Abstract
Helicobacter pylori, a WHO class I carcinogen, is one of the most successful human pathogens colonizing the stomach of over 4.4 billion of the world’s population. Antibiotic therapy represents the best solution but poor response rates have hampered the elimination of H. pylori. A growing body of evidence suggests that H. pylori forms biofilms, but the role of this growth mode in infection remains elusive. Here, we demonstrate that H. pylori cells within a biofilm are tolerant to multiple antibiotics in a manner that depends partially on extracellular proteins. Biofilm-forming cells were tolerant to multiple antibiotics that target distinct pathways, including amoxicillin, clarithromycin, and tetracycline. Furthermore, this tolerance was significantly dampened following proteinase K treatment. These data suggest that H. pylori adapts its phenotype during biofilm growth resulting in decreased antibiotic susceptibility but this tolerance can be partially ameliorated by extracellular protease treatment.
Keywords: Helicobacter pylori, biofilms, antibiotic tolerance, extracellular proteins, proteinase K
1. Introduction
Helicobacter pylori chronically colonizes the stomachs of approximately half of the global population [1]. Although the prevalence differs between nations, infection rates remain high in developing countries (80–90%). Infections are often asymptomatic, although chronic infections can lead to gastritis, peptic ulcers, and may even develop to mucosa-associated lymphoid tissue lymphoma and gastric cancer. Gastric cancer kills over 700,000 people per year worldwide and has an estimated economic burden of over 6 billion dollars in the United State of America [1,2].
Helicobacter pylori infections are treatable through antibiotic therapy, which can reduce the incidence of cancers and ulcerations [3]. Despite the availability of antibiotic interventions, the successful eradication of H. pylori remains challenging [4]. Typical therapy consists of a 10–14-day course of proton pump inhibitor (PPI) and two to three antibiotics (clarithromycin, metronidazole, amoxicillin, levofloxacin, or tetracycline). Although combination therapies are effective in the majority of patients, ~15–25% of cases require additional rounds of treatment to resolve the infection [5,6,7].
There are two leading theories as to why H. pylori infections are difficult to cure: antibiotic resistance and antibiotic tolerance. Resistance of commonly used antibiotics to treat H. pylori infections has significantly increased in recent years, and more than doubling during the past 20 years in Europe [8,9]. In some countries such as Austria, Hungary, and Portugal, resistance to clarithromycin has increased substantially (36.6%, 33.3%, and 31.5%, respectively), and has limited the viability of this drug for routine treatment [8]. In the United States, the prevalence of clarithromycin resistance exceeds 40% in Northwestern hospitals [10]. To address the alarming rate at which these microbes are developing resistance, the World Health Organization (WHO) recently classified clarithromycin-resistant H. pylori as a high priority for antibiotic research and the development of alternative therapies [10].
Conditional antibiotic tolerance also certainly plays a role. H. pylori are rarely resistant to all antibiotics used [6], and therapies are dramatically improved by the addition of PPI [11]. Although the PPI mechanism is not fully understood, one role for PPIs seems to be to promote H. pylori growth and thus render it more susceptible to antibiotic treatment [12].
The in vivo growth state of H. pylori is not well understood, but a common state of growth associated with antibiotic tolerance is formation of biofilm [13,14,15,16]. Biofilm-forming cells of many microbes can be 10 to 10,000 times more tolerant to antibiotics than their planktonic counterparts [13,17,18]. Recent studies have suggested that biofilm-grown H. pylori are tolerant to the commonly used antibiotics clarithromycin, amoxicillin, and metronidazole, and these conditions lead H. pylori to express high levels of antibiotic-associated efflux pumps [19,20]. These studies examined a limited set of H. pylori clinical strains, so it is not yet clear whether this tolerance mechanism is widespread. This finding suggests, however, that biofilm formation may contribute to H. pylori persistence and treatment failure.
Biofilms are a major global challenge to modern medicine and their role in antibiotic tolerance and resistance is no longer questioned. In H. pylori, however, little has been done to explore the role and mechanisms involved in biofilm-mediated antibiotic tolerance. In the present study, we evaluated the antibiotic susceptibility of the H. pylori model strain G27, which is a robust biofilm-forming strain and tested the hypothesis that extracellular proteins might play a role in antibiotic tolerance.
