Highlights
-
•
Cytokine release syndrome seems to play a pivotal role in COVID-19 pathogenesis.
-
•
Tocilizumab is one of the most promising drugs for COVID-19.
-
•
Tocilizumab halved the mortality in critical patients compared to standard of care.
-
•
A transient respiratory function worsening is observed soon after administration.
-
•
Severe infective complications are more common than in rheumatologic setting.
Keywords: Tocilizumab, COVID-19, SARS-CoV2, IL-6, Orotracheal tube
Abstract
Background
As the novel SARS-CoV-2 pandemic occurred, no specific treatment was yet available. Inflammatory response secondary to viral infection might be the driver of severe diseases. We report the safety and efficacy (in terms of overall survival and hospital discharge) of the anti-IL6 tocilizumab (TCZ) in subjects with COVID-19.
Methods
This retrospective, single-center analysis included all the patients consecutively admitted to our Hospital with severe or critical COVID-19 who started TCZ treatment from March 13th to April 03rd, 2020. A 1:2 matching to patients not treated with TCZ was performed according to age, sex, severity of disease, P/F, Charlson Comorbidity Index and length of time between symptoms onset and hospital admittance. Descriptive statistics and non-parametric tests to compare the groups were applied. Kaplan Meier probability curves and Cox regression models for survival, hospital discharge and orotracheal intubation were used.
Results
Seventy-four patients treated with TCZ were matched with 148 matched controls. They were mainly males (81.5%), Caucasian (82.0%) and with a median age of 59 years. The majority (69.8%) showed critical stage COVID-19 disease. TCZ use was associated with a better overall survival (HR 0.499 [95% CI 0.262–0.952], p = 0.035) compared to controls but with a longer hospital stay (HR 1.658 [95% CI 1.088–2.524], p = 0.019) mainly due to biochemical, respiratory and infectious adverse events.
Discussion
TCZ use resulted potentially effective on COVID-19 in terms of overall survival. Caution is warranted given the potential occurrence of adverse events.
Financial support
Some of the tocilizumab doses used in the subjects included in this analysis were provided by the “Multicenter study on the efficacy and tolerability of tocilizumab in the treatment of patients with COVID-19 pneumonia” (EudraCT Number: 2020-001110-38) supported by the Italian National Agency for Drugs (AIFA). No specific funding support was planned for study design, data collection and analysis and manuscript writing of this paper.
Background
Following the emergence of a novel coronavirus (SARS-Cov-2) in Wuhan, China, in December 2019, a pandemic of COVID-19 (Coronavirus Disease 2019) spread globally. According to the ECDC, as of April 18th, SARS-CoV-2 infected 2,114,269 subjects in 180 Countries and caused 145,144 of deaths.1 So far, Italy – especially the Lombardy Region — showed one of the largest concentration of infected and deceased individuals cases in Europe and worldwide. In the largest published case series including 1099 patients in Wuhan, 173 subjects (15.7%) showed a severe disease, 55 (5.0%) required admission to Intensive Care Unit (ICU) and 15 (1.4%) died.2 Nevertheless, the clinical impact of COVID-19 outside China seems dramatically worse with the estimated case fatality rate in Italy well above 10%.1
So far, no established treatment for SARS-CoV-2 has been approved. Since the clinical severity of COVID-19 appears to be related to a cytokines storm with an overproduction of soluble inflammatory mediators, several immune-modulatory agents are currently under investigation.3 Interleukin-6 (IL-6) is a cytokine involved in the physiological inflammatory reaction and immune response4 but it has also been recognized also as a major driver in severe diseases such as chimeric antigen receptor T-cell (CAR-T)-associated cytokine release syndrome (CRS)5 and KSHV-associated inflammatory cytokine syndrome (KICS).6 Moreover, plasma IL-6 levels are elevated in ICU patients with COVID-197 and they appear to be positively correlated with mortality.8 As a consequence of these evidences, tocilizumab (TCZ), a recombinant humanized monoclonal antibody acting as IL-6 receptor agonist, has been proposed for the treatment of patients affected by COVID-199. Few clinical experiences have been published and no randomized clinical trial is currently available. Luo and colleagues recently reported the treatment of 15 subjects10 showing that one single dose of TCZ failed to improve the disease in critically ill patients, while adding a second dose provided better outcomes. Of note, clinical improvement was observed only in 1 patient (6.7%), while two worsened (13.4%) and three died (20.1%).
