Abstract
Background:
Knowledge regarding the morphologic spectrum of pediatric melanoma (PM) is sparse and this may in part contribute to delay in detection and thicker tumors.
Objective:
to analyze the clinical-dermoscopic characteristics of PM.
Methods:
retrospective study of 52 melanomas diagnosed before age of 20.
Results:
Based on clinical, dermoscopic and histopathological characteristics, PM can be classified as Spitzoid and Non-Spitzoid Non-Spitzoid (37, 72.3%) melanomas presented at a mean age of 16.3 (8–20) and were associated with a high-risk phenotype and a preexisting nevus (62.2%). Spitzoid melanomas (15, 27.7%) were diagnosed at a mean age of 12.5 (2–19) and were mostly de novo lesions (73.3%) located on the limbs (73.3%). While less than 25% of PM fulfilled the modified clinical ABCD criteria, 40% of spitzoid melanomas did. Non-Spitzoid tended to be multicomponent (58.3%) or nevus-like patterns (25%). Dermoscopic melanoma criteria were found in all cases. Spitzoid melanomas revealed atypical vascular patterns with shiny-white lines (46.2%) or atypical pigmented spitzoid pattern (30.8%). There was good correlation between Spitzoid subtype histopathologically and dermoscopically (kappa 0.66).
Limitations:
Retrospective study without re-review of pathology.
Conclusion:
Dermoscopy in addition to conventional and modified clinical ABCD criteria helps in detecting PM. Dermoscopy assists in differentiating Spitzoid from non-Spitzoid melanomas.
Keywords: melanoma, detection, childhood, Spitzoid, Spitz, dermoscopy, pediatric melanoma
Graphical abstract:
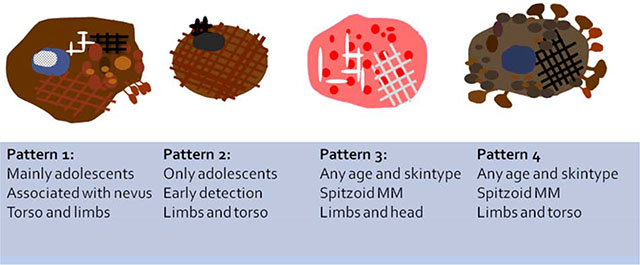
Dermoscopic patterns found in Pediatric Melanoma: Pattern 1, Multicomponent pattern, mostly found in Non-Spitzoid melanomas associated with nevus. Asymmetric polychromic multicomponent pattern, with irregular globules, negative network and structureless areas and some regression features. Pattern 2, Nevus-like; only found in adolescents with Non-Spitzoid melanomas. Symmetric 1 or 2 patterns, with a few melanoma local features. Pattern 3, Pink vascular Spitzoid pattern. Polymorphic vascular pattern and shiny white structures. Pattern 4, Atypical pigmented Spitzoid pattern. Asymmetrical distributed starburst or globular pattern with pseudopods at the periphery.
Capsule summary:
Pediatric melanomas (PM) can be Spitzoid or non-Spitzoid, which have different dermoscopic features.
-Non-Spitzoid melanomas bear morphologic similarity to superficial spreading melanoma, whereas Spitzoid melanomas usually present as amelanotic bumps.
-Both the classic and modified melanoma ABCD criteria can assist in detecting PM, and dermoscopic criteria for melanoma are generally present.
Introduction
Pediatric melanoma (PM) is a melanoma diagnosed during childhood or adolescence. It accounts for less than 3% of pediatric cancers and 1–4% of all melanoma cases. The incidence is not constant across the pediatric age spectrum with melanomas during adolescence (15–19 years old) being 10 times more frequent than those in children (5–9 years old) (1,2). Similar to adult melanoma, the prognosis of pediatric melanoma depends on its AJCC staging (3–5).
PMs are often thicker tumors at time of detection, in part due to delay in diagnosis and/or differences in growth dynamics. The low incidence of PM and the lack of conventional melanoma ABCD criteria, in a subset of these lesions, may help explain the delay in diagnosis (6). Undoubtedly, dermatologists see an overwhelming number of nevi among pediatric patients, and rarely if ever will encounter a melanoma in childhood. Approximately 30% of pediatric dermatological consultations are due to concerns regarding nevi (7). And even among lesions in children that are sufficiently concerning to warrant a biopsy, the nevus to melanoma ratio is about 1:1000 (8).
To improve melanoma early detection, a modified clinical ABCD rule was proposed by Cordoro et al, consisting of ‘Amelanotic, Bleeding bump, Color uniformity and De novo lesion of any diameter’ (9). In addition, dermoscopy improves early detection of melanomas, at least in adults (10). Scant literature exists describing the dermoscopic findings of pediatric melanoma. We present the clinical and dermoscopic findings from a cohort of PM cases.
Methods
The Institutional Review Board at Memorial Sloan Kettering Cancer Center approved this study. We solicited members of the International Dermoscopy Society (IDS) to submit images and clinical data of PM cases. We asked contributors to send, via a secure file transfer system, the clinical and dermoscopic images of histopathologically confirmed PM in patients younger than 20 years old. Patient demographic information and histopathologic information, including melanoma subtype, thickness, ulceration status, mitotic index, and whether there was an associated nevus, were also collected.
A total of 52 melanoma cases were collected from pigmented lesion clinics across 9 countries (Australia, Belgium, Brazil, France, Israel, Italy, Serbia, Spain and USA). One patient presented with two primary melanomas at the age of 17 and 18. All lesions were diagnosed by dermatopathologists specialized in the diagnosis of melanocytic neoplasms at the originating institution. In line with the current classification of PM (11), the dataset of melanomas were classified into Spitzoid melanomas and non-Spitzoid (also termed “conventional” or “adult-like” melanomas, most of which are of the superficial spreading type) melanomas, based on the original institutional histopathological reports.
Clinical and dermoscopic images were evaluated jointly by two experienced reviewers (CC and AAM). The clinical evaluation included the classic ABCD criteria – axis symmetry (0–2), border regularity (0–8), number and type of colors (black, dark brown, light brown, red, white, blue, grey) and diameter. The overall clinical appearance was categorized, by gestalt impression of the reviewers, into “melanoma-like”, “benign-appearing”, and “nodular / polypoid tumors”. Lesions were also evaluated according to the modified ABCD criteria (9).
Dermoscopic specific features were scored as present or absent. For the global dermoscopic pattern classification, we integrated criteria from pattern analysis (12), from a study on nevus-associated melanomas (13) and from a study of atypical Spitzoid tumors (14). This culminated in the use of the following descriptive patterns: (1) ‘Multicomponent pattern’ – >2 dermoscopic structures (reticular, globular or homogeneous), asymmetrically distributed; (2) ‘Nevus-like pattern’–≤2 dermoscopic structures (reticular, globular or homogeneous), symmetrically distributed; (3) ‘Pink Spitzoid pattern’ – hypomelanotic or amelanotic tumors with diffuse vascular pattern; (4) ‘Pigmented Reed-like pattern’ – starburst pattern of streaks or large globules at the periphery; and (5) ‘Non classifiable nodules’ – exophytic tumors not attributable to any of the above-described categories.
