Abstract
Background:Genista tridentata L. is an endemic species from the Iberian Peninsula used in Portuguese traditional medicine to treat inflammation-related diseases; this and other health-promoting effects are usually associated with the flavonoids produced by this species. In fact, anti-inflammatory properties were established for several of these flavonoid derivatives. Methods: A careful survey of the reported data, using mainly the Scopus database and Genista tridentata and Pterospartum tridentatum as keywords, was done. We have examined the papers involving the plant and those about the most relevant flavonoids anti-inflammatory activity. Results: The literature survey demonstrates that species are used to treat several health problems such as antihyperglycemia, hypertension, and inflammatory episodes. It was also possible to establish its richness in flavonoid derivatives, from which several are potential anti-inflammatory agents. Conclusions: From our described and discussed analysis, it can be concluded that Genista tridentata is an excellent source of bioactive flavonoids. Moreover, its traditional use to treat inflammation episodes may be due to its flavonoid content, from which genistein, biochanin A, rutin, and daidzein can be emphasized.
Keywords: Genista tridentata, Pterospartum tridentatum, isoflavones, flavonols, anti-inflammatory, genistein, biochanin A, rutin, daidzein
1. Introduction
Inflammation is a natural defense mechanism involved in the body’s healing process, in which the body is protected from pathogens or abnormal cells [1]. However, if the inflammation is prolonged in time or serious, it can damage the healthy tissues and cause several diseases, such as cancer [2], Alzheimer’s and Parkinson’s diseases [3]. Therefore, the development of new anti-inflammatory drugs is still a demand, and plant secondary metabolites are considered a priority—in particular, those found in medicinal plants [4].
Among the plants used in Portuguese traditional medicine, Genista tridentata L. can be highlighted due to the important applications reported [5]; in fact, the plant, locally named carqueja, is in several regions called the “plant that heals everything” [5], and among its applications is the use to treat inflammatory diseases [6].
Flavonoids, a large family of natural compounds, are usually associated with anti-inflammatory activity [7], and most recently, we demonstrated that G. tridentata is rich in flavonoid derivatives [8], including some for which anti-inflammatory activities have been described. As examples, genistein, daidzein [9,10], and biochanin A [11,12] can be highlighted.
The most promising anti-inflammatory flavonoids that can be isolated from G. tridentata will be discussed in this review, emphasizing their mode of action and in vivo studies. Hopefully, this will help the scientific community to understand their involvement in inflammatory processes and consequently endorse the design for novel derivatives. Furthermore, the traditional medicine applications of G. tridentata will also be addressed and discussed. To accomplish this survey, we used mainly the Scopus database (69 articles), but also Web of Science (61 articles) and PubMed, mostly for the anti-inflammatory activity. The keywords used were the accepted name (Genista tridentata), the most common synonym (Pterospartum tridentatum), and also the less common one (Chamaespartium tridentatum). Naturally, in the survey there were also the flavonoid names combined with anti-inflammatory activity. Relevance was given to the most recent biological evaluations and the in vivo studies and the clinical trials. In all cases, the papers involving both the plant and the most relevant flavonoids anti-inflammatory activities.
2. Genista tridentata: Traditional Applications and Biological Activities
Genista tridentata L. is a bush endemic to the Iberian Peninsula where it grows wildly. Unfortunately, its taxonomy is a little controversial, and consequently, the literature survey is more complicated. The most found scientific name is Pterospartum tridentatum (L.) Willk., which is considered by some taxonomists [13] as the correct name, but other authors used Chamaespartium tridentatum (L.) P.E. Gibbs [14]. However, according to the Plant List database [15], these are synonyms of Genista tridentata L. and there are eleven other synonyms and three infraspecific taxa [15]. However, in our survey, only the abovementioned synonyms were found—it seems that the other synonyms and infraspecific taxa are not used in articles involving chemical profile and/or anti-inflammatory evaluations. Although we used all names in the literature survey, herein, we will refer the species by the accepted name reported in the Plant Lista database [15].
Genista tridentata is an Angiosperm belonging to the Leguminosae family [15], which grows spontaneously under Mediterranean thermal conditions, where it is known as carqueja [16]. G. tridentata is a perennial shrub that can reach up to one meter in height, with stems of woody and rigid consistency. The roots are well-liked and quite long and sometimes intertwine in the roots of other companion species. The stems are woody, erect or prostrate with laterally winged branches, forming false leaves of dark green color, cut out and of coriaceous consistency. Thus the branches have a flattened shape with two or three wing-shaped expansions, with an articulated appearance, ending with two or three teeth. The leaves, persistent, alternating, unifoliolate and triangular, appear to be tridentate, by the leaflets being united to the stipulations. The flowers are of an intense yellow and are arranged in corymbiform inflorescences, in groups of 3 to 10, gathered in small and tight bouquets. They have an induction in the sepals that line them. The fruit is an oblong-linear pod 10 to 12 mm long [17].
Despite the abovementioned disagreement in the G. tridentata taxonomy, the vernacular designation, carqueja, is referred to in the ethnopharmacological surveys. Consequently, it is possible to mention here that G. tridentata is used in the Iberian Peninsula, particularly in Portugal, in traditional medicine, mainly to treat influenza, cold, cough, stomach troubles, and nervousness, and is also used as a tonic, hepatic protector, sedative, cicatrizant, and diuretic [6,14,18,19]. In these applications, the population mainly uses extracts of the plant flowers, leaves, or the aerial parts. Consequently, it is suggested that the plant presents several therapeutic properties, from which antispasmodic, antihypertensive, and anti-inflammatory properties can be emphasized [6,14].
The flowers are used in folk medicine for the treatment of various disorders, including those relating to the respiratory system, digestive tract, nervous system, urinary system and dermatology; it has also been indicated for diabetes control [16,20] and is sometimes used in mixtures with other plants for this purpose [20]. Some authors referred to the use of P. tridentatum for the treatment of colds, stomach pains, intestinal problems, kidney disease, liver and gallbladder problems and also for rheumatism [21]. It was also indicated for pneumonia, bronchitis and tracheitis, headaches, cough, for low blood pressure levels and high levels of cholesterol, diabetes and even in weight loss programs. This species is known for its diuretic, purgative, laxative, hypotensive, hypoglycemic effects, and for its digestive properties [14,22]. The infusion of dried flowers is considered an excellent emollient [21].
One vital point that should be herein mentioned is the obligation to have scientific validations of the claimed properties, an aspect that it is not at all strange to the scientific community [23]. In this regard, several evaluation studies involving G. tridentata extracts were reported and will be herein presented and discussed. Most of the studies were performed using the flowers or the aerial parts extracted with polar solvents and in vitro antioxidant evaluations (Table 1).
Table 1.
Biological assays of Genista tridentata extracts.
| Plant Part | Solvent | Activity Tested | Method | Ref. |
|---|---|---|---|---|
| Aerial parts | Ethanol and water | Antioxidant (ethanol, IC50 = 60.39 ± 1.79 μg/mL; water, IC50 = 42.97 ± 1.69 μg/mL) | DPPH scavenging β-Carotene bleaching test |
[24] |
| Flowers, stems and leaves |
Methanol | Antioxidant (flowers, IC50 = 26.1 ± 1.3 mg/L; stems and leaves, IC50 = 69.7 ± 11.9 mg/L) | DPPH scavenging β-Carotene bleaching test |
[25] |
| Flowers | Methanol | Antioxidant | DPPH scavenging (IC50 = 0.15 ± 0.01 mg/mL) β-Carotene bleaching test (IC50 = 0.14 ± 0.02 mg/mL) Reducing power (IC50 = 0.13 ± 0.00 mg/mL) TBARS inhibition (IC50 = 0.12 ± 0.02 mg/mL) |
[26] |
| Flowers and leaves | Hydroethanolic | Antioxidant (flowers, IC50 = 1016 mg/L; leaves, IC50 = 704 mg/L | DPPH scavenging β-Carotene bleaching test Reducing power ABTS scavenging |
[27] |
| Purchased plant material |
Water | Antioxidant (%AA = 169.5 ± 17.2) | β-Carotene bleaching test ABTS scavenging |
[28] |
| Purchased plant material |
Methanol | Antioxidant | DPPH scavenging (IC50 = 0.18 ± 0.01 mg/mL) β-Carotene bleaching test (IC50 = 0.48 ± 0.09 mg/mL) Reducing power (IC50 = 0.11 ± 0.00 mg/mL) TBARS inhibition (IC50 = 1.18 ± 0.06 mg/mL) |
[29] |
| Purchased plant material |
Hot water | Antioxidant | DPPH scavenging (IC50 = 50 ± 1 μg/mL) β-Carotene bleaching test (IC50 = 266 ± 25 μg/mL) Reducing power (IC50 = 105 ± 2 μg/mL) TBARS inhibition (IC50 = 93 ± 4 μg/mL) |
[30] |
| Flowers | Hot water | Antioxidant (TABARS, IC50 = 8.4 ± 0.2 μg/mL; OxHLIA, IC50 = 37.7 ± 0.9 μg/mL) | TBARS inhibition Oxidative haemolysis inhibition |
[31] |
| Flowers | Hydromethanolic | Antifungal (Candida albicans, 10 mm inhibition zone; Candida glabrata, 11 mm inhibition zone) | Disc diffusion test | [32] |
| Aerial parts | Hydromethanolic | Antibacterial (Staphylococcus aureus, MIC = 39.1 μg/mL) | Microplate bioassay | [33] |
| Flowers | Hot water | Antimicrobial (Escherichia coli, MIC = 0.5 mg/mL; Salmonela typhimurium, MIC = 1 mg/mL; Bacillus cereus, MIC = 1 mg/mL; Listeria monocytogenes, MIC = 1 mg/mL; Aspergillus niger, MIC = 8 mg/mL; Aspergillus versicolor, MIC = 0.5 mg/mL; Penicillium funiculosum, MIC = 0.5 mg/mL; Penicillium verrucosum, MIC = 0.5 mg/mL) | Disc diffusion test | [31] |
| Flowers | Hot water | Cytotoxicity (HeLa, GI50 = 242 ± 10 μg/mL; HepG2, GI50 = 262 ± 11 μg/mL) | Against tumor cells HeLa, HepG2, MCF-7 and NCi-H460 and non-tumor cells PLP2 | [31] |
| Inflorescences | Hot water | Immunostimulatory (significant activity for 200 μg/mL) | Macrophage cell viability and NO production | [34] |
| Purchased plant material |
Water | Toxicity (non toxic at 375 mg/L) | MTT assay; mitochondrial swelling, | [28] |
| Flowers, leaves, stems and roots |
Ethanol | Toxicity (non toxic at 100 μg/mL) | Resazurin assay | [8] |
| Flowers | Hot water | Anti-inflammatory (>400 μg/mL) | Determination of LPS-induced NO production by Murine macrophage (RAW 264.7) cell lines | [31] |
| Flowers, leaves, stems and roots |
Ethanol | Anti-inflammatory (significantat 100 μg/mL) | LPS-induced transcription of pro-inflammatory genes IL-1β, Nos2, Ptgs2, IL-6, and TNF-α; Western blot analysis | [8,35] |
AA, antioxidant activity; GI50, values correspond to the concentration that causes 50% inhibition of cell proliferation; IC50, values corresponded to the extract concentration that inhibits in 50% the oxidation and inflammatory process; MIC, minimum inhibitory concentration.