Aims of present study are to evaluate the efficacy of TCZ in severe and critical COVID-19 subjects comparing survival and hospital discharge with controls matched for disease severity. We also aimed to assess clinical safety, especially in terms of respiratory and blood biochemical recovery and infectious complications in treated individuals.
Methods
This retrospective, single center analysis included all the patients consecutively admitted to our Hospital in Milan, in the Lombardy Region, with a diagnosis of severe or critical COVID-19 and who started TCZ treatment from March 13th to April 03rd, 2020. The approved local protocol for an off-label TCZ administration enrolled subjects aged 18 years or older with a real-time PCR on rhino-pharyngeal swab positive for SARS-CoV-2; CT scan findings of severe, bilateral interstitial pneumonia; presence of an active inflammatory status alternatively defined by abnormal C reactive protein (CRP) levels (>1 mg/dL), IL-6 >40 pg/mL, d-dimer >1.5 mcg/mL, or ferritin >500 ng/mL. Only individuals with severe or critical clinical picture according to the Chinese Guidelines for the management of COVID-19 were eligible.11 A “severe” case was defined as the presence of respiratory distress (respiratory rate ≥30 per min), oxygen saturation on room air at rest ≤93% or P/F (or Horowitz Index, partial pressure of oxygen in arterial blood / fraction of inspired oxygen) ≤300 mmHg; a “critical” case was defined as the presence of respiratory failure with need of ventilation (either invasive or not), septic shock or any other organ dysfunction requiring ICU monitoring and treatment. Exclusion criteria were as follows: alanine aminotransferase (ALT) value >5 x ULN; neutrophil cell count <500 cell/mmc; platelet count <50,000 cell/mmc; data suggesting the presence of an active bacterial infection or a complicated intestinal diverticulitis (including perforated diverticulitis, as indicated in the TCZ summary of product); a positive pregnancy test; a positive HBsAg status; any concomitant disease not defined as “under control”. In analogy with what performed with CAR-T-associated CRS, TCZ dose was 8 mg/kg infused over 60 min (maximum dose of 800 mg); a second dose would be administered after 12 h in case of fever persistence. Clinical and laboratory features were collected at baseline, Day 1, Day 3, Day 5 and Day 7. IL-6 level was measured at baseline, 12 h after the first dose (just before the possible second dose), then at Day 1, Day 3, Day 5 and Day 7. Potential infective complications were assessed over the whole hospital stay.
The local Ethics Committee approved the protocol under the special conditions indicated by the Italian 648/96 law. All subjects provided written informed consent.
For survival and hospital discharge analyses, a paired group of controls was selected. They were matched 2:1 to patients treated with TCZ according to age, sex, severity of disease, P/F, Charlson Comorbidity Index (CCI) and length of time between symptoms onset and hospital admittance. The severity of disease was established according to the worst condition observed over the entire length of hospital stay.
Descriptive statistics (median and interquartile range [IQR] for continuous variables, absolute and relative [%] values for categorical variables) and non-parametric tests (Mann Whitney U for continuous and Chi-square for categorical variables) to compare the groups were applied. ANOVA test for repeated measures was used to compare laboratory parameters trend after treatment. Mean IL-6 levels change after TCZ administration were estimated with ANCOVA; the relationship between IL-6 and P/F values was calculated with the Pearson correlation coefficient. Kaplan Meier probability curves and Cox regression models for survival, hospital discharge and orotracheal tube (OTT) were used. Two-tailed p-values were calculated and a value <0.05 was considered statistically significant. Data management and analysis were performed using SPSS version 25.
