Abstract
Extracellular vesicles (EVs) are involved in intercellular communication, transporting proteins and nucleic acids to proximal and distal regions. There is evidence of glycosylation influencing protein routing into EVs; however, the impact of aberrant cellular glycotransferase expression on EV protein profiles has yet to be evaluated. In this study, we paired extracellular vesicle characterization and quantitative proteomics to determine the systemic impact of altered α(1,6)fucosyltranferase (FUT8) expression on prostate cancer-derived EVs. Our results showed that increased cellular expression of FUT8 could reduce the number of vesicles secreted by prostate cancer cells as well as increase the abundance of proteins associated with cell motility and prostate cancer metastasis. In addition, overexpression of FUT8 resulted in altered glycans on select EV-derived glycoproteins. This study presents the first evidence of altered cellular glycosylation impacting EV protein profiles and provides further rationale for exploring the functional role of glycosylation in EV biogenesis and biology.
Keywords: prostate cancer, extracellular vesicles, proteomics, glycoproteins, core fucosylation
Graphical Abstract

INTRODUCTION
Prostate cancer is the most commonly diagnosed oncogenic malignancy in men worldwide, resulting in almost 30,000 cancer-related deaths in the United States each year.1 Although the initial adoption of prostate-specific antigen (PSA) screening has been associated with reduced mortality and better disease management,2,3 the low diagnostic sensitivity and specificity for discriminating low-risk patients with clinically indolent tumors from those with aggressive disease have resulted in overdiagnosis and treatment.4 Since PSA is a glycoprotein, there have been extensive efforts investigating differential PSA glycoforms for improved diagnostic accuracy of prostate cancer patients.5–8 Wider examination of aberrant glycosylation in prostate cancer has revealed increased expression of the glycotransferase involved in core fucosylation, α(1,6)fucosyltranferase (FUT8) associating with aggressive (AG) prostate cancer and castration resistance, with functional analyses revealing FUT8 impacting cell motility and invasiveness in prostate cancer cells.9–11
Extracellular vesicles (EVs) are secreted microvesicles involved in both proximal and distal intercellular communication via the transport of proteins and nucleic acids (mRNA, miRNA, and DNA).12–16 EVs are representative of several heterogeneous populations of vesicles, including exosomes (30–100 nm) and ectosomes (100–1000 nm), with distinct mechanisms of biogenesis and release into the extracellular space.17 Exosome biogenesis originates in the endocytic pathway, with proteins involved in multivesicular body (MVB) formation (i.e., ALIX and CHMP4) having been shown to modulate exosome biogenesis.18 Ectosomes, which are also referred to as oncosomes and microparticles, directly “bud” from the plasma membrane.19 Previous studies have shown that cancer-derived EVs can induce phenotypic transformation when internalized by recipient cells,20–23 indicating a role in cancer progression. Interestingly, factors associated with oncogenesis such as hypoxia and the pH of the tumor microenvironment have been shown to influence EV biogenesis and the resulting EV protein cargo profile.24,25
Recently, it has been shown that engineering a glycosylation site onto a protein could stabilize and increase recruitment of a glycoprotein into EVs,26 and inhibition of N-linked glycan processing was found to reduce the localization of proteins into EVs.27 These results suggest that glycosylation can influence EV protein profiles; however, the systemic impact of aberrant, oncogenic-associated glycotransferase expression has not been explored. To gain insight into the impact of increased cellular FUT8 expression on EV biogenesis and protein cargo profiles in prostate cancer cells, we paired nanoparticle tracking analysis (NTA) and stable isotope labeling with amino acids in cell culture (SILAC) quantitative proteomics. Utilizing isogenic prostate cancer cell line models and focusing on EVs with a size distribution of <150 nm, our analysis showed that cellular FUT8 expression could significantly impact the number of vesicles observed in the extracellular compartment, while proteins associated with metastasis were increased in abundance in EVs derived from prostate cancer cells overexpressing FUT8. Together, these results highlight the functional impact of altered glycosylation in prostate carcinogenesis via modulated extracellular vesicle production and provide rationale for exploring EV-based diagnostics for prostate cancer screening.
MATERIALS AND METHODS
Materials
Prostate cancer cell lines LAPC4 and LNCaP were purchased from American Type Culture Collection (ATCC, Manassas, VA). RPMI media, SILAC RPMI media, fetal bovine serum (FBS), dialyzed fetal bovine serum (dFBS), Geneticin (G418), puromycin, 100 U/mL penicillin/streptomycin were from Life Technologies (Grand Island, NY). SILAC isotopes: 13C6 14N4-l-lysine (k6) and 13C6 15N4-L-arginine (R10) were from Cambridge Isotope Laboratories, Inc. (Tewksbury, MA). OptiPrep was from Sigma-Aldrich (St. Louis, MO). Mouse anti-ALIX (1A12) was from Santa Cruz Biotechnology (Dallas, TX), and the rabbit anti-FUT8 was a kind gift from Drs. Ruey-Bing Yang and Chen-Fen Tu (Institute of Biomedical Sciences, Academia Sinica, Taipei).28 NuPAGE precast gels and NuOAFE MES running buffer were from Thermo Fisher Scientific (Waltham, MA). Trypsin was from Promega (Madison, WI), and lysyl endopeptidase was from Wako Laboratory Chemicals USA (Richmond, VA). BCA assay kit Pierce was from Thermo Fisher Scientific (Rockford, IL). C18 SepPak columns and Oasis MAX extraction columns were purchased from Waters Corporation (Milford, MA). C18 solid-phase extraction disks were from 3M (St. Paul, MN).
Cell Culture
LAPC4 and LNCaP cell lines were grown and maintained in RPMI media with 10% FBS and 100 U/mL penicillin/streptomycin; LAPC4 cell media were also supplemented with 5 nM R1881. For stably transfected cell lines, LAPC4-FUT8 and LNCAP-FUT8 overexpression (OE) cell media were additionally supplemented with 0.6 mM G418, while LNCaP-FUT8 knockdown (KD) cell media included 2 μg/mL puromycin. For SILAC labeling, LAPC4 cells were grown in SILAC media with 10% dFBS, 5 nM 1881, and 100 U/mL penicillin/streptomycin supplemented with 13C6 14N4-L-lysine (K6) and 13C6 15N4-l-arginine (R10) (heavy). After 10 cell doublings, the incorporation of the stable isotopes was >98% as determined by LC–MS/MS analysis of tryptic peptides isolated from heavy Lys6/Arg10-labeled cells. All cell lines were maintained at 37 °C and 5% CO2 in a humidified incubator. For EV-related experiments, all cell lines were further maintained in the media described above, with bovine-derived EV-depleted FBS (DFBS) or bovine-derived EV-depleted dFBS (DdFBS), in the same incubation conditions. Bovine-derived EV depleted FBS and dFBS were generated by ultracentrifugation for 16 h at 100,000 × g twice, prior to filtration through a 0.22 μm filter. Cell counting was performed using Countess II FL (Life Technologies); an equal volume of intact cells and 0.2% trypan blue were mixed and pipetted onto a cell counting chamber slide.
Extracellular Vesicle Isolation
All cells were grown approximately to 90% confluency in their respective media conditions. The media were removed, and the cells were rinsed three times with phosphate-buffered saline (PBS) and grown in their respective media conditions supplemented with 5% DFBS or DdFBS. After 48 h, the conditioned media were collected and processed for EV isolation as previously described.29 To obtain sufficient material for proteomic analysis, conditioned media from each cell line grown in parallel were pooled and centrifuged at 300 × g for 10 min, 2000 × g for 20 min, and 10,000 ×g for 30 min at 4 °C to remove whole cells and cell debris. The supernatant was then spun at 100,000 × g for 70 min at 4 °C, and the resulting pellet was washed in PBS and spun at 100,000 × g for 70 min at 4 °C. The crude EV pellet was then resuspended in 25 M sucrose and 10 mM Tris-HCl (pH 7.5) and layered on top of a discontinuous OptiPrep density gradient. The formed gradient was centrifuged at 100,000 × g for 18 h at 4 °C. After the centrifugation was completed, 12 aliquots (1 mL) were collected (from the top to the bottom) and diluted with 5 mL of PBS. The fractions were centrifuged at 100,000 × g for 70 min, the supernatants were discarded, and the resulting pellets were washed in PBS. Fractions containing EVs were pooled, centrifuged at 100,000 × g for 70 min, and resuspended in 50 μL.
Electron Microscopy
For negative staining microscopy, 10 μL of EVs was layered and absorbed on a formvar coated 400 mesh copper grid and stained with 1% PTA. Samples were imaged using a Philips/FEI BioTwin CM120 transmission electron microscope (FEI, Hillsboro, OR) equipped with a thermionic LaB6 filament and operated at an acceleration of 80 kV. Images were taken with a pixel size range of 0.47–0.60 nm and a direct magnification range of 65,000–93,000× using an AMT XR80 high-resolution camera.
