Abstract
A new class of compounds, which include s-triazine with pyrimidinetrione or thiopyrimidinedione moiety through a hydrazone linkage, were synthesized and characterized. The newly synthesized s-triazine hydrazone derivatives were evaluated in vitro against four cancer cell lines: A549, HepG2, HCT-116, and MCF-7. Several derivatives showed growth inhibition activity in the low microgram range. The results reveal that the barbiturate derivatives showed poor to no activity, while thiobarbiturate derivatives showed better activity than the analogues barbiturate derivatives. The substituents on the s-triazine moiety have a great effect on the antiproliferative activity, where derivatives with the piperidino and diethylamino on the s-triazine ring (5h) showed the highest activity against all of the tested cell lines (IC50 1.6 ± 0.6, 3.8 ± 0.3, 1.9 ± 0.4, and 1.2± 0.5 μg/mL for the tested cell lines A549, HepG2, HCT-116, and MCF-7, respectively). These results indicate that thiobarbiturates-s-triazine hydrazone derivatives may provide an excellent scaffold for the development of an anticancer drug candidate.
Introduction
Chemotherapy is one of the major strategies for the treatment of cancer. It is estimated that cancer is the second leading cause of morbidity and mortality after the clinical practice has been increasingly limited due to their risk of toxicity, drug resistance, and lack of specificity. This scenario thus calls for a new class of agents for the treatment of cancer and converting it into a chronic disease.
Barbituric acid (BA) and thiobarbituric (TBA) derivatives have become increasingly attractive to medicinal chemists as they possess a wide range of biological activities, such as the capacity to act as enzyme inhibitors,1 as well as antibacterial, anticancer, antiangiogenic, immunomodulatory, antifungal, and antioxidant agents.2−5 These activities are associated with several structural changes at positions N1, N3, and C5, the latter being the most effective/disrupting position.6−12 Among the most important C5-functionalized BAs and TBAs are the 5-benzylidene or 5-methylene derivatives, which have been biologically evaluated, resulting in several compounds of this class being found to possess xanthine oxidase (XO) inhibitory effect13 and antimicrobial activity as well as against fungal strains.14−18
On the other hand, 1,3,5-triazine (s-triazine) is extensively studied because it shows a wide range of biological activities, including antimicrobial,19,20 antiviral,21 and anti-inflammatory22 effects. Moreover, many of s-triazine derivatives showed great promise for further development as new hits for antitumor agents.23−38
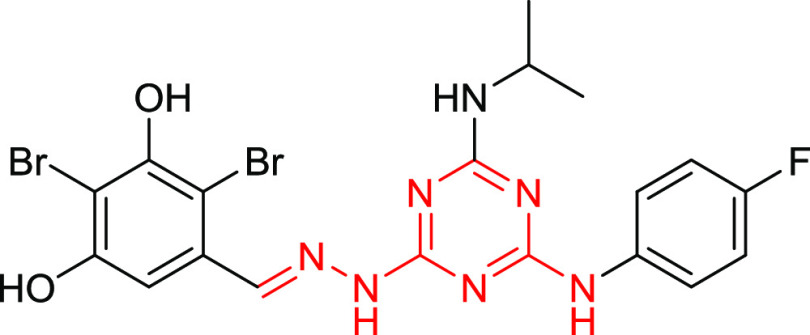
Recently, Bai et al. identified a series of s-triazine hydrazone derivatives as dual-effective inhibitors against both wild-type (WT) and mutant EGFR TKIs.39 Of these derivatives, the compound represented in Figure 1 shows the most potent activity against epidermal growth factor receptor (WTEGFR, IC50 = 25.9 μM) and mutant epidermal growth factor receptor (EGFR T790M/L858R, IC50 = 6.5 μM). Moreover, it also exhibited considerable antiproliferative activity against A549, A431, and NCIH1975 cell lines, with IC50 = 7.7, 8.0, and 10.5 μM, respectively.
Figure 1.
s-Triazine hydrazone derivatives as dual-effective inhibitors against both wild-type (WT) and mutant EGFR TKIs.
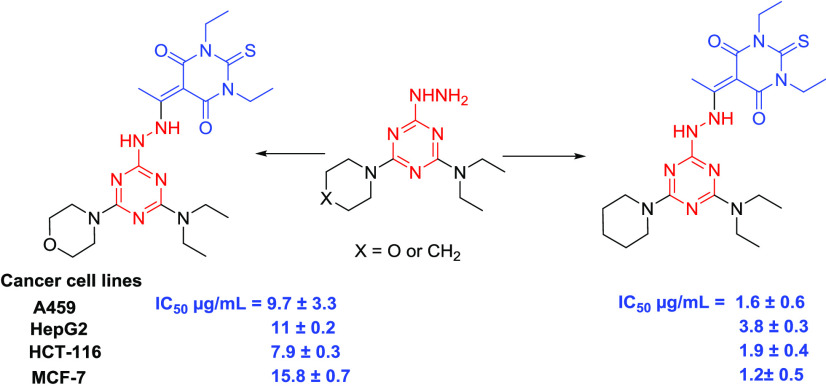
In our recent publications,37,40 we reported the synthesis of several s-triazine hydrazone derivatives (Figure 2) and their corresponding antiproliferative activities against lung carcinoma A549, hepatocyte carcinoma HepG2,40 breast cancer (MCF-7), and colon cancer (HCT-116).37 Our results showed that the substituents at both the s-triazine and benzylidine moieties have a great effect on the antiproliferative activities.
Figure 2.
Structure of s-triazine hydrazone derivatives with anticancer activity.
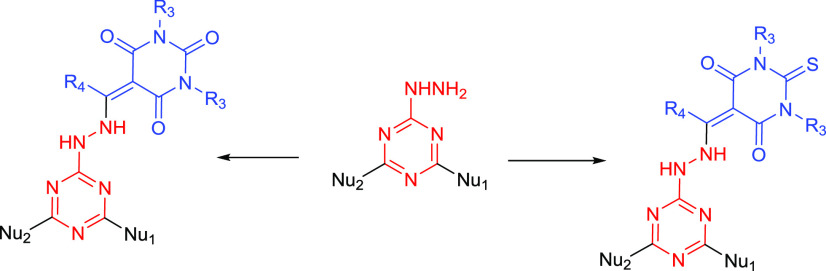
Based on our previous results and searching for new compounds with promising anticancer activity, we set about developing a new class of compounds (Figure 3) bearing the s-triazine core and either a pyrimidinetrione (barbiturate) or thiopyrimidinedione (thiobarbiturate) moiety attached via a hydrazone linkage. The antiproliferative activities of these new compounds against four cancer cell lines, namely, hepatocyte carcinoma HepG2, colon carcinoma HCT-116, lung carcinoma A549, and breast cancer cell lines MCF-7, were studied.