The authors achieved the extract antioxidant activity index or antioxidant potential through several assays, from which DPPH● (2,2-diphenyl-1-picrylhydrazyl radical) scavenging assay and β-carotene bleaching test are the most common. However, it is interesting to note that some authors used other less common tests, such as lipid peroxidation inhibition, through the decrease in TBARS (thiobarbituric acid reactive substances) [26,29,30,31], and, more recently, the oxidative hemolysis inhibition assay [31]. These diversifications in the assays are, in our opinion, very good because they can establish in more detail the G. tridentata health-promoting potential. Altogether, the reported results show that this species presents moderate to strong antioxidant activity, and apparently, the flower extracts and the water extracts are more active [25,27].
Another interesting feature in these reports is the fact that all authors obtained the total phenolic content and/or the total flavonoid content, and some established the polyphenolic profile or identified some of the phenolic compounds present [25,28,29,30,31]. In doing so, they associated the antioxidant activity to the polyphenolic content. On the other hand, some aspects of these reports are less enthusiastic, since the reported values are in different units; the positive controls used are different, making it impossible to perform comparisons.
Other evaluations, such as antifungal [32], antibacterial [31,33] agents, cytotoxicity activity in tumor and non-tumor cells [31], and even the immunostimulatory activity of the G. tridentata polysaccharides [34] were also performed. Additionally, Ferreira et al. also performed in vivo and in vitro toxicological assays and concluded that short-term use is safe [8,28].
The anti-inflammatory evaluation of the G. tridentata extracts and mainly those reports which were recently achieved [8,31,35] will be the focus of this review. In the most recent evaluations, the authors tested parts of the plant separately and established that the anti-inflammatory effects of plant extracts could occur through different mechanisms. Moreover, the roots, which are not used in traditional medicine, also presented strong anti-inflammatory activity [8]. Likewise, the antioxidant and anti-inflammatory activity is associated with the species richness in polyphenolic compounds, particularly flavonoids.
3. Structural Pattern of the Flavonoids Isolated from Genista tridentata
Several authors demonstrated that G. tridentata produces several flavonoids; these metabolites are those that most contribute to the plant anti-inflammatory activity. Therefore, herein the flavonoids that were isolated from G. tridentata extracts or identified in will be discussed.
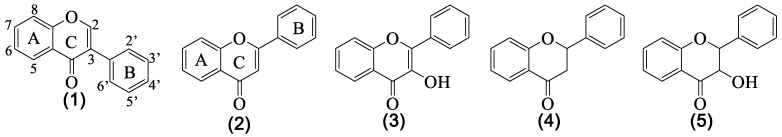
From the several established profiles, it is evident that the only classes of flavonoids detected were isoflavones 1, flavones 2, flavonols 3, flavanones 4 and flavanonols 5 (Figure 1), and the major ones are isoflavones and flavonols (Table 2 and Table 3).
Figure 1.
Structure of the classes of flavonoid derivatives found in G. tridentata.
Table 2.
Isoflavones and flavones produced by G. tridentata.
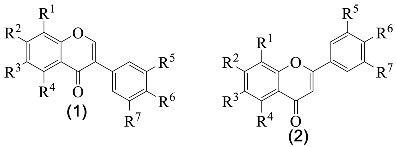
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nº | Name | R1 | R2 | R3 | R4 | R5 | R6 | R7 | Ref. |
| 1a | Sissotrin | H | OGlc | H | OH | H | OMe | H | [8,20,29,30,36] |
| 1b | Genistin | H | OGlc | H | OH | H | OH | H | [20,29,30,33,36] |
| 1c | 5,5′-Dihydroxy-3′-metoxi- -isoflavone-7-O-β-glucoside |
H | OGlc | H | OH | OMe | H | OH | [8,20,29,30,31,36] |
| 1d | Prunetin | H | OMe | H | OH | H | OH | H | [8,20,29,30,36] |
| 1e | Genistein | H | OH | H | OH | H | OH | H | [8,27,29,30,31,33,36] |
| 1f | 7-Methylorobol | H | OMe | H | H | OH | OH | H | [29,30,36] |
| 1g | Genistein-8-C-glucoside | Glc | OH | H | OH | H | OH | H | [29,30,31] |
| 1h | Biochanin A | H | OH | H | OH | H | OMe | H | [8,29,30] |
| 1i | 5-Hydroxy-4′,7-dimethoxy- -isoflavone |
H | OMe | H | OH | H | OMe | H | [8] |
| 1j | Daidzein | H | OH | H | H | H | OH | H | [8] |
| 2a | Luteolin-O-glucuronide | H | OGlc | H | OH | OH | OH | H | [28] |
| 2b | Luteolin-O-(O-acetyl)glucuronide | H | OGlcA-Ac | H | OH | OH | OH | H | [28] |
| 2c | Apigenin | H | OH | H | OH | H | OH | H | [33] |
Glc = glucoside unit; GlcA = glucuronide unit; Ac = acetyl.
Table 3.
Flavonols produced by G. tridentata.
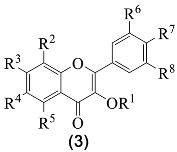
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nº | Name | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | Ref. |
| 3a | Isoquercitrin | Glc | H | OH | H | OH | OH | OH | H | [20,29,30,31,33,36] |
| 3b | Myricetin-6-C-glucoside | H | H | OH | Glc | OH | H | OH | OH | [8,29,30,36] |
| 3c | Rutin | Rha-Glc | H | OH | H | OH | OH | OH | H | [29,30,31,33,36] |
| 3d | Isorhamnetin-O-glucoside | Glc | H | OH | H | OH | OMe | OH | H | [28] |
| 3e | Myricetin-3,4′-di-O- -glucoside |
Glc | H | OH | H | OH | OH | OGlc | OH | [28] |
| 3f | Astragalin | Glc | H | OH | H | OH | H | OH | H | [8] |
| 3g | Isorhamnetin-3-O- -glucoside |
Glc | H | OH | H | OH | OMe | OH | H | [8] |
| 3h | Kaempferol | H | H | OH | H | OH | H | OH | H | [8] |
Glc = glucoside unit; Rha = rhamnoside unit.
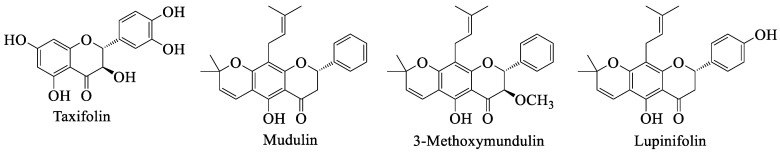
As far as we could find, the first report on the G. tridentata flavonoids allowed the isolation of four isoflavone derivatives 1a to 1d, and one flavonol 3a (Table 2 and Table 3) [20]. Four years later, the same research group found two other isoflavones 1e and 1f, and flavonols 3b and 3c, (Table 2 and Table 3) [36]. The first flavone derivatives were just reported in 2012 and were luteolin derivatives 2a and 2b (Table 2) [28]. Flavanonols were just uncovered, for the first time, in 2014 and are taxifolin derivatives, whereas flavanone derivatives were only reported in 2020 (Figure 2) [8,29].
Figure 2.
Structure of some flavonoid derivatives found in G. tridentata.
Our literature survey showed that the compounds indicated in Table 2 were found by several authors, with the exception of isoflavones 1i and 1j, and all the flavone derivatives that were just reported once [8,28,33]. Furthermore, through the analysis of Table 2, it is possible to detect that most of the flavonoids present one or more hydroxy groups and almost all are linked to saccharide units. Usually, this substitution pattern is associated with the anti-inflammatory property of a flavonoid [37].
It is important to complement the information listed in Table 2 with the information that other isoflavone glycosides were described, namely biochanin A hexoside [8,29,31,33] and genistein hexoside [8,31], but the authors did not identify the hexose nor its position in the isoflavone ring. There are also references describing the presence of a methylbiochanin A or a methylprunetin [8,29,30,31] derivative. In all of these cases, although this is important information about the G. tridentata profile, it was not included in Table 2 because its structure is not fully established. This suggests that some investment in phytochemical studies involving G. tridentata extracts is still needed.