Western Blotting
For immune detection, the EV pellet was lysed in 8 M urea, 50 mM Tris-HCl (pH 8.0), 1 mM EDTA, 2 μg/mL aprotinin, 10 μg/mL leupeptin, 1 mM PMSF, and 10 mM NaF and homogenized using a Branson Sonifier 250 probe sonicator (Danbury, CT) with a duty cycle of 10%, output control 1 with 10s/50s on/off cycle (n = 5), and the protein concentration of the EV lysate was measured via the BCA protein assay. Lysate (10 μg) was mixed with Laemmli buffer with 50 mM DTT and separated using NuPAGE 4–12% bis-Tris precast gels with NuOAFE MES SDS running buffer. Following electrophoresis, proteins were electrotransferred onto a polyvinylidene fluoride (PVDF) membrane using the rapid dry transfer iBlot 2 gel transfer system (Thermo Fisher Scientific). After being blocked in 5% bovine serum albumin in TBST for 1 h at RT, the membrane was probed with the primary antibody (1:2000 dilution) overnight at 4 °C. HRP-conjugated secondary antibodies (goat anti-mouse or goat anti-rabbit) were diluted 1:20,000 and detected using ECL western blotting detection (Sigma, St. Louis, MO).
NanoSight Vesicle Characterization
For extracellular vesicle size and number determination, 10 μL of EVs was analyzed using a NanoSight NS300 (Malvern Panalytical) nanoparticle tracking system. Samples were diluted in a volume of filtered PBS and flowed past a 532 nm laser beam for three 1 min cycles at room temperature. The samples were analyzed using the nanoparticle tracking analysis (NTA software (ver 3.2.16)).
Peptide Digestion and Basic RP-HPLC Fractionation
EVs from each cell line were subjected to lysis and tryptic digestion as described previously,30 with minor modification as noted. First, EVs were resuspended in lysis buffer (8 M urea, 50 mM Tris-HCl (pH 8.0), 1 mM EDTA, 2 μg/mL aprotinin, 10 μg/mL leupeptin, 1 mM PMSF, and 10 mM NaF) and homogenized using a Branson Sonifier 250 probe sonicator (Danbury, CT) with a duty cycle of 10%, output control 1 with 10s/50s on/off cycle (n = 5), and the protein concentration of the EV lysate was measured via the BCA protein assay. Each EV lysate (125 μg) from LAPC4-FUT8 and LAPC4-K6R10 was mixed in a 1:1 ratio and subjected to tryptic digestion via insolution digestion. Samples were reduced with 10 mM tris 2-carboxyethyl phosphine (TCEP) and alkylated with 50 mM iodoacetamide. Tryptic digestion was performed overnight at 37 °C. Peptides were recovered, lyophilized, and subjected to cleanup using C18 SepPak columns and dried down. The peptide material (50 μg) was reconstituted in a volume of 20 mM ammonium formate (pH 10) and 2% acetonitrile (ACN) and loaded onto a 4.6 mm × 250 mm RP ZORBAX 300 A Extend-C18 column with 3.5 μm size beads (Agilent). Peptides were separated using an Agilent 1200 Series HPLC instrument using basic reversed-phase chromatography with solvent A (2% ACN, 5 mM ammonium formate, pH 10) and a nonlinear gradient of solvent B (90% ACN, 5 mM ammonium formate, pH 10) at 1 mL/min as follows: 0% solvent B (9 min), 6% solvent B (4 min), 6 to 28.5% solvent B (50 min), 28 to 34% solvent B (5.5 min), 34 to 60% solvent B (13 min), and then held at 60% solvent B for 8.5 min. Collected fractions were concatenated into 12 fractions, dried down, and resuspended in 3% ACN and 0.1% formic acid (FA) prior to ESI-LC–MS/MS analysis.
Intact Glycopeptide Enrichment
For intact glycopeptide analysis, 100 μg of peptide material was subjected to intact glycopeptide enrichment using Oasis MAX cartridges (particle size, 25–35 μm; 30 mg sorbent per cartridge; Waters). Peptides were resuspended in 80% ACN and 0.1% trifluoroacetic acid (TFA) and adjusted to 95% ACN and 1% TFA. The MAX columns were equilibrated three times with 1 mL of ACN, three times with 100 mM triethylammonium acetate, three times with water, and finally three times with 95% ACN and 1% TFA. The samples were loaded onto MAX columns and washed four times with 1 mL of 95% ACN and 1% TFA. Bound intact glycopeptides were eluted in 400 μL of 50% ACN and 0.1% TFA, dried down, and resuspended in 3% ACN and 0.1% FA prior to ESI-LC–MS/MS analysis.
Nano-ESI-LC-MS/MS Analysis
For data acquisition, peptide samples were analyzed using an Orbitrap Lumos Fusion system. Peptide (1 μg) was separated using an EASY-nLC 1200 UHPLC system (Thermo Scientific) on an in-house packed 20 cm × 75 μm diameter C18 column (1.9 μm ReproSil-Pur C18-AQ beads (Dr. Maisch GmbH); Picofrit 10 μm opening (New Objective)). The column was heated to 50 °C using a column heater (Phoenix-ST). The flow rate was 0.300 μL/min with 0.1% formic acid and 2% acetonitrile in water (A) and 0.1% formic acid and 90% acetonitrile (B). The peptides were separated with a 6–30% B gradient in 84 min and analyzed using a Thermo Fusion Lumos mass spectrometer (Thermo Scientific). Parameters were as follows: MS1: resolution, 60,000; mass range, 350–800 m/z, RF lens, 30%, AGC target, 4.0 × 105; Max IT, 50 ms; charge state, 2–6; dynamic exclusion, 45 s; top 20 ions selected for MS2; MS2: resolution, 50,000; high-energy collision dissociation activation energy (HCD), 34 (global peptide) or 36 (intact glycopeptide); isolation width (m/z), 0.7; AGC target, 2.0 × 105; Max IT, 105 ms. Three replicates were analyzed for the proteomic and glycoproteomic analyses.
Global Protein Identification and Quantification
Global peptide .RAW files were searched against the UniProtKB Swiss-Prot human protein database (version June 2017; 20,192 reviewed sequences) using MaxQuant (version 1.5.3.30) and the most default search parameter settings: 20 ppm peptide and fragment mass tolerance; variable modification: methionine +15.99492, lysine +6.02013 (13C6), and arginine +10.00827 (13C6 15n4); fixed modification: cysteine +57.02510 and semitryptic peptides with up to two missed cleavages; minimum ratio count of two peptides for protein quantitation; and 1% FDR at the protein level.31,32 For intensity-based absolute quantitation (iBAQ), search parameters were the same, except for lysine +6.02013 (13C6) and arginine +10.00827 (13C6 15N4) being included as fixed modifications for LAPC4-WT characterization and excluded for LAPC4-FUT8 OE characterization. All the mass spectrometry proteomics data (both global proteome and N-linked glycoproteome), including raw MS data, processed peak lists, and respective search results, have been deposited to the ProteomeXChange Consortium via the PRIDE33 partner repository with the dataset identifier PXD013340. Proteins quantified in at least three replicates were considered for statistical analysis.
Intact N-Linked Glycopeptide Identification and Quantification
Intact N-linked glycopeptide identification was performed as previously described with some modifications.34 In brief, intact glycopeptide .RAW files were converted to .mzML format using ProteoWizard35 with the “Peak Picking” option selected for all MS levels and subjected to search using GPQuest 2.0 against a customized human database containing over 30,000 known N-linked glycopeptide sequences (including global variable and fixed modifications) and 181 N-linked glycan compositions and can be accessed at the following website: http://nglycositeatlas.biomarkercenter.org/. Prior to intact glycopeptide quantitation using PyQuant,36 GPQuest 2.0 results were filtered to remove peptides above 1% FDR, peptides without the N-linked consensus sequence [N-X-T/S], and peptides without corresponding N-glycan structures. Following PyQuant quantitation, nonquantified peptide spectral matches (PSMs) were removed, and median-normalized PSM H/L ratios were reported. For peptide-level quantitation, the median H/L ratios of matching PSMs (peptide + N-glycan structure; glycoforms) were calculated, and peptide median-normalized H/L ratios were reported for individual glycoforms. Intact N-linked glycopeptides quantified in at least three replicates were considered for statistical analysis.
