Abstract
The amount of energy necessary to place an implant in its seat, described as the integral of the torque-depth curve at insertion (I), has been validated as a reliable measure of primary stability. This study aimed to investigate whether (I) may detect the variations in primary stability caused by changes in the implant length or diameter better than the insertion torque (IT). Cylindric implants featuring a double-etched, sandblasted surface with different diameters or lengths were placed into monolithic polyurethane foam blocks with different densities that mimicked human bone. (I)-, (I)*-, IT-, IT*-diameter and -length plots ((I)* and IT* were the derived values corrected for undersizing) were drawn and the relation between (I), (I)*, IT, and IT* and the fixture diameter or length was investigated with correlation analysis. (I)* and IT* correlated better than (I) and IT with the fixture diameter; (I), (I)*, IT, and IT* correlated equally well with the fixture length. In all cases, the slopes of the lines best fitting the experimental data were greater for (I) or (I)* than IT or IT*, respectively. (I) or (I)* were better detectors than IT or IT* of the changes in primary stability that can be achieved by increasing the fixture diameter or length.
Keywords: primary stability, torque-depth curve integral, insertion torque, undersizing, fixture length, fixture diameter
1. Introduction
Primary implant stability is one of the determinants of implant success, as micromovements exceeding the 50–150 µm threshold may hinder osseointegration and lead to implant failure [1,2,3,4]. Further, certain primary stability thresholds are necessary to apply early or immediate after loading in order to rehabilitate the patient earlier. There are many factors affecting primary stability [4,5]. Some are patient-related, such as the bone density at the insertion site or the thickness of the cortical bone layer; others include the micro- and macro-morphology of the implant being placed. The extent of under preparation in low-density sites or of tapping in high-density sites are other operative choices. The implant diameter and length are among the first parameters the surgeon chooses when planning the whole rehabilitation process [6,7]. Fully understanding how these parameters may affect primary stability is therefore of paramount importance. When the effect of these two parameters on primary stability were investigated in a clinical setting, the results were inconclusive. When Degidi et al. [8] retrospectively assessed 4135 implants in 1045 consecutive patients, they did not find any significant correlation between the implant diameter and the insertion torque (IT), nor between the implant length and IT; they observed the implant stability quotient (ISQ), measured with resonance frequency analysis (RFA), to correlate with the implant length, but they observed no correlation between the ISQ and the implant diameter. Mesa et al. [9] retrospectively assessed the primary stability of 1084 implants placed in 316 patients. The Periotest Value (PTV) was used for measurement and they found it was significantly associated with implant length, but not with implant diameter. Ostman et al. [10] retrospectively assessed 905 implants in 267 patients and found that their ISQ correlated significantly with both their diameter and length. Aragoneses et al. [11] prospectively placed 559 implants in 159 patients and found no correlation between the ISQ and their diameter. Schiffler et al. [12] retrospectively assessed 200 implants in 143 patients and observed that their ISQ did not correlate with the implant diameter or its length. Similar varied observations were reported by other authors [13,14,15,16,17,18,19,20,21,22,23,24]. Some of this discordance may arise from the effect of confounding factors; indeed, in the studies mentioned above, different implant brands and models were used, the insertion protocol was either that provided by the manufacturer or a different protocol was used, and implants were placed in different zones of the two arches. Yet, some authors have also highlighted how different primary stability measuring parameters (e.g., IT, ISQ, and PTV) actually measure different physical quantities. For example, Degidi et al. [8] found no correlation between IT and ISQ values at insertion and explained this finding by remarking how IT is correlated to rotational friction and ISQ to lateral displacement [25]. It follows that changes in the implant diameter or length may affect primary stability differently, and results of studies using different parameters may therefore be inconsistent. Indeed, such inconsistency in results concerning the effect of the implant diameter and length on different primary stability parameters has already been observed in several in vitro investigations [26,27,28]. To overcome the intrinsic ambiguity in using parameters measuring different physical quantities, some authors have suggested the insertion energy (IE) may be a more informative primary stability parameter [29,30]. IE, that is the total work needed to place the implant into its seat, may be more informative than the IT and the ISQ or the PTV. While these measure the stability of the implant after it has been placed, IE measures the implant stability while it is being placed, as a sum of all the interactions the implant has while descending into the pre-drilled tunnel. Indeed, in a study on bovine ribs, IE was shown to better detect the situations where sufficient primary stability can be achieved even in softer bone [29] when bone quality is classified according to Lekholm and Zarb [31]. IE might also detect primary stability enhancements provided by under preparation better than ISQ or IT measurements [32]. In a clinical setting, the IE may be measured as the integral of the torque-depth curve (I), a quantity differing from IE only by a multiplicative constant under the conditions that the implant threads are evenly spaced and the implant rotation speed is constant. These conditions are very often met in a clinical setting. (I) has been shown to be more sensitive to bone density variations than IT, ISQ, and the reverse torque (RT) both on polyurethane foam [33] and equine blocks [34]. (I) was also observed in human and bovine rib studies to correlate significantly with the bone to the implant contact (BIC) area [35,36]. On bovine ribs, (I) was found to discriminate between different implant geometries (cylindric, cylindric-modified, and tapered-cylindric) [37] and to detect different preparation conditions, namely undersizing and tapping, better [38]. On photoelastic resin blocks, mimicking D1 bone, (I) was shown to correlate significantly with the mechanical stress the material was subjected to when implants were placed [39]. When (I) was used in a study involving implants placed in low-density posterior maxillary sites concomitantly to sinus augmentation, it was observed it provided different information than IT concerning primary stability in relation to the residual ridge bone height (RBH) [40]. Overall, these studies suggested that to determine the implant primary stability achieved at insertion, measuring (I) at implant placement might be more useful, clinically, than measuring the IT and the ISQ. To date, no studies have determined whether (I) or IE detects variations in primary stability caused by changes in the implant length or diameter better than other primary stability measuring parameters. This study aimed to investigate this question on polyurethane foam blocks under conditions introducing the fewest confounding factors as possible.
2. Materials and Methods
In this in vitro study, four homogeneous synthetic blocks were used, mimicking human bone. They were solid rigid polyurethane 13 × 18 × 4 cm foam blocks (Sawbones, Vashon Island, Washington, USA; Figure 1) produced as an alternative test medium for human cancellous/cortical bone. The blocks had different densities (0.26, 0.33, 0.49, and 0.65 g/cm3).
Figure 1.
An example of the solid, monolithic rigid polyurethane 13 × 18 × 4 cm foam blocks used in the present study.
Even though polyurethane foam does not replicate the human bone structure, it does display mechanical properties in the range of human cancellous bone as described in the ASTM F-1839-08 standard [41]. The implants were cylindrical fixtures (Stone, IDI Evolution, Concorezzo, Italy) featuring a double-etched, sandblasted surface (Figure 2). These implants feature an evenly-threaded body and a micro-threaded head. Implants used in the present study had different diameters and lengths, as detailed in Table 1. Diameters and length were those available for this implant system. Ten implants for each size were placed into each block, according to the manufacturer’s instructions. Details about the final (body) and countersink drills diameters are also provided in Table 1. Implants were placed with no irrigation.