Figure 3.
Structure of the target products based on previous reported results.
Results and Discussion
Chemistry
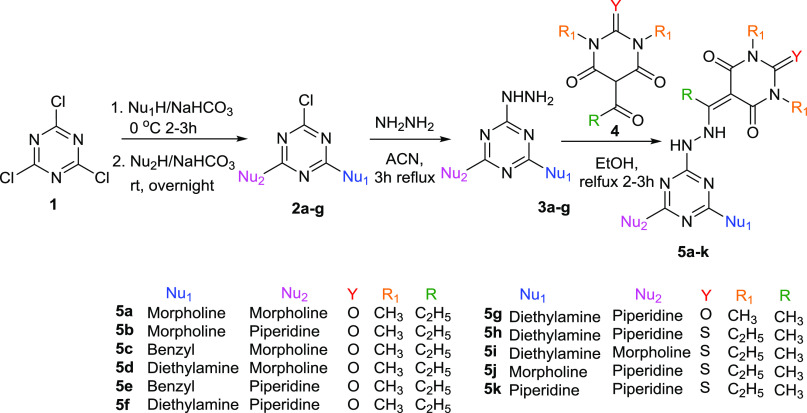
The synthesis of the target compounds was achieved in several consecutive steps as outlined in Scheme 1. Cyanuric chloride 1 was reacted first with a different amine in a one-pot method, as described previously,32 to afford the disubstituted s-triazine derivatives. The chloro derivatives 2a–g were then treated with hydrazine hydrate to afford the 2-hydrazino-4,6-disubstituted-s-triazine derivatives 3a–g in excellent yields and purities.40
Scheme 1. Synthesis of Pyrimidinetrione/Thiopyrimidimetrione-s-Triazine Derivatives.
Compounds 3a–g were reacted with 1,3-dimethyl-5-propionyl pyrimidin-2,4,6-trione 4a, 1,3-dimethyl-5-acetyl pyrimidin-2,4,6-trione 4b, or 5-acetyl-1,3-diethyl-2-thioxodihydropyrimidine-4,6 (1H,5H)-dione 4c in ethanol (EtOH) in the presence of drops of glacial acetic acid as shown in Scheme 1 to afford the target products 5a–k in excellent yields and purities as indicated from their spectral data (Figures S1–S12, Supporting Information).
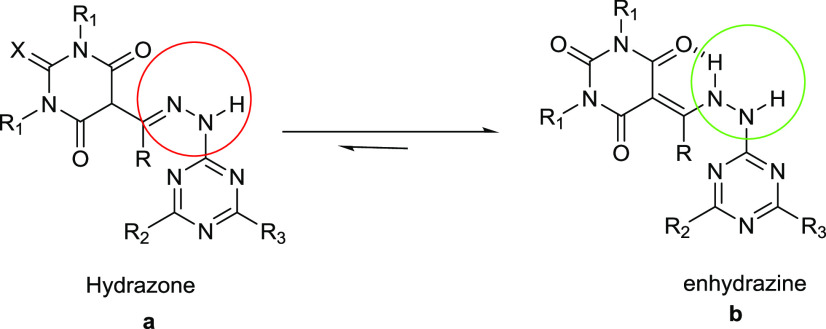
The NMR data for all of the target products 5a–k show that they are in the enhydrazine a form rather than the hydrazone b form (Figure 4), where the two peaks in the range of δ 90.0 and 198.0 ppm are related to the two carbon atoms similar to those of the enhydrazine form (C=C–NH–NH−). In addition, the hydrogen bond in the enhydrazine form stabilizes structure b more than it does the hydrazone isomer a (Figure 4). This observation is also in good agreement with our previously reported data40 and those reported by Giziroglu et al.41
Figure 4.
Tautomeric structure of hydrazone-enhydrazine.
Antiproliferative Activities
The antiproliferative activities of the target compounds (Figure 5) were tested against four cancer cell lines at concentrations ranging between 616 pg/mL and 1 mg/mL using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The results are represented in Table 1.
Figure 5.
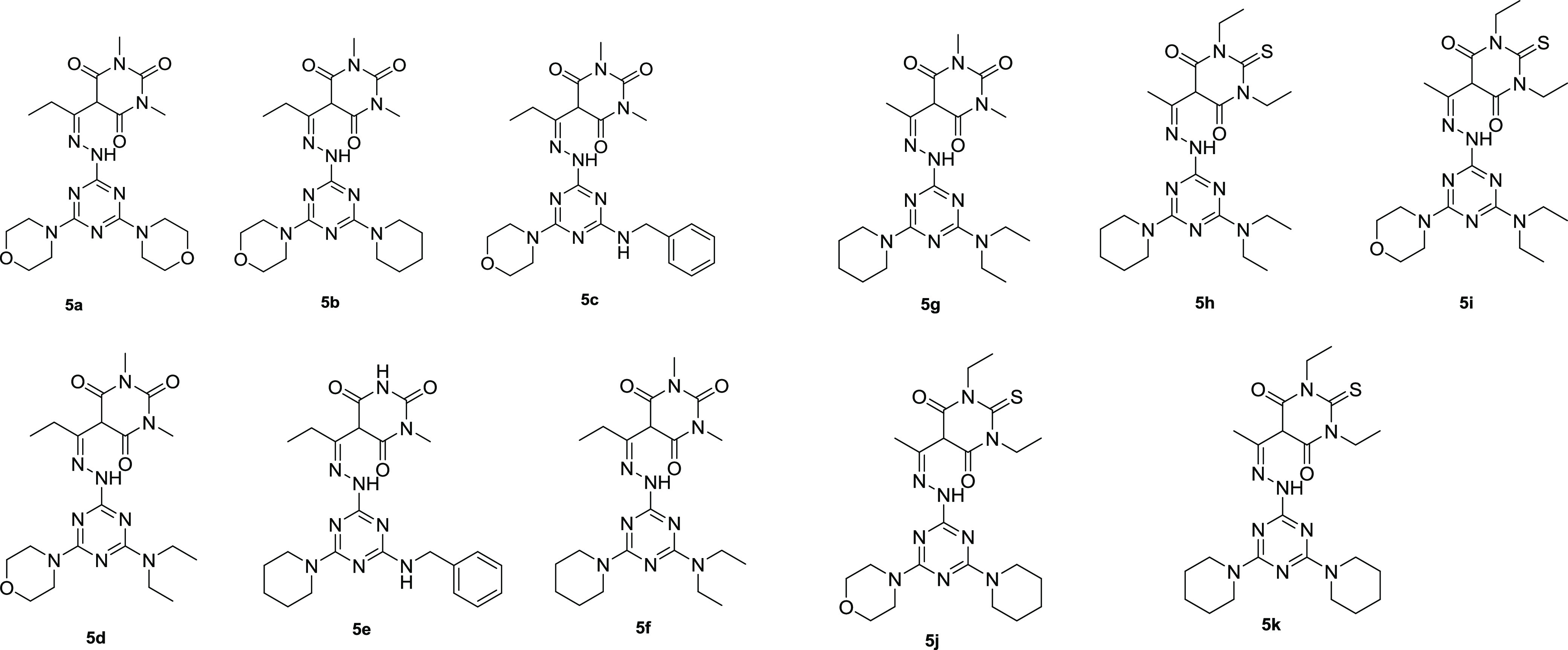
Structure of the target products.