The substitution pattern of flavanonol derivatives includes several hydroxy groups and glucosides, as well as a disaccharide unit. The most referred derivatives were isoquercitrin 3a and rutin 3c (Table 3), and again we are in the presence of compounds having the required substitution pattern for being promising anti-inflammatory agents [37]. Additionally, quercetin hexoside derivatives were also found, but the authors were again unable to identify the hexose or its position [29,30,31].
Finally, we can find the identification and isolation of flavanonols and flavanones (Figure 2). Some authors have reported the presence of taxifolin [33] or its glucosides [8], whereas others just mention hexoside derivatives [29,30,31]. One fact is consistent—G. tridentata produces taxifolin derivatives. The last examples were recently reported and apart from being slightly different, they were isolated from the plant roots [8], which is also uncommon due to the fact that most of the works were performed using flowers or aerial parts. This highlights that some parts of the plant should still be studied.
4. Flavonoids with Anti-Inflammatory Activity
In the previous section, we showed the richness of at G. tridentata in flavonoids; additionally, the major class, that is isoflavones, is commonly associated with beneficial anti-inflammatory properties [10]. Yu et al. discussed, in their excellent review [10], the possible isoflavones anti-inflammatory mechanisms, of which herein we highlight the main points (Table 4). Still, we suggest that our readers consult the original review for details. According to the authors, isoflavones may be involved in the scavenging of reactive oxygen species and, in doing so, they prevent the production of peroxynitrite, species that can oxidize low-density lipoproteins. With this effect, isoflavones can prevent cell membrane damage. However, they can also act by inhibiting the production of pro-inflammatory cytokines and chemokine species such as IL-1β, IL-6, IL-12 and TNF-α, or by inhibiting pro-inflammatory enzymes, such as cyclooxygenase, nitric oxide synthases, lipoxygenase and phospholipase A2, enzymes involved in the production of inflammatory mediators. Finally, there is also evidence that isoflavones can be involved in the regulation of NF-κB factor signaling and, through that regulation, decrease the production of pro-inflammatory cytokines (Table 4) [10,38,39].
Table 4.
Anti-inflammatory effects of the selected flavonoids.
| Flavonoid | Model | Mechanisms |
|---|---|---|
| Biochanin A | In vitro: cytokine release from keratinocytes and HMEC-1 endothelial cells in serum from patients with Behçet’s disease [41] In vitro: LPS-induced inflammation in HUVED cells [42] In vivo: focal cerebral ischemia–reperfusion model [43] In vitro: LPS-induced pro-inflammatory responses in murine BV2 microglial cells [44] In vitro: LPS-induced inflammatory cytokines and mediators production in murine BV2 microglial cells [45] In vivo: LPS/GalN-induced liver injury [46] Ex vivo: interleukin-1β-induced catabolic inflammation through the modulation of NFκB cellular signaling in primary rat chondrocytes [47] In vitro and in vivo: LPS-induced damage of dopaminergic neurons [48] In vivo: cisplatin induced acute kidney injury in mice [49] In vivo: ritonavir induced hepatotoxicity [50] In vivo: transient coronary ligation in Sprague-Dawley rats [51] In vivo: LPS-induced acute lung injury in mice [52] In vitro: LPS-induced NO production, LPS-induced IKK activity, LPS-induced phosphorylation of IκBα and p38 MAPK [53] In vitro: CCl4-induced hepatotoxicity in rats [54] In vivo: Sprague-Dawley rat subarachnoid hemorrhage [55] In vitro: barrier function of intestinal epithelial CaCo-2/TC-7 cells via TEER measurements [56] In vitro: LPS-stimulated macrophages [57] In vivo: focal cerebral ischemia established by middle cerebral artery occlusion [58] |
↓IL-8 ↓IL-8, TNF-α, VCAM-1, ICAM-1, E-selection ↑PPAR-γ ↓IL-8, TNF-α, P38 expression ↓IL-1β, TNF-α, NO, phosphorylation of JNK, ERK and p38 ↓IL-1β, TNF-α, NO, PGE2, NF-κB ↑PPAR-γ IL-1β, TNF-α, ALT, AST, MDA, TXNIP, NLRP3 inflammasome ↑SOD, GPx, catalase, HO-1, Nrf2 ↓IL-1β, TNF-α, IL-6, IL-1α, INFγ, IL- 2, GM-CSF, fractalkine, MCP-1, MIP-3α, LIX ↓IL-1β, TNF-α, IL-6, phosphorylation of JNK, ERK and p38, ↓IL-1β, TNF-α, caspase-3, p53 protein ↓IL-1β, IL-6 ↑IL-10 ↓IL-1β, IL-18, IL-6, TNF-α IL-1β, IL-6, TNF-α, TLR4/NF-κB ↑PPAR-γ IL-6, TNF-α PPAR-γ, PPAR-α iNOS, COX2, TNF-α sTNFR1, TNF-α, NF-κB, ERK, tyrosine phosphorylation ↑SOD, GSH-Px, HO-1, Nrf2 ↓iNOS, phosphorylation of IκBα and p38 MAPK ↓TLR/NF-κB |
| Prunetin | In vitro: barrier function of intestinal epithelial CaCo-2/TC-7 cells via TEER measurements [56] In vitro: LPS-stimulated RAW 264.7 macrophage [59] In vivo: LPS-induced septic shock [59] In vitro: LPS-induced in- flammatory response and MUC5AC expression [60] |
↓sTNFR1, TNF-α, NF-κB, ERK, tyrosine phosphorylation ↓iNOS, PGE2, COX2, NF-κB, p38, IL-1β, TNF-α IL-1β, TNF-α IL-8, IL-6, MUC5AC, TLR4/MyD88 |
| Daidzein | In vitro: LPS-stimulated macrophages [57] In vivo: angiotensin II-induced AAA [61] In vivo: 5-fluorouracil-induced intestinal mucositis [62] In vivo: cisplatin-induced kidney injury [63] In vivo: ischemia/reperfusion injury-induced neurological function deficits in Sprague-Dawley [64] |
↓IL-6 ↓IL-1β, TNF-α, NF-κB, iNOS, COX-2, p38MAPK, TGF-β1 ↓IL-1β, IL-6, TNF-α, NO, COX-2 ↓IL-6, TNF-α, MDA, NO, COX-2, MAPK ↑SOD, GSH ↓TNF-α, NF-κB subunit p65 |
| Genistein | In vitro: LPS-stimulated macrophages [57] In vitro: homocysteine-induced endothelial cell inflammation [65] In vivo: cyclophosphamide - induced hepatotoxicity [66] In vivo: LPS-induced microglial activation in murine BV2 microglial cell line and primary microglial culture [67] In vivo: imiquimod- induced psoriasis-like lesions in mice [68] In vivo: DSS-induced murine colitis [69] In vivo: NASH mouse model [70] In vivo: chronic sleep deprivation [71] In vitro: barrier function of intestinal epithelial CaCo-2/TC-7 cells via TEER measurements [56] In vivo: mouse model of periodontitis [72] In vivo: high-fat high-fructose diet-induced NASH rats [73] In vitro: angiotensin II-stimulated CRP and MMP-9 expression in VSMC [74] |
↓IL-6, TNF-α PPAR-γ, PPAR-α NF-κB subunit p65, IL-6, ICAM-1 ↓IL-1β, COX-2, MPO ↓IL-1β, IL-6, COX-2, iNOS, TNF-α, NF-κB, MAPK ↓IL-1β, IL-6, IL-8, TNF-α, IL-17, IL-23, CCL2, NF-κB, VEGFA ↓IL-1β, IL-18, TNF-α, MPO, NLRP3 inflammasome ↓IL-6, TNF-α, ↓IL-1β, IL-6, COX-2, iNOS, TNF-α, NF-κB p65 ↑HO-1, Nrf2 ↓sTNFR1, tyrosine phosphorylation ↓TNF-α, COX-2, Nos2, ICAM-1, MMP-2, MMP-9 ↓TNF-α, NF-κB ↓p-ERK1/2, p-p38, NF-κB ↑PPAR-γ, |
| Rutin | In vivo: HMGB1-induced inflammation and CLP-induced sepsis model [75] In vivo: LPS-induced acute endotoxemic kidney injury in C57BL/6 mice [76] In vivo: NaF-induced neurotoxicity [77] In vivo: HgCl2-induced nephrotoxicity [78] In vivo: HgCl2-induced hepatotoxicity [79] In vitro: PMA-induced neutrophil stimulation [80] |
↓TLR 4, RAGE, p38 MAPK, VCAM-1, ICAM-1, ERK1/2, NF-κB ↓TLR 4, COX-2, TNF-α, IL-6, SIRT1, NF-κB ↓IL-1β, IL-6, TNF-α ↓IL-1β, IL-33, TNF-α, NF-κB, Bcl-3 ↓IL-1β, TNF-α, NF-κB, Bcl-3, Bcl-2, Bax, p53, p38 MAPK, caspase-3 ↓NO, TNF-α, MPO |
| Taxifolin | In vitro: osteoclastogenesis [81] In vivo: and ovariectomy-induced osteoporosis [81] In vivo: osteolysis model [82] In vitro: on IgE/Ag-stimulated mast cells including BMMCs [83] In vivo: acetaminophen-induced liver injury [84] |
↓AKT, RANKL ↓TNF-α, IL-1β, NF-κB, MAPK, NFATc1, MMP-9, cathepsin K, TRAP ↓MAPK, p38, ERK, JNK; RANKL, NF-κB ↓LTC4, IL-6, COX-2, TNF-α, NF-κB ↓ inhibiting metabolic activation mediated by CYP450 enzymes |
Spagnuolo et al. discussed the flavonoids neuroprotective potential, in particular flavonols, another family well represented in G. tridentata [40]. There is some evidence, at least in in vitro studies, that these flavonoids reduce neuroinflammation also by regulating important signaling pathways such as NF-κB and MAPKs (Table 4) [40].