Results
A total of 222 subjects were selected: 74 patients treated with TCZ and 148 matched controls treated with standard of care (SOC, represented by hydroxychloroquine plus lopinavir/ritonavir or remdesivir according to regional recommendations, drug availability in the Lombardy Region during the study period and remdesivir compassionate use program). They were mainly males (81.5%), Caucasian (82.0%) and with a median age of 59 (IQR, 51–70) years. The respiratory function was heavily impaired as described by a median P/F value of 180 mmHg (IQR 96–259). The majority (69.8%) exhibited a critical clinical picture as defined according to the Chinese recommendations. Table 1 shows baseline demographic and clinical features of treated and matched controls: they did not show any statistically significant difference.
Table 1.
Baseline features of study population.
| Tocilizumab (N = 74) | Standard of care (N = 148) | p | ||
|---|---|---|---|---|
| Age (years), median [IQR] | 59 [51–71] | 59 [52–70] | 0.865 | |
| Male sex,% | 82.4 | 81.1 | 0.807 | |
| Ethnicity,% | Caucasian | 78.4 | 84.5 | 0.267 |
| MENA Region | 9.5 | 3.4 | ||
| South American | 10.8 | 7.4 | ||
| Asian | 1.3 | 3.4 | ||
| Black African | – | 1.3 | ||
| Critical disease,% | 79.7 | 69.6 | 0.109 | |
| P/F (mmHg), median [IQR] | Severe disease | 229 [183–276] | 295 [222–375] | 0.009 |
| Critical disease | 136 [93–197] | 159 [93–246] | 0.459 | |
| Charlson Comorbidity Index, median [IQR] | 2 [1–3] | 2 [1–4] | 0.631 | |
| Time from symptoms onset to hospital admittance (days), median [IQR] | 7 [5–10] | 6 [4–8] | 0.080 | |
| Antiviral treatment,% | LPV/rtv | 75.7 | 85.1 | 0.084 |
| Hydroxychloroquine | 90.5 | 89.9 | 0.644 | |
| Remdesivir | 9.5 | 8.1 | 0.689 | |
IQR: interquartile range; MENA: Middle East and North Africa; P/F: Horowitz Index, defined as the ratio of partial arterial oxygen pressure and fraction of oxygen in the inhaled air; LPV/rtv: lopinavir co-formulated with ritonavir.
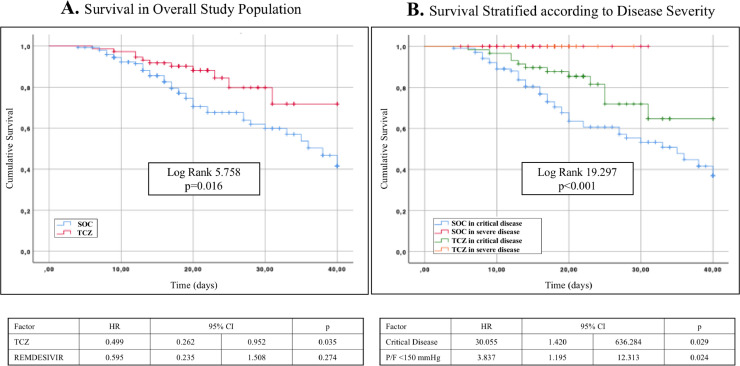
In the overall survival analysis TCZ administration showed an advantage over SOC (Fig. 1 A) with a Hazard Ratio (HR) of 0.499 (95% CI 0.262–0.952, p = 0.035) according to Cox regression model. In subjects with severe disease TCZ did not seem to have any beneficial effect, while it exerted an evident improvement in critical patients (Fig. 1B). The number of doses of TCZ did not appear to have an impact on survival (HR 0.748, 95% CI 0.201–2.785, p = 0.665) as well as baseline IL-6 value (HR 0.448, 95% CI 0.100–2.386, p = 0.376).
Fig. 1.
Kaplan Meier probability curves and Cox regression models for survival in the overall population (A) and stratified according to disease severity (B).
