Key Points
Questions
What should be first-line treatment in the management of insomnia disorder, and is there an added value to providing a second treatment for those who fail initial therapy?
Findings
In a randomized clinical trial of 211 adults with insomnia disorder, first-stage treatment involving behavior therapy or zolpidem medication produced similar response and remission rates. Adding a second-stage therapy significantly increased the percentage of responders and remitters among patients treated initially with behavior therapy but not among those treated initially with medication.
Meaning
Sequential treatments involving cognitive behavioral therapy and medication are an effective strategy for insomnia management.
Abstract
Importance
Despite evidence of efficacious psychological and pharmacologic therapies for insomnia, there is little information about what first-line treatment should be and how best to proceed when initial treatment fails.
Objective
To evaluate the comparative efficacy of 4 treatment sequences involving psychological and medication therapies for insomnia and examine the moderating effect of psychiatric disorders on insomnia outcomes.
Design, Setting, and Participants
In a sequential multiple-assignment randomized trial, patients were assigned to first-stage therapy involving either behavioral therapy (BT; n = 104) or zolpidem (zolpidem; n = 107), and patients who did not remit received a second treatment involving either medication (zolpidem or trazodone) or psychological therapy (BT or cognitive therapy [CT]). The study took place at Institut Universitaire en Santé Mentale de Québec, Université Laval, Québec City, Québec, Canada, and at National Jewish Health, Denver, Colorado, and enrollment of patients took place from August 2012 through July 2017.
Main Outcomes and Measures
The primary end points were the treatment response and remission rates, defined by the Insomnia Severity Index total score.
Results
Patients included 211 adults (132 women; mean [SD] age, 45.6 [14.9] years) with a chronic insomnia disorder, including 74 patients with a comorbid anxiety or mood disorder. First-stage therapy with BT or zolpidem produced equivalent weighted percentages of responders (BT, 45.5%; zolpidem, 49.7%; OR, 1.18; 95% CI, 0.60-2.33) and remitters (BT, 38.03%; zolpidem, 30.3%; OR, 1.41; 95% CI, 0.75-2.65). Second-stage therapy produced significant increases in responders for the 2 conditions, starting with BT (BT to zolpidem, 40.6% to 62.7%; OR, 2.46; 95% CI, 1.14-5.30; BT to CT, 50.1% to 68.2%; OR, 2.09; 95% CI, 1.01-4.35) but no significant change following zolpidem treatment. Significant increase in percentage of remitters was observed in 2 of 4 therapy sequences (BT to zolpidem, 38.1% to 55.9%; OR, 2.06; 95% CI, 1.04-4.11; zolpidem to trazodone, 31.4% to 49.4%; OR, 2.13; 95% CI, 0.91-5.00). Although response/remission rates were lower among patients with psychiatric comorbidity, treatment sequences that involved BT followed by CT or zolpidem followed by trazodone yielded better outcomes for patients with comorbid insomnia. Response and remission rates were well sustained through the 12-month follow-up.
Conclusions and Relevance
Behavioral therapy and zolpidem medication produced equivalent response and remission rates. Adding a second treatment produced an added value for those whose insomnia failed to remit with initial therapies.
Trial Registration
ClinicalTrials.gov Identifier: NCT01651442
This randomized clinical trial evaluates the comparative efficacy of 4 treatment sequences involving psychological and medication therapies for insomnia and examines the moderating effect of psychiatric disorders on insomnia outcomes.
Introduction
Insomnia is a prevalent and often persistent condition1,2,3 that carries significant burden for the individual and society.4,5,6,7,8 Treatment options include various classes of medications (eg, benzodiazepine-receptor agonists and sedating antidepressants), psychological therapies (ie, cognitive behavioral therapies [CBT]), and complementary and alternative therapies (eg, acupuncture).9,10 Medication produces rapid relief and is widely available, but there are concerns about potential adverse effects (eg, daytime sedation) and risks of tolerance and dependence. Furthermore, there are limited data documenting sustained benefits with prolonged use. In contrast, CBT has minimal adverse effects, is preferred by many patients, and results in sustained sleep improvements. However, CBT is more time consuming and has a slower action than medications. In addition, CBT is not readily available despite efforts to increase access and facilitate its implementation through abbreviated and digital programs.11 Evidence from meta-analyses and practice guidelines indicates that CBT should be the first-line therapy, whereas medications may be indicated when CBT is not effective or not available.12,13,14,15,16
The few comparative studies of CBT and medications for insomnia showed little difference in short-term outcomes but superior longer-term outcomes with CBT.17,18,19,20 In contrast, a sequential treatment starting with combined CBT/medication followed by an extended CBT while tapering medication proved superior to continued long-term combined therapy or CBT provided without medication.21 These studies, while informative, are limited by their use of single-agent strategies and their focus on patients with primary insomnia. Hence, these findings provide limited guidance for deciding on optimal first-stage therapy and for the more difficult patients with comorbid psychiatric illnesses. In addition, given that a substantial proportion of patients have insomnia that fails to remit with first-stage therapy, switching from 1 therapy to another on a trial-error basis is common practice. To our knowledge, no study has examined which first-stage treatment is optimal for different insomnias with and without comorbidity and which second-stage treatment offers the best added value for patients whose insomnia does not remit with psychological or medication first-stage therapy.
This study evaluated the short-term and long-term outcomes of 4 treatment sequences using psychological (behavioral and cognitive) and pharmacologic therapies (zolpidem and trazodone) for insomnia with and without comorbid psychiatric disorders. Research questions included (1) what the initial treatment should be, (2) whether there is an added value to offering a second treatment to those who do not achieve remission with initial therapy, and (3) whether there is a moderating effect of psychiatric comorbidity on outcomes.