Considering all these pieces of evidence and the fact that several flavonoids were found in G. tridentata, we selected some significative examples to discuss their anti-inflammatory potential, and Table 4 summarizes the effect and mechanism of action of the selected flavonoids.
4.1. Biochanin A and Prunetin
Biochanin A 1h and prunetin 1d are isomeric natural isoflavones (Figure 3) produced by G. tridentata not as the major components, but in small amounts, 4.8% (μg/g) for biochanin A 1h and 4.1% (μg/g) for prunetin 1d [29]. Some derivatives are also reported, and in particular, the methyl derivative that was not fully identified [29]; in fact, if there is no evidence of mass spectra fragments containing the characteristic A ring fragment [8] or the compound was isolated [20], it is possible to confuse these isomers. One fact is consistent—G. tridentata produced one or both.
Figure 3.
Biochanin A 1h and prunetin 1d structures.
As far as we could find, prunetin 1d was isolated for the first time in 1952 from Pterocarpus angolensis DC. [85] and biochanin A 1h was isolated from Cicer arietinum L. in 1945 [86]. Although these isoflavones’ natural occurrence seems to be similar, from the biological evaluation point of view, biochanin A 1h has been extensively studied, and several health benefits were attributed to its consumption as well as its possible use to develop new drugs [87,88], and anti-inflammatory activity is among those biological properties.
In this century, several evaluations regarding the biochanin A 1h anti-inflammatory activity have been performed (Table 4), and the first example is the study of Kalayciyan et al. [41], in which the compound potential to treat the Behçet’s disease was established. The main anti-inflammatory effect of the compound is to decrease the secretion of interleukin-8 (IL-8), a potent leukocyte chemotactic factor known to induce inflammation [41]. More recently, it was also proved that biochanin A 1h inhibits the IL-8 expression in lipopolysaccharides (LPS)-stimulated human vascular endothelial cells in a dose-dependent manner [42], as well as in focal cerebral ischemia/reperfusion in rats [43]. The biochanin A 1h effects on other interleukins levels, such as IL-1β, IL-6, IL-10, and IL-18, were evaluated in the last feew years, with IL-1β being the most studied one [44,45,46,47,48,49,50,51,52,53,54]. All these studies proved the inhibitory effect that biochanin A 1h has on these inflammatory cytokines. However, the most important aspect is the fact that some of the studies were performed in vivo [43,46,49,50,51,52], which is a forward step to establish this compound pharmacological potential.
The inhibition of another important pro-inflammatory species, such as TNF-α, was also evaluated by several authors [42,43,44,45,46,47,48,49,52,53,54,55,56], as well as the inhibiting pro-inflammatory enzymes [49,55] and key phosphorylation steps [44,48,56,57]. All of these studies suggested that biochanin A 1h’s anti-inflammatory effect occurs by suppressing the pathways NF-κB and MAPK [53,56,57,58], but is also associated with the up-regulation of PPAR expression [43,45,53,54]. Prunetin 1d, a much less studied compound, also presents potent in vitro [56,59,60] and in vivo [59] anti-inflammatory activity, and apparently, its mechanism of action is also associated with the inhibition of the NF-κB pathway [59].
It should be highlighted that several of the studies mentioned above included the evaluation of cytotoxic effects, and all demonstrated that both isoflavones do not affect the viability of the cells, and in the subsequent tests the authors used noncytotoxic concentrations. From these studies, essential facts arose—prunetin 1d should be subjected to more evaluations. Moreover, pharmacodynamic and pharmacokinetic parameters of both isoflavones should be evaluated in order to implement some clinical trials in the future.
4.2. Daidzein
Daidzein 1j (Figure 4) is a natural isoflavone with a significant occurrence, mainly in fruits and nuts [89], which is the reason why humans are exposed to it and also to its health benefits [90]. In fact, several pharmacological properties are attributed to this isoflavone [91], including anti-inflammatory potential [10,91]. Although daidzein 1j occurrence in G. tridentata is rare, only one report on its identification was reported (Table 2), we decided to include here the most recent works on its anti-inflammatory activity, since its occurrence seems to be exclusively in the plant roots [8]. This fact gives importance to that part of the plant, while importance usually is only given to the flowers and aerial parts, which are the ones used traditionally.
Figure 4.
Daidzein 1j structure.
The most recent studies involved in vivo studies with daidzein 1j—the reasons why are herein highlighted. Due to its occurrence in common fruits [89], daidzein 1j is present in mankind’s diet, and it is a nontoxic compound [52]. These recent studies confirmed daidzein 1j’s strong anti-inflammatory activity as well as settling on its mechanism of action (Table 4). Mainly, daidzein 1j strongly affects various pathways, including NF-κB, p38MAPK, and TGF-β1. Regardless of this potential as an anti-inflammatory drug, as far as we could find, daidzein 1j is not involved in clinical trials.
4.3. Genistein
Genistein 1e (Figure 5), like daidzein 1j, occurs naturally in everyday food, such as fruits and nuts [89], and as far as we could find, it is non-toxic for humans [92], which was also recently reinforced by Kumar et al. [93]. The pharmacological potential of genistein 1e is well documented [94]; more recently, an overview regarding their mechanism of action in cancer models was published [95], and in some aspects, the anticancer and the anti-inflammatory activities are associated.
Figure 5.
Genistein 1e structure.
Regarding the anti-inflammatory activity studies, it should be emphasized that, recently, there are more in vivo studies, meaning that scientists are interested in giving this natural isoflavone new medicinal applications. From the reported results, we select a few (Table 4) that demonstrategenistein 1e’s potential to become an anti-inflammatory drug.
It can be seen that like the isoflavones mentioned above, genistein 1e targets the same pathways, with an emphasis on the upregulation of the PPARγ signaling pathway and downregulation of the NF-κB signaling pathway, as well as the decrease in several inflammatory mediators (Table 4). In light of the referred studies, genistein 1e is a candidate to be used in the prevention or treatment of inflammation-related diseases. For example, it could be used to target microRNAs, which is considered a therapeutic target for liver disease. In fact, the results show that the anti-inflammatory activity of genistein 1e downregulated microRNA expression of liver inflammation [70] but also pro-inflammatory cytokines species such as IL-1β and TNF-α [70,73]. Another interesting example is its ability to attenuate NF-κB inflammatory signaling in the brain with consequent inhibition of pro-inflammatory cytokines release, which gives genistein 1e the possibility to become a new drug able to relieve chronic sleep deprivation’s adverse effects [71]. Furthermore, there is some evidence supporting that genistein 1e can, through its anti-inflammatory activity, prevent cardiovascular diseases [74]. Altogether, these findings suggest that genistein is a good candidate for future clinical trials.
4.4. Rutin
Rutin 3c (Figure 6) is amongst the most found flavonoids in G. tridentata (Table 3), for which several biological and pharmacological properties have been established and reviewed through the years [96,97,98,99,100]. Some more specific activities, such as antidiabetic effects [101], reestablishment of the immune homeostasis [96,102], neuroprotective effects [98,103,104] and anticancer effects [98,99,105] were also addressed. Furthermore, some toxicological studies were also performed [98,106] as well as pharmacokinetic [98], bioavailability [99] and formulation development [100]. It should be emphasized that the mentioned properties prompted some clinical trials using rutin 3c [107,108] and although the results are not remarkable, they at least confirm that it is safe to use rutin 3c.
Figure 6.
Rutin 3c structure.
Obviously, rutin 3c‘s anti-inflammatory activity was also evaluated and several interesting results were reported (Table 4). It is known that in general, flavonoids decrease the production of pro-inflammatory interleukins, mainly IL-1β, IL-6, and IL-8, but also tumor necrosis factor α (TNF-α). There is evidence that rutin 3c anti-inflammatory mechanism also involves the downregulation of these pro-inflammatory species [76,77,78,79,80]. The results show that rutin 3c can also exert its anti-inflammatory activity through other mechanisms (Table 4), from which can be highlighted the inhibition of the HMGB1 signaling pathway through the downregulation of TLR4 and RAGE expressions [75] and also the inhibition of the MPO activity [80]. The last one is an important example because it provides evidence that rutin 3c can be a possible therapeutic agent for autoimmune diseases [80].
Collectively, the results demonstrate that rutin 3c attenuates inflammation through several mechanisms and is a nontoxic compound, so clinical trials more focused on its anti-inflammatory potential should be implemented. In this regard, Kalita and Das [109] studied the efficiency of a rutin 3c formulation to be used in the treatment of inflammations through the long-term delivery via the skin. Their results, although preliminary, are sufficiently good to encourage future investigations.
4.5. Taxifolin
Our last example is taxifolin (Figure 2), which, as shown in the previous section, occurs in G. tridentata, mainly linked to sugar moieties. Nevertheless, we specify here some interesting studies due to the fact that in a living organism, it is possible to obtain the aglycone. The taxifolin anti-inflammatory potential has been known, at least, since 1971 [110] and recently Sunil and Xu published an interesting review on taxifolin’s health benefits [111]. Some important aspects arose from this review: the first is the broad biological potential of taxifolin, mainly using in vitro evaluations, but also that the anti-inflammatory and toxicological evaluations are still scarce. The few examples (Table 4) suggest that its mechanism of action is similar to the one reported for the other flavonoids, that is also mainly targets the NF-κB and MAPK pathways. Although, the anti-inflammatory assessments are scarce, they suggest taxifolin’s potential to be a drug candidate for the treatment of inflammations, suggesting that it should be further investigated.