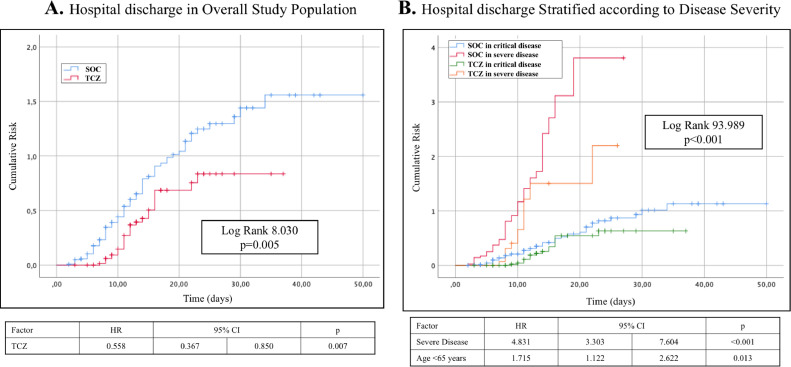
On the contrary, TCZ administration was associated with a longer hospital stay (Fig. 2 ) with a HR of 1.658 (95% CI 1.088–2.524, p = 0.019).
Fig. 2.
Kaplan Meier probability curves and Cox regression models for hospital discharge in the overall population (A) and stratified according to disease severity (B).
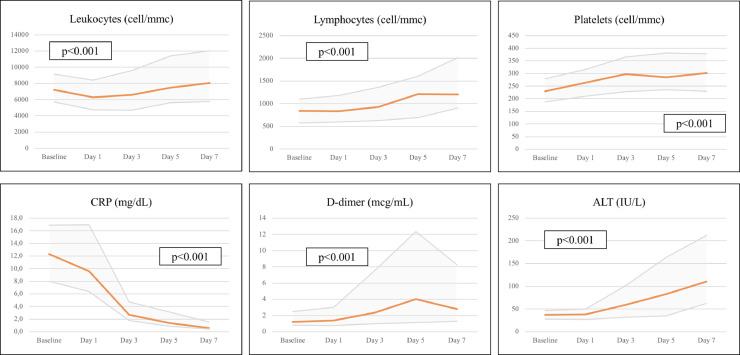
Fig. 3 shows blood cell count and biochemistry trends over the first 7 days from TCZ administration. As expected, CRP values decreased significantly while total leukocytes, lymphocytes, and platelets increased. d-dimer showed a dramatic rise by Day 5, then it decreased but without returning to baseline values. Moreover, a significant ALT increase was observed passing from a baseline value of 37 IU/L to 110 IU/L by Day 7.
Fig. 3.
Trends of hematologic and biochemical parameters (median values with interquartile ranges) after tocilizumab administration (ANOVA test for repeated measures).
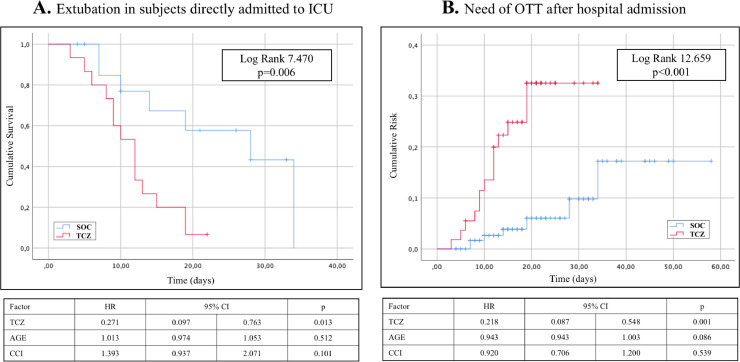
Respiratory function displayed a complex trend after TCZ administration. In patients who underwent OTT immediately after admission TCZ had a beneficial effect (Fig. 4 A). On the contrary, subjects who received TCZ outside the ICU showed a sudden need of OTT after the administration (Log Rank 12.659 with p<0.001, Fig. 4B).
Fig. 4.
Kaplan Meier probability curves and Cox regression models for orotracheal intubation requirement. (A): extubation achievement in subjects directly hospitalised in ICU because of the baseline critical respiratory failure. (B): intubation requirement during hospital stay for those who were admitted in general medical wards with no need of invasive mechanical ventilation at admittance.