Descriptive statistics and graphical methods were used to assess demographics, clinical and dermoscopic variables. The primary outcome variable is the dichotomous lesion classification as spitzoid versus non-spitzoid lesions. Student’s t-test and rank sum-tests were used to compare distributions of continuous study variables by the outcome variable. Pearson’s Chi Square analyses were used to assess cross tabulation comparisons between categorical variables with the primary outcome. Crude and adjusted odds ratios and corresponding 95% confidence intervals (95% CI) were calculated by univariate and multivariate logistic regression. The Type I error probability associated with all tests in this study was set to 0.05. All statistical calculations were made with the SPSS 22.0 (Statistical Package for Social Sciences, SPSS Inc., Chicago, Ill.).
Results
Demographics and Histopathological Data
The study included 52 melanomas from 51 patients. Ten lesions were in situ (19.2%); and 42 were invasive (80.8%) with mean Breslow thickness of 1.8mm (SD 1.4, range 0.30–5.5mm).
The demographic and pathological data are summarized in Table 1. The mean age was 14.8 (range 2–20, SD 4.4) and no difference was observed between the 26 female (52%) and the 25 male (48%) patients. Notably, 6 out of 41 patients (14.6%) had family history of melanoma, and 4 out of 41 (9.5%) were diagnosed with a second primary melanoma during follow-up (mean of 62.6 months, SD 43).
Table 1.
DEMOGRAPHICS OF 52 PEDIATRIC MELANOMAS ACCORDING TO HISTOPATHOLOGICAL SUBTYPE
| VARIABLE╲HISTOPATH. SUBTYPE | Total (n=52) | Spitzoid melanoma (n=15) | non-Spitzoid melanoma (n=37) | P-value |
|---|---|---|---|---|
| AGE (years), mean (range) | 14.8 (2–20) | 12.5 (2–19) | 16.3 (8–20) | 0.004 |
| SEX, n (%) | ||||
| Male | 25 (48.1) | 5 (33.3) | 20 (54.0) | |
| Female | 26 (51.9) | 10 (66.7) | 17 (46.0) | 0.18 |
| RISK FACTORS, n (%)* | ||||
| Multiple primary MM (Yes) | 4 / 41 (9.5) | 0 /14 (0) | 3/27 (11.1) | 0.5 |
| Familial MM (Yes) | 6 / 41 (14.6) | 2/14 (14.3) | 4 / 27 (14.8) | 1.0 |
| Fair skin - Photo-type I-II | 21 / 35 (60.0) | 7/15 (46.7) | 14/20 (70) | 0.16 |
| ANATOMIC LOCATION, n (%) | ||||
| Trunk | 20 (38.5) | 1 (6.7) | 19 (51.4) | 0.01 |
| Head/neck | 7 (13.5) | 3 (20.0) | 4 (10.8) | |
| Limbs: | 25 (48.1) | 11 (73.3) | 14 (37.8) | |
| ∎Upper limbs | 6 (24.0) | 3 (27.3) | 3 (21.4) | P=0.4 |
| ∎Hand / subungual | 1 (4.0) | 1 (9.1) | 0 | |
| ∎Lower Limbs | 16 (64.0) | 7 (64.3) | 9 (64.3) | |
| ∎Foot | 2 (8) | 0 | 2 (14.3) | |
| HISTOPATHOLOGICAL PARAMETERS | ||||
| Ulceration, n (%) | 7 (13.7) | 4 (26.7) | 3 (8.1) | 0.06 |
| De novo melanoma, n (%) | 25 (48.1) | 11 (73.3) | 14 (37.8) | P=0.02 |
| melanoma associated with a nevus, n (%) | 27 (51.9) | 4 (26.7) | 23 (62.2) | |
| • Congenital nevus | 25 (49) | 3 (21.4) | 22 (59.5) | |
| • Dysplastic nevus | 1 (2) | 0 | 1 (2.7) | |
| • Spitz nevus | 1 (2) | 1 (7.1) | 0 | |
| In situ melanoma, n (%) | 10 (19.2) | 0 (0) | 10 (27) | 0.04 |
| Invasive melanoma, n (%) | 42 (80.8) | 15 (100) | 27 (73) | |
| Mean Breslow thickness, millimeters, mean (SD) | 1.8 (1.4) | 2.6 (1.6) | 1.2 (0.9) | P=0.004 |
| OUTCOME, n (%) | ||||
| SNB performed | 13 (25) | 9 (60) | 4 (10.8) | P=0.005 |
| Lymph node metastases** | 6 (11.5) | 4 (26.6) | 2(5.4) | N.S. |
| Visceral metastasis | 1 (2) | 1 (7.1) | 0 |
These parameters were not available for the entire study group, and hence are displayed with the denominator of the numbers of cases with available information. The patient included with two primary tumors in this series was considered only once.
Lymph node metastases were identified at either initial melanoma diagnosis or during follow-up (mean 62.6, SD43 months)
Based on the histopathology report, melanomas were classified into Spitzoid (15 cases, 27.7%) and non-Spitzoid melanoma (37 cases, 72.3%). Patients with a Spitzoid melanoma were significantly younger than patients with non-Spitzoid melanoma (12.5 vs. 16.3 years, p=0.004). Non-Spitzoid melanomas were mostly occurred in the second decade of life. All Spitzoid tumors were invasive, and significantly thicker than the non-Spitzoid melanomas (2.6mm vs. 1.2mm, respectively, p=0.004). These lesions were also more frequently ulcerated compared to non-Spitzoid melanomas (28.6% vs. 8.1%, respectively, p=0.06). Melanomas were associated with a nevus more frequently among non-Spitzoid than Spitzoid melanomas (62.2% vs. 26.7%, p= 0.02). The most common associated nevus was the congenital type (n=25/27, 92.6%).
Clinical Presentation (Tables 1 and 2)
TABLE 2.
CLINICAL PRESENTATION BY HISTOPATHOLOGICAL CLASSIFICATION AS SPITZOID VS NON SPITZOID MELANOMA
| CLINICAL ASPECT╲HISTOPATH. SUBTYPE | Total (n=52) | Spitzoid melanoma (n=15) | non-Spitzoid melanoma (n=37) | P-value |
|---|---|---|---|---|
| Diameter, millimeters, mean (SD) | 8 (3.6) Range 4–18 | 7.5 (2.7) | 8.2 (4) | NS |
| N (%) | N (%) | N (%) | ||
| Overall clinical presentation | ||||
| Melanoma-like (Classic ABCD criteria) | 27 (51.9) | 5 (33.3) | 22 (59.5) | 0.08 |
| Benign-appearing | 15 (28.2) | 3 (20) | 12(32.4) | 0.5 |
| Nodular / polypoid tumor | 10 (19.2) | 7(46.7) | 3 (8.1) | 0.003 |
| Amelanotic | 5 (9.6) | 4 (26.7) | 1 (2.7) | 0.02 |
| Modified ABCD criteria | 11 (21.2) | 6 (40) | 5 (13.5) | 0.05 |
| Colors: | ||||
| Mean number of colors (SD) | 2.3 (1.1) | 2.7 (1.2) | 2.1 (1.0) | 0.1 |
| ∎Light Brown n(%) | 24 (46.2) | 5 (33.3) | 19 (51.4) | 0.24 |
| ∎Dark brown n(%) | 39 (75) | 8 (53.3) | 31 (83.8) | 0.03 |
| ∎Black n(%) | 15 (28.8) | 4 (26.7) | 11 (29.7) | 1 |
| ∎Blue n(%) | 5 (9.6) | 3 (20) | 2 (5.4) | 0.14 |
| ∎Grey n(%) | 9 (17.3) | 5 (35.7) | 4 (10.8) | 0.09 |
| ∎Red/Pink n(%) | 20 (38.5) | 12 (80) | 8 (21.6) | P<0.001 |
The most frequent anatomic location was the lower extremities (16/52, 30.8%), followed by the back (14/52, 26.9%). The overall clinical morphology was categorized as fulfilling the classic melanoma ABCD criteria (called “melanoma-like”) in a majority of cases (n=27, 51.9%). Those not fulfilling the conventional ABCD criteria were called “benign-appearing”(n=15, 28.2%), or “nodular / polypoid tumors” (n=10, 19.2%). Five melanomas (9.6%) were clinically pink and only 11 (21.2%) fulfilled the modified melanoma ABCD criteria (9).
