (no. of patients analyzed at final follow-up); LR—leukocyte rich; LP—leukocyte poor; ROM—range of movement; PROM—patient related outcome measures; VAS—visual analog scale; KSS—Knee Society Score; Lysholm—Lysholm Knee Scoring Scale.
Table 4.
Platelet-rich plasma (PRP) compared with control intervention for TKA.
| LOE | Type of Study | Exp. | Cont. | Follow-up | Control | Preparation Kit | LR/LP | Platelet Concentration | Number of Injections | PROMs | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Guerreiro et al. (2015) |
I | 2 arms | 20(20) | 20(20) | 2 months | no injection | Fanem | LP | 988,250 | 1 | VAS WOMAC HgB drop ROM Ht Wound |
[19] |
| Guerreiro et al. (2019) | I | 4 arms | PRP 20(16) PRP + TXA 20(18)TXA 23(13) |
21(21) | 2 years | saline | Fanem | LP | 618,500 | 1 | VAS WOMAC HgB drop ROM Wound |
[20] |
| Horstman et al. | I | 2 arms | 20(20) | 20(20) | 10 days | no injection | GPS (Biomet) | LR | N/R | 1 | VAS HgB drop ROM wound |
[16] |
| Mochizuk et al. | I | 2 arms | 109 | 206 | 14 days | no injection | N/R | N/R | N/R | 1 | HgB drop ROM BL |
[21] |
| Morishita et al. | I | 2 arms | 20(20) | 20(20) | 28 days | no injection | ACS (Exactech) | LR | 23.4 × 104/µL | 1 | KOOS KSS HgB drop ROM BL CRP |
[17] |
| Peerboom et al. | II | 2 arms | 50(32) | 52(41) | 3 months | no injection | GPS (Biomet) | N/R | N/R | 1 | VAS WOMAC HgB drop ROM wound |
[18] |
LOE—level of evidence; exp.—no. of patients receiving treatment in experimental group (no. of patients analyzed at final follow-up); cont.—no. of patients receiving treatment in control group (no. of patients analyzed at final follow-up); LR—leukocyte rich; LP—leukocyte poor; ROM—range of movement; BL—blood loss; wound—wound healing; CRP—C reactive protein; HgB—hemoglobin; Ht—hematocrit; PROM—patient related outcome measures; VAS—visual analog scale; WOMAC—Western Ontario and McMaster Universities Osteoarthritis Index; KSS—Knee Society Score; KOOS—Knee injury and Osteoarthritis Outcome Score.
Table 5.
Platelet-rich plasma (PRP) compared with control intervention as adjunct treatment for arthroscopy.
| LOE | Type of Study | Exp | Cont | Follow-up | Control | Preparation Kit | LR/LP | Platelet Concentration | Number of Inj. | PROM | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duif et al. | II | 2 arms | 24(21) | 34(32) | 12 months | no injection | ACP (Arthrex) | LP | N/R | 1 | VAS IKDC Lysholm SF-36 |
[23] |
| Kim et al. (2015) | III | 2 arms | MCS + PRP 71(20) | 94(20) | 24 months | MSC + fibrin glue | Process Protocol | N/R | 1.28 × 106/µL | 1 | IKDC Tegner ICRS |
[28] |
| Lee et al. | I | 2 arms | 24(24) | 25(25) | 24 months | microfracture | Magellan APS (MBTD) | N/R | N/R | 1 | VAS IKDC Lysholm |
[24] |
| Manunta et al. | II | 2 arms | 10 | 10 | 12 months | microfracture | GPS II (Biomet) | N/R | N/R | 3 | VAS IKDC |
[25] |
| Manco et al. | III | 2 arms | 14 | 13 | 24 months | microfracture | Manual | N/R | 0.3–1.5 × 106 | 1 | VAS IKDC SF-36 |
[27] |
| Nguyen et al. | III | 2 arms | 15(15) | 15(15) | 18 months | microfracture | New-PRP Pro Kit (GeneWorld) | N/R | N/R | 1 | WOMAC VAS Lysholm Outerbridge |
[26] |
| Vasavilbaso et al. | I | 5 arms | 10(10) | control 10(10) HA 3 10(10) HA 4 10(10) HA 5 10(10) |
18 months | no injection HA |
GPS II (Biomet) | N/R | N/R | 1 | WOMAC | [22] |
LOE—level of evidence; exp.—no. of patients receiving treatment in experimental group (no. of patients analyzed at final follow-up); cont.—no. of patients receiving treatment in control group (no. of patients analyzed at final follow-up); LR—leukocyte rich; LP—leukocyte poor; ROM—range of movement; BL—blood loss; wound—wound healing; CRP—C reactive protein; HgB—hemoglobin; Ht—hematocrit; PROM—patient related outcome measures; VAS—visual analog scale; WOMAC—Western Ontario and McMaster Universities Osteoarthritis Index; SF-36—36-Item Short Form Survey; Outerbridge—Outerbrige cartilage injury scale; Lysholm—Lysholm Knee Scoring Scale; IKDC—International Knee Documentation Committee.
Table 6.
Platelet-rich plasma (PRP) compared with control intervention as adjunct treatment for ACL reconstruction.
| LOE | Type of Study | Exp | Cont | Follow-up | Control | Preparation Kit | LR/LP | Platelet Concentration | Number of Injections | PROM | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Almeida et al. | I | 2 arms | 12(10) | 15(12) | 6 months | no injection | 995-E (Haemonetics Corp, Braintree) | 0.91/mm3± 0.81/mm3 | 1,185,166/mm3± 404,472/mm3 | 1 | Kujala VAS IKDC Lysholm Tegner |
[32] |
| Azcarate et al. | II | 3 arms | 50(50) PG 50(50) Endoret |
50(50) | 12 months | no injection | Beckman J-6B BTI System II |
LP/LR | 837 × 106/mL 504 × 106/mL |
1 | CRP VAS KT-1000 IKDC MRI |
[34] |
| Cervellin et al. | I | 2 arms | 20(20) | 20(20) | 12 months | small blood sample | GPS II (Biomet) | LR | N/R | 1 | VAS VISA |
[29] |
| Mirzatolooei et al. | I | 2 arms | 25(23) | 25(23) | 3 months | no injection | ACP (Arthrex) | LP | N/R | 1 | CT tunnel widening VAS ROM KT-1000 |
[41] |
| Orrego et al. | II | 4 arms | PC 29(26) BP 29(28) PC + BP 29(27) |
29 (27) | 6 months | no injection | GPS II (Biomet) | LR | N/R | 1 | MRI graft maturation IKDC Lysholm |
[39] |
| Radice et al. | III | 2 arms | 25 | 25 | 1 year | no injection | GPS (Biomet) | N/R | N/R | 1 | graft integration MR | [33] |
| Rupreht et al. | II | 2 arms | 25(21) | 25(20) | 6 months | no injection | N/R | N/R | 978 × 103/ mm3 | 1 | tunnel healing MRI |
[37] |
| Sanchez et al. | III | 2 arms | 22(21) | 15 (15) | 24 months | no injection | BTI System II | LP | 2–3 × NPC | 1 | Histology—remodeling graft 2nd arthroscopy |
[40] |
| Seijas et al. (2013) | I | 2 arms | 49(48) | 49(48) | 12 months | no injection | BTI System | N/R | N/R | 1 | MRI graft remodeling |
[35] |
| Seijas et al. (2016) | I | 2 arms | 23 | 20 | 2 years | no injection | N/R | N/R | N/R | 1 | VAS | [31] |
| Silva et al. | I | 4 arms | 10 1xprp 10 3xprp 10 Clotalys |
10 | 3 months | no injection | GPS III (Biomet) | LR | N/R | 3 | graft integration MR | [36] |
| Starantzis et al. | II | 2 arms | 30(25) | 30(26) | 1 year | placebo sample | GPS III (Biomet) | LR | N/R | 1 | MRI CT tunnel diameter Lysholm KT-1000 |
[42] |
| Sözkesen et al. | III | 2 arms | 18 | 26 | 12 months | no injection | Prosys PRS bio kit (Prodizen) | N/R | N/R | 1 | IKDC Lysholm Tegner KT-1000 CT tunnel healing |
[43] |
| Vadala et al. | II | 2 arms | 20 | 20 | 10 months | no injection | PRP Fast Biotech kit (MyCells) | N/R | N/R | 1 | Tegner Lysholm IKDC KT-1000 CT tunnel enlargement |
[44] |
| Ventura et al. | I | 2 arms | 10(10) | 10(10) | 6 months | No injection | GPS (Biomet) | N/R | N/R | 1 | KOOS IKDC KT-1000 Tegner |
[38] |
| Walters et al. | II | 2 arms | 27(17) | 23(12) | 24 months | bone chips with no injection | ACP (Arthrex) | LP | 2–3 × NPC (<750,000 platelets/µL) | 1 | VAS VAS ADL IKDC |
[30] |
LOE—level of evidence; exp.—no. of patients receiving treatment in experimental group (no. of patients analyzed at final follow-up); cont.—no. of patients receiving treatment in control group (no. of patients analyzed at final follow-up); LR—leukocyte rich; LP—leukocyte poor; ROM—range of movement; CRP—C reactive protein; MRI—magnetic resonance imaging; CT—computer tomography; PROM—patient related outcome measures; VAS—visual analog scale; WOMAC—Western Ontario and McMaster Universities Osteoarthritis Index; Tegner—Tegner Activity Score; Lysholm—Lysholm Knee Scoring Scale; IKDC—International Knee Documentation Committee.
Table 7.
Platelet-rich plasma (PRP) compared with control intervention as adjunct treatment for meniscus repair.
| LOE | Type of Study | Exp | Cont | Follow-up | Control | Preparation Kit | LR/LP | Platelet Concentration | Number of Inj. | PROM | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dai et al. | III | 2 arms | 14(13) | 15(13) | 1 year | no injection | N/R | LR | 6.4 ± 1.6 ×NPC | 1 | Lysholm Ikeushi VAS Failure |
[53] |
| Everhart et al. | III | 3 arms | 203(164) 148 55 |
347(294) | 3 years | no injection | GPS III (Biomet)/ Angel (Arthrex) |
LR | 1343 ± 670 k/µL 2064 ± 526 k/µL |
1 | Failure | [52] |
| Griffin et al. | III | 2 arms | 15(11) | 20(15) | 2 years | no injection | Cascade Platelet Rich Fibrin Matrix | N/R | N/R | 1 | IKDC Tegner Lysholm ROM Failure |
[50] |
| Kemmochi et al. | II | 2 arms | 17 | 5 | 6 months | no injection | N/R | LR 3.6 × NPC (2.0–7.3) |
5.5 × NPC (3.4–9.1) | 1 | Tegner Lysholm IKDC |
[49] |
| Kamiński et al., 2018 | I | 2 arms | 19(18) | 18(17) | 45 months | saline | N/R | LR | N/R | 1 | VAS KOOS WOMAC IKDC Failure |
[47] |
| Kamiński et al., 2019 | I | 2 arms | 42(40) | 30(29) | 54 months | trephination | N/R | LR | 823 (320–1659) × 103/µL | 1 | VAS KOOS IKDC WOMAC Failure |
[48] |
| Pujol et al. | III | 2 arms | 17(16) | 17(15) | 2 years | no injection | GPS III (Biomet) | N/R | N/R | 1 | KOOS IKDC ROM Failure |
[51] |
LOE—level of evidence; exp.—no. of patients received treatment in experimental group (no. of patients analyzed at final follow-up); cont.—no. of patients receiving treatment in control group (no. of patients analyzed at final follow-up); LR—leukocyte rich; LP—leukocyte poor; ROM—range of movement; PROM—patient related outcome measures; VAS—visual analog scale; WOMAC—Western Ontario and McMaster Universities Osteoarthritis Index; Tegner—Teger Activity Score; Lysholm—Lysholm Knee Scoring Scale; KOOS—Knee injury and Osteoarthritis Outcome Score; Ikeushi—The knee rating scale of Ikeuchi; IKDC—International Knee Documentation Committee.
Table 8.
Platelet-rich plasma (PRP) compared with control intervention as adjunct treatment for osteoarthritis (blinded RCTs).
