Abstract

Two luminescent MOFs, Mn@MOF and Cd@MOF, have been reported herein, which are capable of selectively detecting 2,4,6-trinitrophenol (TNP), one of the potent organic water pollutants in the class of mutagenic explosive nitroaromatic compounds (epNACs). It is perceived that the d10-based Cd(II)-constituting MOF shows a better response in the realm of TNP-like nitroaromatic sensing in comparison to the d5-based Mn@MOF which may possess lower electron density over the conjugated building blocks. The sensing competences of these chemosensors have been explored by means of various spectroscopic experimentations, and it is observed that for both d5 and d10-containing MOFs, the initial fluorescence intensity is significantly quenched in response to an aqueous solution of TNP. However, Cd@MOF is more selective and sensitive toward TNP over several other epNACs than Mn@MOF. The high chemical stability of the MOF samples, as well as its amusing sensing efficiency of Cd@MOF, further instigated to investigate the sensing ability in various environmental specimens like soil and water culled from several zones of West Bengal, India.
Introduction
In the realm of supramolecular chemistry and crystal designing, the judicious engineering and synthesis of metal–organic frameworks (MOFs) have engrossed great interest for their incomparable degree of tenability and structural diversity, which provides a wide range of chemical and physical properties. The judicious choice and stippling of the building blocks can deliver a desired framework with suitable properties that is evidencing the potential application in discriminating gas separation and storage, drug delivery, catalysis, and sensing.1−10 In this domain, luminescent MOFs (LMOFs) have evolved as selective and efficient chemosensors through strategic selection of nodes and luminescent organic linkers. Furthermore, the thermal and chemical endurance in the harsh environment is also steeping its feasibility.11−15 During this period of development, researchers found that inert metal ions and π-conjugated organic ligands are the suitable combination for its functionalization to be an effective fluorescence-based sensor for the detection of perilous substances.16 There are a plenty of chemicals, and their widespread uses are bringing adverse consequences to the environment and human health. In this perspective, it is highly necessary to rapidly detect chemicals like nitroaromatics (NACs), volatile organic compounds (VOCs), and metal ions to protect and maintain the sustainability of the environment. In particular, the sensual detection of potential explosive and mutagenic pollutant materials like nitroaromatic compounds (NACs) is highly needed for forensic and criminal investigation in order to safeguard human health and security.17−23 Amongst the nitroaromatic compounds, the explosion power of 2,4,6-trinitrophenol (TNP) is even higher than that of the most known explosive materials like 2,4,6-trinitrotoluene (TNT), but surprisingly, very little attention has been levied toward the detection of TNP.24 Furthermore, nitroaromatic compounds are widely used in the production of germicides, fungicides, staining agents, analytical reagents, fireworks, pesticides, and their incautious release into environment leads to severe detrimental impacts to the harmony of soil or aquatic ecosystems.25 Primarily, NACs are introduced into the ecosystem during the manufacturing and degradation of these commercial products where pesticides and germicides like 4,6-dinitro-2-methylphenol (DNoc) and 2-butyl-4,6-dinitrophenol (Dinoseb) are considered to be a major source. The use of nitro derivatives as the explosive material in the land mines is expressing the possibility of nitro-compound accumulation from unexploded and used land mines into the soil as well as the aquatic system. The bio-accumulation of nitrophenols into our food chain can cause serious lethal effects to the living organisms where a level of 150 μg/L nitrophenols26,27 is harmful for the ecosystem. Inside the human cells, like other organic pollutants, a small amount of phenol and its nitro-substituted derivatives show hematotoxicity, hepatotoxicity, along with carcinogenesis. The digestive cycle of mammalians metabolized substituted phenols like TNP into an extremely hazardous and mutagenic species, picramic acid.28−30 The conventional detection methods of NACs are relying mostly on the smelling ability of sniffer dogs’ and expensive sophisticated instrumental techniques that are not portable. Therefore, rapid, easy, and instant detection of TNP is of substantial necessity for the sake of environment, human health, and homeland security.31−33
In this perspective, the unique features like accessibility, high sensitivity, portability, and real-time response of luminescent MOFs embody its efficiency and feasibility to become a promising alternative for discerning and instant detection of TNP in both the solid and solution phases. In this regard, the primary strategy is the establishment of interligand charge transition (ILCT) within the organic building block through introduction of the electron donor and acceptor groups in the same linkers for the development a luminescent MOF.34 These conjugated frameworks, having delocalized π-conjugation in organic ligands, make it an excellent electron donor.38 The potential electrostatic interaction with electron-deficient nitroaromatic analytes through the donor–acceptor electron transfer mechanism is mainly responsible for the sharp and instant quenching of fluorescence. However, the detection of TNP with profound selectivity is considerably ponderous because of the competitive interactions with other nitro analytes that may possess false signals. The effect of other intrusions like the working medium and the presence of other interfering analytes make the sensing phenomenon immensely challenging for the selective detection of NACs from the aqueous medium and soil samples.35 Nevertheless, the tailoring nature of MOFs is suggesting the specific selection and arrangement of nodes, and effective building blocks can overcome the false responses and make the LMOFs precisely selective toward the targeted analyte with high sensitivity. Together with high selectivity and sensitivity, the stability and satisfying solvent resistance of the resulting framework is very important, especially against a harsh aqueous environment, as it can cause the degradation of MOF structures through the hydrolysis of the secondary building units.
Sometimes, the introduction of interpenetration to the MOFs for achieving enhanced stability can consequentially cause reduction in its porosity. Analogously, the construction of helical structures can also enhance the stability of the MOFs through extensive stacking of the subunits. Moreover, this ubiquitous helical structure of MOFs can assist in availing the retained porosity with significant stabilization of frameworks. MOFs implanted with helical structures show fascinating properties to become a promising material for various applications like gas absorption and separation, catalysis, and detection of toxic elements.36 Based on the reported rigid MOFs and the preceding considerations, the introduction of helicity can be achieved by the incorporation of the V-shaped linker to the framework. The geometries and coordination behavior of V-shaped polycarboxylates suggest that the mix ligand synthetic approach will be beneficial for the generation of unique architecture with considerable stability.37,38
Inspired by the aforementioned facts, in this present work, we have synthesized two MOFs (Scheme 1) with the mix ligand approach containing V-shaped polycarboxylates, 4,4′-oxybisbenzoic acid (H2OBA) and isonicotinic acid (HINA), with divalent transition-metal salts to achieve a fascinating helical structure. The 4,4′-oxybisbenzoic acid (H2OBA) contains a flexible −O– group which has a free bending and rotating nature that provide flexibility to the framework for feasible interactions with the guest analytes.34 In continuation, these luminescent MOFs are employed for the investigation of selective detection of TNP from the aqueous medium. In addition, the versatile chemical components of the water sample instigate us to evaluate the interference of various chemical components in the water medium. Therefore, several real-field samples have been collected from different geographical locations for experimentation, and interestingly, these LMOFs shows high efficacy toward selective and sensual detection of TNP, irrespective of its geolocations. Moreover, as far as we are aware, there are a few literature studies on the utilization of transition metals containing luminescent MOFs with a helical structure employed for selective detection of TNP from soil samples. Therefore, the selective detection capability of these MOFs with high sensitivity even in the presence of different interfering agents has further motivated us to evaluate the selective detection ability of TNP from different soil samples, and amusingly, Cd@MOF shows astonishing sensitivity toward TNP in various soil specimens.
Scheme 1. Reaction Methodology of Mn@MOF and Cd@MOF.