Table 1. IC50 Values of the Derivatives in Three Cancer Cell Lines, as Measured Using the MTT Assay.
| compound | A549 | HepG2 | HCT-116 | MCF-7 |
|---|---|---|---|---|
| IC50 (μg/mL) | IC50 (μg/mL) | IC50 (μg/mL) | IC50 (μg/mL) | |
| 5a | NDa | NDa | NDa | NDa |
| 5b | 48.2 ±1.8 | NDa | 15.5 ± 2.1 | 34.5 ± 2.3 |
| 5c | NDa | NDa | NDa | 54.6 ± 3.5 |
| 5d | 71.5 ± 2.3 | 60.3 ± 7.9 | 41.6 ± 2.0 | 48.4 ± 2.1 |
| 5e | 16.1 ± 3.1 | 13.8 ± 1.0 | 14.2 ± 0.3 | 12.9 ± 0.3 |
| 5f | 6.9 ± 0.6 | 10.5 ± 0.3 | 3.9 ± 0.4 | 16.1 ± 1.5 |
| 5g | 9.4 ± 7.2 | 15.2 ± 1.8 | 13.1 ± 1.2 | 19.1 ± 0.7 |
| 5h | 1.6 ± 0.6 | 3.8 ±0.3 | 1.9 ± 0.4 | 1.2 ± 0.5 |
| 5i | 9.7 ± 3.3 | 11.0 ± 0.2 | 7.9 ± 0.3 | 15.8 ± 0.7 |
| 5j | 18.2 ± 1.8 | 20.2 ± 0.9 | 15.5 ± 0.2 | 24.5 ± 2.3 |
| 5k | 12.2 ± 1.5 | 13.2 ± 0.9 | 10.5 ± 2.1 | 18.2 ± 2.3 |
ND not active.
The barbiturate series 5a–g showed poor to no activity against the four cancer cell lines (Table 1). Only derivatives 5f and 5g showed moderate activity against the four tested cell lines. These two derivatives contained both a diethylamine and a piperidine moiety on the s-triazine ring with a methyl or an ethyl group at the azomethene group (C=N–NH−) in the hydrazone moiety. Based on these results, it can be concluded that the presence of diethylamine with the piperidine on the s-triazine moiety enhances the antiproliferative activities, as shown in 5h (thio series) and 5f and 5g (oxa series). Moreover, the piperidine core clearly emerges as preferable to the structurally similar morpholine one (5bvs5a, 5evs5c, 5fvs5d, 5hvs5i, 5kvs5j). These results indicate that the substituent on the s-triazine core has a great effect on the antiproliferative activity.
The presence of thiopyrimidinetrione increased the activity of both, as shown in Table 1. Compound 5h with the piperidine-diethylamine substituent on the s-triazine ring gave excellent results for the four cancer cell lines (Table 1) and showed better performance than compound 5i with the morpholine as shown in Table 1.
The obtained results here agreed with the previously reported results, where the presence of piperidine ring on the triazine moiety increases the activity against the cancer cell line. In addition, the dipiperidino derivatives showed higher activity than the morpholino-piperidino derivatives 5kvs5j, respectively. These observations agreed also with our reported results for derivatives shown in Figure 2(35−37,40)
These results led us to study the induction of apoptosis in HepG2 cells after treatment with the most potent derivatives 5h. The percentages of early and late apoptotic cells were determined by double staining with annexin V and propidium iodide (PI) via flow cytometry. The positioning of quadrants on dot plots was designated, and living cells (annexin V–/PI−), early apoptotic cells (annexin V+/PI−), late apoptotic cells (annexin V+/PI+), and necrotic cells (annexin V–/PI+) were identified. Representative results are shown in Figure 6. These data demonstrate that incubation of HepG2 cells with 5h at concentrations of 15 and 5 μg/mL, respectively, for 24 h markedly decreased the population of viable cells and increased the percentage of apoptotic cells. In the untreated controls and solvent controls, the percentage of apoptotic cells was negligible. When HepG2 cells were exposed to 5 μg/mL (about 5× IC50 value) of 5h for 24 h, the percentage of early apoptotic cells increased from 1.23% in the dimethyl sulfoxide (DMSO) control (Figure 6b) to 15.8% in the treated cells (Figure 6c) and that of late apoptotic cells increased from 1.08% in the DMSO control (Figure 6b) to 4.5% (Figure 6c) in the treated cells.
Figure 6.
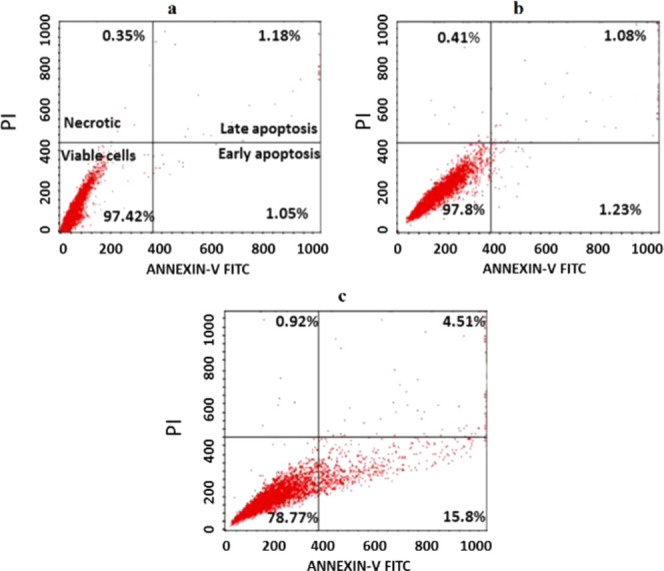
Flow cytometric analysis (annexin V-FTIC/PI assay) of HepG2 cells exposed for 24 h to 15 μg/mL of 5h (c): (a) untreated control and (b) vehicle control (DMSO). The represented dot plots showing percentage of viable, early apoptotic, late apoptotic, and necrotic cells.