Statistical and Functional Pathway Analysis
Quartiles were established for SILAC (LAPC4-WT) and nonSILAC (LAPC4-FUT8 OE) data separately based on reported iBAQ intensity values. To establish a threshold cutoff for confidently identified extracellular vesicle proteins in our dataset, we used a consensus list of 139 extracellular vesicle proteins previously described.37 The reported iBAQ intensity values of these 139 proteins in our dataset were log10 transformed and then fitted with a Gaussian probability density function for both the SILAC (LAPC4-WT) and nonSILAC (LAPC4-FUT8 OE) data separately. The low intensity cutoff is the Gaussian mean, with three standard deviations, which estimates that 99.865% of confidently identified extracellular vesicle protein intensities will be above this cutoff. For gene ontology (GO) assignments, respective protein groups were subjected to functional annotation using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) bioinformatics tool.38,39 Proteins and intact N-linked glycopeptides considered to be significantly increased or decreased include the criteria of an average log 2 median-normalized fold change of ±0.5 and an Benjamini–Hochberg adjusted p value of <0.05.40 Proteins categorized as significantly differentially abundant and above our threshold cutoff were subjected to over-representation enrichment analysis (ORA) using the bioinformatics tool, WebGestalt,41,42 and mapped to REAC-TOME and KEGG pathways. For pathway visualization, we employed KEGG Mapper.43 Nanoparticle tracking analysis results were reported as mode ± s.d., subjected to statistical analyses using Student’s paired t test, and statistical significance was reported as p values and is indicated in the figure legends.
RESULTS AND DISCUSSION
EV Enrichment and Characterization
To investigate the impact of altered FUT8 expression on EV biogenesis and the resulting EV proteome in prostate cancer, we established the cell line models LAPC4 and LNCaP stably overexpressing the FUT8 gene (LAPC4-FUT8 OE and LNCaP-FUT8 OE). In addition, for the LNCaP model, we also established an FUT8 knockdown model by stably expressing shRNA targeting FUT8 mRNA transcripts (LNCaP-FUT8 KD). We evaluated FUT8 expression in each of the cell lines to verify overexpression or knockdown relative to endogenous FUT8 expression in the wild-type (WT) LAPC4 and LNCaP prostate cancer cell lines, respectively (Figure 1A). For EV enrichment, we employed differential ultracentrifugation followed by density gradient separation using an OptiPrep/iodixanol gradient, as shown in Figure 1B and described previously.29 Electron microscopy revealed the morphology associated with exosomes and a vesicle size range of 40–150 nm (Figure 1C). Western blot analysis showed positive staining for the EV marker protein ALIX in all generated preparations (Figure 1D), although we did observe differential abundance in the EV lysates.
Figure 1.
Overview of prostate cancer cell line models with altered FUT8 expression and EV characterization. (A) Western blots of FUT8 overexpression and knockdown in whole cell lysates from LAPC4 and LNCaP prostate cancer cell models. (B) Differential ultracentrifugation strategy for EV enrichment. (C) Electron microscopy image of negatively stained LAPC4-WT and LAPC4-FUT8 EVs. Scale bars = 100 nm. (D) Western blots of known EV marker ALIX in EV lysates derived from LAPC4 and LNCaP prostate cancer cell models.
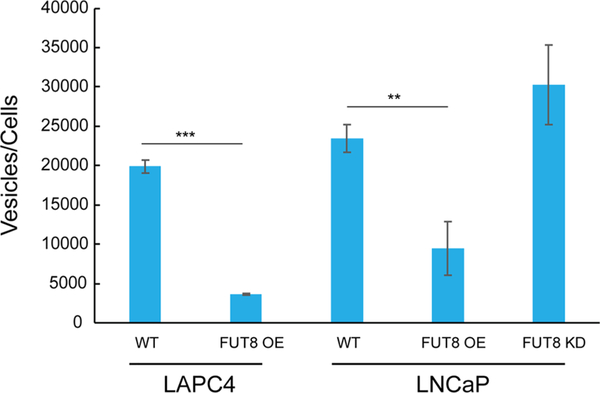
Next, we utilized particle tracking via a NanoSight device to determine vesicle size and number, revealing an overall mode vesicle size of 113 nm (±6 nm) for LAPC4-FUT8 OE EVs and 114 nm (±2 nm) for LAPC4-WT EVs (Figure S1 and Table S1). EVs from LNCaP cells were slightly larger, observing an overall mode vesicle size of 132 nm (±2 nm), 143 nm (±3 nm), and 131 nm (±5 nm) for WT, FUT8-OE, and FUT8-KD EVs, respectively. To evaluate the number of vesicles actively released from each cell line, we measured the total number of vesicles observed in the extracellular space per number of cells in the corresponding cell culture (Figure 2 and Table S2). In both cell models, overexpression of cellular FUT8 resulted in a significant (LAPC4: p < 0.0001; LNCaP: p < 0.005) decrease in the number of vesicles observed in the extracellular space relative to the WT cell lines. Interestingly, in the LNCaP cell model, when cellular FUT8 was reduced by FUT8 knockdown, a corresponding increase in the number of vesicles in the extracellular space was observed; however, this was not found to be significant. Taken together, these results indicate that aberrant cellular FUT8 expression did not affect vesicle size but could impact the number of vesicles being released into the extracellular space.
Figure 2.
Impact of FUT8 expression of extracellular vesicle abundance. The EV number was normalized to the cell number. Values are mean ± standard deviation; all values are representative of three independent experiments with three replicates (***p < 0.0001; **p < 0.005).
Proteomic Analysis of FUT8 Overexpression in LAPC4 EVs
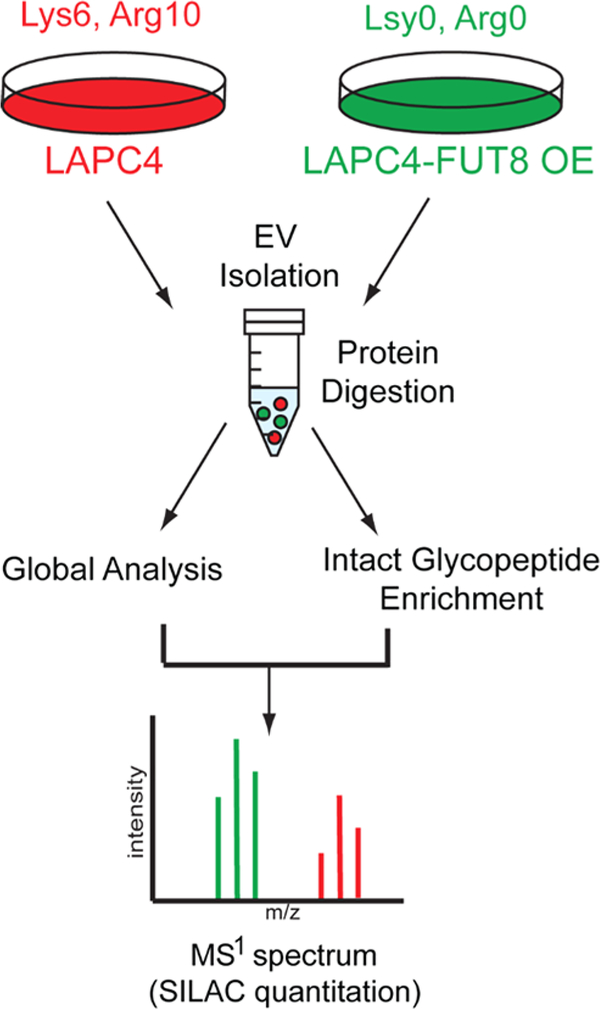
To further characterize the impact of aberrant cellular FUT8 expression on the EV proteome in prostate cancer, we employed a double SILAC quantitation strategy (Figure 3). LAPC4-FUT8 OE cells were considered “light” (L-K0R0) containing endogenous lysine and arginine, while LAPC4-WT cells were isotopically labeled with “heavy” (H-K6R10) amino acids. EVs were enriched from the media supernatant of each cell line, mixed in a 1:1 ratio at the protein level, and subjected to tryptic digestion. To facilitate integrated “global” proteome and glycoproteome analyses, a portion of the tryptic peptides was subjected to basic reversed-phase fractionation prior to LC–MS/MS analysis, while the remaining sample was utilized for intact N-linked glycopeptide enrichment prior to LC–MS/MS analysis. In total, 6043 proteins were identified in the global analysis (protein FDR < 1%; Table S3), with 4157 proteins quantified in all three replicates, which reflects another study that reported comparable number of EV protein identifications when pairing sample fractionation with advanced mass spectrometry instrumentation (2D-LC–MS/MS).37
Figure 3.
Schematic of double SILAC quantitative analysis strategy. LAPC4-WT were isotopically labeled, EVs enriched, and mixed with EVs derived from LAPC4-FUT8 OE cells prior to proteolytic digestion. An aliquot of peptides was subjected to fractionation (global analysis), and the remaining sample was subjected to intact glycopeptide enrichment prior to ESI-LC–MS/MS analysis.
