Abstract
Young people are disproportionately affected by sexually transmitted infections (STIs). The risk of STIs in young people following first-episode schizophrenia is unknown. This study using Taiwan’s National Health Insurance Research Database enrolled 44 109 adolescents and young adults with first-episode schizophrenia and 176 436 age- and sex-matched controls without schizophrenia from 2001 through 2009 and followed to the end of 2011. New-onset STIs were identified. Survival analysis was performed. Cox regression analysis was used to examine the effects of comorbid substance use disorder (SUD), schizophrenia medications, and schizophrenia severity. The E value for causality of evidence was calculated. We found that young people had a higher risk of STIs following first-episode schizophrenia compared with controls without schizophrenia (hazard ratio [HR] = 2.35, 95% CI = 2.08–2.64); these STIs included human immunodeficiency virus (HIV) (3.70, 2.60–5.28) and syphilis (5.35, 3.96–7.23). They also showed a disproportionate distribution of STIs, with an increased proportion of syphilis (20.4% vs 8.2%) and HIV (9.1% vs 6.0%). When presenting with SUD, the risks of HIV (11.00, 7.02–17.25) and syphilis (9.11, 6.16–13.47) were further increased. The severe schizophrenia group had an extremely high risk of syphilis (41.26, 27.69–61.47) and HIV (7.50, 3.85–14.62). Schizophrenia medications may provide beneficial effects against contracting STIs (0.77, 0.68–0.89). We concluded that following first-episode schizophrenia, young patients are at higher risk of STIs, particularly HIV and syphilis. The risk further increased when subjects presented with SUD or severe schizophrenia. Importantly, antipsychotic treatment may lower the risk of STIs.
Keywords: first-episode schizophrenia, adolescent, young adults, sexually transmitted infection, human immunodeficiency virus, syphilis
Introduction
Sexually transmitted infections (STIs) continue to be a global public health problem. According to the World Health Organization, more than 1 million people around the world contract STIs every day.1 Beyond the immediate impact of the infections, STIs may cause tremendous health and social consequences, such as cancer, human immunodeficiency virus (HIV) infection, reproductive health problems, and social stigma. STIs are an economic drain on the healthcare system. For example, they cost billions annually in the United States.2
Patients with psychiatric disorders, including schizophrenia, are at high risk for STIs. A cross-sectional study reported an odds ratio (OR) of 1.51 of HIV infection in patients with schizophrenia, while this association was confounded by comorbid substance use disorders (SUDs).3 Another cross-sectional study found that patients with schizophrenia and comorbid SUD had a higher risk of HIV infection compared with those without comorbid SUD.4 Three cohort studies also reported similar comorbid effects of SUD on HIV infection in patients with schizophrenia.5–7
Although STIs are on the rise in people of all ages, STIs take an especially heavy toll on young people. Evidence suggests that young people aged 15–24 account for half of the 20 million new STIs in the United States each year.8 Importantly, schizophrenia typically begins in late adolescence or early adulthood, periods when sexual drive and sexual activity are peaking.9,10 Following first-episode schizophrenia (FES), these patients’ cognition, perception, emotion, behaviors, and sense of self are profoundly influenced.9,11,12 Propensities for poor decision-making13 and risk-taking10 that follow FES imply that young people with schizophrenia are an underestimated population highly vulnerable to STIs.10,14 However, the disproportionate distribution of STIs in young people has not previously been considered when investigating the association between STIs and schizophrenia. Moreover, whether the management of schizophrenia itself may affect the risk of STIs is also unknown.
In the current study, we used a national longitudinal database to investigate the risk of STIs following FES. We also examined the effects of comorbidities and antipsychotic treatment on the risk of incident STIs. To our knowledge, this is the first study addressing STIs in adolescents and young adults with schizophrenia.
Methods
Data Source
Taiwan’s National Health Insurance, a mandatory universal health insurance program, offers comprehensive medical care coverage to all Taiwanese residents (more than 23 million people). The National Health Research Institute manages the National Health Insurance Research Database (NHIRD), which is the entire insurance claims database and consists of healthcare data from >99% of the entire population of Taiwan. The National Health Research Institute audits and releases the NHIRD for scientific and study purposes. The NHIRD has been used extensively in several epidemiologic studies.15–18 Individual medical records included in the NHIRD are anonymized to protect patient privacy. Comprehensive information of insured individuals is included in the database, including demographic data, dates of clinical visits, diagnoses, and medical interventions. The diagnostic codes used are based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). Taipei Veterans General Hospital institutional review board approved this study (2018-07-016AC).