Conclusions
In summary, the newly synthesized s-triazine hydrazone pyrimidinetrione (thiopyrimidinetrione) derivatives described in this study showed weak to high activity against the tested four cancer cell lines. The results reveal that the pyrimidinetrione (barbituric) series show poor to no activity against cancer cell lines. However, only one compound 5f (with the diethylamino-pieridino derivatives on the triazine moiety from the barbituric series) inhibited the growth of the four cell lines, being the most effective and more selective to HCT-116 and having IC50 of 3.9 ± of 0.4 μg/mL. This observation indicates that the substituent on the s-triazine core has a great effect on the antiproliferative activity of these compounds. Indeed, the combination between the piperidino and diethylamine on the s-triazine core is the optimal configuration.
Thiobarbiturate showed better activity than the analogous barbiturate derivatives (5h–kvs5a–g). The piperidino-benzyl derivatives 5e showed higher activity than its analogues morpholino-benzyl 5c derivatives. These results agreed with our previous results. Derivatives with the piperidino and diethylamino on the s-triazine ring showed promising and highest activity against all tested cell lines. Again, the piperidino derivatives have higher activity than the morpholino derivatives, e.g., 5h showed IC50 of 1.6 ± 0.6, 3.8 ± 0.3, 1.9 ± 0.4, and 1.2 ± 0.5 μg/mL, while 5i showed IC50 of 9.7 ± 3.3, 11 ± 0.2, 7.9 ± 0.3, and 15.8 ± 0.7 μg/mL for the tested cell lines A549, HepG2, HCT-116, and MCF-7, respectively. The obtained results agreed with the previously reported results, where the presence of piperidine on the s-triazine ring increases the activity against the cancer cell. In addition, the dipiperidino derivatives showed higher activity than the morpholino-piperdino derivatives 5kvs5j, respectively. These observations agreed also with our previously reported results35−37,40 for the derivatives shown in Figure 2.
Finally, our findings demonstrate that the derivative 5h at concentrations of 15 and 5 μg/mL, respectively, is able to induce apoptosis in HepG2 cells. These results indicate that s-triazine may provide an excellent scaffold for the development of anticancer drug candidates.
Materials and Methods
All solvents were used directly from the vendor. The 1H-NMR and 13C-NMR spectra were recorded on a JEOL 400 MHz spectrometer (JEOL, Ltd., Tokyo, Japan); at room temperature in CDCl3 and/or DMSO-d6 using an internal standard, δ values were expressed in ppm. Elemental analyses were performed on PerkinElmer 2400 elemental analyzer (PerkinElmer, Inc. 940 Winter Street, Waltham, MA). Melting points were recorded on a Gallenkamp melting point apparatus (Sigma-Aldrich Chemie GmbH, 82024 Taufkirchen, Germany) and are uncorrected.
Chemistry
Synthesis of Compounds 3a–g
Compounds 3a–g were synthesized as previously described by our group42 and characterized and used without further purification.
5-Acetyl-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (4c)
Compound 4c was prepared following the reported method for 4a,b33 and obtained as an off-white solid from EtOH in yield 78%; mp 58–59 °C, 1H-NMR (CDCl3) δ = 1.23–1.30 (m, 6H, 2CH3), 2.71 (s, 3H, CH3), 4.47–4.55 (m, 4H, 2CH2), 17.73 (s, 1H, OH); 13C-NMR (CDCl3) δ = 11.89, 12.19, 25.41, 42.92, 43.25, 97.29, 158.76, 167.57, 177.32, 198.08. Anal. calcd for C10H14N2O3S (242.29): C, 49.57; H, 5.82; N, 11.56. Found C, 49.81; H, 5.98; N, 11.82.
General Method for the Synthesis of 4,6-Disubstituted 1,3,5-Triazine-2-hydrazone Derivatives (5a–k)
To a solution of pyrimidinetrione (or thiopyrimidinetrione) 4a–c (5 mmol) in EtOH (10 mL) containing two to three drops of acetic acid, 4,6-disubstituted 1,3,5-triazine-2-hydrazino 3a–g (5 mmol) was added and the reaction mixture was stirred under reflux for 2–3 h. The solvent was reduced under vacuum and the precipitated product was filtered off and dried at room temperature. The products were collected by simple filtration and then recrystallized to afford the target compounds.
5-(1-(2-(4,6-Dimorpholino-1,3,5-triazin-2-yl)hydrazinyl)propylidene)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (5a)
White solid from EtOH in yield 88%; mp 143–145 °C; 1H-NMR (CDCl3) δ = 1.24 (t, 3H, J = 7.6 Hz, CH3), 3.29 (m, 8H, CH2, 2CH3), 3,68 (m, 8H, 4CH2-N), 3.75 (m, 8H, 4CH2-O), 14.21 (s, 1H, NH); 13C-NMR (CDCl3) δ = 11.6, 22.4, 27.7, 43.8, 66.6, 88.5, 151.3, 162.1, 163.8, 164.3, 166.3, 175.5. Anal. calcd for C20H29N9O5 (475.51): C, 50.52; H, 6.15; N, 26.51. Found C, 50.66; H, 6.31; N, 26.76.
1,3-Dimethyl-5-(1-(2-(4-morpholino-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)hydrazinyl)propylidene)pyrimidine-2,4,6(1H,3H,5H)-trione (5b)
Yellow solid from ethylacetate in yield 87%; mp 210–212 °C; 1H-NMR (CDCl3) δ = 1.24 (t, 3H, J = 7.8 Hz, CH3), 1.5–1.61 (m, 6H, 3CH2), 3.29 (m, 8H, CH2, 2CH3), 3,68–3.77 (m, 12H, 6CH2), 14.2 (s, 1H, NH); 13C-NMR (CDCl3) δ = 11.6, 22.4, 24.2, 27.7, 43.8, 59.8, 66.8, 88.7, 151.2, 162.2, 163.6, 164.5, 166.3, 175.6. Anal. calcd for C21H31N9O4 (473.54): C, 53.27; H, 6.60; N, 26.62. Found C, 53.51; H, 6.73; N, 26.88.
5-(1-(2-(4-(Benzylamino)-6-morpholino-1,3,5-triazin-2-yl)hydrazinyl)propylidene)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (5c)
White solid from ethylacetate in yield 82%; mp 222–223 °C; 1H-NMR (CDCl3) δ = 1.23 (t, 3H, J = 7.2 Hz, CH3), 3.27 (m, 8H, CH2, 2CH3), 3.67 (brs, 8H, 4CH2), 3,78 (brs, 8H, 4CH2), 4.56 (d, 2H, J = 6.0 Hz, CH2-Ph), 7.26–7.30 (m, 5H, C6H5-), 14.2 (brs, 1H, NH); 13C-NMR (CDCl3) δ = 11.7, 22.4, 27.9, 44.9, 59.8, 66.6, 88.8, 127.5, 128.6, 151.2, 162.2, 163.6, 164.5, 166.3, 175.6. Anal. calcd for C23H29N9O4 (495.54): C, 55.75; H, 5.90; N, 25.44. Found C, 55.99; H, 5.83; N, 25.69.
