Clinical Implications.
-
•
A patient with X-linked agammaglobulinemia was infected with severe acute respiratory syndrome coronavirus 2 and presented a 40-day long and stagnant clinical evolution refractory to treatment. The patient received a single dose of coronavirus disease 2019 convalescent plasma and experienced a rapid clinical improvement within 1 week of infusion.
The high infectivity of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the lack of preexisting immunity among the population, and the complexity of the coronavirus disease 2019 (COVID-19) clinical presentation are some of the underlying reasons of the ongoing worldwide health crisis. Antibodies play relevant roles in antiviral responses,1 and hence, patients with compromised specific humoral immunity may be at increased risk to suffer from severe forms of the disease. In fact, 3 recent studies described the clinical evolution of 6 patients with common variable immunodeficiency (CVID), 3 patients with x-linked agammagloblulinemia (XLA) (OMIM# 300755; Bruton's disease), and 1 patient with autosomal agammaglobulinemia.2, 3, 4 However, the mild clinical presentation in agammaglobulinemic patients, as opposed to the former, prompted speculations that anti–SARS-CoV-2 antibodies may be dispensable in clearing the disease.4 This would highlight the relevance of T-cell responses and the significance of anti–SARS-CoV-2 T-cell exhaustion in the ultimate progression of the disease.5 However, administration of hyperimmune plasma was beneficial in some immunocompetent patients with COVID-19.6
We report the case of a 39-year-old male patient with XLA, receiving monthly immunoglobulin replacement therapy, admitted to our hospital with dyspnea, dry cough, and fever of 2-week duration. Chest X-rays showed unilateral alveolar condensation, and quantitative PCR (qPCR) amplification of SARS-CoV-2 from a nasopharyngeal exudate sample confirmed the diagnosis of COVID-19. The patient received hydroxychloroquine (400 mg/12 h, the first day; 200 mg/12 h, 4 days, orally), azithromycin (500 mg/24 h, 5 days, orally), and ceftriaxone (2 g/24 h, 7 days, intravenous [IV]) and a single dose (0.3 g/kg) of IV immunoglobulins. No supplemental oxygen was required.
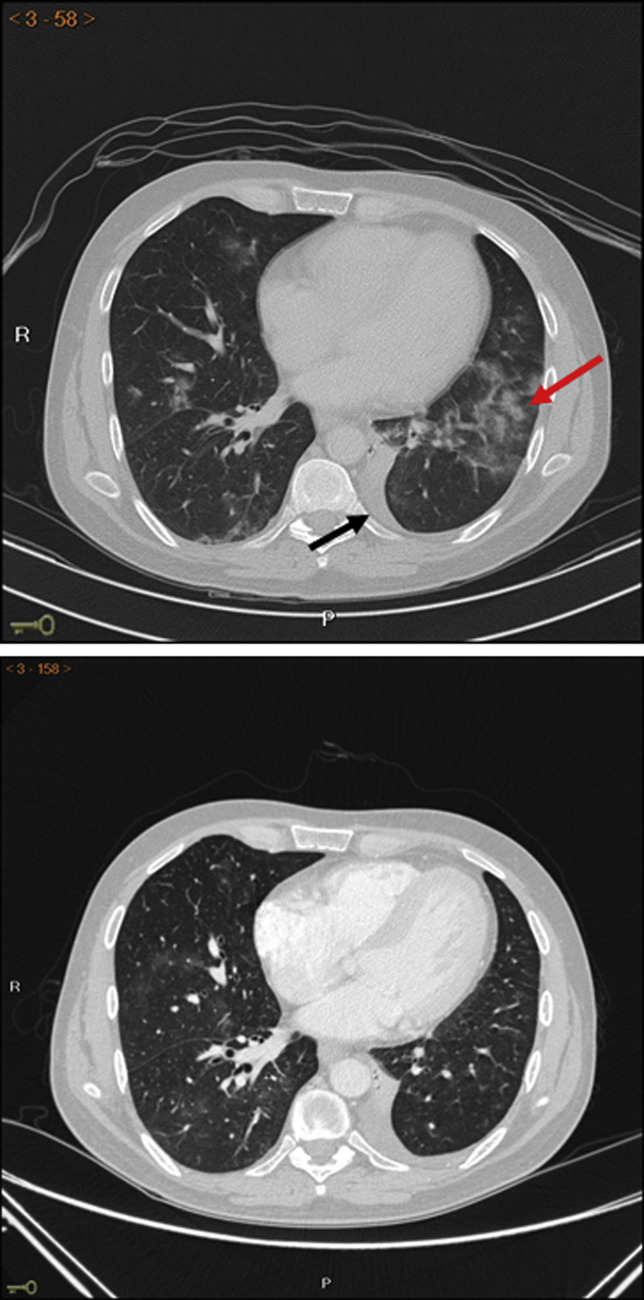
In spite of a favorable initial clinical evolution, fever persisted and intense asthenia appeared. Therefore, empirical therapy against nosocomial germs was started with piperacillin/tazobactam (4 g/0.5 g/8 h, IV) and linezolid (600 mg/12 h, IV). SARS-CoV-2 qPCR test result remained positive on day 11 after admission. On day 15 computed tomography showed extensive bilateral pulmonary involvement in the form of nodular consolidations in ground glass with underlying interstitial pattern (Figure 1 , upper panel). Blood, sputum, stool, and urine cultures were negative. Infections by other respiratory viruses (adenovirus, metapneumovirus, rhinovirus, and respiratory syncytial virus), as well as the presence of opportunistic germs such as Pneumocystis jiroveci, cytomegalovirus, EBV, Mycoplasma pneumoniae, aspergillus, leishmania, and brucella, were all excluded. A third SARS-CoV-2 qPCR was carried out on day 21 after admission and the result remained positive.