Inclusion Criteria
Six STIs were included in this study: HIV (ICD-9-CM codes: 042, V08), syphilis (ICD-9-CM codes: 091–F097), genital warts (ICD-9-CM code: 078.11), gonorrhea (ICD-9-CM code: 098), chlamydial infection (ICD-9-CM codes: 078.8, 078.88), and trichomoniasis (ICD-9-CM code: 131). The schizophrenia cohort comprised adolescents aged 12–17 years and young adults aged 18–29 years who were first diagnosed with schizophrenia (ICD-9-CM code: 295) by board-certified psychiatrists between January 1, 2001 and December 31, 2009. The time of schizophrenia diagnosis was defined as the time of enrollment in the study, and the schizophrenia cohort did not have any history of STIs and schizophrenia before enrollment. The control cohort was matched by age, sex, and time of enrollment (1:4). The controls were randomly selected after eliminating study cases, those who had been given a diagnosis of schizophrenia at any time, and those with any known STI before enrollment. The outcome of interest was any of the 6 STIs identified during follow-up (from enrollment to December 31, 2011, or death).
Psychiatric comorbidities, including disruptive behavior disorders, alcohol use disorders (AUDs), and SUD, were examined as confounding factors. The average times of psychiatric admission for schizophrenia per year during the follow-up period was recorded and served as the scale of disease severity of schizophrenia in our study. Disease severity was divided into 3 categories: mild (<1/year), moderate (1–2/year), and severe (>2/year). The use of atypical antipsychotics during the follow-up period, including amisulpride, aripiprazole, clozapine, olanzapine, paliperidone, quetiapine, risperidone, and ziprasidone, was also examined. The participants were divided into 3 subgroups: nonusers (cumulative defined daily dose [cDDD] during the follow-up < 30), short-term users (cDDD = 30–364), and long-term users (cDDD ≥ 365). Level of urbanization (from level 1, most urbanized region, to level 5, least urbanized region) was also assessed for each subject in this study.
Statistical Analysis
For between-group comparisons, the F test was used for continuous variables and Pearson’s χ 2 test for nominal variables. Cox regression analyses were performed with adjustment for demographic data (age, sex, income, level of urbanization), psychiatric comorbidities, and medication use. Firstly, for the investigation of FES with the risk of any STI, the presence of any STI, including HIV, syphilis, genital warts, gonorrhea, chlamydial infection, and trichomoniasis was defined as the event, and the period between enrollment and the diagnosis date of firstly diagnosed STI or study end or death was defined as the follow-up duration. Secondly, for the assessment of FES with the risk of each STI, the presence of each STI (ie, HIV) was defined as the event, and the period between enrollment and the event (ie, HIV) or study end or death was defined as the follow-up duration. Lastly, regarding the multiple STIs in single individual, Andersen–Gill model for the recurrent time-to-event analysis (HIV, syphilis, genital warts, gonorrhea, chlamydial infection, and trichomoniasis) was applied for the association between FES and STIs.19 The hazard ratio (HR) with a 95% confidence interval (CI) of any STI was calculated. The survival curves of the schizophrenia and control cohorts were examined by Kaplan–Meier analysis with a log-rank test.
Furthermore, subanalyses stratified by sex and age group (adolescent or young adult) were assessed for the relationship between schizophrenia and STI risk. Cox regression analyses were also used to clarify dose–response relationships between psychiatric comorbidities and STI risk and between schizophrenia severity (frequency of psychiatric admission) and STI risk. In addition, in order to further clarify the impact of schizophrenia severity with STI risk, we specifically examined the STI risk among young patients with FES and defined those with mild disease severity as the reference group. We also examined whether long-term use (cDDD ≥ 365) of atypical antipsychotics may lower STI risk compared with short-term use (cDDD = 30–364). Furthermore, regarding the important role of intravenous (IV) drug abuse/dependence in the risk of HIV infection, we specifically examined the IV drug abuse/dependence with HIV infection risk among young patients with FES. We used the Hepatitis C virus infection as a marker of exposure to IV drug abuse/dependence, because the exact data of IV drug abuse/dependence were not available in NHIRD.
Sensitivity analyses were performed to investigate the associations between schizophrenia and any STI after excluding the first year or first 3 years of observation. The E value for causality of evidence was calculated. The E value is defined as the minimum strength of association on the risk ratio scale that an unmeasured confounder would need to have with both the exposure and the outcome to fully explain away a specific exposure–outcome association, conditional on the measured covariates.20,21 A large E value implies that considerable unmeasured confounding would be needed to explain away an effect estimate. A small E value implies little unmeasured confounding would be needed to explain away an effect estimate. A 2-tailed P value of less than .05 was considered statistically significant. All data processing and statistical analyses were performed with Statistical Package for Social Science (SPSS) version 17 software (Chicago: SPSS, Inc.) and Statistical Analysis Software (SAS) version 9.1 (SAS Institute, Cary, NC).