5-(1-(2-(4-(Diethylamino)-6-morpholino-1,3,5-triazin-2-yl)hydrazinyl)propylidene)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (5d)
Pale yellow solid from EtOH in 85% yield; mp 171–173 °C; 1H-NMR (CDCl3) δ = 1.13 (t, 6H, J = 6.8 Hz, 2CH3), 1.26 (t, 3H, J = 7.2 Hz, CH3), 3.29–3.34 (m, 8H, CH2, 2CH3), 3.52–3.54 (m, 4H, 2CH2), 3,70 (m, 4H, 2CH2-N), 3.77 (m, 4H, 2CH2), 14.2 (s, 1H, NH); 13C-NMR (CDCl3) δ = 11.7, 12.9, 22.4, 27.7, 41.6, 43.8, 66.7, 88.4, 151.2, 162.2, 169.5, 175.6. Anal. calcd for C20H31N9O4 (461.53): C, 52.05; H, 6.77; N, 27.31. Found C, 52.29; H, 6.96; N, 27.56.
5-(1-(2-(4-(Benzylamino)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)hydrazinyl)propylidene)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (5e)
Off-white solid from ethylacetate in 86% yield; mp 226–227 °C; 1H-NMR (DMSO-d6) δ = 1.11 (t, 3H, J = 6.4 Hz, CH3), 1.46 (brs, 4H, 2CH2), 1.58 (brs, 2H, CH2), 3.67–3.9 (m, 6H, 3CH2), 4.46 (brs, 2H, CH2-Ph), 7.26–7.30 (m, 5H, C6H5−), 14.85 (brs, 1H, NH); 13C-NMR (CDCl3) δ = 11.2, 21.4, 24.2, 25.5, 27.2, 44.0, 78.6, 78.9, 79.2, 126.8, 127.4, 128.2, 150.8, 162.2, 161.5, 163.6, 166.3, 174.9. Anal. calcd for C24H31N9O3 (493.57): C, 58.40; H, 6.33; N, 25.54. Found C, 58.65; H, 6.54; N, 25.80.
5-(1-(2-(4-(Diethylamino)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)hydrazono)propyl)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (5f)
Off-white solid from EtOH in 83% yield; mp 135–137 °C; 1H-NMR (CDCl3) δ = 1.12 (t, 6H, J = 7.2 Hz, 2CH3), 1.55 (brs, 4H, 2CH2), 2.75(s, 3H, CH3), 3.29 (s, 6H, 2CH3), 3.52 (q, 4H, J = 6.8, 7.2 Hz, 2CH2), 3.70 (t, 4H, J = 6.0 Hz, 2CH2-N), 14.37 (s, 1H, NH); 13C-NMR (CDCl3) δ = 13.2, 16.8, 24.7, 25.7, 27.7, 41.6, 44.5, 89.0, 151.5, 162.4, 169.7, 175.6, 189.0. Anal. calcd for C20H31N9O3 (455.53): C, 53.92; H, 7.01; N, 28.30. Found C, 54.12; H, 7.20; N, 28.55.
5-(1-(2-(4-(Diethylamino)-6-morpholino-1,3,5-triazin-2-yl)hydrazinyl)ethylidene)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (5g)
Pale yellow solid from EtOH in 88% yield; mp 149–150 °C; 1H-NMR (CDCl3) δ = 1.15 (t, 6H, J = 7.2 Hz, 2CH3), 1.26 (t, 6H, J = 7.2 Hz, 2CH3), 2.78 (s, 3H, CH3), 3.52–3.57(q, 4H, J = 6.4, 7.6 Hz, 2CH2), 3.70 (m, 4H, 2CH2–N), 3.79 (m, 4H, 2CH2–O), 4.54 (q, 4H, J = 7.6, 6.4 Hz, 2CH2), 14.8 (s, 1H, NH); 13C-NMR (CDCl3) δ = 12.3, 17.2, 41.9, 43.1, 43.9, 66.6, 91.3, 162.9, 169.5, 177.4; Anal. calcd for C21H33N9O3S (491.24): C, 51.31; H, 6.77; N, 25.64; O; found: C, 51.16; H, 6.51; N, 25.88.
5-(1-(2-(4-(Diethylamino)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)hydrazono)ethyl)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (5h)
Yellow solid from ethylacetate in 81% yield; mp 222–224 °C; 1H-NMR (CDCl3) δ = 1.17 (t, 6H, J = 7.6 Hz, 2CH3), 1.26 (t, 6H, J = 7.2 Hz, 2CH3), 1.59 (brs, 4H, 2CH2), 1.65 (brs, 2H, CH2), 3.58 (brs, 4H, 2CH2), 3.75 (brs, 4H, 2CH2), 4.55 (q, 4H, J = 7.2, 6.8 Hz, 2CH2), 15.11 (s, 1H, NH); 13C-NMR (CDCl3) δ = 12.3, 17.2, 24.5, 25.7, 42.1, 43.1, 44.9, 91.2, 151.5, 162.4, 169.7, 177.3. Anal. calcd for C22H35N9O2S (489.64): C, 53.97; H, 7.21; N, 25.75. Found C, 54.18; H, 7.44; N, 25.98.
5-(1-(2-(4-(Diethylamino)-6-morpholino-1,3,5-triazin-2-yl)hydrazono)ethyl)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (5i)
Yellow crystals from EtOH in 90% yield; mp 198–200 °C; 1H-NMR (CDCl3) δ = 1.17 (t, 6H, J = 7.2 Hz, 2CH3), 1.26 (t, 6H, J = 6.8 Hz, 2CH3), 2.78 (s, 3H, CH3), 3.52–3.57 (m, 4H, 2CH2), 3.69 (brt, 4H, 2CH2), 3.71 (brt, 4H, 2CH2), 4.54 (q, 4H, 2CH2), 14.89 (s, 1H, NH); 13C-NMR (CDCl3) δ = 12.3, 17.1, 41.9, 43.1, 43.9, 66.6, 91.3, 162.9, 177.3. Anal. calcd for C21H33N9O3S (491.24): C, 51.31; H, 6.77; N, 25.64. Found C, 51.43; H, 6.91; N, 25.82.