Figure 1.
Upper panel: CT displaying bilateral pulmonary involvement in the form of nodular consolidations and ground-glass opacities primarily in the left lower lobe (red arrow) and small-size left pleural effusion (black arrow). Lower panel: CT of the chest 6 days after COVID-19 convalescent donor plasma transfusion showing almost complete resolution of bilateral lung lesions pattern. CT, Computed tomography.
Stagnancy in the patient's evolution, as represented by the lack of response to any of the treatments dispensed (including a second pulse of nonspecific immunoglobulins at 0.8 g/kg/24 h and aggressive antibiotic therapy), together with persistent fever and bilateral pneumonia, prompted us to consider the possibility that natural killer (NK)-cell and T-cell exhaustion might be an underlying cause for immunologic failure, as shown elsewhere.5 We assessed this possibility in lung lymphoid populations obtained by bronchoalveolar lavage on day 21 after admission, and they consisted of CD3+CD8+ (86%) and CD3+CD4+ T lymphocytes (11.5%) and CD56+CD16+ NK cells (2.5%). Staining of intracellular cytokines in stimulated CD8+ cells showed negligible production of TNF-α or IFN-γ (Figure 2 , A, middle and bottom panels).
Figure 2.
(A) Flow cytometric double fluorescence analysis of CD8 cells showing coexpression of surface CD3 (upper panel) and intracytoplasmic TNF-α (middle panel) or IFN-γ (lower panel) after cell stimulation. (B) Blood peripheral T lymphocytes and NK-cell counts determined on days 0, 3, and 7 postinfusion of COVID-19 convalescent plasma.
Because of the resulting lymphocyte phenotype, we reasoned that, in the absence of functional B cells, viral clearance would not be possible. Hence, we administered on day 23 after admission compassionate-use approved COVID-19 convalescent donor plasma (200 mL, single dose, IV). The plasma was analyzed by an ELISA (COVID-19 ELISA IgG, Vircell SL, Granada, Spain), which detected IgG antibodies against either the spike or nucleocapside viral proteins with a titer greater than or equal to 1/320. After 24 hours of infusion, fever ceased without subsequent reappearance and with progressive improvement of asthenia. After 48 hours of infusion, qPCR from nasopharyngeal exudate failed to amplify SARS-CoV-2 sequences.
On day 30 after admission, that is, 1 week after administration of COVID-19 convalescent donor plasma, the patient remained asymptomatic and a chest tomography showed almost complete resolution of bilateral lung lesions (Figure 1, lower panel). Furthermore, a vigorous recovery in the peripheral T-lymphocyte subpopulations (CD3+, CD4+, and CD8+) was observed, whereas NK-cell counts slightly increased (Figure 2, B). Hence, the patient was subsequently discharged and remains COVID-19 free.
