Abstract
Pyruvate kinase deficiency (PKD) is the most common enzyme defect of glycolysis and an important cause of hereditary, nonspherocytic hemolytic anemia. The disease has a worldwide geographical distribution but there are no verified data regarding its frequency. Difficulties in the diagnostic workflow and interpretation of PK enzyme assay likely play a role. By the creation of a global PKD International Working Group in 2016, involving 24 experts from 20 Centers of Expertise we studied the current gaps in the diagnosis of PKD in order to establish diagnostic guidelines. By means of a detailed survey and subsequent discussions, multiple aspects of the diagnosis of PKD were evaluated and discussed by members of Expert Centers from Europe, USA, and Asia directly involved in diagnosis. Broad consensus was reached among the Centers on many clinical and technical aspects of the diagnosis of PKD. The results of this study are here presented as recommendations for the diagnosis of PKD and used to prepare a diagnostic algorithm. This information might be helpful for other Centers to deliver timely and appropriate diagnosis and to increase awareness in PKD.
1 |. RATIONALE FOR THE GUIDELINES
Selwin and Dacie described in 1956 a form of nonspherocytic hemolytic anemia which resulted in ex vivo hemolysis that could be restored by adding adenosine triphosphate (ATP) to the red cells but not by adding glucose. Five years later pyruvate kinase (PK) deficiency (OMIM 266200) was the first glycolytic enzyme disorder identified and described.1 Since its first description PK deficiency has been recognized as the major glycolytic enzymopathy and the most common cause of chronic hereditary nonspherocytic hemolytic anemia. To date, more than 600 families have been reported in the literature and more than 300 mutations have been identified in the causative gene PKLR.2,3 In spite to what these numbers perhaps suggest, PK deficiency is likely underdiagnosed. In part, this can be ascribed to problems generally associated with diagnosing an autosomal recessive red cell disorder that is, parents that are hematologically normal, or the fact that patients with PK deficiency, as in other hereditary hemolytic disorders, may receive regular transfusions, thereby complicating interpretation of diagnostic tests. The wide variability of PK deficiency is another important complicating factor, as many people live undiagnosed with compensated anemia.4 In addition, there is general lack of familiarity with this diagnosis even among hematologists. Finally, difficulties in the performance and interpretation of PK enzymatic activity assays is also likely to play a role. For example, in 1979 a survey by Beutler revealed that only 6 out of 13 PK deficient patients had been diagnosed correctly.5 That same year, the first guidelines on the diagnosis of PK deficiency were established by the working group of the International Committee for Standardization in Hematology Expert Panel on Red Cell Enzymes.6 The main purpose of these guidelines was to harmonize the variety of techniques used for the analysis of PK variants into a set of standard methods. By 1988, the situation had not improved, as demonstrated by a second review of cases: only 4 out of 17 patients assayed for PK activity in various laboratories had been diagnosed correctly.7 This may, at least in part, explain the approximately 5- to 15-fold difference between genetic estimates on the prevalence of PK deficiency and the actual number of diagnosed cases reported.8–10 Consequently, it seems reasonable to assume that probably many cases of PK deficiency remain unrecognized, with the prevalence of PK deficiency largely unknown.11
Since the first description of PK deficiency, significant progress has been made towards a better understanding of clinical course, its physiopathology and diagnostic approaches (Supporting Information Figure S1). However, there is currently a lack of consensus on when and how to test for PK deficiency when approaching a patient with hemolytic anemia. For instance, some diagnostic algorithms for hemolytic anemia do not include testing for PK deficiency.12
It is important to correctly assign the diagnosis of PK deficiency to affected patients. For patients, to understand what they are suffering from, and for treating hematologists to decide on the appropriate therapy. Furthermore, proper diagnosis enables genetic counseling of additional family members and/or future offspring (Supporting Information: A patient’s experience).
In order to increase awareness of PK deficiency, and to identify the current diagnostic gaps, we followed formal consensus development techniques. A global PK deficiency International Working Group was established in 2016, comprising 24 experts from 20 different Centers. A detailed questionnaire based on the comments/criticisms from the International Working Group was prepared and distributed to 13 expert Centers directly involved in laboratory diagnosis of PK deficiency from 7 European, 5 USA, and 1 Asian Countries.
Questionnaires were evaluated in order to prepare recommendations. All the items addressed were given a score and compared with the group as a whole. None of the core statements achieved a mean degree of consensus below 85%.
The writing of these recommendations has made use of evidence-based material in peer-reviewed publications from online literature search using key words relevant to the subject. The unpublished data presented herein are merely for illustration purposes.
The presented diagnostic recommendations are endorsed by EuroBloodNet (European Network in Rare Hematological Diseases, www.eurobloodnet.eu).
2 |. PYRUVATE KINASE DEFICIENCY
2.1 |. Pathophysiology
PK (ATP:pyruvate 2-O-phosphotransferase, EC 2.7.1.40) catalyzes the conversion of phosphoenolpyruvate (PEP) to pyruvate, coupled with the synthesis of one ATP molecule (Supporting Information Figure S2). This reaction, the last step of the glycolytic pathway, is irreversible under physiological conditions. The enzyme requires both monovalent and divalent cations, usually K+, and Mg2+ or Mn2+ 13,14 for its activity.
The tight regulation of PK activity is of great importance not only for glycolysis itself, but also for the entire metabolism (Supporting Information Figure S3). For the red blood cell, PK plays a central role in cellular energy metabolism because it catalyzes one of the two major steps of ATP production. Since mature RBCs lack mitochondria, they are completely dependent on glycolysis for the production of ATP to maintain cell integrity and several critical functions. PK deficiency is thought to lead to ATP depletion, which ultimately affects the viability of the cell. PK deficiency also results in the accumulation of glycolytic intermediates proximal to the metabolic block, particularly 2-phosphoglycerate, 3-phosphoglycerate and 2, 3-diphosphoglycerate (2, 3-DPG), which may increase up to 3fold and further impair the glycolytic flux.15
Four PK isozymes are present in mammalian tissues.16,17 The L-type (mainly expressed in liver, renal cortex and small intestine) and the R-type (expressed in the erythrocytes), are both encoded by PKLR gene18 under the control of 2 tissue-specific promoters.19 The M1-type (skeletal muscle, heart and brain) and M2- type (leukocytes, platelets, lung, spleen, kidney and adipose tissue) are encoded by the PKM gene20 and generated by alternative mRNA splicing.21–23 PK-M2 is the principal form in leukocytes, platelets, lung, spleen, kidney, and adipose tissue. In the liver, PK-M2 represents a minor component since, as the hepatocytes mature, the predominant isoenzyme becomes PK-L. During erythroid differentiation, the M2 isoenzyme is progressively replaced by the erythrocyte isoform PK-R.24–26 PK is a homotetramer in almost all organisms,27 although it may exist in different forms, from monomer to decamer.28 A high degree of structural homology among PKs from different species has been reported based on published crystal structures29–32 including human erythrocytes.33 The PK monomer encompasses 3 principal domains: the A domain, with a classic (α/β)8 barrel topology, the small B domain characterized by an irregular β barrel, and the COOH-terminal C domain by an α/β topology. A fourth small NH2-terminal domain is formed by a helix-turn-helix motif.34 The active site lies in a cleft between the A domain and the flexible B domain. The C domain contains the binding site for fructose 1,6-bisphosphate (FBP).30,31,35–37
2.2 |. Frequency/ethnic distribution
PK deficiency is the most frequent enzyme abnormality of the glycolytic pathway, and the most common cause of hereditary nonspherocytic hemolytic anemia.4,7,9 The disease is transmitted as an autosomal recessive trait, with clinical symptoms confined to compound heterozygous and homozygous deficiency patients.
PK deficiency has a worldwide geographical distribution. There are no precise figures indicating the frequency of the disorder. Based on published literature, the estimated PK deficiency prevalence in the Caucasian population was calculated to be 5:100 000.9 There are other estimates based on patient registries suggesting a lower incidence of about 1:100 000.10 This discrepancy may be explained by a high number of mildly affected patients with PK deficiency who are not referred to Centers of Expertise (CE) and, hence, remain undiagnosed.
PK deficiency has been shown to have a protective effect against replication of the malaria parasite in mouse38 and human red cells; although it is not clear whether PK-LR mutant alleles are more prevalent in malaria endemic areas.39–41
2.3 |. Clinical aspects
Clinical manifestations of PK deficiency comprise the usual hallmarks of lifelong chronic hemolysis that are also seen in other forms of hereditary hemolytic anemias, like red cell membrane disorders, unstable hemoglobin variants, or other more rare red cell enzyme disorders. The degree of anemia varies widely, ranging from very mild anemia or fully compensated hemolysis to nonimmune hydrops fetalis or life-threatening neonatal anemia. This implies that patients may present any time from very early in life to in young adulthood.