The large number of proteins identified in our study was unanticipated, prompting our examination of how many of the proteins reported in our study have been identified previously in EVs. Using the data repository, Vesiclepedia,44,45 we evaluated only those entries with protein evidence observing ~94% of the proteins in our entire dataset that were previously identified in EVs, and 96% of the proteins leveraged for downstream analysis (quantified across all three replicates) were previously identified in EVs. Next, we employed the intensity-based absolute quantitation (iBAQ) algorithm from the MaxQuant software suite31,32,46 to rank protein abundance of EV proteins in our wild-type and FUT8 overexpression conditions independently and determined the distribution of proteins considered to be positive and negative EV markers in our dataset.47 Dividing our dataset into four equal groupings based on reported iBAQ intensities revealed that the majority of positive EV markers (CD81, CD9, CD63, PDCD6IP (aLIX), TSG101, SDCBP, and FLOT1) were in the highest abundance quartile (Figure S2A, and Table S4). Negative markers showed a wider distribution, with HSP90B1, CANX, and CYC1 observed in the highest abundance quartile, while GOLGA2 and AGO2 were detected in lower abundance quartiles. Considering carcinogenesis results in a high degree of cellular dysregulation, including impacting protein localization48,49 and designation of negative markers based on their respective canonical cellular localization, may misrepresent the degree of EV analyte purity. Further investigation of the gene ontology (GO) annotations of the proteins in each quartile revealed that proteins associated with extracellular exosome were among the top 15 enriched pathways (adjusted p > 0.05; Figure S2C and Table S5) in the first three abundance quartiles and may suggest that lower abundant proteins identified in the remaining quartile are not EV-associated.
Previously, Crescitelli et al. generated a list of 139 proteins commonly identified in EVs,37 and we explored the relative distribution of these consensus EV-associated proteins in our dataset. As shown in Figure S3A,B, the large majority of these consensus EV proteins were identified in the highest abundance quartile, while proteins such as ITGA6, TKT, GSN, APOE, AGO2, and THBS1 were distributed among the remaining quartiles (Table S4). Inferring that the distribution of these consensus proteins might enable a stratification of confident EV-associated protein identifications in our dataset, we plotted the reported iBAQ intensities of consensus EV proteins against protein count and found that the consensus EV proteins displayed a normal distribution (Figure S3C,D). Selecting a threshold cutoff of three standard deviations from the Gaussian mean, we found that ~2100 proteins in our dataset were above this threshold (Figure S3A,B and Table S4). GO annotation analysis revealed that proteins above the threshold were primarily associated with extracellular exosome and focal adhesion, while those below the threshold were associated with cell components including the Golgi apparatus and nucleoplasm (Figure S3E and Table S6). When combined with our quartile analysis, these results further support the hypothesis that lower abundant proteins in our dataset may not be EV-associated, and those proteins below our established threshold warrant further examination or validation before inferring biological relevance.
We examined the proteins that displayed significant differences in abundance between the EV populations, identifying 387 proteins increased in abundance and 603 proteins decreased in abundance in LACP4-FUT8 OE-derived EVs relative to LAPC4-WT-derived EVs (log2 FC > 0.5, adjusted p < 0.05; Table S7). Employing our threshold cutoff revealed that 671 proteins showed differential abundance, including 211 proteins increased in abundance and 460 proteins decreased in abundance in LACP4-FUT8 OE-derived EVs relative to LAPC4-WT-derived EVs (Table S8). Using the bioinformatics tool WebGestalt,41 we performed ontology and pathway analysis of the differentially abundant EV proteins above our cutoff (Table S9). We observed a significant enrichment (FDR ≤ 0.05) of proteins associated with the endosome, including clathrin-mediated endocytosis components (EPN1, AP2A1, ARPC5, and CLTA/B)50 and the endosomal sorting complex required for transport (ESCRT) complexes (ESCRT-0: STAM; ESCRT-I: TSG101, VPS28, VPS37, MVB12, and PDCD6IP (ALIX); ESCRT-II: VPS25 and VPS36; ESCRT-III: all CHMP proteins (1–6) and IST1; VPS4B complex: VPS4A/B and VTA1)51 increased in WT EVs relative to FUT8 OE EVs (Figure S4). Parallel to this result was the observed decreased abundance of extracellular vesicle markers CD63, CD9, and CD81 in FUT8 OE EVs. Considering that EVs are reflective of their parental cell of origin, these results suggest dysregulation of the endocytic pathway in prostate cancer cells overexpressing FUT8. In an independent study, our group showed that overexpression of FUT8 in prostate cancer cells was associated with reduced expression of lysosome biogenesis-associated proteins and increased EGFR expression,11 further supporting the notion of FUT8 expression impacting endosomal activity. Directly related to exosome biogenesis, a previous study indicated that silencing of select ESCRT components impacted the number of vesicles secreted by cells,52 and aberrant ESCRT activity resulting from increased cellular FUT8 expression may explain the reduced number of vesicles secreted by FUT8-transformed prostate cancer cells observed in our study.
Previously, our laboratory results showed that increased FUT8 expression is associated with aggressive, higher-grade prostate carcinoma and can increase the motility and invasiveness of prostate cancer cells.9,10 We observed that several regulators of integrin expression and cell invasiveness increased in FUT8 OE EVs (Figure S5), including ILK, PSAP, PARVB, SPP1, and TLN1,53–57 and the differential abundance of these proteins could serve as EV-based surrogates for inferring elevated cell motility potential. Paradoxically, we observed a significant decrease (FDR ≤ 0.05) of cell surface proteins associated with “focal adhesion” and “ECM–receptor interaction” in FUT8 OE EVs (Figure S5 and Table S9), including multiple integrins, CD47 and CD44. Although the increased abundance of these proteins in WT EVs relative to FUT8 OE exosomes would seem contrary to an aggressive, invasive phenotype associated with FUT8 overexpression,9,10 we have to consider that increased integrin expression is a common feature of cancer cells, and these relative differences would have to be further evaluated when compared to normal or benign prostate epithelial-derived EVs. In addition, the lower abundance of these cell surface receptors in FUT8-transformed EVs may be more reflective of dysregulated receptor internalization via endocytosis, thus impacting select protein routing to exosome EV populations. Future studies will have to examine whether FUT8-transformed EVs display a differential impact on the proliferative and migrative phenotypes of recipient cells relative to EVs derived from cell types expressing basal levels of FUT8. Interestingly, several proteins with a functional role in bone matrix homeostasis, or have been shown to modulate the bone microenvironment, were increased in abundance in FUT8 OE EVs, including TNC and TLN1.58,59 Prostate cancer primarily metastasizes to the bone, and with evidence of EVs having a functional role in priming the metastatic niche,60,61 the presence of these proteins in FUT8-transformed EVs may further support the mechanism of EV-mediated prostate cancer metastasis.
Our data showed that FUT8 OE EVs were significantly enriched for proteins associated with the nucleosome and the cell cycle regulation (FDR ≤ 0.05; Table S9). This included multiple chromatin modifiers such as an ISWI complex component (SMARCA5), those involved in histone methylation (HDAC2), and proteins involved in DNA topology (TOP1 and TOP2A), which have all been previously associated with invasive or aggressive phenotypes in prostate cancer or other cancer types when overexpressed.62–65 We also observed that the CSNK2 complex was elevated in FUT8 OE EVs, along with several proteins associated with the DNA damage response (BANF1, PARP1, RAD50, and MRE11). CSNK2 has been shown to modulate multiple pathways including cell cycle progression and DNA damage repair,66 and the identification of these proteins in EVs potentially reflects active DNA fidelity mechanisms to promote prostate cancer cell survival. Finally, we detected a significant increased abundance of histone proteins in FUT8 OE EVs. Although these proteins are usually associated with the nuclear compartment and chromatin structure, recent reports have indicated that they are enriched in EVs and have a role in EV uptake by recipient cells.67,68 Future studies will have to investigate the spatial distribution of histone proteins in EVs as well as determine if differential abundance of histones in EVs can impact recipient cell internalization. Overall, these results suggest that FUT8 overexpression results in dysregulation of cellular pathways associated with an aggressive prostate cancer phenotype that is consistent with the observed proteome profile of secreted extracellular vesicles.