| LOE | Type of Study | Exp. | Cont. | Follow-up | Control | Preparation Kit | LR/LP | Platelet Concentration | Number of inj. | PROM | K-L | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahmad et al. | I | 2 arms | PRP 45(45) | 45(44) | 6 months | HA | N/R | LR | N/R | 3 | VAS IKDC USG |
1–3 | [66] |
| Bastos et al. (2018) | II | 2 arms | PRP+MSC 9(9) |
MSC 9(9) | 12 months | MSC | N/R | N/R | 106/μL | 1 | KOOS ROM CFU-F |
1–4 | [81] |
| Bastos et al. (2019) | II | 3 arms | MSCs 16(15) MSCs + PRP 14(14) |
17(16) | 12 months | CS | FalconTM | LP | 106/µL | 1 | KOOS ROM |
1–4 | [74] |
| Buendia-Lopez et al. | II | 3 arms | PRP 35(33) | HA 36(32) NSAIDs 35(33) |
52 weeks | HA NSAIDs |
N/R | LP | 1,095,000 ± 23,200/mm3 | 1 | WOMAC VAS X-ray MRI |
1–2 | [69] |
| Cole et al. | I | 2 arms | PRP 52(49) | 59(50) | 52 weeks | HA | ACP | LP | 1.73 ± 0.053 xNPC | 3 | WOMAC IKDC VAS Lysholm |
1–3 | [63] |
| Duymus et al. | I | 3 arms | PRP 39(33) | HA 39(34) Ozone 39(35) |
12 months | HA Ozone |
Ycellbio kit | LP | >1,500,000/µL | 2 | WOMAc VAS |
2–3 | [62] |
| Elik et al. | I | 2 arms | PRP 30(30) | 30(27) | 6 months | saline | Revmed, VERSUS-5000 i2 | LR | N/R | 3 | VAS WOMAC SF-36 USG |
1–3 | [57] |
| Filardo et al. (2012) | II | 2 arms | PRP 54 | 55 | 12 months | HA | N/R | LR | 5 × NPC | 3 | IKDC EQ-VAS Tegner KOOS ROM |
1–3 | [78] |
| Filardo et al. (2015) | I | 2 arms | PRP 96(94) | 93(89) | 12 months | HA | N/R | LR 1.1 ± 0.5 × NLC |
4.6 ± 1.4 x NPC | 3 | IKDC KOOS EQ-VAS Tegner ROM |
0–3 | [60] |
| Görmeli et al. | I | 4 arms | PRP 3 44(39) PRP 1 45(44) |
HA 44(39) Saline 43(40) |
6 months | HA saline |
N/R | N/R | 5.2 × (1,118,000 µL) 5.3 × (1,152,000 µL) |
3 1 |
EQ-VAS IKDC |
1–3 or 4 | [54] |
| Jubert et al. | II | 2 arms | PRP 35(34) | 30(30) | 6 months | CS | N/R | LP | 0.99 × 106/µL (0.34–1.54 × 106/ µL) | 1 | VAS KOOS SF-36 |
3–4 | [58] |
| Kavadar et al. | I | 3 arms | PRP1 34(33) PRP2 34(32) PRP3 34(33) |
-- | 6 months | - | N/R | LR | 4–5 × NPC | 1 2 3 |
VAS WOMAC TUG |
3 | [80] |
| Kon et al. | II | 2 arms | APS 31(29) | 15(15) | 12 months | saline | nSTRIDE APS Kit (Biomet) | LR | N/R | 1 | VAS WOMAC KOOS SF-36 CGI-S/C PGI-S/C OMERACT –OARSI MRI RTG MRI |
2 or 3 | [56] |
| Lana et al. | I | 3 arms | PRP 36(36)HA + PRP 33(33) | 36(36) | 12 months | HA | N/R | LR | 800,000–1,600,000/mm3 | 3 | WOMAC, VAS |
1–3 | [79] |
| Lin et al. | I | 3 arm | PRP 31(31) | HA 29(29) S 27(27) |
12 months | HA Saline |
RegenKit-THT | LP | 1.81 ± 0.34 × NPC | 3 | WOMAC IKDC |
Ahlbäck 1–3 |
[89] |
| Lisi et al. | I | 2 arm | PRP 28(25) | 22(22) | 12 months | HA | N/R | N/R | N/R | 3 | WOMAC Lysholm Tegner AKSS Lequesne VAS ROM |
2–3 | [68] |
| Louis et al. | II | 2 arms | PRP 26(17) | 28(17) | 6 months | Durolane, HA | MultifugeHeraus R | LP | 800 ± 276 × 109/L | 1 | WOMAC VAS RTG ROM |
2–4 | [65] |
| Di Martino et al. | I | 2 arms | PRP 96(85) | 93(82) | 24 months | HA | N/R | 1.1 ± 0.5 × NLC | 4.6 ±1.4 × NPC | 3 | IKDC EQVAS Tegner |
1–3 | [70] |
| Montañez-Heredia et al. | I | 2 arms | PRP 28(27) | 27(26) | 6 months | HA | N/R | LP | 952 × 109/L | 1 | VAS KOOS EQoL |
1–3 | [76] |
| Patel et al. | I | 3 arms | PRP1 27(26) PRP2 25(25) |
23(23) | 6 months | saline | N/R | LP | 310.14 × 103/µL | 1 2 |
WOMAC VAS |
Ahlbäck 1–2 |
[86] |
| Paterson et al. | I | 2 arms | PRP 11(10) | 10(9) | 12 weeks | HA | Premiere XC-2000 |
LR | N/R | 3 | VAS KOOS KQoL Functional tests |
2–3 | [61] |
| Raeissadat et al. (2017) | II | 2 arms | PRGF-Endoret 41(36) | 36(33) | 6 months | HA | Rooyagen Kit |
LR | 4.6 ± 0.7 × NPC | 2 | WOMAC Lequesne VAS |
2–3 | [71] |
| Rahimzadeh et al. | I | 2 arms | PRP 21(21) | 21(21) | 6 months | PRL (dextrose) | Standard kit, Iran | N/R | N/R | 2 | WOMAC | 1–2 | [73] |
| Sánchez et al. | I | 2 arms | PRGF-Endoret 89(79) | 87(74) | 6 months | HA | BTI Biotechnology Institute system |
LP | N/R | 3 | WOMAC Lequesne OMERACT–OARSI |
Ahlbäck 1–3 |
[90] |
| Simental- Mendía et al. (2016) | I | 2 arms | PRP 33(33) | 32(32) | 24 weeks | acetaminophen | N/R | LP | 513.25 ± 189.3 K/µL | 3 | VAS WOMAC, SF-12 |
1 or 2 | [55] |
| Simental-Mendía (2019) | I | 2 arms | 1 prp 18 3 prp 17 |
- | 48 weeks | - | NR | LP | 99.3 ± 162.0 × 106/μL | 1 3 |
VAS WOMAC SF-12 |
1–2 | [85] |
| Smith et al. | I | 2 arms | ACP 15(15) | 15(15) | 1 year | saline | Hettich ROTOFIX 32 A; Arthrex | LP | N/R | 3 | WOMAC | 2–3 | [72] |
| Su et al. | I | 3 arms | io 28(27) ia 26(25) |
32(30) | 18 months | HA | N/R | LR 29.92 ± 1.54 × 109/L. |
789.68 ± 17.80 × 109/L | 2 | VAS WOMAC |
2–3 | [67] |
| Tavassoli et al. | II | 3 arms | PRP 1 31(28) PRP 2 33(28) |
31(27) | 12 weeks | HA | Rooyagen kit | LR | N/R | 1 2 |
WOMAC VAS |
Ahlbäck 1–4 |
[87] |
| Uslu-Guvendi et al. | II | 3 arms | PRP 1 19(19) PRP3 19(14) |
19(17) | 6 months | CS | N/R | 8.67 109/L | 875 109/L | 1 3 |
VNS WOMAC Lequesne |
3 | [59] |
| Vaquerizo et al. | I | 2 arms | 48(48) PRGF-Endoret |
48(42) | 48 weeks | Durolane HA | BTI Biotechnology Institute system |
LP | N/R | 3 | WOMAC Lequesne OMERACT–OARSI |
2–4 | [75] |
| Wu et al. | I | 2 arms | 20(20) | 20(20) | 6 months | saline | RegenKit-THT-1, Regen Lab | LR | N/R | 1 | WOMAC Isokineticfunction |
Ahlbäck 1–2 |
[88] |
| Yu et al. | II | 4 arms | PRP 104 PRP + HA 96 |
HA 88 saline 72 |
1 year | HA saline |
N/R | N/R | N/R | 1 | WOMAC Kanofsky |
- | [91] |
LOE—level of evidence; exp.—no. of patients receiving treatment in experimental group (no. of patients analyzed at final follow-up); cont.—no. of patients receiving treatment in control group (no. of patients analyzed at final follow-up); LR—leukocyte rich; LP—leukocyte poor; ROM—range of movement; PROM—patient related outcome measures; MRI—magnetic resonance imaging; VAS—visual analog scale; WOMAC—Western Ontario and McMaster Universities Osteoarthritis Index; TUG—tug lesion; Tegner—Teger Activity Score; SF-36—36-Item Short Form Survey; SF-12—12-Item Short Form Survey; PGI-S/C—Patient Global Impression of Severity Scale; OMERACT–OARSI—OMERACT–OARSI osteoarthritis pain measure; Lysholm—Lysholm Knee Scoring Scale; Lequesne—Lequesne index of severity for osteoarthritis; KQol—knee-related quality of life; KOOS—Knee injury and Osteoarthritis Outcome Score; K-L—Kellgren–Lawrence scale; Karnofsky—Karnofsky Performance Status Scale; EQ-VAS—EuroQol Visual analogue scale; Eqol—EuroQol quality of life scale; CGI-S/C—The Clinical Global Impressions Scale; CFU—colony forming unit; AKSS—American Knee Society Score.
Table 9.
Platelet-rich plasma (PRP) compared with control intervention as adjunct treatment for osteoarthritis (non-blinded RCTs).
| LOE | Type of Study | Exp | Cont | Follow-up | Control | Preparation Kit | LR/LP | Plateletconcentration | Number of Inj. | PROM | K-L | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cerza et al. | I | 2 arms | ACP 60(60) | 60(60) | 24 weeks | HA | ACP (Arthrex) | LP | N/R | 4 | WOMAC | 1–3 | [84] |
| Huang et al. | I | 3 arms | 40(40) | HA 40(40) CS 40(40) |
12 months | HA CS |
N/R | LP | N/R | 4 | WOMAC VAS |
1–2 | [77] |
| Raeissadat et al. (2015) | II | 2 arms | PRP 87(77) | 73(62) | 12 months | HA | Rooyagen Kit | LR | 4.8 ± 1.80 × NPC | 2 | WOMAC SF-36 |
1–4 | [82] |
| Rayegani et al. | I | 2 arms | 32(31) | 33(31) | 6 months | acetaminophen | Rooyagen kit | LR | 5.6 × NPC | 2 | WOMAC SF-36 |
1–4 | [64] |
| Spakova et al. | II | 2 arms | PRP 60 | 60 | 6 months | HA | Labofuge 400R, Heraeus | LR 6.4 ± 2.3 × 103/µL |
680 ± 132 × 106/mL | 3 | WOMAC NRS-11 |
1–3 | [83] |
LOE—level of evidence; exp.—no. of patients receiving treatment in experimental group (no. of patients analyzed at final follow-up); cont.—no. of patients receiving treatment in control group (no. of patients analyzed at final follow-up); LR—leukocyte rich; LP—leukocyte poor; ROM—range of movement; PROM—patient related outcome measures; MRI—magnetic resonance imaging; VAS—visual analog scale; WOMAC—Western Ontario and McMaster Universities Osteoarthritis Index; SF-36—36-Item Short Form Survey; NRS-11—numerical rating scale.
One injection of platelet-rich plasma was performed in 55 studies, two injections in 14 studies, three injections in 21 studies and four injections in two studies. Platelet concentration was provided in 48 articles, 33 studies used leukocyte-rich PRP, 25 studies used leukocyte-poor PRP, and in 25 studies no information was provided.
In addition, 41 studies compared the application of PRP versus other treatments (25 versus hyaluronic acid (HA), 4 versus corticosteroids, 4 versus microfractures, 10 versus other substances), 42 studies compared the use of PRP versus placebo (12 versus saline and 30 versus no injection), and 7 studies compared single injection of PRP versus multiple injections. Primary outcomes included pain measurement (visual analog scale (VAS)) in 48 studies and functional outcomes in 73 studies: International Knee Documentation Committee (IKDC), 24 studies; Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), 32; Victorian Institute of Sport Assessment for patella tendonitis (VISA-P), 5; 36-Item Short Form Survey (SF-36), 7; Knee injury and Osteoarthritis Outcome Score (KOOS), 12; The Lysholm Knee Scoring Scale, 14; Teger Activity Score, 10; Lequesne score, 6; and others (meniscal repair failure, 6; time for return to sport (RTS), 4; re-injury, 3; knee stability, 6; graft integration, 5; tunnel widening, 4; hemoglobin drop, 6; range of movement (ROM), 9). Radiographic outcomes were presented in 15 studies (computed tomography, X-ray, magnetic resonance imaging, ultrasonography).
A total of 75 studies was included into quantitative synthesis: VAS was analyzed in 5 subgroups, IKDC, 5; WOMAC, 3; Tegner, 1; KOOS (activities of daily living (ADL), 1; pain, 1; quality of life (QoL), 1; sport, 1; symptoms, 1); VISA-P, 1; SF-36, 1; graft integration, 1; tunnel widening, 1; re-injury rate, 1; RTS, 1; repair failure, 1; blood loss, 1; KT-1000 (knee arthrometer), 1; adverse events, 1.
2.3. Patellar Tendinitis (PT)
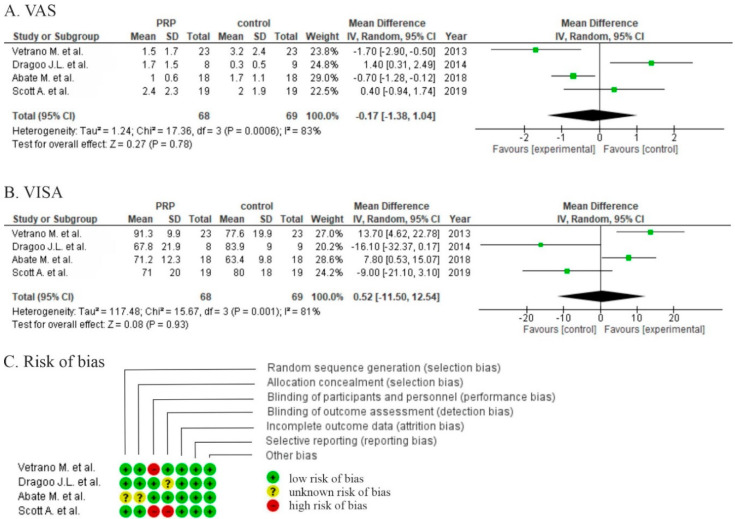
Four studies reported data from 137 patients. Inclusion criteria required randomization, control groups, use of VAS for pain as well as VISA-P with a minimum of 6 months follow-up. We included RCTs comparing the use of PRP in patellar tendinopathy versus saline, dry needling (DN) or extracorporeal shockwave therapy (ESWT) (Table 1).
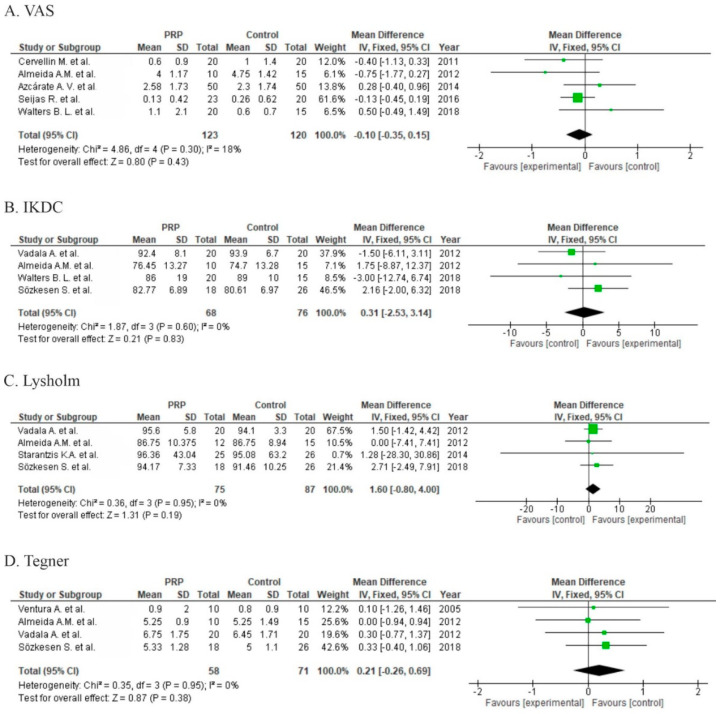
Two studies showed non-significant differences in favor of PRP (p > 0.05) in VAS comparing PRP with saline injection after 1 year [6] or DN after 6 months [7]. Two studies also reported pain scales (VAS) with significant differences at, respectively, 1 year compared to ESWT (p = 0.009) [8] and 6 months compared to high volume image guided injections (HVIGIs) [9]. The pooled estimate for these 4 studies demonstrated non-significant differences in favor of PRP (p = 0.80) (Figure 2A).
Figure 2.
Forest plot for (A) visual analog scale (VAS) scores and (B) Victorian Institute of Sport Assessment (VISA) (CI: confidence interval; IV: inverse variance; SD: standard deviation.). (C) Risk of bias analysis.
The same authors measured the severity of jumper’s knee via VISA-P score. Two studies [6,7] proved no differences in symptom severity after 6 months and 1 year with statistical significance greater than 0.05. Another study showed significant differences between groups of PRP injection and ESWT (p = 0.026) after 1 year [8] and significant differences as compared to HVIGI (p = 0.03) [9]. Pooled data estimated for these studies demonstrated non-significant differences in favor of PRP (p = 0.93) (Figure 2B).
Functional outcomes with Tegner, Lysholm, and SF-12 scores were analyzed in one study. Dry needling showed significant improvement at >26 weeks when compared to PRP group (p = 0.006) [7]. In another study, a modified Blazina scale showed significant improvement at 12 months in favor of the PRP group (p = 0.015) [8].
Two studies were at high risk of bias for one or more domains [6,8], and two studies were at an unclear risk of bias for one or more domains (Figure 2C). Moderate risk of performance bias was identified in two studies [6,8]. Similarly, two were at risk of detection bias [6,7]. No data concerning the generation of random sequence and allocation were provided thus increasing risk of selection bias [9].
2.4. Muscle Injuries around the Knee
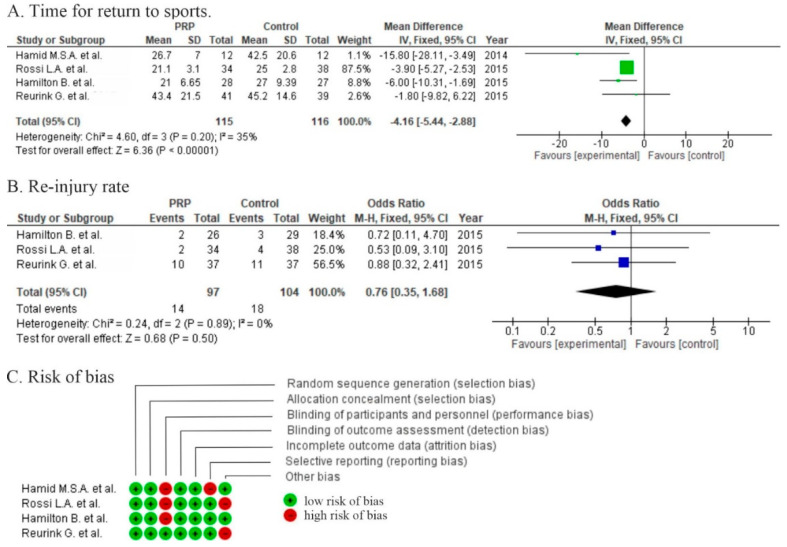
Four studies including 224 patients measured time for return to sport after a muscle injury (hamstring, quadriceps, gastrocnemius). In all reported studies PRP was delivered intralesionally. Two studies performed the injection under the guidance of ultrasound [10,11] and the other two used magnetic resonance imaging (MRI) prior to the injection to detect the damaged area [12,13]. Three studies reported re-injury incidences, and only two provided patient reported outcome measures (pain). Each study compared rehabilitation programs with/without PRP injection. All reported shorter time for return to sport in favor of PRP in comparison to control groups (Table 2). One study included only professional athletes [12] and three studies recruited both competitive and recreational athletes [10,11,13].
The mean time for return to sport ranged from 21 to 43 days in the PRP group and from 25 to 45 days in the control groups. Two studies [10,11] showed significant differences in RTS (p = 0.001; p = 0.02) and two studies [12,13] showed shorter RTS, but no significant differences between PRP and control groups (p > 0.05). The pooled estimate for these 4 studies demonstrated significant differences in favor of PRP (p ≤ 0.00001) with a mean difference of −4.16 (−5.44, −2.88) (Figure 3A). Due to the high heterogeneity of patient recruitment and only small differences in the time to return to sport, an analysis of cost-effectiveness should be accomplished to evaluate whether the results are worth the cost.