Figure 2.
The implant used in the present study. The fixture is cylindrical and features a double-etched, sandblasted surface.
Table 1.
Sizes of implants used in the present study. To assess how changes in the fixture diameter affected either IT or (I), 12-mm long implants were used, featuring four different (thread) diameters: 3.5, 3.75, 5.0 and 6.0 mm. To assess the effect of length changes, 3.75-mm (thread) wide implants were used with lengths of 8.0, 12.0 or 15.5 mm.
| Block Density (g/cm3) | Implant Size (Thread Diameter × Length, mm) | Final Drill Diameter (mm) | Countersink Drill Diameter (mm) |
|---|---|---|---|
| 0.26 | 3.5 × 12.0 | 2.8 | None |
| 3.75 × 8.0 | 3.0 | 3.5 | |
| 3.75 × 12.0 | 3.0 | 3.5 | |
| 3.75 × 15.5 | 3.0 | 3.5 | |
| 5.0 × 12.0 | 3.8 | 4.3 | |
| 6.0 × 12.0 | 4.2 | 5.3 | |
| 0.33 | 3.5 × 12.0 | 2.8 | None |
| 3.75 × 8.0 | 2.8 | 3.7 | |
| 3.75 × 12.0 | 2.8 | 3.7 | |
| 3.75 × 15.5 | 2.8 | 3.7 | |
| 5.0 × 12.0 | 4.0 | 4.8 | |
| 6.0 × 12.0 | 4.8 | 6.0 | |
| 0.49 | 3.5 × 12.0 | 3.2 | 3.5 |
| 3.75 × 8.0 | 3.2 | 4.0 | |
| 3.75 × 12.0 | 3.2 | 4.0 | |
| 3.75 × 15.5 | 3.2 | 4.0 | |
| 5.0 × 12.0 | 4.2 | 5.0 | |
| 6.0 × 12.0 | 5.0 | 6.0 | |
| 0.65 | 3.5 × 12.0 | 3.2 | 3.5 |
| 3.75 × 8.0 | 3.2 | 4.0 | |
| 3.75 × 12.0 | 3.2 | 4.0 | |
| 3.75 × 15.5 | 3.2 | 4.0 | |
| 5.0 × 12.0 | 4.8 | 5.0 | |
| 6.0 × 12.0 | 5.0 | 6.0 |
An instantaneous torque-measuring micromotor (TMM2, IDI Evolution, Concorezzo, Italy) was used for primary stability measurement at the time of implant placement. The micromotor controlling console is sized 310 × 325 × 180 mm; the micromotor delivers 100 Ncm maximum torque. The medical touch screen of the controlling console displays the instantaneous torque with a 1-Ncm precision.
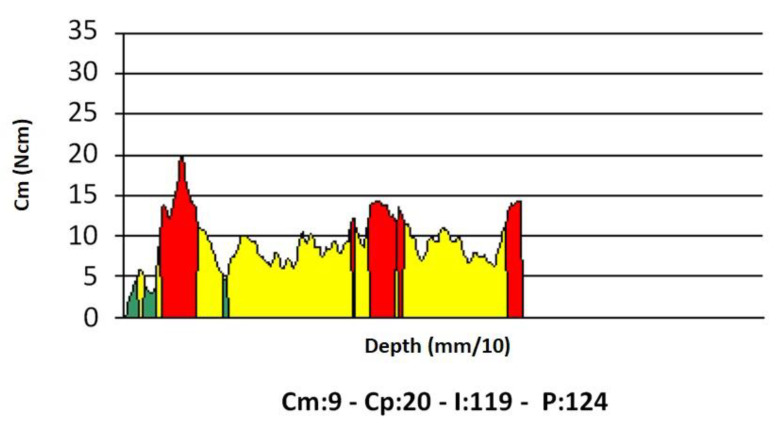
Implant insertion was carried out at a preset rotation speed of 35 rpm. During measurement, the device displayed a torque/depth graph showing how the instantaneous torque varied according to the implant depth, together with the average torque (Cm), peak torque (Cp), and (I) (the torque-depth curve integral; Figure 3).
Figure 3.
An example of a torque-depth plot generated from the micromotor measurements during implant insertion. Cm, average torque; Cp, peak torque (i.e., the insertion torque IT); (I), integral of the torque-depth curve; p, depth reached by the probe/implant in tenths of a millimeter.
The Cp is commonly known as the IT. It should be noted that, under the conditions of the present study (the insertion speed constant, and evenly spaced implant threads), (I) was equal to IE multiplied by a constant factor [33]. A summary of the values provided by the micromotor is given in Table 2. All measurements were stored in the device’s solid-state memory and were later downloaded for statistical analysis.
Table 2.
A summary of the measures provided by the micromotor.
| Measure | Symbol as Provided by the Micromotor | Unit of Measure | Information Provided |
|---|---|---|---|
| Average torque | Cm | Ncm | When recorded at probing, it is a quantification of bone density. |
| Peak torque | Cp | Ncm | When recorded at implant insertion, it is the maximum torque that was exerted by the micromotor during implant placement. In the present work, it has been indicated by the acronym IT (insertion torque). |
| Integral | I | Ncm2/100 | When measured at implant insertion, it provides the area bounded by the torque-depth curve (Figure 3). If the implant threads are evenly spaced and the rotation speed at insertion is constant (two conditions that are met in this study), the integral (I) is equal to the insertion energy, IE, multiplied by a constant factor. |
| Depth | p | mm/10 | Indicates the depth reached by the probe, when density is being measured, or by the implant when it is being placed. |
Data Analysis
IT and (I) data that were collected with each insertion condition were first checked for normality using the Shapiro–Wilk test. As their distribution was found to be normal, a single IT or (I) average value ± SD (standard deviation) was calculated for each insertion condition. These values were then used to draw IT-diameter, IT-length, (I)-diameter, and (I)-length plots. The correlation between the two variables, under each implant geometry and bone density condition, was studied by calculating the corresponding Pearson’s correlation coefficient and drawing the best fitting lines according to the least square method, assuming the relation between the two variables was linear (i.e., described by a y = mx + q equation, x and y being the two variables of interest). Site preparation according to the manufacturer instructions involves a certain degree of undersizing, that is preparing a final implant seat having a diameter smaller than that of the implant. Such undersizing is expected to have an effect both on IT and on I measurements. To investigate the effect of undersizing on the two stability parameters, IT and (I) data were also normalized by an undersizing coefficient C. This was calculated as C = (1 − r) = 1 − rd/ri where rd was the diameter of the final (body) drill, and ri was the diameter of the implant threads (C was dependent on the specific insertion condition of interest; we omitted indexing it for the sake of clarity). Normalized IT and (I) data, from now on indicated as IT* and (I)*, were then calculated as IT* = IT/C and (I)* = (I)/C, and their error was calculated using the propagation of error formula [42]. All formulas used in the present study are summarized in Table 3.
Table 3.