2. Results
2.1. H. Pylori G27 Biofilm Cells are Antibiotic Tolerant
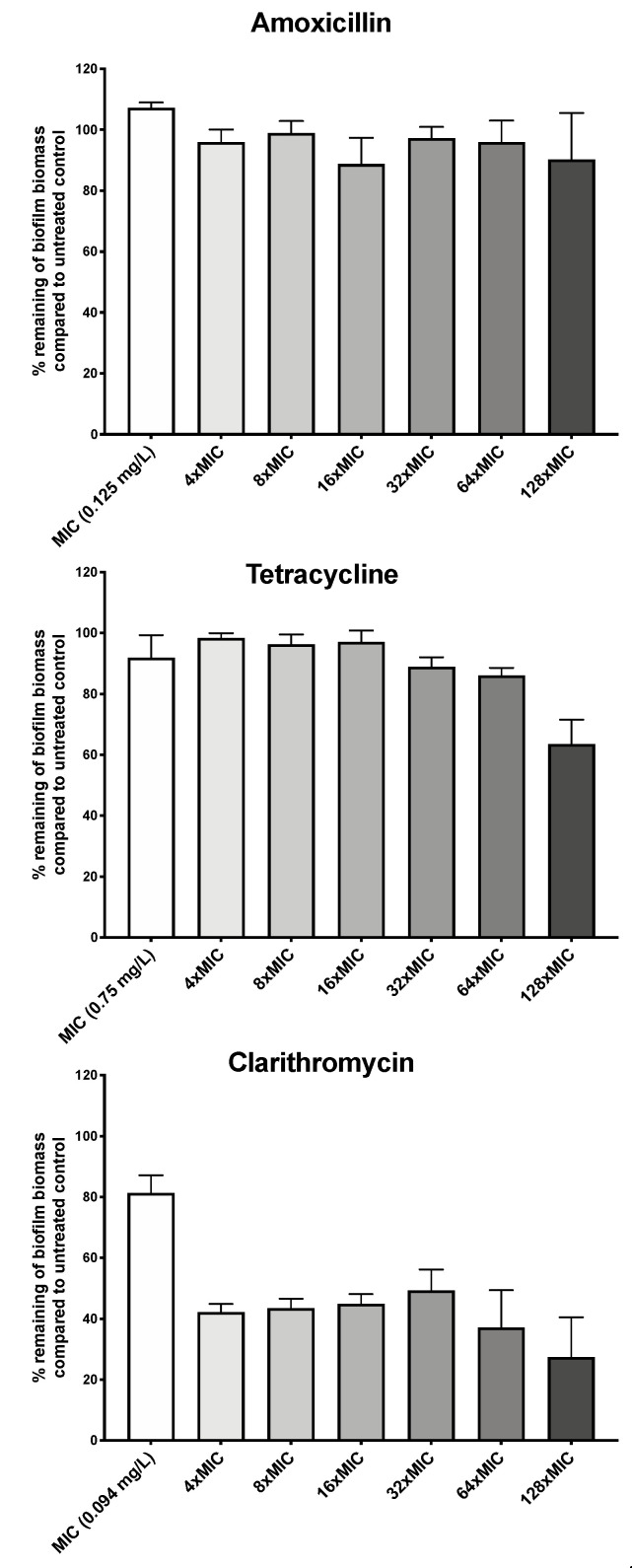
We first asked whether H. pylori G27 biofilms were antibiotic tolerant by determining susceptibility of biofilm and planktonic cells to three clinically relevant antibiotics: the macrolide clarithromycin, the β-lactam amoxicillin, and tetracycline. H. pylori G27 biofilms were formed for 48 h, left untreated or treated with antibiotic for another 24 h, and then biofilm biomass was evaluated using the crystal violet assay as described in the Methods. Prior to biofilm treatment, we determined the H. pylori G27 minimum inhibitory concentrations (MIC) for each antibiotic using the ETEST strip method. We found that the MICs for clarithromycin was 0.094 mg/L, amoxicillin was 0.125 mg/L, and tetracycline was 0.75 mg/L. We then used these amounts to guide treatment of H. pylori G27 biofilm. Generally, antibiotic treatment had some effect but did not fully eliminate the biofilms. Amoxicillin treatment did not clearly diminish the biofilm biomass at up to 128 times the MIC (Figure 1), whereas tetracycline caused no effect at up to 64 times the MIC and a significant 30% decrease at 128 times the MIC (Figure 1). Clarithromycin was somewhat more effective, causing a 60% decrease in biomass at even four times the MIC, but still leaving 30% of the biomass at even the highest dose (Figure 1). Overall, these data suggest that biofilm H. pylori are not eliminated by high antibiotic dose.
Figure 1.
H. pylori biofilm growth is associated with high level tolerance towards amoxicillin, tetracycline, and clarithromycin. The percentage of remaining H. pylori G27 biofilm biomass after exposure to different concentration of amoxicillin, tetracycline, or clarithromycin compared to untreated control. Biofilms were grown for 48 h and then, exposed to antibiotics at the indicated concentrations for 24 h. Control/untreated biofilms were exposed to fresh media without antibiotic for the same time period. Biofilm were quantified using crystal violet. The percent biomass remaining was determined relative to the untreated control. Experiments were performed three independent times with at least 8 technical replicates for each. Error bars represent standard errors of the mean.