Results and Discussion
Crystal Structure and Morphology
The LMOFs (Mn@MOF and Cd@MOF) have been synthesized using the solvothermal method, and the X-ray Crystallographic studies (Tables S3 and S6) revealed that both of the crystals are isostructural and attribute to 2D frameworks and are crystallized in the triclinic crystal system with the space group of P-1. Typically, these LMOFs are composed of Mn(II)/Cd(II) nodes which are associated with 4,4′-oxybisbenzoic acid (H2OBA) and 4-isonicotinic acid (HINA) ligands to form a 2D net-like topology.
The secondary building unit (SBU) for both of the LMOFs are quite identical, which contain trinuclear metal ions to make the clusters and are connected by the association of four OBA strut to constitute the one-dimensonal metal chains. Furthermore, the metal nodes are connected by four INA building blocks to propagate as the 2D net-like architecture (Figure 1a). All the metal centers are hexacoordinated, where two solvent molecules are satisfying one coordination site for each terminal metal centers. Moreover, the central metal ion (Mn1, Cd1) has different coordination environments compared to the two terminal metal (Mn2 and Cd2) centers (Figures 1c,d and 2b,c). The connectivity of two carboxylate groups of each OBA unit possesses various coordination modes, which leads to different coordination geometries around the mental centers (Figure 1b).
Figure 1.
(a) Ball and stick diagram of the crystal structure of Mn@MOF is depicting the coordination environment with the atom numbering; (b) representation for metal and ligand connectivity; (c) asymmetric unit of terminal Mn (depicted as Mn2); (d) asymmetric unit of central Mn (depicted as Mn1); (H atoms are omitted for better understanding.).
Figure 2.
(a) Ball and stick diagram of the crystal structure of Cd@MOF is depicting the coordination environment with atom numbering; (b) asymmetric unit of terminal Cd (depicted as Cd1); (c) asymmetric unit of central Cd (depicted as Cd2); (H atoms are omitted for better understanding.).
The coordination site of the central metal atom is consisted of four flexible OBA ligands and two INA ligands, where the equatorial coordination sites are occupied with two μ-1,1 bridged carboxylate oxygens (i.e., Mn1–O3, Cd1–O3) of two carboxylate groups from two different OBA ligands. Other two sites are attached with monodentate oxygen centers (i.e., Mn1–O7, Cd1–O8) which are also contributed by the OBA moiety (Figures 1d and 2b). One of the OBA ligand having the syn-syn coordination mode involves two oxygen atoms of the OBA moiety (Mn1–O7, Mn2-O6; Cd1–O8, Cd2-O7), whereas second OBA moiety having syn-anti coordination mode with the chelating carboxylate oxygen atom (Mn1–O3; Cd1–O3) and one monodentate oxygen atom (Mn2-O4; Cd2-O6). The apical position is occupied by monodentate carboxylate oxygen (Mn1–O1; Cd1–O5) associated with the INA unit (Figures 1d and 2b). Similarly, the two terminal metal atoms are coordinated with bridged (Mn2–O3 and Cd2–O3) and monodentate (Mn2–O6,O4 and Cd2–O7,O6) carboxylate oxygen of the OBA unit, as likely to the central metal node. However, the major difference in the coordination environment is the nitrogen center of the pyridine ring of the INA moiety which occupies that one axial site. However, the sixth coordination site is fully filled by the amide oxygen (Mn2–O5 and Cd2–O2) of the solvent molecule (DMF) (Figures 1c and 2c). In a nutshell, the chemical formula is also quite similar, as for Mn@MOF, that is, C46H38Mn3N4O16, which possess the [Mn3(OBA)4(INA)4(DMF)2]n repeating unit with a trimetallic secondary building unit (SBU) of [Mn3(CO2)6N2O2]; similarly, Cd@MOF also has the formula unit of C46H38Cd3N4O16 which also propagates like [Cd3(OBA)4(INA)4(DMF)2]n with a trimetallic secondary building unit (SBU) [Cd3(CO2)6N2O2].
Nevertheless, these three metal nodes are linked together with μ-1,1-O of the carboxlate group of OBA in a equidistance manner (Mn1–Mn2 = 3.655 Å; Cd1– Cd2 = 3.685 Å). Even though the coordination geometry is quite similar for the respective metal nodes in case of both of the LMOFs, but the different size of metal nodes possesses different bond angles and bond distance with the constituting atoms. The bond distance-associated bridged oxygen atom (Mn–O3; Cd1–O3) of the OBA ligand lies between 2.197 and 2.289(3) Å (Mn1–O3, Mn2–O3) for Mn@MOF (Table S4), whereas for Cd@MOF (Table S7), it lies between 2.285 and 2.363(3) Å (Cd1–O3, Cd2–O3). Likewise, other bond distances like the monodentate oxygen center ranging from 2.085(3) to 2.295(3) Å (Mn2–O2, Mn2–O4, Mn2–O5, Mn2–O6, Mn1–O1, Mn1–O7) changes to 2.208(4) to 2.387(3) Å (Cd2–O2, Cd2–O5, Cd2–O6, Cd2–O7, Cd1–O4, Cd1–O8). In case of terminal metal nodes, the metal–N bond distance has been elongated slightly from 2.335(4) Å (Mn2–N1) to 2.351(4)Å (Cd2–N). Interestingly, the bond angles between the central metal-bridge oxygen atom-terminal metal are also shifted largely, and the value of Mn1–O3–Mn2 angles is 109.15°(12), whereas Cd1–O3–Cd2 angles are 104.89°(12) (Tables S5 and S8).
Surprisingly, the central metal ions in both LMOFs have adopted an octahedral (Oh) geometry, whereas the terminal centers have a highly distorted octahedral geometry. Two equilateral triangles are exactly parallel and staggered for an ideal octahedral geometry, while the triangles are not equilateral in a distorted one. According to the Bailer twist, the side of the triangle (s), the inter-triangular distance (h), and the twist angle (ϕ), have a specific order, like regular octahedron having s/h = 1.22, ϕ = 60° (Figure S1).40,41 Herein, Mn@MOF, for Mn1, the s/h value and the average twist angle are 1.24 and 89.25°, respectively, representing a regular octahedron geometry, whereas for Mn2, these values are s/h = 1.13 and ϕ = 83.87°, respectively, which is signifying a highly distorted trigonal (Figure S2) octahedron geometry (Figure 3a).
Figure 3.
Coordination polyhedral around the constituting metal ions; representation of the coordination geometry around the central and terminal metal ions, respectively, for (a) Mn@MOF and (b) Cd@MOF.
Interestingly, for Cd@MOF, Cd1 has also espoused an octahedral (Oh) geometry. The s/h value and the average twist angle are 1.22 and 91.13°, respectively, for the central Cd(II) atom, indicating a regular octahedron geometry, whereas for the terminal one (Cd2), these values are s/h = 1.17 and ϕ = 88.32°, respectively, which is signifying a highly distorted trigonal octahedron geometry (Figure 3b).
The crystal architectures are suggesting that the terminal pyridyl rings of the INA ligand facilitate to a double wall of the 2D net framework with the formation of a propagating 2D chain with rectangular channels (Figure 4a,b). Although there are some deviations in the bond distance and bond angles between the LMOFs, the size of the cavity formed by the propagating chains of MOFs is quite similar (Figure 4a,b). The packing pattern has disclosed that the 2D nets are propagating almost through the diagonal to the bc/ac plane (Figure S3) and are stacked to make effective and identical C–H···π bonding interactions in the supramolecular assembly with the satisfactory distance (2.541 AÅ) (Figure S4). Further simplification of the crystal structure of LMOFs (Figure 5a) suggests a zigzag-like 2D sheet (Figure S5) which is formed by a three-dimensional net-like topology (Figure 5c,d) with effective stacking of layers (Figure 5b).