Spitzoid melanomas were frequently located on the limbs (n=11/15, 73.3%), while non-Spitzoid melanomas tended to occur on the torso (19/37, 51.4%). The most frequent clinical morphology of Spitzoid melanomas was “nodular / polypoid” (46.7%). In contrast, most non-Spitzoid melanomas (59.4%) had a “melanoma-like” clinical morphology. Spitzoid melanomas more often displayed the modified-ABCD criteria as compared to non-Spitzoid melanomas (40% vs. 13.5%, OR=4.3; 95% CI: 1.1–17.2, p=0.05).
Dermoscopic attributes(Table 3)
TABLE 3.
DERMOSCOPIC ATTRIBUTES BY HISTOPATHOLOGICAL CLASSIFICATION AS SPITZOID VS NON SPITZOID MELANOMA
| DERMOSCOPY╲HISTOPATH. SUBTYPE | Overall (n=49) | Spitzoid melanoma (n=13) | non-Spitzoid melanoma (n=36) | p-value |
|---|---|---|---|---|
| mean (SD) | mean (SD) | mean (SD) | ||
| Overall dermoscopic pattern: | ||||
| ∎Multicomponent | 24 (49) | 3(23.1) | 21 (58.3) | 0.029 |
| ∎Nevus-like | 9 (18.4) | 0 | 9 (25) | 0.2 |
| ∎Atypical Pigmented Reed-like | 7 (14.3) | 4 (30.8) | 3 (8.3) | 0.07 |
| ∎Pink Spitz-like | 7 (14.3) | 6 (46.2) | 1 (2.8) | 0.001 |
| ∎Non-classifiable nodules | 2 (4.1) | 0 | 2 (5.6) | 1.0 |
| Number of dermoscopic colors mean (SD) | 3.7 (1.3) | 3.8 (1.6) | 3.6 (1.2) | 0.57 |
| Colors: | ||||
| ∎Light Brown | 41 (83.7) | 10 (76.9) | 31 (86.1) | 0.44 |
| ∎Dark Brown | 42 (85.7) | 9 (69.2) | 33 (91.7) | 0.04 |
| ∎Black | 20 (40.8) | 3 (23.1) | 17 (47.2) | 0.13 |
| ∎White | 19 (38.8) | 9 (69.2) | 10 (27.8) | 0.009 |
| ∎Blue | 24 (49) | 7 (53.9) | 17 (47.2) | 0.68 |
| ∎Grey | 23 (46.9) | 8 (61.5) | 15 (41.7) | 0.22 |
| ∎Red/Pink | 25 (51) | 11 (84.6) | 14 (38.9) | 0.005 |
| Structures | ||||
| Atypical Network | 21 (42.9) | 2 (15.4) | 19 (52.8) | 0.02 |
| Negative Network | 11 (22.5) | 1 (7.7) | 10 (27.8) | 0.14 |
| Streaks/pseudopods | 11 (22.5) | 3 (23.1) | 8 (22.2) | 0.95 |
| Irregular dots | 22 (44.9) | 6 (46.2) | 16 (44.4) | 0.92 |
| Irregular blotches | 15 (30.6) | 4 (30.8) | 11 (30.6) | 0.99 |
| Irregular globules | 27 (55.1) | 5 (38.5) | 22 (61.1) | 0.16 |
| Shiny-white areas | 10 (20.4) | 6 (46.2) | 4 (11.1) | 0.007 |
| Blue/white veil | 19 (38.8) | 6 (46.2) | 13 (36.1) | 0.52 |
| Regression | 9 (18.4) | 1 (7.7) | 8 (22.2) | 0.25 |
| Structureless areas | 30 (61.2) | 5 (38.5) | 25 (69.4) | 0.05 |
| Sharp demarcation | 10 (20.4) | 4 (30.8) | 6 (16.7) | 0.28 |
| Vascular patterns | ||||
| Corkscrew | 3 (6.1) | 2 (15.4) | 1 (2.8) | 0.10 |
| Dotted | 11 (22.5) | 5 (38.5) | 6 (16.7) | 0.11 |
| Linear/irregular | 8 (16.3) | 4 (30.8) | 4 (11.1) | 0.10 |
| Hairpin | 3 (6.1) | 2 (15.4) | 1 (2.8) | 0.10 |
| Milky-red areas | 9 (18.4) | 5 (38.5) | 4 (11.1) | 0.03 |
| Polymorphous | 9 (18.4) | 6 (46.2) | 3 (8.3) | 0.003 |
Dermoscopic images were available for 49 of 52 cases (94.2%). The global dermoscopic patterns observed included: (1) multicomponent pattern (n=24, 49%), mostly associated with non-Spitzoid melanoma (87.5%, p<0.03); (2) nevus-like pattern (n=9, 18.4%), observed only among non-Spitzoid melanomas; and (3) vascular pink Spitz-like pattern (n=7, 14.3%), mostly associated with Spitzoid melanomas (85.7%, p<0.002). Pigmented Reed-like pattern (n=7, 14.3%) was seen at a higher frequency among Spitzoid melanomas, albeit not reaching statistical significance. Two tumors were categorized as non-classifiable nodules (4.1%).
On univariate analysis, red and white colors, milky red areas, polymorphous vessels and shiny white structures were associated with Spitzoid melanoma. Presence of both red color and shiny white structures was strongly associated with histopathological Spitzoid subtype (OR=16.5, 95% CI; 1.7–163.3, p=0.02). Overall dermoscopic Spitzoid patterns (pink or pigmented) were associated with Spitzoid histopathologic diagnosis (78.6% were Spitzoid vs 11.1% were non-spitzoid, p<0.001). Lesions with a spitzoid dermoscopic pattern were much more likely to be classified as a spitzoid lesion histologically (OR 29.3; 95CI 5.6–152.1).
Dark brown color, atypical network and structureless areas were associated with non-Spitzoid melanomas. In addition, presence of irregular globules was associated with melanomas arising within a nevus (73.1% vs 34.8%, p=0.007).
The distribution of demographic and clinical attributes by global dermoscopic pattern are shown in Table 4. Melanomas presenting with multicomponent or nevus-like patterns were more likely to be histopathologically classified as non-Spitzoid. These lesions were also associated with older age, fair-skin phenotype, family history of melanoma and the presence of a pre-existing nevus. Melanomas showing pink-Spitzoid or Reed-like dermoscopic patterns were more likely to be histopathologically classified as Spitzoid (kappa 0.66) and associated with younger age, anatomic location on the limbs and de novo development.