Results
Table 1 shows the demographic and clinical characteristics of the schizophrenia cohort and the controls. In total, 44 109 adolescents and young adults with schizophrenia and 174 436 age- and sex-matched controls were enrolled in this study. The average age of study subjects was 23.28 ± 4.38 years and males predominated (58.2% males vs 41.8% females). During the follow-up period, the schizophrenia cohort had a higher incidence of contracting any of 6 STIs than the control cohort (3.0% vs 1.3%, P < .001), including HIV, syphilis, genital warts, gonorrhea, chlamydial infection, and trichomoniasis. Additionally, the schizophrenia cohort contracted STIs earlier (3.97 ± 2.80 vs 6.33 ± 2.58 years, P < .001) and at younger ages (27.41 ± 4.96 vs 31.57 ± 4.26 years, P < .001). The psychiatric comorbidities examined were more common in the schizophrenia cohort, including disruptive behavior disorder (2.3% vs 0.1%, P < .001), AUD (7.4% vs 2.0%, P < .001), and SUD (14.1% vs 2.9%, P < .001). The schizophrenia cohort resided in less urbanized regions (P < .001) and had lower incomes (P < .001).
Table 1.
Demographic Data and Incidence of Any STI Among Adolescents and Young Adults With Schizophrenia and Controls
| Demographics | Schizophrenia (n = 44 109) | Controls (n = 176 436) | P value |
|---|---|---|---|
| Age at enrollment (years, SD, n, %) | 23.28 (4.38) | 23.29 (4.39) | .805 |
| Male: female (%) | 58.2%: 41.8% | 58.2%: 41.8% | 1.000 |
| Atypical antipsychotics (n, %) | <.001 | ||
| <30 cDDD | 10 268 (23.3) | 175 866 (99.7) | |
| 30–364 cDDD | 9518 (21.6) | 464 (0.3) | |
| ≥365 cDDD | 24 323 (55.1) | 106 (0.1) | |
| Frequency of admission for schizophrenia (n, %) | |||
| <1/year | 38 972 (88.4) | ||
| 1–2/year | 2706 (6.1) | ||
| >2/year | 2431 (5.5) | ||
| Incidence of any STI (n, 100,000 person-years) | 1306 (363.40) | 2216 (152.10) | <.001 |
| HIV | 119 (32.59) | 133 (9.09) | <.001 |
| Syphilis | 266 (73.05) | 181 (12.38) | <.001 |
| Genital warts | 250 (68.52) | 406 (27.77) | <.001 |
| Gonorrhea | 145 (39.73) | 189 (12.92) | <.001 |
| Chlamydial infection | 188 (51.50) | 483 (33.04) | <.001 |
| Trichomoniasis | 456 (125.44) | 912 (62.45) | <.001 |
| Age at any STI (years, SD) | 27.41 (4.96) | 31.57 (4.26) | <.001 |
| Time to first STI (years, SD) | 3.97 (2.80) | 6.33 (2.58) | <.001 |
| Psychiatric comorbidities (n, %) | |||
| Disruptive behavior disorders | 1002 (2.3) | 219 (0.1) | <.001 |
| Alcohol use disorders | 3244 (7.4) | 3561 (2.0) | <.001 |
| Substance use disorders | 6205 (14.1) | 5089 (2.9) | <.001 |
| IV drug abuse/dependence | 191 (0.4) | 107 (0.1) | <.001 |
| Level of urbanization | <.001 | ||
| 1 (most urbanized) | 8877 (20.1) | 56 535 (32.0) | |
| 2 | 14 055 (31.9) | 55 371 (31.4) | |
| 3 | 6531 (14.8) | 33 262 (18.9) | |
| 4 | 5550 (12.6) | 20 888 (11.8) | |
| 5 (most rural) | 9096 (20.6) | 10 380 (5.9) | |
| Income-related insured amount | <.001 | ||
| ≤15 840 NTD/month | 27 447 (62.2) | 48 674 (27.6) | |
| 15 841–25 000 NTD/month | 13 701 (31.1) | 66 244 (37.5) | |
| ≥25 001 NTD/month | 2961 (6.7) | 61 518 (34.9) | |
| Follow-up duration (years) | 8.15 (2.81) | 8.26 (2.71) | <.001 |
Note: STI, sexually transmitted infection; NTD, new Taiwan dollar; SD, standard deviation; IV, intravenous; cDDD, cumulative defined daily dose.
The Kaplan–Meier survival analysis with log-rank test showed a significantly higher risk of STIs in the adolescents and young adults with schizophrenia (P < .001; supplementary figure 1). Andersen–Gill model showed the consistent finding that FES was related to an increased risk (HR = 4.30) of contracting STIs during the follow-up. Table 2 shows that AUD (HR = 1.40, 95% CI = 1.18–1.58) and SUD (2.15, 1.93–2.40) were related to the risk of total subsequent STIs in the adolescents and young adults with schizophrenia, and the risk was still higher when adjusting for the psychiatric comorbidities (2.35, 2.08–2.64). Subanalyses revealed that in the schizophrenia cohort, adolescents had a higher STI risk than young adults (4.35 vs 2.17), and males had a higher STI risk than females (3.24 vs 1.86).
Table 2.