Figure 3.
Forest plot for (A) time for return to sport (RTS) and (B) re-injury rate (CI: confidence interval; IV: inverse variance; SD: standard deviation). (C) Risk of bias analysis.
The re-injury rate ranged from 6% to 27% in the PRP group and from 10% to 31% in the control groups. Three studies [10,12,13] reported lower re-injury rate in favor of the PRP group but with non-significant differences (p = 0.47) (Figure 3B).
Two studies [10,11] showed significantly lower pain severity (beta regression coefficient = −0.272, 95% confidence interval (CI) (−0.5, −0.045), p = 0.019 during motion and −0.390, 95% CI (−0.67, −0.11), p = 0.007, respectively) but non-significant differences in pain intensity (p = 0.157) [13].
Two studies were at high risk of bias for two domains, and two studies were at high risk for one domain (Figure 3C). Moderate risk of performance bias was identified in three studies [10,11,12]. One was at risk of reporting bias [11]. Discrepancies between the number of patients undergoing final follow up in Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) chart versus manuscript was detected in two studies [10,13].
2.5. High Tibial Osteotomy (HTO)
Two RCTs including 80 patients evaluated the intraoperative use of PRP as an adjunct to HTO with or without the addition of other myeloid stromal cells [14,15] (Table 3).
Koh et al. injected PRP into the medial joint space under arthroscopic visualization and afterwards performed HTO. This study showed significant differences in KOOS and VAS in favor of PRP with the addition of Mesenchymal Stem Cells (MSC) in a 2-year follow up (p < 0.05). Second-look arthroscopy during plate removal reported a significant difference between the groups with respect to cartilage healing again in the PRP + MSC groups (p = 0.023) [14].
Dallari et al. added lyophilized bone chips with platelet gel and with/without bone marrow (BM) to the osteotomy hole. This study showed better osseointegration in X-ray analysis after 1-year follow up and histologically more active osteogenic processes in favor of PRP+/−BM groups (p < 0.05) [15].
Both studies were at high risk of bias for one domain (performance bias).
2.6. Total Knee Arthroplasty (TKA)
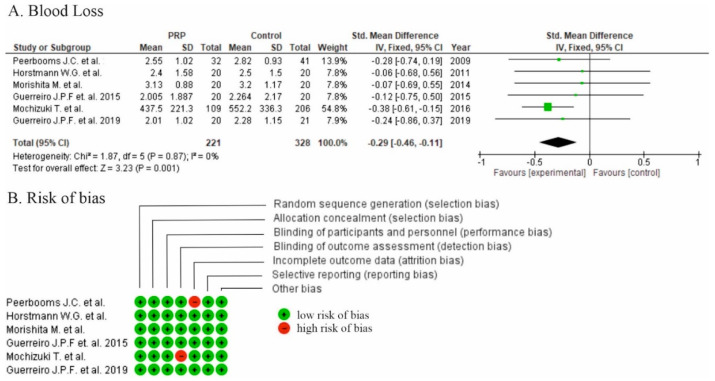
Six RCT studies including 621 patients evaluated the use of PRP as an adjunct to TKA (Table 4). All studies applied PRP intraoperatively: three sprayed platelet gel onto the exposed surface of the wound [16,17,18] and the other three injected PRP into the joint [19,20,21]. The aim of all these studies was to assess potential blood loss during the procedure after TKA.
Lower hemoglobin drop was reported in all six studies [16,17,18,19,20,21] with significant differences and two studies reported lower calculated blood loss in the PRP group (p > 0.05; p < 0.001) [17,21]. There were significant differences in favor of PRP in comparison to the control groups for the overall effect on blood parameters (standardized mean difference −0.29, 95% CI (−0.46, −0.11), p = 0.001) for the pooled estimates for all six studies (Figure 4A).
Figure 4.
(A) Forest plot of time for blood loss analysis after total knee arthroplasty (TKA) (CI: confidence interval; IV: inverse variance; SD: standard deviation). (B) Risk of bias analysis.
Four studies reported better pain control in the PRP group (VAS) for a short time period after surgery [16,18,19,20]. No effect was observed in long-term follow-up.
Functional outcome was measured using the WOMAC [18,19,20] score in three studies and Knee Society Score (KSS) and KOOS in another study [17], but with non-significant differences between the groups. Range of motion was measured in all studies with non-significant differences between the groups [16,17,18,19,20,21]. Thromboembolism was absent in all studies. However, Morishita et al. reported one patient requiring a secondary skin suture in the PRP group [17]. Peerbooms et al. and Guereirro et al. reported superficial wound infection in one and two patients, respectively, all treated successfully with antibiotics [18,19]. A subsequent study performed by Guerreiro reported two cases of deep infection treated by debridement and TKA review [20].
Two studies were at high risk of bias for one domain (Figure 4B). Low risk of performance bias was identified in four studies [16,17,19,20]. A moderate risk of performance bias was identified in two studies [18,21] (Figure 4B).
2.7. Arthroscopy
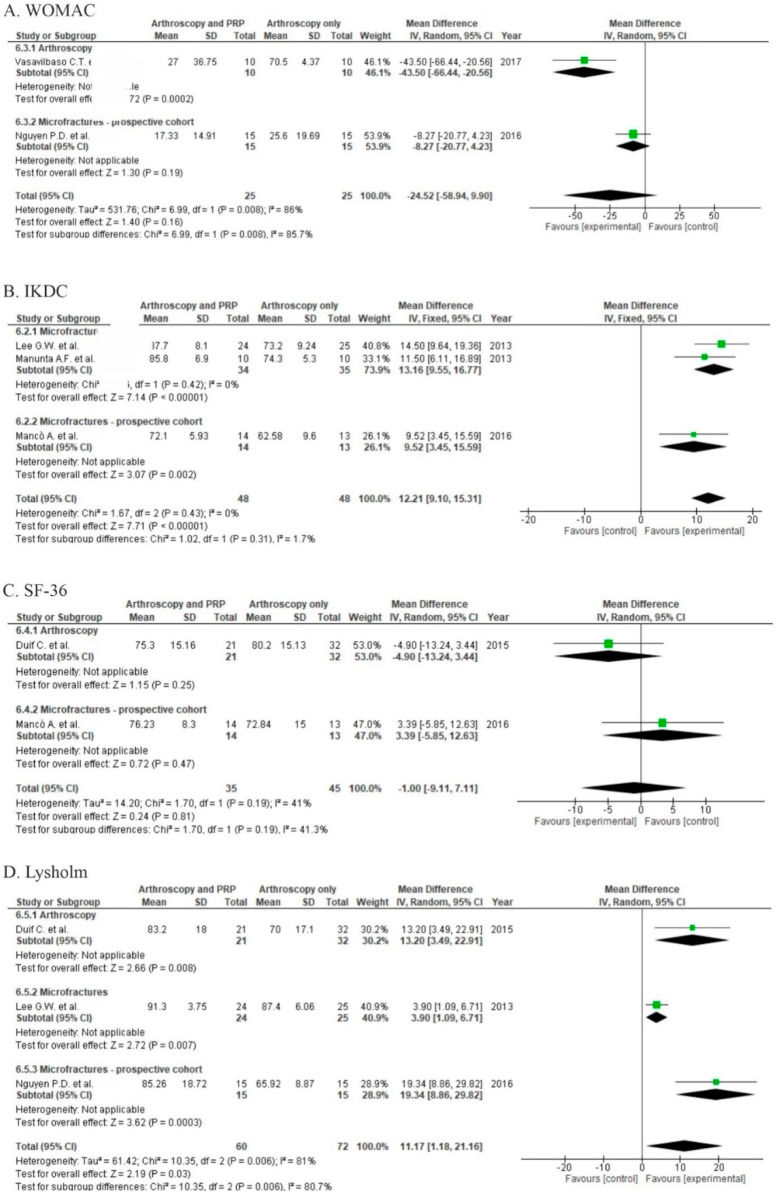
Four RCTs [22,23,24,25,26,27] and three prospective cohort [26,27,28] studies, including 199 patients, evaluated the use of PRP as an adjunct to knee arthroscopy treatment for cartilage and/or meniscal pathology: two studies included patients with osteoarthritis (OA) changes according to the Kellgren and Lawrence (KL) classification system with concomitant meniscal lesions [22,23]; two studies included patients with cartilage lesions of grade III–IV according to the Outerbridge classification system and early OA stages I–II according to the KL classification system [24,27]; one study included patients with chondral defects of medial femoral condyle grade II–III according to the Outerbridge classification system [25]; and one study included patients with OA stage II–III according to the KL classification system [26]. In five studies [23,24,26,27,28] PRP was used intraoperatively, and in another two studies PRP was used after surgery [22,25]. Kim et al. was excluded from the meta-analysis. The study analyzed PRP effectiveness when applied as an adjuvant to injection of MSC versus surgical implantation of MSCs [28].
Two studies reported functional outcome in the WOMAC score [22,26]. One of them (RCT) showed significant differences in WOMAC scores (p = 0.0002) when comparing PRP to a control group at 18 months and reported hyaluronic acid injections to be more effective than PRP [22]. The pooled estimate for these two studies showed significant differences in favor of the PRP group (p = 0.0040, Figure 5A). Four studies [24,25,27,28] reporting outcomes measured in IKDC (Figure 5B), showed significant differences in favor of PRP (p < 0.00001). In subgroup analysis, two RCTs presented significant differences in favor of PRP when applied with microfractures [24,25]. Additionally, one prospective cohort trial also showed significant differences in favor of PRP [27]. Another two studies [23,27] showed better outcomes in patient self-assessment SF-36 scale, one of them in favor of the control [27] and the other in favor of the PRP group [23]; but differences were not significant (p = 0.81, Figure 5C). Functional outcome was also measured by the Lysholm score by three studies [23,24,26] (Figure 5D), showing non-significant differences in favor of PRP (p = 0.03).
Figure 5.
Forest plot for (A) Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), (B) International Knee Documentation Committee (IKDC), (C) 36-Item Short Form Survey (SF-36), and (D) Lysholm scores (D) (CI: confidence interval; IV: inverse variance; SD: standard deviation).
Three randomized studies [23,24,25] and two prospective cohort studies [26,27] used VAS to assess pain level. Two studies [24,25] with the addition of PRP to arthroscopic microfractures showed significant differences in pain severity in favor of PRP (p < 0.0001); although two cohort studies report non-significant differences in favor of PRP (p = 0.81). Arthroscopy without microfractures showed lower pain levels when complemented with PRP, but the differences were not significant (p = 0.07) [23]. The pooled estimate for these five studies demonstrated non-significant differences in favor of the PRP group (p = 0.13) (Figure 6A). Due to the large variety of patient recruitment regimens, any conclusions should be stated carefully. However, all subgroups showed positive effects of PRP during synthesis. There is a need for more RCTs to allow for definitive conclusions with low heterogeneity.
Figure 6.
(A) Forest plot pain intensity for VAS (CI: confidence interval; IV: inverse variance; SD: standard deviation). (B) Risk of bias analysis.
Two studies were at high risk of bias for four domains [26,27] and three studies were at high risk of bias for one domain [23,25,28]. High risk of performance bias was identified in two studies [26,27], moderate risk of performance bias was identified in three studies [23,25,28], and a low risk of performance bias was identified in two studies [22,24] (Figure 6B).
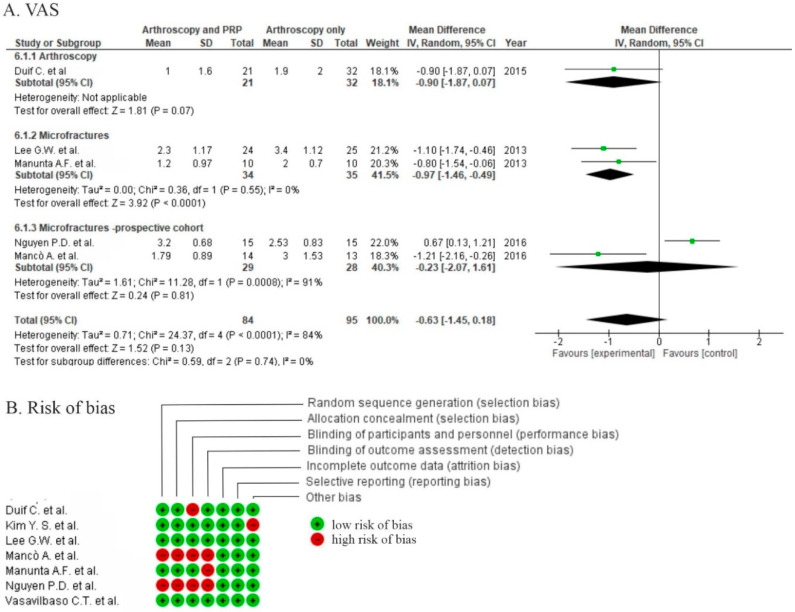
2.8. Anterior Cruciate Ligament Reconstruction (ACL)
Sixteen RCTs, including 740 patients, evaluated the use of PRP as an adjunct to ACL reconstruction with patellar ligament (Bone-Patella Tendon-Bone-BPTB) autograft [29,30,31,32,33,34,35] or hamstrings graft [33,36,37,38,39,40,41,42,43,44] (Table 6).
Five studies reported pain assessment with the VAS [29,30,31,32,34]. The overall effect showed no significant differences with respect to pain (p = 0.43); however, two studies showed significant differences in short-term follow-up in favor of the PRP group (2–6 months) [31,32] (Figure 7A).
Figure 7.
Forest plot for (A) VAS, (B) IKDC, (C) Lysholm score, and (D) Tegner scores (CI: confidence interval; IV: inverse variance; SD: standard deviation).
Seven studies reported functional results via IKDC scores [30,32,34,38,39,43,44], but only four of them provided data allowing for synthesis [30,32,43,44] (Figure 7B) and no significant differences (p = 0.83) were detected. A further four studies provided only categorical output data (excellent, good, regular, poor) with non-significant odds ratio (1.39 (0.27, 7.21), p = 0.7). Functional outcome was measured by the Lysholm score in four studies and provided insignificant results (p = 0.19, Figure 7C). Five studies used the Tegner scale for activity assessment [38,42,43,44,45]. Pooled estimates for these studies showed no significant differences (p = 0.38) in favor of the control (Figure 7D). Three studies showed no significant differences [38,42,43] in functional outcomes, one study did not report functional outcome results [42], and one study reported worse outcomes in both groups when compared to baseline [32].
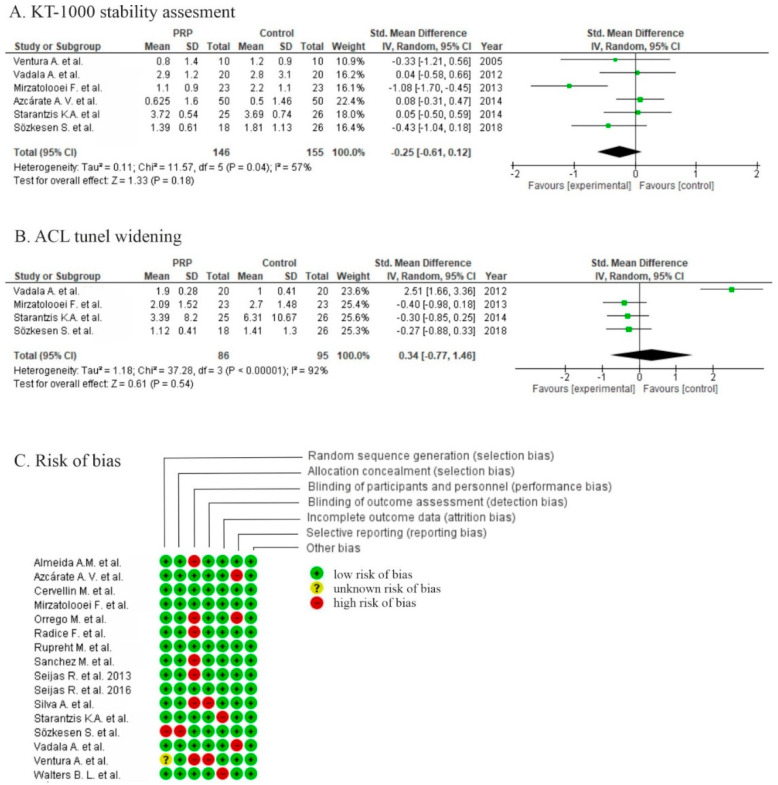
Six studies reported the outcomes of anterior tibial translation assessments [34,38,41,42,43,44] with no significant differences between groups (p = 0.18) in meta-analysis. Only one study showed a significant difference in favor of PRP using KT-1000 (Figure 8) [41].