Although cumulative cases of SARS-CoV-2–infected patients suffering from primary antibody immunodeficiencies are limited, it appears that individuals devoid of B cells (agammaglobulinemia) have a milder presentation and course than those with a partial B-cell repertoire (CVID).2, 3, 4 This observation is strengthened by the finding that pharmacological blockade of Bruton tyrosine kinase results in diminished lung inflammatory response by inhibiting overproduction of macrophage-mediated cytokines.7 The evolution of our patient was consistent with those observations until day 15 of admission, when he suffered a rapid deterioration in his condition accompanied by a lack of cytokine production by lung T and NK cells. If this lack of functional responses was the consequence of cell exhaustion, we should bear in mind that T-cell responses involve triggering of multiple epitope-specific clones, and not all may be equally prone to functional blockade.8 Therefore, local silencing of immunodominant clones may hamper the overall immune response.
The remarkable and prompt recovery observed after infusion of convalescent plasma suggests that certain patients may have stringent dependency on specific antibodies to achieve viral clearance. Although this is a single case and therefore the observation could be coincidental and we have not formally established a cause-effect relationship, we nevertheless propose that administration of COVID-19 hyperimmune plasma should be further explored as first line of treatment for patients with congenital agammaglobulinemia, especially while SARS-CoV-2–specific antiviral drugs remain unavailable. Previous clinical cases suggest that B cells may have a prominent role in triggering lung inflammation3; in this scenario, defective germinal center formation due to the primary immunodeficiency would divert IL-6 intended for that process toward lung inflammation, thus raising the possibility of a B-cell interference as therapeutic intervention.3 However, our data suggest a critical role of B cells in clearing the disease when certain immunologic conditions are present.
In summary, even though administration of passive immunity for patients with primary immunodeficiency has just been proposed,9 this is the first report showing a rapid recovery of a patient with a primary antibody immunodeficiency after administration of hyperimmune plasma. Although it should be kept in mind whether the patient reported here was idiosyncratic and might not represent a prototypical patient with XLA (despite carrying a common Bruton tyrosine kinase gene mutation), our data merit further investigations to determine the precise clinical benefit of hyperimmune plasma in other antibody-deficient patients, as well as optimal dose and timing of its administration.
Acknowledgments
We thank Professor J.L. Martinez for invaluable help with microbiology studies. We gratefully acknowledge Red Andaluza de Medicina Transfusional, Tejidos y Células, Servicio Andaluz de Salud for supplying and analyzing the COVID-19 convalescent plasma.
Footnotes
This work was supported by Consejería de Universidades, Junta de Andalucía (grant no. CT208 to M.S.) and Action for A-T (Haslemere, UK) (grant no. AAT- 8GRA02 to I.J.M.).
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Burton D.R. Antibodies, viruses and vaccines. Nat Rev Immunol. 2002;2:706–713. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- 2.Fill L., Hadney L., Graven K., Persaud R., Hostoffer R. The clinical observation of a CVID patient infected with COVID-19. Ann Allergy Asthma Immunol. 2020;125:112–114. doi: 10.1016/j.anai.2020.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinti I., Lougaris V., Milito C., Cinetto F., Pecoraro A., Mezzaroma I. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. 2020;146:211–213.e4. doi: 10.1016/j.jaci.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soresina A., Moratto D., Chiarini M., Paolillo C., Baresi G., Foca E. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr Allergy Immunol. 2020;31:565–569. doi: 10.1111/pai.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang B., Liu S., Tan T., Huang W., Dong Y., Chen L. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020;158:e9–e13. doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roschewski M., Lionakis M.S., Sharman J.P., Roswarski J., Goy A., Monticelli M.A. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci Immunol. 2020;5:eabd0110. doi: 10.1126/sciimmunol.abd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahan S.M., Wherry E.J., Zajac A.J. T cell exhaustion during persistent viral infections. Virology. 2015;479-80:180–193. doi: 10.1016/j.virol.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammarstrom L., Abolhassani H., Baldanti F., Marcotte H., Pan-Hammarstrom Q. Development of passive immunity against SARS-CoV-2 for management of immunodeficient patients—a perspective. J Allergy Clin Immunol. 2020;146:58–60. doi: 10.1016/j.jaci.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]