TABLE 4.
DEMOGRAPHIC AND CLINICAL ATTRIBUTES BY THE GLOBAL DERMOSCOPIC PATTERNS
| PRESENTATION╲DERMOSCOPIC PATTERNS | Multicomponent (n=24) | Nevus-like (n=9) | Pink Spitzoid (n= 7) | Reed-like (n=7) |
|---|---|---|---|---|
| Age, mean (SD) | 15.7 (3.5) | 17.4 (1.6) | 13 (6) | 12.8 (6.3) |
| Age<12 years, n (%) | 5 (20.8%) | 0 | 2 (28.6) | 2 (28.6) |
| Fair skin - Photo-type I-II, n (%) | 8/12 (66.7%) | 4/6 (66.7%) | 3 (42.9%) | 3 (50%) |
| Anatomic location on torso, n (%) | 13 (54.2%) | 4 (44.4%) | 0 | 1 (14.3%) |
| Anatomic location on limbs, n (%) | 8 (33.3%) | 5 (55.6%) | 5 (71.4%) | 5 (71.4%) |
| Family history, n (%) | 3 /17 (17.6%) | 3 (33.3%) | 0 | 0 |
| In situ MM, n (%) | 4 (16.7%) | 5 (55.6%) | 0 | 1 (14.3%) |
| Breslow thickness, in mm, mean (SD) | 1.4 (1.4) | 1.3 (1.2) | 2 (1.4) | 2.6 (1.8) |
| Histopathological classification as Spitzoid melanoma, n (%) | 3 (12.5%) | 0 | 6 (85.7%) | 4 (57.1%) |
| Melanoma arising in a nevus, n (%) | 18 (75%) | 3 (33.3%) | 1 (14.3%) | 3 (42.9%) |
| Amelanotic clinically, n (%) | 1 (4.2%) | 0 | 2 (33.3%) | 0 |
| Modified ABCD clinical criteria, n (%) | 2 (8.3%) | 0 | 6 (85.7%) | 0 |
| Ulceration, n (%) | 3 (12.5%) | 0 | 2 (28.2%) | 0 |
| Predominant dermoscopic colors (%) | Dark brown (91.7%) Light brown (95.8%) |
Dark brown (100%) Light brown (88.9%) |
Pink (100%) Red (57.1%) |
Dark brown (100%) Blue-grey (100%) Black (71.4%) |
| Most frequent melanoma-specific dermoscopic criteria, (%) | 70.8% irregular globules 50% atypical network 50% blue white veil 50% visible atypical vessels 29% negative network |
70% atypical network 50% irregular globules 30% negative network |
100% blue white veil 71.4% pseudopods / streaks 71.4% irregular globules 57% shiny white structures |
85.7% visible vessels 42.9% milky red areas 28.6% shiny white structures |
Discussion
Pediatric melanoma is rare, but this potentially fatal disease is curable if diagnosed early. Few studies have investigated the clinical morphology of pediatric melanoma due to the rarity of the condition, and limited data with clinical and dermoscopic images. By collaborating with the international community of pigmented lesion experts, we amassed a series of 52 clinically-imaged, biopsy-proven pediatric melanomas, 49 of which also had dermoscopic images.
We have shown that the clinical and dermoscopic features are associated with the histopathological sub-categorization of melanomas as Spitzoid or non-Spitzoid. Comparing these two histopathologic subsets of melanomas, we observed differences in the demographics and morphological presentation of each.
The majority of melanomas in our series were non-Spitzoid (70%) and most of these patients were adolescents. Their melanomas were more likely to occur on the back, to be associated with a nevus, and were clinically pigmented with a suspicious morphology (“melanoma like”). Non-Spitzoid melanoma patients harbored melanoma risk factors including fair skin (phototype I-II) in 70%, and a family history of melanoma in 14.8% (4/27). In addition, 11.1% (3/27) of these patients eventually developed a second primary melanoma; in line with prior estimates of 25% risk for a second primary melanoma within 20 years (4).
Dermoscopically, the global patterns associated with non-Spitzoid melanoma were multicomponent pattern (58%) and nevus-like pattern (25%). The multicomponent pattern is the “classic” dermoscopic melanoma pattern encountered in superficial spreading type melanoma, with most cases revealing irregular globules, atypical network, blue white veil, atypical vessels, and up to 30% revealed a negative network. Nevus-like pattern occurred only in adolescence after 12 years of age, was associated more commonly with family history of melanoma, and included a high proportion of in situ melanomas (55%).
We speculate that these were melanomas caught at an earlier stage, in high-risk adolescents being screened at pigmented lesion clinics(15–17). All nevus-like melanomas in our series presented at least one melanoma-specific dermoscopic feature that identified the lesion as suspicious including atypical network (70%), irregular globules (50%) and negative network (30%). The latter was previously found to be predictive of melanoma arising in nevi (13), however, in the present study we were not able to demonstrate this association, probably due to the fact that Spitzoid melanomas can present with a negative network without an associated nevus being present.
Preliminary studies have demonstrated that non-Spitzoid pediatric melanoma present similarly to superficial spreading melanoma, with a high burden of somatic single-nucleotide variations, mainly affecting TERT promoter and activating BRAF mutations. Most of these somatic variations show fingerprints of ultraviolet damage, implying that sun-exposure may play a role(18). These data suggest that children and adolescents, at high-risk for melanoma, may benefit from sun-protection and periodic skin examination, similar to at risk adults (19,20).
Spitzoid melanomas comprised the minority (30%) in the present series. These patients were younger and rarely had associated risk factors. They most commonly occurred on the extremities and head, were nodular or polypoid, arose de-novo, and 25% were amelanotic. These melanomas were all invasive, thicker than non-Spitzoid melanomas, and more frequently ulcerated. Spitzoid melanomas were previously shown to be associated with chromosomal rearrangements, resulting in activated kinase signaling (11,21), suggesting a different pathogenesis from non-Spitzoid melanomas.
The dermoscopic ‘vascular pink Spitz-like pattern’, seen in amelanotic tumors, was strongly associated with a Spitzoid histopathological classification, and the dermoscopic clues included the presence of atypical vessels (e.g., polymorphic vessels, dotted vessels, and/or milky red areas) and shiny white structures. The presence of both red color and shiny white structures greatly increased the likelihood of the lesion being a Spitzoid melanoma. A recent expert consensus paper (22) determined that all nodular melanocytic neoplasms with a dermoscopic Spitzoid morphology, clinically or dermoscopically asymmetric, occurring after the age of 12, should be managed cautiously.
The ‘pigmented Reed-like’ dermoscopic pattern was also seen more commonly among melanomas with Spitzoid histopathology, but this association did not reach statistical significance. Dermoscopic clues to the ‘pigmented Reed-like’ melanomas included the presence of black, blue-grey and dark brown colors and peripheral streaks and dark blotches. In agreement with Moscarella et al (14) findings, ulcerated nodules, atypical vessels, shiny white structures and blue white veil were associated with the diagnosis of Spitzoid melanoma or atypical Spitzoid tumor, and the symmetric starburst pattern was associated with the diagnosis of Spitz / Reed nevus.