Methods
Study Design and Overview
This was a single-blinded, randomized clinical trial conducted at 2 sites: Institut Universitaire en Santé Mentale de Québec, Université Laval, Québec City, Québec, Canada, and at National Jewish Health, Denver, Colorado. Local ethics committees approved the protocol, and all participants provided written informed consent. Enrollment of patients took place from August 2012 through July 2017. A complete description of the study protocol is available elsewhere and in Supplement 1.22
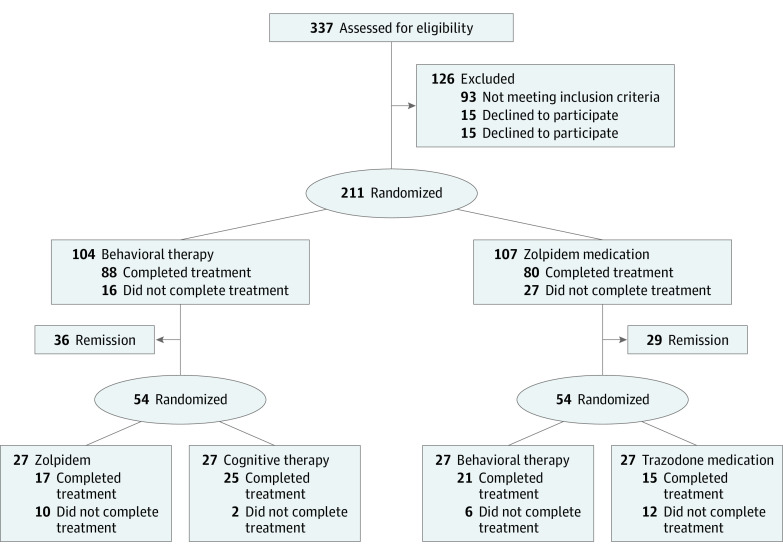
There were 2 treatment stages and 2 treatment arms (psychological therapy: behavior therapy [BT] and cognitive therapy [CT]; and medication: zolpidem and trazodone for each stage, leading to 4 treatment sequences) (Figure 1). Participants meeting criteria (N = 211) were randomly assigned in a 1:1 ratio to BT (n = 107) or zolpidem (n = 104), stratified by sex, age (<55 years vs ≥55 years), and presence of a comorbid psychiatric disorder. The first randomization was based on a stratified (site, sex, and age) computer-generated (SAS PROC PLAN; SAS Institute Inc) list of numbers for participants’ allocation. After the initial 6-week therapy, patients in remission were followed up for the next 12 months receiving maintenance therapy, while nonremitters were randomized (stratified by first randomization and comorbidity) to a second-stage psychological (BT or CT) or drug therapy (zolpidem or trazodone). Measurements were taken at baseline, end of first-stage and second-stage therapies (ie, weeks 6 and 12), and at 3-month, 6-month, and 12-month follow-ups.
Figure 1. CONSORT Patient Flowchart.
The 54 patients randomized to second-stage therapy included all patients not in remission after first-stage therapy who accepted second randomization and a few patients who did not complete first-stage treatment according to study protocol but were available for second treatment.
Participants
A total of 211 adults meeting criteria for an insomnia disorder were recruited from the community through media advertisements and from outpatient clinics. Selection criteria and assessment instruments are described in the eMethods in Supplement 2.
Outcome Measures
The primary study end points were the percentages of individuals achieving a treatment response or remission from insomnia, using total scores from the Insomnia Severity Index (ISI).23,24 The ISI was completed at baseline, weekly throughout treatment, and at each follow-up. Response was defined at each assessment as a reduction of 8 points or more on the ISI compared with baseline score. Remission was defined at each assessment as an ISI score less than 8. Secondary outcomes were derived from sleep diaries.
Treatments
Cognitive Behavioral Therapy
First-stage psychological therapy consisted of BT, which included sleep restriction25 and stimulus control procedures.26 Although full CBT is the treatment of choice, we used only its core behavioral elements as first-stage therapy because it directly targets behavioral factors that exacerbate insomnia and because it is briefer and easier to deliver and transfer to clinical settings.
Second-stage psychological treatment consisted of CT, which focuses on sleep- and mood-disruptive cognitions (ie, thoughts and beliefs) that exacerbate the vicious cycle of insomnia, such as misconceptions about sleep needs, perceived consequences, and worries about sleep loss.27,28 We chose CT as second-stage therapy because it requires more time and training to implement than BT and may not be essential for all patients. Yet, because of its unique features in targeting perpetuating mechanisms (eg, worries) shared with some comorbid psychiatric disorders (eg, anxiety and depression)29 and not addressed by BT, the addition of CT as second-stage therapy provided an opportunity to evaluate its unique contribution to outcomes among patients with comorbid psychiatric disorders.
Medication
The first-stage medication treatment involved zolpidem, sublingual, 5 mg to 10 mg, taken nightly at bedtime. zolpidempidem was selected as a first-stage therapy because of its documented efficacy and because it is among the most prescribed medications for insomnia.14,30,31,32 All participants started with an initial dose of 5 mg, which was titrated up to 10 mg based on therapeutic response, adverse effects, and patient’s age and sex (per US Food and Drug Administration recommendations, dosage was limited to 5 mg in women). The clinician provided support during medication consultation visits, but no behavioral or cognitive intervention was allowed. Medications were dispensed by the pharmacy at each site.
The second-stage pharmacotherapy consisted of trazodone (50-150 mg) taken 30 minutes before bedtime. We chose trazodone because it is among the most commonly prescribed medications for insomnia (off-label).33,34 It has a different mechanism of action than benzodiazepine receptor agonists and has shown efficacy for insomnia cooccurring with major depression.35,36
Additional information about treatment implementation and monitoring of compliance is provided in the eMethods in Supplement 2.