1,3-Diethyl-5-(1-(2-(4-morpholino-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)hydrazono)ethyl)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (5j)
Yellow powder from ethylacetate in 89% yield; mp 199–203 °C; 1H-NMR (CDCl3) δ = 1.16–1.23 (m, 6H, 2CH3), 1.57–1.64 (m, 6H, 3CH2), 2.72 (s, 3H, CH3), 3.66 (m, 12H, 6CH2), 4.51 (q, 4H, J = 6.4 Hz, 2CH2), 14.89 (brs, 1H, NH); 13C-NMR (CDCl3) δ = 12.3, 17.2, 18.4, 24.5, 25.7, 43.1, 43.9, 44.8, 58.4, 66.6, 91.4, 168.2, 177.4. Anal. calcd for C22H33N9O3S (503.63): C, 52.47; H, 6.60; N, 25.03. Found C, 52.54; H, 6.83; N, 25.25.
5-(1-(2-(4,6-Di(piperidin-1-yl)-1,3,5-triazin-2-yl)hydrazono)ethyl)-1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione (5k)
Yellow powder from ethylacetate in 92% yield; mp 189–193 °C; 1H-NMR (CDCl3) δ = 1.16–1.24 (m, 6H, 2CH3), 1.53–1.60 (m, 6H, 3CH2), 2.74 (s, 3H, CH3), 3.64 (m, 4H, 2CH2), 4.51 (q, 4H, J = 6.4 Hz, 2CH2), 14.89 (brs, 1H, NH); 13C-NMR (CDCl3) δ = 12.3, 17.2, 18.4, 24.6, 25.7, 43.1, 44.8, 58.4, 91.2, 167.4, 177.3. Anal. calcd for C23H35N9O2S (501.65): C, 55.07; H, 7.03; N, 25.13. Found C, 55.25; H, 7.29; N, 25.39.
Antiproliferative Activity
Cell Proliferation Assay
As described previously,32 growth inhibitions were measured in 96-well plates. Aliquots of 120 μL of the suspended cells (50 000 mL–1) were added to 60 μL of a serial dilution of the inhibitor (S) and incubated for five days. After the incubation time, growth was determined the MTT assay. Briefly, 20 μL of MTT (5 mg/mL in phosphate-buffered saline (PBS)) was added to each well, and the plates were incubated for 2 h at 37 °C, and 5% CO2-atmosphere in the cell incubator. The supernatants were removed, and the cells were washed two times, each with 100 μL of PBS. The formed water-insoluble formazan crystals were dissolved in 200 μL of isopropanol/HCl mixture. The absorbance was read at 595 nm using a microplate reader (Thermo Scientific). The metabolic activities of cells were calculated by dividing the absorbance of the treated cells by the absorbance of the control cells multiplied by 100%. The IC50 values were defined as the sample concentration inhibiting 50% of cell growth.
Detection of Apoptosis by Flow Cytometry
Apoptosis was measured in HepG2 cells by annexin V–propidium iodide (PI) double staining method using the annexin V-FITC apoptosis detection kit (BD Biosciences, San Diego). Briefly, the cells were exposed to 5h at a concentration of about 5× IC50 values in six-well plates for 24 h. At the end of the exposure, the cells were washed with cold PBS, trypsinized, and centrifuged at 1000 rpm; then, the cell pellet was washed again with PBS and resuspended in 100 μL of 1× binding buffer (1 × 106 cells/mL). Then, 5 μL each of annexin V-FITC and PI were added to the cell suspension and the cells were gently vortexed. After incubation for 20 min at room temperature (25 °C) in the dark, the samples were diluted by adding 400 μL of 1× binding buffer. Annexin V/PI fluorescence was analyzed for each sample using a BD FACSCalibur flow cytometer. A total of 10 000 events were acquired for each sample, and the data were analyzed using Cell Quest Pro software (BD Biosciences).
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project at King Saud University, Riyadh, Saudi Arabia, for funding this work through project no. (RSP-2020/50).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00468.
Figures S1–S12 present the NMR (1H and 13C) spectra for the prepared compounds 5a–k (PDF)
Author Contributions
The synthesis was carried out by H.A.R., K.D., and A.S.; the series was designed and supervised by A.E.-F., B.G.d.l.T., and F.A.; and the antiproliferative activities were carried by E.S. All authors contributed to the results and discussion. The first draft of the manuscript was prepared by H.A.R., K.D., A.S., and E.S.; all authors contributed in the final version.
The authors declare no competing financial interest.
Supplementary Material
References
- Wang J.; Radomski M. W.; Medina C.; Gilmer J. F. MMP inhibition by barbiturate homodimers. Bioorg. Med. Chem. Lett. 2013, 23, 444–447. 10.1016/j.bmcl.2012.11.063. [DOI] [PubMed] [Google Scholar]
- Shaker R. M.; Ishak E. A. Barbituric acid utility in multi-component reactions. Z. Naturforsch. 2011, 66, 1189–1201. 10.1515/znb-2011-1201. [DOI] [Google Scholar]
- Ziarani G. M.; Aleali F.; Lashgari N. Recent applications of barbituric acid in multicomponent reactions. RSC Adv. 2016, 6, 50895–50922. 10.1039/C6RA09874F. [DOI] [Google Scholar]
- Mahmudov K. T.; Kopylovich M. N.; Maharramov A. M.; Kurbanova M. M.; Gurbanov A. V.; et al. Pombeiro, Barbituric acids as a useful tool for the construction of coordination and supramolecular compounds, A.J.L. Coord. Chem. Rev. 2014, 265, 1–37. 10.1016/j.ccr.2014.01.002. [DOI] [Google Scholar]
- Kobra N.; Zahra K. Barbituric acids in organic transformations, an outlook to the reaction media. Mini-Rev. Org. Chem. 2017, 14, 143–173. 10.2174/1570193X14666170206122416. [DOI] [Google Scholar]
- Barakat A.; Islam M. S.; Al-Majid A. M.; Soliman S. M.; Mabkhot Y. N.; Al-Othman Z. A.; Ghabbour H. A.; Fun H.-K. Synthesis of novel 5-monoalkylbarbiturate derivatives: new access to 1,2-oxazepines. Tetrahedron Lett. 2015, 56, 6984–6987. 10.1016/j.tetlet.2015.10.108. [DOI] [Google Scholar]