In contrast to previous reports (6,9), the majority of pediatric melanomas did not meet the modified ABCD criteria (Amelanotic, Bleeding bump, Color uniformity and De novo any diameter). Only 13% of non-Spitzoid melanomas, and 40% of Spitzoid melanomas fulfilled the modified ABCD criteria. While some of these melanomas may manifest the conventional ABCD criteria, it should be underscored that all of the cases here did reveal dermoscopic features to aid in their detection.
Our study has limitations. This study provides information that can improve the sensitivity for detecting PM but it does not address the impact on specificity. The size of dataset is relatively small, which reflects the rarity of this entity. These cases originated from specialized pigmented lesion clinics and this introduces potential referral bias of individuals with a high-risk phenotype. These specialized clinics likely use digital surveillance techniques resulting in the diagnosis of a higher proportion of non-rapidly growing melanomas. Finally, histopathological diagnoses were not secondarily re-reviewed; we relied on solely on the review from the contributing institution’s dermatopathologist.
The correlation between the clinical morphology and the histopathological classification supports the existence of distinct, reproducibly recognized subsets of PM. In contrast to previous reports, the present study highlights that the majority of melanomas diagnosed under the age of 20 look similar to melanomas found in adults. Non-Spitzoid melanomas were associated with adolescence and with the presence of melanoma risk factors. All non-spitzoid melanomas reveal dermoscopic features associated with melanoma. Spitzoid melanomas were associated with younger age, location on the extremities, and nodular/polypoid clinical morphology. Dermoscopy of Spitzoid melanomas revealed atypical vessels and shiny white lines (if amelanotic) or asymmetric starburst pattern (if pigmented). The addition of dermoscopy to classic and modified melanoma ABCD criteria can all act in concert to help in the detection of melanoma in children as early as possible.
Figure 1. Pattern 1, Multicomponent pattern. Non-Spitzoid melanomas in adolescents.
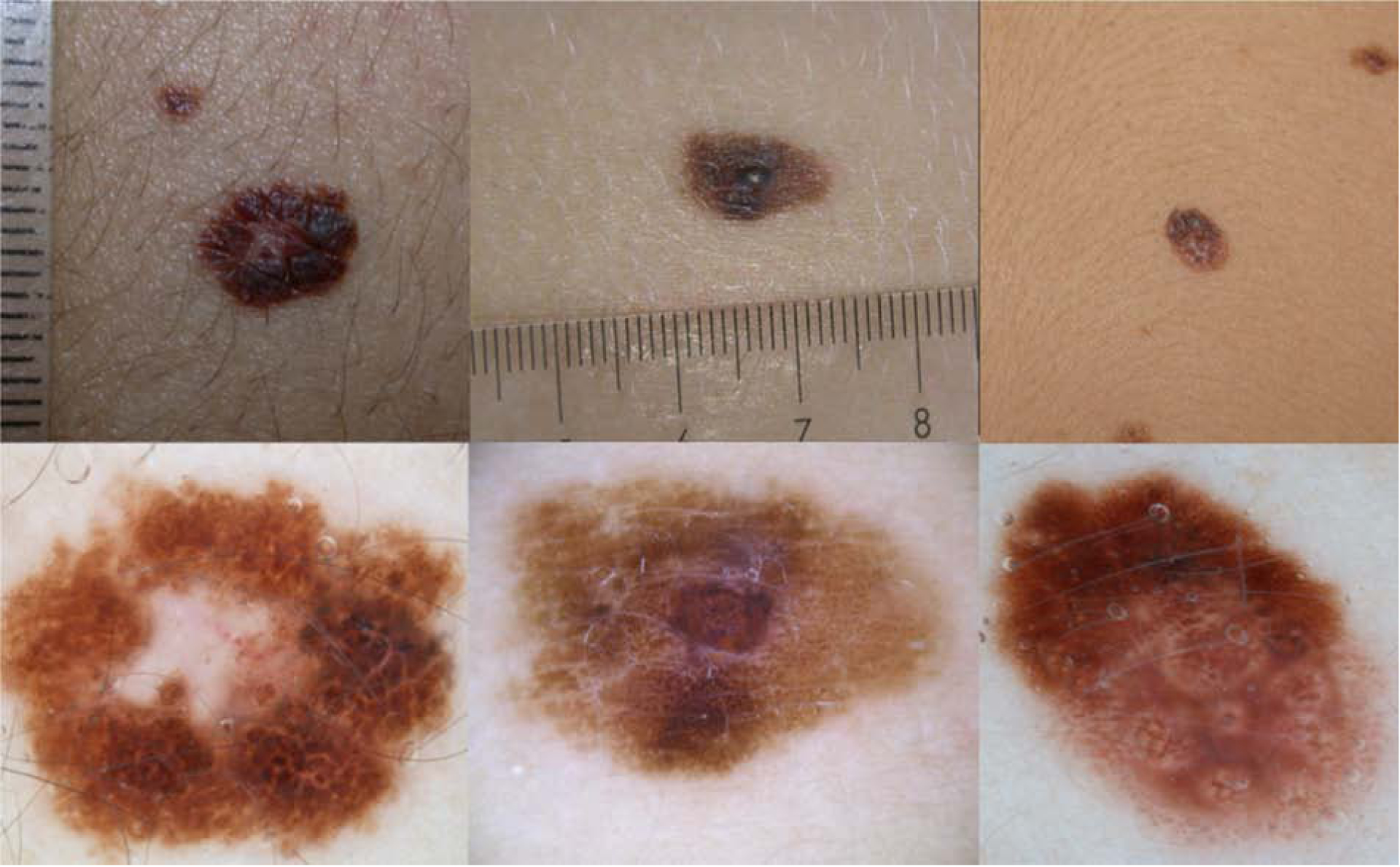
a) Clinically, symmetric 6mm pigmented lesion on the upper limb of 18yo girl.
b) Dermoscopy: Asymmetric polychromic (white, blue-grey, light and dark brown) multicomponent pattern, with irregular globules, negative network and structureless white regression central area. Melanoma arising in a nevus, Breslow 0.4mm.
c) Clinically, asymmetric 12mm lesion on the back of a 13year old girl affected by multiple atypical nevi and familial melanoma.
d) Dermoscopy: Asymmetric multicomponent polychromic pattern, irregular globules and negative network and focal bluegrey regression. Melanoma arising in a nevus, Breslow 0.3mm.
e) Clinically symmetric pigmented lesion on the trunk of 14 year old girl presenting multiple nevi.
f) Dermoscopy: asymmetric multicomponent pattern, with network, irregular globules, islands of negative network and structureless areas. Superficial spreading Melanoma, Breslow 0.7mm.
Figure 2. Pattern 2, Nevus-like. Non-Spitzoid melanomas in adolescents.