Figure 4.
Cavity size and different inter-atomic distance (projected along the b axis, ac plane) of LMOFs (a) Mn@MOF and (b) Cd@MOF.
Figure 5.
(a) Simplified structure of LMOFs (projected along the bc plane); (b) propagation of 2D sheets and there close packed condition; and (c,d) simple representation of 2D net like topology of Mn@MOF and Cd@MOF, respectively.
There is no significant change in the cell parameters except for the fact that the Cd (II)-containing MOF had larger cell volume, and more distorted bond angles arose because of the accommodation of larger atoms compared to the Mn(II)-containing sample.
Field-Emission Scanning Electron Microscopy
The structural similarity is also observed in the surface morphological studies, which suggest that both of the MOF samples possess a block-shaped morphology, and propagated in a polycrystalline manner. The SEM image of the synthesized Mn@MOF (Figure S6a) exhibits the block-shaped polycrystalline form, which also reassembles with the simplified 3D net-like geometry (Figure 5c) propagated through a block-shaped polycrystalline form. The surface of Mn@MOF is representing the tip of a flower stamen, where the pollen grain-like spike-shaped constituent on the top face of the crystal block (Figure S6b,c) may be serving for the propagation of the repeating phase of the crystal. The similar polycrystalline form and structural features for the Cd@MOF samples have also been observed where the crystal block grows like an “Aragonite Cave Flowers” (Figure S7b,c). The coordination environment and medium of crystal growth is influenced by the packing/stacking of different phases, which leads to a diverse form of orientation.42 Herein, in spite of having similar coordination geometry, the difference in the growth solvent results in a dissimilar growth and crystal shape.
Thermal Stability and Purity
Powder XRD measurements are exhibiting almost all the major peaks in the PXRD pattern of the bulk samples and are matched with the simulated PXRD patterns of the as-synthesized LMOF samples (Figures S8 and S9) which confirms the phase clarity and uniformity of the bulk material. Furthermore, both LMOF samples were soaked in different harsh medium [NaOH (pH ≈ 12), HCl (pH ≈ 4), boiling water, and so forth.] to investigate its vulnerability for real-world applications. The PXRD patterns of the soaked samples have no significant change in the major peaks which indicates its high stability under different working conditions.
Thermogravimetric analysis (TGA) is also performed to examine the thermal stability of both the MOFs, and it has revealed that around 14% of weight loss has been experienced between ca. 50–300 °C, signifying the expulsion and followed by the liberation of coordinated solvent molecules (DMFs) for Mn@MOF (Figure S10). Furthermore, the framework starts to decompose at 300 °C and completely ruptures at 400 °C through the breakdown of the crystallographic arrangement. After 550 °C, complete breakdown of the framework occurs with the formation of metallic char. However, Cd@MOF (Figure S10) shows a lower mass loss (6.6%) within the temperature range of 50–300 °C that may be associated to the elimination of coordinated DMF molecules from the crystal structure. In continuation, a complete rupture of the crystal framework is also observed at around 400 °C with a mass loss of 44.7%. This mass loss is similar to that of the earlier case which is signifying the rupture of organic building blocks. However, a continuous mass loss is observed up to 800 °C because of the conversion of metallic char. Therefore, a high thermal stability of the LMOFs has been perceived from the TGA analysis.43−45
The IR spectroscopic experiments were performed using KBR pellets, which further (Figure S11) confirm the formation of LMOFs. It can be perceived that Mn@MOF has three characteristic IR spectra at 3400, 1600, and 1400 cm–1, attributing to the existence of −OH, −C=O, and −C–O functional groups, respectively. Similar IR spectra have also been observed for Cd@MOF where the C=O and C–O peaks are slightly shifted.34−51
Luminescence Behaviour
Previously, MOFs were modified depending upon their porosity, surface area, band gap, and so forth for sensing of epNACs from different solvent medium. For practical applications, epNACs detection from aqueous media becomes one of the essential criteria. In order to evaluate the luminescence behavior, 1 mg of each MOF samples (Mn@MOF & Cd@MOF) were dispersed in 5 mL of acetonitrile (ACN) to make a homogenous suspension. Furthermore, upon photo excitation at 360 nm (Figure S12), emissions of both of these samples were observed at 430 nm (Figure 6a,b). The homogenous suspension shows an excellent emissive property of the LMOFs under UV light; however, Cd@MOF exhibits slightly higher intense fluorescence compared to Mn@MOF (Figure S13). Conventionally, d10 metal containing MOFs, consisting of conjugated organic linkers, are considered as the excellent sensor toward the detection of electron-deficient nitro aromatic compounds.
Figure 6.
UV–vis absorption spectra and the photoluminescence spectra of (a) Mn@MOF; (b) Cd@MOF (inset: the digital photograph of the samples before and after excitation).
The stable d10 electronic configuration of the metal ion basically indicates the non-oxidizable or non-reducible52,53 behavior of the metal nodes, and that further signifies that the d10 metal nodes do not have any influence on the fluorescence properties of the MOF samples.54 Therefore, one of the reasons for the resulting fluorescence is subjected to the ligand-to-ligand charge-transfer (LLCT) (π*–n or π*−π) transition between the organic linkers in the LMOFs.55,56 Furthermore, there is significant reduction in the nonradiative decay because of the mix ligand complexation with the metal nodes.57−59 In order to examine the effect of half-filled d orbital configuration on the fluorescence of the resulting MOF and its detection ability toward the epNACs, the Mn(II)-based MOF (Mn@MOF) has been chosen to serve the purpose.
The origin of the luminescence suggests that the paramagnetic transition metal ions result in the quenching of the luminescence. In general, the charge-transfer process based on d–d transition is considered to be the mechanism of quenching. Herein, the Mn2+ ion having high-spin d5 electronic configuration, which is paramagnetic in nature, is incorporated within the MOFs. A study by Ma et al.60 where it is shown that incorporation of Mn2+ ions into a Cd-MOF by gradual postsynthetic metal node metathesis can lead to corresponding reduction in the fluorescence quantum yield of the Cd-MOF from 74.8 to 9.7%. Therefore, the Mn2+ containing MOF may have diverse photoluminescence (PL) properties than a d10 Cd2+—MOF, which may lead to distinct differences in sensitivity and selectivity of these luminescent probes.61
Fluorescence Titration Study for epNAC Detection
The high fluorescence emissive property of these MOFs instigated us to explore its potentiality towards epNAC detection from water for justifiable practical applicability. Indeed, the dispersed MOF samples (Mn@MOF and Cd@MOF) were used in order to examine their recognition efficiencies toward a series of electron-deficient nitroaromatic epNACs such as TNP, DNB (1,3-dinitro benzene), DNT (2,6-dinitro toluene), NB (nitro benzene), DNBA (1,3-dinitro benzoic acid), NBA (4-nitro benzoic acid), and so forth in 10–4 M aqueous solution. First, the effect of water on the inherent fluorescence intensity of both the LMOFs at 430 nm is checked, and there is no significant influence of water on the fluorescence intensity of the synthesized MOFs. In case of Mn@MOF, a highly selective and significant fluorescence quenching (∼55%) is perceived upon the gradual addition of 415 μL aqueous TNP solution (10–4 M) (Figure 7), whereas for other epNACs, there is no subtle changes in the fluorescence spectra (Figure 8).
Figure 7.

Fluorescence titration of Mn@MOF upon the gradual addition (0–415 μL) of TNP (10–4 M) aqueous solution.
Figure 8.