Neonatal jaundice requiring phototherapy and/or exchange transfusion is common. Early onset of anemia is usually associated with a more severe clinical course. Infants and young children can be transfusion dependent from an early age. The anemia tends to improve with age, and is relatively constant in adulthood, although exacerbations requiring occasional transfusion may occur because of the stress of acute infections or pregnancy.4,42
Anemia may be surprisingly well tolerated in PK-deficient patients43 probably because of the increased red cell 2, 3-DPG content, which is responsible for a rightward shift in the oxygen dissociation curve of hemoglobin. Splenomegaly is a common finding, reported in about 80% of patients,4,44 and in the author’s experiences may worsen over time. In severely affected patients, splenectomy may be required. Splenectomy, while not arresting hemolysis, results in a hemoglobin increase of 1–3 g/dL, and may reduce or even eliminate transfusion requirements.4,42,44,45 Notably, since splenectomy is not indicated in some other forms of chronic hemolytic anemia, such as hereditary stomatocytosis,46 the diagnosis of PK deficiency should be established and comorbidity of stomatocytosis or other thrombophilic disorders should be excluded before splenectomy is performed.
Iron overload is common in both chronically transfused as well as transfusion-independent individuals. In transfusion-independent PK deficient patients, the cause of iron overload is unclear but may involve a degree of ineffective erythropoiesis.47,48 Coinheritance of hereditary hemochromatosis mutations has also been described in iron-overloaded, PK-deficient patients.49–53 Inappropriately low levels of hepcidin were detected in PK-deficient patients with increased ferritin and no mutations in genes associated with hereditary hemochromatosis, thereby confirming the predominant effect of accelerated erythropoiesis on hepcidin production.48
Gallstones are detected with increased frequency after the first decade of life and may occur even after splenectomy. The coinheritance of UGT1A1 TA promoter polymorphism also contributes to their occurrence.42
Other complications of PK deficiency include aplastic crisis following parvovirus infections and, more rarely, kernicterus, chronic leg ulcers, acute pancreatitis secondary to biliary tract disease, splenic abscess, spinal cord compression by extramedullary hematopoietic tissue, pulmonary hypertension and thromboembolic events particularly in splenectomized patients.54–57 Rare cases of fulminant hepatic failure related to PK deficiency have been described.58,59
3 |. LABORATORY DIAGNOSIS OF PKD
3.1 |. The position of PKD in the field of hereditary non immune-hemolytic anemia
The hematological features of PK deficiency are shared with all other hereditary hemolytic diseases that is, red cell enzyme deficiencies, membrane disorders or unstable hemoglobins, and congenital dyserythropoietic anemias.15,60–69 Patients usually have features of chronic hemolysis such as an increased reticulocyte count, increased lactate dehydrogenase (LDH), reduced haptoglobin and elevated bilirubin. However, in contrast to membrane disorders and unstable hemoglobins, red cell morphology in PK deficiency is usually unremarkable, generally displaying some degree of anisocytosis and poikilocytosis. A variable proportion (3%−30%) of echinocytes is occasionally observed, particularly after splenectomy. In the most severe cases, some erythroblasts may be observed in peripheral blood smear as a consequence of ineffective erythropoiesis. Dyserythropoietic features may also been observed at bone marrow examination, resulting in possible misdiagnosis with congenital dyserythropoietic anemias.70–72
The reticulocyte count in not-splenectomized patients is usually increased. However, reticulocytosis is not proportional to the severity of hemolysis, likely due to a decreased erythropoietic drive since the oxygen delivery to tissues is relatively improved by the increase in 2, 3-DPG and because younger PK defective erythrocytes are selectively sequestered by the spleen.25,26,73–75 Splenectomy therefore results in a conspicuous rise of reticulocytes even if the anemia becomes less severe. Unconjugated bilirubin concentration is very often increased, but usually <5 mg/dL, and may show a slight rise after splenectomy. Red cell osmotic fragility can be either normal or altered, and thus is not informative.4,76 Iron status parameters, in particular serum ferritin and transferrin saturation, may be increased disproportionally to the history of blood transfusions or even in nontransfused patients.4
Since the hematological features of PK deficiency are not specific, the possibility of PK deficiency and other metabolic abnormalities should be considered in all patients displaying chronic hemolysis where an immune-mediated hemolytic process, red cell membrane defect, unstable hemoglobin, or paroxysmal nocturnal hemoglobinuria has been excluded. Establishing the diagnosis of PK deficiency is therefore the final step of a diagnostic workup based not only on laboratory investigations, but also on the patient’s personal and family medical history and clinical examination. A proposed diagnostic flowchart of hemolytic anemia is shown in Supporting Information Figure S4. In light of a suspected erythroenzymopathy, the diagnosis of PK deficiency ultimately depends upon the demonstration of decreased enzyme activity and/or the identification of causative mutations in PKLR gene.
PK deficiency should also be considered in transfusion-dependent patients without obvious etiology, in neonates with unexplained severe hyperbilirubinemia, when reticulocytosis increases after splenectomy of an undiagnosed hemolytic patient or in patients with positive family history of PKD.42 When available, it is very helpful to study parents and other family members to confirm the presumed heterozygous state of the enzyme deficiency,77 particularly in transfusion-dependent cases. This approach is almost always adopted in EU Reference Centers, but is not always considered elsewhere. This discrepancy may be related to the lack of insurance coverage for genetic tests in some. As revealed by the results of the questionnaire there are currently 2 general approaches employed by Centers in the diagnosis of PK deficiency: (1) to screen for PK deficiency by measuring PK enzymatic activity in red blood cell lysates and to confirm a suspected PK deficiency by DNA sequence analysis of PKLR; (2) to screen for PK deficiency by NGS panels and to confirm the suspected pathogenic nature of an identified novel mutation by measuring PK enzymatic activity. These techniques, as well as their advantages/disadvantages are described next.
3.2 |. Diagnostic tools—biochemical analysis
3.2.1 |. Spectrophotometric assay of red blood cell PK activity
For many years, a standardized assay for measuring PK activity has been used. It was established by the International Committee for Standardization in Hematology (ICSH).6,78 It was adopted as the reference method for the diagnosis of this disease and it is still the method of choice in most reference laboratories. In this assay, PK activity is calculated from the rate of formation of pyruvate, linked to the oxidation of NADH in an LDH-mediated reaction (Supporting Information Figure S2)). The decrease in optical density that occurs as NADH is oxidized is measured by a spectrophotometer at 340 nm. A brief description of the original method is reported in Table S1.
Care should be taken in interpreting the assay results. Falsely normal levels are sometimes encountered due to: (a) markedly increased number of reticulocytes (similar to other red cell enzymes, PK activity is strongly influenced by red cell age); (b) interference from normal donor red cells in recently transfused patients; (c) incomplete platelet and leukocyte removal (the leukocyte isozyme is more active than that of the red cell); (d) compensatory expression of M2 isoenzyme79; or (e) kinetically abnormal mutant PKs that, although ineffective in vivo, may display almost normal features under the laboratory conditions (eg, low vs high PEP conditions). Moreover, the degree of PK enzymatic activity reduction does not predict disease severity.80
The key aspect of the enzymatic assay, and its role in diagnosing PK deficiency, were addressed in the survey (Supporting Data). For a number of aspects of the PK enzymatic assay all centers showed broad consensus. These include the method of choice (spectrophotometric assay as described by Beutler78), and the anticoagulant used to collect blood samples in ethylenediaminetetraacetic acid (EDTA).
For some other aspects, differences in standard operating procedure were observed.
3.2.2 |. Sample storage time
Storage times of samples before measurement in different laboratories ranged from 48 hours to 20 days at 4C.78 A wide consensus on this aspect is of the utmost importance to reduce the possibility of falsely positive results. For illustration purpose, the influence of storage time on activity of PK and hexokinase (HK, whose activity is often measured along with PK as a means to evaluate mean red cell age) was evaluated by 3 independent laboratories on 9 samples from normal subjects. PK and HK activity were measured at T = 0, 7, 14, and 21 days after collection. After 7 days of storage a 20% reduction in PK enzymatic activity was observed, which remained for up to 21 days of storage. Median loss of HK activity after 7 days of storage was 8% which further decreased to 30% after 21 days of storage.