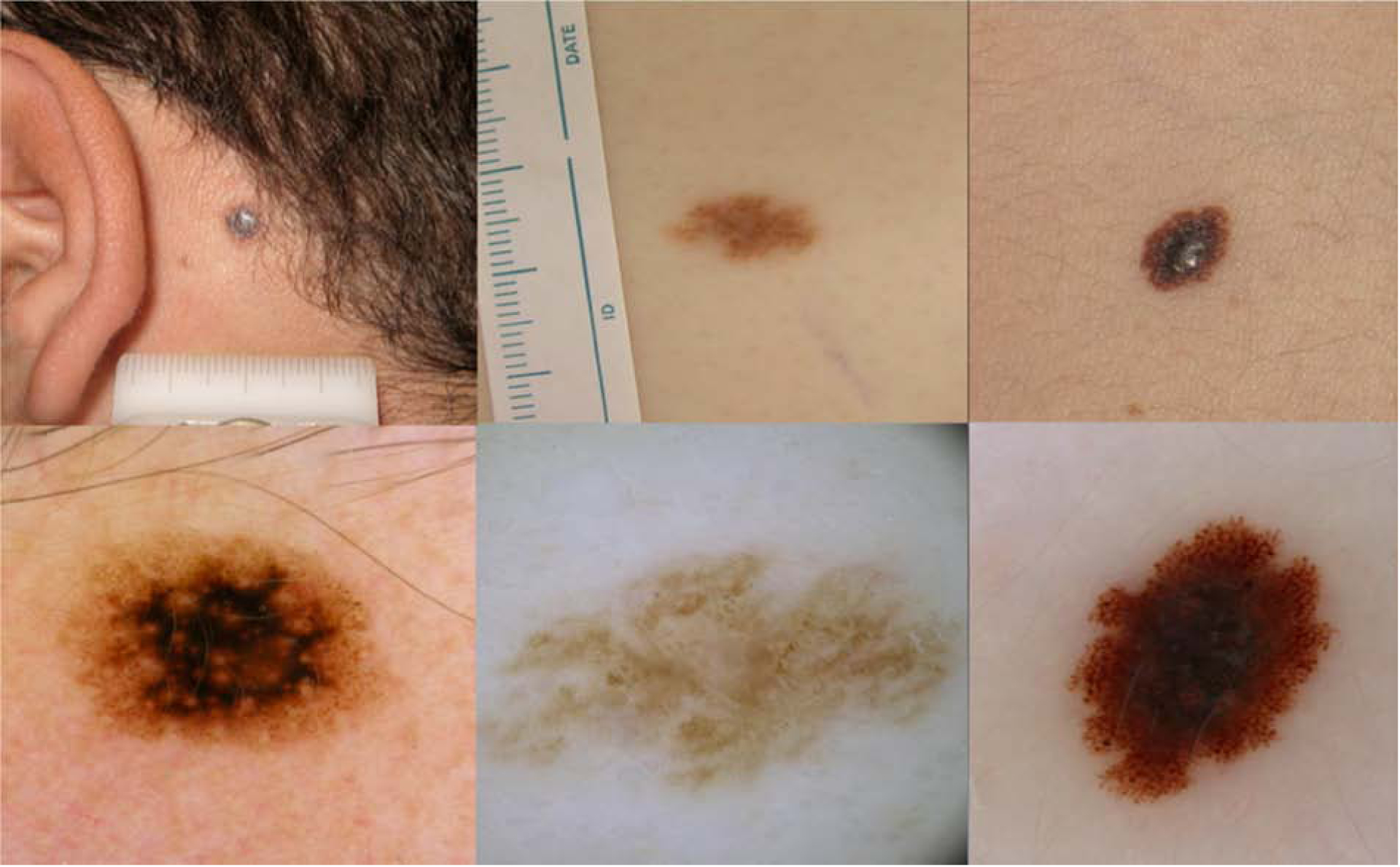
a) Clinically, symmetric 5mm lesion on a 19 year old boy.
b) Dermoscopy: Symmetric globular pattern, with structureless central black area. Superficial spreading Melanoma, Breslow 0.6mm.
c) Clinically, symmetric light brown macule on the chest of a 17year old boy affected by multiple atypical nevi and previous melanoma.
d) Dermoscopy: Symmetric light brown lesion showing irregular globules and negative network. Melanoma arising in a nevus, Breslow 1.0mm.
e) Clinically asymmetric lesion on the trunk of a 19 year old boy.
f) Dermoscopy: symmetric reticular-globular pattern, with atypical network, irregular pigmented globules and dots at the periphery and central blue-white veil. Melanoma arising in a nevus, Breslow 3.1mm.
Figure 3. Pattern 3, Pink vascular Spitzoid pattern.
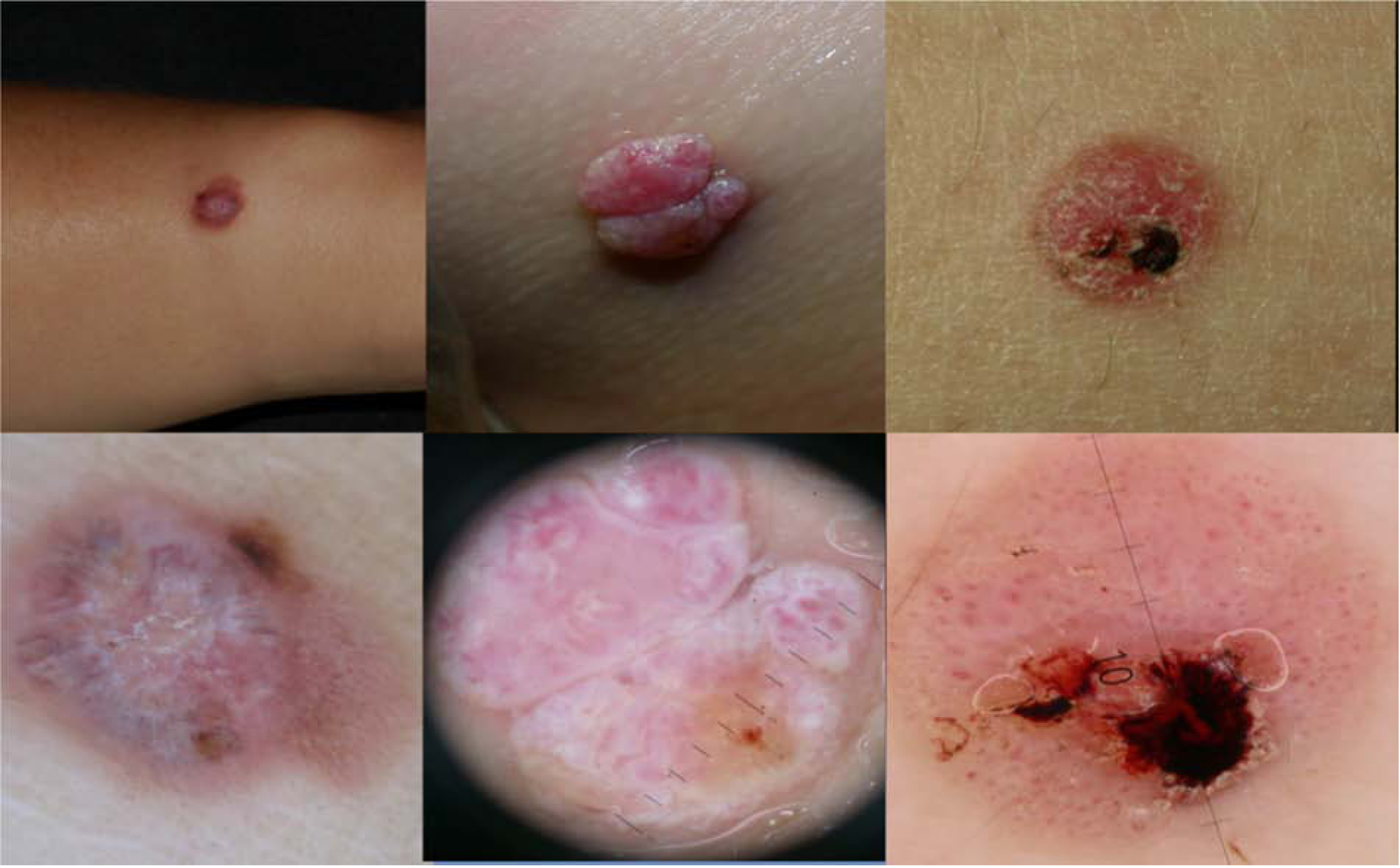
a) Clinically, hypomelanotic rapid growing tumor on a 14 year old girl.