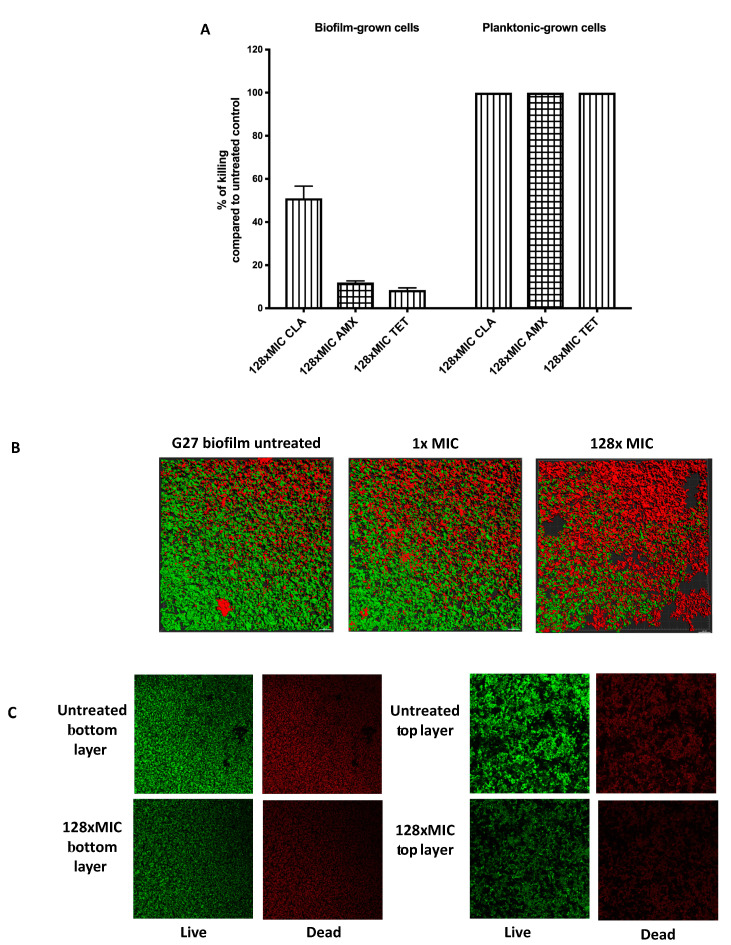
We next assessed the viability of biofilm and planktonic cells after exposure to the antibiotics. This analysis was accomplished by plating the samples to analyze live cells by colony-forming units (CFU), compared to untreated controls to determine the percent killing (Figure 2A). As expected, planktonic cells were completely killed by exposure to 128 times MIC levels of each antibiotic, resulting in no viable cells (Figure 2A). Conversely, cells grown as biofilms tolerated exposure to amoxicillin or tetracycline up to 128 times MIC, with only 12.5% or 7.5% of biofilm cell populations killed, respectively (Figure 2A). Clarithromycin was more able to kill biofilm cells with exposure at 128 times MIC, eliminating approximately half the population (Figure 2A). These results suggest that a substantial number of H. pylori biofilm cells survive high level antibiotic exposure.
Figure 2.
Viability of H. pylori biofilm and planktonic cells after antibiotic exposure. (A) H. pylori G27 were grown in biofilm or planktonic conditions for 48 h and then treated or not treated with either clarithromycin (CLR), amoxicillin (AMX), and tetracycline (TET) for an additional 24 h, as described in Figure 1. After treatment, samples were plated to determine colony-forming units (CFU) and compared to the untreated control, to determine the % killing. (B) Live and dead biomasses in H. pylori G27 biofilm treated or not treated with clarithromycin. Biofilms were visualized by confocal laser scanning microscopy (CLSM) with LIVE/DEAD viability strain. Viable cells exhibit green fluorescence, whereas dead and damaged cells exhibit red fluorescence. These images are three-dimensional (3D) reconstructions from an average of 19 image stacks assembled using Imaris software. (C) Images were taken from the bottom and top layers of H. pylori G27 biofilm treated or not treated with 128× MIC of clarithromycin.
We further evaluated the impact of clarithromycin on biofilms using confocal microscopy (Figure 2B,C). As shown previously [21], untreated H. pylori biofilms contain both live and dead cells (Figure 2B,C). After high dose treatment, we observed a large number of dead cells. We analyzed the distribution of dead and surviving cells after clarithromycin exposure, but did not observe any noticeable differences between cells at the top or bottom layers (Figure 2C). This result suggests that treatment with up to 128 times of the MIC of clarithromycin increases dead cells however, dead population are homogeneously distributed within the biofilm (Figure 2C). Additionally, we determined that around 50% of the population survived the clarithromycin treatment (Figure 2B,C), similar to the finding with CFU analysis. Together, these data show that H. pylori G27 biofilm cells show high tolerance towards antimicrobials, and combined with published data [19,20], suggests that antibiotic tolerance may be a general property of H. pylori biofilm cells.
2.2. Biofilm-Associated Proteins Restrict Clarithromycin Effects
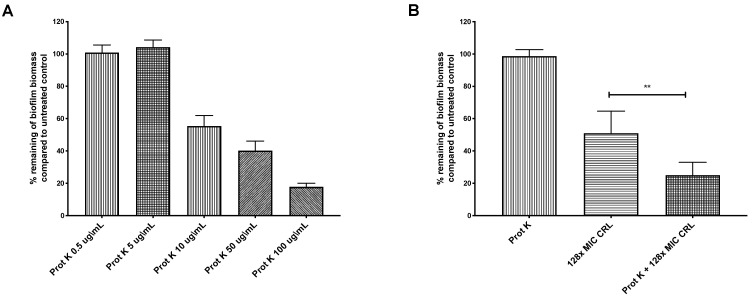
Biofilms can constitute physical and chemical diffusion barriers to antimicrobial penetration [13,17]. H. pylori is known to use a matrix composed mainly of proteins [21,22,23,24]. We thus tested whether H. pylori’s extracellular proteins limit antibiotic action. The biofilms were treated with proteinase K, which was shown previously to disperse preformed H. pylori G27 biofilms [22]. We first determined the biofilm proteinase K dose response, to identify doses that would largely leave preformed biofilms intact, with the idea that the biofilm structure would be partially destabilized and allow diffusion of antibiotics. Doses between 0.5 and 5 μg/mL of proteinase K left the biofilm intact (Figure 3A) and did not affect the viability of strain G27 (data not shown). Combining proteinase K (0.5 μg/mL) with clarithromycin at 128 times the MIC resulted in a significantly higher biofilm eradication compared to treatment with clarithromycin alone (Figure 3B). This outcome suggests that some target of proteinase K, such as the extracellular matrix or outer membrane proteins, limits clarithromycin exposure. These combined treatments did not completely eradicate H. pylori biofilms, so there may be other properties associated with H. pylori biofilm growth that contribute to clarithromycin resistance, such as low metabolism or overexpression of efflux pumps [19,21,25]. Our data thus show that H. pylori biofilms are antibiotic tolerant, and this phenotype is partially dependent on extracellular proteins.
