Abstract
5-Fluorouracil-nicotinamide (5-FU-NCM), a co-crystal with a 2D layer structure formed by hydrogen bonds, was synthesized by solvent evaporation and liquid phase-assisted grinding at room temperature. Compared to 5-FU alone, the results of solubility, oil–water partition coefficient, anti-tumor effect in vivo and vitro, acute toxicity, and pharmacokinetic parameters indicate that the co-crystal is a potential anti-tumor drug.
Introduction
Interdisciplinary research is critical to the development of new therapeutics that will improve human health and quality of life. Whether it is developing new drugs or modifying the current ones, generating new therapies are technically and intellectually challenging, time-consuming, and costly.1 Among the most difficult challenges is maximizing the efficacy while limiting adverse side effects to the greatest extent possible. Modifying the existing compounds represents a highly promising approach to identifying potential new therapeutic uses.
Design and synthesis of pharmaceutical co-crystals, crystalline single-phase materials composed of two or more different molecular and/or ionic compounds generally in a stoichiometric ratio, is one of the methods to achieve this goal.2,3 They are neither solvates nor simple salts. At least, one of the crystals in the co-crystal is an active pharmaceutical ingredient (API) and the other is pharmaceutically acceptable, known as co-crystal former (CCF).4 Because no substantial electron transfer occurs, co-crystals retain the greatest degree of API integrity. By adding a CCF, the physicochemical properties of co-crystals can be enhanced beyond those exhibited by the API alone. Co-crystals have been drawing scientists’ attention because of their fundamental properties such as improving the physical and chemical stability, increasing the dissolution and solubility, and improving the bioavailability of APIs.5−12 Valsartan/sacubitril (brand name Entresto), a drug used in the treatment of heart failure and developed by Novartis, has a co-crystallized structure of valsartan and sacubitril in a 1:1 molar ratio.13 It was approved by the FDA in 2015 and has been a co-crystallized drug available on the market. Another co-crystallized anti-diabetic drug named Ertugliflozin, the active form of which is a 1:1 cocrystal of ertugliflozin with pyroglutamic acid, is now FDA-approved and marketed as Steglatro.14 These compounds indicate the potentially promising future of co-crystal drugs. The purpose of the present study was using this approach to design and test a new co-crystal compound with anti-tumor properties.
5-Fluorouracil (5-FU), one of the anti-tumor drugs used in 1960s, is a cell cycle inhibitor that has been widely used clinically against many solid tumors, including advanced colorectal cancer.15 It has also been administered with other drugs to treat ovarian, gastric, and head and neck cancers.16−18 However, because of rapid metabolism and low oral bioavailability, 5-FU is rarely used in oral dosage forms. The most common form of administration is continuous intravenous (IV) infusion. In addition to the inconvenience of IV administration, it is also accompanied by severe side effects such as bone marrow inhibition, cardiotoxicity, abdominal pain, and diarrhea.19 As the analogue of pyrimidine, 5-FU has several N–H donors and C=O acceptors.12,20 Nicotinamide (NCM), a water-soluble vitamin, is present in a variety of foods, which is normally looked at as a co-former because of its N–H donor and C=O and N-containing pyridyl ring acceptors.21 The characteristics of their structures enable them to easily form a co-crystal. To optimize the potency and minimize the side effects, we sought to generate a co-crystal compound of 5-FU and NCM.
Results and Discussion
Chemistry
The 5-fluorouracil-nicotinamide (5-FU-NCM) co-crystal was synthesized at room temperature using the solvent evaporation technique and liquid phase-assisted grinding. Single-crystal X-ray diffraction analysis indicates that the molecular structure of the co-crystal comprises three 5-FU, one NCM, and one water molecule attached together by hydrogen bonding and lone pair electron−π stacking (see Figure S2 in Supporting Information). The adjacent 5-FU molecules form a Z-type chain A in the c-direction through the N–H···O–hydrogen bond, which is connected in the same manner as the reported 5-FU form (see Figure S3 in Supporting Information).22 Furthermore, chain B is formed along the c direction by 5-FU, NCM, and water molecules through the N–H···O, O–H···O, and N–H···N hydrogen bonds, which interacts with chain A to form a layered structure (Figure 1).
Figure 1.
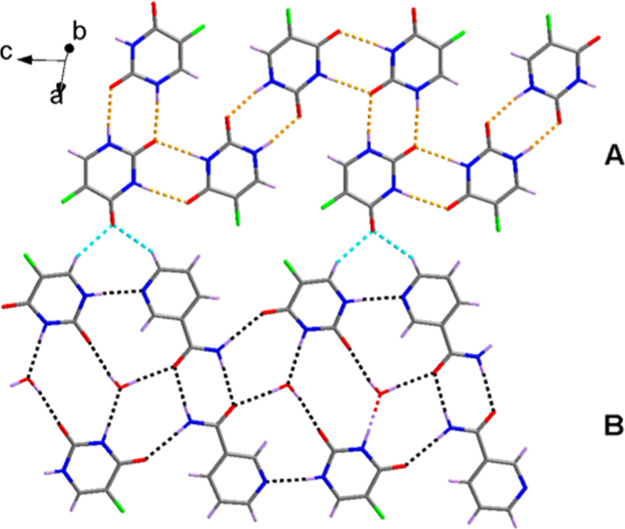
Layered structure of 5-FU-NCM. (A) 5-FU molecules forming a Z-type chain. (B) 5-FU, NCM, and water molecules held through hydrogen bonds forming a chain along the c direction.