Intact N-Linked Glycopeptide Analysis of FUT8 Overexpression in LAPC4 EVs
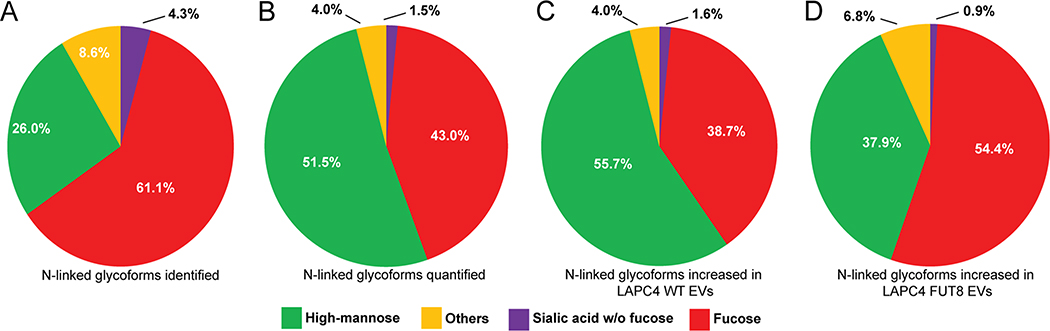
Investigation of the N-linked glycoproteome of LAPC4-WT and LAPC4-FUT8 OE EVs resulted in the identification of 2813 unique intact glycopeptides from 283 glycoproteins (peptide FDR < 1%; Table S10), with quantitation information reported for 1080 intact glycopeptides from 173 glycoproteins in at least one replicate (Table S11) and 328 glycopeptides from 92 glycoproteins quantified in all three replicates (Table S12). We examined the glycan composition of the intact glycopeptides in our dataset, observing that a majority of the identified glycans were fucosylated (61%; Figure 4A). Filtering our data to include only those intact glycopeptides identified in all three replicates, we found high-mannose structures (glycan branching with 5–9 mannose residues) to be the predominant species (51%), with 43% of the quantified glycoforms fucosylated (Figure 4B). In this subset of glycoform species, 124 intact glycopeptides were found to be increased in abundance in WT EVs, while 103 intact glycopeptides were increased in FUT8-transformed EVs (log2 FC > 0.5), observing disparate, inverse high-mannose and fucosylated glycan compositions between the two EV populations (Figure 4C,D). The elevated abundance of fucosylated N-linked glycopeptides in FUT8-transformed EVs relative to WT-derived EVs reflects the cellular-level alterations of N-linked glycosylation and would provide rationale for future exploration of leveraging discriminatory N-linked glycan and glycoproteome profiles for EV-based diagnostics in prostate cancer. Differential abundance of intact glycoforms can be influenced by several factors, including differential abundance of global protein expression and glycosite occupancy.69 To discriminate between these two stoichiometries, we integrated global protein and intact glycopeptide measurements (Table 1). We found that the significant differential abundance (p < 0.05) of glycopeptides derived from EGFR, ITGA1, LGALS3BP, TNC, and IGF2R mirrored the trends of their respective global protein measurements, indicative of global abundance influencing the observe glycosylation measurements. In contrast, the significant differential abundance (p < 0.05) of glycopeptides derived from LAMP1, LAMP2, HSP90B1, and SLIT2 was not detected in the global analysis and reflects alterations in glycosite occupancy. Delineated in our analysis was the degree of microheterogeneity of several glycoproteins at specific glycosites, comprising differences in both glycan composition and abundance. CD63, MFGE8, and FN1 displayed differential global protein abundance between LAPC4-WT and LAPC4-FUT8 EV populations, while glycosite occupancy was much more variable and included disparate patterns of abundance of glycan composition on the same glycosylation site (Table 1). Although previous, independent studies have indicated that the glycosylation of CD63 and FN1 can impact their functionality and cancer cell malignancy,70,71 future studies will have to determine whether altered glycan branching at specific sites can influence the glycoprotein function in a similar manner. Overall, these results show that FUT8 overexpression in prostate cancer cells results in differential glycosite occupancy on glycoproteins as well as glycoprotein expression in secreted extracellular vesicles.
Figure 4.
(A–D) Composition of N-linked glycans on intact glycopeptides (A) identified, (B) quantified, and (C, D) increased in (C) LAPC4-WT EVs (log2 FC > 0.5) and (D) LAPC4-FUT8 EVs (log2 FC > 0.5).
Table 1.
Intact Glycopeptide and Global Protein Abundance Differences between EV Populations Derived from LAPC4-WT and LAPC4-FUT8 OE Cells
| sprotein accession | protein name | gene names | glycoform (N-glycan) | glyco ratio FUT8/WT | global ratio FUT8/WT |
|---|---|---|---|---|---|
| P00523 | epidermal growth factor receptor | EGFR | DSISINATNIK-N2H5F0S0 | −2.41 | −0.73 |
| DSISINATNIK-N2H6F0S0 | −1.59 | ||||
| NCTSISGDLHILPVAFR-N2H6F0S0 | −1.74 | ||||
| P56199 | integrin alpha-1 | ITGA1 | NTTFNVESTKK-N2H6F0S0 | −2.84 | −1.44 |
| NTTFNVESTKK-N2H8F0S0 | −0.66 | ||||
| NTTFNVESTK-N2H6F0S0 | −3.03 | ||||
| SENA SIVISSSNQK-N2H9F0S0 | −1.90 | ||||
| SQNDKFNVSITVK-N2H6F0S0 | −2.26 | ||||
| YNHTGQVIIYR-N2H9F0S0 | −1.86 | ||||
| P11717 | cation-independent mannose-6-phosphate receptor | IGF2R | NGSSIVDLSPLIHR-N2H5F1S0 TNITIVCKPGDIESAPVIR-N2H6F0S0 |
5.46 0.67 |
0.61 |
| Q08380 | galectin-3-binding protein | LGALS3BP | AAIPSAIDTNSSK-N5H6F1S1 | 3.49 | 2.74 |
| P24821 | tenascin | TNC | NTTSYVLR-N4H5F1S0 | 3.86 | 0.75 |
| NTTSYVLR-N4H5F1S1 | 1.48 | ||||
| P11279 | lysosome-associated membrane glycoprotein 1 | LAMP1 | GHTITINFTR-N2H6F0S0 | 0.72 | 0.28 |
| P13473 | lysosome-associated membrane glycoprotein 2 | LAMP2 | VASVININPNTTHSTGSCR-N2H3F1S0 | 2.01 | 0.40 |
| P14625 | endoplasmin | HSP90B1 | HNNDTQHIWESDSNEFSVIADPR-N6H3F0S0 | 1.01 | 0.15 |
| HNNDTQHIWESDSNEFSVIADPR-N2H5F0S0 | −0.79 | ||||
| O94813 | slit homolog 2 protein | SLIT2 | ITCVGNDSFIGLSSVR-N5H4F1S0 | 1.44 | −0.01 |
| ITCVGNDSFIGLSSVR-N5H4F2S0 | −4.64 | ||||
| QAPGQNGTSFHGCIR-N5H4F2S0 | −2.96 | ||||
| P08962 | CD63 antigen | CD63 | NNHTASIIDR-N5H6F1S2 | −2.14 | −1.48 |
| NNHTASIIDR-N2H3F1S0 | 2.06 | ||||
| NNHTASIIDR-N3H3F1S0 | 3.61 | ||||
| NNHTASIIDR-N3H6F1S0 | 3.38 | ||||
| NNHTASIIDR-N3H6F1S1 | 2.34 | ||||
| NNHTASIIDR-N4H5F3S0 | 1.49 | ||||
| NNHTASIIDR-N5H6F2S0 | 1.40 | ||||
| Q08431 | lactadherin | MFGE8 | NNSIPDK-N3H6F0S1 | −3.63 | −0.60 |
| NNSIPDK-N3H6F1S0 | −2.30 | ||||
| NNSIPDK-N4H5F2S0 | −0.67 | ||||
| NNSIPDKQITASSSYK-N2H5F0S0 | −1.08 | ||||
| NNSIPDKQITASSSYK-N3H6F1S0 | −2.21 | ||||
| NNSIPDK-N3H6F2S0 | 2.12 | ||||
| VAYSNDSANWTEYQDPR-N4H4F3S0 | 3.73 | ||||
| VAYSNDSANWTEYQDPR-N4H5F1S0 | 1.03 | ||||
| VAYSNDSANWTEYQDPR-N7H4F0S0 | 1.19 | ||||
| P02751 | fibronectin | FN1 | LDAPTNIQFVNETDSTVIVR-N4H5F1S0 | 3.16 | 0.54 |
| LDAPTNIQFVNETDSTVIVR-N5H5F2S0 | −3.12 | ||||
| LDAPTNIQFVNETDSTVIVR-N6H3F2S0 | −3.41 |
CONCLUSIONS
In this study, we evaluated the impact of increased cellular FUT8 expression on EV biogenesis and the EV proteome in a prostate cancer cell model. Applying an additional criterion of protein abundance in our dataset, quantitative proteomics revealed differential abundance of proteins between LAPC4-WT and LAPC4-FUT8-overexpressed EV populations, with proteins associated with an aggressive prostate cancer phenotype increased in the latter. In contrast, prostate cancer cells overexpressing cellular FUT8 produce fewer extracellular vesicles, and proteomic analysis reveals that a lower abundance of endocytic-related machinery in LAPC4-FUT8 EVs reflects dysregulation of endocytosis in FUT8-transformed prostate cancer cells. Integrating the results obtained in our global and intact glycopeptide analyses delineated glycosite occupancy differences between the two EV populations, concordant global and glycoexpression patterns of select glycoproteins as well as the feature of elevated fucosylated N-linked glycopeptide abundance in the EVs derived from FUT8 overexpressing cells. Overall, this study further supports that aberrant glycosylation can systematically impact cellular processes and highlights the unique insight that is gained by examining glycosylation patterns, in addition global protein profiles, to identify glycosylation-specific features associated with tumorigenesis.