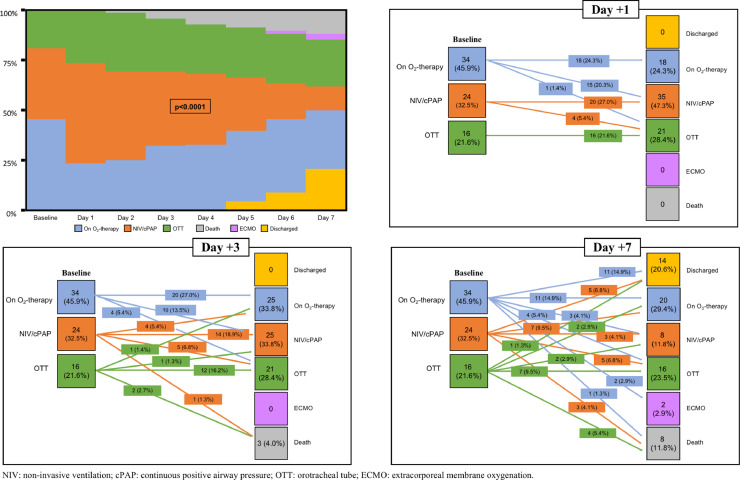
Fig. 5 summarizes the trajectories of oxygen and ventilation requirements within 7 days from anti-IL6 receptor administration: a large number of patients became critical after TCZ with a rapid need of assisted ventilation (either invasive or not).
Fig. 5.
Trajectories of oxygen and ventilation requirements within 7 days from tocilizumab administration (Chi-square test). Soon after drug start oxygen requirement increased significantly with 28.4% of subjects worsening their respiratory condition and only 8.1% improving by Day 3. At Day 7, 30.0% worsened while 38.3% improved baseline clinical status.
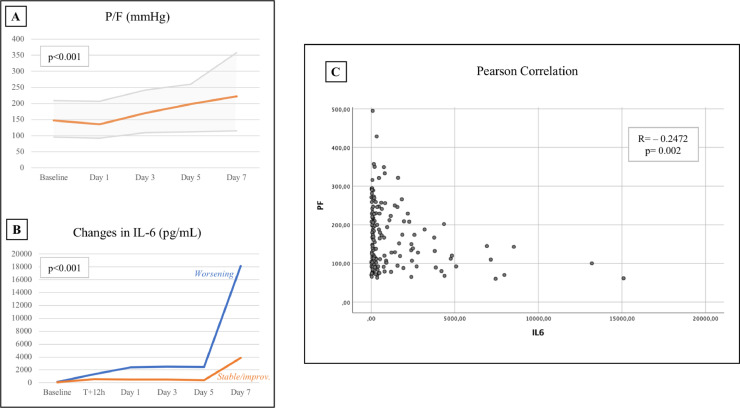
Respiratory deterioration seemed generally temporary with a return to baseline P/F values by Day 3 and an improvement by Day 5 and 7. At the end of the follow up period, one third of the 74 treated individuals (35.1%) showed a worsening of clinical conditions (in terms of ventilation requirements or death), while the majority (64.9%) had a stable or improved state. Comparing IL-6 trends in these two subgroups, both displayed an increased 12 h after TCZ administration, but later on the curves diverged significantly: those with a worsening condition continued to increase their IL-6 values, while the others showed a less steep increase. When calculating the Pearson correlation coefficient between IL-6 and P/F, an inverse correlation with a R=−0.2472 was observed (p = 0.002) (Fig. 6 ).
Fig. 6.
P/F (A) and IL-6 (B) trend during the first seven days from tocilizumab administration (ANOVA for repeated measures and ANCOVA tests). P/F slightly decreased by Day 1, while it improved significantly by Day 5 and 7. IL-6 values exhibited a different trend between those who improved and those who worsened baseline clinical condition. The relationship between IL-6 and P/F values (C) showed a statistically significant inverse correlation (Pearson correlation coefficient).