Statistical Analyses
To evaluate each treatment sequence while taking into account the nature of the sequential multiple assignment randomized trial design (ie, 2 randomizations, where the second is conditional on the response to the first), the analytic strategy was based on Nahum-Shani recommendations (2012).37 Percentages of response/remission according to 4 treatment sequences and 5 times (assessment after first-stage treatment [post1] to follow-up at 12 months) were analyzed using a weighted logistic (binary outcome) generalized estimating equations model.38 Strata variables (site, age, sex, and comorbidity status) and baseline ISI were included as covariates.39 A priori contrasts within the weighted logistic generalized estimating equations models were used to test significance for comparisons between and within sequences.
Power analyses were computed for weighted generalized estimating equations models40 using standard power settings (2-tailed 5% α; 80% power) and estimates from previous studies (10% attrition rate and 30%-45% remission rate). Because effect size estimates were not available for sequential therapies, sensitivity analyses were preferred. Sample size for expected differences for the primary hypotheses was estimated at 224 participants for first-stage randomization (additional information on statistical and power analyses is available in the study protocol in Supplement 1).22
Results
Patients
Patients were 211 adults (132 women; mean [SD] age, 45.6 [14.9] years) meeting criteria for insomnia disorder (mean duration, 13.2 years). Seventy-four patients (35%) had a comorbid psychiatric disorder (eg, anxiety and depression), and 137 (68%) presented with at least 1 comorbid medical disorder (eg, hypertension) (Table 1).
Table 1. Sociodemographic and Clinical Characteristics of Participants.
| Characteristic | No (%) | ||
|---|---|---|---|
| Behavioral therapy (n = 104) | Zolpidempidem (n = 107) | Total sample (n = 211) | |
| Study site | |||
| Université Laval, Québec | 59 (57) | 62 (58) | 121 (57) |
| National Jewish Health, Denver | 45 (43) | 45 (42) | 90 (43) |
| Age, mean (SD), y | 45.9 (14.4) | 45.4 (15.5) | 45.6 (14.9) |
| Sex | |||
| Female | 64 (62) | 68 (64) | 132 (63) |
| Male | 40 (39) | 39 (36) | 79 (37) |
| Race/ethnicity | |||
| White | 92 (88) | 90 (84) | 182 (86) |
| Black | 6 (6) | 8 (8) | 14 (7) |
| Other | 2 (2) | 2 (2) | 4 (2) |
| Hispanic | 4 (4) | 7 (7) | 11 (5) |
| Education, mean (SD), y | 16.3 (2.6) | 16.0 (3.8) | 16.1 (3.3) |
| Occupation | |||
| Employed | |||
| Full time | 53 (52) | 57 (53) | 110 (52) |
| Part time | 11 (11) | 25 (23) | 36 (17) |
| Student | 6 (6) | 4 (4) | 10 (5) |
| Unemployed | 13 (13) | 4 (4) | 17 (8) |
| Retired | 20 (19) | 17 (16) | 37 (18) |
| Duration of insomnia, mean (SD), y | 13.9 (12.3) | 12.5 (12.6) | 13.2 (12.5) |
| Psychiatric comorbidity | 36 (35) | 38 (36) | 74 (35) |
| Medical comorbidity | 66 (67) | 71 (69) | 137 (68) |
| Use of sleep-promoting prescribed medication in the last year | 25 (26) | 24 (23) | 49 (25) |
| Use of psychotropic medication at baseline (other than sleep-promoting) | 18 (18) | 17 (16) | 35 (17) |
Of the 211 randomized patients, 43 did not complete first-stage therapy, including 15.3% in BT (16 of 104) and 25.2% (27 of 107) in the zolpidem condition (χ21, 2.03; P = .15). Of the unremitted and available participants after stage 1 therapy, 108 participants accepted randomization to second-stage treatment (27 per condition). Of those, 30 participants (27.8%) did not complete second-stage treatment (BT+zolpidem, 10; BT+CT, 2; zolpidem+BT, 6; and zolpidem+trazodone, 12). Cumulative attrition was significantly higher for the 2 treatment sequences starting with zolpidem treatment relative to those starting with BT (χ23, 10.96; P = .01).
Treatment Response and Remission
Response Rates
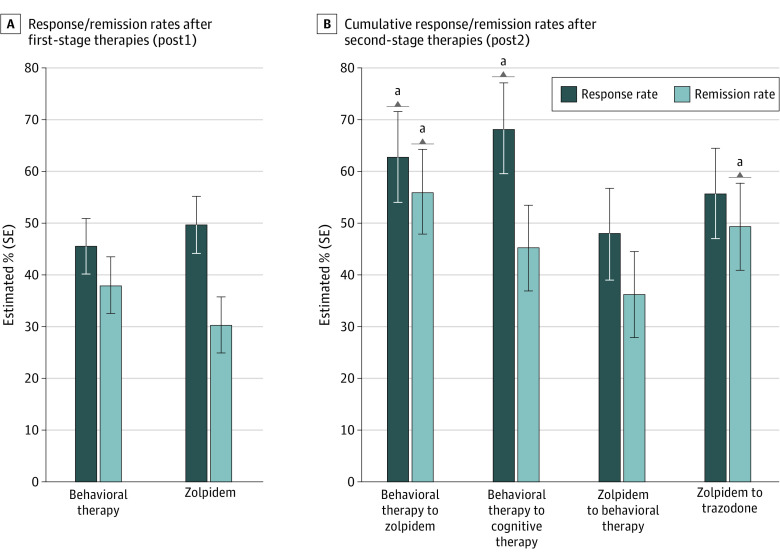
Percentages of responders (adjusted means and standard errors) by conditions and assessments are shown in Figure 2. After controlling for weighted covariates and missing data, both first-stage therapies produced similar percentages of responders (BT, 45.5%; zolpidem, 49.7%; OR, 1.18; 95% CI, 0.60-2.33). For patients whose insomnia did not remit with first-stage therapy, significant increases in responders were observed with second-stage therapy for the BT+zolpidem sequence (from 40.6% to 62.7%; OR, 2.46;95% CI, 1.14-5.30) and the BT+CT sequence (from 50.6% to 68.2%; OR, 2.09; 95% CI, 1.01-4.35). Conversely, no significant change was observed in response rates for the zolpidem+BT condition (52.9%-47.9%; P = .59) and the zolpidem+trazodone condition (46.4%-55.7%; P = .44). Overall comparisons of cumulative response rates across all 4 sequences after second-stage therapy (post2) failed to reach significance, although percentages of responders tended to be higher for the 2 sequences starting with BT (BT+zolpidem, 62.7%; BT+CT, 68.2%) than those starting with zolpidem (zolpidem+BT, 47.9%; zolpidem+trazodone, 55.7%; OR, 1.76; 95% CI, 0.85-3.65). The largest difference was between BT+CT and zolpidem+BT (OR, 2.33; 95% CI, 1.10-5.52).