b) Dermoscopy: Polymorphic vascular pattern, with dotted vessels, milky red areas and remnants of pigment at the periphery, central shiny white structures. Spitzoid melanoma, Breslow 1.9 mm.
c) Clinically, amelanotic pink bump on the lower limb of a 3 year old girl.
d) Dermoscopy: Polymorphic vascular pattern, milky red areas and globules and shiny white structures. Spitzoid melanoma Breslow 5.5 mm
e) Clinically ulcerated amelanotic bump on the lower limb of a 17 year old girl.
f) Dermoscopy: Vascular pattern showing dotted vessels and milky red globules, and ulceration. Spitzoid melanoma, Breslow 1.9 mm.
Figure 4. Pattern 4, Pigmented Spitzoid pattern.
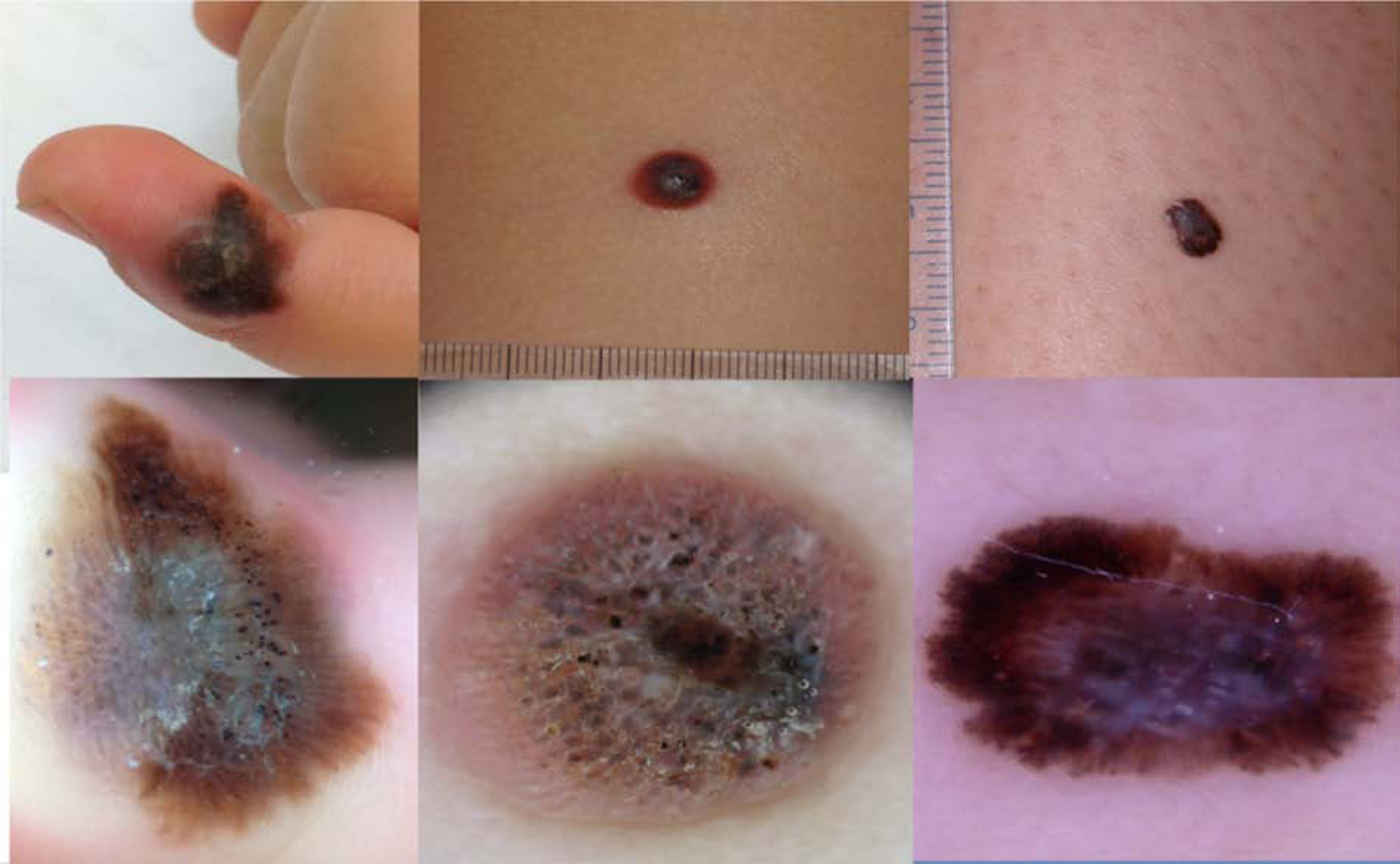
a) Clinically, asymmetric polychromic lesion on the finger of a 4 year old boy.
b) Dermoscopy: Multicomponent pattern on acral site, showing Spitzoid features with peripheral streaks, blue white veil in the center and multiple irregular black globules and dots. Spitzoid melanoma, Breslow 2.8 mm.
c) Clinically, symmetric pigmented tumor on the lower limb of a 9 year old boy.
d) Dermoscopy: Asymmetric globular pattern with ulceration, blue white veil and irregular black dots. Invasive Spitzoid melanoma
e) Clinically pigmented lesion on the lower limb of a 17 year old girl.
f) Dermoscopy: Asymmetric pigmented Spitzoid pattern, with irregular pseudopods, blue white veil, shiny white structures. Spitzoid melanoma, Breslow 0.42 mm.
Acknowledgement:
We thank the International Dermoscopy Society members who contributed with less than 3 cases of pediatric melanomas who are not included as coauthors; Zeljko Mijuskovic MD, Danika Fietz MD, Cristiane Comparin MD, Irene Giam MD, Alan C. Halpern MD and Pedro Zaballos MD. We thank to Shirin Bajaj MD who helped in the international collection of the cases. We are in debt to all our colleagues of Dermatology Departments who referred their atypical and difficult melanocytic tumors to Pigmented Lesion Units and allowed us the continuing learning and research in this field.
Funding resources of the work:
This research was funded in part through the NIH/NCI Cancer Support Grant P30 CA008748. The research at the Melanoma Unit in Barcelona is partially funded by Spanish Fondo de Investigaciones Sanitarias grants PI12/00840, PI15/00716 and PI15/00956; CIBER de Enfermedades Raras of the Instituto de Salud Carlos III, Spain, co-financed by European Development Regional Fund “A way to achieve Europe” ERDF; AGAUR 2014_SGR_603 of the Catalan Government, Spain; European Commission under the 6th Framework Programme, Contract No. LSHC-CT-2006-018702 (GenoMEL) and by the European Commission under the 7th Framework Programme, Diagnoptics; A grant from “Fundació La Marató de TV3, 201331-30”, Catalonia, Spain; a grant from Telemaraton of Spain “Todos somos raros” and a grant from “Asociación Española Contra el Cáncer (AECC)”.