Cox Regression Analyses for Any STI and Subanalyses Stratified by Age Group and Sex Among Adolescents and Young Adults With Schizophrenia and Controlsa
| Risk factor | Adolescents <18 years | Young Adults 18–29 years | Males | Females | Total |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Schizophrenia (presence vs absence) | 4.35 (3.14–6.02) | 2.17 (1.91–2.47) | 3.24 (2.73–3.86) | 1.86 (1.58–2.19) | 2.35 (2.08–2.64) |
| Psychiatric comorbidities (presence vs absence) | |||||
| Disruptive behavior disorders | 1.70 (0.94–3.08) | 0.92 (0.58–1.47) | 1.02 (0.62–1.68) | 1.56 (0.92–2.65) | 1.21 (0.84–1.73) |
| Alcohol use disorders | 1.58 (0.78–3.21) | 1.39 (1.20–1.61) | 1.20 (0.98–1.47) | 1.63 (1.33–2.01) | 1.40 (1.18–1.58) |
| Substance use disorders | 2.08 (1.32–3.28) | 2.21 (1.97–2.47) | 1.77 (1.52–2.07) | 2.63 (2.25–3.06) | 2.15 (1.93–2.40) |
Note: Bold type indicates that the data are statistically significant. STI, sexually transmitted infection; HR, hazard ratio; CI, confidence interval.
aAdjusted for demographic data, psychiatric comorbidities, and medications.
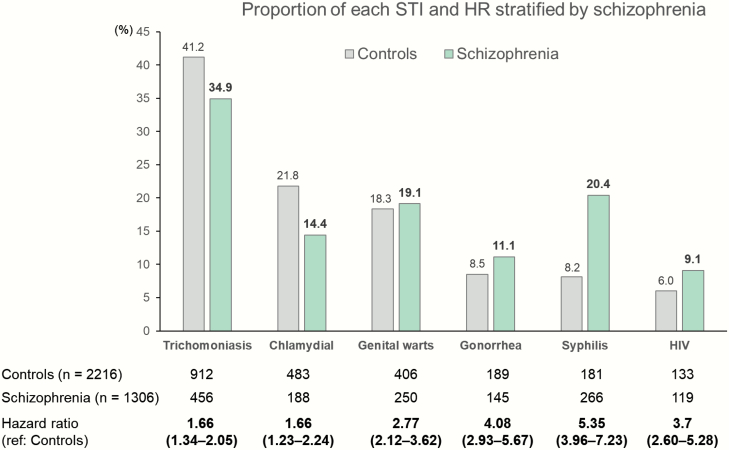
Figure 1 shows the risk of each STI and their proportional distribution in the schizophrenia vs control cohorts. The schizophrenia cohort had higher risks for all 6 STIs (P < .001). The schizophrenia cohort were at highest risk of syphilis (HR = 5.35), followed by gonorrhea (4.08), HIV (3.70), genital warts (2.77), chlamydial infection (1.66), and trichomoniasis (1.66). Moreover, each group had a distinct distribution of STIs. The schizophrenia cohort had notably higher proportions of syphilis (20.4% vs 8.2%) and HIV (9.1% vs 6.0%).
Fig. 1.
Proportion of each STI and hazard ratio (adjusted for demographic data, psychiatric comorbidities, and medications). STI, sexually transmitted infection; HR, hazard ratio. Bold type indicates that the data are statistically significant.
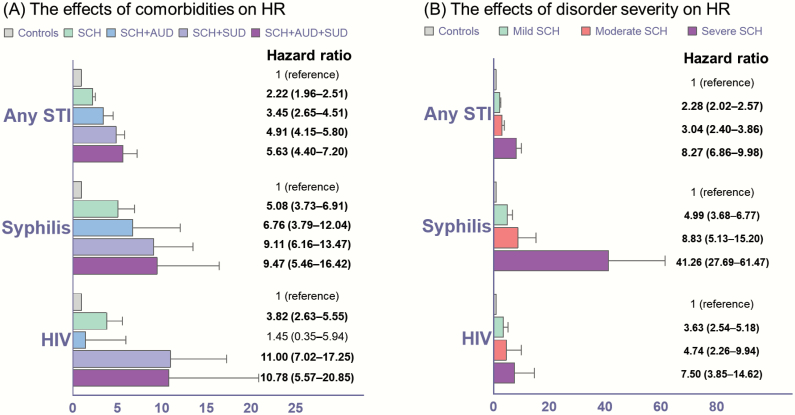
Figure 2A illustrates dose–response relationships between AUD, SUD, and the risk of STIs. Compared with controls, the schizophrenia cohort had an increased risk of STIs (HR = 2.22). The STI risk in schizophrenia cohort was further increased when presenting with AUD (3.45), SUD (4.91), or both (5.63). The increasing trend of STI risk in association with comorbid AUD and SUD was also observed for syphilis and HIV infections, with the highest risk of HIV infection in young people with schizophrenia comorbid with both AUD and SUD (10.78). Figure 2B reveals another dose–response relationship, namely, between schizophrenia severity and STI risk in young people with schizophrenia. Compared with controls, the mild schizophrenia group had an increased risk of any STI (2.28). The risk escalated with increased schizophrenia severity, with the highest risk of any STI in the severe schizophrenia group (8.27). The increasing trend of STI risk in association with schizophrenia severity was also observed for syphilis and HIV infection, with the highest risk of syphilis in young people with severe schizophrenia (41.26). Compared with young patients with mild schizophrenia severity, those with severe schizophrenia severity had the highest risk of contracting any STI (4.36), including HIV (2.72) and syphilis (9.98), during the follow-up (supplementary table 1). In addition, IV drug abuse/dependence extremely increased the risk of HIV infection (42.15, 27.40–64.84) among patients with FES compared with the controls.