Figure 2. Response and Remission Rates After Stage 1 and Stage 2 Therapies.
aP <.05.
Remission Rates
Percentages of patients in remission according to conditions and assessments are displayed in Figure 3. At post1, there was no significant difference in percentage of remitters between BT (38.0%) and zolpidem (30.3%), after controlling for covariates and missing data (OR, 1.41; 95% CI, 0.75-2.65). However, the addition of second-stage therapy produced significant increases of remitters in the BT+zolpidem condition (from 38.1% to 55.9%; OR, 2.06; 95% CI, 1.04-4.11) and a nonsignificant increase in the zolpidem+trazodone condition (from 31.4% to 49.4%; OR, 2.13; 95% CI, 0.91-5.00). Small but nonsignificant increases were observed in the BT+CT condition (38.0% to 45.2%; P = .33) and the ZOL+BT condition (29.2% to 36.2%; P = .41). There were no significant overall differences in cumulative remission rates at post2 (BT+zolpidem, 55.9%; BT+CT, 45.2%; zolpidem+BT, 36.2%; zolpidem+trazodone, 49.4%; P = .39).
Figure 3. Response and Remission Rates According to Condition and Assessment.
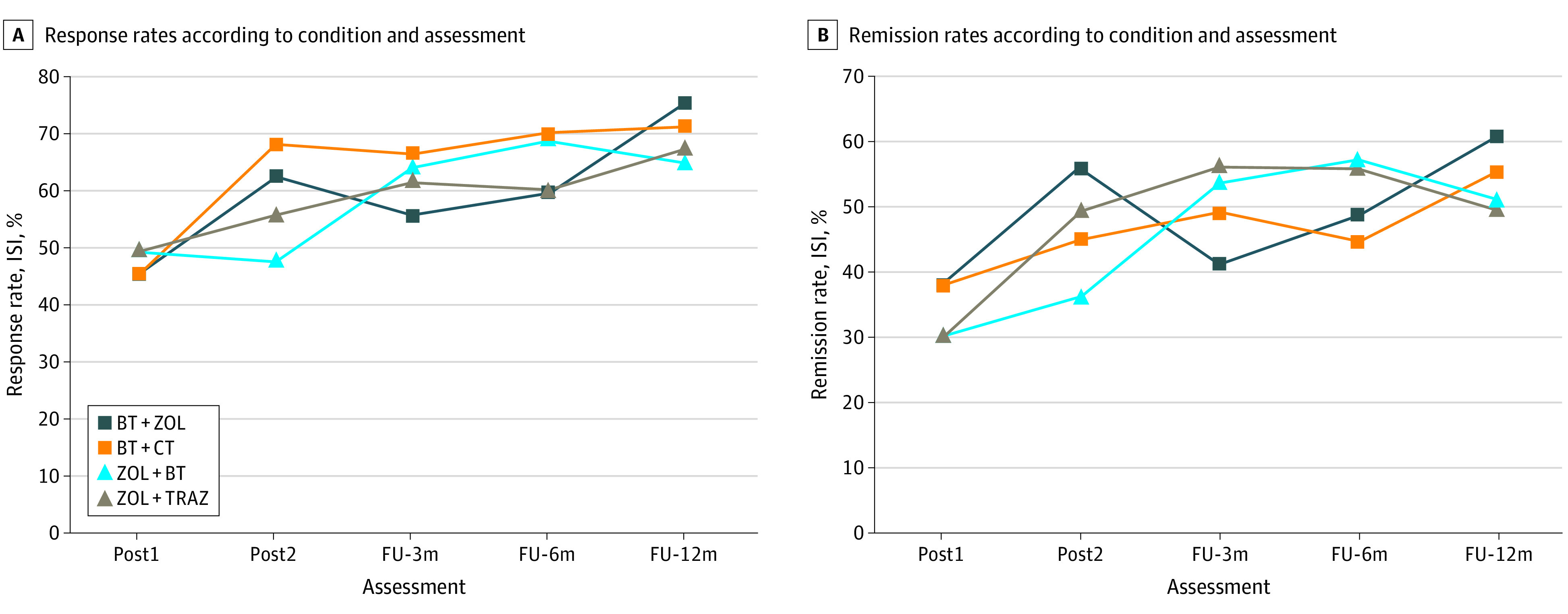
A, Standard errors are 0.06 at post1 and ranged from 0.07 to 0.09 for latter assessments. B, Standard errors are 0.05 at post1 and ranged from 0.07 to 0.09 for latter assessments. BT indicates behavioral therapy; CT, cognitive therapy; FU, follow-up; ISI, Insomnia Severity Index; TRAZ, trazodone; ZOL, zolpidem.