Formulas used in the present study.
| Quantity | Formula |
|---|---|
| Undersizing coefficient, C | C = (1 − r) = 1 − rd/ri where rd: final (body) drill diameter ri: implant threads diameter |
| Normalized IT, IT* | IT* = IT/C |
| Normalized (I), (I)* | (I)* = I/C |
| Propagation of error formula [42] | σ2y = (df/dx1)2σ21 + … + (df/dxn)2σ2n where y = f(x1, … xn) is a function of multiple variables and σy is the standard deviation of y σi for (i = 1 … n) is the standard deviation of the xi independent variable |
| Standard deviation of IT*, σIT* | σIT* = σIT/C (being σc = 0) |
| Standard deviation of (I)*, σ(I)* | σ(I)* = σ(I)/C (being σc = 0) |
The relation between IT*, (I)*, and the implant diameter or length was again investigated by calculating the corresponding Pearson’s correlation coefficients and assessing whether the IT*-diameter and (I)*-diameter, IT*-length, and (I)*-length plots could be best fitted by straight lines (i.e., if a linear relationship could be found between each pair of variables). The slopes of the best fitting lines were then plotted versus the block density to investigate how changes in bone (block) density affected the variation in primary stability consequent to a unitary (1 mm) change either in the fixture diameter or length.
All values in this work are the mean ± standard deviation (SD). Statistical calculations were performed using standard statistical software (Origin 2020, OriginLab, Northampton, MA, USA). The methodology was reviewed by an independent statistician.
3. Results
Data for each insertion condition are provided in Table 4.
Table 4.
Implant primary stability parameters corresponding to each insertion condition.
| Diameter (mm) | Bone Density (g/cm3) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.26 | 0.33 | 0.49 | 0.65 | |||||
| IT | IT* | IT | IT* | IT | IT* | IT | IT* | |
| 3.5 | 21.20 ± 1.10 | 26.80 ± 3.27 | 14.40 ± 1.67 | 22.80 ± 3.27 | 70.00 ± 3.62 | 148.89 ± 18.17 | 330.49 ± 38.40 | 389.27 ± 55.85 |
| 3.75 | 23.60 ± 2.30 | 29.80 ± 1.30 | 24.80 ± 2.68 | 51.20 ± 3.03 | 73.98 ± 7.22 | 156.84 ± 6.86 | 327.27 ± 35.41 | 625.24 ± 37.04 |
| 5.0 | 29.40 ± 1.34 | 29.60 ± 1.82 | 53.60 ± 1.14 | 34.60 ± 3.57 | 144.12 ± 6.58 | 193.04 ± 11.85 | 582.61 ± 12.39 | 1482.86 ± 152.80 |
| 6.0 | 49.80 ± 1.79 | 38.20 ± 0.45 | 69.20 ± 0.45 | -- | 195.29 ± 7.02 | 263.45 ± 3.08 | 673.30 ± 4.35 | -- |
| (I) | (I)* | (I) | (I)* | (I) | (I)* | (I) | (I)* | |
| 3.5 | 100.20 ± 9.98 | 145.20 ± 10.83 | 43.80 ± 6.10 | 91.00 ± 9.17 | 330.85 ± 32.97 | 806.67 ± 60.14 | 1005.25 ± 139.98 | 1553.66 ± 156.48 |
| 3.75 | 87.00 ± 6.28 | 121.00 ± 4.95 | 86.40 ± 10.53 | 182.00 ± 19.74 | 272.73 ± 19.70 | 636.84 ± 26.05 | 1140.18 ± 138.91 | 2222.52 ± 241.01 |
| 5.0 | 166.60 ± 9.13 | 131.40 ± 5.86 | 222.00 ± 6.32 | 145.70 ± 11.81 | 816.67 ± 44.74 | 856.96 ± 38.20 | 2413.04 ± 68.75 | 6244.29 ± 506.31 |
| 6.0 | 256.80 ± 6.14 | 182.80 ± 3.96 | 333.60 ± 1.14 | -- | 1007.06 ± 24.08 | 1260.69 ± 27.33 | 3245.84 ± 11.09 | -- |
| Length (mm) | IT | IT* | IT | IT* | IT | IT* | IT | IT* |
| 8.0 | 11.20 ± 1.79 | 17.60 ± 2.61 | 21.80 ± 1.48 | 41.80 ± 3.49 | 28.97 ± 4.63 | 69.47 ± 10.29 | 109.00 ± 7.42 | 285.00 ± 23.81 |
| 12.0 | 18.60 ± 1.67 | 26.60 ± 1.82 | 46.40 ± 5.94 | 62.00 ± 4.80 | 48.10 ± 4.33 | 105.00 ± 7.17 | 232.00 ± 29.71 | 422.73 ± 32.70 |
| 15.5 | 25.20 ± 4.60 | 27.60 ± 0.55 | 60.00 ± 4.64 | 58.80 ± 10.43 | 65.17 ± 11.91 | 108.95 ± 2.16 | 300.00 ± 23.18 | 400.91 ± 71.09 |
| (I) | (I)* | (I) | (I)* | (I) | (I)* | (I) | (I)* | |
| 8.0 | 11.80 ± 1.79 | 25.00 ± 5.61 | 33.00 ± 3.74 | 66.60 ± 8.88 | 30.52 ± 4.63 | 98.68 ± 22.15 | 165.00 ± 18.71 | 454.09 ± 60.52 |
| 12.0 | 59.00 ± 10.75 | 103.00 ± 8.37 | 162.60 ± 17.18 | 240.60 ± 20.18 | 152.59 ± 27.79 | 406.58 ± 33.03 | 813.00 ± 85.92 | 1640.45 ± 137.60 |
| 15.5 | 144.40 ± 45.87 | 159.20 ± 4.44 | 321.20 ± 25.66 | 361.80 ± 67.34 | 373.45 ± 118.64 | 628.42 ± 17.52 | 1606.00 ± 128.28 | 2466.82 ± 459.14 |
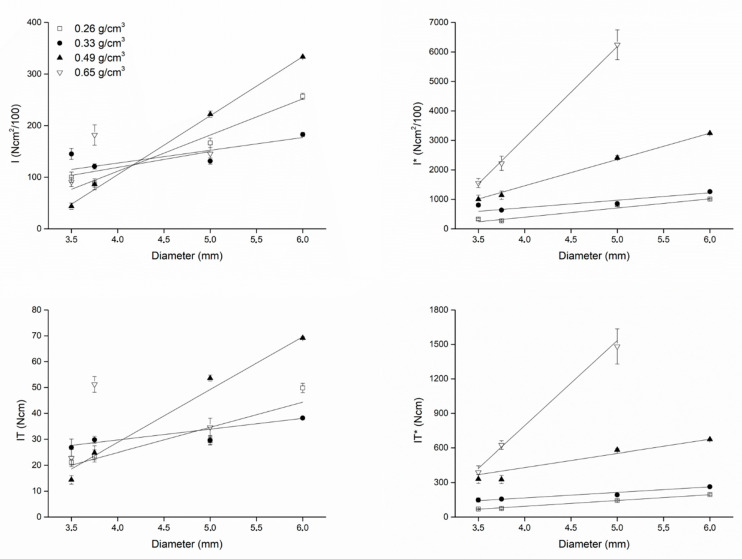
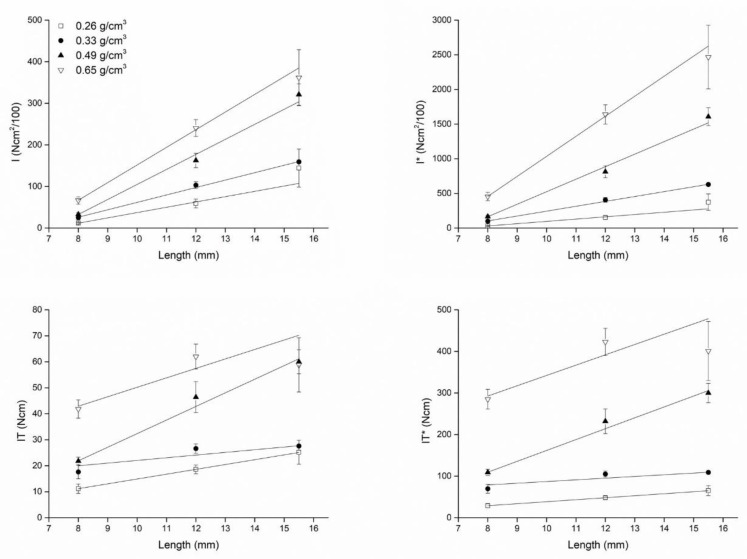
Plots showing how IT, (I), IT*, (I)* varied as a function of the fixture diameter and length are shown in Figure 4 (diameter) and Figure 5 (length).