Twenty-seven infectious complications in 24 patients (32.4%) were observed. The majority of them (21 out of 27) was recorded in ICU patients. Of note, 11 severe events (14.9%) were registered: 6 sepsis cases due to gram-negative bacteria; 2 sepsis cases due to gram-positive bacteria; 1 candidemia; 1 lung abscess and 1 epidural abscess, both requiring surgical drainage. Among these subjects, one death from septic shock after ICU discharge was observed.
Discussion
Overproduction of soluble inflammatory mediators leading to CRS seems to have a pivotal role in the pathogenesis of COVID-19,12 so several interleukin antagonists or cytokine signaling inhibitors are currently under investigation. For example, anakinra, an anti-interleukin-1 receptor, proved to be effective in preliminary studies in critical patients.13 , 14 Additionally, also Janus kinase (JAK)1/JAK2 inhibitors, such as baricitinib, have been speculated to be active against COVID-19 and small clinical experiences have been published.15 , 16 We used TCZ in severely and critically ill patients infected by SARS-CoV-2 with a significant improvement in terms of overall survival. To date, few data are available about TCZ efficacy in COVID-19. To date, there are only some case reports in special population (oncologic, onco-hematologic and rheumatologic subjects, or other small case series10 , 17, 18, 19, 20, 21, 22). The mentioned paper by Luo and colleagues is a small case series and provides unsatisfactory and conflicting results. One paper recently published by Guaraldi and colleagues evaluated a large group (n = 179) of unselected patients treated with TCZ23 but the comparison was performed with 222 individuals with significantly less severe baseline features of disease. Nevertheless, the improvement in terms of mortality was similar to what we observed. Our data suggest that TCZ may be beneficial in critically ill patients, while its effect in severe cases does not appear to affect survival, even though it may prevent diseases progression requiring mechanical ventilation.
Unexpectedly, TCZ use was associated with a longer hospital stay. This finding might be the result of several biochemical, respiratory and infectious complications. In Phase III and IV trials conducted with TCZ in rheumatologic subjects24, 25, 26, 27, 28, 29, 30, 31 a decrease in total leukocytes was recorded: these studies describe a common and reversible neutropenia, usually mild (grade 1–2), while more severe decreases in neutrophils (grade 3–4) occur less frequently (largely below 5%). This outcome is the result of repeated administrations of TCZ over a longer follow up (generally of 24 weeks). We observed a transient decrease in total leukocytes in the first days, but later on they increased mainly as a result of the rise in lymphocytes. The different administration schedule and the shorter follow up could explain this apparent contradiction with existing literature. Indeed, we observed a significant rise in platelets and lymphocytes, which may reflect an improvement of the inflammatory status. On the contrary, d-dimer showed a significant increase despite the improvement in other inflammatory markers, an observation which may suggest a persistent alteration of the coagulation. The perturbation of coagulative homeostasis has already been related to death in COVID-19 patients8 , 32. Our data indicate that the risk of thrombotic complications after treatment might not be completely reduced. Additionally, liver enzyme elevation is widely described in previous rheumatologic literature where it is generally recorded in about 30% of treated subjects, while in our population an increase in ALT values appeared with higher frequency and greater severity than what hitherto reported: an additive effect of drug and viral toxicity could be hypothesized.
The consequences of TCZ on lung function were complex. Soon after the drug administration, a severe worsening of P/F with a large need of OTT was observed. Such worsening appeared transitory but could hamper the clinical advantages of this treatment. Although the majority of patients improved their respiratory conditions by Day 7, in the first hours after TCZ administration an urgent need of non-invasive and mechanical ventilation emerged. From a practical point of view, clinicians should be aware that soon after TCZ administration respiratory failure could progress to a critical state. Thus, a continuous monitoring of respiratory parameters after TCZ seems warranted.