5. Conclusions
This survey demonstrates beyond any doubt that G. tridantata is a source of bioactive metabolites, some of which present interesting anti-inflammatory activities which, in turn, contribute to the extracts’ anti-inflammatory activity. Amongst our findings, the toxicological evaluations of both extracts and pure compounds are important and contribute to establishing G. tridentata’s medicinal value as well as the secondary metabolites’ pharmacological value. However, in our opinion, some efforts on the plant taxonomy should be made to prevent confusion in the data reported. Moreover, we think that an extra effort on clinical trials, mainly concerning the pure compounds used as drugs, should be performed.
Acknowledgments
Thanks are due to the University of Aveiro and FCT/MCT for the financial support for the LAQV-REQUIMTE (UIDB/50006/2020) through national founds and, where applicable, co-financed by the FEDER, within the PT2020 Partnership Agreement.
Abbreviations
| AAA | abdominal aortic aneurysm |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| Ag | antigen |
| AKT | serine/threonine kinase |
| Bax | Bcl-2 associated X protein |
| Bcl-2 | B-cell lymphoma-2 |
| Bcl-3 | B-cell lymphoma-3 |
| BMMCs | bone marrow derived mast cells |
| caspase-3 | cysteine aspartate specific protease-3 |
| CCL2 | chemokine ligand 2 |
| CLP | cecal ligation and puncture |
| CRP | C-reactive protein |
| CXC | α-chemokines |
| CYP450 | Cytochrome P450 |
| DPPH● | 2,2-diphenyl-1-picrylhydrazyl radical |
| DSS | dextran sulfate sodium |
| E-selection | endothelial cells |
| ERK | extracellular signal-regulated protein kinase |
| G. | Genista |
| GalN | D-galactosamine |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| GPx | glutathione peroxidase |
| HMEC-1 | human dermal microvascular endothelial cell-1 |
| HMGB1 | high mobility group box 1 |
| HO-1 | heme oxygenase-1 |
| HUVEC | human umbilical vein endothelial |
| ICAM-1 | intercellular adhesion molecule-1 |
| IFNγ | interferon gamma |
| IgE | immunoglobulin E |
| IKK | IκB kinase |
| IL-10 | interleukin-10 |
| IL-12 | interleukin-12 |
| IL-18 | interleukin-18 |
| IL-1α | interleukin-1α |
| IL-1β | interleukin-1β |
| IL-2 | interleukin-2 |
| IL-6 | interleukin-6 |
| IL-8 | interleukin-8 |
| iNOS | inducible nitric oxide synthase |
| JNK | c-jun N-terminal kinase |
| LIX | lipopolysaccharide-induced CXC chemokine |
| LPS | lipopolysaccharides |
| LTC4 | cysteinyl leukotriene 4 |
| MAPK | mitogen-activated protein kinases |
| MCP-1 | monocyte chemoattractant protein-1 |
| MDA | malondialdehyde |
| MIP-3α | macrophage inflammatory protein 3 α |
| MMP | matrix metalloproteinases |
| MPO | myeloperoxidase |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MUC5AC | mucin 5AC glycoprotein |
| MyD88 | myeloid differentiation primary response 88 |
| NASH | nonalcoholic steatohepatitis |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NFATc1 | nuclear factor-activated T cells c1 |
| NLRP3 | NRL pyrin domain containing 3 |
| Nos2 | nitric oxide synthase 2 |
| Nrf2 | nuclear factor erythroid 2 |
| NRL | nucleotide-binding, leucine-rich repeat containing proteins |
| PGE2 | prostaglandin E2 |
| PMA | phorbol 12-myristate 13-acetate |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| Ptgs2 | prostaglandin-endoperoxide synthase 2 |
| RAGE | receptor for advanced glycation end-products |
| RANKL | receptor activator of nuclear factor-κB ligand |
| SIRT1 | sirtuin 1 |
| SOD | superoxide dismutase |
| sTNFR1 | soluble tumor necrosis factor receptor-1 |
| TBARS | thiobarbituric acid reactive substances |
| TEER | transepithelial electrical resistance |
| TGF-β1 | transforming growth factor β1 |
| TLR 4 | toll-like receptors 4 |
| TNF-α | tumor necrosis factor alpha |
| TRAP | tartrate-resistant acid phospha- tase |
| TXNIP | thioredoxin-interacting protein |
| VCAM-1 | vascular cytoadhesion molecule-1 |
| VEGF | vascular endothelial growth factor |
| VEGFA | vascular endothelial growth factor A |
| VSMC | vascular smooth muscle cells |
Author Contributions
D.C.G.A.P. and M.A.M.S. performed the literature survey; D.C.G.A.P. and A.M.S.S. conceived and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ahmed A.U. An overview of inflammation: Mechanism and consequences. Front. Biol. 2011;6:274–281. doi: 10.1007/s11515-011-1123-9. [DOI] [Google Scholar]
- 2.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 3.Glass C.K., Saijo K., Winner B., Marchetto M.C., Gage F.H. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veeresham C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Tech. Res. 2012;3:200–201. doi: 10.4103/2231-4040.104709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neves J.M., Matos C.M., Moutinho C.G., Gomes L.R. Usos Populares de Plantas Medicinais da Flora Transmontana. Edições Universidade Fernando Pessoa; Porto, Portugal: 2008. pp. 226–235. [Google Scholar]
- 6.Novais M.H., Santos I., Mendes S., Pinto-Gomes C. Studies on pharmaceutical ethnobotany in Arrabida Natural Park (Portugal) J. Ethnopharm. 2004;93:183–195. doi: 10.1016/j.jep.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Ferrándiz M.L., Alcaraz M.J. Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids. Agents Actions. 1991;32:283–288. doi: 10.1007/BF01980887. [DOI] [PubMed] [Google Scholar]
- 8.Simões M.A.M., Pinto D.C.G.A., Neves B.M.R., Silva A.M.S. Flavonoid profile of the Genista tridentata L., a species used traditionally to treat inflammatory processes. Molecules. 2020;25:812. doi: 10.3390/molecules25040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hämäläinen M., Nieminen R., Vuorela M., Moilanen E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007 doi: 10.1155/2007/45673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J., Bi X., Yu B., Chen D. Isoflavones: Anti-inflammatory benefit and possible caveats. Nutrients. 2016;8:361. doi: 10.3390/nu8060361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H.Q., Jin Z.Y., Li G.H. Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage through inhibition of microglia activation and proinflammatory factors generation. Neurosci. Lett. 2007;417:112–117. doi: 10.1016/j.neulet.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 12.Tan J.W., Kim M.K. Neuroprotective effects of biochanin A against b-amyloid-induced neurotoxicity in PC12 cells via a mitochondrial-dependent apoptosis pathway. Molecules. 2016;21:548. doi: 10.3390/molecules21050548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teixeira G., Pereira A.L. Winged stems in Pterospartum tridentatum: Morphoanatomical study. Acta Bot. Gall. 2004;151:103–109. doi: 10.1080/12538078.2004.10516023. [DOI] [Google Scholar]
- 14.Neves J.M., Matos C., Moutinho C., Queiroz G., Gomes L.R. Etgnopharmacological notes about ancient uses of medicinal plants in Trás-os-Montes (northern of Portugal) J. Ethnopharmacol. 2009;124:270–283. doi: 10.1016/j.jep.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 15.The Plant List Database. [(accessed on 26 May 2020)]; Available online: http://www.theplantlist.org/
- 16.Carvalho A.M. Biblioteca de Ciencias No 35. Consejo Superior de Investigaciones Científicas; Madrid, Spain: 2010. Plantas y sabiduría popular del Parque Natural de Montesinho: Un estudio etnobotánico en Portugal; p. 496. [Google Scholar]
- 17.Flora-On. [(accessed on 26 May 2020)]; Available online: https://flora-on.pt/index.php#/0BJwn.