Figure 8.
Forest plot for (A) KT-1000 (knee arthrometer) stability assessment and (B) tunnel widening (CI: confidence interval; IV: inverse variance; SD: standard deviation). (C) Risk of bias analysis.
Five studies reported the outcome of tunnel widening after graft fixation, two of them used computer tomography (CT) [43,44] and three used MRI [39,41,42] to evaluate tunnel enlargement. The pooled estimates for four studies included in meta-analyses showed non-significant differences in favor of the control (p = 0.54) (Figure 8).
Eight studies assessed the outcomes of ACL graft integration in the femoral or tibial tunnel. Six of them evaluated signal intensity of the graft on MRI [33,34,35,36,37,39], one reported significant difference in ACL density measured on CT (p < 0,01) [38], and one explored better remodeling using histologic parameters (p = 0.024) [40]. Three studies [33,39,40] reported faster graft remodeling (p < 0.001; p = 0,036; p = 0.024), and the remaining four [34,35,36,37] showed no significant differences during the final follow-up. We included in the meta-analysis four studies [35,36,39,40] and the pooled estimates for these studies showed non-significant differences in favor of PRP (p = 0.06).
Three studies were at high risk of bias for two domains [36,39,43], eight studies were at high risk of bias for one domain [30,32,33,34,35,40,42,44], and one study was at high risk of bias for two domains with a risk of reporting bias for one domain [38]. High risk of performance bias was identified in one study [38], a moderate risk of performance bias was identified in twelve studies [30,32,33,34,35,36,39,40,42,43,44,46], and a low risk of performance bias was identified in three studies [29,31,41] .
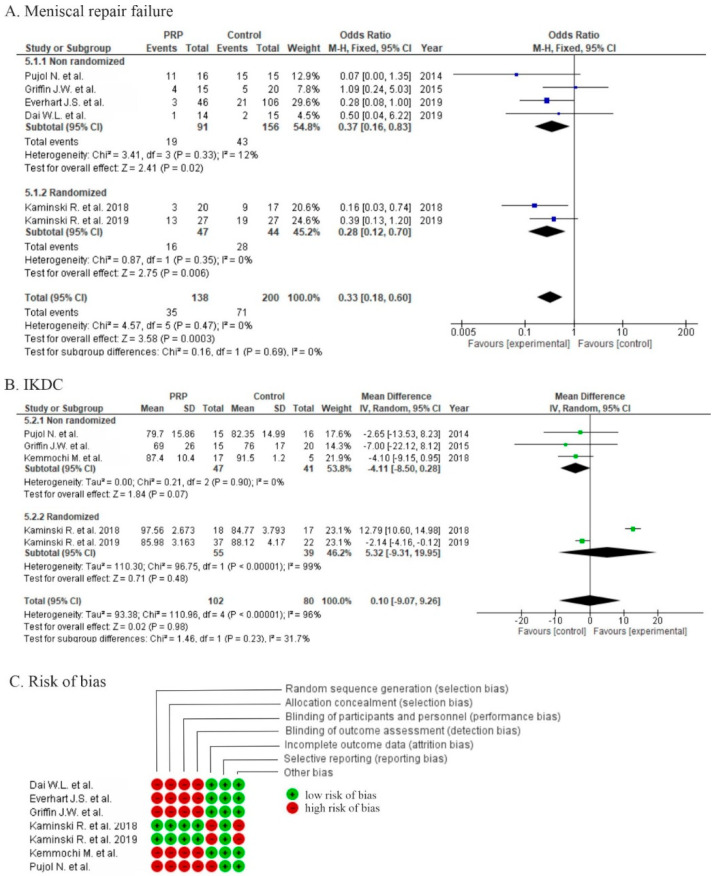
2.9. Meniscal Repair
Two RCTs [47,48] and five non-randomized studies [49,50,51,52,53] evaluated the use of PRP in meniscus healing. In five studies, PRP was injected after arthroscopic meniscus repair [47,49,50,52,53], in one study patients underwent open meniscal repair with an adjunct of PRP [51], and in another the authors compared percutaneous meniscal trephination with or without PRP [6].
Six studies reported failure rates of meniscus healing, two randomized studies using MRI and second-look arthroscopy showed significant differences in favor of PRP (p = 0.006) [47,48], and another four non-randomized studies also showed significant differences in favor of PRP (p = 0.02) [50,51,52,53]. In three studies the failure rate was defined by the need for revision surgery [50,52,53] and in the final study, MRI was used to assess meniscus healing [51]. One study did not provide any objective radiographic outcomes, only commenting “some” MRIs [49]. The pooled estimates for all six studies showed significant differences in favor of PRP (p = 0.0003), but due to the diversity of clinical trial types, synthesis provided only level of evidence III type data (retrospective cohort studies) with low heterogeneity (I2 12%) (Figure 9A). Only one study reported outcomes after meniscus repair with concomitant ACLR, and the authors concluded that PRP healing effect depended upon the ACLR [52].
Figure 9.
Forest plot for (A) meniscal repair failure and (B) IKDC (CI: confidence interval; IV: inverse variance; SD: standard deviation). (C) Risk of bias analysis.
Five studies [47,48,49,50,51] reported functional results via IKDC scores; the pooled estimates for these studies showed non-significant differences in favor of the control (p = 0.98), although two randomized trials [5,6] showed non-significant differences in favor of PRP (p = 0.48) (Figure 9B).
Functional outcome was also recorded by the Lysholm score in three studies [49,50,53], by the KOOS score in three studies [47,48,51], and by the Tegner score in two studies [49,50].
Unfortunately, there is a large variety of clinical trial designs in this section, which may introduce a higher percentage of heterogeneity. Additionally, there could be an increase in heterogeneity via the study of Kaminski et al. [47] as the final assessment was made by two different methods. There is a strong need for more RCTs allowing for the performance of meta-analysis with low heterogeneity.
One study was at high risk of bias for five domains [51], four studies were at high risk of bias for four domains [49,50,52,53], and two studies were at high risk of bias for two domains [47,48]. High risk of performance bias was identified in five studies [49,50,51,52,53] and moderate risk of performance bias was identified in two studies [47,48] (Figure 9C).
2.10. Osteoarthritis
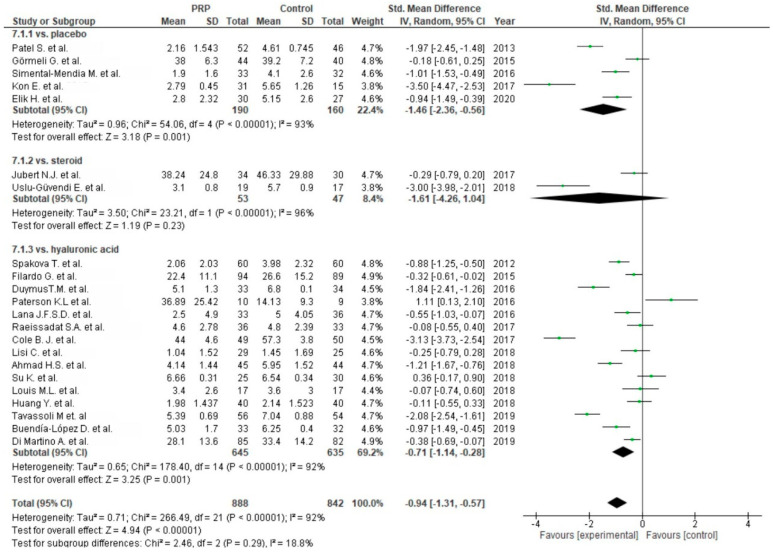
Thirty-eight studies, including 2962 patients, evaluated the use of PRP in osteoarthritis treatment. Thirty-three articles included patients with Kellgren–Laurence radiographic classifications system [54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85], five studies included patients with Ahlbäck radiographic classification system [86,87,88,89,90], and one study did not specify the osteoarthritis grade [91] (Table 8 and Table 9). Follow-up ranged from 6 months up to 2 years; thus, for such a large group as OA, heterogeneity will be too high due to our inability to compare outcomes at the same time point.
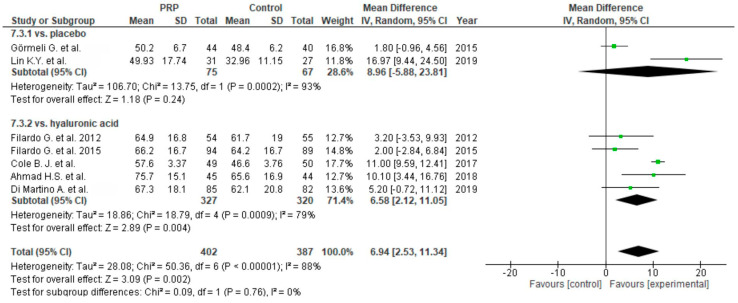
Twenty-eight studies compared PRP versus control groups [54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,73,76,77,78,79,82,83,86,87,88,89,91], five studies compared PRP with the addition of another substance (MSC or HA) versus the control groups [74,79,81,91], six studies compared multiple injections of PRP [54,59,80,85,86,87], three studies compared PRGF-Endoret versus control groups [71,75,90], two studies compared autologous conditioned plasma (ACP) versus control groups [72,84], and one study compared intraosseous injection versus intra-articular injection versus the control group [67]. In twenty-three studies, HA [54,60,61,62,63,65,66,67,68,69,70,71,76,77,78,79,82,83,84,87,90,91] was used as a control, in ten studies placebo was used as a control (saline, no injection, physical therapy) [54,56,57,73,79,80,85,86,88,91], in four studies corticosteroids [58,59,74,77] were used as the control, and in two studies acetaminophen [55,64] was used as the control.
Thirty-three trials were included in the meta-analysis and another five were excluded due to being non-blinded [64,77,82,83,84].
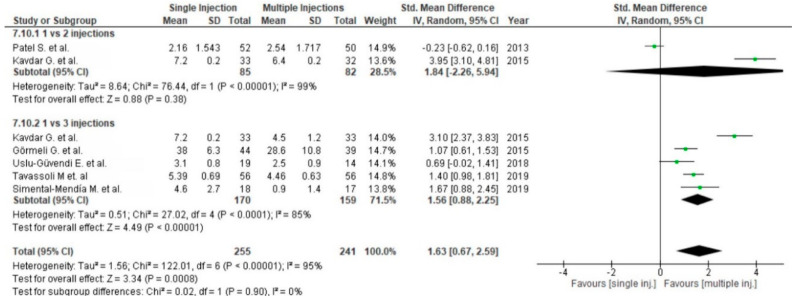
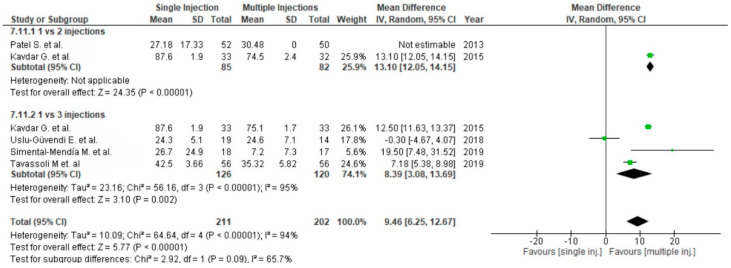
Twenty-three studies reported pain via the VAS comparing PRP versus placebo [54,55,56,57,69,86], corticosteroids [58,59,77] or HA [54,60,61,62,63,65,66,67,68,69,70,71,76,77,78,79,87] (Figure 10). Placebo and HA subgroups showed significant differences in favor of PRP (p < 0.00001), despite the steroid subgroup showing non-significant differences in favor of PRP (p = 0.23). The pooled estimates for these studies also showed significant differences in favor of PRP (p < 0.00001). Six studies comparing single versus multiple (two or three times) injections of PRP assessed significant differences in favor of multiple injections (p = 0.0008) (Figure 11). However, only three injections of PRP showed significant differences compared to a single injection (p < 0.00001).
Figure 10.
Forest plot for VAS comparing platelet-rich plasma (PRP) versus control (CI: confidence interval; IV: inverse variance; SD: standard deviation).
Figure 11.
Forest plot for VAS comparing single versus multiple injections of PRP (CI: confidence interval; IV: inverse variance; SD: standard deviation).
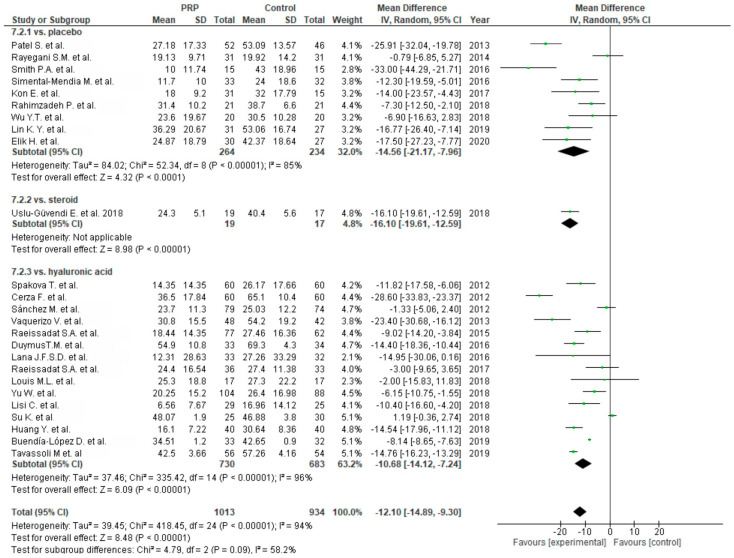
Functional outcome was measured in twenty-eight studies via the WOMAC scale. One study was excluded from meta-analysis due to the reporting of only WOMAC pain scores [63]. Twenty-five studies compared PRP versus control groups: placebo [55,56,57,64,69,72,73,86,88,89,91], corticosteroids [59,77] or HA [62,63,65,67,68,69,71,75,77,79,82,83,84,87,89,90,91] (Figure 12). The pooled estimates for these studies showed significant differences in favor of PRP (p < 0.00001); furthermore each subgroup showed significant differences in favor of PRP (p < 0.00001). Functional outcomes were also analyzed in five studies [59,80,85,86,87] comparing single versus multiple injections and showed significant differences in favor of multiple injections (p < 0.00001), both in all studies and subgroups (Figure 13).
Figure 12.
Forest plot for WOMAC scores (CI: confidence interval; IV: inverse variance; SD: standard deviation).
Figure 13.
Forest plot for WOMAC scores comparing single PRP injection versus multiple PRP injections (CI: confidence interval; IV: inverse variance; SD: standard deviation).
Six studies evaluated functional outcomes in IKDC rating scores [54,60,63,66,70,78,89] and showed significant differences in favor of PRP (p = 0.002) (Figure 14). Five studies showed significant differences in favor of PRP compared to HA as a control group (p = 0.004) [60,63,66,70,78], and two studies showed non-significant differences in favor of PRP when compared with placebo (p = 0.24) [54,89].
Figure 14.
Forest plot for IKDC scores (CI: confidence interval; IV: inverse variance; SD: standard deviation).
Eight studies evaluated osteoarthritis outcomes via KOOS scores [56,58,60,61,74,76,78,81] (Figure 15). We excluded from the meta-analysis two of these studies, due to the lack of measurements in one [56] and division of the results according to the physician in another [76]. The pooled estimates for these studies showed non-significant differences in KOOS sport (p = 0.60), quality of life (p = 0.78), and ADL (p = 0.69) sub-scales in favor of PRP (p > 0.05), but in KOOS symptoms (p = 0.23) and pain (0.97) sub-scales were in favor of the control groups (p > 0.05).
Figure 15.
Forest plot for Knee injury and Osteoarthritis Outcome Score (KOOS) sub-scores: (A) pain; (B) symptoms; (C) activities of daily living (ADL); (D) sports; (E) quality of life (QoL). (CI: confidence interval; IV: inverse variance; SD: standard deviation).
Functional outcomes were also measured with the KSS score in one study [68], Lysholm score in two studies [63,68], Tegner score in four studies [60,68,70,79], Outcome Measures in Arthritis Clinical Trials–Osteoarthritis Research Society International (OMERACT–OARSI) pain measure in three studies [56,75,90], and Lequesne score in five studies [59,68,71,75,90]. Quality of life was measured with SF-36 scores in five studies [56,57,64,82], SF-12 in two studies [55,76], and European Quality of Life (EQoL) in two studies [61,76]. Significant improvement was shown in all KSS and Lysholm scores (p < 0.05), in 3/5 studies for the Lequesne score [59,68,75], and 1/3 study for OMERACT–OARSI scores [75]. No study detected significant difference in Tegner scores.
