to the editor
In the APPROACH trial (Aug. 8 issue),1 volanesorsen — an antisense oligonucleotide targeting APOC3 messenger RNA — reduced circulating triglycerides in patients with familial chylomicronemia syndrome, but thrombocytopenia developed in 76% of treated patients. This unexpected adverse event raises a key question regarding inhibition of apolipoprotein C-III synthesis as a therapeutic strategy: is thrombocytopenia an on-target effect of reduced apolipoprotein C-III activity or an off-target effect of volanesorsen?
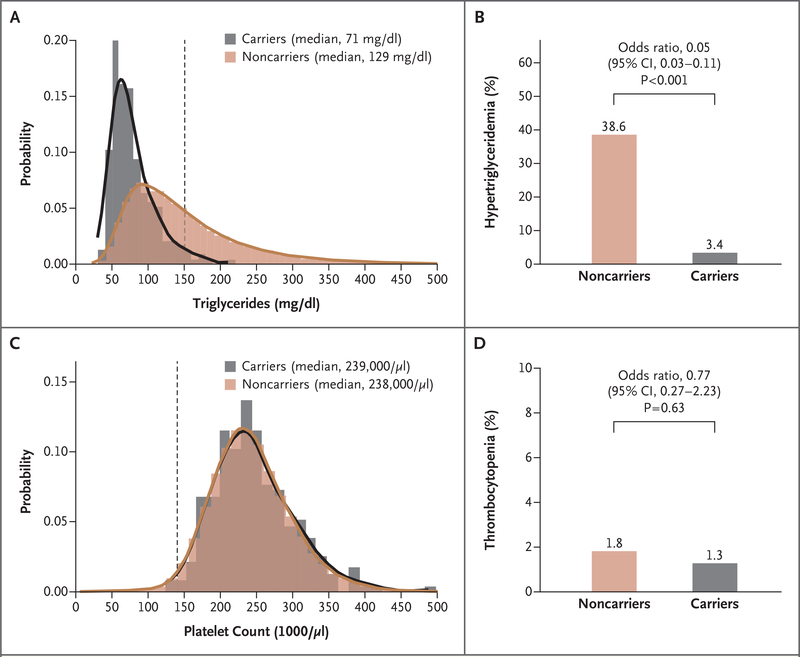
We therefore asked whether heterozygous carriers of an inactivating mutation in APOC3 are at increased risk for thrombocytopenia. Among 42,503 participants in the UK Biobank, 235 (0.6%) were found to carry any of four previously described inactivating mutations in APOC3.2 As expected, carriers had significantly lower levels of triglycerides than noncarriers (median, 71 mg per deciliter and 129 mg per deciliter, respectively; P<0.001), as well as a lower risk of hypertriglyceridemia (odds ratio, 0.05; 95% CI, 0.03 to 0.11; P<0.001) (Fig. 1A and 1B). We found no significant difference between carriers and noncarriers in platelet count (median, 239,000 and 238,000 per microliter, respectively; P = 0.21) or in the prevalence of thrombocytopenia (1.3% and 1.8%, respectively; P = 0.63) (Fig. 1C and 1D). These results suggest that the effect of volanesorsen is medication- or class-specific rather than an inherent property of apolipoprotein C-III inhibition.
Figure 1. Inactivating Mutations in APOC3 and the Risk of Hypertriglyceridemia or Thrombocytopenia.
Gene sequencing of specimens from 42,503 participants in the UK Biobank identified an inactivating mutation in APOC3 in 235 (0.6%). Panel A shows the distribution of triglyceride levels in carriers and noncarriers. The vertical dashed line indicates a triglyceride level of 150 mg per deciliter, the cutoff value for hypertriglyceridemia. Panel B shows the percentage of participants with hypertriglyceridemia. In a logistic-regression model with adjustment for age, sex, fasting duration at time of blood collection, and genetic ancestry, carriers had a significantly lower risk of hypertriglyceridemia than noncarriers. Panel C shows the distribution of platelet counts in carriers and noncarriers. In a linear regression model with adjustment for age, sex, fasting duration at time of blood collection, and genetic ancestry, there was no significant difference in platelet counts between carriers and noncarriers. The vertical dashed line indicates 140,000 per microliter, the cutoff value for thrombocytopenia. Panel D shows the percentage of participants with thrombocytopenia. In a logistic-regression model with adjustment for age, sex, fasting duration at time of blood collection, and genetic ancestry, there was no evidence of a higher risk of thrombocytopenia among carriers than among noncarriers. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129.
Acknowledgments
Dr. Khera reports being supported by an institutional grant from the Broad Institute of MIT and Harvard (BroadIgnite), grant 1K08HG010155 from the National Human Genome Research Institute, a Hassenfeld Scholar Award from Massachusetts General Hospital, having served as a consultant to Color Genomics and Navitor Pharmaceuticals, having received honoraria from Illumina, having received grant support from the Novartis Institute for Biomedical Research and IBM Research, and having a patent application entitled “Genetic Risk Predictor” (20190017119) owned by the General Hospital Corporation. No other potential conflict of interest relevant to this letter was reported.
Contributor Information
Sumeet A. Khetarpal, Massachusetts General Hospital Boston, MA
Minxian Wang, Broad Institute of MIT and Harvard Cambridge, MA
Amit V. Khera, Massachusetts General Hospital Boston, MA
References
- 1.Witztum JL, Gaudet D, Freedman SD, et al. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med 2019;381:531–42. [DOI] [PubMed] [Google Scholar]
- 2.The TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med 2014;371:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]