Figure 4.
Plots describing how (I) and (I)* (top) and IT and IT* (bottom) varied as a function of the fixture diameter when implants were inserted in blocks with different densities. When (I) and IT were normalized for the undersizing coefficient (right), the effect of bone density on the correlation between (I)* or IT* and the fixture diameter could be appreciated: the greater the density, the greater (I)* or IT*. Such an effect could not be appreciated for the non-normalized variables (I) and IT, because of the effect of different site preparations (that is, different undersizing) when placing the implants according to the implant manufacturer’s instructions. The linear fit was better (i.e., the Pearson’s r coefficients were higher) for normalized variables than for non-normalized ones (see Table 5).
Figure 5.
Plots show how (I) and (I)* (top) and IT and IT* (bottom) varied as a function of the fixture length when implants were inserted in blocks with different densities. The effect of bone density on the correlation between (I) or IT and the implant length could be appreciated even considering non-normalized variables (left): the greater the density, the greater (I) and IT. Such effect was equally observed when considering the normalized (I)* and IT* parameters (right). Regression lines fitted equally well (i.e., the Pearson’s r coefficients were similar) both the non-normalized and the normalized variables (see Table 5).
The plots also show the best fitting lines to the experimental data, whose equations are provided in Table 5, together with the corresponding Pearson’s coefficients describing the correlation between IT, IT*, (I), and (I)* and the implant diameter and length.
Table 5.
Coefficients (m, slope; q, intercept) of the y = mx + q best fitting lines to the implant primary stability data (IT, IT*, (I), (I)*) when plotted versus either the fixture diameter or length, for each block density. The table also shows the corresponding Pearson’s r linear correlation coefficients.
| Plot | Density (g/cm3) | m (Slope) | q (Intercept) | r (Pearson’s) |
|---|---|---|---|---|
| IT-Diameter | 0.26 | 9.71 ± 2.71 | −13.94 ± 12.16 | 0.93003 |
| 0.33 | 4.17 ± 0.99 | 13.03 ± 5.70 | 0.94774 | |
| 0.49 | 20.41 ± 1.90 | −52.86 ± 10.86 | 0.99148 | |
| 0.65 | 0.81 ± 19.45 | 33.83 ± 79.06 | 0.04176 | |
| IT*-Diameter | 0.26 | 50.49 ± 2.18 | −108.29 ± 9.28 | 0.99814 |
| 0.33 | 48.00 ± 4.15 | −25.38 ± 23.28 | 0.99261 | |
| 0.49 | 122.65 ± 20.63 | −60.48 ± 120.77 | 0.97285 | |
| 0.65 | 740.11 ± 89.34 | −2167.77 ± 333.79 | 0.99279 | |
| (I)-Diameter | 0.26 | 70.14 ± 7.86 | −168.86 ± 38.08 | 0.98767 |
| 0.33 | 24.75 ± 8.77 | 28.52 ± 44.72 | 0.89414 | |
| 0.49 | 114.34 ± 1.84 | −352.37 ± 10.85 | 0.99974 | |
| 0.65 | 30.82 ± 37.99 | −4.17 ± 155.17 | 0.63003 | |
| (I*)-Diameter | 0.26 | 311.62 ± 38.67 | −850.46 ± 178.62 | 0.98495 |
| 0.33 | 251.49 ± 54.47 | −285.72 ± 265.16 | 0.95615 | |
| 0.49 | 892.91 ± 28.47 | −2110.76 ± 169.51 | 0.99898 | |
| 0.65 | 3106.65 ± 137.61 | −9347.63 ± 506.67 | 0.99902 | |
| IT-Length | 0.26 | 1.86 ± 0.01 | −3.67 ± 0.12 | 0.99998 |
| 0.33 | 1.03 ± 0.51 | 11.75 ± 7.62 | 0.89729 | |
| 0.49 | 5.23 ± 0.40 | −19.97 ± 3.66 | 0.99709 | |
| 0.65 | 3.64 ± 1.68 | 13.81 ± 16.97 | 0.90775 | |
| IT*-Length | 0.26 | 4.80 ± 0.03 | −9.49 ± 0.30 | 0.99998 |
| 0.33 | 4.07 ± 2.00 | 46.37 ± 30.07 | 0.89729 | |
| 0.49 | 26.17 ± 2.00 | −99.83 ± 18.30 | 0.99709 | |
| 0.65 | 24.81 ± 11.46 | 94.18 ± 115.71 | 0.90775 | |
| (I)-Length | 0.26 | 12.76 ± 2.19 | −90.35 ± 17.88 | 0.98554 |
| 0.33 | 17.85 ± 0.67 | −116.65 ± 8.74 | 0.99929 | |
| 0.49 | 36.15 ± 3.02 | −256.49 ± 25.43 | 0.99654 | |
| 0.65 | 42.43 ± 1.93 | −272.47 ± 17.15 | 0.99897 | |
| (I*)-Length | 0.26 | 33.01 ± 5.68 | −233.66 ± 46.25 | 0.98554 |
| 0.33 | 70.46 ± 2.66 | −460.47 ± 34.50 | 0.99929 | |
| 0.49 | 180.73 ± 15.08 | −1282.47 ± 127.15 | 0.99654 | |
| 0.65 | 289.28 ± 13.13 | −1857.72 ± 116.95 | 0.99897 |
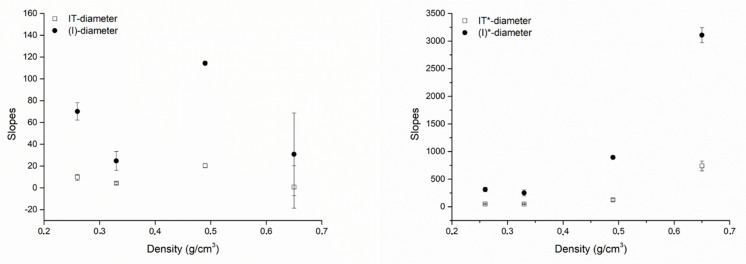
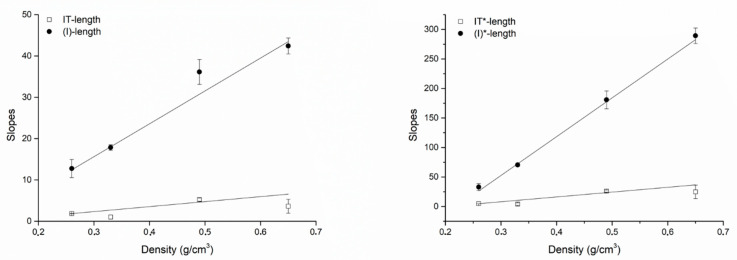
The correlation coefficients for IT* or (I)* and the fixture diameter were greater than the correlation coefficients for IT or (I) and the fixture diameter. However, the correlation coefficients for IT* or (I)* and the fixture length were not greater than the correlation coefficients for IT or (I) and the fixture length. The slopes of all best fitting lines to the experimental data were systematically greater considering (I) or (I)* compared to IT or IT*, indicating that a unitary change (1 mm) in the fixture diameter or length had a greater effect on (I) or (I)* than on IT or IT*. Figure 6 (diameter) and Figure 7 (length) show the angular coefficients (i.e., the slopes) of the best fitting lines to the experimental data plotted against density. Concerning the diameters, slopes describing how a unitary change (1 mm) in the fixture diameter affects primary stability seemed to not be a clear function of density. Concerning IT (r = 0.82944) and (I) (r = 0.81343), while they seemed to vary as a non-linear function of density both for IT* (r = 0.38289) and (I)* (r = 0.83095; Figure 6), a linear correlation was found, instead, between the IT (r = 088582), (I) (r = 0.99024), IT* (r = 0.94239) and (I)* (r = 0.99707) slopes describing how a unitary change (1 mm) in the fixture length affects primary stability and the block density (Figure 7). In all cases, the increase concerning (I)* was greater than that concerning IT*, indicating that a unitary change in density (1 g/cm3) causes a greater effect on (I)* than on IT*. When considering how the slopes concerning (I) or IT and length varied as a function of the block densities, this was also true for (I) when compared to IT.
Figure 6.