Figure 3.
Proteinase K treatment sensitizes H. pylori G27 biofilms to clarithromycin. H. pylori G27 was grown as a biofilm for 48 h (as done in Figure 1) before exposure to varying doses of proteinase K (Prot K) ± clarithromycin (CRL) for 24 h. The biofilm biomass was determined by crystal violet staining, and compared to untreated controls (fresh media without antibiotic and/or proteinase K). (A) Proteinase K (Prot K) dose response. (B) Proteinase K at 5 µg/mL (Prot K), clarithromycin (CRL), or both treatments. Experiments were performed three independent times with at least eight technical replicates for each. Error bars represent standard errors of the mean. Statistical analyses were performed using ANOVA (**, p < 0.01).
3. Discussion
We report here that H. pylori biofilm formation leads to antibiotic tolerance in a manner that is partially dependent on extracellular proteins. Biofilm cells are highly tolerant to amoxicillin and tetracycline and moderately tolerant to clarithromycin. This clarithromycin tolerance can be alleviated by low-level treatment with proteinase K, suggesting the antibiotic tolerance state of the biofilms is at least partially reversible one.
Despite recent interest, H. pylori biofilms are still relatively understudied in general, with little information about their direct and indirect roles in antibiotic tolerance and resistance. The reason for this lack of attention is probably multifactorial. First, H. pylori is a highly fastidious microorganism that requires complex growth media containing blood or serum, low-oxygen condition, and long incubations. Growing H. pylori as a biofilm makes things even more difficult, since it requires additional specific conditions and longer incubation time. It was recently shown that H. pylori SS1, a strain model for studying disease pathogenesis in murine experiment, requires low levels of serum to form a robust biofilm. Low levels of serum are not optimal for planktonic growth but it seems to trigger a stress or signaling that drastically increases the biofilm formation and thus sessile mode of growth [21]. As shown here and previously, these conditions are not necessary for H. pylori G27 to form biofilms. To date, there is the lack of consensus about the best strain with which to study H. pylori biofilms and also about the ideal methodology. Indeed, several approaches have been developed to investigate H. pylori biofilms across laboratories including glass frits [26], glass slides [27,28], and borosilicate glass bottles [29] for the study of biofilms formed at the air–liquid interface, and plastic tissue culture plates [21,22] or biotic surface such as MDCK epithelial cells [27] for biofilms formed at the solid–liquid interface. These variations in strains and approaches make the comparisons challenging.
Here, we used H. pylori strain G27, which has benefits because it is commonly available and well characterized with reliable growth and biofilm formation [21,22]. We used the 96-well plate model, which allows the formation of biofilm at the solid–liquid and air–liquid interfaces. Within this biofilm, DNA and proteins were the most important components of the matrix described to be unevenly distributed [22]. G27 biofilms have been previously shown to be inhibited by high dose of proteinase K (25–200 µg/mL) [22].
Proteinase K is a relatively nonspecific protease that does not breach the H. pylori outer membrane at doses below 400 µg/mL [30]. We, thus, predict that our 5 µg/mL proteinase K dose largely targets proteins outside the outer membrane such as those secreted in biofilm matrix or associated with the outer membrane. Within H. pylori, matrix proteins have been suggested to be the main and the primary structural component, based on confocal microscopy and enzymatic assays [22]. However, the matrix does not have a substantial appearance in electron microscopy [21], raising the question of how well it would actually limit small molecule diffusion. Indeed, recent evidence suggests that even thick biofilm matrices do not substantially slow diffusion of small molecules such as antibiotics [31,32,33]. Another possibility for the proteinase K targets is outer membrane proteins [34]. These proteins could directly limit antibiotic penetration by coating the outer membrane, act as adhesins to connect H. pylori cells into structures that block antibiotic exposure of inner biofilm layers, or could be a component of tripartite efflux pumps [35,36,37,38]. Outer membrane proteins constitute a diverse group that includes porins, which fulfill multiple roles, including diffusion of small molecules such as antibiotics including β-lactams and fluoroquinolones [36,37,38,39]. In a study of H. pylori outer membrane proteins, the Hop and Hom family proteins were susceptible to proteolytic cleavage, whereas the Hor and Hof family proteins are resistant to proteinase K treatment [34]. Hop proteins are designated as porins [40], although their influence on antibiotic susceptibility in H. pylori remains largely unexplored.
In other microbes, there are known biofilm-promoting surface proteins that are sensitive to proteinase K. Specifically, the biofilm-associated protein (Bap) are cell-anchored proteins that promote biofilm development in both Gram-negative and Gram-positive bacteria [41]. In Staphylococcus aureus, 2 μg/mL proteinase K significantly inhibited biofilm development of Bap-positive strains but did not affect Bap-deficient mutant strains [42]. Orthologs to Bap exist in other bacterial species [43], including LapA in Pseudomonas fluorescens. LapA is described as an cell-anchored adhesin that is required for attachment and biofilm formation and is necessary to adhere to abiotic surfaces [44]. LapA is found in the G27 genome and shares a 37% domain identity to P. fluorescens LapA, providing a potential target for proteinase K cleavage in H. pylori. Thus, it seems likely that proteinase K acts on H. pylori-anchored surface proteins, but their identity and function remain to be investigated.