Fig. 2.
The effects of comorbidities and disorder severity on the risk of STIs among adolescents and young adults after first-episode schizophrenia. STI, sexually transmitted infection; HR, hazard ratio; CI, confidence interval. (A) Adjusted for demographic data and medications. (B) Adjusted for demographic data, psychiatric comorbidities, and medications. Bold type indicates that the data are statistically significant.
Table 3 shows the beneficial effects of atypical antipsychotics against STI incidence in the schizophrenia cohort. Compared with nonusers, long-term antipsychotic use was associated with a lower risk of contracting STIs (HR = 0.77, 95% CI = 0.68–0.89). Subanalyses found that the beneficial effects for long-term users were more pronounced in the adolescent group than the young adult group (0.59 vs 0.80). Moreover, increased effectiveness for long-term users was observed in the comorbid SUD group compared with those without comorbid SUD (0.68 vs 0.81).
Table 3.
Schizophrenia Medications and the Risk of Any STI Among Adolescents and Young Adults With Schizophreniaa
| Subgroup | Schizophrenia | ||||
|---|---|---|---|---|---|
| Adolescents <18 years | Young Adults 18–29 years | With Substance Use Disorder | Without Substance Use Disorder | Total | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Atypical antipsychotics | |||||
| <30 cDDD | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| 30–364 cDDD | 1.29 (0.87–1.92) | 1.05 (0.88–1.24) | 0.92 (0.66–1.26) | 1.13 (0.94–1.34) | 1.08 (0.92–1.26) |
| ≥365 cDDD | 0.59 (0.40–0.87) | 0.80 (0.69–0.92) | 0.68 (0.51–0.90) | 0.81 (0.69–0.94) | 0.77 (0.68–0.89) |
Note: Bold type indicates that the data are statistically significant. STI, sexually transmitted infection; HR, hazard ratio; CI, confidence interval; cDDD, cumulative defined daily dose.
aAdjusted for demographic data and psychiatric comorbidities.
Supplementary figure 2 shows the E values for the statistically significant HRs in the present study. The first E value of 4.13 means that the minimum strength of an unmeasured confounder to explain away the FES–STI association should be a relative risk of more than 4.13 in an association with both FES and any STI infection. Supplementary table 2 shows the sensitivity analyses of excluding the first year (HR = 2.00, 95% CI = 1.76–2.27) and the first 3 years (1.58, 1.36–1.83). A decreasing trend of STI risk was observed in the follow-up period. The risk of STIs appeared to be lower after 3 years.
Discussion
We showed that after FES, adolescents and young adults without comorbid AUD or SUD still had an increased risk of STIs. When they had either comorbid AUD or comorbid SUD, the risk increased, and those comorbid with both AUD and SUD had the highest risk of STIs. Compared with the control cohort, the schizophrenia cohort had a disproportionate distribution of STIs, with high proportions of syphilis and HIV infection. The mean first time of diagnosis of any STI was 3.97 years following FES, and the increased risk of STIs included (from highest to lowest) syphilis, gonorrhea, HIV, genital warts, chlamydial infection, and trichomoniasis. The severe schizophrenia group had particularly higher risks of HIV and syphilis compared with the mild schizophrenia group. Among patients with FES having IV drug abuse/dependence, the risk of HIV infection was tremendously increased as compared with the controls. Importantly, atypical antipsychotic treatment may provide beneficial effects against STI risk. These beneficial effects were more prominent in the adolescent schizophrenia group and in the schizophrenia comorbid SUD group.
Substantial evidence has indicated an increased risk of STIs in patients with schizophrenia.3,4,7,14,22 The increased STI risk may be mainly associated with the comorbid SUD.4–7 These triply diagnosed persons (schizophrenia, SUD, and STIs) has raised awareness of a syndemic between mental illness, SUD, and HIV.23,24 Importantly, our study found that an interaction between FES and SUD may produce a higher risk for the 6 STIs, particularly for syphilis, gonorrhea, and HIV. Besides, our study found that young people following FES had a disproportionate distribution of STIs compared with control cohort without schizophrenia, showing the largest increased proportion in syphilis (increased 12.2%), followed by HIV (3.1%), and gonorrhea (2.6%). Although the mechanism underlying this phenomenon is unclear, it could be partially explained by shared genetic factors. A study investigating the common genetic factors between schizophrenia and HIV infection found significant pleiotropy and a positive genetic correlation for schizophrenia and HIV infection.25 It is also suggested that the genetic underpinnings of schizophrenia may increase the risk of substance use in adolescence, which may both enhance the risk for developing a later SUD, and also serve an additional risk factor for schizophrenia.26 Another explanation for the disproportionate distribution of STIs is related to crosstalk between syphilis and HIV. Concordant syphilis and HIV infection is common, particularly among men having sex with men.27,28 HIV may affect the presentation, disease progression, and therapy of syphilis, while syphilis may facilitate transmission and acquisition of HIV. Importantly, evidence suggests that patients with schizophrenia have higher probability of engaging in homosexual activity than people with other mental disorders,29–31 thereby increasing their risk of concordant HIV infection and syphilis.