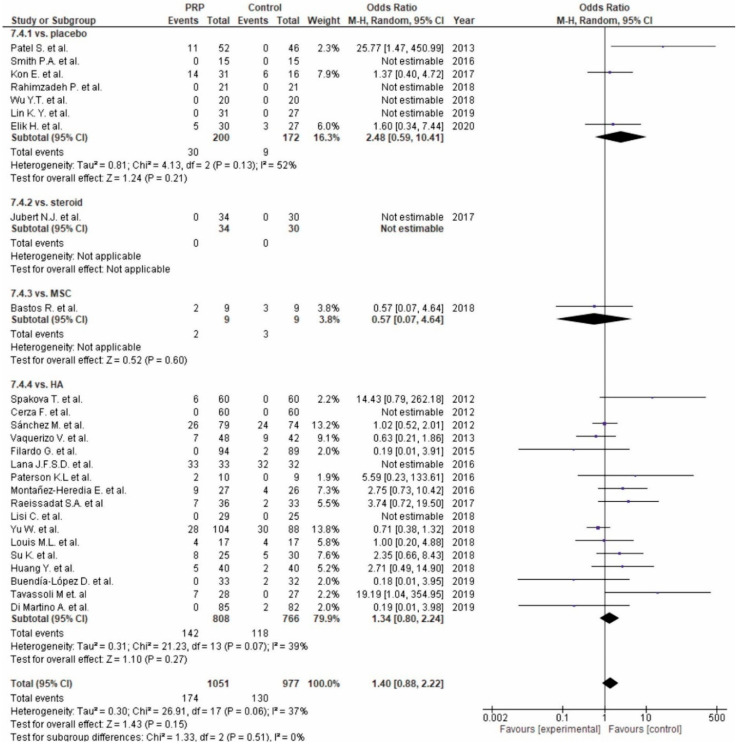
Twenty-six studies reported adverse events (Figure 16). Seven studies [56,57,72,73,86,88,89] comparing PRP versus placebo reported non-significant differences in favor of the control groups (p = 0.21), fourteen studies [60,61,65,67,68,69,70,71,75,76,77,79,83,84,87,90,91] comparing PRP versus HA reported non-significant differences in favor of the control groups (p = 0.27), one study [58] comparing PRP versus steroids reported no adverse events [81], and one study comparing PRP versus MSC showed non-significant differences in favor of PRP (p = 0.60) [81]. The pooled estimates for these studies showed non-significant differences in favor of the control groups (p = 0.15).
Figure 16.
Forest plot for adverse events (CI: confidence interval).
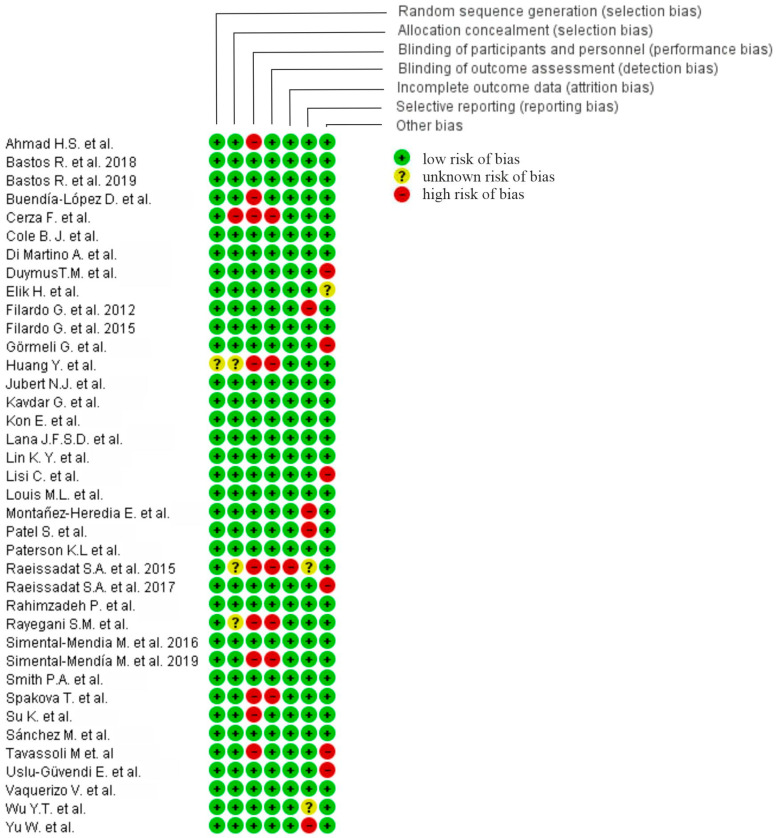
One study was at high risk of bias for three domains [84], two studies were at high risk of bias for two domains [83,85,87], and twelve studies were at high risk of bias for one domain [54,59,62,66,67,68,69,71,76,78,86,91]. One study was at high risk of performance bias for three domains with the risk of reporting bias for two domains [82], one study was at high risk of performance bias for two domains with risk of reporting bias for two domains [77], one study was at high risk of performance bias for two domains with risk of reporting bias for one domain [64], and two studies were at risk of reporting bias for one domain [57,88]. Low risk of performance bias was identified in seventeen studies, moderate risk of performance bias was identified in seventeen studies, and high risk of performance bias was identified in four studies (Figure 17).
Figure 17.
Risk of bias analysis for PRP application in osteoarthritis.
3. Discussion
In recent years, blood derived products have been gaining more popularity in orthopedic treatment—especially platelet-rich plasma—due to their mechanism of action leading to stem cell proliferation, modulation of inflammatory processes, and angiogenesis [92]. It has resulted in an increased number of publications regarding the use of PRP in both conservative and intraoperative treatment, including systematic reviews and meta-analysis. However, this meta-analysis is the first concerning PRP applications in all knee diseases.
The most important finding of our study was that PRP has some benefits in almost all analyzed subgroups. PRP improves outcomes in osteoarthritis applications, as well as in arthroscopic treatment of cartilage degeneration. PRP also has an influence on meniscus healing, faster return to sport after muscle injuries, and reduces blood loss after total knee replacement.
Dupley et al. included two RCTs in their meta-analysis comparing PRP injection to ESWT or dry needling. They reported no significant differences in mean VISA-P scores at early follow-up (two or three months; difference in means, 11.9; standard error (SE), 7.4; 95% CI (–2.7, 26.4); p = 0.109). However, PRP was statistically better than the control at longer assessment periods (at six months or more than six months; difference in means, 12.7; SE, 4.4; 95% CI (4.1, 21.3); p = 0.004) [93]. Chen et al. included 11 studies in their meta-analysis, but only two RCTs [7,8], the same as Dupley et al. [93], both comparing the application of PRP to control in VISA-P and VAS. The mean difference in functional outcome was 13.22 (95% CI (2.37, 24.07)). In the pain scale, the mean difference comparing PRP with control groups was −1.87 (95% CI (−3.28, −0.46)) and showed that leukocyte-rich PRP (LR-PRP) has better functional improvement and pain reduction for patellar tendinitis compared with corticosteroids, treatment ultrasound, autologous blood injection (ABI) or topical glyceryl trinitrate (TGT) compared to control groups [94]. In our meta-analysis, four RCTs were included [6,7,8,9]. The results showed no significant differences in VAS (p = 0.78, 95% CI −0.17 (−1.38, 1.04)) and VISA-P scores (p = 0.97, 95% CI 0.52 (−11.50,12.54)). Two studies [8,9] provided better outcomes in both functional and pain scales. This may be the result of two injections of PRP over two weeks compared with a single injection in other studies [6,7]. Further research using a higher number of subjects and with lower biases is needed to state unequivocally that the number of injections positively influences the effect of PRP on PT treatment.
Grassi et al. performed a meta-analysis evaluating outcomes after PRP application in acute muscle injuries [95]. Six RCTs showed significantly shorter time for return to sport in the PRP group (p = 0.006, 95% CI −7.17 (−12.26, −2.08)); however, in three studies with hamstring injuries the difference was not significant (p = 0.07, 95% CI −5.95 (−12.48, 0.57)). No other significant differences for fixed-effect meta-analysis among the group were found including re-injury rate, complications, pain, muscle strength, function, ROM, and imaging. Three studies reported better pain outcomes in the PRP group (p < 0.05). Bubnov et al. reported a greater ROM and higher strength in the PRP group [96]. In our review we analyzed four RCTs which included hamstring injuries. Time for return to sport was significantly shorter in PRP versus control groups (p < 0.00001, 95% CI −4.16 (−5.44, −2.88)). The differences in hamstring meta-analyses may be the result of a narrow range of 95% CI in one additional study (−3.90 (−5.27, −2.53)) or it could be the result of the evaluation of other muscle injures except the hamstring (quadriceps, gastrocnemius) [10]. We also failed to find any significant differences in re-injury rate (p = 0.50). In two studies we also reported lower pain severity. However, more prospective studies for PRP application after muscle injuries are needed as current research shows promising results for a faster return to sport, which can be a major advantage especially for athletes.
In our synthesis we included only two RCTs with PRP application in high tibial osteotomy; this was due to a lack of similar studies. Both RCTs evaluated different outcomes, with significant differences in functional and pain scales, as well as in radiological bone healing and second-look arthroscopy cartilage healing. Roffi et al. performed a systematic review on the application of PRP in bone healing, identifying forty-five pre-clinical in-vivo studies and nineteen clinical studies. Nine clinical studies addressed the role of PRP in the treatment of fractures. Six of them showed improved results in PRP groups regarding radiological parameters. Only five trials reported functional outcomes, with two studies providing improved outcomes. Another ten studies addressed the treatment of delayed or non-unions. Eight of them suggested a positive role for PRP in stimulating bone healing [97]. The results are promising; however, further research is necessary to confirm the effectiveness of PRP in accelerating bone healing and to exclude bias. Good outcomes in these two studies could be the result of an addition of other myeloid stromal cells.
Muchedzi et al. included seventeen RCTs for evaluation of PRP in both osteoarthritis and following total knee arthroplasty. Primary outcomes after TKA were presented in five studies and included less pain in short-term follow-up in the PRP group (p = 0.05, heterogeneity 91%), but no improvements in functional outcome in WOMAC scores. Secondary outcomes were evaluated in ten studies with no significant differences in blood loss (p = 0.07). Three studies provided no benefits in length of hospital stay (p = 0.31) [98]. We included six RCTs and analyzed blood loss after TKA to show significant reduction in blood loss in the PRP group (p = 0.001, 95% CI −0.29 (−0.46, −0.11)). Four studies reported better outcomes in VAS in the PRP group in short-term follow up. None of the studies showed differences in functional outcomes or range of motion. The differences in outcomes may be a result of study choice as we analyzed only RCTs. Promising results in decreasing pain and blood loss after TKA should encourage further well-planned RCTs with a higher number of patients.
There exists no previous meta-analysis evaluating the use of PRP in addition to arthroscopic surgery. Good outcomes in cartilage healing after PRP injection to knee joint is known [99]. This suggests that the addition of PRP to surgical treatment might also have a satisfactory effect. Comparing microfractures to PRP injection showed significantly better outcomes in IKDC and Lysholm scores (p < 0.00001; p = 0.03) for the PRP group, although the results are only level of evidence III. The positive effects of microfractures in arthroscopic surgery are known, so further extensive research for arthroscopy with concomitant PRP treatment should be encouraged.
Davey et al. analyzed anterior cruciate ligament reconstruction with augmentation of PRP. Thirteen RCTs showed neither significant improvement in any of the clinical outcomes (Tegner, Lysholm, KOOS, IKDC) nor in pain reduction (p = 0.18). PRP also does not support graft healing or donor-site morbidity [100]. In our study including 16 RCTS, we also did not find significant improvements in functional outcomes (IKDC, Lysholm, Tegner), pain reduction (VAS), stability assessment (KT-1000) or tunnel widening. Every outcome crosses the zero line in the forest plot. Currently there is no evidence for supporting ACLR by PRP injection, despite the numerous positive effects of PRP in other diseases.
Haunschild et al. performed a systematic review, including five studies (two prospective and three retrospective) comparing PRP augmentation of meniscus repair to meniscus repair alone. Three studies showed no significant differences in outcome or failure, another two had improvements at the final follow up (KOOS, IKDC, WOMAC, failure). Three studies assessed radiographic findings using MRI: Pujol et al. [51] showed a significantly improved healing rate (p < 0.01); Kaminski et al. [47] showed insignificant findings on MRI, although significant improvement was reported in second-look arthroscopy; and Kemmochi et al. [49] failed to show clearly any improvement only revealing a tendency toward healing. We included in the meta-analysis two additional studies (two RCTs and five non-randomized). Six studies of meniscal repair failure reported significant differences in favor of the PRP group (p = 0.003, 95% CI 0.33 (0.18; 0.60)), one study [49] was excluded because of unclear criteria of improvements in a follow-up MRI and lack of exact outcomes. When comparing with previous analysis where improvements in failure rate were not clearly demonstrated, it is probable that we added two more studies with high weight in our meta-analysis (29.6%, 24.6%). Six studies reported functional outcomes, but only three of them reported significant improvements in some of the scores (KOOS, IKDC, WOMAC). There is insufficient evidence for the addition of PRP to meniscus repair treatment, however, we conclude there are some promising results which should encourage more randomized clinical trials in the near future.
Zhang et al. performed a systematic review of thirteen studies (ten RCTs, three prospective) comparing PRP application in osteoarthritis versus hyaluronic acid. Pain outcomes estimated by VAS did not reveal significant differences and WOMAC pain was significantly decreased after 6 and 12 months of follow-up (p < 0.01; mean difference (MD = −15.25; 95% CI: −22.17 to −8.32). In addition, WOMAC physical function showed significant differences in favor of the PRP group (p < 0.01; MD 11.17; 95% CI (–16.37, –5.98). Functional outcomes in IKDC scale was significant at 6 months of follow-up (p < 0.01), however differences were not significant among the groups after 12 months (p = 0.13) [101]. Similarly, Vilchez-Cavazos et al. evaluated the treatment of knee osteoarthritis comparing a single PRP injection versus multiple PRP injections. Five RCTs measured pain and functional outcomes showing insignificant differences in favor of multiple injections in pain scores (p = 0.19; 95% CI 0.65 (−0.31; 1.60)) but significantly better results on joint function in the WOMAC score when comparing multiple injections versus single injection (p < 0.0001; 95% CI 2.24 (1.12, 3.36)) [102]. Our analysis of PRP injection in the treatment of an osteoarthritic knee included thirty-eight studies. Hyaluronic acid, corticosteroids, saline, no injection, and acetaminophen were evaluated as control groups. The most significant conclusion was that multiple injections were significantly more effective than a single injection with respect to pain (VAS, p = 0.0008, 95% CI 1.63 (0.67; 2.59)) and functional outcomes (WOMAC, p < 0.00001, 95% CI 9.46 (6.25; 12.67)). However, we did not find any correlation between injection intervals and clinical outcomes. PRP application was repeated after 1, 2, 3 or 4 weeks. Only one study assessed the effects of a different number of PRP injections. Kavadar et al. compared one, two, and three injections of PRP in grade 3 OA. Mean differences in VAS and TUG (Timed Up and Go Test) significantly favored multiple injections (1 inj. vs. 2 inj., 1 inj. vs. 3 inj., and 2 inj. vs. 3 inj.), but WOMAC mean differences were significant only in comparison of single versus multiple injections [80]. There is a strong need for more RCTs evaluating the effectiveness of multiple injections of PRP and answering the question: Is twice the applications of PRP injection satisfactory for an optimal clinical effect or does effectiveness improve with the number of injections? Our significant results in pain outcomes derive from the accurate VAS measurements; Vilchez-Cavazos used change in pain data for comparison, despite the fact that even a single injection of PRP significantly improved pain (p < 0.00001) and functional outcomes in WOMAC and IKDC (p < 0.00001; p = 0.002) versus control groups. Only in KOOS scores were differences not significant for pooled estimates studies. This might be a result of the small group of included studies. Furthermore, 50% of these studies (3/6) included hyaluronic acid as a control group which was the only one showing insignificant differences in favor of control groups. Some authors concluded that this could be the reason for using LR-PRP or an older population of patients [60]. The analysis of adverse events also showed the advantage of PRP over other treatments. Differences were not significant but still PRP seems to be the safer option for patients (p = 0.15; 95% CI 1.40 (0.88, 2.22)).