- Bayat M.; Bayat Y.; Asayesh S. S. One-pot synthesis of 2H-pyrano[2,3-d]pyrimidine derivatives. Monatsh. Chem. – Chem. Mon. 2012, 143, 479–483. 10.1007/s00706-011-0602-7. [DOI] [Google Scholar]
- Ghahremanzadeh R.; Fereshtehnejad F.; Bazgir A. Chromeno[2,3-d]pyrimidine-triones synthesis by a three-component coupling reaction. Chem. Pharm. Bull. 2010, 58, 516–520. 10.1248/cpb.58.516. [DOI] [PubMed] [Google Scholar]
- Ismail M. A.; Al-Shihry S.; Arafa R. K.; El-Ayaan U. Synthesis, antimicrobial activity and molecular modeling study of substituted 5-aryl-pyrimido[5,4-c]quinoline-2,4-diones. J. Enzyme Inhib. Med. Chem. 2013, 28, 530–538. 10.3109/14756366.2011.654113. [DOI] [PubMed] [Google Scholar]
- Pati A.; Majumdar P.; Garnayak S.; Behera A. K.; Behera R. K. Regiospecific ring closure reactions of 1,3-diphenylthiobarbituric acid and dimedone: Formation of spiro vs fused heterocycles. Indian J. Chem., Sect. B: Org. Med. Chem. 2013, 53, 384–391. 10.1002/chin.201440163. [DOI] [Google Scholar]
- Girgis A. S.; Farag H.; Ismail N. S. M.; George R. F. Synthesis, hypnotic properties and molecular modeling studies of 1,2,7,9-tetraaza-spiro[4.5]dec-2-ene-6,8,10-triones. Eur. J. Med.Chem. 2011, 46, 4964–4969. 10.1016/j.ejmech.2011.07.058. [DOI] [PubMed] [Google Scholar]
- Zhao H.-W.; Tian T.; Li B.; Yang Z.; Pang H.-L.; Meng W.; Song X.-Q.; Chen X.-Q. Diastereoselective synthesis of dispirobarbiturates through Et3N-catalyzed [3+2] cycloaddition of barbituratebased olefins with 3-isothiocyanato oxindoles. J. Org. Chem. 2015, 80, 10380–10385. 10.1021/acs.joc.5b01810. [DOI] [PubMed] [Google Scholar]
- Khan K. M.; Khan M.; Karim A.; Taha M.; Ambreen N.; Gojayev A.; Perveen S.; Choudhary M. I. Xanthine oxidase inhibition by 5-aryledene N,N′-dimethylbarbituric acid derivatives. J. Chem. Soc. Pak. 2013, 35, 495–498. [Google Scholar]
- Khan K. M.; Khan M.; Ahmad A.; Irshad A.; Kardono L. B. S.; Rahim F.; Haider S. M.; Ahmed S.; Parveen S. Antibacterial and antifungal activities of 5-arylidene-N,N-dimethylbarbiturates derivatives. J. Chem. Soc. Pak. 2014, 36, 1153–1157. [Google Scholar]
- Aly H. M.; Kamal M. M. Efficient one-pot preparation of novel fused chromeno[2,3-d]pyrimidine and pyrano[2,3-d]pyrimidine derivatives. Eur. J. Med. Chem. 2012, 47, 18–23. 10.1016/j.ejmech.2011.09.040. [DOI] [PubMed] [Google Scholar]
- Figueiredo J.; Serrano J. L.; Cavalheiro E.; Keurulainen L.; Yli-Kauhaluomac J.; Moreira V. M.; Ferreira S.; Domingues F. C.; Silvestre S.; Almeida P. Trisubstituted barbiturates and thiobarbiturates: synthesis and biological evaluation as xanthine oxidase inhibitors, antioxidants, antibacterial and anti-proliferative agents. Eur. J. Med. Chem. 2018, 143, 829–842. 10.1016/j.ejmech.2017.11.070. [DOI] [PubMed] [Google Scholar]
- Rauf A.; Shahzad S.; Bajda M.; Yar M.; Ahmed F.; Hussain N.; Akhtar M. N.; Khan A.; Jonczyk J. Design and synthesis of new barbituric- and thiobarbituric acid derivatives as potent urease inhibitors: Structure activity relationship and molecular modeling studies. Bioorg. Med. Chem. 2015, 23, 6049–6058. 10.1016/j.bmc.2015.05.038. [DOI] [PubMed] [Google Scholar]
- Neumann D. M.; Cammarata A.; Backes G.; Palmer G. E.; Jursic B. S. Synthesis and antifungal activity of substituted 2,4,6-pyrimidinetrione carbaldehyde hydrazones. Bioorg. Med. Chem. 2014, 22, 813–826. 10.1016/j.bmc.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Bahar A. A.; Liu Z.; Garafalo M.; Kallenbach N.; Ren D. Controlling persister and biofilm cells of gram-negative bacteria with a new 1, 3, 5-triazine derivative. Pharmaceuticals 2015, 8, 696–710. 10.3390/ph8040696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K.; Pieterse C. M. J.; Bakker P. A. H. M.; Berendsen R. L. Beneficial microbes going underground of root immunity. Plant Cell Environ 2019, 42, 2860–2870. 10.1111/pce.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mibu N.; Yokomizo K.; Aki H.; Ota N.; Fujii H.; Yuzuriha A.; Saneyoshi S.; Tanaka A.; Koga A.; Zhou J.; et al. Synthesis and Antiviral Evaluation of Some C3-Symmetrical Trialkoxy-Substituted 1, 3, 5-Triazines and Their Molecular Geometry. Chem. Pharm. Bull. 2015, 63, 935–944. 10.1248/cpb.c15-00309. [DOI] [PubMed] [Google Scholar]
- Zacharie B.; Abbott S. D.; Bienvenu J.-F. O.; Cameron A. D.; Cloutier J. E.; Duceppe J.-S.; Ezzitouni A.; Fortin D.; Houde K.; Lauzon C.; et al. 2, 4, 6-trisubstituted triazines as protein a mimetics for the treatment of autoimmune diseases. J. Med. Chem. 2010, 53, 1138–1145. 10.1021/jm901403r. [DOI] [PubMed] [Google Scholar]
- Liu B.; Sun T.; Zhou Z.; Du L. A systematic review on antitumor agents with 1, 3, 5-triazines. Med. Chem. 2015, 5, 131–148. 10.4172/2161-0444.1000255. [DOI] [Google Scholar]
- Zhao H.; Liu Y.; Cui Z.; Beattie D.; Gu Y.; Wang Q. Design, synthesis, and biological activities of arylmethylamine substituted chlorotriazine and methylthiotriazine compounds. J. Agric. Food. Chem. 2011, 59, 11711–11717. 10.1021/jf203383s. [DOI] [PubMed] [Google Scholar]
- Shah D. R.; Modh R. P.; Chikhalia K. H. Privileged s-triazines: structure and pharmacological applications. Future Med. Chem. 2014, 6, 463–477. 10.4155/fmc.13.212. [DOI] [PubMed] [Google Scholar]
- Hu Z.; Ma T.; Chen Z.; Ye Z.; Zhang G.; Lou Y.; Yu Y. Solid-phase synthesis and antitumor evaluation of 2, 4-diamino-6-aryl-1, 3, 5-triazines. J. Comb. Chem. 2009, 11, 267–273. 10.1021/cc800157k. [DOI] [PubMed] [Google Scholar]