The reasons for this evolution are not clear. Previous published data7 , 8 , 33 indicate that critical patients have higher levels of IL-6 leading to an augmented risk of death. We observed a steep increase in IL-6 levels in subjects with a worse outcome compared to those with a stable or improving clinical condition. Moreover, IL-6 increase seems to be inversely correlated with the P/F value. In animal models, IL-6 values are associated with a reduced lung function affecting several functional parameters (residual capacity, lung compliance, inspiratory capacity, and so on).34 Additionally, this cytokine could induce a significant, dose-dependent increase in the respiratory system resistance mechanisms.35 Thus, IL-6 seems to play a role by itself in the pathogenesis of lung diseases. A sudden rise in IL-6 level over the first hours after drug infusion is expected: it might be speculated that, in some conditions, this trigger leads to a worse inflammatory state that TCZ is not able to completely contain. A worse cytokine storm compartmentalized in the lung may result in the observed decrease in P/F. For this reason, a co-administration of TCZ with steroids might be taken into account.
In the above-mentioned studies performed in rheumatological setting, the overall rate of infective complications is high (around 70%), but the proportion of severe events is relatively low (3%). Indeed, we observed a rate of severe infections well above 10%, showing that these complications are probably the main drawback of TCZ, especially in the ICU where the majority of infectious events occurred. Compared to historical data,36 the characteristics of observed infections were similar, but our rate of septic complications seems higher. Since the effect of TCZ on fever and inflammatory markers might delay the diagnosis of an underlying infection leading to severe manifestations, exclusion of active bacterial infection is mandatory before treatment and microbiological monitoring in absence of clear symptoms should be taken into account.
The main limitations of our study are common to all case-control analyses compared to randomized controlled trials: although the choice of matched controls seems effective, selection biases could not be completely ruled out. Additionally, the retrospective nature of the study could hamper the strength of the observations. Finally, any inference should be assessed prudently because the small sample size in a single center did not allow to draw strong conclusions.
This study confirms the potentially effectiveness of TCZ on COVID-19 — especially in critically ill patients — with a reliable comparison group that allows to weigh the potential clinical impact of this treatment. Nevertheless, we suggest using it cautiously due to drug-related adverse events, remarkably transitory respiratory worsening and bacterial infections. Several trials are currently registered on ClinicalTrials.gov and many are still enrolling patients: study designs, drug combinations and target population are very different and could provide in the forthcoming months a detailed overview of TCZ use in the COVID-19 setting.
Declaration of Competing Interest
None.
Acknowledgments
NIGUARDA COVID-19 WORKING GROUP: Donatella Bambacini, Stefania Chiappetta, Fulvio Crippa, Maria Cristina Moioli, Davide Motta, Benedetta Maria Nocita, Carloandrea Orcese, Annamaria Pazzi, Alessandro Raimondi, Alice Sacco, Marta Vecchi, Beniamino Piero Vigo, Laura Ciceri, Silvia Colombo, Davide Ferrazzi, Claudia Galli, Linda Guarnieri, Enrica Silvia Periti, Lorenzo Porta, Valeria Tombini, Silvia Bondini, Alessandra Cernuschi, Anna Gandino, Marta Molaro, Lucrezia Rovati, Gabriele Bassi, Giampaolo Casella, Andrea Degasperi, Riccardo Giudici, Gianpaola Monti, Anna Rossi, Claudia Alteri, Stefania Carta, Luna Colagrossi, Diana Fanti, Ester Mazzola, Alice Nava, Chiara Vismara.
References
- 1.European Center for Disease Prevention and Control. Available at:https://www.ecdc.europa.eu/en/covid-19-pandemic. Accessed on April 29th, 2020.