3.2.3 |. Sample preparation
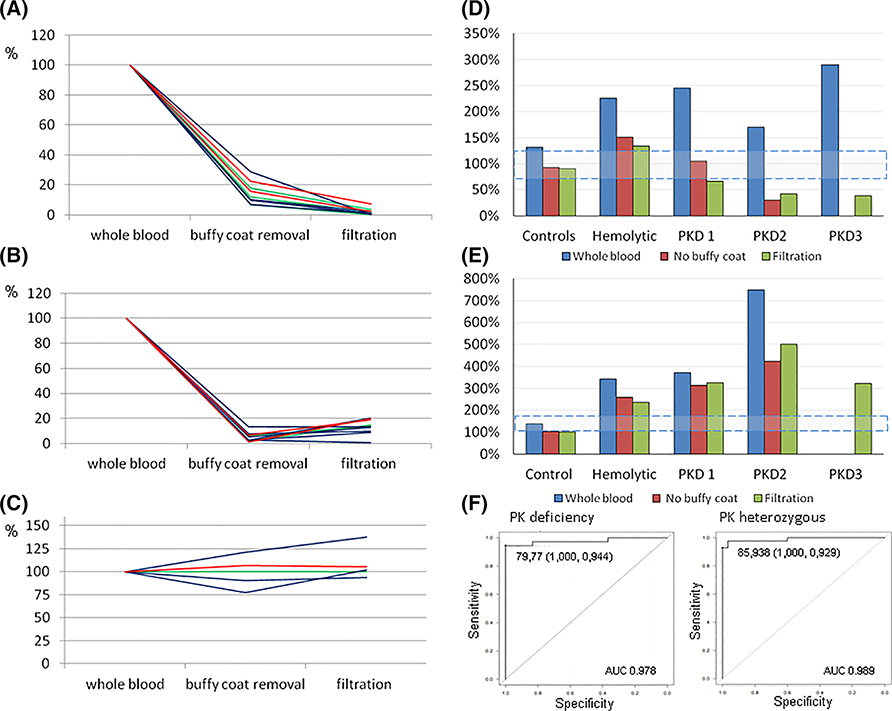
Platelet and leukocyte contamination is considered by all centers to be one of the most common causes of false negative results, consequently, careful RBC purification is considered necessary.78,81 All but 2 centers purify RBCs by filtration over α-cellulose/microcrystalline cellulose column. However, this procedure, reported to have a leukocyte contamination in the final red cell suspension usually <4 cells/108 erythrocytes,81,82 has some limitations. In particular, it requires manual preparation of syringes is time consuming and requires a minimum of 1 mL of sample. The latter is a limiting factor for neonatal blood samples. Simply removing the buffy-coat, as performed by 2 centers, could be considered a possible alternative. It reduces preparation time and allows for smaller sample volumes, but it is difficult to standardize. Measurements—performed in parallel on RBCs obtained from normal controls, patients with other hemolytic anemias and reticulocytosis, and PK deficient patients - showed that white cell and platelet count are reduced to 5% and 14%, respectively by filtration, and to 15% and 5% by buffy coat removal (Figure 1A,B). The observed differences, however, did not substantially affect the final PK activity (Figure 1D), suggesting that buffy coat removal could be considered an alternative method for RBC purification. It is worth noting that enzyme activities were always increased in activity assays performed directly on whole blood, thereby preventing PK deficiency from being detected in all the cases examined. None of the 2 methods (filtration or buffy coat removal) results in reticulocyte depletion (Figure 1C).
FIGURE 1.
Effect of buffy-coat removal and RBC filtration on: (A) WBC, (B) platelets, and (C) reticulocytes (expressed as % of cells in whole blood). Effect of buffy-coat removal and RBC filtration on: (D) PK activity and (E) HK activity (expressed as % of normal values). (F) ROC curve analysis of PK activity assay
3.2.4 |. Reticulocytosis
The interpretation of the results of PK enzyme assay of RBC, as well as other erythroenzymopathies, may also be confounded by the fact that the blood of patients with hemolytic anemia is enriched with reticulocytes and young erythrocytes. Since many PKLR mutations result in the production of unstable enzymes, the young circulating erythrocytes may contain normal or near-normal levels of enzyme.83 In contrast to what is observed in normal individuals, in PK deficient patients there is no correlation between PK activity and reticulocyte number.4 Nevertheless, it is important to take red cell age into account when interpreting results, particularly when low-normal PK activity levels are detected in a suspected PK-deficient patient. Various approaches may be used to evaluate the influence of reticulocytosis on overall PK activity84,85 that is, by comparing a patient’s enzyme activity to that from a control sample with the same degree of reticulocytosis, or by calculating the ratio of PK activity to another cell age dependent enzyme (eg, HK, PK activity/HK activity, or G6PD). As an example, in PK-deficient patient 1 (Figure 1D,E), characterized by a very high number of reticulocytes (55%, absolute number 1105 × 109/L), the PK activity falls inside the reference limits; however, the PK/HK ratio was drastically reduced in respect to that calculated on normal controls (PK/HK activity: 3.9 in patient 1 vs 18.5 detected in normal controls).
3.2.5 |. Interference of donor red blood cells
The contribution of PK activity from normal donor red cells in recently transfused patients is also a relatively common cause of false negative results. The results of the survey showed marked heterogeneity in opinion on the number of days allowed between the last transfusion and the collection of blood samples for the PK enzymatic assay (40–120 days). In 2/12 centers this aspect was not considered at all.
The lifespan of erythrocytes is 120 days, and 120 days could be considered the minimum time required before analyzing PK activity (common practice in some laboratories) after RBC transfusion. In patients requiring transfusion every few weeks this is obviously not possible. However, the lifespan of transfused red cells, as studied by Cr51 labeling,86,87 ranges from 90 to 110 days88 and the mean erythrocyte loss is considered to be approximatively 1% /day.86,89 This implies that for 1 unit of packed red blood cells (about 180 mL of RBC) infused into an adult weighing 60 kg (with Hb pretransfusion Hb level at 8 g/dL that is, Hct 25%, and RBC mass around 1125 mL), the final RBC donor contamination is about 12% at the time of transfusion (about 24% for 2 RBCs units transfused). Due to the 1% daily loss of transfused RBCs, the expected contamination with donor RBCs 50 days after the transfusion ranges from 6% (1 U) to 12% (2 U) or even less. Therefore, 50 days after transfusion, we can expect an over-estimation of PK activity due to donor RBC contamination of about 6%−12%. This overestimate should probably not result in a missed diagnosis.
In general, assays should be delayed for as long as possible after a red cell transfusion, and the laboratory should record the time since transfusion. Clinical urgency may require an assay to be performed when transfused red cells are still present; these results need to be interpreted with caution, for example, enzyme activity level slightly below normal range in presence of transfused red cells supports the diagnosis of PK-deficiency.
3.2.6 |. PK assay reference intervals
The results of enzyme assay are reported by all centers as IU/gHb. However, despite all centers use the same methodology, the reference ranges are different from one center to another. One reason could be the different assay temperatures at which the assay is done (eg, room temperature vs 30C or 37C). We recommend performing the analysis at 37C. However, as an alternative, PK activity should be expressed as the percentage of normal activity.
3.2.7 |. False positive results
False positive results are probably less of a problem and may for instance be due to bad sample storage conditions (concomitant testing of a normal control treated in the same conditions may avoid this). Notably, a decreased activity of PK may be secondary to other pathologies, such as mutations in KLF1 gene90 and acquired (acute myeloid leukemias, or myelodysplastic syndromes).91–93
3.2.8 |. Specificity/Sensitivity
No systematic studies on sensitivity and specificity of PK activity assays are available. A receiver operator characteristic (ROC) curve of % decrease activity has been performed in one center to identify the cut-off limit to discriminate carriers (n = 41) from true PK deficient patients (n = 34) compared with hemolytic patients not PKD (n = 30). The mean erythrocyte PK activity of the PKD cases was 38.5% of controls that of heterozygotes was 56.6%, whereas that of hemolytic patients not PKD was 155% of low normal reference range. PK activity of the PKD patients and the heterozygotes was significantly different (P = .0001914). ROC curve showed a cut-off limit of 81.5% and 96.9% to discriminate PKD patients and heterozygotes from hemolytic patients not PKD, respectively (Figure 1F).94
As could be expected from the wide heterogeneity of PKLR mutations there is a diversity in activities measured in RBCs from PK-deficient patients. To avoid misdiagnosis95,96 genotyping of the PKLR gene to confirm PK deficiency is therefore recommended (see below), particularly prior to splenectomy.96
Advantages and limitations of biochemical PK enzyme assay retrieved from inter-laboratory survey are summarized in Table 1.
TABLE 1.
Advantages and limitations of biochemical PK enzyme assay
| Advantages | Limitations |
|---|---|
| • Fast; time-frame 2 hours Results available in 2–10 working days | • Inability, on itself, to discriminate homozygous/compound heterozygous from heterozygote carriers |
| • Cheap; costs of analysis (15–80€/$) | |
| • Recent transfusions interference | |
| • Test availability in different centers | |
| • Reticulocytosis interference | |
| • Sensitivity/ specificity | • Leucocyte/platelet interference |
| • Evidence of functional abnormality in PK activity | • Neonates: Minimal amount of blood 1 mL is required |
3.3 |. Other biochemical tests
In some cases, kinetically abnormal mutant PKs, although ineffective in vivo, may display normal or even higher catalytic activity under the optimal, artificial conditions of laboratory assay. Although rare, in the authors experience some cases of true PK deficiency were nondeficient on repeated occasions using the standard enzymatic assay. These patients had dysfunctional thermolabile enzyme variants that were revealed by the thermal stability test (PK activity assay performed after 1 hour incubation at 53°C).6 Performing additional biochemical tests other than PK activity assay itself may therefore be useful to identify a metabolic block at the PK step, or bring to light altered biochemical properties. Such tests include the detection of elevated upstream glycolytic intermediates (2-phosphoglycerate, 3-phosphoglycerate, and 2, 3-biphosphoglycerate), PK thermostability test, PK activity assay performed at low substrate concentration. These methods, described in detail by the International Committee for Standardization in Hematology (ICSH),6 are now rarely performed in laboratory practice but may contribute to establish the diagnosis in specific cases.