- 18.Camejo-Rodrigues J., Ascensão L., Bonet M.À., Vallès J. An ethnobotanical study of medicinal and aromatic plants in the natural park of “Serra de São Mamede”)Portugal) J. Ethnopharmacol. 2003;89:199–209. doi: 10.1016/S0378-8741(03)00270-8. [DOI] [PubMed] [Google Scholar]
- 19.Rivera D., Verde J., Fajardo J., Obón C., Consuegra V., García-Botía J., Ríos S., Alcaraz F., Valdés A., del Moral A., et al. Ethnopharmacology in the upper Guadiana river área (Castile-La Mancha, Spain) J. Ethnopharmacol. 2019;241:111968. doi: 10.1016/j.jep.2019.111968. [DOI] [PubMed] [Google Scholar]
- 20.Vitor R.F., Mota-Filipe H., Teixeira G., Borges C., Rodrigues A.I., Teixeira A., Paulo A. Flavonoids of na extract of Pterospartum tridentatum showing endothelial protection against oxidative injury. J. Ethnopharmacol. 2004;93:363–370. doi: 10.1016/j.jep.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Grosso A.C., Costa M.M., Ganço L., Pereira A.L., Teixeira G., Lavado J.M.G., Figueireido A.C., Pedro L.G. Essential oil composition of Pterospartum tridentatum grown in Portugal. Food Chem. 2007;102:1083–1088. doi: 10.1016/j.foodchem.2006.06.049. [DOI] [Google Scholar]
- 22.Coelho M.T., Gonçalves J.C., Alves V., Martins M.M. Antioxidant activity and phenolic content of extracts from different Pterospartum tridentatum populations growing in Portugal. Procedia Food Sci. 2011;1:1454–1458. doi: 10.1016/j.profoo.2011.09.215. [DOI] [Google Scholar]
- 23.Taylor J.L.S., Rabe T., McGaw L.J., Jäger A.K., van Staden J. Towards the scientific validation of traditional medicinal plants. Plant. Growth Reg. 2001;34:23–37. doi: 10.1023/A:1013310809275. [DOI] [Google Scholar]
- 24.Luís Â., Domingues F., Gil C., Duarte A.P. Antioxidant activity of extracts of Portuguese shrubs: Pterospartum tridentatum, Cytisus scoparius and Erica spp. J. Med. Plant. Res. 2009;3:886–893. [Google Scholar]
- 25.Luís Â., Domingues F., Duarte A.P. Bioactive compounds, RP-HPLC analysis of phenolics, and antioxidant activity of some Portuguese shrub species extracts. Nat. Prod. Commun. 2011;6:1863–1872. doi: 10.1177/1934578X1100601219. [DOI] [PubMed] [Google Scholar]
- 26.Pinela J., Barros L., Carvalho A.M., Ferreira I.C.F.R. Influence of the drying method in the antioxidant potential and chemical composition of four shrubby flowering plants from the tribe Genisteae (Fabaceae) Food Chem. Toxicol. 2011;48:2983–2989. doi: 10.1016/j.fct.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 27.Martínez A., Estévez J.C., Silva-Pando F.J. Antioxidant activity, total phenolic content and skin care properties of 35 selected plants from Galicia (NW Spain) Front. Life Sci. 2012;6:77–86. doi: 10.1080/21553769.2013.776994. [DOI] [Google Scholar]
- 28.Ferreira F.M., Dinis L.T., Azedo P., Galhano C.I.C., Simões A., Cardoso S.M., Domingues M.R.M., Pereira O.R., Palmeira C.M., Peixoto F.P. Antioxidant capacity and toxicological evaluation of Pterospartum tridentatum flower extracts. CyTA J. Food. 2012;10:92–102. doi: 10.1080/19476337.2011.590233. [DOI] [Google Scholar]
- 29.Roriz C.L., Barros L., Carvalho A.M., Santos-Buelga C., Ferreira I.C.F.R. Pterospartum tridentatum, Gomphrena globosa and Cymbopogon citratus: A phytochemical study focused on antioxidant compounds. Food Res. Int. 2014;62:684–693. doi: 10.1016/j.foodres.2014.04.036. [DOI] [Google Scholar]
- 30.Roriz C.L., Barros L., Carvalho A.M., Santos-Buelga C., Ferreira I.C.F.R. Scientific validation of synergistic antioxidant effects in commercialized mixtures of Cymbopogon citratus and Pterospartum tridentatum or Gomphrena globose for infusions preparation. Food Chem. 2015;185:16–24. doi: 10.1016/j.foodchem.2015.03.136. [DOI] [PubMed] [Google Scholar]
- 31.Caleja C., Finimundy T.C., Pereira C., Barros L., Calhelha R.C., Sokovic M., Ivanov M., Carvalho A.M., Rosa E., Ferreira I.C.F.R. Challenges of traditional herbal teas: Plant infusions and their mixtures with bioactive properties. Food Funct. 2019;10:5939–5951. doi: 10.1039/C9FO01473J. [DOI] [PubMed] [Google Scholar]
- 32.Martins N., Ferreira I.C.F.R., Barros L., Carvalho A.M., Henriques M., Silva S. Plants used in folk medicine: The potential of their hydromethanolic extracts against Candida species. Ind. Crops Prod. 2015;66:62–67. doi: 10.1016/j.indcrop.2014.12.033. [DOI] [Google Scholar]
- 33.Aires A., Marrinhas E., Carvalho R., Dias C., Saavedra M.J. Phytochemical composition and antibacterial activity of hydroalcoholic extracts of Pterospartum tridentatum and Mentha pulegium against Staphylococcus aureus isolates. BioMed Res. Int. 2016;2016 doi: 10.1155/2016/5201879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martins V.M.R., Simões J., Ferreira I., Cruz M.T., Domingues M.R., Coimbra M.A. In vitro macrophage nitric oxide production by Pterospartum tridentatum (L.) Willk. inflorescence polysaccharides. Carbohydr. Polym. 2017;157:176–184. doi: 10.1016/j.carbpol.2016.09.079. [DOI] [PubMed] [Google Scholar]
- 35.Pinto D.C.G.A., Silva A.M.S. Valorisation of Portuguese natural resources. Phytochem. Rev. 2020 doi: 10.1007/s11101-020-09666-9. [DOI] [Google Scholar]
- 36.Paulo A., Martins S., Branco P., Dias T., Borges C., Rodrigues A.I., Costa M.C., Teixeira A., Mota-Filipe H. The opposing effects of the flavonoids isoquercitrin and sissotrin, isolated from Pterospartum tridentatum, on oral glucose tolerance in rats. Phytother. Res. 2008;22:539–543. doi: 10.1002/ptr.2403. [DOI] [PubMed] [Google Scholar]
- 37.Silva C.F.M., Pinto D.C.G.A., Silva A.M.S. Chromones: A promising ring-system for new anti-inflammatory drugs. ChemMedChem. 2016;11:2252–2260. doi: 10.1002/cmdc.201600359. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Rojas B., Gutiérrez-Venegas G. Flavonoids exert multiple periodontic benefits including anti-inflammatory, periodontal ligament-supporting, and alveolar bone-preserving effects. Life Sci. 2018;209:435–454. doi: 10.1016/j.lfs.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 39.Choy K.W., Murugain D., Leong X.-F., Abas R., Alias A., Mustafa M.R. Flavonoids as natural anti-inflammatory agents targeting nuclear factor-kappa B (NFκB) signaling in cardiovascular diseases: A mini review. Front. Pharmacol. 2019;10:1295. doi: 10.3389/fphar.2019.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spagnuolo C., Moccia S., Russo G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018;153:105–115. doi: 10.1016/j.ejmech.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Klayciyan A., Orawa H., Fimmel S., Perschel F.H., González J.-B., Fitzner R.G., Orfanos C.E., Zouboulis C.C. Nicotine and biochanin A, but not cigarette smoke, induce anti-inflammatory effects on keratinocytes and endothelial cells in patients with Behçet’s disease. J. Investig. Dermatol. 2007;127:81–89. doi: 10.1038/sj.jid.5700492. [DOI] [PubMed] [Google Scholar]
- 42.Ming X., Ding M., Zhai B., Xiao L., Piao T., Liu M. Biochanin A inhibits lipopolysaccharide-induced inflammation in human umbilical vein endothelial cells. Life Sci. 2015;136:36–41. doi: 10.1016/j.lfs.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 43.Wang W., Tang L., Li Y., Wang Y. Biochanin A protects against focal cerebral ischemia/reperfusion in rats via inhibition of p38-mediated inflammatory responses. J. Neurol. Sci. 2015;348:121–125. doi: 10.1016/j.jns.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 44.Wu W.-Y., Wu Y.-Y., Huang H., He C., Li W.-Z., Wang H.-L., Chen H.-Q., Yin Y.-Y. Biochanin A attenuates LPS-induced pro-inflammatory responses and inhibits the activation of the MAPK pathway in BV2 microglial cells. Int. J. Mol. Med. 2015;35:391–398. doi: 10.3892/ijmm.2014.2020. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y., Chen W. Biochanin A inhibits lipopolysaccharide-induced inflammatory cytokines and mediators production in BV2 microglia. Neurochem. Res. 2015;40:165–171. doi: 10.1007/s11064-014-1480-2. [DOI] [PubMed] [Google Scholar]
- 46.Liu X., Wang T., Liu X., Cai L., Qi J., Zhang P., Li Y. Biochanin A protects lipopolysaccharide/D-galactosamine-induced acute liver injury in mice by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Int. Immunopharmacol. 2016;38:324–331. doi: 10.1016/j.intimp.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Oh J.-S., Cho I.-A., Kang K.-R., You J.-S., Yu S.-J., Lee G.-J., Seo Y.-S., Kim C.S., Kim D.K., Kim S.-G., et al. Biochanin-A antagonizes the interleukin-1b-induced catabolic inflammation through the modulation of NFκB cellular signaling in primary rat chondrocytes. Biochem. Biophys. Res. Commun. 2016;477:723–730. doi: 10.1016/j.bbrc.2016.06.126. [DOI] [PubMed] [Google Scholar]
- 48.Wang J., Wu W.-Y., Huang H., Li W.-Z., Chen H.-Q., Yin Y.-Y. Biochanin A protects against lipopolysaccharide-induced damage of dopaminergic neurons both in vivo and in vitro via inhibition of microglial activation. Neurotox. Res. 2016;30:486–498. doi: 10.1007/s12640-016-9648-y. [DOI] [PubMed] [Google Scholar]