There is no single method of PRP preparation and there are many devices and protocols being used. In our synthesis, the most frequently applied centrifuge was the GPS System III (Zimmer Biomet). In addition, there is no evidence for improved outcomes after leukocyte addition to PRP. Most of the studies used leukocyte-rich platelet-rich plasma. There were only two studies in our metanalysis comparing Leukocyte-poor and leukocyte-rich PRP. The first, in the patellar tendinopathy section, showed a non-significant difference in VISA and VAS scores in favor of leucocyte-rich PRP [6]. A second study which compared PRGF (leukocyte poor) with leukocyte-rich PRP, showed only a significant difference in swelling scores on the first day and CRP ten days after surgery in favor of PRGF. There is a lack of studies comparing leukocyte concentration in PRP to clinical outcomes. Hanish et al. did not find any significant differences between leukocyte-poor (LP)-PRP and LR-PRP in treatment of Achilles tendinopathy in VISA-A and VAS [103]. Further, Yerlikali et al. showed no significant differences in pain, functional parameters, and inflammatory reaction between LR-PRP and LP-PRP in patients with lateral epicondylitis [104]. Riboh et al. performed a meta-analysis including six RCTs and three prospective comparative studies comparing efficacy of leukocyte concentration in OA treatment. The final analysis included WOMAC score, IKDC score, and adverse events. They showed a slight advantage in functional outcomes favoring LP-PRP, but leukocyte concentration did not influence upon adverse reactions [105]. Despite the small amount of studies, leukocytes may be important factors in supporting the action of PRP. They play a role in regeneration through stimulation of immune processes. Lana et al. suggested leukocyte-rich PRP may have some benefits over leukocyte-poor PRP, due to macrophage inclusion, which are “like instructors of the healing orchestra”, because of their role in remodeling and repair phases [106]. On the other hand, Braun et al. concluded that leukocytes provide acute inflammatory response and LP-PRP leads to better outcomes of synovial cell treatment than LR-PRP [107]. There is a strong need for further research on the effectiveness of LP and LR-PRP.
Our metanalysis showed and summarized many positive effects of PRP. However, there are still many unsolved questions and issues requiring specific studies that should be performed according to the “DOSES” cell-therapy communication tool [108]. This standardized system for describing cell therapies allows the systematic performance of RCTs and full clinical outcome assessment of PRP in knee disorders.
3.1. Strengths
Our major strength is that this synthesis includes comprehensive analysis of PRP application in the treatment of major knee lesions. We performed level of evidence I analysis in all types of lesions but two, which included level of evidence III studies. Our promising results could be a result of a large amount of included RCTs and wide array of control groups. This reduces the risk of bias and provides a more complete and reliable analysis.
3.2. Limitations
English language trials were included, but non-English language studies were excluded; they also may contain relevant research. The PRP preparation kits were heterogeneous and not always clearly defined, furthermore platelet count and leukocyte content differed. Level of evidence of included studies varied (I–II). Five of seven included studies in one subgroup were non-randomized (level of evidence III). Some studies with a high risk of bias could have influenced the final results of this synthesis. The diversity of scales used did not allow us to perform meta-analysis of every outcome. Additionally, some of the syntheses consisted of high heterogeneity studies (above 40%).
4. Materials and Methods
4.1. Search Strategy
In this review we concentrated on PRP application in knee lesions compared with placebo- or other treatment control groups. This study was completed in compatibility with the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. A systematic review of the use of platelet-rich plasma in knee lesions was completed with a comprehensive published literature search through PubMed, Embase, Cochrane Database of Systematic Reviews, and Clinicaltrials.gov. The references of the investigations found in this search were cross-referenced to identify additional pertinent studies not identified in the original searches. All searches were performed in February 2020. The searches were performed combining the following keywords: (1) “PRP” or “platelet-rich plasma” or “plasma rich in growth factors” or “platelet derived growth factor” or “platelet derived” or “platelet gel” or “platelet concentrate” or “PRF” or “platelet rich fibrin” or “ACP” or ”autologous conditioned plasma” or ”PRGF” or “platelet lysate”, and (2) “knee” or “knee osteoarthritis” or “meniscus” or “menisci” or “chondral” or “cartilage” or “ligament” or “patella” or “patellar” or “PCL” or “MCL” or “iliotibial” or “osteochondritis” or “hamstring” or “quadriceps” or “epicondyle” or “osteonecrosis” or “arthroscopy” or “tibia” or “tibial” or “femur” or “femoral” or “trochlea” or “posterolateral” or “posteromedial” or “chondrocyte” or “articular” or “arthroplasty” or “osteotomy” or “red zone” or “white zone” or “extrusion” or “red-white” or “intra-meniscal”. Systematic review registration was performed on 10.02.2020 using PROSPERO (International Prospective Register of Systematic Reviews, ID 167715).
4.2. Inclusion and Exclusion Criteria
This review included all clinical studies meeting the following inclusion criteria: PRP utilization as conservative treatment in knee lesions or as support in knee surgery, English language, human subjects, paper published in a peer-reviewed journal, and full text available. Only randomized controlled trials were included, in addition to the meniscus and microfractures section where only a small number of RCTs was identified. Exclusion criteria included all animal studies, basic scientific investigations, case reports, review articles, expert opinions, letters to editor, studies without control groups, studies not using PRP, papers not peer reviewed, papers not in English, trials evaluating platelet-poor plasma, and investigations on other diseases unrelated to the knee joint. The investigations included in this study were independently reviewed by two orthopedic surgeons/authors for inclusion and exclusion criteria.
4.3. Types of Interventions
We compared intralesional, injected PRP preparation with:
-
-
placebo injection (low volume saline injection, matching the prp volume);
-
-
high volume saline image guided injection;
-
-
local steroids injection;
-
-
hyaluronic acid injection;
-
-
exercise and other physical therapies (e.g., low-dose radiation therapy, eccentric loading program, dry needling);
-
-
any other medications given locally or systemically aimed at treating pain; and
-
-
combinations of the active interventions listed above.
4.4. Outcomes
Primary outcomes included:
-
-
pain as measured by standard validated pain scale, such as visual analogue score (VAS), EQ-VAS or numerical rating scale (NRS);
-
-
functional measurement by any standard validated scale, such as the International Knee Documentation Committee (IKDC), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Knee Society Score (KSS), Victorian Institute of Sport Assessment (VISA), 36-Item Short Form Survey (SF-36), Knee injury and Osteoarthritis Outcome Score (KOOS), Lysholm Knee Scoring Scale, Teger Activity Score, and Ikeuchi grade knee rating scale;
-
-
meniscal repair failure;
-
-
time for return to sport;
-
-
re-injury;
-
-
knee stability, measured as tibial translation;
-
-
graft integration; and
-
-
tunnel widening.
Adverse events were also evaluated and analyzed. If multiple time points were reported within our time frames, we extracted the last time point (e.g., if data were reported at six weeks, three months, six months, and one year, we extracted outcomes at one year).
4.5. Data Collection and Analysis
For each study included in the analysis, the following data were extracted by two independent reviewers: authors, year of publication, type of knee lesions, details of interventions in the study, sample size (randomized and analyzed), outcome measurements, follow-up period, main results, and percentage and type of adverse events included in the publication. Each study’s level of evidence was examined and evaluated based on criteria established by Oxford Centre for Evidence-Based Medicine Levels of Evidence Working Group [109]. Measures of treatment effect at a final point were the mean and standard deviation for continuous outcome measures. When studies reported other measures (e.g., median) and other dispersion measures such as standard error (SE) of the mean or 95% CI of the mean, range or interquartile range (IQR) we calculated the SD in order to perform the relevant meta-analytical pooling according to previous studies (see [110,111]).
The study weight was calculated using the Mantel–Haenszel method. We assessed statistical heterogeneity using Tau2 or Chi2, df and I2 statistics. The I2 statistic describes the percentage of total variation across trials that is due to heterogeneity. In the case of low heterogeneity (I2 < 40%), studies were pooled using a fixed-effects model, otherwise a random-effects analysis was made.
Subgroup analysis was undertaken for the type of clinical trial and for the type of control intervention.
4.6. Assessment of Risk of Bias
Revised Cochrane risk-of-bias tool was used to evaluate risk. Disagreement in the risk of bias assessment was resolved by consensus and, if necessary, by the opinion of a third reviewer. A study was deemed to be:
-
-
“low risk” if all items were scored as “low risk”
-
-
“moderate risk” if up to two items were classified as “high risk” or “unclear risk”
-
-
“high risk” if more than two items were scored as “high risk”
We presented our assessment of risk of bias using two “Risk of Bias” summary figures for every sub-section of the manuscript.
4.7. Statistical Analysis
Qualitative statistical analysis and meta-analysis were performed using R software and REVMAN 5.3 [112,113] with p-values of < 0.05.
5. Conclusions
Our systematic review and meta-analysis of the clinical use of platelet-rich plasma in knee lesions show some promising results. First of all, our study confirms significant benefits in the use of PRP in osteoarthritis compared with various control groups. PRP is also safe for patients when compared to control groups; there is an insignificant difference in adverse events in favor of control groups. In other subgroups, differences in functional or pain scales or other measured parameters were significant in favor of the PRP group (blood loss in TKA, time to return to sport in hamstring injuries, microfractures augmentation). Some analyzed results showed an advantage of PRP compared to control groups and should lead to further research with a higher number of subjects and with lower biases to state unequivocally that PRP is a necessary component of the treatment (meniscal repair failure). Only one area clearly showed no differences in ACL reconstruction with or without PRP, thus we conclude so far that PRP has not been proved beneficial in ACLR. However, we do recommend PRP application in knee osteoarthritis and suggest performing more clinical trials concerning PRP application in other knee lesions. The optimal protocol (e.g., number of injections, timeframe) for the most effective treatment should be determined. Methods of preparation of platelet-rich plasma need further standardization. Studies should be performed to establish adequate cost–benefit of PRP compared with other standard, less expensive, treatments.
Author Contributions
Conceptualization, R.K. and K.K.-K.; data curation, E.T., K.K., and R.K.; formal analysis, R.K. and K.K.-K.; funding acquisition, R.K. and S.P.; investigation E.T. and K.K.; methodology, R.K., K.K.-K., and S.P.; project administration, E.T. and K.K.; resources, R.K.; software, R.K.; supervision, R.K. and S.P.; validation, R.K. and K.K.-K.; visualization, R.K.; writing—original draft, E.T., R.K., and K.K.-K.; writing—review and editing, R.K., E.T., K.K., K.K.-K., and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Centre of Postgraduate Medical Education Grant, grant number 501–1–007–18–20.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Gobbi A., Espregueira-Mendes J., Lane J.G., Karahan M. Bio-Orthopaedics: A New Approach. Springer; Berlin/Heidelberg, Germany: 2018. [Google Scholar]
- 2.Andia I., Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat. Rev. Rheumatol. 2013;9:721–730. doi: 10.1038/nrrheum.2013.141. [DOI] [PubMed] [Google Scholar]
- 3.Alves R., Grimalt R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Skin Appendage Disord. 2018;4:18–24. doi: 10.1159/000477353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cozma C.N., Raducu L., Jecan C.R. Platelet Rich Plasma—Mechanism of action and clinical applications. J. Clin. Invest. Surg. 2016;1:41–46. doi: 10.25083/2559.5555.12.16. [DOI] [Google Scholar]
- 5.Canata G.L., D’Hooghe P., Hunt K.J. Muscle and Tendon Injuries: Evaluation and Management. Springer; Berlin/Heidelberg, Germany: 2017. [Google Scholar]
- 6.Scott A., LaPrade R.F., Harmon K.G., Filardo G., Kon E., Della Villa S., Bahr R., Moksnes H., Torgalsen T., Lee J., et al. Platelet-Rich Plasma for Patellar Tendinopathy: A Randomized Controlled Trial of Leukocyte-Rich PRP or Leukocyte-Poor PRP Versus Saline. Am. J. Sports Med. 2019;47:1654–1661. doi: 10.1177/0363546519837954. [DOI] [PubMed] [Google Scholar]
- 7.Dragoo J.L., Wasterlain A.S., Braun H.J., Nead K.T. Platelet-Rich Plasma as a Treatment for Patellar Tendinopathy: A Double-Blind, Randomized Controlled Trial. Am. J. Sports Med. 2014;42:610–618. doi: 10.1177/0363546513518416. [DOI] [PubMed] [Google Scholar]
- 8.Vetrano M., Castorina A., Vulpiani M.C., Baldini R., Pavan A., Ferretti A. Platelet-Rich Plasma Versus Focused Shock Waves in the Treatment of Jumper’s Knee in Athletes. Am. J. Sports Med. 2013;41:795–803. doi: 10.1177/0363546513475345. [DOI] [PubMed] [Google Scholar]
- 9.Abate M., Di Carlo L., Verna S., Di Gregorio P., Schiavone C., Salini V. Synergistic activity of platelet rich plasma and high volume image guided injection for patellar tendinopathy. Knee Surg. Sports Traumatol. Arthrosc. 2018;26:3645–3651. doi: 10.1007/s00167-018-4930-6. [DOI] [PubMed] [Google Scholar]
- 10.Rossi L.A., Molina Rómoli A.R., Bertona Altieri B.A., Burgos Flor J.A., Scordo W.E., Elizondo C.M. Does platelet-rich plasma decrease time to return to sports in acute muscle tear? A randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2017;25:3319–3325. doi: 10.1007/s00167-016-4129-7. [DOI] [PubMed] [Google Scholar]
- 11.A Hamid M.S., Mohamed Ali M.R., Yusof A., George J., Lee L.P.C. Platelet-Rich Plasma Injections for the Treatment of Hamstring Injuries: A Randomized Controlled Trial. Am. J. Sports Med. 2014;42:2410–2418. doi: 10.1177/0363546514541540. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton B., Tol J.L., Almusa E., Boukarroum S., Eirale C., Farooq A., Whiteley R., Chalabi H. Platelet-rich plasma does not enhance return to play in hamstring injuries: A randomised controlled trial. Br. J. Sports Med. 2015;49:943–950. doi: 10.1136/bjsports-2015-094603. [DOI] [PubMed] [Google Scholar]
- 13.Reurink G., Goudswaard G.J., Moen M.H., Weir A., Verhaar J.A.N., Bierma-Zeinstra S.M.A., Maas M., Tol J.L. Rationale, secondary outcome scores and 1-year follow-up of a randomised trial of platelet-rich plasma injections in acute hamstring muscle injury: The Dutch Hamstring Injection Therapy study. Br. J. Sports Med. 2015;49:1206–1212. doi: 10.1136/bjsports-2014-094250. [DOI] [PubMed] [Google Scholar]