Abbreviation and acronym list:
- PM
pediatric melanoma
- Clinical ABCD
asymmetry, border irregularity, multiple colors, diameter >6mm
- Modified ABCD
amelanotic, bleeding bump, color uniformity, any diameter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interests to declare
References
- 1.Campbell LB, Kreicher KL, Gittleman HR, Strodtbeck K, Barnholtz-Sloan J, Bordeaux JS. Melanoma Incidence in Children and Adolescents: Decreasing Trends in the United States. J Pediatr [Internet]. 2015. June [cited 2015 Aug 26];166(6):1505–13. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25866386 [DOI] [PubMed] [Google Scholar]
- 2.Han D, Zager JS, Han G, Marzban SS, Puleo CA, Sarnaik AA, et al. The unique clinical characteristics of melanoma diagnosed in children. Ann Surg Oncol [Internet]. 2012. November [cited 2016 Dec 5];19(12):3888–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22864798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brecht IB, Garbe C, Gefeller O, Pfahlberg A, Bauer J, Eigentler TK, et al. 443 paediatric cases of malignant melanoma registered with the German Central Malignant Melanoma Registry between 1983 and 2011. Eur J Cancer [Internet]. 2015 May [cited 2015 Aug 26];51(7):861–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25794606 [DOI] [PubMed] [Google Scholar]
- 4.Stanelle EJ, Busam KJ, Rich BS, Christison-Lagay ER, Dunkel IJ, Marghoob AA, et al. Early-stage non-Spitzoid cutaneous melanoma in patients younger than 22 years of age at diagnosis: long-term follow-up and survival analysis. J Pediatr Surg [Internet]. 2015. June [cited 2015 Jul 15];50(6):1019–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25819019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Averbook BJ, Lee SJ, Delman KA, Gow KW, Zager JS, Sondak VK, et al. Pediatric melanoma: analysis of an international registry. Cancer [Internet]. 2013. November 15 [cited 2016 Dec 5];119(22):4012–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24022819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitkov M, Chrest M, Diehl NN, Heckman MG, Tollefson M, Jambusaria-Pahlajani A. Pediatric melanomas often mimic benign skin lesions: A retrospective study. J Am Acad Dermatol [Internet]. 2016. October [cited 2016 Dec 6];75(4):706–711.e4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27430613 [DOI] [PubMed] [Google Scholar]
- 7.Aguilera P, Puig S, Guilabert A, Julià M, Romero D, Vicente A, et al. Prevalence study of nevi in children from Barcelona. Dermoscopy, constitutional and environmental factors. Dermatology [Internet]. 2009. January [cited 2013 May 25];218(3):203–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19060476 [DOI] [PubMed] [Google Scholar]
- 8.Moscarella E, Zalaudek I, Cerroni L, Sperduti I, Catricalà C, Smolle J, et al. Excised melanocytic lesions in children and adolescents - a 10-year survey. Br J Dermatol [Internet]. 2012. August [cited 2013 May 25];167(2):368–73. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22428965 [DOI] [PubMed] [Google Scholar]
- 9.Cordoro KM, Gupta D, Frieden IJ, McCalmont T, Kashani-Sabet M. Pediatric melanoma: Results of a large cohort study and proposal for modified ABCD detection criteria for children. J Am Acad Dermatol [Internet]. 2013. June [cited 2017 Feb 15];68(6):913–25. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23395590 [DOI] [PubMed] [Google Scholar]
- 10.Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol [Internet]. 2002. March [cited 2013 May 22];3(3):159–65. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11902502 [DOI] [PubMed] [Google Scholar]
- 11.Lu C, Zhang J, Nagahawatte P, Easton J, Lee S, Liu Z, et al. The genomic landscape of childhood and adolescent melanoma. J Invest Dermatol [Internet]. 2015. March [cited 2015 Aug 26];135(3):816–23. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4340976&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Argenziano G, Soyer HP, Chimenti S, Talamini R, Corona R, Sera F, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol [Internet]. 2003. May [cited 2015 Mar 14];48(5):679–93. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12734496 [DOI] [PubMed] [Google Scholar]
- 13.Shitara D, Nascimento M, Ishioka P, Carrera C, Alós L, Malvehy J, et al. Dermoscopy of Naevus-associated Melanomas. Acta Derm Venereol [Internet]. 2014. November 14 [cited 2015 Mar 24]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/25394499 [DOI] [PubMed] [Google Scholar]
- 14.Moscarella E, Lallas A, Kyrgidis A, Ferrara G, Longo C, Scalvenzi M, et al. Clinical and dermoscopic features of atypical Spitz tumors: A multicenter, retrospective, case-control study. J Am Acad Dermatol [Internet]. 2015. November [cited 2016 Nov 21];73(5):777–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26475536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salerni G, Carrera C, Lovatto L, Puig-Butille JA, Badenas C, Plana E, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol [Internet]. 2011/June/21 2011; Available from: http://www.ncbi.nlm.nih.gov/pubmed/21683472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salerni G, Carrera C, Lovatto L, Martí-Laborda RM, Isern G, Palou J, et al. Characterization of 1152 lesions excised over 10 years using total-body photography and digital dermatoscopy in the surveillance of patients at high risk for melanoma. J Am Acad Dermatol [Internet]. 2012. November [cited 2013 May 16];67(5):836–45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22521205 [DOI] [PubMed] [Google Scholar]
- 17.Kittler H, Guitera P, Riedl E, Avramidis M, Teban L, Fiebiger M, et al. Identification of clinically featureless incipient melanoma using sequential dermoscopy imaging. Arch Dermatol [Internet]. 2006. September [cited 2013 May 27];142(9):1113–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16982998 [DOI] [PubMed] [Google Scholar]
- 18.Rabbie R, Rashid M, Arance AM, Sánchez M, Tell-Marti G, Potrony M, et al. Genomic analysis and clinical management of adolescent cutaneous melanoma. Pigment Cell Melanoma Res [Internet]. 2017; Available from: http://doi.wiley.com/10.1111/pcmr.12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Argenziano G, Cerroni L, Zalaudek I, Staibano S, Hofmann-Wellenhof R, Arpaia N, et al. Accuracy in melanoma detection: a 10-year multicenter survey. J Am Acad Dermatol [Internet]. 2012. July [cited 2013 May 25];67(1):54–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21982636 [DOI] [PubMed] [Google Scholar]
- 20.Salerni G, Terán T, Puig S, Malvehy J, Zalaudek I, Argenziano G, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol [Internet]. 2012. November 26 [cited 2013 May 18]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/23181611 [DOI] [PubMed] [Google Scholar]
- 21.Pappo AS. Pediatric melanoma: the whole (genome) story. Am Soc Clin Oncol Educ Book [Internet]. 2014. January [cited 2015 Aug 26];e432–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24857134 [DOI] [PubMed] [Google Scholar]
- 22.Lallas A, Moscarella E, Longo C, Kyrgidis A, de Mestier Y, Vale G, et al. Likelihood of finding melanoma when removing a Spitzoid-looking lesion in patients aged 12 years or older. J Am Acad Dermatol [Internet]. 2015. January [cited 2016 Feb 29];72(1):47–53. Available from: http://www.sciencedirect.com/science/article/pii/S0190962214020003 [DOI] [PubMed] [Google Scholar]