Follow-up Assessments
Improvements achieved at the end of first-stage and second-stage therapies were well sustained over time (Figure 3A and B). The percentages of responders remained stable from post2 to 12-month follow-ups for the BT+CT (post2, 68.2%; 3-month follow-up, 66.6%; 6-month follow-up, 69.9%; and 12-month follow-up, 71.4%; time effect P = .96) and zolpidem+trazodone sequences (55.7%, 61.8%, 60.3%, and 67.4%, respectively; P = .73). Further increases of response rates were observed for the other 2 sequences, BT+zolpidem (62.7%, 55.8%, 59.8%, and 75.4%; P = .07) and zolpidem+BT (47.9%, 64.2%, 69.1%, and 65.0%; P = .04). Remission in 3 sequences remained stable through follow-ups for BT+CT (45.2%, 49.1%, 44.8%, and 55.5%; P = .40), zolpidem+BT (36.2%, 53.8%, 57.3%, and 51.1%; P = .35), and zolpidem+trazodone (49.4%, 56.2%, 56.1%, and 49.7%; P = .82). Between-group comparisons of responder and remitter rates were nonsignificant at each follow-up.
Effect of Psychiatric Comorbidity on Treatment Outcome
The moderating role of psychiatric comorbidity on response/remission rates was investigated by adding a comorbidity × condition × assessment interaction. Simple effects were examined to compare response and remission rates according to comorbidity status for each treatment sequence. At post1, there was a nonsignificant higher response among patients without comorbidity relative to those with comorbidity (53.9% vs 36.4%; OR, 2.04; 95% CI, 0.96-4.35). This trend was present for both patients treated with BT (52.1% vs 34.3%; OR, 2.09; 95% CI, 0.75-5.83) and zolpidem (55.6% vs 38.5%; OR, 2.00; 95% CI, 0.70-5.70). At post2, the inverse result was found as patients with comorbidity showed a significantly higher response rate than those without comorbidity (74.2% vs 53.6%; OR, 2.48; 95% CI, 1.01-6.22). This result was best explained by the simple main effects showing that patients with psychiatric comorbidity and receiving a treatment sequence that involved 2 therapies within the same modality (ie, 2 psychological or 2 medication treatments) did best overall. Hence, patients with psychiatric comorbidity who received BT+CT (58.3% vs 85.3%; OR, 27.02; 95% CI, 2.24-322.6) or zolpidem+trazodone (40.0% vs 77.5%; OR, 4.80; 95% CI, 0.66-35.0) had higher response rates than patients who switched treatment (ie, BT to zolpidem or zolpidem to BT).
With regard to remission at post1, there were also higher overall remission rates among patients without comorbidity, regardless of treatment (41.6% vs 19.7%; OR, 2.90; 95% CI, 1.34-6.26). Higher remission rates were observed in BT (44.2% vs 26.2%, although it did not reach significance; OR, 2.24; 95% CI, 0.83-6.01), and in zolpidem (39.01% vs 14.5%; OR, 3.77; 95% CI, 1.21-11.69). At post2, similar remission rates were observed in patients with or without comorbidity (47.1% vs 45.6%; P = .89). Consistent with the response rates, within the subgroup of patients with comorbidity, those who received 2 treatments within the same modality (ie, 2 psychological or 2 medication treatments) had slightly higher remission rates, albeit nonsignificant, relative to those who switched modality: BT+CT (51.50% vs 42.50%) or zolpidem+trazodone (61.41% vs 39.81%), BT+zolpidem (62.61% vs 42.58%), and zolpidem+BT (37.50% vs 33.25%) (eResults and eFigure in Supplement 2).
Sleep Diary Data
Table 2 shows adjusted means and standard errors for sleep/wake variables derived from the patients’ electronic daily diaries. Both first-stage therapies produced significant reduction of sleep onset latency (SOL) and time awake after sleep onset (WASO), with significantly larger effects for BT relative to zolpidem (−21.1 vs −11.7 for SOL; P = .04, Cohen d = 0.37; and −33.0 vs −16.6; P = .01; d = 0.53 for WASO). A further WASO reduction was observed at post2 in the zolpidem+BT sequence (mean [SE], −10.5 [5.7]; P = .05; d = 0.34), while a significant increase was observed in the BT+CT condition (9.1 [4.3]; P = .03; d = 0.30). No additional changes observed in the other conditions. Comparisons of the 4 sequences at post2 failed to yield significant group differences for either SOL or WASO.
Table 2. Estimated Means (SE) and Significance of Temporal Changes for Sleep Diary Data.