Plots showing how the slopes of the best fitting lines to the experimental data concerning the fixture diameters varied as a function of the block densities the fixtures were placed in. Left, slopes of the best fitting lines to the (I)-diameter and the IT-diameter plots; right, slopes of the lines best fitting the (I)*-diameter and the IT*-diameter plots. When (I) or IT were considered (left) they seemed to be not related to density in any way. When (I)* and IT* were considered (right), instead, a relation seemed to exist between bone density and them: the (I)*-diameter and IT*-diameter slopes appeared to vary as a non-linear function of bone density. Changes in (I)* plot slopes were always greater than those of the IT* plot slopes.
Figure 7.
Plots showing how the slopes of the best fitting lines to the experimental data concerning the fixture length varied as a function of the block densities the fixtures were placed in. Left, slopes of the best fitting lines to the (I)-length and the IT-length plots; right, slopes of the best fitting lines to the (I)*-length and the IT*-length plots. Even with no correction for undersizing, the slopes always varied as a linear function of bone densities. Changes in (I) or (I)* plot slopes were always greater than those of IT or IT* plot slopes.
4. Discussion
Results of the present study provide evidence that (I) at implant insertion might better detect changes in the implant diameter or length than IT. In all conditions that were assessed, in fact, the relation between (I) and the implant diameter or length was described by linear equations having a greater angular coefficient (slope) than those relating to IT. This means, in other words, that (I) is more sensitive than IT to changes in the implant diameter or length. It follows that, using (I) instead of IT to measure the implant primary stability in the clinical setting, the oral surgeon might gain a more precise and objective assessment of his/her choices concerning the size of the fixture being placed, which can actually allow the achievement of the desired primary implant stability. This should be the subject of further studies.
The observation that (I) is more sensitive than IT to dimensional changes of the fixture, are consistent with the fact that (I) measures a cumulative quantity; i.e., the sum of all interactions the fixture had while descending into its tunnel, while IT measured only the maximum friction encountered during such descent [33]. These results, therefore, indicated that (I) might be more useful than IT to validate pre-surgical choices concerning implant dimensions aimed to achieve enhanced primary stability.
Results of the present study also suggested that, to appropriately assess the effect of a unitary increase in diameter on primary stability, the extent of undersizing, measured by the ratio between the final (body) drill diameter and the diameter of the fixture threads, needs to be taken into account. When IT or (I) measurements were corrected with the undersizing coefficient C, in fact, both the correlation coefficients between IT* or (I)* and the implant diameter were greater than those concerning IT or (I), indicating IT* and (I)* describe the relation between the implant diameter and its stability better. This finding was expected based on the observation that an increase in undersizing should be, on first approximation, physically equivalent to an increase in the fixture diameter. The C coefficient is calculated as the complement to 1 of the ratio between the implant thread diameter and the final (body) drill diameter. Given this ratio is constant, C will also be constant. Therefore, undersizing may be visualized, in the clinical setting, as having a simple linear effect on primary stability, determined by such a ratio. Conversely, the increase in primary stability that the oral surgeon should expect when increasing the implant diameter will be proportional to the diameter increase only if the coefficient C is constant. The effect of undersizing seems to be less relevant when considering how the fixture length affects its primary stability, as the correlation coefficients between IT, IT*, (I), (I)* and length observed in the present study were quite similar. This observation might be explained by considering the relative effect that a change in diameter or length has on the implant surface: as a first approximation, the implant may be regarded as a cylinder contacting bone through its lateral and bottom surfaces. Indicating the cylinder radius as R and its length L, the total area contacting bone would be A = πR2 + 2πRL = πR(R + 2L). Supposing R or L are increased by the same quantity k, an increase in diameter would provide an increase in area ΔAdiam = π(R + k)(R + k +2L) − πR(R + 2L) = π(k2 + 2 k R + 2 k L); an increase in length would instead provide a surface increase equal to ΔAlength = πR(R + 2(L+ k) − πR(R + 2L) = 2 π k R, with ΔAdiam − ΔAlength = π(k2 + 2 k R + 2 k L) − 2 π k R = π(k2 + 2 k L). It follows that an increase in the implant diameter will always provide a greater increase in the area contacting bone than an increase in its length, and that the surface increase will be proportional to the square of the increase in diameter. These considerations may also explain why a unitary increase in the fixture diameter stabilizes the fixture more, by rule, than an equal increase in length, as observed in the present study and by other authors in in vitro studies [43,44,45]. These observations suggested that using shorter and larger implants in a clinical setting may be as viable as, or more viable than, using longer ones; this piece of information may be useful when dealing with delicate anatomic structures, such as the inferior alveolar nerve or the maxillary sinus, and it is consistent with current clinical evidence concerning short implants [46,47,48]. These observations also provide theoretical support for the application of immediate loading protocols on short implants; indeed, the current clinical evidence indicates that the immediate loading of short implants is comparable to conventional length implants in terms of implant survival, marginal bone level change, and ISQ [49,50,51].