Supplementary Material
Figure S1, NanoSight-determined size distribution of extracellular vesicles derived from LAPC4 and LNCaP prostate cancer cells Figure S2, abundance distribution of EV marker proteins in LAPC4-WT and LAPC4-FUT8 OE extracellular vesicle populations; Figure S3, abundance distribution of consensus EV proteins in LAPC4-WT and LAPC4-FUT8 OE extracellular vesicle populations; Figure S4, KEGG annotation of differentially abundant “endocytosis” proteins; and Figure S5, KEGG annotation of differentially abundant ECM–receptor interaction and focal adhesion proteins (PDF)
Table S1, NanoSight measurements of vesicle size; Table S2, cell count numbers and NanoSight measurements of vesicle number; Table S3, reported MaxQuant search results (including identification and quantitation information); Table S4, reported MaxQuant intensity-based absolute quantitation (iBAQ) results; Table S5, DAVID cell component enrichment analysis results of protein quartiles in LACP4-FUT8 OE and SILAC-labeled LAPC4-WT derived extracellular vesicles; Table S6, DAVID cell component enrichment analysis results of LACP4-FUT8 OE and SILAC-labeled LAPC4-WT derived extracellular vesicle proteins above and below the Gaussian threshold; Table S7, list of significantly differentially abundant proteins between LAPC4-WT and LAPC4-FUT8 extracellular vesicle populations; Table S8, Significantly differentially abundant proteins between LACP4-FUT8 OE and SILAC-labeled LAPC4-WT derived extracellular vesicles above cutoff; Table S9, WebGestalt pathway analysis results; Table S10, GPQuest intact glycopeptide search results; Table S11, PyQuant intact glycopeptide quantitation results; and Table S12, list of differentially abundant intact glycopeptides between LAPC4-WT and LAPC4-FUT8 extracellular vesicle populations (XLSX)
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health, National Cancer Institute, the Early Detection Research Network (EDRN, U01CA152813), and the Clinical Proteomic Tumor Analysis Consortium (CPTAC, U24CA210985). Electron microscopy services reported in this publication were performed at The Johns Hopkins University School of Medicine Microscope Facility supported by the National Center for Research Resources of the National Institutes of Health under award number S10RR026445.
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jproteome.9b00578.
Contributor Information
David J. Clark, Department of Pathology, The Johns Hopkins University School of Medicine, Baltimore 21231, Maryland, United States
Michael Schnaubelt, Department of Pathology, The Johns Hopkins University School of Medicine, Baltimore 21231, Maryland, United States.
Naseruddin Hoti, Department of Pathology, The Johns Hopkins University School of Medicine, Baltimore 21231, Maryland, United States.
Yingwei Hu, Department of Pathology, The Johns Hopkins University School of Medicine, Baltimore 21231, Maryland, United States.
Yangying Zhou, Department of Pathology, The Johns Hopkins University School of Medicine, Baltimore 21231, Maryland, United States.
Mahta Gooya, Department of Pathology, The Johns Hopkins University School of Medicine, Baltimore 21231, Maryland, United States.
Hui Zhang, Department of Pathology, The Johns Hopkins University School of Medicine, Baltimore 21231, Maryland, United States.
REFERENCES
- (1).Siegel RL; Miller KD; Jemal A Cancer Statistics, 2017. CA-Cancer J. Clin 2017, 67, 7–30. [DOI] [PubMed] [Google Scholar]
- (2).Etzioni R; Tsodikov A; Mariotto A; Szabo A; Falcon S; Wegelin J; diTommaso D; Karnofski K; Gulati R; Penson DF; Feuer E Quantifying the Role of PSA Screening in the US Prostate Cancer Mortality Decline. Cancer, Causes Control 2008, 19, 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Catalona WJ; Smith DS; Ratliff TL; Basler JW Detection of Organ-Confined Prostate Cancer Is Increased through Prostate-Specific Antigen-Based Screening. JAMA 1993, 270, 948–954. [PubMed] [Google Scholar]
- (4).Tabayoyong W; Abouassaly R Prostate Cancer Screening and the Associated Controversy. Surg. Clin. North Am. 2015, 95, 1023–1039. [DOI] [PubMed] [Google Scholar]
- (5).Meany DL; Zhang Z; Sokoll LJ; Zhang H; Chan DW Glycoproteomics for Prostate Cancer Detection : Changes in Serum PSA Glycosylation Patterns. J. Proteome Res. 2009, 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Li QK; Chen L; Ao M-H; Chiu JH; Zhang Z; Zhang H; Chan DW Serum Fucosylated Prostate-Specific Antigen (PSA) Improves the Differentiation of Aggressive from Non-Aggressive Prostate Cancers. Theranostics 2015, 5, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Fujita K; Hayashi T; Matsuzaki K; Nakata W; Masuda M; Kawashima A; Ujike T; Nagahara A; Tsuchiya M; Kobayashi Y; Nojima S; Uemura M; Morii E; Miyoshi E; Nonomura N Decreased Fucosylated PSA as a Urinary Marker for High Gleason Score Prostate Cancer. Oncotarget 2016, 7, 56643–56649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Pihikova D; Kasak P; Kubanikova P; Sokol R; Tkac J Aberrant Sialylation of a Prostate-Specific Antigen: Electrochemical Label-Free Glycoprofiling in Prostate Cancer Serum Samples. Anal. Chim. Acta 2016, 934, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Shah P; Wang X; Yang W; Toghi Eshghi S; Sun S; Hoti N; Chen L; Yang S; Pasay J; Rubin A; Zhang H Integrated Proteomic and Glycoproteomic Analyses of Prostate Cancer Cells Reveal Glycoprotein Alteration in Protein Abundance and Glycosylation. Mol. Cell. Proteomics 2015, 14, 2753–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Wang X; Chen J; Li QK; Peskoe SB; Zhang B; Choi C; Platz EA; Zhang H Overexpression of α (1,6) Fucosyltransferase Associated with Aggressive Prostate Cancer. Glycobiology 2014, 24, 935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Höti N; Yang S; Hu Y; Shah P; Haffner MC; Zhang H Overexpression of α (1,6) Fucosyltransferase in the Development of Castration-Resistant Prostate Cancer Cells. Prostate Cancer Prostatic Dis. 2018, 21, 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Théry C Exosomes: Secreted Vesicles and Intercellular Communications. F1000 Biol. Rep. 2011, 3, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Vlassov AV; Magdaleno S; Setterquist R; Conrad R Exosomes: Current Knowledge of Their Composition, Biological Functions, and Diagnostic and Therapeutic Potentials. Biochim. Biophys. Acta, Gen. Subj 2012, 1820, 940–948. [DOI] [PubMed] [Google Scholar]
- (14).Patel GK; Khan MA; Zubair H; Srivastava SK; Khushman M; Singh S; Singh AP Comparative Analysis of Exosome Isolation Methods Using Culture Supernatant for Optimum Yield, Purity and Downstream Applications. Sci. Rep 2019, 9, 5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Trams EG; Lauter CJ; Salem N Jr.; Heine U Exfoliation of Membrane Ecto-Enzymes in the Form of Micro-Vesicles. Biochim. Biophys. Acta, Biomembr. 1981, 645, 63–70. [DOI] [PubMed] [Google Scholar]
- (16).Raposo G; Stoorvogel W Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Kao C-Y; Papoutsakis ET Extracellular Vesicles: Exosomes, Microparticles, Their Parts, and Their Targets to Enable Their Biomanufacturing and Clinical Applications. Curr. Opin. Biotechnol. 2019, 60, 89–98. [DOI] [PubMed] [Google Scholar]
- (18).Baietti MF; Zhang Z; Mortier E; Melchior A; Degeest G; Geeraerts A; Ivarsson Y; Depoortere F; Coomans C; Vermeiren E; Zimmermann P; David G Syndecan–syntenin–ALIX Regulates the Biogenesis of Exosomes. Nat. Cell Biol. 2012, 14, 677–685. [DOI] [PubMed] [Google Scholar]
- (19).Pap E; Pállinger É; Falus A The Role of Membrane Vesicles in Tumorigenesis. Crit. Rev. Oncol. Hematol. 2011, 79, 213–223. [DOI] [PubMed] [Google Scholar]