3.4 |. Diagnostic tools—genetic analysis
3.4.1 |. DNA sequence analysis of PKLR
The gene encoding red blood cell PK (PKLR) is located on chromosome 1q21. It consists of 12 exons and is approximately 9.5 kb in size.97 By the use of tissue-specific promoters19,97 PK-L and PK-R subunits are transcribed. Exon 1 is exclusively expressed in erythroid cells (transcript ENST00000342741, NM_000298) whereas expression of exon 2 is confined to the liver (ENST00000392414, NM_181871). Hence, the PK-R monomer is composed of 574 amino acids,98 NP_000289.
To date, more than 300 mutations in PKLR have been associated with PKD.2,3,99 The large majority of these are missense substitutions affecting residues critical to the enzyme’s structure and/or function. The most commonly reported mutations include Arg510Gln (16%) and Arg486Trp (12%),100 the former being mainly found in Northern Europe and the USA, the latter in Southern Europe.80 A particularly high frequency exists among the Pennsylvania Amish (Arg479His)101 and a 1149 base pair deletion, which results in loss of exon 11, in the Gypsy communities.102
With the advent of next generation sequencing (NGS) techniques for diagnostic purposes, PKLR is usually included in gene panels designed for the diagnosis of hereditary hemolytic anemia.71,72,103,104 Indeed, 1 EU Center only performs PKLR gene analysis in this context, whereas 5 Centers perform standard Sanger sequencing methods for the detection of mutations in patients in which decreased PK activity was found. The erythroid-specific promoter is generally included in Sanger sequencing protocols, whereas sequence analysis of the liverspecific exon 2 is not commonly performed. Usually, NGS analysis permits a more extensive gene sequencing with than Sanger sequencing (generally including all coding DNA regions, intronic flanking, 3’ up-stream, and 5’ down-stream regions; some of them [Japan] including the entire intronic region).
At least 2 EU Centers perform additional Multiplex Ligation-dependent Probe Amplification analysis or other methods for the detection of (large) deletions in PKLR. Assays of copy number variations (eg, CGH array or digital PCR) may also be considered.
European and Japan Centers generally consider DNA sequence analysis mandatory to confirm a suspected deficiency of PK (6/7 EU Centers always perform DNA analysis of PKLR, and 1 EU Center only performs it in atypical cases or transfused patients). In contrast, DNA analysis in the USA has not previously been widely done (only 2/5 Centers performs it on a regular basis) and this may be due to lack of insurance coverage for genetic testing. However, genetic testing is now becoming more available in numerous laboratories.
3.4.2 |. Advantages and limitations of DNA analysis
DNA analysis offers obvious advantages when compared with biochemical testing, such as less complicated handling and shipping of samples. It may also allow for smaller sample volumes. Furthermore, DNA analysis is not hampered by the presence of transfused red blood cells. Finally, it facilitates family screening and prenatal testing. However, DNA analysis has historically been of limited use as a first step in the diagnosis of PK deficiency because conventional Sanger sequencing is time consuming and relatively expensive.
Importantly, not every mutation detected by DNA analysis can immediately be classified as a disease-causing variant, even after in silico analysis by mutation prediction programs.77 They should be considered Variants of Unknown Clinical Significance (VUS) until their pathogenic nature is confirmed by functional analysis such as PK enzymatic assays, Western Blot, RT-PCR analysis, or gene reporter assays. This is especially important when patient samples are not accompanied by clinical and laboratory information.
In line with this, and in contrast to US Centers, all but one EU Center performs such complementary biochemical methods to confirm the pathogenic nature of detected mutations. Indeed, 4 EU Centers reported a few patients homozygous or compound heterozygous for PKLR mutations that showed normal PK activity.
The currently employed methodologies by most Centers limit the detection of (large) deletions, or mutations in deep intronic regions or other105 unknown regulatory regions affecting PKLR gene expression.106 Such mutations could be involved in suspected cases of PK deficiency that fail to demonstrate the “classical” PKLR genotype of 2 in trans mutations. In fact, PK deficient (via enzymatic testing) patients displaying either only one mutation or no mutations at all are regularly encountered.
On the other side, a decrease in PK activity in absence of PKLR mutations may be attributable to other causes of decreased PK activity, such as mutations in KLF1 gene.90 In addition, heterozygosity for a mutation in PKLR may accompany other red cell pathologies such as congenital dyserythropoietic anemia,107 hereditary spherocytosis,95,108 or hereditary xerocytosis.96
The role of DNA analysis is rapidly changing as new technologies are developed and it becomes easier, cheaper, and faster to rapidly sequence large numbers of genes. This trend is likely to continue, and it seems probable that in the near future whole exome or whole genome sequencing will replace targeted NGS panels. The limiting factor will be in bioinformatic interpretation of these data. In parallel, databases will also contain increasing amounts of data to facilitate the interpretation of VUS, although it seems likely that PK biochemical assays will become increasingly important as a means of confirming the significance of identified mutations.
We conclude that enzyme analyses and DNA studies are complementary techniques for the diagnosis of PK deficiency. Their use is dictated by clinical and laboratory findings, and in some cases by availability.
4 |. SUMMARY/RECOMMENDATIONS FOR LABORATORY DIAGNOSIS OF PK DEFICIENCY
4.1 |. Essential laboratory data and clinical parameters in the diagnosis of PK deficiency
Panel recommendations are presented in Table 2. The degree of agreement was expressed as a percentage (0% not agree, 100% fully agree) and reported in the table as mean, median and range. A wide (>95%) consensus was reached on what patients’ information should be requested with sample and on technical aspects of the diagnosis of PK deficiency. Different positions were expressed about the need to confirm a biochemical PK deficiency by molecular testing (88.3% of agreement); 2 centers reserved molecular testing only to atypical cases. Different approaches among centers may be due to inability to obtain reimbursement by national insurances.
TABLE 2.
Recommendation on essential laboratory data and clinical parameters in the diagnosis of PK deficiency
| Recommendation | Evidence | |
|---|---|---|
| Clinical presentation | PK deficiency may be suspected in: - patients with variable chronic anemia and/or splenomegaly and/or jaundice, with normal or near-normal red cell morphology. - transfusion dependent cases of unknown etiology - haemolytic patients with unexplained severe neonatal indirect hyperbilirubinemia - presence of high reticulocyte number in splenectomised patients with no diagnosis |
Mean: 95% Median:100% (75–100) |
| Clinical data | -information on clinical history (both recent as well as from infancy, ie neonatal jaundice), family history should always be requested together with samples, as well as the time of last blood transfusion | Mean: 98.6% Median:100% (90–100) |
| Laboratory data (mandatory in bold) | -complete blood count -RBC morphology -markers of haemolysis (reticulocyte count, LDH, unconjugated bilirubin, haptoglobina,b) |
Mean: 97% Median:100% (90–100) |
| Differential diagnosis | Acquired haemolytic anemia, membranopathies, CDAs, unstable haemoglobins, red cell enzymopathies other than PK deficiency should be excluded (see figure 5) | Mean: 92.1% Median:100% (50–100) |
| Biochemical testing | ||
| Reference test for biochemical assay | RBC PK activity assay by spectrophotometry (Beutler, 84) | Mean: 98.7% Median:100% (80–100) |
| Storage time of sample | PK enzyme assay may be considered stable at 4°C until up to 21 days after collection.c A maximum of 14 days storage is recommended if PK activity is related to HK activity due to different stability of HK activity | Mean: 95% Median:100% (80–100) |
| Sample anticoagulant | Citrtate-dextrose solution (ACD); EDTA, citrate phosphate dextrose (CPD), heparin could be considered for the enzyme assay (Beutler, 84): EDTA is the main anticoagulant used in daily practice. |
Mean: 100% Median:100% |
| Sample preparation | Purification on α-cellulose/microcrystalline cellulose column is recommended. Buffy coat removal may be considered as an alternative. PK enzyme activity cannot be performed on whole blood |
Mean: 96.7% Median:100% (80–100) |
| Reticulocytes interference | Reticulocyte number must be taken into account when interpreting results of PK enzyme assay, particularly when of low-normal PK activity levels. Results could be compared with enzyme activities obtained from a control sample with the same degree of reticulocytosis, or by calculating the ratio of PK activity to another cell age dependent enzyme (eg, hexokinase). |
Mean: 96.1% Median:100% (70–100) |
| Interference of donor red blood cells | The enzyme assay should be performed as far as possible after a red cell transfusion. The laboratory should record the time since transfusion. A minimum of 50 days from last transfusion is considered a “safe” period for testing of PK activity, leading to an estimated donor RBC contamination of about 7–14%. Results of enzyme activity need to be interpreted with caution in transfused patients.d | Mean: 96.9% Median:100% (60–100) |
| Confirmatory tests | In case of decreased PK activity, sequencing of PKLR gene is highly recommended to confirm the diagnosis | Mean: 88.3% Median:100% (10–100) |
| Molecular testing | ||
| Indication | -molecular testing is highly recommended to confirm a suspected case of PK deficiency based on decreased enzyme activity. -molecular testing of PKLR gene by Sanger is suitable for patients with (relatively) decreased PK activity - use of NGS panels is a reliable alternative method for diagnosis of PK deficiency. It is particularly relevant for: - neonates (if family study is not available) - transfusion dependent patients/recently transfused patients - samples with prolonged shipping times |
Mean: 91.2% Median:100% (10–100) |
| PKLR genotype discrepancies | In case of genotype discrepancies (patients with suspected PKD and one or none mutations detected) further investigation are required: -assays for detection of large deletions -re-evaluation of other causes of haemolysis by specific tests or NGS platform In absence of any mutation and decreased PK activity: - NGS tools or, KLF1 gene mutations should be considered |
Mean: 92.5% Median:100% (40–100) |
The degree of agreement was expressed as percentage of agreement and reported as mean, median and range.