Several groups have documented increased expression of efflux pumps in H. pylori biofilm cells. Since some of these efflux pumps span the outer membrane, these might be rendered nonfunctional by proteinase K. Previous work showed that H. pylori clinical strains, TK1049 and TK1402, became antibiotic tolerant in the biofilm state to amoxicillin, metronidazole, and clarithromycin [19,20]. These results generally agreed well with those presented here, although there were some differences. The most substantial was that TK1049 and TK1402 biofilms did not become completely tolerant to amoxicillin, with amoxicillin treatment resulting in ~40% reduction in biofilm mass as compared to no reduction with the strain used here, G27 [20]. Clarithromycin treatment gave similar results with TK1402 and G27; in that, the antibiotic partially removed and killed biofilm cells [19]. Overall, these studies all converge on the idea that biofilm growth renders H. pylori antibiotic tolerant, but the degree of tolerance appears to vary between strain backgrounds and the antibiotic used.
Biofilm cells are also known to have altered physiology in addition to expressing surface structures [45]. Recent work showed that H. pylori biofilms have indicators of slowed growth. Transcriptome analysis revealed that key metabolic genes of the TCA cycle were downregulated in biofilm cells compared to their planktonic counterparts [21]. Biofilms are well known to generate heterogeneous gradients of nutrients and oxygen with microniches that drastically mitigate cell growth and metabolic states [45]. Growth and metabolic states are crucial determinants for antibiotic susceptibility [46], however, in H. pylori, the biofilm slow-growth impact on antibiotic susceptibilities has yet to be determined.
Tolerance is well known to play a crucial role in the survival of the bacterial population [14]. Antibiotic tolerance and persistence also represent a reservoir for bacteria to acquire mutations [14]. In a recent study using an evolution experiment and mathematical modeling, tolerance preceded and enhanced the evolution of resistance when cells were subjected to intermittent antibiotic exposure [47]. Knowing that H. pylori treatments require long-term regimen that include several antibiotics, and that we are facing alarming levels of antibiotic resistance, tolerance and its impact on therapies in H. pylori must be taken into consideration in the future.
In summary, our study suggests that H. pylori biofilm growth creates H. pylori cells that are significantly antibiotic tolerant. Our data suggest that this phenotype arises from a combination of extracellular proteins as well as other mechanism that has yet to be fully characterized. It will be exciting to learn in more detail how antibiotic tolerance is created, how it affects antibiotic resistance, and how it can be prevented to promote more effective H. pylori treatments.
4. Materials and Methods
4.1. Bacterial Strain and Growth Conditions
This study employed H. pylori G27 strain, generously provided by Nina Salama [48]. H. pylori was grown on Columbia Horse Blood Agar (CHBA) (Difco), containing: 0.2% β-cyclodextrin, 10 μg/mL vancomycin, 5 μg/mL cefsulodin, 2.5 U/mL polymyxin B, 5 μg/mL trimethoprim, and 8 μg/mL amphotericin (all chemicals are from Thermo Fisher or Gold Biotech), or Brucella Broth (Difco) containing 10% heat-inactivated fetal bovine serum (BB10) (Gibco/BRL). Culture were grown under microaerobic conditions (10% CO2, 5% O2, and 85% N2) at 37 °C. For planktonic growth, constant shaking (180 rpm) was used.
4.2. Biofilm Formation and Crystal Violet Assays
For biofilm formation, H. pylori strains were grown overnight with shaking in BB10, diluted to an OD600 of 0.15 with fresh BB10 media, and 200 µL of the bacterial suspension was added to the wells of a sterile 96-well polystyrene microtiter plates (Costar, 3596). Experiments were performed in biological and technical triplicates. Following 3 days of static incubation under microaerobic and static conditions, the culture medium was removed by aspiration and the wells were washed twice with phosphate-buffered saline (PBS). Wells were then filled with 200 μL of crystal violet (0.1%, wt/vol), and plates were incubated for 2 min at room temperature. The crystal violet solution was removed by aspiration, the wells were washed twice with PBS, and then, air dried for 20 min at room temperature. To visualize biofilms, 200 μL of 70% ethanol (vol/vol) was added to each well, and the absorbance was measured at 590.
4.3. Antibiotic Minimum Inhibitory Concentration (MIC) Determination
The antimicrobial susceptibilities for amoxicillin, clarithromycin, and tetracycline were determined using ETEST strips (bioMérieux). BB10 cultures of H. pylori were adjusted overnight to an OD600 of 0.5, and 50 µL of culture was spread onto a CHBA plate. ETEST strips were aseptically placed onto each plate and incubated for 72 h. MICs were identified as the point at which bacterial growth intersected the strip. The experiments were conducted in triplicate for each antibiotic.