- 49.Suliman F.A., Khodeer D.M., Ibrahiem A., Mehanna E.T., El-Kherbetawy M.K., Mohmmad H.M.F., Zaitone S.A., Moustafa Y.M. Renoprotective effect of the isoflavonoid biochanin A against cisplatin induced acute kidney injury in mice: Effect on inflammatory burden and p53 apoptosis. Int. Immunopharmacol. 2018;61:8–19. doi: 10.1016/j.intimp.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 50.Alauddin, Chaturvedi S., Malik M.Y., Azmi L., Shukla I., Naseem Z., Rao C.V., Agarwal N.K. Formononetin and biochanin A protects against ritonavir induced hepatotoxicity via modulation of NfκB/pAkt signaling molecules. Life Sci. 2018;213:174–182. doi: 10.1016/j.lfs.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 51.Bai Y., Li Z., Liu W., Gao D., Liu M., Zhang P. Biochanin A attenuates myocardial ischemia/reperfusion injury through the TLR4/NF-κB/NLRP3 signaling pathway. Acta Cir. Bras. 2019;34:e201901104. doi: 10.1590/s0102-865020190110000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu X., Qin H., Li Y., Li J., Fu L., Li M., Jiang C., Yun J., Liu Z., Feng Y., et al. Biochanin A protect against lipopolysaccharide-induced acute injury in mice by regulating TLR4//NF-κB and PPAR-γ parhway. Microb. Pathogen. 2020;138:103846. doi: 10.1016/j.micpath.2019.103846. [DOI] [PubMed] [Google Scholar]
- 53.Kole L., Giri B., Manna S.K., Pal B., Ghosh S. Biochanin A, an isoflavone, showed anti-proliferative and anti-inflammatory activities through the inhibition of iNOS expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB nuclear translocation. Eur. J. Pharmacol. 2011;653:8–15. doi: 10.1016/j.ejphar.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 54.Breikaa R.M., Algandaby M.M., El-Demerdas E., Abdel-Naim A.B. Biochanin A protects against acute carbon tetrachloride-induced hepatotoxicity in rats. Biosci. Biotechnol. Biochem. 2013;77:909–916. doi: 10.1271/bbb.120675. [DOI] [PubMed] [Google Scholar]
- 55.Wu L., Ye Z., Zhuang Z., Gao Y., Tang C., Zhou C., Wang C., Zhang X., Xie G., Liu J., et al. Biochanin A reduces inflammatory injury and neuronal apoptosis following subarachnoid hemorrhage via suppression of the TLRs/TIRAP/MyD88/NF-κB pathway. Behav. Neurol. 2018 doi: 10.1155/2018/1960106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piegholdt S., Pallauf K., Esatbeyoglu T., Speck N., Reiss K., Ruddigkeit L., Stocker A., Huebbe P., Rimbach G. Biochanin A and prunetin improve epithelial barrier function in intestinal CaCo-2 cells via downregulation of ERK, NF-κB, and tyrosine phosphorylation. Free Radic. Biol. Med. 2014;70:255–264. doi: 10.1016/j.freeradbiomed.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 57.Qiu L., Lin B., Lin Z., Lin Y., Lin M., Yang X. Biochanin A ameliorates the cytokine secretion profile of lipopolysaccharide-stimulated macrophages by a PPARγ-dependent pathway. Mol. Med. Repor. 2012;5:217–222. doi: 10.3892/mmr.2011.599. [DOI] [PubMed] [Google Scholar]
- 58.Guo M., Lu H., Qin J., Qu S., Wang W., Guo Y., Liao W., Song M., Chen J., Wang Y. Biochanin A provides neuroprotection against cerebral ischemia/reperfusion injury by Nrf2-mediated inhibition of oxidative stress and inflammation signaling pathway in rats. Med. Sci. Monit. 2019;25:8975–8983. doi: 10.12659/MSM.918665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang G., Ham I., Choi H.-Y. Anti-inflammatory effect of prunetin via suppression of NF-κB pathway. Food Chem. Toxicol. 2013;58:124–132. doi: 10.1016/j.fct.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 60.Hu H., Li H. Prunetin inhibits lipopolysaccharide -induced inflammatory cytokine production and MUC5AC expression by inactivating the TLR4/MyD88 pathway in human nasal epithelial cells. Biomed. Pharmacother. 2018;106:1469–1477. doi: 10.1016/j.biopha.2018.07.093. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y.-F., Bai Y.-Q., Qi M. Daidzein attenuates abdominal aortic aneurysm through NF-κB, p38MAPK and TGF-β1 pathways. Mol. Med. Rep. 2016;14:955–962. doi: 10.3892/mmr.2016.5304. [DOI] [PubMed] [Google Scholar]
- 62.Atiq A., Shal B., Naveed M., Khan A., Ali J., Zeeshan S., Al-Sharari S.D., Kim Y.S., Khan S. Diadzein ameliorates 5-fluorouracil-induced intestinal mucositis by suppressing oxidative stress and inflammatory mediators in rodents. Eur. J. Pharmacol. 2019;843:292–306. doi: 10.1016/j.ejphar.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 63.Tomar A., Kaushik S., Khan S.I., Bisht K., Nag C.N., Arya D.S., Bhatia J. The dietary isoflavone daidzein mitigates oxidative stress, apoptosis, and inflammation in CDDP-induced kidney injury in rats: Impact on the MAPK signaling pathway. J. Biochem. Mol. Toxicol. 2020;34:e22431. doi: 10.1002/jbt.22431. [DOI] [PubMed] [Google Scholar]
- 64.Zhang F., Ru N., Shang X.-H., Chen J.-F., Yan C., Li Y., Liang J. Daidzein ameliorates spinal cord ischemia/reperfusion injury-induced neurological function deficits in Sprague-Dawley rats through PI3K/Akt signaling pathway. Exp. Ther. Med. 2017;14:4878–4886. doi: 10.3892/etm.2017.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han S., Wu H., Li W., Gao P. Protective effects of genistein in homocysteine-induced endothelial cell inflammatory injury. Mol. Cell Biochem. 2015;403:43–49. doi: 10.1007/s11010-015-2335-0. [DOI] [PubMed] [Google Scholar]
- 66.Mansour D.F., Saleh D.O., Mostafa R.E. Genistein ameliorates cyclophosphamide-induced hepatotoxicity by modulation of oxidative stress and inflammatory mediators. Open Access Maced. J. Med. Sci. 2017;5:836–843. doi: 10.3889/oamjms.2017.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Du Z.-R., Feng X.-Q., Li N., Qu J.-X., Feng L., Chen L., Chen W.-F. G protein-coupled estrogen receptor is involved in the anti-inflammatory effects of genistein in microglia. Phytomedicine. 2018;43:11–20. doi: 10.1016/j.phymed.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 68.Wang A., Wei J., Lu C., Chen H., Zhong X., Lu Y., Li L., Huang H., Dai Z., Han L. Genistein suppresses psoriasis-related inflammation through a STAT3-NF-κB-dependent mechanism in keratinocytes. Int. Immunopharmacol. 2019;69:270–278. doi: 10.1016/j.intimp.2019.01.054. [DOI] [PubMed] [Google Scholar]
- 69.Chen Y., Le T.H., Du Q., Zhao Z., Liu Y., Zou J., Hua W., Liu C., Zhu Y. Genistein protects against DSS-induced colitis by inhibiting NLRP3 inflammasome via TGR5-cAMP signaling. Int. Immunopharmacol. 2019;71:144–154. doi: 10.1016/j.intimp.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 70.Gan M., Shen L., Fan Y., Tan Y., Zheng T., Tang G., Niu L., Zhao Y., Chen L., Jiang D., et al. MicroRNA-451 and genistein ameliorate nonalcoholic steatohepatitis in mice. Int. J. Mol. Sci. 2019;20:6084. doi: 10.3390/ijms20236084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu C., Lv J., Jiang N., Wang H., Huang H., Zhang L., Li S., Zhang N., Fan B., Liu X., et al. Protective effects of genistein on the cognitive deficits induced by chronic sleep deprivation. Phytother. Res. 2020;34:846–858. doi: 10.1002/ptr.6567. [DOI] [PubMed] [Google Scholar]
- 72.Bhattarai G., Poudel S.B., Kook S.-H., Lee J.-C. Anti-inflammatory, anti-osteoclastic, and antioxidant activities of genistein protect against alveolar bone loss and periodontal tissue degradation in a mouse model of periodontitis. J. Biomed. Mat. Res. 2017;195A:2510–2521. doi: 10.1002/jbm.a.36109. [DOI] [PubMed] [Google Scholar]
- 73.Pummoung S., Werawatganon D., Klaikeaw N., Siriviriyakul P. Genistein-attenuated hepatic steatosis and inflammation in nonalcoholic steatohepatitis with bilateral ovariectomized rats. Pharmacogn. Mag. 2018;14:S20–S24. [Google Scholar]
- 74.Xu L., Liu J., Li K., Wang S., Xu S. Genistein inhibits ang II- induced CRP and MMP-9 generations via Er-p38/ERK1/2-PPARγ-NF-κB signaling pathway in rat vascular smooth muscle cells. Life Sci. 2019;216:140–146. doi: 10.1016/j.lfs.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 75.Yoo H., Ku S.-K., Baek Y.-D., Bae J.-S. Anti-inflammatory effects of rutin on HMGB1-induced inflammatory responses in vitro and in vivo. Inflamm. Res. 2014;63:197–206. doi: 10.1007/s00011-013-0689-x. [DOI] [PubMed] [Google Scholar]