| Stage1 | Mean (SE) | Stage 2 | Mean (SE) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretreatment (a) | Posttreatment 1 (b) | Change (b-a) | P value | Posttreatment 2 (c) | Change (c-b) | P value | Follow-up | ||||
| 3 mo | 6 mo | 12 mo | |||||||||
| Sleep onset latency, min | |||||||||||
| BT | 42.2 (3.6) | 21.1 (1.8) | −21.1 (3.2) | <.001 | BT+ZOL | 20.3 (1.9) | −1.5 (2.6) | .56 | 22.0 (3.0) | 23.6 (3.2) | 21.7 (3.2) |
| BT+CT | 18.7 (2.5) | −1.6 (2.6) | .53 | 19.3 (1.8) | 20.5 (2.4) | 18.4 (2.1) | |||||
| ZOL | 41.6 (4.1) | 29.9 (3.8) | −11.7 (3.5) | <.001 | ZOL+BT | 22.3 (4.1) | −8.4 (4.5) | .06 | 17.0 (2.6) | 16.3 (2.4) | 16.2 (2.0) |
| ZOL+trazodone | 17.6 (4.5) | −11.5 (6.4) | .07 | 18.5 (3.0) | 21.0 (3.3) | 21.2 (3.3) | |||||
| Wake after sleep onset, min | |||||||||||
| BT | 60.6 (3.9) | 27.6 (2.1) | −33.0 (3.6) | <.001 | BT+ZOL | 31.2 (5.3) | 6.2 (5.2) | .23 | 26.5 (3.7) | 26.2 (3.2) | 25.5 (3.2) |
| BT+CT | 39.3 (4.5) | 9.1 (4.3) | .03 | 34.4 (3.9) | 30.6 (3.7) | 30.5 (3.7) | |||||
| ZOL | 59.4 (3.8) | 42.8 (5.1) | −16.6 (5.5) | .003 | ZOL+BT | 31.2 (5.3) | −10.5 (5.7) | .06 | 29.7 (4.8) | 28.9 (4.8) | 32.7 (8.0) |
| ZOL+trazodone | 32.0 (8.1) | −11.9 (9.8) | .22 | 20.9 (3.5) | 22.0 (4.4) | 19.2 (3.7) | |||||
| Total sleep time, min | |||||||||||
| BT | 360.4 (8.6) | 370.9 (7.5) | 10.6 (5.9) | .07 | BT+ZOL | 356.3 (16.1) | −9.6 (13.5) | .48 | 398.4 (8.6) | 404.7 (9.3) | 414.9 (7.4) |
| BT+CT | 371.4 (14.0) | −4.7 (9.9) | .64 | 400.8 (9.4) | 401.0 (11.1) | 407.4 (11.8) | |||||
| ZOL | 357.6 (9.6) | 390.7 (12.2) | 33.1 (11.7) | .005 | ZOL+BT | 390.4 (16.7) | 1.9 (17.0) | .91 | 411.6 (12.1) | 413.7 (11.1) | 408.0 (11.7) |
| ZOL+trazodone | 431.5 (19.8) | 38.6 (23.6) | .10 | 455.7 (8.3) | 451.5 (12.4) | 448.1 (10.1) | |||||
| Sleep efficiency, % | |||||||||||
| BT | 71.3 (1.4) | 83.7 (0.9) | 12.5 (1.1) | <.001 | BT+ZOL | 81.5 (1.9) | −2.1 (2.1) | .32 | 83.9 (1.3) | 84.5 (1.4) | 85.7 (1.4) |
| BT+CT | 81.8 (1.9) | −2.1 (1.5) | .14 | 84.2 (1.3) | 81.9 (1.4) | 85.4 (1.2) | |||||
| ZOL | 71.8 (1.4) | 77.6 (2.2) | 5.8 (1.8) | .001 | ZOL+BT | 83.2 (1.9) | 5.9 (2.6) | .02 | 84.4 (1.8) | 85.2 (1.6) | 85.0 (1.8) |
| ZOL+trazodone | 84.5 (3.0) | 6.6 (4.1) | .11 | 87.3 (1.3) | 87.0 (1.4) | 88.1 (1.2) | |||||
Abbreviations: BT, behavior therapy; CT, cognitive therapy; ZOL, zolpidem.
Significant increase in total sleep time was made with first-stage zolpidem therapy (mean [SE], 33.1 [11.7] minutes; P = .005; d = 0.44), but not BT (10.6 [5.9] minutes; P = .07; d = 0.14), and the group difference was not significant (P = .09; d = 0.30). At post2, the largest cumulative increase, although not significant, was observed in the zolpidem+trazodone condition (38.6 minutes; d = 0.51). Comparison of all 4 sequences at post2 yielded a significant overall effect for total sleep time (χ23 = 9.13; P = .03), with the zolpidem+trazodone sleeping longer (431.5 minutes) than any of the other 3 conditions (BT+zolpidem, 356.3 minutes; BT+CT, 371.4 minutes; and zolpidem+BT, 390.4 minutes).
Significant gains in sleep efficiency were made with both first-stage therapy and were higher for BT (mean [SE], 12.5% [1.1%]; P < .001; d = 1.09) relative to zolpidem (5.8% [1.8%], P = .001; d = 0.51). At post2, the largest cumulative increase was in the zolpidem+BT condition (5.9 minutes; P = .02; d = 0.52); no change was observed in the other sequences. Comparison of the 4 sequences at post2 failed to reach significance.
Sleep onset latency and WASO improvements achieved with treatment were well sustained throughout follow-ups for all sequences, with means remaining at less than or near the 30-minute cut point typically used to define insomnia (Table 2). For total sleep time, both conditions starting with BT showed significant improvements at follow-ups (mean, 356.3 at post2 vs 414.9 minutes at 12-month follow-up for BT+zolpidem; P < .001; and mean, 371.4 vs 407.4 for BT+CT; P = .02), while results remained stable for zolpidem+BT (mean, 390.4 vs 408.0; P = .46) and ZOL+trazodone (mean, 431.5 vs 448.1; P = .56). There were significant group differences at each follow-up, with patients in zolpidem+trazodone sleeping significantly longer (from 40 minutes to nearly 1 hour) than patients in the other 3 conditions (Table 2). For sleep efficiency, only the BT+zolpidem sequence showed a significant increase, from 81.5% at post2 to 85.7% at 12-month follow-up (P = .04). No group differences were obtained at follow-ups.