Decreased haptoglobin useful only after 6 months of age.
Or evaluation of carboxyhaemoglobin evaluation as index of haemolysis.
In the impossibility of shipment at 4°C, the assay must be performed by 3–5 day.
If the transfusion history is not available, a statement that recent transfusion can affect results should be added to the results.
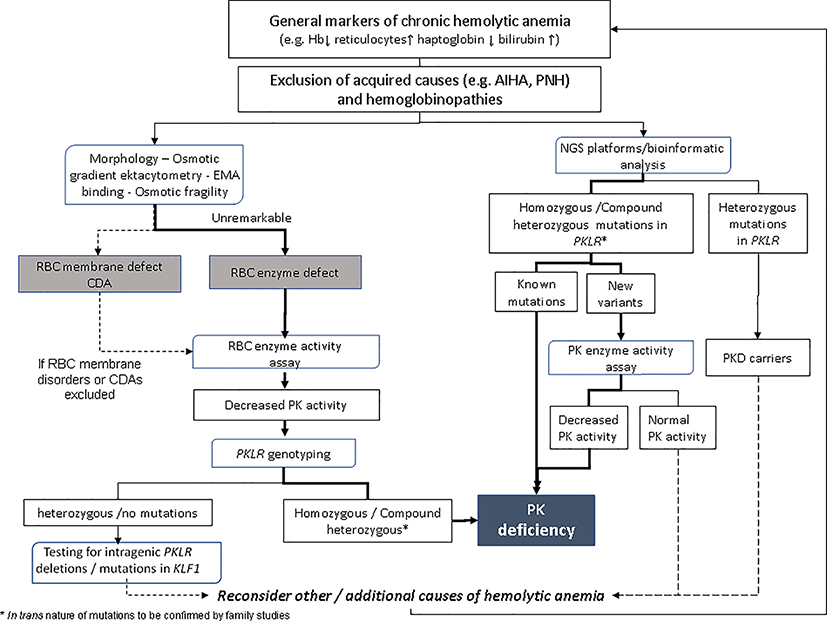
Based on recommendations and consensus group work, an diagnostic algorithm for diagnostic work-up for diagnosis of PK deficiency is reported in Figure 2.
FIGURE 2.
Diagnostic algorithm for diagnostic work-up for diagnosis of PK deficiency based on recommendations and consensus group work. Blue boxes represent the methods used in the diagnostic process
4.2 |. Inter-laboratory standardization—external quality control program
Due to the rarity of the disease, to date no external quality control program exists for the PK enzyme assay. Provisional attempts include the regular exchange of samples from normal controls and PK-deficient patients among laboratories in the USA (4 Centers) and Europe (3 Centers). A quality external control program would be of great benefit and could provide significant added value for the diagnosis of PK deficiency.
4.3 |. National/international reference centers for the diagnosis of PK deficiency
CE are physical expert structures for the management and care of rare disease patients. Each CE is specialized in a specific disease or group of rare diseases, and shares the mission of providing patients with the highest standards of diagnosis, treatment, and longitudinal care. In this sense, recommendations for CE in Rare Anemias (RAs) were collected and published in a White Book by ENERCA,11 the pilot European Network for Rare and Congenital Anemias, currently one of the main arms of the recently established by the European Commission ERN-EuroBloodNet (www.eurobloodnet.eu).
The ENERCA recommendations for laboratories in RAs include:
Laboratory accreditation based on the ISO 15189 standard, by an official national body
Necessary resources (human, technical and management) for achieving a diagnosis (in house or via a national or European network):
Routine tests: For the diagnosis of RAs the basic requirement is a competent hematology laboratory equipped with the following: (1) Automated red cell counters, morphology, and tests for hemolysis and hemoglobin fractionation. (2) Tests considered to be essential for the diagnosis of very RAs include tests for membrane defects, enzyme defects, hemoglobinopathies and erythropoietic defects.
For PK deficiency, analysis of both PK activity quantitative measurement and PKLR gene analysis were considered mandatory for being a CE for PK deficiency diagnosis.
5 |. CONCLUSIONS
PKD is the most common enzyme defect of glycolysis and thereby an important cause of hereditary nonspherocytic hemolytic anemia. In daily practice, diagnosing PKD may be difficult due to incomplete diagnostic algorithms and interpretation of the PK enzyme assay (see appendix). By this study a global PKD International Working Group, identified and evaluated the current gaps in the diagnosis of PKD in order to establish diagnostic guidelines that will improve diagnosis and increase awareness of the disease. A wide consensus on diagnosis of PKD was reached among ECs worldwide. Major outcomes were used to create a diagnostic algorithm. The results of this study are presented as recommendations for the diagnosis of PK deficiency and used in preparing a diagnostic algorithm. Since no external quality control program exists for PK testing, this information might be helpful for other centers to deliver timely and appropriate diagnosis and to increase awareness in PKD. The presented diagnostic guidelines and recommendations are endorsed by EuroBloodNet (European Network in Rare Hematological Diseases, www.eurobloodnet.eu).
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to sincerely thank Pavla Koralkova for preparing Supporting Information Figure S3; Cristina Vercellati (Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milano), Wietske Fennis (UMCU–—Utrecht) and Carolyn Wong CLS, MT(ASCP) (Stanford University School of Medicine) for performing biochemical assays. The authors wish also thanks the participants to the International Working Group organized to identify the current diagnostic gaps in diagnosing PK deficiency and Agios Pharmaceutics for the organizational support. The list of participants and their affiliations is reported in Supporting Information.
APPENDIX: SUMMARY TABLE
What is the test?
The diagnosis PKD is based on the exclusion of the most common causes of hemolytic anemias, and upon the demonstration of decreased enzyme activity and/or the identification of causative mutations in PKLR gene. There are currently 2 general approaches employed in the diagnosis of PKD: (1) to screen for PK deficiency by measuring PK enzymatic activity in red blood cells and to confirm a suspected PK deficiency by DNA sequence analysis of PKLR gene; (2) to screen for PK deficiency by NGS panels and to confirm the suspected pathogenic nature of an identified novel mutation by measuring PK enzymatic activity.
How is it measured?
The spectrophotometric assay of red blood cell PK activity, performed as recommended by the International Committee for Standardization in Hematology (ICSH) (Table S1), is adopted as the reference method for the diagnosis of PKD and it is still the method of choice in most reference laboratories. Care should be taken in interpreting the results of PK activity assay. Falsely normal levels are sometimes encountered due to: (1) markedly increased number of reticulocytes; (2) interference from normal donor red cells in recently transfused patients; (3) incomplete platelet and leukocyte removal; or (4) kinetically abnormal mutant PKs that may display almost normal under the laboratory conditions. Molecular testing is based on the identification of one homozygote or 2 compound heterozygote mutations in PKLR gene by Sanger sequencing. With the advent of NGS techniques for diagnostic purposes, PKLR is usually included in gene panels designed for the diagnosis of hereditary hemolytic anemia. Multiple aspects of the diagnosis of PKD were discussed by a global PKD International Working Group. The results are presented as recommendations (Table 2) and used to prepare a diagnostic algorithm (Figure 2).
What are the normal range values?
The results of enzyme assay are reported as IU/gHb. However, despite the use of the same methodology, the reference ranges are different from one Center to another. To date no external quality control program exists for the PK enzyme assay. Provisional attempts include the regular exchange of samples from normal controls and PK-deficient patients among laboratories in the US and Europe. A quality external control program would be of great benefit for the diagnosis of PK deficiency.
What conditions or types of conditions is it used for?
PK deficiency is a rare hereditary disease and may be suspected in patients with variable chronic anemia and/or splenomegaly and/or jaundice, with normal or near-normal red cell morphology. It may be suspected also in transfusion dependent hemolytic cases of unknown etiology; hemolytic patients with unexplained severe neonatal indirect hyperbilirubinemia, or in presence of high reticulocyte number in splenectomized patients with no diagnosis.
What tests are helpful to do with it for a more complete picture?
Information on clinical history, family history, and the time of last blood transfusion should always be requested together with samples. First level investigations: Complete blood count; RBC morphology; markers of hemolysis. In case of genotype discrepancies (patients with suspected PKD and one or none mutations detected) further investigation are required: assays for detection of large deletions, re-evaluation of other causes of hemolysis by specific tests or NGS platform.
What tests provide similar information?
PK enzyme activity assay and molecular studies are complementary techniques for the diagnosis of PK deficiency. Their use is dictated by clinical and laboratory findings, and in some cases by availability.
How does its use impact treatment?
A timely and appropriate diagnosis of PK deficiency is important for patients, to understand what they are suffering from, for treating hematologists to decide on the appropriate therapy (ie, monitoring iron overload) and to enable genetic counseling in severe cases. The diagnosis is nowadays especially important considering the development of new therapies for PK deficiency that is, gene therapy and activator treatments.