Results of the present study concerning the effect of bone density on changes in primary stability parameters caused by changes in the fixture diameter or length indicated that: (a) at higher densities, a given increase in the fixture diameter or length will provide a greater implant stabilization than at lower densities; (b) increases in primary stability caused by an increase in the fixture diameter and length are better detected using (I) or (I)* than when using IT or IT*; and (c) when densities are higher, the increase in primary stability (either measured by (I), (I)*, IT or IT*) that is achieved by increasing the implant length/diameter is more than proportional to the density increase, while it is proportional to the density increase when the fixture length is increased. This latter finding might be explained by considering greater densities as associated with greater friction levels around the implant. Such friction, when increasing the implant diameter, will increase proportionally to the increase in the area surface; that is, according to the square of the increase in diameter. Instead, the increase in friction due to an increase in density is expected to vary only linearly with an increase in the fixture length. These observations concerning the effect of bone density, when translated in the clinical setting, suggest that, when low bone density is encountered (as, for example, in the posterior maxilla), choosing larger rather than longer implants may be helpful to achieve greater implant stability. This indication is consistent with current clinical evidence [47,48,49,52].
The present study has several limitations. The first concerns tests of the biomaterials being used; while the blocks displayed some macroscopic features similar to human bone, there was no trabecular structure. The material’s elastic response to mechanical stress is therefore expected to be unlike that of human cancellous bone [34,53]. Indeed, Orlando et al. [34] showed that, while (I)-density plots are still linear when equine bone is used instead of polyurethane foam, the linear relation between these two quantities is described by a different equation. Further, data in the present study were collected under no irrigation; Di Stefano et al. [33] showed that, on polyurethane foam blocks, irrigation had virtually no effect on primary stability parameters, such as (I), IT, RT, and ISQ. However, the experiments described in the present study should be repeated under irrigation and on testing materials that better mimic human bone (such as, equine bone blocks, bovine ribs or cadaver bone). Moreover, the present study compared (I) and (I)* to IT and IT*, respectively, but not to ISQ. The comparison between (I) and ISQ, as far as changes in the fixture length and diameter are concerned, should be the subject of additional investigations.
Finally, it is known that (I) and IT vary according to bone density depending on the implant shape and other macro- and micro-morphological characteristics [37]. Accordingly, primary stability values as well as equations and correlation coefficients calculated in the present study should be regarded as valid only for the specific implant model that was used to perform the experiments. While the overall picture that emerges from the present study; i.e., that (I) and (I)* are more sensitive to changes in the fixture diameter and length than IT and IT*, and that increasing the implant diameter stabilizes the implant more than increasing its length, different implant models and brands are expected to be characterized by different (I)-, (I)*-, IT-, and IT-diameter/length plots.
5. Conclusions
(I), as a primary stability measuring parameter, is more sensitive than IT to changes in stability that can be achieved by increasing the fixture diameter or length. When used to assess changes in stability given by varying the fixture diameter, (I) should be normalized for undersizing, as undersizing increases primary stability proportionally to (1-r), with r being the ratio between the final (body) drill diameter and that of the implant threads. All other conditions being the same, an increase in the implant diameter stabilizes the fixture more than an equal increase in length.
Author Contributions
Conceptualization, D.A.D.S., P.A. and F.A.; methodology, D.A.D.S., P.A. and F.A.; formal analysis, D.A.D.S., P.A. and F.A.; investigation, D.A.D.S., P.A. and F.A.; writing—original draft preparation, D.A.D.S., P.A. and F.A.; writing—review and editing, D.A.D.S., P.A. and F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Danilo Alessio Di Stefano and Paolo Arosio have a scientific consultancy relationship with IDI Evolution S.r.l. All other authors declare that they have no conflicts of interest. Data belonged to the authors, and none of the manufacturers cited in the study interfered with its execution or results.
References
- 1.Szmukler-Moncler S., Salama H., Reingewirtz Y., Dubruille J.H. Timing of loading and effect of micromotion on bone-dental implant interface: Review of experimental literature. J. Biomed. Mater. Res. 1998;43:192–203. doi: 10.1002/(SICI)1097-4636(199822)43:2<192::AID-JBM14>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 2.Trisi P., Perfetti G., Baldoni E., Berardi D., Colagiovanni M., Scogna G. Implant micromotion is related to peak insertion torque and bone density. Clin. Oral Implants Res. 2009;20:467–471. doi: 10.1111/j.1600-0501.2008.01679.x. [DOI] [PubMed] [Google Scholar]
- 3.Javed F., Romanos G.E. The role of primary stability for successful immediate loading of dental implants. A literature review. J. Dent. 2010;38:612–620. doi: 10.1016/j.jdent.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Javed F., Ahmed H.B., Crespi R., Romanos G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013;5:162–167. doi: 10.1556/IMAS.5.2013.4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinemann F., Hasan I., Bourauel C., Biffar R., Mundt T. Bone stability around dental implants: Treatment related factors. Ann. Anat. 2015;199:3–8. doi: 10.1016/j.aanat.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Buser D., Sennerby L., De Bruyn H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol 2000. 2017;73:7–21. doi: 10.1111/prd.12185. [DOI] [PubMed] [Google Scholar]
- 7.Morton D., Gallucci G., Lin W.S., Pjetursson B., Polido W., Roehling S., Sailer I., Aghaloo T., Albera H., Bohner L., et al. Group 2 ITI Consensus Report: Prosthodontics and implant dentistry. Clin. Oral Implants Res. 2018;29(Suppl. 16):215–223. doi: 10.1111/clr.13298. [DOI] [PubMed] [Google Scholar]