Quenching efficiency of different epNACs toward the fluorescence intensity of Mn@MOF
A similar type of quenching fashion is also observed for Cd@MOF during fluorescence titration experimentation with the same solution of TNP (Figure 9) in a similar environment. The comparison of the titration graphs affirms that a higher quenching response is observed for Cd@MOF than the prior one. In case of Cd@MOF, a higher quenching efficiency is also noticed for TNP while for the other competent, the epNAC response is very feeble (Figure 10).
Figure 9.

Fluorescence titration of Cd@MOF upon the gradual addition (0–280 μL) of TNP (10–4 M) aqueous solution.
Figure 10.
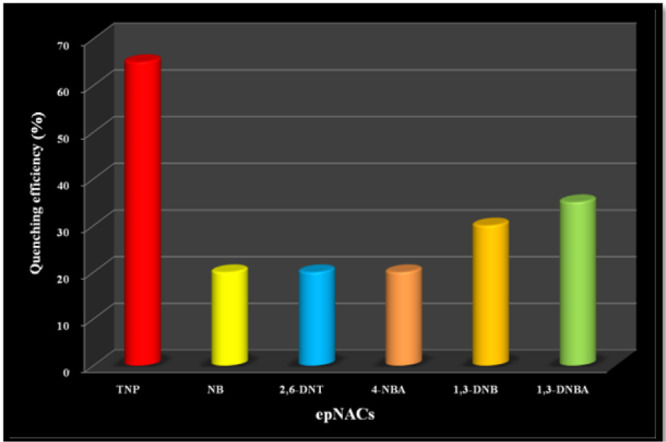
Quenching efficiency of different epNACs towards the fluorescence intensity of Cd@MOF
Insights into TNP Sensing Mechanism
In order to explicate the plausible mechanistic course of sensing, different interactive pathways have been explored, and it is found that resonance energy transfer (RET) and photo-induced charge transfer (PET) are the best possible mechanisms. These phenomena well acclaimed in fluorescence spectroscopy where PET is subjected to the enhancement of easy electron transfer to the excited electronic state, which possess the quenching of fluorescence. In contrast, RET signifies the facile energy transmission from an electronically excited donor chromophore to the acceptor moiety. Generally, the resonance energy transfer occurs when the emitted energy of the donor and absorbance spectrum of the acceptor eventually overlaps in a significant way. The extent of overlap of the two spectra (absorbance and emission spectra) signifies the stratum of sensing. The overlapping shows that emission of Mn@MOF has the maximum overlap with the absorbance band of TNP compared to other epNACs (Figure 11a). Therefore, the fluorescence quenching is maximum for TNP over other analytes. The emission profile of Mn@MOF shows a shift in the fluorescence wavelength upon interaction with TNP, revealing a charge transfer between the host and guest analyte. In particular, after interaction with TNP, the distance of the host and guest becomes within the favorable region, which in turn makes the charge transfer facile, and thus, the fluorescence energy lowers caused by the red-shifted emission wavelength. As previously discussed, Cd@MOF expresses a better fluorescence quenching response over Mn@MOF, but there is also a shift in the position of the characteristic fluorescence emission upon the addition of the TNP solution. Therefore, the possibility of charge transfer is directing the possible mechanism of the fluorescence-quenching phenomenon. Similar to Mn@MOF, the overlap between the emission band of Cd@MOF and the absorbance band of TNP is also observed to be maximum compared to other epNACs (Figure 11b).
Figure 11.
Overlap of the absorption spectra of epNACs with the emission spectra of (a) Mn@MOF (b) Cd@MOF.
Additionally, IR spectra suggest that there are three characteristic peaks at 3400, 1600, and 1400 cm–1 attributing to the presence of −OH, −C=O, and −C–O functional groups respectively, which are eventually unaltered in the presence of TNP, affirming a nonbonding π–π host–guest interaction between the chemosensor and TNP (Figure S14a,b).49−51 As a consequence, it can be inferred that both of these methodologies are collaboratively responsible for the fluorescence quenching of the emission of both the MOF samples.
Furthermore, the Stern–Volmer (SV) plot of Mn@MOF (Figure 12a) is nonlinear in nature, which implies the existence of static quenching for both of the cases [where (I0/I) = Ksv(Q) + 1; I0 = initial fluorescence intensity of MOF sample and I = fluorescence intensity of MOF in the presence of epNACs; {Q} = molar concentration of epNACs; and Ksv = quenching constant (M–1)]. The nature of the SV plot also indicates that an energy transfer or an integrated way of forming a charge transfer complex causes fluorescence quenching. In this regard, the modified SV equation, (Io/I – 1) = Ksv [Q], is employed to determinate the quenching constant [where Io is initial fluorescence intensity and I is fluorescence intensity of LMOFs in the presence of epNACs, [Q] is the molar concentration of epNACs, and Ksv is the quenching constant (M–1)]. The slope of the (Io/I – 1) versus [Q] plot can assist in obtaining the mathematical value of Ksv. It is found that the calculated value of Ksv is 9.92 × 104 M–1 which is nearly comparable with Ksv reported in the recent literature (Table S9) The detection limit of Mn@MOF has also been calculated to be 0.77 × 10–6 M.
Figure 12.
SV plot of (a) Mn@MOF; (b) Cd@MOF with epNACs (conc. 10–4 M).
In case of Cd@MOF, the SV plot (Figure 12b) is also nonlinear in nature with the significant shift of the initial fluorescence, which corroborates with the fact that integrated self-adsorption and an energy transfer pathway are responsible for quenching.33 The calculated quenching constant (Ksv) for Cd@MOF is 21.76 × 104 M–1, whereas the limit of detection for Cd@MOF is 0.54 × 10–6 M, which also suggests that Cd(II)-based system is more selective and sensitive towards TNP than the Mn(II) based system (Mn@MOF). However, the Cd@MOF possesses better sensing efficiency towards TNP than some recently related reports (Table S9).
To investigate the characteristic of the static or dynamic quenching phenomenon upon the interaction with TNP, the TCSPC experimentation is also performed. The TCSPC plot of Mn@MOF (Figure S19a) suggests that there is significant change in the average lifetime, indicating dynamic quenching upon the interaction with TNP. However, there is no significant change in the average lifetime for Cd@MOF (Figure S19b), and an upward curvature has been observed in the SV plot (Figure 12b), suggesting the formation of a ground state complex.60−64 Upon the addition of TNP, a prominent red shifting in the fluorescence spectra of Cd@MOF (Figure 9) has also been observed, confirming the formation of the GS complex.
Selective of Sensing TNP with the Interference of Other epNACs
With respect to selectivity, both of the MOF samples were selective toward TNP sensing, but Cd@MOF showed extensively higher sensitivity compared to Mn@MOF. Therefore, further detection studies were restricted with Cd@MOF only. The higher sensitivity of Cd@MOF toward TNP instigated us to explore its profound selectivity in the competitive environment with the coexistence of other interfering epNACs. The expedition started with the emission intensity of Cd@MOF dispersed in ACN, which shows its value to be 380 a.u. After that, a concentrated solution of other epNACs (NB, 4-NBA, 1,3-DNB, 1,3-DNBA, and 2,6-DNT) was added to that dispersed MOF solution in different sets of samples. The selectivity and interference study with epNACs were carried out with a dispersed solution of the MOF sample (1 mg/5 mL in ACN) in a similar environment. The corresponding emission of each set of samples was measured, and there were no subtle changes observed in the emission intensity profile. Therefore, in conclusion, it can be stated that there was no noticeable effect on emission intensity of Cd@MOF in response of epNACs except TNP. Furthermore, in the selectivity exploration, finally, an aqueous solution of TNP was added to each set of samples containing individual epNACs in the dispersed solution of Cd@MOF. Upon the investigation of the emission intensities, appreciable quenching was observed for each set of epNACs (Figure 13). Therefore, these experimental findings validated the extensive selective sensitivity of Cd@MOF toward TNP over other epNACs in the aqueous medium.