- (20).Skog J; Würdinger T; van Rijn S; Meijer DH; Gainche L; Curry WT Jr.; Carter BS; Krichevsky AM; Breakefield XO Glioblastoma Microvesicles Transport RNA and Proteins That Promote Tumour Growth and Provide Diagnostic Biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Clark DJ; Fondrie WE; Yang A; Mao L Triple SILAC Quantitative Proteomic Analysis Reveals Differential Abundance of Cell Signaling Proteins between Normal and Lung Cancer-Derived Exosomes. J. Proteomics 2016, 133, 161–169. [DOI] [PubMed] [Google Scholar]
- (22).Luga V; Zhang L; Viloria-Petit AM; Ogunjimi AA; Inanlou MR; Chiu E; Buchanan M; Hosein AN; Basik M; Wrana JL Exosomes Mediate Stromal Mobilization of Autocrine Wnt-PCP Signaling in Breast Cancer Cell Migration. Cell 2012, 151, 1542–1556. [DOI] [PubMed] [Google Scholar]
- (23).Xiao X; Yu S; Li S; Wu J; Ma R; Cao H; Zhu Y; Feng J Exosomes: Decreased Sensitivity of Lung Cancer A549 Cells to Cisplatin. PLoS One 2014, 9, No. e89534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Kucharzewska P; Christianson HC; Welch JE; Svensson KJ; Fredlund E; Ringnér M; Mörgelin M; Bourseau-Guilmain E; Bengzon J; Belting M Exosomes Reflect the Hypoxic Status of Glioma Cells and Mediate Hypoxia-Dependent Activation of Vascular Cells during Tumor Development. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 7312–7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Parolini I; Federici C; Raggi C; Lugini L; Palleschi S; De Milito A; Coscia C; Iessi E; Logozzi M; Molinari A; Colone M; Tatti M; Sargiacomo M; Fais S Microenvironmental pH Is a Key Factor for Exosome Traffic in Tumor Cells. J. Biol. Chem. 2009, 284, 34211–34222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Hung ME; Leonard JN Stabilization of Exosome-Targeting Peptides via Engineered Glycosylation. J. Biol. Chem. 2015, 290, 8166–8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Liang Y; Eng WS; Colquhoun DR; Dinglasan RR; Graham DR; Mahal LK Complex N-Linked Glycans Serve as a Determinant for Exosome/Microvesicle Cargo Recruitment. J. Biol. Chem. 2014, 289, 32526–32537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Tu C-F; Wu M-Y; Lin Y-C; Kannagi R; Yang R-B FUT8 Promotes Breast Cancer Cell Invasiveness by Remodeling TGF-β Receptor Core Fucosylation. Breast Cancer Res. 2017, 19, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Clark DJ; Fondrie WE; Liao Z; Hanson PI; Fulton A; Mao L; Yang AJ Redefining the Breast Cancer Exosome Proteome by Tandem Mass Tag Quantitative Proteomics and Multivariate Cluster Analysis. Anal. Chem. 2015, 87, 10462–10469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Clark DJ; Hu Y; Bocik W; Chen L; Schnaubelt M; Roberts R; Shah P; Whiteley G; Zhang H Evaluation of NCI-7 Cell Line Panel as a Reference Material for Clinical Proteomics. J. Proteome Res. 2018, 17,2205–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Cox J; Mann M MaxQuant Enables High Peptide Identification Rates, Individualized P.p.b.-Range Mass Accuracies and Proteome-Wide Protein Quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [DOI] [PubMed] [Google Scholar]
- (32).Tyanova S; Temu T; Cox J The MaxQuant Computational Platform for Mass Spectrometry-Based Shotgun Proteomics. Nat. Protoc. 2016, 11, 2301–2319. [DOI] [PubMed] [Google Scholar]
- (33).Perez-Riverol Y; Csordas A; Bai J; Bernal-Llinares M; Hewapathirana S; Kundu DJ; Inuganti A; Griss J; Mayer G; Eisenacher M; Pérez E; Uszkoreit J; Pfeuffer J; Sachsenberg T; Yilmaz Ş; Tiwary S; Cox J; Audain E; Walzer M; Jarnuczak AF; Ternent T; Brazma A; Vizcaíno JA The PRIDE Database and Related Tools and Resources in 2019: Improving Support for Quantification Data. Nucleic Acids Res. 2019, 47, D442–D450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Hu Y; Shah P; Clark DJ; Ao M; Zhang H Reanalysis of Global Proteomic and Phosphoproteomic Data Identified a Large Number of Glycopeptides. Anal. Chem. 2018, 90, 8065–8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Chambers MC; Maclean B; Burke R; Amodei D; Ruderman DL; Neumann S; Gatto L; Fischer B; Pratt B; Egertson J; Hoff K; Kessner D; Tasman N; Shulman N; Frewen B; Baker TA; Brusniak M-Y; Paulse C; Creasy D; Flashner L; Kani K; Moulding C; Seymour SL; Nuwaysir LM; Lefebvre B; Kuhlmann F; Roark J; Rainer P; Detlev S; Hemenway T; Huhmer A; Langridge J; Connolly B; Chadick T; Holly K; Eckels J; Deutsch EW; Moritz RL; Katz JE; Agus DB; MacCoss M; Tabb DL; Mallick P A Cross-Platform Toolkit for Mass Spectrometry and Proteomics. Nat. Biotechnol. 2012, 30, 918–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Mitchell CJ; Kim M-S; Na CH; Pandey A PyQuant: A Versatile Framework for Analysis of Quantitative Mass Spectrometry Data. Mol. Cell. Proteomics 2016, 15, 2829–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Crescitelli R; Lässer C; Jang SC; Cvjetkovic A; Malmhäll C; Karimi N; Höög JL; Johansson I; Fuchs J; Thorsell A; Gho YS; Olofsson Bagge R; Lötvall J Subpopulations of Extracellular Vesicles from Human Metastatic Melanoma Tissue Identified by Quantitative Proteomics after Optimized Isolation. J. Extracell. Vesicles 2020, 9, 1722433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Huang DW; Sherman BT; Lempicki RA Systematic and Integrative Analysis of Large Gene Lists Using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [DOI] [PubMed] [Google Scholar]
- (39).Huang DW; Sherman BT; Lempicki RA Bioinformatics Enrichment Tools: Paths toward the Comprehensive Functional Analysis of Large Gene Lists. Nucleic Acids Res. 2009, 37, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Benjamini Y; Hochberg Y Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar]
- (41).Wang J; Vasaikar S; Shi Z; Greer M; Zhang B WebGestalt 2017: A More Comprehensive, Powerful, Flexible and Interactive Gene Set Enrichment Analysis Toolkit. Nucleic Acids Res. 2017, 45, W130–W137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Liao Y; Wang J; Jaehnig EJ; Shi Z; Zhang B WebGestalt 2019: Gene Set Analysis Toolkit with Revamped UIs and APIs. Nucleic Acids Res. 2019, W199–W205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Kanehisa M; Sato Y KEGG Mapper for Inferring Cellular Functions from Protein Sequences. Protein Sci. 2020, 29, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Kalra H; Simpson RJ; Ji H; Aikawa E; Altevogt P; Askenase P; Bond VC; Borraàs FE; Breakefield X; Budnik V; Buzas E; Camussi G; Clayton A; Cocucci E; Falcon-Perez JM; Gabrielsson S; Gho YS; Gupta D; Harsha HC; Hendrix A; Hill AF; Inal JM; Jenster G; Krämer-Albers E-M; Lim SK; Llorente A; Lötvall J; Marcilla A; Mincheva-Nilsson L; Nazarenko I; Nieuwland R; Nolte-’t Hoen ENM; Pandey A; Patel T; Piper MG; Pluchino S; Keshava Prasad TS; Rajendran L; Raposo G; Record M; Reid GE; Sánchez-Madrid F; Schiffelers RM; Siljander P; Stensballe A; Stoorvogel W; Taylor D; Thery C; Valadi H; van Balkom BWM; Vázquez J; Vidal M; Wauben MHM; Yáñez-Mó M; Zoeller M; Mathivanan S Vesiclepedia: A Compendium for Extracellular Vesicles with Continuous Community Annotation. PLoS Biol. 2012, 10, 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Pathan M; Fonseka P; Chitti SV; Kang T; Sanwlani R; Van Deun J; Hendrix A; Mathivanan S Vesiclepedia 2019: A Compendium of RNA, Proteins, Lipids and Metabolites in Extracellular Vesicles. Nucleic Acids Res. 2019, 47, D516–D519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Schwanhäusser B; Busse D; Li N; Dittmar G; Schuchhardt J; Wolf J; Chen W; Selbach M Global Quantification of Mammalian Gene Expression Control. Nature 2011, 473, 337–342. [DOI] [PubMed] [Google Scholar]