From a neurobiological perspective, adolescence is a transitional period of development characterized by heightened vulnerability to sensation-seeking and risk-taking behaviors.32 The neurobiological underpinnings of risky sexual behavior (RSB) in adolescents and young adults are different from those of adults. A comprehensive review reported that the neurocognitive constructs of RSB in adolescents and young adults are associated with working memory, decision-making, and risk-taking propensity.33 However, in adults, poor executive function-associated RSB is strongly related to SUD. These findings support our hypothesis that adolescents and young adults having schizophrenia may be directly associated with an increased risk of STIs because of their working memory deficits and risky decision making.10,11,13 Moreover, a comorbid SUD or AUD can be an important contributing factor.
While young people with schizophrenia were sexually active,10 their level of sexual health awareness may not be high. A previous study found that 58.1% of patients with schizophrenia never used condoms, 47.8% had multiple sex partners, 35.2% used drugs during sex, 29.7% traded sex for drugs, money, or other goods, and 17.5% had drug-injection histories.34 Another study reported similar RSB in patients with schizophrenia, finding that 62% had multiple sex partners, 50% had exchanged sex for money or goods, 22% participated in homosexual activity, and 12% had at least one partner who was HIV positive or injected drugs.22 A study comparing patients with schizophrenia, bipolar disorder, and heroin addiction found that the schizophrenia group had the highest frequency of participating homosexual acts.31 Clearly, in addition to comorbid SUD, poor sexual health awareness is an important factor leading to RSB and, subsequently, to STIs in adolescents and young adults with schizophrenia.
The current study also suggests an association between schizophrenia severity, atypical antipsychotic use, and risk of STIs. We found a lower risk of STIs in association with long-term antipsychotic use in the adolescents and young adults with schizophrenia. Substantial evidence suggests that antipsychotics may induce sexual dysfunction via postsynaptic dopamine antagonism, prolactin elevation, and alpha 1-adrenergic receptor blockade.35 The beneficial effects of long-term antipsychotic use may be also related to psychopathological improvement. Other evidence suggests that sexual activity was positively correlated with greater psychopathological symptoms in patients with schizophrenia.22,34 A meta-analysis reported that following FES, patients demonstrated medium-to-large impairments across all neurocognitive domains.36 Psychopathological symptoms and neurocognitive deficits could severely interfere with patients’ ability to assess risk or to adopt risk reduction strategies. Indeed, a dose–response relationship between schizophrenia severity and the risk of STIs was observed in the present study. Adherence to medication is another important factor contributing to the increased risk of STIs. A study investigated the association between adherence to medication and cognitive function in patients with schizophrenia.37 The authors found that executive function was a strong predictor for nonadherence to medication in schizophrenia, suggesting that patients with poor adherence to medications may have more severe cognitive impairment. Taking these findings together, adolescent and young adults with schizophrenia who can be adequately adherent to long-term antipsychotic treatment may have stable psychopathology and cognitive function, thereby reducing the possibility of RSB and subsequent STI risk.
The strengths of this study are its unbiased patient inclusion, large sample size, and use of E values to support causal evidence. However, this study also has limitations. First, the NHIRD does not provide information on the level of sexual health awareness. We do not have information about the subjects’ number of sex partners, sexual orientation, or condom use. Second, many STIs are relatively asymptomatic in their early phase, and patients might feel stigmatized and refuse to seek medical help and consultation. Besides, the prodromal phase of schizophrenia may be undetected for a long time. Therefore, the discovery bias may partially explain the higher risk of STIs in the more severe cases of schizophrenia in whom both conditions were more likely to be diagnosed. Third, the NHIRD does not provide information on the severity of schizophrenia symptoms, such as the Positive and Negative Syndrome Scale; therefore, we used psychiatric admission rates as the scale of disease severity in our study. Fourth, the NHIRD does not provide information on smoking status, body mass index, psychosocial stress, family history, personal lifestyle, blood/urine samples for SUDs, environment, and sociodemographic status of the parents during the childhood of the cohort members; therefore, we could not investigate potential influence of these factors. Fifth, the relative prevalence and risk of developing specific STIs can vary widely across different regional settings. Therefore, the generalizability of our study findings to other countries or cultures may be limited.