Figure 13.

Selectivity of Cd@MOF for TNP in the presence of other epNACs.
Reversibility
Because of better response, the Cd@MOF has been chosen for further investigation where reversibility is one of the concerning issues for effective and efficient use of the material. Herein, the Cd@MOF shows excellent reversibility (Figure 14) of its fluorescence intensity upto three cycles, and regeneration is only done by centrifuging the MOF sample with three repeated times of washing with the organic solvent.
Figure 14.

Selectivity of Cd@MOF for TNP in the presence of other epNACs.
Detection of TNP in Environmental Specimens
Detection of TNP from the Water Sample
There were vast sources of TNP like epNACs, and consequently, it was being continuously generated and simultaneously accumulated into the environment. Most of the toxic pollutants enter into the food chain or human body through water. The natural water sources like rivers, lakes, sea, and oceans are the only major resources to meet the societal demand. Major cities like Kolkata, Durgapur, and so forth in West Bengal, India, are established on the bank of rivers like Ganga, Damodor, from where water is supplied to the corresponding Municipalities. Therefore, for frequent monitoring on the accumulation pathways of organic explosives and mutagenic pollutants, discerning the detection of TNP from natural water resources is highly important. In bid to investigate the selective sensing ability of our synthesized MOF, several water specimens were culled from the diverse area of West Bengal state, India. The water sample compositions varied widely depending on its geological positions and that variation of composition may affect the sensitivity of our sensor. The collected natural water sample has been used to prepare TNP solutions (1 mM) for further investigation. In the beginning of this investigation, the effect on the fluorescence intensity of each water waster specimen without the addition of TNP was checked, and no effect had been observed (Figure S20a). First, two natural water samples were collected: (i) water collected from the industrial site of Durgapur (Specimen I) and (ii) water specimens collected from Ganga (Specimen II) were taken in consideration to examine the influence of these simulated specimen on the fluorescence emission of Cd@MOF. The fluorescence emission of Cd@MOF was considerably quenched after the addition of Specimen I (Figure 15a) and Specimen II (Figure 15b). The remaining water was also regulating the similar quenching fashion irrespective of its variation in composition. Now, it can be concluded that the fluorescence emission of Cd@MOF will always be quenched in the presence of TNP in any water sample, irrespective of its source.
Figure 15.
Fluorescence quenching of Cd@MOF upon the gradual addition of TNP into water Specimen I (a) and specimen II (b).
Detection of TNP from the Soil Sample
In most of the agriculture-prone countries like India, the major agricultural zone is resided on the basin of a river for easy irrigation facilities. Currently, the release of industrial waste into the rivers increases the possibility of soil contamination in agriculture fields, that is, it also simultaneously enhances the accumulation of TNP like epNACs and mutagenic water pollutants in the crops. Thus, these pollutants are directly accumulated into the food chain followed by the cell of living organisms, causing severe fatal dieses like cancer, anaemia, infertility, respiratory track, and so forth. In this regard, selective detection of TNP from the soil sample for continuous tracking of the accumulation of TNP in the ecosystem is highly appreciable. Nevertheless, the detection from the soil sample is much more difficult than the water sample because of the presence of excessive variable interfering agents. As Cd@MOF shows, its potential toward the detection of TNP with high selectivity and sensitivity, even irrespective of its water sample sources, has stimulated us to investigate its effectiveness toward TNP detection from the soil sample.
Soil samples has been collected from different sites in which soil specimen I was collected from the agricultural field situated at the basin of Ganga, and another soil specimen II was collected from the Durgapur industrial area. After natural drying, these soil samples were taken for further measurement where the soil sample (1.0 g) was extracted with 5.00 mL of water under continuous mechanical shaking for 10 min followed by centrifuging for next 5 min at 5000–6000 rpm. The resultant supernatants were further used as the stock solution to prepare the simulated TNP solution.39 The influence on the fluorescence intensity of the soil supernatant without the addition of TNP was checked, and no significant effect had been observed (Figure S20b). As likely to the water specimen, soil specimens were prepared with same concentration of TNP.
The influence of each specimen on the fluorescence emission of Cd@MOF was monitored, and an appreciable quenching was observed for each specimen (Figure 16, two representative fluorescence experiments with Cd@MOF and TNP–soil specimens). It is now obviously interesting to conclude that the quenching of Cd@MOF emission always occurred in the presence of TNP, irrespective of its competitive environment. Thereby, Cd@MOF can be contemplated as a universal luminescent sensor for easy, instant, and discerning detection of TNP like organic explosives and mutagenic water pollutants.
Figure 16.
Fluorescence quenching of Cd@MOF upon the gradual addition of TNP into soil specimen I (a) and specimen II (b).
Conclusions
In summary, this report provides strategic assistance for designing and engineering luminescent MOFs for discerning detection of explosive nitroaromatic compounds (epNACs). The consequence on judicious choice of the constituent of LMOFs, especially different metal ions, reflected in diverse structural features as well as sensing response. Conventionally, the metal center with half or full-filled electronic configuration are considered to have no influence on the sensing response. However, this exploration proposes a significant effect of these metal ions on the fluorescence as well as its sensing responses. The soft, Cd2+-based LMOF is more sensitive toward the TNP sensor than the hard center containing the Mn2+-based MOF. The high sensitivity and chemical stability exert a high potential of Cd@MOF toward sensing of epNACs in real world. Consequently, this MOF is can efficiently detect TNP from various environmental matrixes (soil and water) which were collected from the diverse zones of West Bengal, a state in eastern India. Cd@MOF can be contemplated as a universal luminescent sensor for easy, instant, and discerning detection of TNP like organic explosives and mutagenic water pollutants from environmental specimens.
Experimental Sections
Materials and Methods
The analytical-grade chemicals are used in this work without further purification. All chemicals have been purchased from Sigma Aldrich. A Bruker AXS (D8) PXRD instrument has been employed for measuring the powder X-ray diffraction (PXRD) patterns. The Fourier transform infrared (FTIR) spectra have been obtained with KBr pellets using the Perkin Elmer spectrum 100 model instrument. A Pyris Diamond TG/DTA (Perkin Elmer, STA-6000) thermal analyzer was used for thermogravimetric analysis (TGA) in the temperature range of 40–800 °C at the heating rate of 10.0 °C/min under a N2 atmosphere with the flow rate of 20.0 mL/min. The single-crystal X-ray analysis is carried out in Bruker AXS. UV–vis studies have been carried out in CARY-60. Perkin Elmer LS-45 has been used for fluorescence titration at an excitation wavelength 360 nm. The surface morphology was investigated by field-emission scanning electron microscopy (FE-SEM) equipped with energy-dispersive spectroscopy (EDS) (Make: Zeiss, Germany). TCSPC studies were carried out in a HORIBA Jobin Yvon picosecond time-correlated single photon counting spectrometer (model no. Fluorocube-01-NL). For the soil sample analysis pre-treatment, the supernatant is prepared as per the standard reported method.39
Synthesis of Mn@MOF
The solvothermal method has been employed for synthesizing Mn@MOF under mild conditions (Scheme 1). H2OBA (0.1 mmol, 25.82 mg), HINA (0.1 mmol, 12.31 mg), and MnCl2·4H2O (0.2 mmol, 39.58 mg) were dissolved in the mixture of 4 mL of DMF and 1 mL of chlorobenzene solution periodically with ultrasonication for 5 min. The resulting solution was filtered and placed in a sealed Teflon-lined autoclave (14 mL capacity), which was then kept in an air oven heated at 393 K, for 3 days under autogenous pressure. The reaction mixture was cooled down for obtaining suitable colorless block-shaped single crystals (Mn@MOF), and further solid-state elemental analysis was performed with the dried sample. Elemental analysis resulted C46H38Mn3N4O16 calcd (%): C, (51.75); H, (3.59); N, (5.25); found (%): C, (50.96); H, (4.08); N, (5.62).