4.4. Planktonic and Biofilm Antimicrobial Susceptibility
To determine the antibiotic susceptibility of biofilm cells, they were cultured as described above in 96-well plates. After 48 h of incubation, the supernatant was removed by aspiration and 200 μL of BB10 media containing the desired antibiotic concentration was added to each well. Wells containing media without antibiotic supplementation were used as positive controls, and wells containing antibiotic supplementations without culture were used as negative control. Plates were incubated for 24 h before biofilm-containing wells were washed, stained, and quantified with crystal violet staining as described above. Alternatively, biofilms with and without exposure to clarithromycin were washed in 2X PBS, resuspended in PBS, and serially diluted to count colony-forming units (CFUs). For planktonic growth assays, H. pylori cultures were grown for 18–72 h and then exposed to the desired antibiotic concentration for 24 h. Cultures were then plated for enumeration by CFU counting.
4.5. Biofilm Dispersion Assay
Proteinase K treatment of established biofilms was performed as described previously [21,22]. Briefly, biofilms were grown as above. After 48 h of incubation, supernatants were replaced with either fresh media containing either proteinase K alone (200–0.05 μg/mL, Sigma-Aldrich) or fresh media containing proteinase K (200–0.05 μg/mL) and clarithromycin (MIC or 128× MIC). For control wells, supernatants were replaced with fresh media. Wells were treated for 24 h under the desired conditions, and biofilms were quantified using crystal violet staining as described above.
4.6. Confocal Laser Scanning Microscopy
Biofilms of H. pylori G27 WT were prepared as described above using BB10 media. To use confocal laser scanning microscopy (CLSM), μ-Slide 8-well glass bottom chamber slides (ibidi, Germany) were used in place of 96-well microtiter plates. Growth and treatment were the same for other antibiotic exposure experiments. After 3 days, biofilms were stained using a FilmTracer™ FM®1–43 (Invitrogen) or FilmTracer™ LIVE/DEAD Biofilm Viability Kit (Invitrogen) according to the manufacturer’s instructions. Stained biofilms were visualized by CLSM with an LSM 5 Pascal Laser Scanning Microscope (Zeiss), and images were acquired using Imaris software (Bitplane). Biomass analysis of biofilm was carried out using FM®1–43 stained z-stack images (0.15 μm thickness) obtained by CLSM from randomly selected areas. The biomass of biofilms was determined using COMSTAT [49].
4.7. Statistical Analysis
Biofilm data were analyzed statistically using GraphPad Prism software (version 8, GraphPad Software Inc., San Diego, CA) by application of Wilcoxon–Mann–Whitney test or one-way ANOVA with Bartlett’s test. p < 0.05 or < 0.01 were considered to reflect statistical significance.
Author Contributions
Conceptualization, K.M.O. and S.H.; investigation, S.H. and J.Z.; writing—review and editing, K.M.O., S.H., and A.C.; and funding acquisition, K.M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National institute of Allergy and Infectious Diseases (NIAID) grant RO1AI116946 (to K.M.O), funds from the Santa Cruz Cancer Benefits Group (to KMO), and NIH S10 grant 1S10OD023528 (to F. Yildiz). We thank Ben Abrams (University of California, Santa Cruz) for confocal microscope training and assistance. Finally, we thank members of the Ottemann Lab, particularly Yasmine Elshenawi and Shuai Hu, for helpful discussions and comments on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Testerman T.L., Morris J. Beyond the stomach: An updated view ofHelicobacter pyloripathogenesis, diagnosis, and treatment. World J. Gastroenterol. 2014;20:12781–12808. doi: 10.3748/wjg.v20.i36.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin D.M., Pisani P., Ferlay J. Global cancer statistics. CA A Cancer J. Clin. 1999;49:74–108. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 3.Ford A.C., Gurusamy K., Delaney B., Forman D., Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter pylori-positive people. Cochrane Database Syst. Rev. 2016;2016:CD003840. doi: 10.1002/14651858.CD003840.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vakil N., Vaira B. Treatment for H. pylori Infection. J. Clin. Gastroenterol. 2013;47:383–388. doi: 10.1097/MCG.0b013e318277577b. [DOI] [PubMed] [Google Scholar]
- 5.Chey W.D., Leontiadis G.I., Howden C.W., Moss S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017;112:212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 6.Fallone C.A., Chiba N., Van Zanten S.V., Fischbach L., Gisbert J.P., Hunt R.H., Jones N.L., Render C., Leontiadis G.I., Moayyedi P., et al. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology. 2016;151:51–69.e14. doi: 10.1053/j.gastro.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Crowe S.E. Helicobacter pylori Infection. N. Engl. J. Med. 2019;380:1158–1165. doi: 10.1056/NEJMcp1710945. [DOI] [PubMed] [Google Scholar]
- 8.Mégraud F., Coenen S., Versporten A., Kist M., Lopez-Brea M., Hirschl A.M., Andersen L.P., Goossens H., Glupczynski Y. Helicobacter pyloriresistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2012;62:34–42. doi: 10.1136/gutjnl-2012-302254. [DOI] [PubMed] [Google Scholar]