- 8.Degidi M., Daprile G., Piattelli A. Primary stability determination by means of insertion torque and RFA in a sample of 4135 implants. Clin. Implant Dent. Relat. Res. 2012;14:501–507. doi: 10.1111/j.1708-8208.2010.00302.x. [DOI] [PubMed] [Google Scholar]
- 9.Mesa F., Muñoz R., Noguerol B., de Dios Luna J., Galindo P., O’Valle F. Multivariate study of factors influencing primary dental implant stability. Clin. Oral Implants Res. 2008;19:196–200. doi: 10.1111/j.1600-0501.2007.01450.x. [DOI] [PubMed] [Google Scholar]
- 10.Ostman P.O., Hellman M., Wendelhag I., Sennerby L. Resonance frequency analysis measurements of implants at placement surgery. Int. J. Prosthodont. 2006;19:77–83. [PubMed] [Google Scholar]
- 11.Aragoneses J.M., Suárez A., Brugal V.A., Gómez M. Frequency Values and Their Relationship with the Diameter of Dental Implants. Prospective Study of 559 Implants. Implant Dent. 2019;28:279–288. doi: 10.1097/ID.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 12.Shiffler K., Lee D., Rowan M., Aghaloo T., Pi-Anfruns J., Moy P.K. Effect of length, diameter, intraoral location on implant stability. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016;122:e193–e198. doi: 10.1016/j.oooo.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Friberg B., Sennerby L., Meredith N., Lekholm U. A comparison between cutting torque and resonance frequency measurements of maxillary implants. A 20-month clinical study. Int. J. Oral Maxillofac. Surg. 1999;28:297–303. doi: 10.1016/S0901-5027(99)80163-5. [DOI] [PubMed] [Google Scholar]
- 14.Aparicio C. The use of the Periotest value as the initial success criteria of an implant: 8-year report. Int. J. Periodontics Restor. Dent. 1997;17:150–161. [PubMed] [Google Scholar]
- 15.Bischof M., Nedir R., Szmukler-Moncler S., Bernard J.P., Samson J. Implant stability measurement of delayed and immediately loaded implants during healing. Clin. Oral Implants Res. 2004;15:529–539. doi: 10.1111/j.1600-0501.2004.01042.x. [DOI] [PubMed] [Google Scholar]
- 16.Degidi M., Daprile G., Piattelli A., Carinci F. Evaluation of factors influencing resonance frequency analysis values, at insertion surgery, of implants placed in sinus-augmented and nongrafted sites. Clin. Implant Dent. Relat. Res. 2007;9:144–149. doi: 10.1111/j.1708-8208.2007.00042.x. [DOI] [PubMed] [Google Scholar]
- 17.Karl M., Graef F., Heckmann S., Krafft T. Parameters of resonance frequency measurement values: A retrospective study of 385 ITI dental implants. Clin. Oral Implants Res. 2008;19:214–218. doi: 10.1111/j.1600-0501.2007.01462.x. [DOI] [PubMed] [Google Scholar]
- 18.Alsabeeha N.H., De Silva R.K., Thomson W.M., Payne A.G. Primary stability measurements of single implants in the midline of the edentulous mandible for overdentures. Clin. Oral Implants Res. 2010;21:563–566. doi: 10.1111/j.1600-0501.2009.01890.x. [DOI] [PubMed] [Google Scholar]
- 19.Sim C.P., Lang N.P. Factors influencing resonance frequency analysis assessed by Osstell mentor during implant tissue integration: I. Instrument positioning, bone structure, implant length. Clin. Oral Implants Res. 2010;21:598–604. doi: 10.1111/j.1600-0501.2009.01878.x. [DOI] [PubMed] [Google Scholar]
- 20.González-García R., Monje F., Moreno-García C. Predictability of the resonance frequency analysis in the survival of dental implants placed in the anterior non-atrophied edentulous mandible. Med. Oral Patol. Oral Cir. Bucal. 2011;16:e664–e669. doi: 10.4317/medoral.16982. [DOI] [PubMed] [Google Scholar]
- 21.Pommer B., Hof M., Fädler A., Gahleitner A., Watzek G., Watzak G. Primary implant stability in the atrophic sinus floor of human cadaver maxillae: Impact of residual ridge height, bone density, and implant diameter. Clin. Oral Implants Res. 2014;25:e109–e113. doi: 10.1111/clr.12071. [DOI] [PubMed] [Google Scholar]
- 22.Maiorana C., Farronato D., Pieroni S., Cicciu M., Andreoni D., Santoro F. A Four-Year Survival Rate Multicenter Prospective Clinical Study on 377 Implants: Correlations between Implant Insertion Torque, Diameter, and Bone Quality. J. Oral Implantol. 2015;41:e60–e65. doi: 10.1563/AAID-JOI-D-13-00206. [DOI] [PubMed] [Google Scholar]
- 23.Lozano-Carrascal N., Salomó-Coll O., Gilabert-Cerdà M., Farré-Pagés N., Gargallo-Albiol J., Hernández-Alfaro F. Effect of implant macro-design on primary stability: A prospective clinical study. Med. Oral. Patol. Oral Cir. Bucal. 2016;21:e214–e221. doi: 10.4317/medoral.21024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang H., Xu Z., Shao X., Wismeijer D., Sun P., Wang J., Wu G. Multivariate linear regression analysis to identify general factors for quantitative predictions of implant stability quotient values. PLoS ONE. 2017;12:e0187010. doi: 10.1371/journal.pone.0187010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sennerby L., Meredith N. Implant stability measurements using resonance frequency analysis: Biological and biomechanical aspects and clinical implications. Periodontol 2000. 2008;47:51–66. doi: 10.1111/j.1600-0757.2008.00267.x. [DOI] [PubMed] [Google Scholar]
- 26.Hsu J.T., Shen Y.W., Kuo C.W., Wang R.T., Fuh L.J., Huang H.L. Impacts of 3D bone-to- implant contact and implant diameter on primary stability of dental implant. J. Formos. Med. Assoc. 2017;116:582–590. doi: 10.1016/j.jfma.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Vayron R., Nguyen V.H., Lecuelle B., Haiat G. Evaluation of dental implant stability in bone phantoms: Comparison between a quantitative ultrasound technique and resonance frequency analysis. Clin. Implant Dent. Relat. Res. 2018;20:470–478. doi: 10.1111/cid.12622. [DOI] [PubMed] [Google Scholar]
- 28.Park K.J., Kwon J.Y., Kim S.K., Heo S.J., Koak J.Y., Lee J.H., Lee S.J., Kim T.H., Kim M.J. The relationship between implant stability quotient values and implant insertion variables: A clinical study. J. Oral Rehabil. 2012;39:151–159. doi: 10.1111/j.1365-2842.2011.02255.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim S.H., Lee S.J., Cho I.S., Kim S.K., Kim T.W. Rotational resistance of surface-treated mini-implants. Angle Orthod. 2009;79:899–907. doi: 10.2319/090608-466.1. [DOI] [PubMed] [Google Scholar]
- 30.Degidi M., Daprile G., Piattelli A., Iezzi G. Development of a new implant primary stability parameter: Insertion torque revisited. Clin. Implant Dent. Relat. Res. 2013;15:637–644. doi: 10.1111/j.1708-8208.2011.00392.x. [DOI] [PubMed] [Google Scholar]
- 31.Lekholm U., Zarb G.A. Patient selection and preparation. In: Brånemark P.I., Zarb G.A., Albrektsson T., editors. Tissue-Integrated Prostheses: Osseointegration in Clinical Dentistry. Quintessence Publishing Co.; Chicago, IL, USA: 1985. pp. 199–209. [Google Scholar]