Synthesis of Cd@MOF
H2OBA (0.1 mmol, 25.82 mg), HINA (0.1 mmol, 12.31 mg) and Cd(NO3)2·4H2O (0.2 mmol, 61.68 mg) were dissolved in the mixture of 5 mL of DMF solution periodically with proper ultrasonication for 5 min (Scheme 1). The resulting solution was filtered, followed by pouring in a sealed Teflon-lined autoclave (14 mL capacity) which was kept in an air oven at 393 K, for 3 days, under autogenous pressure. The hot reaction mixture was then cooled to room temperature, which helped obtain suitable colorless block-shaped single crystals (Cd@MOF). The SCXRD analysis and solid-state elemental analysis were then performed with the dried sample. Elemental analysis resulted C46H38Cd3N4O16 calcd (%): C, (44.55); H, (3.08); N, (4.51); found (%): C, (43.25); H, (4.38); N, (4.22).
Acknowledgments
The Department of Science and Technology (DST)-WTI-sponsored project (GAP 214312 vide DST/TM/WTI/2k16/277) is hereby acknowledged for financial assistance. A.H. and S.B. thank the DST for their fellowship. A.M. thanks the UGC for her fellowship. The authors are thankful to Dr. Tapan Kumar Pal, Pandit Deendyal Petroleum University, Gandhinagar, for his immense help in resolving crystallograpic data.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01194.
Crystal structure and simplified structure of LMOFs; characterizations of the LMOFs, like FE-SEM, PXRD, TGA, and FTIR spectra; and all spectroscopic experimentations and corresponding plots, optical images of the samples before and after excitation, crystallographic details, and comparison table (PDF)
Crystallographic data of Mn@MOF (CIF)
Crystallographic data of Cd@MOF (CIF)
Accession Codes
CCDC 1990522–1990523 contain the supplementary crystallographic data for this paper.
Author Contributions
∥ S.B. and A.M. contributed equally to the work. All the authors have contributed throughout the work. All authors have approved the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Lan A.; Li K.; Wu H.; Olson D. H.; Emge T. J.; Ki W.; Hong M.; Li J. A Luminescent Microporous Metal–Organic Framework for the Fast and Reversible Detection of High Explosives. Angew. Chem., Int. Ed. 2009, 48, 2334–2338. 10.1002/anie.200804853. [DOI] [PubMed] [Google Scholar]
- Pramanik S.; Zheng C.; Zhang X.; Emge T. J.; Li J. New Microporous Metal–Organic Framework Demonstrating Unique Selectivity for Detection of High Explosives and Aromatic Compounds. J. Am. Chem. Soc. 2011, 133, 4153–4155. 10.1021/ja106851d. [DOI] [PubMed] [Google Scholar]
- Lan A.; Li K.; Wu H.; Kong L.; Nijem N.; Olson D. H.; Emge T. J.; Chabal Y. J.; Langreth D. C.; Hong M.; Li J. RPM3: A Multifunctional Microporous MOF with Recyclable Framework and High H2Binding Energy†. Inorg. Chem. 2009, 48, 7165–7173. 10.1021/ic9002115. [DOI] [PubMed] [Google Scholar]
- Chen B.; Xiang S.; Qian G. Metal–Organic Frameworks with Functional Pores for Recognition of Small Molecules. Acc. Chem. Res. 2010, 43, 1115–1124. 10.1021/ar100023y. [DOI] [PubMed] [Google Scholar]
- O’Keeffe M.; Yaghi O. M. Deconstructing the Crystal Structures of Metal–Organic Frameworks and Related Materials into Their Underlying Nets. Chem. Rev. 2012, 112, 675–702. 10.1021/cr200205j. [DOI] [PubMed] [Google Scholar]
- Stock N.; Biswas S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. 10.1021/cr200304e. [DOI] [PubMed] [Google Scholar]
- Suh M. P.; Park H. J.; Prasad T. K.; Lim D.-W. Hydrogen Storage in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 782–835. 10.1021/cr200274s. [DOI] [PubMed] [Google Scholar]
- Ghosh P.; Roychowdhury A.; Corbella M.; Bhaumik A.; Mitra P.; Mobin S. M.; Mukherjee A.; Basu S.; Banerjee P. Designed synthesis of CO2-promoted copper(II) coordination polymers: synthesis, structural and spectroscopic characterization, and studies of versatile functional properties. Dalton Trans. 2014, 43, 13500–13508. 10.1039/c4dt00183d. [DOI] [PubMed] [Google Scholar]
- Song X.-Z.; Song S.-Y.; Zhao S.-N.; Hao Z.-M.; Zhu M.; Meng X.; Wu L.-L.; Zhang H.-J. Single-Crystal-to-Single-Crystal Transformation of a Europium(III) Metal–Organic Framework Producing a Multi-responsive Luminescent Sensor. Adv. Funct. Mater. 2014, 24, 4034–4041. 10.1002/adfm.201303986. [DOI] [Google Scholar]
- Xiao J.-D.; Qiu L.-G.; Ke F.; Yuan Y.-P.; Xu G.-S.; Wang Y.-M.; Jiang X. Rapid synthesis of nanoscale terbium-based metal–organic frameworks by a combined ultrasound-vapour phase diffusion method for highly selective sensing of picric acid. J. Mater. Chem. A 2013, 1, 8745–8752. 10.1039/c3ta11517h. [DOI] [Google Scholar]
- Nagarkar S. S.; Joarder B.; Chaudhari A. K.; Mukherjee S.; Ghosh S. K. Highly Selective Detection of Nitro Explosives by a Luminescent Metal–Organic Framework. Angew. Chem., Int. Ed. 2013, 52, 2881–2885. 10.1002/anie.201208885. [DOI] [PubMed] [Google Scholar]
- Banerjee D.; Hu Z.; Pramanik S.; Zhang X.; Wang H.; Li J. Vapor phase detection of nitroaromatic and nitroaliphatic explosives by fluorescence active metal–organic frameworks. CrystEngComm 2013, 15, 9745–9750. 10.1039/c3ce41680a. [DOI] [Google Scholar]
- Hu Z.; Pramanik S.; Tan K.; Zheng C.; Liu W.; Zhang X.; Chabal Y. J.; Li J. Selective, Sensitive, and Reversible Detection of Vapor-Phase High Explosives via Two-Dimensional Mapping: A New Strategy for MOF-Based Sensors. Cryst. Growth Des. 2013, 13, 4204–4207. 10.1021/cg4012185. [DOI] [Google Scholar]
- Banerjee D.; Hu Z.; Li J. Luminescent metal–organic frameworks as explosive sensors. Dalton Trans. 2014, 43, 10668–10685. 10.1039/c4dt01196a. [DOI] [PubMed] [Google Scholar]
- Kreno L. E.; Leong K.; Farha O. K.; Allendorf M.; Van Duyne R. P.; Hupp J. T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. 10.1021/cr200324t. [DOI] [PubMed] [Google Scholar]