Discussion
The findings show that first-stage treatment with BT or zolpidem yielded equivalent and relatively modest outcomes, while the addition of a second-stage therapy produced a significant value added in enhancing overall rates of response and remission. Of all 4 treatment sequences tested in this trial, the best sequences were those that used BT as initial treatment, followed by either CT or zolpidem. These findings are consistent with practice guidelines of several professional organizations that CBT should be the first-line treatment for the management of insomnia.12,14
The presence of psychiatric comorbidity was associated with less favorable outcome during initial therapy, but within the subgroup of patients with comorbid psychiatric disorders, response and remission rates were higher for patients who received either CT or trazodone as second-stage treatment. Thus, patients who received 2 treatments within the same modality (ie, 2 psychological or 2 medication treatments) had slightly better outcomes than those who switched modality. Unlike BT and zolpidem, which specifically focus on sleep/insomnia symptoms, CT and trazodone have a broader spectrum of action by targeting psychological/mood symptoms; granted, the trazodone dosage used in our study was probably subclinical to expect a direct effect on mood. Nonetheless, the implication of these findings is that treatment sequence for patients with comorbid insomnia and psychiatric disorders should incorporate a therapeutic component that also addresses psychological/mood disturbances. This is an important finding given the high prevalence of psychiatric comorbidity, particularly depression and anxiety, among individuals with insomnia.4 This finding is also in line with emerging evidence from the transdiagnostic literature suggesting that some key psychological constructs (eg, worries) play a contributory role across different psychiatric/sleep disorders.41,42
Secondary end points derived from sleep diaries showed that behavioral and cognitive therapies were effective in reducing sleep latency and time awake after sleep onset as well increasing sleep efficiency, whereas medications had their strongest effect by increasing TST. This benefit of sleeping longer was particularly noticeable for the treatment sequence involving zolpidem followed by trazodone. Given the emerging literature on insomnia phenotypes and the higher risk for cardiovascular morbidity among individuals with insomnia and short sleep durations, such findings could guide the development of personalized therapies for insomnia management. It is also noteworthy that treatment benefits (eg, reduced insomnia severity and improved sleep continuity and duration) were well maintained at follow-ups in all 4 treatment sequences. While long-term benefits of CBT are well documented,17,21 such long-term outcome with medication therapies is relatively novel.
Limitations
Two important methodologic limitations, such as the lack of a control group and the small sample sizes for each treatment sequence, may have reduced the power to detect more significant differences among the groups. In addition, even with cumulative response rates of 65% to 70%, such outcomes are far from optimal and leave room for more effective therapies.
Conclusions
In summary, this study documented the benefits of 4 treatment sequences involving psychological and medication therapies for insomnia disorder. Additional studies are needed to validate best treatment algorithms for insomnia disorder, and rather than randomizing patients to treatment options, perhaps a more effective strategy would involve a personalized approach matching patients with their preferred treatment, while also taking into account their insomnia phenotypes (ie, presence of hyperarousal and TST).
Trial Protocol.
eMethods.
eResults.
eFigure. Response and remission rates among patients with comorbid psychiatric disorders
Data Sharing Statement.
References
- 1.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed American Psychiatric Association; 2013. [Google Scholar]
- 2.Roth T, Jaeger S, Jin R, Kalsekar A, Stang PE, Kessler RC. Sleep problems, comorbid mental disorders, and role functioning in the national comorbidity survey replication. Biol Psychiatry. 2006;60(12):1364-1371. doi: 10.1016/j.biopsych.2006.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morin CM, Bélanger L, LeBlanc M, et al. . The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169(5):447-453. doi: 10.1001/archinternmed.2008.610 [DOI] [PubMed] [Google Scholar]
- 4.Baglioni C, Battagliese G, Feige B, et al. . Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1-3):10-19. doi: 10.1016/j.jad.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 5.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17(4):241-254. doi: 10.1016/j.smrv.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction: a population study. Circulation. 2011;124(19):2073-2081. doi: 10.1161/CIRCULATIONAHA.111.025858 [DOI] [PubMed] [Google Scholar]
- 7.Parthasarathy S, Vasquez MM, Halonen M, et al. . Persistent insomnia is associated with mortality risk. Am J Med. 2015;128(3):268-75.e2. doi: 10.1016/j.amjmed.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daley M, Morin CM, LeBlanc M, Grégoire JP, Savard J. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep. 2009;32(1):55-64. [PMC free article] [PubMed] [Google Scholar]
- 9.Buysse DJ. Insomnia. JAMA. 2013;309(7):706-716. doi: 10.1001/jama.2013.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379(9821):1129-1141. doi: 10.1016/S0140-6736(11)60750-2 [DOI] [PubMed] [Google Scholar]
- 11.van Straten A, Cuijpers P. Self-help therapy for insomnia: a meta-analysis. Sleep Med Rev. 2009;13(1):61-71. doi: 10.1016/j.smrv.2008.04.006 [DOI] [PubMed] [Google Scholar]
- 12.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians . Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133. doi: 10.7326/M15-2175 [DOI] [PubMed] [Google Scholar]