Footnotes
CONFLICT OF INTEREST
PB, BG, DJK, MMP, WB, EvB, PPG, RvW are scientific advisors to Agios Pharmaceuticals. The remaining authors declare no competing financial interests.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Valentine WN, Tanaka KR, Miwa S. A specific erythrocyte glycolytic enzyme defect (pyruvate kinase) in three subjects with congenital non-spherocytic hemolytic anemia. Trans Assoc Am Physicians. 1961; 74:100–110. [PubMed] [Google Scholar]
- 2.Canu G, De Bonis M, Minucci A, Capoluongo E. Red blood cell PK deficiency: an update of PK-LR gene mutation database. Blood Cells Mol Dis. 2016;57:100–109. [DOI] [PubMed] [Google Scholar]
- 3.van Wijk PKLR Leiden Open Variation Database. https://grenada.lumc.nl/LOVD2/mendelian_genes/home.php?select_db=PKLR
- 4.Zanella A, Fermo E, Bianchi P, Valentini G. Red cell pyruvate kinase deficiency: molecular and clinical aspects. Br J Haematol. 2005; 130(1):11–25. [DOI] [PubMed] [Google Scholar]
- 5.Beutler E Red cell enzyme defects as nondiseases and as diseases. Blood. 1979;54(1):1–7. [PubMed] [Google Scholar]
- 6.Miwa S, Boivin P, Blume KG, et al. Recommended methods for the characterization of red cell pyruvate kinase variants. Br J Haematol.1979;43(2):275–286. [DOI] [PubMed] [Google Scholar]
- 7.Hirono A, Forman L, Beutler E. Enzymatic diagnosis in nonspherocytic hemolytic anemia. Medicine (Baltimore). 1988;67(2):110–117. [DOI] [PubMed] [Google Scholar]
- 8.de Medicis E, Ross P, Friedman R, et al. Hereditary nonspherocytic hemolytic anemia due to pyruvate kinase deficiency: a prevalence study in Quebec (Canada). Hum Hered. 1992;42(3):179–183. [DOI] [PubMed] [Google Scholar]
- 9.Beutler E, Gelbart T. Estimating the prevalence of pyruvate kinase deficiency from the gene frequency in the general white population. Blood. 2000;95(11):3585–3588. [PubMed] [Google Scholar]
- 10.Carey PJ, Chandler J, Hendrick A, et al. Prevalence of pyruvate kinase deficiency in northern European population in the north of England. Northern region Haematologists group. Blood. 2000;96(12):4005–4006. [PubMed] [Google Scholar]
- 11.Vives-Corrons JL, Manu Pereira M, Romeo-Casabona C, et al. ENERCA. WhiteBook for the creation of a European Reference Network of Centres of Expertise on Rare anemias; 2014. 10.4081/thal.2014.4878 [DOI]
- 12.Dhaliwal G, Cornett PA, Tierney LM Jr. Hemolytic anemia. Am Fam Physician. 2004;69(11):2599–2606. [PubMed] [Google Scholar]
- 13.Boyer PD, Lardy HA, Phillips PH. The role of potassium in muscle phosphorylation. J Biol Chem. 1942;146:673–682. [Google Scholar]
- 14.Gupta RK, Oesterling RM, Mildvan AS. Dual divalent cation requirement for activation of pyruvate kinase: essential roles of both enzyme- and nucleotide-bound metal ion. Biochemistry. 1976;15(13):2881–2887. [DOI] [PubMed] [Google Scholar]
- 15.Zanella A, Bianchi P. Red cell pyruvate kinase deficiency: from genetics to clinical manifestations. Baillieres Best Pract Res Clin Haematol. 2000;13(1):57–81. [DOI] [PubMed] [Google Scholar]
- 16.Imamura K, Tanaka T. Multimolecular forms of pyruvate kinase from rat and other mammalian tissues. I electrophoretic studies. J Biochem. 1972;71(6):1043–1051. [DOI] [PubMed] [Google Scholar]
- 17.Ibsen KH. Interrelationships and functions of the pyruvate kinase isozymes and their variant forms: a review. Cancer Res. 1977;37(2):341–352. [PubMed] [Google Scholar]
- 18.Satoh H, Tani K, Yoshida MC, Sasaki M, Miwa S, Fujii H. The human liver-type pyruvate kinase (PKL) gene is on chromosome 1 at band q21. Cytogenet Cell Genet. 1988;47(3):132–133. [DOI] [PubMed] [Google Scholar]
- 19.Noguchi T, Tamada K, Inoue H, Matsuda T, Tanaka T. The L- and R-type isozymes of rat pyruvate kinase are produced from a single gene by use of different promoters. J Biol Chem. 1987;262(29): 14366–14371. [PubMed] [Google Scholar]
- 20.Tani K, Yoshida MC, Satoh H, et al. Human M2-type pyruvate kinase: cDNA cloning, chromosomal assignment and expression in hepatoma. Gene. 1988;73(2):509–516. [DOI] [PubMed] [Google Scholar]
- 21.Black JA, Rittenberg MB, Standerfer RJ, Peterson JS. Hereditary persistence of fetal erythrocyte pyruvate kinase in the basenji dog. Prog Clin Biol Res. 1978;21:275–295. [PubMed] [Google Scholar]
- 22.Noguchi T, Inoue H, Tanaka T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J Biol Chem. 1986;261(19):13807–13812. [PubMed] [Google Scholar]
- 23.Whitney KM, Goodman SA, Bailey EM, Lothrop CD Jr. The molecular basis of canine pyruvate kinase deficiency. Exp Hematol. 1994;22(9):866–874. [PubMed] [Google Scholar]
- 24.Marie J, Garreau H, Kahn A. Evidence for a postsynthetic proteolytic transformation of erythrocyte pyruvate kinase into L-type enzyme. FEBS Lett. 1977;78(1):91–94. [DOI] [PubMed] [Google Scholar]
- 25.Takegawa S, Fujii H, Miwa S. Change of pyruvate kinase isozymes from M2- to L-type during development of the red cell. Br J Haematol. 1983;54(3):467–474. [DOI] [PubMed] [Google Scholar]
- 26.Max-Audit I, Testa U, Kechemir D, Titeux M, Vainchenker W, Rosa R. Pattern of pyruvate kinase isozymes in erythroleukemia cell lines and normal human erythroblasts. Blood. 1984;64(4):930–936. [PubMed] [Google Scholar]
- 27.Fothergill-Gilmore LA, Michels PA. Evolution of glycolysis. Prog Biophys Mol Biol. 1993;59(2):105–235. [DOI] [PubMed] [Google Scholar]
- 28.Muñoz ME, Poncem E. Pyruvate kinase: current status of regulatory and functional properties. Comp Biochem Physiol B Biochem Mol Biol. 2003;135(2):197–218. [DOI] [PubMed] [Google Scholar]
- 29.Muirhead H, Clayden DA, Barford D, et al. The structure of cat muscle pyruvate kinase. EMBO J. 1986;5(3):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattevi A, Valentini G, Rizzi M, Speranza ML, Bolognesi M, Coda A. Crystal structure of Escherichia coli pyruvate kinase type I: molecular basis of the allosteric transition. Structure. 1995;3(7):729–741. [DOI] [PubMed] [Google Scholar]
- 31.Jurica MS, Mesecar A, Heath PJ, Shi W, Nowak T, Stoddard BL. The allosteric regulation of pyruvate kinase by fructose-1,6-bisphosphate. Structure. 1998;6(2):195–210. [DOI] [PubMed] [Google Scholar]
- 32.Rigden DJ, Phillips SE, Michels PA, Fothergill-Gilmore LA. The structure of pyruvate kinase from Leishmania mexicana reveals details of the allosteric transition and unusual effector specificity. J Mol Biol. 1999;291(3):615–635. [DOI] [PubMed] [Google Scholar]
- 33.Valentini G, Chiarelli LR, Fortin R, et al. Structure and function of human erythrocyte pyruvate kinase. Molecular basis of nonspherocytic hemolytic anemia. J Biol Chem. 2002;277(26):23807–23814. [DOI] [PubMed] [Google Scholar]
- 34.Mattevi A, Bolognesi M, Valentini G. The allosteric regulation of pyruvate kinase. FEBS Lett. 1996;389(1):15–19. [DOI] [PubMed] [Google Scholar]
- 35.Valentini G, Chiarelli L, Fortin R, Speranza ML, Galizzi A, Mattevi A. The allosteric regulation of pyruvate kinase. J Biol Chem. 2000; 275(24):18145–18152. [DOI] [PubMed] [Google Scholar]
- 36.Wooll JO, Friesen RH, White MA, et al. Structural and functional linkages between subunit interfaces in mammalian pyruvate kinase. J Mol Biol. 2001;312(3):525–540. [DOI] [PubMed] [Google Scholar]
- 37.Fenton AW, Blair JB. Kinetic and allosteric consequences of mutations in the subunit and domain interfaces and the allosteric site of yeast pyruvate kinase. Arch Biochem Biophys. 2002;397(1):28–39. [DOI] [PubMed] [Google Scholar]
- 38.Min-Oo G, Fortin A, Tam MF, Nantel A, Stevenson MM, Gros P. Pyruvate kinase deficiency in mice protects against malaria. Nat Genet. 2003;35(4):357–362. [DOI] [PubMed] [Google Scholar]