- Senthilkumar S.; Goswami R.; Smith V. J.; Bajaj H. C.; Neogi S. Pore Wall-Functionalized Luminescent Cd(II) Framework for Selective CO2 Adsorption, Highly Specific 2,4,6-Trinitrophenol Detection, and Colorimetric Sensing of Cu2+ Ions. ACS Sustainable Chem. Eng. 2018, 6, 10295–10306. 10.1021/acssuschemeng.8b01646. [DOI] [Google Scholar]
- Toal S. J.; Trogler W. C. Polymer sensors for nitroaromatic explosives detection. J. Mater. Chem. 2006, 16, 2871–2883. 10.1039/b517953j. [DOI] [Google Scholar]
- Senesac L.; Thundat T. G. Nanosensors for trace explosive detection. Mater. Today 2008, 11, 28–36. 10.1016/s1369-7021(08)70017-8. [DOI] [Google Scholar]
- Salinas Y.; Martínez-Máñez R.; Marcos M. D.; Sancenón F.; Costero A. M.; Parra M.; Gil S. Optical chemosensors and reagents to detect explosives. Chem. Soc. Rev. 2012, 41, 1261–1296. 10.1039/c1cs15173h. [DOI] [PubMed] [Google Scholar]
- McQuade D. T.; Pullen A. E.; Swager T. M. Conjugated Polymer-Based Chemical Sensors. Chem. Rev. 2000, 100, 2537–2574. 10.1021/cr9801014. [DOI] [PubMed] [Google Scholar]
- Balakrishnan V. K.; Halasz A.; Hawari J. Alkaline Hydrolysis of the Cyclic Nitramine Explosives RDX, HMX, and CL-20: New Insights into Degradation Pathways Obtained by the Observation of Novel Intermediates. Environ. Sci. Technol. 2003, 37, 1838–1843. 10.1021/es020959h. [DOI] [PubMed] [Google Scholar]
- Germain M. E.; Knapp M. J. Optical explosives detection: from color changes to fluorescence turn-on. Chem. Soc. Rev. 2009, 38, 2543–2555. 10.1039/b809631g. [DOI] [PubMed] [Google Scholar]
- Nagarkar S. S.; Desai A. V.; Ghosh S. K. A fluorescent metal–organic framework for highly selective detection of nitro explosives in the aqueous phase. Chem. Commun. 2014, 50, 8915–8918. 10.1039/c4cc03053b. [DOI] [PubMed] [Google Scholar]
- Venkatramaiah N.; Kumar S.; Patil S. Fluoranthene based fluorescent chemosensors for detection of explosive nitroaromatics. Chem. Commun. 2012, 48, 5007–5009. 10.1039/c2cc31606d. [DOI] [PubMed] [Google Scholar]
- He G.; Peng H.; Liu T.; Yang M.; Zhang Y.; Fang Y. A novel picric acid film sensor via combination of the surface enrichment effect of chitosan films and the aggregation-induced emission effect of siloles. J. Mater. Chem. 2009, 19, 7347–7353. 10.1039/b906946a. [DOI] [Google Scholar]
- Wasi S.; Tabrez S.; Ahmad M. Toxicological effects of major environmental pollutants: an overview. Environ. Monit. Assess. 2013, 185, 2585–2593. 10.1007/s10661-012-2732-8. [DOI] [PubMed] [Google Scholar]
- USEPA . Ambient Water Quality Criteria for Nitrophenol; U. S. Environmental Protection Agency: Washington, DC, 1980. [Google Scholar]
- Michalowicz J.; Duda W. Phenols – Sources and Toxicity. Pol. J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]
- Thorne P. G.; Jenkins T. F. A field method for quantifying ammonium picrate and picric acid in soil. Field Anal. Chem. Technol. 1997, 1, 165–170. . [DOI] [Google Scholar]
- Wollin K. M.; Dieter H. H. Toxicological Guidelines for Monocyclic Nitro-, Amino- and Aminonitroaromatics, Nitramines, and Nitrate Esters in Drinking Water. Arch. Environ. Contam. Toxicol. 2005, 49, 18–26. 10.1007/s00244-004-0112-2. [DOI] [PubMed] [Google Scholar]
- Dey B.; Saha R.; Mukherjee P. A luminescent-water soluble inorganic co-crystal for a selective pico-molar range arsenic(III) sensor in water medium. Chem. Commun. 2013, 49, 7064–7066. 10.1039/c3cc43574a. [DOI] [PubMed] [Google Scholar]
- Gole B.; Bar A. K.; Mukherjee P. S. Modification of Extended Open Frameworks with Fluorescent Tags for Sensing Explosives: Competition between Size Selectivity and Electron Deficiency. Chem.—Eur. J. 2014, 20, 2276–2291. 10.1002/chem.201302455. [DOI] [PubMed] [Google Scholar]
- Ghosh P.; Saha S. K.; Roychowdhury A.; Banerjee P. Recognition of an Explosive and Mutagenic Water Pollutant, 2,4,6-Trinitrophenol, by Cost-Effective Luminescent MOFs. Eur. J. Inorg. Chem. 2015, 2851–2857. 10.1002/ejic.201500233. [DOI] [Google Scholar]
- Kim T. K.; Lee J. H.; Moon D.; Moon H. R. Luminescent Li-Based Metal–Organic Framework Tailored for the Selective Detection of Explosive Nitroaromatic Compounds: Direct Observation of Interaction Sites. Inorg. Chem. 2013, 52, 589–595. 10.1021/ic3011458. [DOI] [PubMed] [Google Scholar]
- Pramanik S.; Zheng C.; Zhang X.; Emge T. J.; Li J. New Microporous Metal–Organic Framework Demonstrating Unique Selectivity for Detection of High Explosives and Aromatic Compounds. J. Am. Chem. Soc. 2011, 133, 4153–4155. 10.1021/ja106851d. [DOI] [PubMed] [Google Scholar]
- Liu X.-J.; Zhang Y.-H.; Chang Z.; Li A.-L.; Tian D.; Yao Z.-Q.; Jia Y.-Y.; Bu X.-H. A Water-Stable Metal–Organic Framework with a Double-Helical Structure for Fluorescent Sensing. Inorg. Chem. 2016, 55, 7326–7328. 10.1021/acs.inorgchem.6b00935. [DOI] [PubMed] [Google Scholar]
- Gong Y.-N.; Zhong D.-C.; Lu T.-B. Interpenetrating metal–organic frameworks. CrystEngComm 2016, 18, 2596–2606. 10.1039/c6ce00371k. [DOI] [Google Scholar]
- Hu J.-S.; Huang X.-H.; Pan C.-L.; Zhang L. Photochemical and Magnetic Properties of Seven New Metal–Organic Frameworks Constructed by Flexible Tetrapyridines and V-Shaped Polycarboxylate Acids. Cryst. Growth Des. 2015, 15, 2272–2281. 10.1021/acs.cgd.5b00067. [DOI] [Google Scholar]
- Liu J.; Yang S.; Li F.; Dong L.; Liu J.; Wang X.; Pu Q. Highly fluorescent polymeric nanoparticles based on melamine for facile detection of TNT in soil. J. Mater. Chem. A 2015, 3, 10069–10076. 10.1039/c5ta00185d. [DOI] [Google Scholar]
- Alvarez S. Distortion Pathways of Transition Metal Coordination Polyhedra Induced by Chelating Topology. Chem. Rev. 2015, 115, 13447–13483. 10.1021/acs.chemrev.5b00537. [DOI] [PubMed] [Google Scholar]
- Ghorai P.; Dey A.; Hazra A.; Dutta B.; Brandão P.; Ray P. P.; Banerjee P.; Saha A. Cd(II) Based Coordination Polymer Series: Fascinating Structures, Efficient Semiconductors, and Promising Nitro Aromatic Sensing. Cryst. Growth Des. 2019, 19, 6431–6447. 10.1021/acs.cgd.9b00891. [DOI] [Google Scholar]