- 32.Degidi M., Daprile G., Piattelli A. Influence of underpreparation on primary stability of implants inserted in poor quality bone sites: An in vitro study. J. Oral Maxillofac. Surg. 2015;73:1084–1088. doi: 10.1016/j.joms.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 33.Di Stefano D.A., Arosio P., Gastaldi G., Gherlone E. The insertion torque-depth curve integral as a measure of implant primary stability: An in vitro study on polyurethane foam blocks. J. Prosthet. Dent. 2018;120:706–714. doi: 10.1016/j.prosdent.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Orlando F., Arosio F., Arosio P., Di Stefano D.A. Bone Density and Implant Primary Stability. A Study on Equine Bone Blocks. Dent. J. (Basel) 2019;7:73. doi: 10.3390/dj7030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capparé P., Vinci R., Di Stefano D.A., Traini T., Pantaleo G., Gherlone E.F., Gastaldi G. Correlation between Initial BIC and the Insertion Torque/Depth Integral Recorded with an Instantaneous Torque-Measuring Implant Motor: An in vivo Study. Clin. Implant Dent. Relat. Res. 2015;17(Suppl. 2):e613–e620. doi: 10.1111/cid.12294. [DOI] [PubMed] [Google Scholar]
- 36.Iezzi G., Filippone A., Di Stefano D.A., Arosio P., Piattelli A., Scarano A., Perrotti V. A site-specific intraoperative measurement of bone-to-implant contact during implant insertion: A study on bovine ribs using a computerized implant motor. J Dent. Sci. 2015;10:21–27. doi: 10.1016/j.jds.2014.11.002. [DOI] [Google Scholar]
- 37.Di Stefano D.A., Arosio P., Perrotti V., Iezzi G., Scarano A., Piattelli A. Correlation between Implant Geometry, Bone Density, and the Insertion Torque/Depth Integral: A Study on Bovine Ribs. Dent. J. (Basel) 2019;7:25. doi: 10.3390/dj7010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Stefano D.A., Perrotti V., Greco G.B., Cappucci C., Arosio P., Piattelli A., Iezzi G. The effect of undersizing and tapping on bone to implant contact and implant primary stability: A histomorphometric study on bovine ribs. J. Adv. Prosthodont. 2018;10:227–235. doi: 10.4047/jap.2018.10.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andreasi Bassi M., Arosio P., Di Stefano D.A. Evaluation of Peri-implant Bone Stress on D1 Bone Using a Computerized Torque-Measuring Implant Motor: A Study on Photoelastic Resin Blocks. Int. J. Oral Maxillofac. Implants. 2018;33:770–778. doi: 10.11607/jomi.6079. [DOI] [PubMed] [Google Scholar]
- 40.Arosio P., Greco G.B., Zaniol T., Iezzi G., Perrotti V., Di Stefano D.A. Sinus augmentation and concomitant implant placement in low bone-density sites. A retrospective study on an undersized drilling protocol and primary stability. Clin. Implant Dent. Relat. Res. 2018;20:151–159. doi: 10.1111/cid.12558. [DOI] [PubMed] [Google Scholar]
- 41.ASTM—American Society for Testing and Materials . Standard ASTM F1839-08(2012); Standard Specification for Rigid Polyurethane Foam for Use as A Standard Material for Testing Orthopaedic Devices and Instruments. ASTM; West Conshohocken, PA, USA: 2012. [Google Scholar]
- 42.Ku H.H. Notes on the use of propagation of error formulas. Journal of Research of the National Bureau of Standards. J. Res. Natl. Bur. Stand. 1966;70:263–273. [Google Scholar]
- 43.Bilhan H., Geckili O., Mumcu E., Bozdag E., Sünbüloğlu E., Kutay O. Influence of surgical technique, implant shape and diameter on the primary stability in cancellous bone. J. Oral Rehabil. 2010;37:900–907. doi: 10.1111/j.1365-2842.2010.02117.x. [DOI] [PubMed] [Google Scholar]
- 44.Möhlhenrich S.C., Heussen N., Elvers D., Steiner T., Hölzle F., Modabber A. Compensating for poor primary implant stability in different bone densities by varying implant geometry: A laboratory study. Int. J. Oral Maxillofac. Surg. 2015;44:1514–1520. doi: 10.1016/j.ijom.2015.08.985. [DOI] [PubMed] [Google Scholar]
- 45.Romanos G.E., Delgado-Ruiz R.A., Sacks D., Calvo-Guirado J.L. Influence of the implant diameter and bone quality on the primary stability of porous tantalum trabecular metal dental implants: An in vitro biomechanical study. Clin. Oral Implants Res. 2018;29:649–655. doi: 10.1111/clr.12792. [DOI] [PubMed] [Google Scholar]
- 46.Palacios J.A.V., Garcia J.J., Caramês J.M.M., Quirynen M., da Silva Marques D.N. Short implants versus bone grafting and standard-length implants placement: A systematic review. Clin. Oral Investig. 2018;22:69–80. doi: 10.1007/s00784-017-2205-0. [DOI] [PubMed] [Google Scholar]
- 47.Papaspyridakos P., De Souza A., Vazouras K., Gholami H., Pagni S., Weber H.P. Survival rates of short dental implants (≤6 mm) compared with implants longer than 6 mm in posterior jaw areas: A meta-analysis. Clin. Oral Implants Res. 2018;29(Suppl. 16):8–20. doi: 10.1111/clr.13289. [DOI] [PubMed] [Google Scholar]
- 48.Fan T., Li Y., Deng W.W., Wu T., Zhang W. Short Implants (5 to 8 mm) Versus Longer Implants (>8 mm) with Sinus Lifting in Atrophic Posterior Maxilla: A Meta-Analysis of RCTs. Clin. Implant Dent. Relat. Res. 2017;19:207–215. doi: 10.1111/cid.12432. [DOI] [PubMed] [Google Scholar]
- 49.Weerapong K., Sirimongkolwattana S., Sastraruji T., Khongkhunthian P. Comparative study of immediate loading on short dental implants and conventional dental implants in the posterior mandible: A randomized clinical trial. Int. J. Oral Maxillofac. Implants. 2019;34:141–149. doi: 10.11607/jomi.6732. [DOI] [PubMed] [Google Scholar]
- 50.Han J., Tang Z., Zhang X., Meng H. A prospective, multi-center study assessing early loading with short implants in posterior regions. A 3-year post-loading follow-up study. Clin. Implant Dent. Relat. Res. 2018;20:34–42. doi: 10.1111/cid.12568. [DOI] [PubMed] [Google Scholar]
- 51.Anitua E., Flores J., Flores C., Alkhraisat M.H. Long-term Outcomes of Immediate Loading of Short Implants: A Controlled Retrospective Cohort Study. Int. J. Oral Maxillofac. Implants. 2016;31:1360–1366. doi: 10.11607/jomi.5330. [DOI] [PubMed] [Google Scholar]
- 52.Felice P., Barausse C., Pistilli V., Piattelli M., Ippolito D.R., Esposito M. Posterior atrophic jaws rehabilitated with prostheses supported by 6 mm long × 4 mm wide implants or by longer implants in augmented bone. 3-year post-loading results from a randomised controlled trial. Eur. J. Oral Implantol. 2018;11:175–187. [PubMed] [Google Scholar]
- 53.Grant J.A., Bishop N.E., Götzen N., Sprecher C., Honl M., Morlock M.M. Artificial composite bone as a model of human trabecular bone: The implant-bone interface. J. Biomech. 2007;40:1158–1164. doi: 10.1016/j.jbiomech.2006.04.007. [DOI] [PubMed] [Google Scholar]