Conclusions
Following FES, relapse prevention appears to be the top health care priority. However, physical illnesses are common in individuals with schizophrenia and contribute to a reduction of life expectancy of 10–20 years.38 Premature death among people with schizophrenia has been associated with several health-risk behaviors, including RSB.39 The present study suggests that adolescent and young adult patients with schizophrenia are a population highly vulnerable to contracting STIs, particularly HIV and syphilis. To date, this fragile population has not received specific STI preventive services and health care. Education and counseling on sexual risk reduction should be tailored to the needs of young people with schizophrenia because of their psychopathological symptoms and neurocognitive deficits.40 Importantly, treatment with schizophrenia medications may lower the risk of STIs. Routine and frequent screening for STIs are strongly suggested for young people with schizophrenia, particularly those with comorbid SUD, poor medication adherence, and severe disorder severity.
Funding
The study was supported by grant from Taipei Veterans General Hospital (V106B-020, V107B-010, V107C-181, and V107C-052) and Ministry of Science and Technology, Taiwan (107-2314-B-075-063-MY3). The funding source had no role in any process of study.
Supplementary Material
Acknowledgments
We thank Mr I-Fan Hu for his friendship and support. We thank Dr M.H.C. and Dr J.W.H., who designed the study, wrote the protocol and article, Dr N.Y.K., Dr Y.M.B., and Dr K.L.H., who assisted with the preparation and proof-reading of the article, and Dr Y.M.B., Dr T.J.C., and Dr M.H.C., who provided the advices on statistical analysis. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Low N, Broutet NJ. Sexually transmitted infections—research priorities for new challenges. PLoS Med. 2017;14(12):e1002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Owusu-Edusei K Jr, Chesson HW, Gift TL, Tao G, Mahajan R, Ocfemia MC, Kent CK. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis. 2013;40(3):197–201. [DOI] [PubMed] [Google Scholar]
- 3. Blank MB, Mandell DS, Aiken L, Hadley TR. Co-occurrence of HIV and serious mental illness among Medicaid recipients. Psychiatr Serv. 2002;53(7):868–873. [DOI] [PubMed] [Google Scholar]
- 4. Himelhoch S, McCarthy JF, Ganoczy D, Medoff D, Dixon LB, Blow FC. Understanding associations between serious mental illness and HIV among patients in the VA Health System. Psychiatr Serv. 2007;58(9): 1165–1172. [DOI] [PubMed] [Google Scholar]
- 5. Prince JD, Walkup J, Akincigil A, Amin S, Crystal S. Serious mental illness and risk of new HIV/AIDS diagnoses: an analysis of Medicaid beneficiaries in eight states. Psychiatr Serv. 2012;63(10):1032–1038. [DOI] [PubMed] [Google Scholar]
- 6. Helleberg M, Pedersen MG, Pedersen CB, Mortensen PB, Obel N. Associations between HIV and schizophrenia and their effect on HIV treatment outcomes: a nationwide population-based cohort study in Denmark. Lancet HIV. 2015;2(8):e344–e350. [DOI] [PubMed] [Google Scholar]
- 7. Chen SF, Chiang JH, Hsu CY, Shen YC. Schizophrenia is associated with an increased risk of sexually transmitted infections: a nationwide population-based cohort study in Taiwan. Schizophr Res. 2018;202:316–321. [DOI] [PubMed] [Google Scholar]
- 8. Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187–193. [DOI] [PubMed] [Google Scholar]
- 9. Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388(10039):86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramrakha S, Caspi A, Dickson N, Moffitt TE, Paul C. Psychiatric disorders and risky sexual behaviour in young adulthood: cross sectional study in birth cohort. BMJ. 2000;321(7256):263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leeson VC, Barnes TR, Harrison M, et al. The relationship between IQ, memory, executive function, and processing speed in recent-onset psychosis: 1-year stability and clinical outcome. Schizophr Bull. 2010;36(2):400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Strauss GP, Robinson BM, Waltz JA, et al. Patients with schizophrenia demonstrate inconsistent preference judgments for affective and nonaffective stimuli. Schizophr Bull. 2011;37(6):1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Falcone MA, Murray RM, Wiffen BD, et al. Jumping to conclusions, neuropsychological functioning, and delusional beliefs in first episode psychosis. Schizophr Bull. 2015;41(2):411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gottesman II, Groome CS. HIV/AIDS risks as a consequence of schizophrenia. Schizophr Bull. 1997;23(4): 675–684. [DOI] [PubMed] [Google Scholar]
- 15. Li CT, Bai YM, Huang YL, et al. Association between antidepressant resistance in unipolar depression and subsequent bipolar disorder: cohort study. Br J Psychiatry. 2012;200(1):45–51. [DOI] [PubMed] [Google Scholar]