- 39.Ayi K, Min-Oo G, Serghides L, et al. Pyruvate kinase deficiency and malaria. 2008. New Engl J Med. 2008;358(17):1805–1810. [DOI] [PubMed] [Google Scholar]
- 40.Berghout J, Higgins S, Loucoubar C, Sakuntabhai A, Kain KC, Gros P. Genetic diversity in human erythrocyte pyruvate kinase. Genes Immun. 2012;13(1):98–102. [DOI] [PubMed] [Google Scholar]
- 41.Machado P, Manco L, Gomes C, et al. Pyruvate kinase deficiency in sub-Saharan Africa: identification of a highly frequent missense mutation (G829A; Glu277Lys) and association with malaria. PLoS One. 2012;7(10):e47071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grace RF, Zanella A, Neufeld EJ, et al. Erythrocyte pyruvate kinase deficiency: 2015 status report. Am J Hematol. 2015;90(9):825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oski FA, Marshall BE, Cohen PJ, Sugerman HJ, Miller LD. The role of the left-shifted or right-shifted oxygenhemoglobin equilibrium curve. Ann Intern Med. 1971;74(1):44–46. [DOI] [PubMed] [Google Scholar]
- 44.Grace RF, Bianchi P, van Beers EJ, et al. The clinical spectrum of pyruvate kinase deficiency: data from the pyruvate kinase deficiency natural history study. Blood. 2018;131(20):2183–2192. [DOI] [PubMed] [Google Scholar]
- 45.Iolascon A, Andolfo I, Barcellini W, et al. Recommendations regarding Splenectomy in hereditary hemolytic Anemias. Haematologica. 2017; 102(8):1304–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stewart GW, Amess JA, Eber SW, et al. Thrombo-embolic disease after splenectomy for hereditary stomatocytosis. Br J Haematol. 1996;93(2):303–310. [DOI] [PubMed] [Google Scholar]
- 47.Aizawa S, Kohdera U, Hiramoto M, et al. Ineffective erythropoiesis in the spleen of a patient with pyruvate kinase deficiency. Am J Hematol. 2003;74(1):68–72. [DOI] [PubMed] [Google Scholar]
- 48.Mojzikova R, Koralkova P, Holub D, et al. Iron status in patients with pyruvate kinase deficiency: neonatal hyperferritinaemia associated with a novel frameshift deletion in the PKLR gene (p.Arg518fs), and low hepcidin to ferritin ratios. Br J Haematol. 2014;165(4):556–563. [DOI] [PubMed] [Google Scholar]
- 49.Zanella A, Berzuini A, Colombo MB, et al. Iron status in red cell pyruvate kinase deficiency: study of Italian cases. Br J Haematol. 1993; 83(3):485–490. [DOI] [PubMed] [Google Scholar]
- 50.Zanella A, Bianchi P, Iurlo A, et al. Iron status and HFE genotype in erythrocyte pyruvate kinase deficiency: study of Italian cases. Blood Cells Mol Dis. 2001;27(3):653–661. [DOI] [PubMed] [Google Scholar]
- 51.Dolan LM, Ryan M, Moohan J. Pyruvate kinase deficiency in pregnancy complicated by iron overload. International. J Obstet Gynaecol. 2002;109:844–846. [DOI] [PubMed] [Google Scholar]
- 52.Marshall SR, Saunders PW, Hamilton PJ, Taylor PR. The dangers of iron overload in pyruvate kinase deficiency. Br J Haematol. 2003;120:1090–1091. [DOI] [PubMed] [Google Scholar]
- 53.Finkenstedt A, Bianchi P, Theurl I, et al. Regulation of iron metabolism through GDF15 and hepcidin in pyruvate kinase deficiency. Br J Haematol. 2009;144(5):789–793. [DOI] [PubMed] [Google Scholar]
- 54.Rutgers MJ, van der Lugt PJ, van Turnhout JM. Spinal cord compression by extramedullary hematopoietic tissue in pyruvate-kinase-deficiency-caused hemolytic anemia. Neurology. 1979;29(4):510–513. [DOI] [PubMed] [Google Scholar]
- 55.Pincus M, Stark RA, O’Neill JH. Ischaemic stroke complicating pyruvate kinase deficiency. Intern Med J. 2003;33(9–10):473–474. [DOI] [PubMed] [Google Scholar]
- 56.Christensen RD, Eggert LD, Baer VL, Smith KN. Pyruvate kinase deficiency as a cause of extreme hyperbilirubinemia in neonates from a polygamist community. J Perinatol. 2010;30(3):233–236. [DOI] [PubMed] [Google Scholar]
- 57.Christensen RD, Nussenzveig RH, Yaish HM, Henry E, Eggert LD, Agarwal AM. Causes of hemolysis in neonates with extreme hyperbilirubinemia. J Perinatol. 2014;34(8):616–619. [DOI] [PubMed] [Google Scholar]
- 58.Raphaël MF, Van Wijk R, Schweizer JJ, et al. Pyruvate kinase deficiency associated with severe liver dysfunction in the newborn. Am J Hematol. 2007;82(11):1025–1028. [DOI] [PubMed] [Google Scholar]
- 59.Olivier F, Wieckowska A, Piedboeuf B, Alvarez F. Cholestasis and hepatic failure in a neonate: a case report of severe pyruvate kinase deficiency. Pediatrics. 2015;136(5):e1366–e1368. [DOI] [PubMed] [Google Scholar]
- 60.Bianchi P, Fermo E, Vercellati C, et al. Congenital dyserythropoietic anemia type II (CDAII) is caused by mutations in the SEC23B gene. Hum Mutat. 2009;30(9):1292–1298. [DOI] [PubMed] [Google Scholar]
- 61.Schwarz K, Iolascon A, Verissimo F, et al. Mutations affecting the secretory COPII coat component SEC23B cause congenital dyserythropoietic anemia type II. Nat Genet. 2009;41(8):936–940. [DOI] [PubMed] [Google Scholar]
- 62.Bianchi P, Schwarz K, Högel J, et al. Analysis of a cohort of 101 CDAII patients: description of 24 new molecular variants and genotype-phenotype correlations. Br J Haematol. 2016;175(4):696–704. [DOI] [PubMed] [Google Scholar]
- 63.Russo R, Gambale A, Langella C, Andolfo I, Unal S, Iolascon A. Retrospective cohort study of 205 cases with congenital dyserythropoietic anemia type II: definition of clinical and molecular spectrum and identification of new diagnostic scores. J Hematol. 2014;89(10):E169–E175. [DOI] [PubMed] [Google Scholar]
- 64.Gambale A, Iolascon A, Andolfo I, Russo R. Diagnosis and management of congenital dyserythropoietic anemias. Expert Rev Hematol. 2016;9(3):283–296. [DOI] [PubMed] [Google Scholar]
- 65.Andolfo I, Russo R, Rosato BE, et al. Genotype-phenotype correlation and risk stratification in a cohort of 123 hereditary stomatocytosis patients. Am J Hematol. 2018. 10.1002/ajh.25276 [DOI] [PubMed] [Google Scholar]
- 66.Mariani M, Barcellini W, Vercellati C, et al. Clinical and hematologic features of 300 patients affected by hereditary spherocytosis grouped according to the type of the membrane protein defect. Haematologica. 2008;93(9):1310–1317. [DOI] [PubMed] [Google Scholar]
- 67.Gallagher PG. Diagnosis and management of rare congenital nonimmune hemolytic disease. Hematol Am Soc Hematol Educ Prog. 2015; 392–399. [DOI] [PubMed] [Google Scholar]
- 68.Lux SE 4th. Anatomy of the red cell membrane skeleton: unanswered questions. Blood. 2016;127(2):187–199. [DOI] [PubMed] [Google Scholar]
- 69.King MJ, Garçon L, Hoyer JD, et al. International Council for Standardization in Haematology. ICSH guidelines for the laboratory diagnosis of nonimmune hereditary red cell membrane disorders. Int J Lab Hematol. 2015;37(3):304–325. [DOI] [PubMed] [Google Scholar]
- 70.Haija MA, Qian YW, Muthukumar A. Dyserythropoiesis in a child with pyruvate kinase deficiency and coexistent unilateral multicystic dysplastic kidney. Pediatr Blood Cancer. 2014;61(8):1463–1465. [DOI] [PubMed] [Google Scholar]
- 71.Roy NB, Wilson EA, Henderson S, et al. A novel 33-gene targeted resequencing panel provides accurate, clinical-grade diagnosis and improves patient management for rare inherited anemias. Br J Haematol. 2016;175(2):318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Russo R, Andolfo I, Manna F, et al. Multi-gene panel testing improves diagnosis and management of patients with hereditary anemias. Am J Hematol. 2018;93(5):672–682. [DOI] [PubMed] [Google Scholar]