- 2.Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu C.-.C., Chen M.-.Y., Chang Y.-.L. Potential therapeutic agents against COVID-19: what we know so far. J Chin Med Assoc. 2020 doi: 10.1097/JCMA.0000000000000318. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uciechowski P., Dempke W.C.M. Interleukin-6: a masterplayer in the cytokine network. Oncology. 2020;98:131–137. doi: 10.1159/000505099. [DOI] [PubMed] [Google Scholar]
- 5.Norelli M., Camisa B., Barbiera G. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 6.Katano H. Pathological features of Kaposi's sarcoma-associated herpesvirus infection. Adv Exp Med Biol. 2018;1045:357–376. doi: 10.1007/978-981-10-7230-7_16. [DOI] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020 doi: 10.1002/jmv.25801. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin L., Li T.S. Interpretation of “guidelines for the diagnosis and treatment of novel coronavirus (2019-nCoV) infection by the National Health Commission (trial version 5)”. Zhonghua Yi Xue Za Zhi. 2020;100:805–807. doi: 10.3760/cma.j.cn112137-20200205-00199. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transpl. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimopoulos G., de Mast Q., Markou N. Favourable anakinra responses in severe COVID-19 patients with secondary hemophagocytic lymphohistocytosis. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.05.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavalli G., De Luca G., Campochiaro C. Interleukin-1 blokade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stebbing J., Krishnan V., de Bono S. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol Med. 2020 doi: 10.15252/emmm.202012697. [Epub ahead of printing] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantini F., Niccoli L., Matarrese D. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michot J.-.M., Albiges L., Chaput N. Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.03.300. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X., Song K., Tong F. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020 doi: 10.1182/bloodadvances.2020001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mihai C., Dobrota R., Schröder M. COVID-19 in a patient with systemic sclerosis treated with tocilizumab for SSc-ILD. Ann Rheum Dis. 2020;79:668–669. doi: 10.1136/annrheumdis-2020-217442. [DOI] [PubMed] [Google Scholar]
- 20.Di Gianbenedetto S., Ciccullo A., Borghetti A. Off-label Use of Tocilizumab in Patients with SARS-Cov-2 Infection. J Med Virol. 2020 doi: 10.1002/jmv.25897. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cellina M., Orsi M., Bombaci F., Sala M., Marino P., Oliva G. Favorable changes of CT findings in a patient with COVID-19 pneumonia after treatment with tocilizumab. Diagn Interv Imaging. 2020;101:323–324. doi: 10.1016/j.diii.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X., Han M., Li T. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guaraldi G., Meschiari M., Cozzi-Lepri A. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30173-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabay C., Emery P., Van Vollenhoven R. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381:1541–1550. doi: 10.1016/S0140-6736(13)60250-0. [DOI] [PubMed] [Google Scholar]
- 25.Kremer J.M., Blanco R., Brzosko M. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 2011;63:609–621. doi: 10.1002/art.30158. [DOI] [PubMed] [Google Scholar]
- 26.Jones G., Sebba A., Gu J. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the ambition study. Ann Rheum Dis. 2010;69:88–96. doi: 10.1136/ard.2008.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emery P., Keystone E., Tony H.P. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516–1523. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genovese M.C., McKay J.D., Nasonov E.L. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy. Arthritis Rheum. 2008;58:2968–2980. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 29.Smolen J.S., Beaulieu A., Rubbert-Roth A. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 30.Kivitz A., Olech E., Borofsky M. Subcutaneous tocilizumab versus placebo in combination with disease-modifying antirheumatic drugs in patients with rheumatoid arthritis. Arthritis Care Res. 2014;66:1653–1661. doi: 10.1002/acr.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dougados M., Kissel K., Conaghan P.G. Clinical, radiographic and immunogenic effects after 1 year of tocilizumab-based treatment strategies in rheumatoid arthritis: the ACT-RAY study. Ann Rheum Dis. 2014;3:803–809. doi: 10.1136/annrheumdis-2013-204761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y., Xiao M., Zhang S. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMc2007575. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shieh J.M., Tseng H.Y., Jung F., Yang S.H., Lin J.C. Elevation of IL-6 and IL-33 levels in serum associated with lung fibrosis and skeletal muscle wasting in a bleomycin-induced lung injury mouse model. Mediators Inflamm. 2019;2019 doi: 10.1155/2019/7947596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubini A. Interleukin-6 and lung inflammation: evidence for a causative role in inducing respiratory system resistance increments. Inflamm Allergy Drug Targets. 2013;12:315–321. doi: 10.2174/1871528111312050003. [DOI] [PubMed] [Google Scholar]
- 36.ECDC Annual Epidemiol Report, 2016. Available at https://www.ecdc.europa.eu/sites/default/files/documents/AER-HCAI_ICU_3_0.pdf. Accessed on April 29th, 2020.