- Zheng M.; Xu C.; Ma J.; Sun Y.; Du F.; Liu H.; Lin L.; Li C.; Ding J.; Chen K.; et al. Synthesis and antitumor evaluation of a novel series of triaminotriazine derivatives. Biorg. Med. Chem. 2007, 15, 1815–1827. 10.1016/j.bmc.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Kuo G.-H.; DeAngelis A.; Emanuel S.; Wang A.; Zhang Y.; Connolly P. J.; Chen X.; Gruninger R. H.; Rugg C.; Fuentes-Pesquera A. Synthesis and identification of [1, 3, 5] triazine-pyridine biheteroaryl as a novel series of potent cyclin-dependent kinase inhibitors. J. Med. Chem. 2005, 48, 4535–4546. 10.1021/jm040214h. [DOI] [PubMed] [Google Scholar]
- Sączewski F.; Bułakowska A.; Bednarski P.; Grunert R. Synthesis, structure and anticancer activity of novel 2, 4-diamino-1, 3, 5-triazine derivatives. Eur. J. Med. Chem. 2006, 41, 219–225. 10.1016/j.ejmech.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Sączewski F.; Bułakowska A. Synthesis, structure and anticancer activity of novel alkenyl-1, 3, 5-triazine derivatives. Eur. J. Med. Chem. 2006, 41, 611–615. 10.1016/j.ejmech.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Perspicace E.; Jouan-Hureaux V.; Ragno R.; Ballante F.; Sartini S.; La Motta C.; Da Settimo F.; Chen B.; Kirsch G.; Schneider S.; et al. Design, synthesis and biological evaluation of new classes of thieno [3, 2-d] pyrimidinone and thieno [1, 2, 3] triazine as inhibitor of vascular endothelial growth factor receptor-2 (VEGFR-2). Eur. J. Med. Chem. 2013, 63, 765–781. 10.1016/j.ejmech.2013.03.022. [DOI] [PubMed] [Google Scholar]
- Bojarski J. T.; Mokrosz J. L.; Bartoń H. J.; Paluchowska M. H. Recent progress in barbituric acid chemistry. Adv. Heterocycl. Chem. 1985, 38, 229–297. 10.1016/s0065-2725(08)60921-6. [DOI] [Google Scholar]
- Neumann D. M.; Cammarata A.; Backes G.; Palmer G. E.; Jursic B. S. Synthesis and antifungal activity of substituted 2, 4, 6-pyrimidinetrione carbaldehyde hydrazones. Bioorg. Med. Chem. 2014, 22, 813–826. 10.1016/j.bmc.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Rageot D.; Bohnacker T.; Keles E.; McPhail J. A.; Hoffmann R. M.; Melone A.; Borsari C.; Sriramaratnam R.; Sele A. M.; Beaufils F.; Hebeisen P.; Fabbro D.; Hillmann P.; Burke J. E.; Wymann M. P. (S)-4-(Difluoromethyl)-5-(4-(3-methylmorpholino)-6-morpholino- 1,3,5-triazin-2-yl)pyridin-2-amine (PQR530), a potent, orally bioavailable, and brain-penetrable dual inhibitor of class I PI3K and mTOR Kinase. J. Med. Chem. 2019, 62, 6241–6261. 10.1021/acs.jmedchem.9b00525. [DOI] [PubMed] [Google Scholar]
- El-Faham A.; Farooq M.; Almarhoon Z.; Abd Alhameed R.; Wadaan M. A. M.; de la Torre B. G.; Albericio F. Di- and tri-substituted s-triazine derivatives: Synthesis, characterization, anticancer activity in human breast-cancer cell lines, and developmental toxicity in zebrafish embryos. Bioorg. Chem. 2020, 94, 103397 10.1016/j.bioorg.2019.103397. [DOI] [PubMed] [Google Scholar]
- Farooq M.; Sharma A.; Almarhoon Z.; Al-Dhfyan A.; El-Faham A.; Abu Taha N.; Wadaan M. A. M.; de laTorre B. G.; Albericio F. Design and synthesis of mono-and di-pyrazolyl-s-triazine derivatives, their anticancer profile in human cancer cell lines, and in vivo toxicity in zebrafish embryos. Bioorg. Chem. 2019, 87, 457–464. 10.1016/j.bioorg.2019.03.063. [DOI] [PubMed] [Google Scholar]
- Barakat A.; El-Senduny F. F.; Almarhoon Z.; Al-Rasheed H. H.; Badria F. A.; Al-Majid A. M.; Ghabbour H. A.; El-Faham A. Synthesis, X-ray crystal structures, and preliminary anti-proliferative activities of new s-triazine-hydroxybenzylidene hydrazone derivatives. J. Chem. 2019, 2019, 9403908 10.1155/2019/9403908. [DOI] [Google Scholar]
- Srivastava G. K.; Alonso-Alonso M. L.; Fernandez-Bueno I.; Garcia-Gutierrez M. T.; Rull F.; Medina J.; Coco R. M.; Pastor J. C. Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation. Sci. Rep. 2018, 8, 1425 10.1038/s41598-018-19428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F.; Liu H.; Tong L.; Zhou W.; Liu L.; Zhao Z.; Liu X.; Jiang H.; Wang X.; Xie H.; et al. Discovery of novel selective inhibitors for EGFR-T790M/L858R. Bioorg. Med. Chem. Lett. 2012, 22, 1365–1370. 10.1016/j.bmcl.2011.12.067. [DOI] [PubMed] [Google Scholar]
- El-Faham A.; Soliman S. M.; Ghabbour H. A.; Elnakady Y. A.; Mohaya T. A.; Siddiqui M. R.; Albericio F. Ultrasonic promoted synthesis of novel s-triazine-Schiff base derivatives; molecular structure, spectroscopic studies and their preliminary anti-proliferative activities. J. Mol. Struct. 2016, 1125, 121–135. 10.1016/j.molstruc.2016.06.061. [DOI] [Google Scholar]
- Giziroglu E.; Aygün M.; Sarikurkcu C.; Kazar D.; Orhan N.; Firinci E.; Soyleyici H. C.; Gokcen C. Synthesis, characterization and antioxidant activity of new dibasic tridentate ligands: X-ray crystal structures of DMSO adducts of 1, 3-dimethyl-5-acetyl-barbituric acid o-hydroxybenzoyl hydrazone copper (II) complex. Inorg. Chem. Commun. 2013, 36, 199–205. 10.1016/j.inoche.2013.09.013. [DOI] [Google Scholar]
- Sharma A.; Jad Y.; Ghabbour H.; de la Torre B.; Kruger H.; Albericio F.; El-Faham A. Synthesis, crystal structure and DFT studies of 1,3-dimethyl-5-propionylpyrimidine-2,4,6(1H,3H,5H)-trione. Crystals 2017, 7, 31 10.3390/cryst7010031. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.