- 9.Savoldi A., Carrara E., Graham D.Y., Conti M., Tacconelli E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology. 2018;155:1372–1382.e17. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talarico S., Korson A.S., Leverich C.K., Park S., Jalikis F.G., Upton M.P., Broussard E., Salama N.R. High prevalence of Helicobacter pylori clarithromycin resistance mutations among Seattle patients measured by droplet digital PCR. Helicobacter. 2018;23:e12472. doi: 10.1111/hel.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcus E.A., Inatomi N., Nagami G.T., Sachs G., Scott D. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment. Pharmacol. Ther. 2012;36:972–979. doi: 10.1111/apt.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott D., Sachs G., Marcus E.A. The role of acid inhibition in Helicobacter pylori eradication. F1000Research. 2016;5:1747. doi: 10.12688/f1000research.8598.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hathroubi S., Mekni M.A., Domenico P., Nguyen D., Jacques M. Biofilms: Microbial Shelters against Antibiotics. Microb. Drug Resist. 2017;23:147–156. doi: 10.1089/mdr.2016.0087. [DOI] [PubMed] [Google Scholar]
- 14.Lewis K. Persister cells, dormancy and infectious disease. Nat. Rev. Genet. 2006;5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 15.Bernier S.P., Lebeaux D., DeFrancesco A.S., Valomon A., Soubigou G., Coppée J.-Y., Ghigo J.-M., Beloin C. Starvation, Together with the SOS Response, Mediates High Biofilm-Specific Tolerance to the Fluoroquinolone Ofloxacin. PLoS Genet. 2013;9:e1003144. doi: 10.1371/journal.pgen.1003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciofu O., Tolker-Nielsen T. Tolerance and Resistance of Pseudomonas aeruginosa Biofilms to Antimicrobial Agents-How P. aeruginosa Can Escape Antibiotics. Front. Microbiol. 2019;10:913. doi: 10.3389/fmicb.2019.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart P.S. Antimicrobial Tolerance in Biofilms. Microbiol. Spectr. 2015;3:269–285. doi: 10.1128/microbiolspec.MB-0010-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Høiby N., Bjarnsholt T., Givskov M., Molin S., Ciofu O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Yonezawa H., Osaki T., Hanawa T., Kurata S., Ochiai K., Kamiya S. Impact of Helicobacter pylori Biofilm Formation on Clarithromycin Susceptibility and Generation of Resistance Mutations. PLoS ONE. 2013;8:e73301. doi: 10.1371/journal.pone.0073301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yonezawa H., Osaki T., Hojo F., Kamiya S. Effect of Helicobacter pylori biofilm formation on susceptibility to amoxicillin, metronidazole and clarithromycin. Microb. Pathog. 2019;132:100–108. doi: 10.1016/j.micpath.2019.04.030. [DOI] [PubMed] [Google Scholar]
- 21.Hathroubi S., Zerebinski J., Ottemann K.M., Hengge R., Cover T. Helicobacter pylori Biofilm Involves a Multigene Stress-Biased Response, Including a Structural Role for Flagella. MBio. 2018;9:e01973-18. doi: 10.1128/mBio.01973-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Windham I.H., Servetas S.L., Whitmire J.M., Pletzer D., Hancock R.E.W., Merrell D.S. Helicobacter pylori Biofilm Formation Is Differentially Affected by Common Culture Conditions, and Proteins Play a Central Role in the Biofilm Matrix. Appl. Environ. Microbiol. 2018;84:AEM.00139-18. doi: 10.1128/AEM.00391-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grande R., Di Giulio M., Bessa L.J., Di Campli E., Baffoni M., Guarnieri S., Cellini L. Extracellular DNA in Helicobacter pylori biofilm: A backstairs rumour. J. Appl. Microbiol. 2010;110:490–498. doi: 10.1111/j.1365-2672.2010.04911.x. [DOI] [PubMed] [Google Scholar]
- 24.Grande R., Di Marcantonio M.C., Robuffo I., Pompilio A., Celia C., Di Marzio L., Paolino D., Codagnone M., Muraro R., Stoodley P., et al. Helicobacter pylori ATCC 43629/NCTC 11639 Outer Membrane Vesicles (OMVs) from Biofilm and Planktonic Phase Associated with Extracellular DNA (eDNA) Front. Microbiol. 2015;6:1114. doi: 10.3389/fmicb.2015.01369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge X., Cai Y., Chen Z., Gao S., Geng X., Li Y., Jia J., Sun Y., Li Y. Bifunctional Enzyme SpoT Is Involved in Biofilm Formation ofHelicobacter pyloriwith Multidrug Resistance by Upregulating Efflux Pump Hp1174 (gluP) Antimicrob. Agents Chemother. 2018;62:e00957-18. doi: 10.1128/AAC.00957-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cole S.P., Harwood J., Lee R., She R., Guiney D.G. Characterization of Monospecies Biofilm Formation by Helicobacter pylori. J. Bacteriol. 2004;186:3124–3132. doi: 10.1128/JB.186.10.3124-3132.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson J.K., Huang J.Y., Wreden C., Sweeney E.G., Goers J., Remington S.J., Guillemin K. Chemorepulsion from the Quorum Signal Autoinducer-2 Promotes Helicobacter pylori Biofilm Dispersal. mBio. 2015;6:e00379-15. doi: 10.1128/mBio.00379-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yonezawa H., Osaki T., Kurata S., Fukuda M., Kawakami H., Ochiai K., Hanawa T., Kamiya S. Outer Membrane Vesicles of Helicobacter pylori TK1402 are Involved in Biofilm Formation. BMC Microbiol. 