- 76.Khajevand-Khazaei M.-R., Mohseni-Moghaddam P., Hosseini M., Gholami L., Baluchnejadmojarad T., Roghani M. Rutin, a quercetin glycoside, alleviates acute endotoxemic kidney injury in C57Bl/6 mice via suppression of inflammation and up-regulation of antioxidants and SIRT1. Eur. J. Pharmacol. 2018;833:307–313. doi: 10.1016/j.ejphar.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 77.Nkpaa K.W., Onyeso G.I. Rutin attenuates neurobehavioral deficits, oxidative stress, neuroinflammation and apoptosis in fluoride treated rats. Neurosci. Lett. 2018;682:92–99. doi: 10.1016/j.neulet.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 78.Caglayan C., Kandemir F.M., Yildirim S., Kucukler S., Eser G. Rutin protects mercuric chloride-induced nephrotoxicity via targeting of aquaporin 1 level, oxidative stress, apoptosis and inflammation in rats. J. Trace Elem. Med. Biol. 2019;54:69–78. doi: 10.1016/j.jtemb.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 79.Caglayan C., Kandemir F.M., Darendelioglu E., Yildirim S., Kucukler S., Dortbudak M.B. Rutin ameliorates mercuric chloride-induced hepatotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis. J. Trace Elem. Med. Biol. 2019;56:60–68. doi: 10.1016/j.jtemb.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 80.Nikfarjam B.A., Adineh M., Hajiali F., Nassiri-Asl M. Treatment with rutin—A therapeutic strategy for neutrophil-mediated inflammatory and autoimmune diseases. J. Pharmacopunct. 2017;20:52–56. doi: 10.3831/KPI.2017.20.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cai C., Liu C., Zhao L., Liu H., Li W., Guan H., Zhao L., Xiao J. Effects of taxifolin on osteoclastogenesis in vitro and in vivo. Front. Pharmacol. 2018;9:1286. doi: 10.3389/fphar.2018.01286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang H.-Q., Wang Y.-J., Yang G.-T., Gao Q.-L., Tang M.-X. Taxifolin inhibits receptor activator of NF-kB ligand-induced osteoclastogenesis of Human bone marrow-derived macrophages in vitro and prevents lipopolysaccharide-induced bone loss in vivo. Pharmacology. 2019;103:101–109. doi: 10.1159/000495254. [DOI] [PubMed] [Google Scholar]
- 83.Pan S., Zhao X., Ji N., Shao C., Fu B., Zhang Z., Wang R., Qiu Y., Jin M., Kong D. Inhibitory effect of taxifolin on mast cell activation and mast cell-mediated allergic inflammatory response. Int. Immunopharmacol. 2019;71:205–214. doi: 10.1016/j.intimp.2019.03.038. [DOI] [PubMed] [Google Scholar]
- 84.Hu C., Ye J., Zhao L., Li X., Wang Y., Liu X., Pan L., You L., Chen L., Jia Y., et al. 5,7,3′,4′-Flavan-on-ol (taxifolin) protects against acetaminophen-induced liver injury by regulating the glutathione pathway. Life Sci. 2019;236:116939. doi: 10.1016/j.lfs.2019.116939. [DOI] [PubMed] [Google Scholar]
- 85.King F.E., Jurd L. The chemistry of extractives from hardwoods. Part VIII. *the isolation of 5,4′-dihydroxy-7-methoxyisoflavone (prunetin) from the heartwood of Pterocarpus angolensis and a synthesis of 7,4′dihydroxy-5-methoxyisoflavone hitherto known as prunutsetin. J. Chem. Soc. 1952;1952:3190–3195. [Google Scholar]
- 86.Siddiqui M.T., Siddiqi M. Hypolipidemic principles of Cicer arietinum: Biochanin-A and formononetin. Lipids. 1976;11:243–246. doi: 10.1007/BF02532865. [DOI] [PubMed] [Google Scholar]
- 87.Raheja S., Girdhar A., Lather V., Pandita D. Biochanin A: A phytoestrogen with therapeutic potential. Trends Food Sci. Technol. 2018;79:55–66. doi: 10.1016/j.tifs.2018.07.001. [DOI] [Google Scholar]
- 88.Sarfraz A., Javeed M., Shah M.A., Hussain G., Shafiq N., Sarfraz I., Riaz A., Sadiqa A., Zara S., Kanwal L., et al. Biochanin A: A novel bioactive multifunctional compound from nature. Sci. Total Environ. 2020;722:137907. doi: 10.1016/j.scitotenv.2020.137907. [DOI] [PubMed] [Google Scholar]
- 89.Liggins J., Bluck L.J.C., Runswick S., Atkinson C., Coward W.A., Bingham S.A. Daidzein and genistein content of fruits and nuts. J. Nutr. Biochem. 2000;11:326–331. doi: 10.1016/S0955-2863(00)00085-1. [DOI] [PubMed] [Google Scholar]
- 90.Barlow J., Johnson J.A.P., Scofield L. [(accessed on 29 May 2020)];Fact Sheet on the Phytoestrogen Daidzein. 2007 BCERC COTC Fact. Sheet. Available online: https://www.zerobreastcancer.org/research/bcerc_factsheets_phytoestrogen_daidzein.pdf.
- 91.Sun M.-Y., Ye Y., Xiao L., Rahman K., Xia W., Zhang H. Daidzein: A review of pharmacological effects. Afr. J. Tradit. Complement. Altern. Med. 2016;13:117–132. doi: 10.4314/ajtcam.v13i3.15. [DOI] [Google Scholar]
- 92.Barlow J., Johnson J.A.P., Scofield L. [(accessed on 29 May 2020)];Fact Sheet on the Phytoestrogen Genistein. 2007 BCERC COTC Fact. Sheet. Available online: https://www.zerobreastcancer.org/research/bcerc_factsheets_phytoestrogen_genistein.pdf.
- 93.Kumar M., Singh K., Duraisamy K., Allam A.A., Ajarem J., Chow B.K.C. Protective effect of genistein against compound 48/80 induced anaphylactoid shock via inhibiting MAS related G protein-coupled receptor Χ2 (MRGPRΧ2) Molecules. 2020;25:1028. doi: 10.3390/molecules25051028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Polkowski K., Mazurek A.P. Biological properties of genistein. A review of in vitro and in vivo data. Acta Poloniae Pharm. Drug Res. 2000;57:135–155. [PubMed] [Google Scholar]
- 95.Tuli H.S., Tuorkey M.J., Thakral F., Sak K., Kumar M., Sharma A.K., Sharma U., Jain A., Aggarwal V., Bishayee A. Molecular mechanisms of action of genistein in cancer: Recent advances. Front. Pharmacol. 2019;10:1336. doi: 10.3389/fphar.2019.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Al-Dhabi N.A., Arasu M.V., Park C.H., Park S.U. An up-to-date review of rutin and its biological and pharmacological activities. EXCLI J. 2015;14:59–63. doi: 10.17179/excli2014-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rauf A., Imran M., Patel S., Muzaffar R., Bawazeer S.S. Rutin: Exploitation of the flavonol for health and homeostasis. Biomed. Pharmacother. 2017;96:1559–1561. doi: 10.1016/j.biopha.2017.08.136. [DOI] [PubMed] [Google Scholar]
- 98.Ganeshpurkar A., Saluja A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017;25:149–164. doi: 10.1016/j.jsps.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gullón B., Lú-Chau T.A., Moreira M.T., Lema J.M., Eibes G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017;67:220–235. [Google Scholar]
- 100.Riaz H., Raza S.A., Aslam M.M., Ahmad M.S., Ahmad M.A., Maria P. An updated review of pharmacological, standardization methods and formulation development of rutin. J. Pure App. Microbiol. 2018;12:127–132. doi: 10.22207/JPAM.12.1.16. [DOI] [Google Scholar]
- 101.Ghorbani A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017;96:305–312. doi: 10.1016/j.biopha.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 102.Manzoni A.G., Passos D.F., Leitemperger J.W., Storck T.R., Doleski P.H., Jantsch M.H., Loro V.L., Leal D.B.R. Hyperlipidemia-induced lipotoxicity and immune activation in rats are prevented by curcumin and rutin. Int. Immunopharmacol. 2020;81:106217. doi: 10.1016/j.intimp.2020.106217. [DOI] [PubMed] [Google Scholar]
- 103.Enogieru A.B., Haylett W., Hiss D.C., Bardien S., Ekpo O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid. Med. Cell. Long. 2018;2018 doi: 10.1155/2018/6241017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mazumder M.K., Borah A., Choudhury S. Inhibitory potential of plant secondary metabolites on anti-Parkinsonian drug targets: Relevance to pathophysiology, and motor and non-motor behavioural abnormalities. Med. Hypotheses. 2020;137:109544. doi: 10.1016/j.mehy.2019.109544. [DOI] [PubMed] [Google Scholar]
- 105.Harikrishnan H., Jantan I., Alagan A., Haque M.A. Modulation of cell signaling pathways by Phyllanthus amarus and its major constituents: Potential role in the prevention and treatment of inflammation and cancer. Inflammopharmacology. 2020;28:1–18. doi: 10.1007/s10787-019-00671-9. [DOI] [PubMed] [Google Scholar]
- 106.Hasumura M., Yasuhara K., Tamura T., Imai T., Mitsumori K., Hirose M. Evaluation of the toxicity of enzymatically decomposed rutin with 13-weeks dietary administration to Wistar rats. Food Chem. Toxicol. 2004;42:439–444. doi: 10.1016/j.fct.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 107.Boyle S.P., Dobson V.L., Duthie S.J., Hinselwood D.C., Kyle J.A.M., Collins A.R. Bioavailability and efficiency of rutin as an antioxidant: A human supplementation study. Eur. J. Clin. Nutr. 2000;54:774–782. doi: 10.1038/sj.ejcn.1601090. [DOI] [PubMed] [Google Scholar]
- 108.Ragheb S.R., El Wakeel L.M., Nasr M.S., Sabri N.A. Impact of rutin and vitamin C combination on oxidative stress and glycemic control in patients with type 2 diabetes. Clin. Nutr. ESPEN. 2020;35:128–135. doi: 10.1016/j.clnesp.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 109.Kalita B., Das M.K. Rutin-phospholipid complex in polymer matrix for long-term delivery of rutin via skin for treatment of inflammatory diseases. Artif. Cells NanoMed. Biothechnol. 2018;46:541–556. doi: 10.1080/21691401.2017.1411931. [DOI] [PubMed] [Google Scholar]
- 110.Gupta M.B., Bhalla T.N., Gupta G.P., Mitra C.R., Bhargava K.P. Anti-inflammatory activity of taxifolin. Jpn. J. Pharmacol. 1971;21:377–382. doi: 10.1254/jjp.21.377. [DOI] [PubMed] [Google Scholar]
- 111.Sunil C., Xu B. An insight into the health-promoting effects of taxifolin (dihydroquercetin) Phytochemistry. 2019;166:112066. doi: 10.1016/j.phytochem.2019.112066. [DOI] [PubMed] [Google Scholar]