- 14.Koh Y.-G., Kwon O.-R., Kim Y.-S., Choi Y.-J. Comparative Outcomes of Open-Wedge High Tibial Osteotomy with Platelet-Rich Plasma Alone or in Combination with Mesenchymal Stem Cell Treatment: A Prospective Study. Arthrosc. J. Arthrosc. Relat. Surg. 2014;30:1453–1460. doi: 10.1016/j.arthro.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 15.Dallari D., Savarino L., Stagni C., Cenni E., Cenacchi A., Fornasari P.M., Albisinni U., Rimondi E., Baldini N., Giunti A. Enhanced Tibial Osteotomy Healing with Use of Bone Grafts Supplemented with Platelet Gel or Platelet Gel and Bone Marrow Stromal Cells. J. Bone Jt. Surg. 2007;89:2413–2420. doi: 10.2106/00004623-200711000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Horstmann W.G., Slappendel R., van Hellemondt G.G., Wymenga A.W., Jack N., Everts P.A.M. Autologous platelet gel in total knee arthroplasty: A prospective randomized study. Knee Surg. Sports Traumatol. Arthrosc. 2011;19:115–121. doi: 10.1007/s00167-010-1207-0. [DOI] [PubMed] [Google Scholar]
- 17.Morishita M., Ishida K., Matsumoto T., Kuroda R., Kurosaka M., Tsumura N. Intraoperative Platelet-Rich Plasma Does Not Improve Outcomes of Total Knee Arthroplasty. J. Arthroplast. 2014;29:2337–2341. doi: 10.1016/j.arth.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Peerbooms J.C., de Wolf G.S., Colaris J.W., Bruijn D.J., Verhaar J.A.N. No positive effect of autologous platelet gel after total knee arthroplasty: A double-blind randomized controlled trial: 102 patients with a 3-month follow-up. Acta Orthop. 2009;80:557–562. doi: 10.3109/17453670903350081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerreiro J.P.F., Danieli M.V., Queiroz A.O., Deffune E., Ferreira R.R. Platelet-rich plasma (PRP) applied during total knee arthroplasty. Rev. Bras. Ortop. 2015;50:186–194. doi: 10.1016/j.rbo.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerreiro J.P.F., Lima D.R., Bordignon G., Danieli M.V., Queiroz A.O., Cataneo D.C. Platelet-Rich Plasma (PRP) and Tranexamic Acid (TXA) Applied in Total Knee Arthroplasty. Acta Ortop. Bras. 2019;27:248–251. doi: 10.1590/1413-785220192705214417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mochizuki T., Yano K., Ikari K., Hiroshima R., Kawakami K., Koenuma N., Ishibashi M., Shirahata T., Momohara S. Platelet-rich plasma for the reduction of blood loss after total knee arthroplasty: A clinical trial. Eur. J. Orthop. Surg. Traumatol. 2016;26:901–905. doi: 10.1007/s00590-016-1821-8. [DOI] [PubMed] [Google Scholar]
- 22.Vasavilbaso C.T., Bello C.D.R., López E.M., Granado M.P.C., Álvarez J.M.N., Davalillo C.A.T., Orbezo F.I.G. Benefits of different postoperative treatments in patients undergoing knee arthroscopic debridement. Open Access Rheumatol. Res. Rev. 2017;9:171–179. doi: 10.2147/OARRR.S138353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duif C., Vogel T., Topcuoglu F., Spyrou G., von Schulze Pellengahr C., Lahner M. Does intraoperative application of leukocyte-poor platelet-rich plasma during arthroscopy for knee degeneration affect postoperative pain, function and quality of life? A 12-month randomized controlled double-blind trial. Arch. Orthop. Trauma Surg. 2015;135:971–977. doi: 10.1007/s00402-015-2227-5. [DOI] [PubMed] [Google Scholar]
- 24.Lee G.W., Son J.-H., Kim J.-D., Jung G.-H. Is platelet-rich plasma able to enhance the results of arthroscopic microfracture in early osteoarthritis and cartilage lesion over 40 years of age? Eur. J. Orthop. Surg. Traumatol. 2013;23:581–587. doi: 10.1007/s00590-012-1038-4. [DOI] [PubMed] [Google Scholar]
- 25.Manunta A., Manconi A. The treatment of chondral lesions of the knee with the microfracture technique and platelet-rich plasma. Joints. 2013;1:167–170. doi: 10.11138/jts/2013.1.4.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen P.D., Tran T.D.-X., Nguyen H.T.-N., Vu H.T., Le P.T.-B., Phan N.L.-C., Vu N.B., Phan N.K., Van Pham P. Comparative Clinical Observation of Arthroscopic Microfracture in the Presence and Absence of a Stromal Vascular Fraction Injection for Osteoarthritis: ADSC and PRP Injection for Osteoarthritis. Stem Cells Transl. Med. 2017;6:187–195. doi: 10.5966/sctm.2016-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancò A., Goderecci R., Rughetti A., De Giorgi S., Necozione S., Bernardi A., Calvisi V. Microfracture versus microfracture and platelet-rich plasma: Arthroscopic treatment of knee chondral lesions. A two-year follow-up study. Joints. 2016;4:142–147. doi: 10.11138/jts/2016.4.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y.S., Kwon O.R., Choi Y.J., Suh D.S., Heo D.B., Koh Y.G. Comparative Matched-Pair Analysis of the Injection Versus Implantation of Mesenchymal Stem Cells for Knee Osteoarthritis. Am. J. Sports Med. 2015;43:2738–2746. doi: 10.1177/0363546515599632. [DOI] [PubMed] [Google Scholar]
- 29.Cervellin M., de Girolamo L., Bait C., Denti M., Volpi P. Autologous platelet-rich plasma gel to reduce donor-site morbidity after patellar tendon graft harvesting for anterior cruciate ligament reconstruction: A randomized, controlled clinical study. Knee Surg. Sports Traumatol. Arthrosc. 2012;20:114–120. doi: 10.1007/s00167-011-1570-5. [DOI] [PubMed] [Google Scholar]
- 30.Walters B.L., Porter D.A., Hobart S.J., Bedford B.B., Hogan D.E., McHugh M.M., Klein D.A., Harousseau K., Nicholas S.J. Effect of Intraoperative Platelet-Rich Plasma Treatment on Postoperative Donor Site Knee Pain in Patellar Tendon Autograft Anterior Cruciate Ligament Reconstruction: A Double-Blind Randomized Controlled Trial. Am. J. Sports. Med. 2018;46:1827–1835. doi: 10.1177/0363546518769295. [DOI] [PubMed] [Google Scholar]
- 31.Seijas R., Cuscó X., Sallent A., Serra I., Ares O., Cugat R. Pain in donor site after BTB-ACL reconstruction with PRGF: A randomized trial. Arch. Orthop. Trauma Surg. 2016;136:829–835. doi: 10.1007/s00402-016-2458-0. [DOI] [PubMed] [Google Scholar]
- 32.De Almeida A., Demange M., Sobrado M., Rodrigues M., Pedrinelli A., Hernandez A., Nakamura N. Platelet-Rich Plasma Added to the Patellar Tendon Harvest Site During Anterior Cruciate Ligament Reconstruction Enhanced Healing. J. Bone Jt. Surg.-Am. 2013;95:942. doi: 10.2106/JBJS.9510.EBO450. [DOI] [PubMed] [Google Scholar]
- 33.Radice F., Yánez R., Gutiérrez V., Rosales J., Pinedo M., Coda S. Comparison of Magnetic Resonance Imaging Findings in Anterior Cruciate Ligament Grafts with and Without Autologous Platelet-Derived Growth Factors. Arthrosc. J. Arthrosc. Relat. Surg. 2010;26:50–57. doi: 10.1016/j.arthro.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 34.Azcárate A.V., Lamo-Espinosa J., Beola J.D.A., Gonzalez M.H., Gasque G.M., Nin J.R.V. Comparison between two different platelet-rich plasma preparations and control applied during anterior cruciate ligament reconstruction. Is there any evidence to support their use? Injury. 2014;45:S36–S41. doi: 10.1016/S0020-1383(14)70008-7. [DOI] [PubMed] [Google Scholar]
- 35.Seijas R., Ares O., Catala J., Alvarez-Diaz P., Cusco X., Cugat R. Magnetic Resonance Imaging Evaluation of Patellar Tendon Graft Remodelling after Anterior Cruciate Ligament Reconstruction with or without Platelet-Rich Plasma. J. Orthop. Surg. 2013;21:10–14. doi: 10.1177/230949901302100105. [DOI] [PubMed] [Google Scholar]
- 36.Silva A., Sampaio R. Anatomic ACL reconstruction: Does the platelet-rich plasma accelerate tendon healing? Knee Surg. Sports Traumatol. Arthrosc. 2009;17:676–682. doi: 10.1007/s00167-009-0762-8. [DOI] [PubMed] [Google Scholar]
- 37.Rupreht M., Jevtič V., Serša I., Vogrin M., Jevšek M. Evaluation of the tibial tunnel after intraoperatively administered platelet-rich plasma gel during anterior cruciate ligament reconstruction using diffusion weighted and dynamic contrast-enhanced MRI. J. Magn. Reson. Imaging. 2013;37:928–935. doi: 10.1002/jmri.23886. [DOI] [PubMed] [Google Scholar]
- 38.Ventura A., Terzaghi C., Borgo E., Verdoia C., Gallazzi M., Failoni S. Use of growth factors in ACL surgery: Preliminary study. J. Orthopaed. Traumatol. 2005;6:76–79. doi: 10.1007/s10195-005-0085-6. [DOI] [Google Scholar]
- 39.Orrego M., Larrain C., Rosales J., Valenzuela L., Matas J., Durruty J., Sudy H., Mardones R. Effects of Platelet Concentrate and a Bone Plug on the Healing of Hamstring Tendons in a Bone Tunnel. Arthrosc. J. Arthros. Relat. Surg. 2008;24:1373–1380. doi: 10.1016/j.arthro.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Sánchez M., Anitua E., Azofra J., Prado R., Muruzabal F., Andia I. Ligamentization of Tendon Grafts Treated with an Endogenous Preparation Rich in Growth Factors: Gross Morphology and Histology. Arthrosc. J. Arthrosc. Relat. Surg. 2010;26:470–480. doi: 10.1016/j.arthro.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 41.Mirzatolooei F., Alamdari M.T., Khalkhali H.R. The impact of platelet-rich plasma on the prevention of tunnel widening in anterior cruciate ligament reconstruction using quadrupled autologous hamstring tendon: A randomised clinical trial. Bone Jt. J. 2013;95:65–69. doi: 10.1302/0301-620X.95B1.30487. [DOI] [PubMed] [Google Scholar]
- 42.Starantzis K.A., Mastrokalos D., Koulalis D., Papakonstantinou O., Soucacos P.N., Papagelopoulos P.J. The Potentially Positive Role of PRPs in Preventing Femoral Tunnel Widening in ACL Reconstruction Surgery Using Hamstrings: A Clinical Study in 51 Patients. J. Sports Med. 2014;2014:1–10. doi: 10.1155/2014/789317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sözkesen S., Karahan H.G., Kurtulmus A., Kayali C., Altay T. PRP on Preventıon of Tunnel Enlargement in ACL Reconstructıon. Ortop. Traumatol. Rehabil. 2018;20:285–291. doi: 10.5604/01.3001.0012.6462. [DOI] [PubMed] [Google Scholar]
- 44.Vadalà A., Iorio R., De Carli A., Ferretti M., Paravani D., Caperna L., Iorio C., Gatti A., Ferretti A. Platelet-rich plasma: Does it help reduce tunnel widening after ACL reconstruction? Knee Surg. Sports Traumatol. Arthrosc. 2013;21:824–829. doi: 10.1007/s00167-012-1980-z. [DOI] [PubMed] [Google Scholar]
- 45.De Almeida A.M., Demange M.K., Sobrado M.F., Rodrigues M.B., Pedrinelli A., Hernandez A.J. Patellar Tendon Healing with Platelet-Rich Plasma: A Prospective Randomized Controlled Trial. Am. J. Sports Med. 2012;40:1282–1288. doi: 10.1177/0363546512441344. [DOI] [PubMed] [Google Scholar]
- 46.Magnussen R.A., Flanigan D.C., Pedroza A.D., Heinlein K.A., Kaeding C.C. Platelet rich plasma use in allograft ACL reconstructions: Two-year clinical results of a MOON cohort study. Knee. 2013;20:277–280. doi: 10.1016/j.knee.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaminski R., Kulinski K., Kozar-Kaminska K., Wielgus M., Langner M., Wasko M.K., Kowalczewski J., Pomianowski S. A Prospective, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study Evaluating Meniscal Healing, Clinical Outcomes, and Safety in Patients Undergoing Meniscal Repair of Unstable, Complete Vertical Meniscal Tears (Bucket Handle) Augmented with Platelet-Rich Plasma. BioMed Res. Int. 2018;2018:1–9. doi: 10.1155/2018/9315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaminski R., Maksymowicz-Wleklik M., Kulinski K., Kozar-Kaminska K., Dabrowska-Thing A., Pomianowski S. Short-Term Outcomes of Percutaneous Trephination with a Platelet Rich Plasma Intrameniscal Injection for the Repair of Degenerative Meniscal Lesions. A Prospective, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study. Int. J. Mol. Sci. 2019;20:856. doi: 10.3390/ijms20040856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kemmochi M., Sasaki S., Takahashi M., Nishimura T., Aizawa C., Kikuchi J. The use of platelet-rich fibrin with platelet-rich plasma support meniscal repair surgery. J. Orthop. 2018;15:711–720. doi: 10.1016/j.jor.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griffin J.W., Hadeed M.M., Werner B.C., Diduch D.R., Carson E.W., Miller M.D. Platelet-rich Plasma in Meniscal Repair: Does Augmentation Improve Surgical Outcomes? Clin. Orthop. Relat. Res. 2015;473:1665–1672. doi: 10.1007/s11999-015-4170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pujol N., Salle De Chou E., Boisrenoult P., Beaufils P. Platelet-rich plasma for open meniscal repair in young patients: Any benefit? Knee Surg. Sports Traumatol. Arthrosc. 2015;23:51–58. doi: 10.1007/s00167-014-3417-3. [DOI] [PubMed] [Google Scholar]
- 52.Everhart J.S., Cavendish P.A., Eikenberry A., Magnussen R.A., Kaeding C.C., Flanigan D.C. Platelet-Rich Plasma Reduces Failure Risk for Isolated Meniscal Repairs but Provides No Benefit for Meniscal Repairs with Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2019;47:1789–1796. doi: 10.1177/0363546519852616. [DOI] [PubMed] [Google Scholar]
- 53.Dai W.-L., Zhang H., Lin Z.-M., Shi Z.-J., Wang J. Efficacy of platelet-rich plasma in arthroscopic repair for discoid lateral meniscus tears. BMC Musculoskelet. Disord. 2019;20:113. doi: 10.1186/s12891-019-2500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Görmeli G., Görmeli C.A., Ataoglu B., Çolak C., Aslantürk O., Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: A randomized, double-blind, placebo-controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2017;25:958–965. doi: 10.1007/s00167-015-3705-6. [DOI] [PubMed] [Google Scholar]
- 55.Simental-Mendía M., Vílchez-Cavazos J.F., Peña-Martínez V.M., Said-Fernández S., Lara-Arias J., Martínez-Rodríguez H.G. Leukocyte-poor platelet-rich plasma is more effective than the conventional therapy with acetaminophen for the treatment of early knee osteoarthritis. Arch. Orthop. Trauma Surg. 2016;136:1723–1732. doi: 10.1007/s00402-016-2545-2. [DOI] [PubMed] [Google Scholar]
- 56.Kon E., Engebretsen L., Verdonk P., Nehrer S., Filardo G. Clinical Outcomes of Knee Osteoarthritis Treated with an Autologous Protein Solution Injection: A 1-Year Pilot Double-Blinded Randomized Controlled Trial. Am. J. Sports Med. 2018;46:171–180. doi: 10.1177/0363546517732734. [DOI] [PubMed] [Google Scholar]
- 57.Elik H., Doğu B., Yılmaz F., Begoğlu F.A., Kuran B. The efficiency of platelet-rich plasma treatment in patients with knee osteoarthritis. J.Back Musculoskelet. Rehabil. 2020;33:127–138. doi: 10.3233/BMR-181374. [DOI] [PubMed] [Google Scholar]
- 58.Joshi Jubert N., Rodríguez L., Reverté-Vinaixa M.M., Navarro A. Platelet-Rich Plasma Injections for Advanced Knee Osteoarthritis: A Prospective, Randomized, Double-Blinded Clinical Trial. Orthop. J. Sports Med. 2017;5:232596711668938. doi: 10.1177/2325967116689386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uslu Güvendi E., Aşkin A., Güvendi G., Koçyiğit H. Comparison of Efficiency Between Corticosteroid and Platelet Rich Plasma Injection Therapies in Patients with Knee Osteoarthritis. Arch. Rheumatol. 2018;33:273–281. doi: 10.5606/ArchRheumatol.2018.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Filardo G., Di Matteo B., Di Martino A., Merli M.L., Cenacchi A., Fornasari P., Marcacci M., Kon E. Platelet-Rich Plasma Intra-articular Knee Injections Show No Superiority Versus Viscosupplementation: A Randomized Controlled Trial. Am. J. Sports Med. 2015;43:1575–1582. doi: 10.1177/0363546515582027. [DOI] [PubMed] [Google Scholar]
- 61.Paterson K.L., Nicholls M., Bennell K.L., Bates D. Intra-articular injection of photo-activated platelet-rich plasma in patients with knee osteoarthritis: A double-blind, randomized controlled pilot study. BMC Musculoskelet. Disord. 2016;17:67. doi: 10.1186/s12891-016-0920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duymus T.M., Mutlu S., Dernek B., Komur B., Aydogmus S., Kesiktas F.N. Choice of intra-articular injection in treatment of knee osteoarthritis: Platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg. Sports Traumatol. Arthrosc. 2017;25:485–492. doi: 10.1007/s00167-016-4110-5. [DOI] [PubMed] [Google Scholar]