2009;9:197. doi: 10.1186/1471-2180-9-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong E.H.J., Ng C.G., Goh K.L., Vadivelu J., Ho B., Loke M.F. Metabolomic analysis of low and high biofilm-forming Helicobacter pylori strains. Sci. Rep. 2018;8:1409. doi: 10.1038/s41598-018-19697-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sause W.E., Castillo A.R., Ottemann K.M. The Helicobacter pylori Autotransporter ImaA (HP0289) Modulates the Immune Response and Contributes to Host Colonization. Infect. Immun. 2012;80:2286–2296. doi: 10.1128/IAI.00312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walters M.C., Roe F., Bugnicourt A., Franklin M.J., Stewart P.S. Contributions of Antibiotic Penetration, Oxygen Limitation, and Low Metabolic Activity to Tolerance of Pseudomonas aeruginosa Biofilms to Ciprofloxacin and Tobramycin. Antimicrob. Agents Chemother. 2003;47:317–323. doi: 10.1128/AAC.47.1.317-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh R., Sahore S., Kaur P., Rani A., Ray P. Penetration barrier contributes to bacterial biofilm-associated resistance against only select antibiotics, and exhibits genus-, strain- and antibiotic-specific differences. Pathog. Dis. 2016;74:ftw056. doi: 10.1093/femspd/ftw056. [DOI] [PubMed] [Google Scholar]
- 33.Oubekka S.D., Briandet R., Fontaine-Aupart M.-P., Steenkeste K. Correlative Time-Resolved Fluorescence Microscopy To Assess Antibiotic Diffusion-Reaction in Biofilms. Antimicrob. Agents Chemother. 2012;56:3349–3358. doi: 10.1128/AAC.00216-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voss B.J., Gaddy J.A., McDonald W.H., Cover T.L. Analysis of Surface-Exposed Outer Membrane Proteins in Helicobacter pylori. J. Bacteriol. 2014;196:2455–2471. doi: 10.1128/JB.01768-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi U., Lee C.-R. Distinct Roles of Outer Membrane Porins in Antibiotic Resistance and Membrane Integrity in Escherichia coli. Front. Microbiol. 2019;10:953. doi: 10.3389/fmicb.2019.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iyer R., Moussa S., Durand-Réville T.F., Tommasi R., Miller A.A. Acinetobacter baumanniiOmpA Is a Selective Antibiotic Permeant Porin. ACS Infect. Dis. 2017;4:373–381. doi: 10.1021/acsinfecdis.7b00168. [DOI] [PubMed] [Google Scholar]
- 37.Delcour A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2009;1794:808–816. doi: 10.1016/j.bbapap.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mach T., Neves P., Spiga E., Weingart H., Winterhalter M., Ruggerone P., Ceccarelli M., Gameiro P. Facilitated Permeation of Antibiotics across Membrane Channels—Interaction of the Quinolone Moxifloxacin with the OmpF Channel. J. Am. Chem. Soc. 2008;130:13301–13309. doi: 10.1021/ja803188c. [DOI] [PubMed] [Google Scholar]
- 39.Samsudin F., Ortiz-Suarez M.L., Piggot T.J., Bond P.J., Khalid S. OmpA: A Flexible Clamp for Bacterial Cell Wall Attachment. Structure. 2016;24:2227–2235. doi: 10.1016/j.str.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Odenbreit S., Swoboda K., Barwig I., Ruhl S., Borén T., Koletzko S., Haas R. Outer Membrane Protein Expression Profile in Helicobacter pylori Clinical Isolates. Infect. Immun. 2009;77:3782–3790. doi: 10.1128/IAI.00364-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taglialegna A., Navarro S., Ventura S., Garnett J.A., Matthews S.J., Penades J.R., Lasa I., Valle J. Staphylococcal Bap Proteins Build Amyloid Scaffold Biofilm Matrices in Response to Environmental Signals. PLoS Pathog. 2016;12:e1005711. doi: 10.1371/journal.ppat.1005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao T.S., Shukla S.K. Staphylococcus aureus biofilm removal by targeting biofilm-associated extracellular proteins. Indian J. Med. Res. 2017;146:S1–S8. doi: 10.4103/ijmr.IJMR_410_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Latasa C., Solano C., Penadés J.R., Lasa I. Biofilm-associated proteins. Comptes Rendus Boil. 2006;329:849–857. doi: 10.1016/j.crvi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Boyd C.D., Smith T.J., El-Kirat-Chatel S., Newell P.D., Dufrêne Y.F., O’Toole G.A. Structural Features of the Pseudomonas fluorescens Biofilm Adhesin LapA Required for LapG-Dependent Cleavage, Biofilm Formation, and Cell Surface Localization. J. Bacteriol. 2014;196:2775–2788. doi: 10.1128/JB.01629-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002;292:107–113. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- 46.Cabral D., Wurster J., Belenky P. Antibiotic Persistence as a Metabolic Adaptation: Stress, Metabolism, the Host, and New Directions. Pharmaceuticals. 2018;11:14. doi: 10.3390/ph11010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levin-Reisman I., Ronin I., Gefen O., Braniss I., Shoresh N., Balaban N.Q. Antibiotic tolerance facilitates the evolution of resistance. Science. 2017;355:826–830. doi: 10.1126/science.aaj2191. [DOI] [PubMed] [Google Scholar]
- 48.Baldwin D.N., Shepherd B., Kraemer P., Hall M.K., Sycuro L.K., Pinto-Santini D.M., Salama N.R. Identification of Helicobacter pylori Genes That Contribute to Stomach Colonization. Infect. Immun. 2006;75:1005–1016. doi: 10.1128/IAI.01176-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heydorn A., Nielsen A.T., Hentzer M., Sternberg C., Givskov M., Ersbøll B.K., Molin S. Quantification of biofilm structures by the novel computer program comstat. Pt 10Microbiology. 2000;146:2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]