- 73.Mentzer WC Jr, Baehner RL, Schmidt-Schonbeth H, Robinson SH, Nathan DG. Selective reticulocyte destruction in erythrocyte pyruvate kinase deficiency. J Clin Invest. 1971;50(3):688–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsumoto N, Ishihara T, Nakashima K, Miwa S, Uchino F, Kondo M. Sequestration and destruction of reticulocytes in the spleen in pyruvate kinase deficiency hereditary non-spherocytic hemolytic anemia. Nippon Ketsueki Gakkai zasshi: J Japan Haematol Soc. 1972;35:525–537. [PubMed] [Google Scholar]
- 75.Nijhof W, Wierenga PK, Staal GEJ, Jansen G. Changes in activities and isozyme patterns of glycolytic enzymes during erythroid differentiation in vitro. Blood. 1984;64(3):607–613. [PubMed] [Google Scholar]
- 76.Dacie J Pyruvate-kinase (PK) deficiency In: Dacie J, ed. The Hemolytic Anemias. Vol 1 3rd ed. New York: Churchill Livingstone; 1985:284–320. [Google Scholar]
- 77.Gallagher PG, Glader B. Diagnosis of Pyruvate Kinase Deficiency. Pediatr Blood Cancer. 2016;63(5):771–772. [DOI] [PubMed] [Google Scholar]
- 78.Beutler E Red Cell Metabolism: A Manual of Biochemical Methods. New York: Grune & Stratton, Inc.; 1984. [Google Scholar]
- 79.Kanno H, Wei DC, Chan LC, et al. Hereditary hemolytic anemia caused by diverse point mutations of pyruvate kinase gene found in Japan and Hong Kong. Blood. 1994;84(10):3505–3509. [PubMed] [Google Scholar]
- 80.Zanella A, Fermo E, Bianchi P, Chiarelli LR, Valentini G. Pyruvate kinase deficiency: the genotype-phenotype association. Blood Rev. 2007;21(4):217–231. [DOI] [PubMed] [Google Scholar]
- 81.Mosca A, Tagarelli A, Paleari R, Scarpelli P, Brancati C. Rapid determination of erythrocyte pyruvate kinase activity. Clin Chem. 1993; 39(3):512–516. [PubMed] [Google Scholar]
- 82.Zanella A, Colombo MB, Miniero R, Perroni L, Meloni T, Sirchia G. Erythrocyte pyruvate kinase deficiency: 11 new cases. Br J Haematol. 1988;69(3):399–404. [DOI] [PubMed] [Google Scholar]
- 83.Lakomek M, Schröter W, De Maeyer G, Winkler H. On the diagnosis of erythrocyte enzyme defects in the presence of high reticulocyte counts. Br J Haematol. 1989;72(3):445–451. [DOI] [PubMed] [Google Scholar]
- 84.Rijksen G, Veerman AJ, Schipper-Kester GP, Staal GE. Diagnosis of pyruvate kinase deficiency in a transfusion-dependent patient with severe hemolytic anemia. Am J Hematol. 1990;35(3):187–193. [DOI] [PubMed] [Google Scholar]
- 85.Lakomek M, Neubauer B, von der Lühe A, Hoch G, Winkler H, Schröter W. Erythrocyte pyruvate kinase deficiency: relations of residual enzyme activity, altered regulation of defective enzymes and concentrations of high-energy phosphates with the severity of clinical manifestation. Eur J Haematol. 1992;49(2):82–92. [DOI] [PubMed] [Google Scholar]
- 86.Ebaugh FG Jr, Emerson CP, Ross JF. The use of radioactive chromium 51 as an erythrocyte tagging agent for the determination or red cell survival in vivo. J Clin Invest. 1953;32(12):1260–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mollison PL, Veall N. The use of the isotope 51Cr as a label for red cells. Br J Haematol. 1955;1(1):62–74. [DOI] [PubMed] [Google Scholar]
- 88.Luten M, Roerdinkholder-Stoelwinder B, Bost HJ, Bosman GJ. Survival of the fittest? Survival of stored red blood cells after transfusion. Cell Mol Biol. 2004;50(2):197–203. [PubMed] [Google Scholar]
- 89.Bosman GJ. Survival of red blood cells after transfusion: processes and consequences. Front Physiol. 2013;4:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Viprakasit V, Ekwattanakit S, Riolueang S, et al. Mutations in Kruppel-like factor 1 cause transfusion-dependent hemolytic anemia and persistence of embryonic globin gene expression. Blood. 2014; 123(10):1586–1595. [DOI] [PubMed] [Google Scholar]
- 91.Boivin P, Galand C, Hakim J, Kahn A. Acquired red cell pyruvate kinase deficiency in leukemias and related disorders. Enzyme. 1975; 19(5–6):294–299. [DOI] [PubMed] [Google Scholar]
- 92.Vives Corrons JL, Pujades MA, Sierra J, Ribera JM. Characteristics of red cell pyruvate kinase (PK) and pyrimidine 5’nucleotidase (P5N) abnormalities in acute leukaemia and chronic lymphoid diseases with leukaemic expression. Br J Haematol. 1987;66(2):173–177. [DOI] [PubMed] [Google Scholar]
- 93.Lin G, Xie Y, Liang X, et al. Study on red cell enzymes and isoenzymes in patients with leukemia and myelodysplastic syndromes. Zhonghua Xue Ye Xue Za Zhi. 1997;18(7):350–353. [PubMed] [Google Scholar]
- 94.Iwasaki T, Utsugisawa T, Yamamoto T, et al. Kanno H pyruvate kinase deficiency in Japan: a summary of clinical feature, laboratory data and enzymatic diagnosis. HemaSphere. 2018;2(S1):214856. [Google Scholar]
- 95.Vercellati C, Marcello AP, Fermo E, Barcellini W, Zanella A. Bianchi P. a case of hereditary spherocytosis misdiagnosed as pyruvate kinase deficient hemolytic anemia. Clin Lab. 2013;59(3–4):421–424. [PubMed] [Google Scholar]
- 96.Fermo E, Vercellati C, Marcello AP, et al. Hereditary Xerocytosis due to mutations in PIEZO1 gene associated with heterozygous pyruvate kinase deficiency and Beta-thalassemia trait in two unrelated families. Case Rep Hematol. 2017;2769570:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kanno H, Fujii H, Miwa S. Structural analysis of human pyruvate kinase L-gene and identification of the promoter activity in erythroid cells. Biochem Biophys Res Commun. 1992;188(2):516–523. [DOI] [PubMed] [Google Scholar]
- 98.Kanno H, Miwa S. Single-nucleotide substitution in pyruvate kinase deficiency. Blood. 1991;78(7):1891–1892. [PubMed] [Google Scholar]
- 99.Bianchi P, Zanella A. Haematologically important mutations: red cell pyruvate kinase (third update). Blood Cells Mol Dis. 2000;26(1):47–53. [DOI] [PubMed] [Google Scholar]
- 100.Bianchi P, Fermo E, Lezon-Geyda K, et al. Molecular characterization of 140 patients in the pyruvate kinase deficiency (PKD) natural history study (NHS): report of 20 new variants. Blood. 2015;126:3337. [Google Scholar]
- 101.Kanno H, Ballas SK, Miwa S, Fujii H, Bowman HS. Molecular abnormality of erythrocyte pyruvate kinase deficiency in the Amish. Blood. 1994;83(8):2311–2316. [PubMed] [Google Scholar]
- 102.Baronciani L, Beutler E. Molecular study of pyruvate kinase deficient patients with hereditary nonspherocytic hemolytic anemia. J Clin Invest. 1995;95(4):1702–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Agarwal AM, Nussenzveig RH, Reading NS, et al. Clinical utility of next-generation sequencing in the diagnosis of hereditary haemolytic anemias. Br J Haematol. 2016;174(5):806–814. [DOI] [PubMed] [Google Scholar]
- 104.Del Orbe Barreto R, Arrizabalaga B, De la Hoz AB, et al. Detection of new pathogenic mutations in patients with congenital haemolytic anemia using next-generation sequencing. Int J Lab Hematol. 2016; 38(6):629–638. [DOI] [PubMed] [Google Scholar]
- 105.Jaouani M, Manco L, Kalai M, et al. Molecular basis of pyruvate kinase deficiency among Tunisians: description of new mutations affecting coding and noncoding regions in the PKLR gene. Int J Lab Hematol. 2017;39(2):223–231. [DOI] [PubMed] [Google Scholar]
- 106.van Oirschot BA, Francois JJ, van Solinge WW, et al. Novel type of red blood cell pyruvate kinase hyperactivity predicts a remote regulatory locus involved in PKLR gene expression. Am J Hematol. 2014; 89(4):380–384. [DOI] [PubMed] [Google Scholar]
- 107.Pereira J, Gonzalez A, Vagace J, et al. Congenital dyserythropoietic anemia associated to a GATA1 mutation aggravated by pyruvate kinase deficiency. Ann Hematol. 2016;95(9):1551–1553. [DOI] [PubMed] [Google Scholar]
- 108.van Zwieten R, van Oirschot BA, Veldthuis M, et al. Partial pyruvate kinase deficiency aggravates the phenotypic expression of band 3 deficiency in a family with hereditary spherocytosis. Am J Hematol. 2015;90(3):E35–E39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.