- 63.Cole B.J., Karas V., Hussey K., Merkow D.B., Pilz K., Fortier L.A. Hyaluronic Acid Versus Platelet-Rich Plasma: A Prospective, Double-Blind Randomized Controlled Trial Comparing Clinical Outcomes and Effects on Intra-articular Biology for the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2017;45:339–346. doi: 10.1177/0363546516665809. [DOI] [PubMed] [Google Scholar]
- 64.Rayegani S.M., Raeissadat S.A., Sanei Taheri M., Babaee M., Bahrami M.H., Eliaspour D., Ghorbani E. Does intra articular platelet rich plasma injection improve function, pain and quality of life in patients with osteoarthritis of the knee? A randomized clinical trial. Orthop. Rev. 2014;6 doi: 10.4081/or.2014.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Louis M.L., Magalon J., Jouve E., Bornet C.E., Mattei J.C., Chagnaud C., Rochwerger A., Veran J., Sabatier F. Growth Factors Levels Determine Efficacy of Platelets Rich Plasma Injection in Knee Osteoarthritis: A Randomized Double Blind Noninferiority Trial Compared With Viscosupplementation. Arthrosc. J. Arthrosc. Relat. Surg. 2018;34:1530–1540. doi: 10.1016/j.arthro.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 66.Ahmad H.S., Farrag S.E., Okasha A.E., Kadry A.O., Ata T.B., Monir A.A., Shady I. Clinical outcomes are associated with changes in ultrasonographic structural appearance after platelet-rich plasma treatment for knee osteoarthritis. Int. J. Rheum. Dis. 2018;21:960–966. doi: 10.1111/1756-185X.13315. [DOI] [PubMed] [Google Scholar]
- 67.Su K., Bai Y., Wang J., Zhang H., Liu H., Ma S. Comparison of hyaluronic acid and PRP intra-articular injection with combined intra-articular and intraosseous PRP injections to treat patients with knee osteoarthritis. Clin. Rheumatol. 2018;37:1341–1350. doi: 10.1007/s10067-018-3985-6. [DOI] [PubMed] [Google Scholar]
- 68.Lisi C., Perotti C., Scudeller L., Sammarchi L., Dametti F., Musella V., Di Natali G. Treatment of knee osteoarthritis: Platelet-derived growth factors vs. hyaluronic acid. A randomized controlled trial. Clin. Rehabil. 2018;32:330–339. doi: 10.1177/0269215517724193. [DOI] [PubMed] [Google Scholar]
- 69.Buendía-López D., Medina-Quirós M., Fernández-Villacañas Marín M.Á. Clinical and radiographic comparison of a single LP-PRP injection, a single hyaluronic acid injection and daily NSAID administration with a 52-week follow-up: A randomized controlled trial. J. Orthop. Traumatol. 2018;19:3. doi: 10.1186/s10195-018-0501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Di Martino A., Di Matteo B., Papio T., Tentoni F., Selleri F., Cenacchi A., Kon E., Filardo G. Platelet-Rich Plasma Versus Hyaluronic Acid Injections for the Treatment of Knee Osteoarthritis: Results at 5 Years of a Double-Blind, Randomized Controlled Trial. Am. J. Sports Med. 2019;47:347–354. doi: 10.1177/0363546518814532. [DOI] [PubMed] [Google Scholar]
- 71.Raeissadat S.A., Rayegani S.M., Ahangar A.G., Abadi P.H., Mojgani P., Ahangar O.G. Efficacy of Intra-articular Injection of a Newly Developed Plasma Rich in Growth Factor (PRGF) Versus Hyaluronic Acid on Pain and Function of Patients with Knee Osteoarthritis: A Single-Blinded Randomized Clinical Trial. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2017;10:117954411773345. doi: 10.1177/1179544117733452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith P.A. Intra-articular Autologous Conditioned Plasma Injections Provide Safe and Efficacious Treatment for Knee Osteoarthritis: An FDA-Sanctioned, Randomized, Double-blind, Placebo-controlled Clinical Trial. Am. J. Sports Med. 2016;44:884–891. doi: 10.1177/0363546515624678. [DOI] [PubMed] [Google Scholar]
- 73.Rahimzadeh P., Imani F., Faiz S.H.R., Entezary S.R., Narimani Zamanabadi M., Alebouyeh M.R. The effects of injecting intra-articular platelet-rich plasma or prolotherapy on pain score and function in knee osteoarthritis. Clin. Interv. Aging. 2018;13:73–79. doi: 10.2147/CIA.S147757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bastos R., Mathias M., Andrade R., Amaral R.J.F.C., Schott V., Balduino A., Bastos R., Miguel Oliveira J., Reis R.L., Rodeo S., et al. Intra-articular injection of culture-expanded mesenchymal stem cells with or without addition of platelet-rich plasma is effective in decreasing pain and symptoms in knee osteoarthritis: A controlled, double-blind clinical trial. Knee Surg. Sports Traumatol. Arthrosc. 2019 doi: 10.1007/s00167-019-05732-8. [DOI] [PubMed] [Google Scholar]
- 75.Vaquerizo V., Plasencia M.Á., Arribas I., Seijas R., Padilla S., Orive G., Anitua E. Comparison of Intra-Articular Injections of Plasma Rich in Growth Factors (PRGF-Endoret) Versus Durolane Hyaluronic Acid in the Treatment of Patients with Symptomatic Osteoarthritis: A Randomized Controlled Trial. Arthrosc. J. Arthrosc. Relat. Surg. 2013;29:1635–1643. doi: 10.1016/j.arthro.2013.07.264. [DOI] [PubMed] [Google Scholar]
- 76.Montañez-Heredia E., Irízar S., Huertas P., Otero E., del Valle M., Prat I., Díaz-Gallardo M., Perán M., Marchal J., Hernandez-Lamas M. Intra-Articular Injections of Platelet-Rich Plasma versus Hyaluronic Acid in the Treatment of Osteoarthritic Knee Pain: A Randomized Clinical Trial in the Context of the Spanish National Health Care System. Int. J. Mol. Sci. 2016;17:1064. doi: 10.3390/ijms17071064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang G., Hua S., Yang T., Ma J., Yu W., Chen X. Platelet-rich plasma shows beneficial effects for patients with knee osteoarthritis by suppressing inflammatory factors. Exp. Ther. Med. 2018 doi: 10.3892/etm.2018.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Filardo G., Kon E., Di Martino A., Di Matteo B., Merli M.L., Cenacchi A., Fornasari P.M., Marcacci M. Platelet-rich plasma vs. hyaluronic acid to treat knee degenerative pathology: Study design and preliminary results of a randomized controlled trial. BMC Musculoskelet. Disord. 2012;13:229. doi: 10.1186/1471-2474-13-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lana J.F.S.D., Weglein A., Sampson S.E., Vicente E.F., Huber S.C., Souza C.V., Ambach M.A., Vincent H., Urban-Paffaro A., Onodera C.M.K., et al. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J. Stem Cells Regen. Med. 2016;12:69–78. doi: 10.46582/jsrm.1202011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kavadar G., Demircioglu D.T., Celik M.Y., Emre T.Y. Effectiveness of platelet-rich plasma in the treatment of moderate knee osteoarthritis: A randomized prospective study. J. Phys. Ther. Sci. 2015;27:3863–3867. doi: 10.1589/jpts.27.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bastos R., Mathias M., Andrade R., Bastos R., Balduino A., Schott V., Rodeo S., Espregueira-Mendes J. Intra-articular injections of expanded mesenchymal stem cells with and without addition of platelet-rich plasma are safe and effective for knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2018;26:3342–3350. doi: 10.1007/s00167-018-4883-9. [DOI] [PubMed] [Google Scholar]
- 82.Raeissadat S.A., Rayegani S.M., Hassanabadi H., Fathi M., Ghorbani E., Babaee M., Azma K. Knee Osteoarthritis Injection Choices: Platelet- Rich Plasma (PRP) versus Hyaluronic Acid (A one-year randomized clinical trial) Clin. Med. Insights Arthritis Musculoskelet. Disord. 2015;8:CMAMD.S17894. doi: 10.4137/CMAMD.S17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spaková T., Rosocha J., Lacko M., Harvanová D., Gharaibeh A. Treatment of Knee Joint Osteoarthritis with Autologous Platelet-Rich Plasma in Comparison with Hyaluronic Acid. Am. J. Phys. Med. Rehabil. 2012;91:411–417. doi: 10.1097/PHM.0b013e3182aab72. [DOI] [PubMed] [Google Scholar]
- 84.Cerza F., Carnì S., Carcangiu A., Di Vavo I., Schiavilla V., Pecora A., De Biasi G., Ciuffreda M. Comparison Between Hyaluronic Acid and Platelet-Rich Plasma, Intra-articular Infiltration in the Treatment of Gonarthrosis. Am. J. Sports Med. 2012;40:2822–2827. doi: 10.1177/0363546512461902. [DOI] [PubMed] [Google Scholar]
- 85.Simental-Mendía M., Acosta-Olivo C.A., Hernández-Rodríguez A.N., Santos-Santos O.R., de la Garza-Castro S., Peña-Martínez V.M., Vilchez-Cavazos F. Intraarticular injection of platelet-rich plasma in knee osteoarthritis: Single versus triple application approach. Pilot study. Acta Reumatol. Port. 2019;44:138–144. [PubMed] [Google Scholar]
- 86.Patel S., Dhillon M.S., Aggarwal S., Marwaha N., Jain A. Treatment with Platelet-Rich Plasma Is More Effective Than Placebo for Knee Osteoarthritis: A Prospective, Double-Blind, Randomized Trial. Am. J. Sports Med. 2013;41:356–364. doi: 10.1177/0363546512471299. [DOI] [PubMed] [Google Scholar]
- 87.Tavassoli M., Janmohammadi N., Hosseini A., Khafri S., Esmaeilnejad-Ganji S.M. Single- and double-dose of platelet-rich plasma versus hyaluronic acid for treatment of knee osteoarthritis: A randomized controlled trial. World J. Orthop. 2019;10:310–326. doi: 10.5312/wjo.v10.i9.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu Y.-T., Hsu K.-C., Li T.-Y., Chang C.-K., Chen L.-C. Effects of Platelet-Rich Plasma on Pain and Muscle Strength in Patients with Knee Osteoarthritis. Am. J. Phys. Med. Rehabil. 2018;97:248–254. doi: 10.1097/PHM.0000000000000874. [DOI] [PubMed] [Google Scholar]
- 89.Lin K.-Y., Yang C.-C., Hsu C.-J., Yeh M.-L., Renn J.-H. Intra-articular Injection of Platelet-Rich Plasma Is Superior to Hyaluronic Acid or Saline Solution in the Treatment of Mild to Moderate Knee Osteoarthritis: A Randomized, Double-Blind, Triple-Parallel, Placebo-Controlled Clinical Trial. Arthrosc. J. Arthrosc. Relat. Surg. 2019;35:106–117. doi: 10.1016/j.arthro.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 90.Sánchez M., Fiz N., Azofra J., Usabiaga J., Aduriz Recalde E., Garcia Gutierrez A., Albillos J., Gárate R., Aguirre J.J., Padilla S., et al. A Randomized Clinical Trial Evaluating Plasma Rich in Growth Factors (PRGF-Endoret) Versus Hyaluronic Acid in the Short-Term Treatment of Symptomatic Knee Osteoarthritis. Arthros. J. Arthrosc. Relat. Surg. 2012;28:1070–1078. doi: 10.1016/j.arthro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 91.Yu W., Xu P., Huang G., Liu L. Clinical therapy of hyaluronic acid combined with platelet-rich plasma for the treatment of knee osteoarthritis. Exp. Ther. Med. 2018 doi: 10.3892/etm.2018.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Andia I., Maffulli N. New biotechnologies for musculoskeletal injuries. Surgeon. 2019;17:244–255. doi: 10.1016/j.surge.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 93.Dupley L., Charalambous C.P. Platelet-Rich Plasma Injections as a Treatment for Refractory Patellar Tendinosis: A Meta-Analysis of Randomised Trials. Knee Surg. Relat. Res. 2017;29:165–171. doi: 10.5792/ksrr.16.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen P.-C., Wu K.-T., Chou W.-Y., Huang Y.-C., Wang L.-Y., Yang T.-H., Siu K.-K., Tu Y.-K. Comparative Effectiveness of Different Nonsurgical Treatments for Patellar Tendinopathy: A Systematic Review and Network Meta-analysis. Arthrosc. J. Arthrosc. Relat. Surg. 2019;35:3117–3131.e2. doi: 10.1016/j.arthro.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 95.Grassi A., Napoli F., Romandini I., Samuelsson K., Zaffagnini S., Candrian C., Filardo G. Is Platelet-Rich Plasma (PRP) Effective in the Treatment of Acute Muscle Injuries? A Systematic Review and Meta-Analysis. Sports Med. 2018;48:971–989. doi: 10.1007/s40279-018-0860-1. [DOI] [PubMed] [Google Scholar]
- 96.Bubnov R. Ultrasound guided injections of Platelets Rich Plasma for muscle injury in professional athletes. Comparative study. Med. Ultrason. 2013;15:101–105. doi: 10.11152/mu.2013.2066.152.rb1vy2. [DOI] [PubMed] [Google Scholar]
- 97.Roffi A., Di Matteo B., Krishnakumar G.S., Kon E., Filardo G. Platelet-rich plasma for the treatment of bone defects: From pre-clinical rational to evidence in the clinical practice. A systematic review. Int. Orthop. 2017;41:221–237. doi: 10.1007/s00264-016-3342-9. [DOI] [PubMed] [Google Scholar]
- 98.Muchedzi T.A., Roberts S.B. A systematic review of the effects of platelet rich plasma on outcomes for patients with knee osteoarthritis and following total knee arthroplasty. Surgeon. 2018;16:250–258. doi: 10.1016/j.surge.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 99.Laver L., Marom N., Dnyanesh L., Mei-Dan O., Espregueira-Mendes J., Gobbi A. PRP for Degenerative Cartilage Disease: A Systematic Review of Clinical Studies. Cartilage. 2017;8:341–364. doi: 10.1177/1947603516670709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Davey M.S., Hurley E.T., Withers D., Moran R., Moran C.J. Anterior Cruciate Ligament Reconstruction with Platelet-Rich Plasma: A Systematic Review of Randomized Control Trials. Arthrosc. J. Arthrosc. Relat. Surg. 2020;36:1204–1210. doi: 10.1016/j.arthro.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 101.Zhang H., Wang C., Li H., Huang Y., Li Z. Intra-articular platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: A meta-analysis. Drug Des. Dev. Ther. 2018;12:445–453. doi: 10.2147/DDDT.S156724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vilchez-Cavazos F., Millán-Alanís J.M., Blázquez-Saldaña J., Álvarez-Villalobos N., Peña-Martínez V.M., Acosta-Olivo C.A., Simental-Mendía M. Comparison of the Clinical Effectiveness of Single Versus Multiple Injections of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Systematic Review and Meta-analysis. Orthop. J. Sports Med. 2019;7:232596711988711. doi: 10.1177/2325967119887116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hanisch K., Wedderkopp N. Platelet-rich plasma (PRP) treatment of noninsertional Achilles tendinopathy in a two case series: No significant difference in effect between leukocyte-rich and leukocyte-poor PRP. Orthop. Res. Rev. 2019;11:55–60. doi: 10.2147/ORR.S187638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yerlikaya M., Talay Çaliş H., Tomruk Sütbeyaz S., Sayan H., Ibiş N., Koç A., Karakükçü Ç. Comparison of Effects of Leukocyte-Rich and Leukocyte-Poor Platelet-Rich Plasma on Pain and Functionality in Patients with Lateral Epicondylitis. Arch. Rheumatol. 2018;33:73–79. doi: 10.5606/ArchRheumatol.2018.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Riboh J.C., Saltzman B.M., Yanke A.B., Fortier L., Cole B.J. Effect of Leukocyte Concentration on the Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2016;44:792–800. doi: 10.1177/0363546515580787. [DOI] [PubMed] [Google Scholar]
- 106.Lana J.F., Huber S.C., Purita J., Tambeli C.H., Santos G.S., Paulus C., Annichino-Bizzacchi J.M. Leukocyte-rich PRP versus leukocyte-poor PRP—The role of monocyte/macrophage function in the healing cascade. J. Clin. Orthop. Trauma. 2019;10:S7–S12. doi: 10.1016/j.jcot.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Braun H.J., Kim H.J., Chu C.R., Dragoo J.L. The Effect of Platelet-Rich Plasma Formulations and Blood Products on Human Synoviocytes: Implications for Intra-articular Injury and Therapy. Am. J. Sports Med. 2014;42:1204–1210. doi: 10.1177/0363546514525593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Murray I.R., Chahla J., Safran M.R., Krych A.J., Saris D.B.F., Caplan A.I., LaPrade R.F., Caplan A.I., Cole B.J., Guilak F., et al. International Expert Consensus on a Cell Therapy Communication Tool: DOSES. J. Bone Jt. Surg. 2019;101:904–911. doi: 10.2106/JBJS.18.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.OCEBM Levels of Evidence Working Group Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence Oxford Centre for Evidence-Based Medicine. [(accessed on 30 April 2020)]; Available online: https://www.cebm.net/index.aspx?o=5653.
- 110.Luo D., Wan X., Liu J., Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 111.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Higgins J., Thomas J., Chandler J., Cumpston M., Li T., Page M., Welch V. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019) [(accessed on 30 March 2020)]; Available online: www.training.cochrane.org/handbook.
- 113.R Core Team A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria. [(accessed on 15 March 2020)]; Available online: http://www.R-project.org/