- (47).Lötvall J; Hill AF; Hochberg F; Buzás EI; Di Vizio D; Gardiner C; Gho YS; Kurochkin IV; Mathivanan S; Quesenberry P; Sahoo S; Tahara H; Wauben MH; Witwer KW; Théry C Minimal Experimental Requirements for Definition of Extracellular Vesicles and Their Functions: A Position Statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Wang X; Li S Protein Mislocalization: Mechanisms, Functions and Clinical Applications in Cancer. Biochim. Biophys. Acta, Rev. Cancer 2014, 1846, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Min K-W; Lee S-H; Baek SJ Moonlighting Proteins in Cancer. Cancer Lett. 2016, 370, 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Kaksonen M; Roux A Mechanisms of Clathrin-Mediated Endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [DOI] [PubMed] [Google Scholar]
- (51).Henne WM; Buchkovich NJ; Emr SD The ESCRT Pathway. Dev. Cell 2011, 21, 77–91. [DOI] [PubMed] [Google Scholar]
- (52).Colombo M; Moita C; van Niel G; Kowal J; Vigneron J; Benaroch P; Manel N; Moita LF; Théry C; Raposo G Analysis of ESCRT Functions in Exosome Biogenesis, Composition and Secretion Highlights the Heterogeneity of Extracellular Vesicles. J. Cell Sci. 2013, 126, 5553–5565. [DOI] [PubMed] [Google Scholar]
- (53).Qian Y; Zhong X; Flynn DC; Zheng JZ; Qiao M; Wu C; Dedhar S; Shi X; Jiang B-H ILK Mediates Actin Filament Rearrangements and Cell Migration and Invasion through PI3K/ Akt/Rac1 Signaling. Oncogene 2005, 24, 3154–3165. [DOI] [PubMed] [Google Scholar]
- (54).Hu S; Delorme N; Liu Z; Liu T; Velasco-Gonzalez C; Garai J; Pullikuth A; Koochekpour S Prosaposin down-Modulation Decreases Metastatic Prostate Cancer Cell Adhesion, Migration, and Invasion. Mol. Cancer 2010, 9, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Sakamoto S; McCann RO; Dhir R; Kyprianou N Talin1 Promotes Tumor Invasion and Metastasis via Focal Adhesion Signaling and Anoikis Resistance. Cancer Res. 2010, 70, 1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Eslami A; Miyaguchi K; Mogushi K; Watanabe H; Okada N; Shibuya H; Mizushima H; Miura M; Tanaka H PARVB Overexpression Increases Cell Migration Capability and Defines High Risk for Endophytic Growth and Metastasis in Tongue Squamous Cell Carcinoma. Br. J. Cancer 2015, 112, 338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Khodavirdi AC; Song Z; Yang S; Zhong C; Wang S; Wu H; Pritchard C; Nelson PS; Roy-Burman P Increased Expression of Osteopontin Contributes to the Progression of Prostate Cancer. Cancer Res. 2006, 66, 883–888. [DOI] [PubMed] [Google Scholar]
- (58).San Martin R; Pathak R; Jain A; Jung SY; Hilsenbeck SG; Piña-Barba MC; Sikora AG; Pienta KJ; Rowley DR Tenascin-C and Integrin α9 Mediate Interactions of Prostate Cancer with the Bone Microenvironment. Cancer Res. 2017, 77, 5977–5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Jin J-K; Tien P-C; Cheng C-J; Song JH; Huang C; Lin S-H; Gallick GE Talin1 Phosphorylation Activates β1 Integrins: A Novel Mechanism to Promote Prostate Cancer Bone Metastasis. Oncogene 2015, 34, 1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Abate-Shen C; Shen MM Molecular Genetics of Prostate Cancer. Genes Dev. 2000, 14, 2410–2434. [DOI] [PubMed] [Google Scholar]
- (61).Peinado H; Alečković M; Lavotshkin S; Matei I; Costa-Silva B; Moreno-Bueno G; Hergueta-Redondo M; Williams C; García-Santos G; Ghajar CM; Nitadori-Hoshino A; Hoffman C; Badal K; Garcia BA; Callahan MK; Yuan J; Martins VR; Skog J; Kaplan RN; Brady MS; Wolchok JD; Chapman PB; Kang Y; Bromberg J; Lyden D Melanoma Exosomes Educate Bone Marrow Progenitor Cells toward a pro-Metastatic Phenotype through MET. Nat. Med. 2012, 18, 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Jin Q; Mao X; Li B; Guan S; Yao F; Jin F Overexpression of SMARCA5 Correlates with Cell Proliferation and Migration in Breast Cancer. Tumor Biol. 2015, 36, 1895–1902. [DOI] [PubMed] [Google Scholar]
- (63).Zhang W; Xu J DNA Methyltransferases and Their Roles in Tumorigenesis. Biomarker Res. 2017, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Weichert W; Röske A; Gekeler V; Beckers T; Stephan C; Jung K; Fritzsche FR; Niesporek S; Denkert C; Dietel M; Kristiansen G Histone Deacetylases 1, 2 and 3 Are Highly Expressed in Prostate Cancer and HDAC2 Expression Is Associated with Shorter PSA Relapse Time after Radical Prostatectomy. Br. J. Cancer 2008, 98, 604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Schaefer-Klein JL; Murphy SJ; Johnson SH; Vasmatzis G; Kovtun IV Topoisomerase 2 Alpha Cooperates with Androgen Receptor to Contribute to Prostate Cancer Progression. PLoS One 2015, 10, No. e0142327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Montenarh M Protein Kinase CK2 in DNA Damage and Repair. Transl. Cancer Res. 2016, 5, 49–63. [Google Scholar]
- (67).Ortiz A Not All Extracellular Vesicles Were Created Equal: Clinical Implications. Ann. Transl. Med 2017, 5, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Ochieng J; Nangami G; Sakwe A; Rana T; Ingram S; Goodwin JS; Moye C; Lammers P; Adunyah SE Extracellular Histones Are the Ligands for the Uptake of Exosomes and Hydroxyapatite-Nanoparticles by Tumor Cells via Syndecan-4. FEBS Lett. 2018, 592, 3274–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Sun S; Zhang H Large-Scale Measurement of Absolute Protein Glycosylation Stoichiometry. Anal. Chem. 2015, 87, 6479–6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Hsiao C-T; Cheng H-W; Huang C-M; Li H-R; Ou M-H; Huang J-R; Khoo K-H; Yu HW; Chen Y-Q; Wang Y-K; Chiou A; Kuo J-C Fibronectin in Cell Adhesion and Migration via N-Glycossylation. Oncotarget 2017, 8, 70653–70668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Tominaga N; Hagiwara K; Kosaka N; Honma K; Nakagama H; Ochiya T RPN2-Mediated Glycosylation of Tetraspanin CD63 Regulates Breast Cancer Cell Malignancy. Mol. Cancer 2014, 13, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, NanoSight-determined size distribution of extracellular vesicles derived from LAPC4 and LNCaP prostate cancer cells Figure S2, abundance distribution of EV marker proteins in LAPC4-WT and LAPC4-FUT8 OE extracellular vesicle populations; Figure S3, abundance distribution of consensus EV proteins in LAPC4-WT and LAPC4-FUT8 OE extracellular vesicle populations; Figure S4, KEGG annotation of differentially abundant “endocytosis” proteins; and Figure S5, KEGG annotation of differentially abundant ECM–receptor interaction and focal adhesion proteins (PDF)
Table S1, NanoSight measurements of vesicle size; Table S2, cell count numbers and NanoSight measurements of vesicle number; Table S3, reported MaxQuant search results (including identification and quantitation information); Table S4, reported MaxQuant intensity-based absolute quantitation (iBAQ) results; Table S5, DAVID cell component enrichment analysis results of protein quartiles in LACP4-FUT8 OE and SILAC-labeled LAPC4-WT derived extracellular vesicles; Table S6, DAVID cell component enrichment analysis results of LACP4-FUT8 OE and SILAC-labeled LAPC4-WT derived extracellular vesicle proteins above and below the Gaussian threshold; Table S7, list of significantly differentially abundant proteins between LAPC4-WT and LAPC4-FUT8 extracellular vesicle populations; Table S8, Significantly differentially abundant proteins between LACP4-FUT8 OE and SILAC-labeled LAPC4-WT derived extracellular vesicles above cutoff; Table S9, WebGestalt pathway analysis results; Table S10, GPQuest intact glycopeptide search results; Table S11, PyQuant intact glycopeptide quantitation results; and Table S12, list of differentially abundant intact glycopeptides between LAPC4-WT and LAPC4-FUT8 extracellular vesicle populations (XLSX)