- Ghosh A. K.; Hazra A.; Mondal A.; Banerjee P. Weak interactions: The architect behind the structural diversity of coordination polymer. Inorg. Chim. Acta 2019, 488, 86–119. 10.1016/j.ica.2019.01.008. [DOI] [Google Scholar]
- Dutta B.; Hazra A.; Dey A.; Sinha C.; Ray P. P.; Banerjee P.; Mir M. H. Construction of a Succinate-Bridged Cd(II)-Based Two-Dimensional Coordination Polymer for Efficient Optoelectronic Device Fabrication and Explosive Sensing Application. Cryst. Growth Des. 2020, 20, 765–776. 10.1021/acs.cgd.9b01181. [DOI] [Google Scholar]
- Parmar B.; Rachuri Y.; Bisht K. K.; Suresh E. Mixed-Ligand LMOF Fluorosensors for Detection of Cr(VI) Oxyanions and Fe3+/Pd2+ Cations in Aqueous Media. Inorg. Chem. 2017, 56, 10939–10949. 10.1021/acs.inorgchem.7b01130. [DOI] [PubMed] [Google Scholar]
- Parmar B.; Rachuri Y.; Bisht K. K.; Laiya R.; Suresh E. Mechanochemical and Conventional Synthesis of Zn(II)/Cd(II) Luminescent Coordination Polymers: Dual Sensing Probe for Selective Detection of Chromate Anions and TNP in Aqueous Phase. Inorg. Chem. 2017, 56, 2627–2638. 10.1021/acs.inorgchem.6b02810. [DOI] [PubMed] [Google Scholar]
- Parmar B.; Bisht K. K.; Rachuri Y.; Suresh E. Zn(ii)/Cd(ii) based mixed ligand coordination polymers as fluorosensors for aqueous phase detection of hazardous pollutants. Inorg. Chem. Front. 2020, 7, 1082–1107. 10.1039/c9qi01549c. [DOI] [Google Scholar]
- Rachuri Y.; Parmar B.; Suresh E. Three-Dimensional Co(II)/Cd(II) Metal–Organic Frameworks: Luminescent Cd-MOF for Detection and Adsorption of 2,4,6-Trinitrophenol in the Aqueous Phase. Cryst. Growth Des. 2018, 18, 3062–3072. 10.1021/acs.cgd.8b00204. [DOI] [Google Scholar]
- Rachuri Y.; Parmar B.; Bisht K. K.; Suresh E. Mixed ligand two dimensional Cd(ii)/Ni(ii) metal organic frameworks containing dicarboxylate and tripodal N-donor ligands: Cd(ii) MOF is an efficient luminescent sensor for detection of picric acid in aqueous media. Dalton Trans. 2016, 45, 7881–7892. 10.1039/c6dt00753h. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Tan H.; Zhao J.-x.; Li X.; Ma H.; Chen X.; Yang X. Structural change from homogenous structure to staging in benzoic acid intercalated LDH: experimental and molecular dynamics simulation insights. Phys. Chem. Chem. Phys. 2012, 14, 9067–9075. 10.1039/c2cp40674h. [DOI] [PubMed] [Google Scholar]
- Bardak F.; Atac A.; Kurt M. Infrared and Raman study of some isonicotinic acid metal(II) halide and tetracyanonickelate complexes. Spectrochim. Acta, Part A 2009, 71, 1896–1900. 10.1016/j.saa.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Can N.; Ataç A.; Bardak F.; Can Ş. E. S. Spectroscopic and Luminescence Properties of an Isonicotinic Acid. Turk. J. Chem. 2005, 29, 589–595. [Google Scholar]
- Cui Y.; Yue Y.; Qian G.; Chen B. Luminescent Functional Metal-Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. 10.1021/cr200101d. [DOI] [PubMed] [Google Scholar]
- Vijayakumar C.; Tobin G.; Schmitt W.; Kim M.-J.; Takeuchi M. Detection of explosive vapors with a charge transfer molecule: self-assembly assisted morphology tuning and enhancement in sensing efficiency. Chem. Commun. 2010, 46, 874–876. 10.1039/b921520d. [DOI] [PubMed] [Google Scholar]
- Allendorf M. D.; Bauer C. A.; Bhakta R. K.; Houk R. J. T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. 10.1039/b802352m. [DOI] [PubMed] [Google Scholar]
- Liu F.-J.; Sun D.; Hao H.-J.; Huang R.-B.; Zheng L.-S. Anion-Controlled Assembly of Silver(I)/Aminobenzonitrile Compounds: Syntheses, Crystal Structures, and Photoluminescence Properties. Cryst. Growth Des. 2012, 12, 354–361. 10.1021/cg201159z. [DOI] [Google Scholar]
- Sun D.; Xu H.-R.; Yang C.-F.; Wei Z.-H.; Zhang N.; Huang R.-B.; Zheng L.-S. Encapsulated Diverse Water Aggregates in Two Ag(I)/4,4′-Bipyridine/Dicarboxylate Hosts: 1D Water Tape and Chain. Cryst. Growth Des. 2010, 10, 4642–4649. 10.1021/cg100927k. [DOI] [Google Scholar]
- Zhu Q.; Shen C.; Tan C.; Sheng T.; Hu S.; Wu X. Bright blue emissions with temperature-dependent quantum yields from microporous metal–organic frameworks. Chem. Commun. 2012, 48, 531–533. 10.1039/c1cc15138j. [DOI] [PubMed] [Google Scholar]
- Bai H.-Y.; Ma J.-F.; Yang J.; Liu Y.-Y.; Hua-Wu H.; Ma J.-C. Effect of Anions on the Self-Assembly of Cd(II)-Containing Coordination Polymers Based on a Novel Flexible Tetrakis(imidazole) Ligand. Cryst. Growth Des. 2010, 10, 995–1016. 10.1021/cg901332m. [DOI] [Google Scholar]
- Wang X.-L.; Qin C.; Wang E.-B.; Su Z.-M.; Xu L.; Batten S. R. An unprecedented eight-connected self-penetrating network based on pentanuclear zinc cluster building blocks. Chem. Commun. 2005, 4789–4791. 10.1039/b506398a. [DOI] [PubMed] [Google Scholar]
- Ma J.-x.; Huang X.-f.; Song X.-q.; Liu W.-s. Assembly of Framework-Isomeric 4 d–4 f Heterometallic Metal–Organic Frameworks with Neutral/Anionic Micropores and Guest-Tuned Luminescence Properties. Chem.—Eur. J. 2013, 19, 3590–3595. 10.1002/chem.201204022. [DOI] [PubMed] [Google Scholar]
- Bajpai A.; Mukhopadhyay A.; Krishna M. S.; Govardhan S.; Moorthy J. N. A fluorescent paramagnetic Mn metal–organic framework based on semi-rigid pyrene tetra-carboxylic acid: sensing of solvent polarity and explosive nitroaromatics. IUCrJ 2015, 2, 552–562. 10.1107/s2052252515012506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R.Principles of Fluorescence Spectroscopy, 3rd ed,; Springer Science, 2006. [Google Scholar]
- Gebert H.; Kretzschmar W.; Regenstein W. Ground-State Complex Formation of Perylene with Pyromellitic Dianhydride Studied by Static Fluorescence Quenching. J. Fluoresc. 1998, 8, 67–72. 10.1007/bf02758239. [DOI] [Google Scholar]
- Singh D. K.; Giri P. K.; Iyer P. K. Evidence for Defect-Enhanced Photoluminescence Quenching of Fluorescein by Carbon Nanotubes. J. Phys. Chem. C 2011, 115, 24067–24072. 10.1021/jp207392d. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.