- 16. Chen MH, Pan TL, Li CT, et al. Risk of stroke among patients with post-traumatic stress disorder: nationwide longitudinal study. Br J Psychiatry. 2015;206(4):302–307. [DOI] [PubMed] [Google Scholar]
- 17. Tsai CF, Chen MH, Wang YP, et al. Proton pump inhibitors increase risk for hepatic encephalopathy in patients with cirrhosis in a population study. Gastroenterology. 2017;152(1):134–141. [DOI] [PubMed] [Google Scholar]
- 18. Chen MH. Response to comment on Chen et al. risk of developing type 2 diabetes in adolescents and young adults with autism spectrum disorder: a nationwide longitudinal study. Diabetes Care. 2016;39:788–793. [DOI] [PubMed] [Google Scholar]
- 19. Ozga AK, Kieser M, Rauch G. A systematic comparison of recurrent event models for application to composite endpoints. BMC Med Res Methodol. 2018;18(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–274. [DOI] [PubMed] [Google Scholar]
- 21. Haneuse S, VanderWeele TJ, Arterburn D. Using the E-Value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602–603. [DOI] [PubMed] [Google Scholar]
- 22. Cournos F, Guido JR, Coomaraswamy S, Meyer-Bahlburg H, Sugden R, Horwath E. Sexual activity and risk of HIV infection among patients with schizophrenia. Am J Psychiatry. 1994;151(2):228–232. [DOI] [PubMed] [Google Scholar]
- 23. Blank MB, Eisenberg MM. Registries and syndemics: untapped potential for global health. Lancet HIV. 2015;2(8):e314–e315. [DOI] [PubMed] [Google Scholar]
- 24. Blank MB, Himelhoch S, Walkup J, Eisenberg MM. Treatment considerations for HIV-infected individuals with severe mental illness. Curr HIV/AIDS Rep. 2013;10(4):371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Q, Polimanti R, Kranzler HR, Farrer LA, Zhao H, Gelernter J. Genetic factor common to schizophrenia and HIV infection is associated with risky sexual behavior: antagonistic vs. synergistic pleiotropic SNPs enriched for distinctly different biological functions. Hum Genet. 2017;136(1):75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khokhar JY, Dwiel LL, Henricks AM, Doucette WT, Green AI. The link between schizophrenia and substance use disorder: a unifying hypothesis. Schizophr Res. 2018;194:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lynn WA, Lightman S. Syphilis and HIV: a dangerous combination. Lancet Infect Dis. 2004;4(7):456–466. [DOI] [PubMed] [Google Scholar]
- 28. Zetola NM, Klausner JD. Syphilis and HIV infection: an update. Clin Infect Dis. 2007;44(9):1222–1228. [DOI] [PubMed] [Google Scholar]
- 29. Bai YM, Huang Y, Lin CC, Chen JY. Emerging homosexual conduct during hospitalization among chronic schizophrenia patients. Acta Psychiatr Scand. 2000;102(5):350–353. [DOI] [PubMed] [Google Scholar]
- 30. Bolton SL, Sareen J. Sexual orientation and its relation to mental disorders and suicide attempts: findings from a nationally representative sample. Can J Psychiatry. 2011;56(1):35–43. [DOI] [PubMed] [Google Scholar]
- 31. Hariri AG, Karadag F, Gokalp P, Essizoglu A. Risky sexual behavior among patients in Turkey with bipolar disorder, schizophrenia, and heroin addiction. J Sex Med. 2011;8(8):2284–2291. [DOI] [PubMed] [Google Scholar]
- 32. Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1189–1201; quiz 1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ross JM, Duperrouzel J, Vega M, Gonzalez R. The neuropsychology of risky sexual behavior. J Int Neuropsychol Soc. 2016;22(6):586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McKinnon K, Cournos F, Sugden R, Guido JR, Herman R. The relative contributions of psychiatric symptoms and AIDS knowledge to HIV risk behaviors among people with severe mental illness. J Clin Psychiatry. 1996;57(11):506–513. [DOI] [PubMed] [Google Scholar]
- 35. de Boer MK, Castelein S, Wiersma D, Schoevers RA, Knegtering H. The facts about sexual (dys)function in schizophrenia: an overview of clinically relevant findings. Schizophr Bull. 2015;41(3):674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315–336. [DOI] [PubMed] [Google Scholar]
- 37. El-Missiry A, Elbatrawy A, El Missiry M, Moneim DA, Ali R, Essawy H. Comparing cognitive functions in medication adherent and non-adherent patients with schizophrenia. J Psychiatr Res. 2015;70:106–112. [DOI] [PubMed] [Google Scholar]
- 38. Melle I, Olav Johannesen J, Haahr UH, et al. Causes and predictors of premature death in first-episode schizophrenia spectrum disorders. World Psychiatry. 2017;16(2):217–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McGinty EE, Baller J, Azrin ST, Juliano-Bult D, Daumit GL. Interventions to address medical conditions and health-risk behaviors among persons with serious mental illness: a comprehensive review. Schizophr Bull. 2016;42(1):96–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lukoff D, Gioia-Hasick D, Sullivan G, Golden JS, Nuechterlein KH. Sex education and rehabilitation with schizophrenic male outpatients. Schizophr Bull. 1986;12(4):669–677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.