- 13.Riemann D, Baglioni C, Bassetti C, et al. . European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675-700. doi: 10.1111/jsr.12594 [DOI] [PubMed] [Google Scholar]
- 14.Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307-349. doi: 10.5664/jcsm.6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Straten A, van der Zweerde T, Kleiboer A, Cuijpers P, Morin CM, Lancee J. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3-16. doi: 10.1016/j.smrv.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 16.Wilson SJ, Nutt DJ, Alford C, et al. . British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol. 2010;24(11):1577-1601. doi: 10.1177/0269881110379307 [DOI] [PubMed] [Google Scholar]
- 17.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281(11):991-999. doi: 10.1001/jama.281.11.991 [DOI] [PubMed] [Google Scholar]
- 18.Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia: a randomized controlled trial and direct comparison. Arch Intern Med. 2004;164(17):1888-1896. doi: 10.1001/archinte.164.17.1888 [DOI] [PubMed] [Google Scholar]
- 19.Wu R, Bao J, Zhang C, Deng J, Long C. Comparison of sleep condition and sleep-related psychological activity after cognitive-behavior and pharmacological therapy for chronic insomnia. Psychother Psychosom. 2006;75(4):220-228. doi: 10.1159/000092892 [DOI] [PubMed] [Google Scholar]
- 20.Sivertsen B, Omvik S, Pallesen S, et al. . Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. JAMA. 2006;295(24):2851-2858. doi: 10.1001/jama.295.24.2851 [DOI] [PubMed] [Google Scholar]
- 21.Morin CM, Vallières A, Guay B, et al. . Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301(19):2005-2015. doi: 10.1001/jama.2009.682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morin CM, Edinger JD, Krystal AD, Buysse DJ, Beaulieu-Bonneau S, Ivers H. Sequential psychological and pharmacological therapies for comorbid and primary insomnia: study protocol for a randomized controlled trial. Trials. 2016;17(1):118. doi: 10.1186/s13063-016-1242-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297-307. doi: 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 24.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601-608. doi: 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10(1):45-56. [PubMed] [Google Scholar]
- 26.Bootzin RR, Epstein D, Wood JM. Stimulus Control Instructions In: Hauri P, ed. Case Studies in Insomnia. Plenum Press; 1991:19-28. doi: 10.1007/978-1-4757-9586-8_2 [DOI] [Google Scholar]
- 27.Harvey AG, Sharpley AL, Ree MJ, Stinson K, Clark DM. An open trial of cognitive therapy for chronic insomnia. Behav Res Ther. 2007;45(10):2491-2501. doi: 10.1016/j.brat.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 28.Morin CM, Espie CA. Insomnia: A Clinical Guide to Assessment and Treatment. Kluwer Academic/Plenum; 2003. [Google Scholar]
- 29.Harvey AG. Insomnia, psychiatric disorders, and the transdiagnostic perspective. Curr Dir Psychol Sci. 2008;17(5):299-303. doi: 10.1111/j.1467-8721.2008.00594.x [DOI] [Google Scholar]
- 30.Cascade EF, Kalali AH, Reites J. Current status of hypnotic prescribing habits in the United States. Psychiatry (Edgmont). 2007;4(9):24-25. [PMC free article] [PubMed] [Google Scholar]
- 31.Wilt TJ, MacDonald R, Brasure M, et al. . Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the american college of physicians. Ann Intern Med. 2016;165(2):103-112. doi: 10.7326/M15-1781 [DOI] [PubMed] [Google Scholar]
- 32.Asnis GM, Chakraburtty A, DuBoff EA, et al. . zolpidempidem for persistent insomnia in SSRI-treated depressed patients. J Clin Psychiatry. 1999;60(10):668-676. doi: 10.4088/JCP.v60n1005 [DOI] [PubMed] [Google Scholar]
- 33.Walsh JK. Drugs used to treat insomnia in 2002: regulatory-based rather than evidence-based medicine. Sleep. 2004;27(8):1441-1442. doi: 10.1093/sleep/27.8.1441 [DOI] [PubMed] [Google Scholar]
- 34.Krystal AD. A compendium of placebo-controlled trials of the risks/benefits of pharmacological treatments for insomnia: the empirical basis for U.S. clinical practice. Sleep Med Rev. 2009;13(4):265-274. doi: 10.1016/j.smrv.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 35.Nierenberg AA, Adler LA, Peselow E, Zornberg G, Rosenthal M. trazodoneodone for antidepressant-associated insomnia. Am J Psychiatry. 1994;151(7):1069-1072. doi: 10.1176/ajp.151.7.1069 [DOI] [PubMed] [Google Scholar]
- 36.Walsh JK, Erman M, Erwin C. Subjective hypnotic efficacy of trazodone and zolpidem in DSM-III-R primary insomnia. Hum Psychopharmacol. 1998;13:191-198. doi: [DOI] [Google Scholar]
- 37.Nahum-Shani I, Qian M, Almirall D, et al. . Experimental design and primary data analysis methods for comparing adaptive interventions. Psychol Methods. 2012;17(4):457-477. doi: 10.1037/a0029372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robins JM, Rotnitzky A, Zhao L. Analysis of semiparametric regression models for repeated outcomes in the presence of missing data. J Am Stat Assoc. 1995;90(429):106-121. doi: 10.1080/01621459.1995.10476493 [DOI] [Google Scholar]
- 39.European Medicines Agency (EMA) Guideline on Adjustment for Baseline Covariates in Clinical Trials (No. EMA/CHMP/295050/2013). European Medicines Agency; 2015. [Google Scholar]
- 40.Dahmen G, Rochon J, König IR, Ziegler A. Sample size calculations for controlled clinical trials using generalized estimating equations (GEE). Methods Inf Med. 2004;43(5):451-456. doi: 10.1055/s-0038-1633896 [DOI] [PubMed] [Google Scholar]
- 41.Harvey AG, Dong L, Bélanger L, Morin CM. Mediators and treatment matching in behavior therapy, cognitive therapy and cognitive behavior therapy for chronic insomnia. J Consult Clin Psychol. 2017;85(10):975-987. doi: 10.1037/ccp0000244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harvey AG, Murray G, Chandler RA, Soehner A. Sleep disturbance as transdiagnostic: consideration of neurobiological mechanisms. Clin Psychol Rev. 2011;31(2):225-235. doi: 10.1016/j.cpr.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eMethods.
eResults.
eFigure. Response and remission rates among patients with comorbid psychiatric disorders
Data Sharing Statement.