In contrast to the solubility of 5-FU (10.46 g/L, 0.080 mol/L, 293 K), the co-crystal’s solubility of 17.61 g/L (0.033 mol/L, 293 K) corresponds to the dissolution of 0.099 mol/L 5-FU according to the structure mentioned above (see Figures S7, S8 and Tables S3, S4 in Supporting Information). The log P value, oil–water partition coefficient, is a measure of lipophilicity or hydrophobicity, indicating the solubility of the compound in water or oil.11 Our results show the log P value of the co-crystal as −1.17 (P = 0.068), while the log P of 5-FU is −0.46 (P = 0.35). These results suggest that the co-crystal molecule is more hydrophilic and has lower membrane permeability compared to its API. The low permeability of 5-FU-NCM could potentially limit its transport through lipophilic cell and organellar membranes, thus diminishing its bioavailability.
Biological Activity
To assess the inhibition of cell proliferation, we carried out 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay on different tumor cell lines.13 5-FU-NCM has inhibitory effects on these tumor cell lines, and the inhibition is dose- and time-dependent (see Figure S9). The biochemical half maximal inhibitory concentration (IC50) of the co-crystal is calculated as 72 h and indicates that the IC50 of the 5-FU-NCM co-crystal is less than the IC50 of its corresponding API (Table 1). These results suggest that the co-crystal molecule has the ability to inhibit tumor cell proliferation in cell culture, and its efficacy is superior to its API. Among all the tested cancer cells, the 5-FU-NCM co-crystals show the greatest potency (lowest IC50) against HCT116 cells.
Table 1. IC50 (mol/L) Comparison Between 5-FU and 5-FU-NCM on Tumor Cellsa.
| cell lines | 5-FU | 5-FU-NCM |
|---|---|---|
| HCT116 | 3.25 × 10–8 ± 1.18 × 10–8 | 1.03 × 10–8 ± 1.34 × 10–9 |
| HeLa | 7.96 × 10–6 ± 2.13 × 10–6 | 1.59 × 10–6 ± 1.35 × 10–7 |
| Hep G2 | 3.86 × 10–6 ± 5.56 × 10–7 | 3.43 × 10–6 ± 2.42 × 10–6 |
| JEG3 | 2.18 × 10–6 ± 2.81 × 10–6 | 4.44 × 10–7 ± 2.60 × 10–7 |
| SMMC7721 | 1.89 × 10–5 ± 1.58 × 10–6 | 6.27 × 10–8 ± 3.41 × 10–7 |
The same molar concentration of API (5-FU) was applied to a variety of cell lines in different forms: 5-FU and 5-FU-NCM.
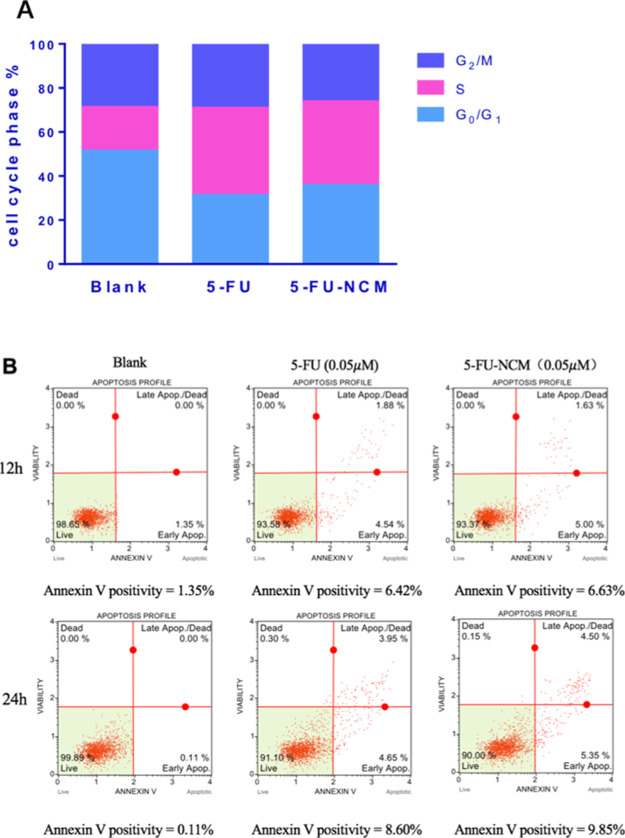
5-FU is effective in the treatment of multiple cancers primarily by inhibiting transition through the G1/S phase of the cell cycle.14 In addition, cell cycle arrest may also trigger apoptosis, thus contributing further to the anti-tumor activity of 5-FU.15 Using flow cytometry, we investigated the effect of the 5-FU-NCM co-crystal on HCT116 cells. Our data indicate a block in the G1/S phase. The efficacy of the co-crystal is similar to the API group (Figure 2A). When examining apoptosis, the results indicate a modest increase in apoptosis with time; however, there is no difference in this parameter when compared to the API group (Figure 2B). No significant difference in potency is observed either, suggesting that the 5-FU-NCM co-crystal and the API may work via the same mechanism of action.
Figure 2.
Cell cycle and cytometric analysis. (A) Effect of 5-FU-NCM and 5-FU at the concentration of 5 μM for 12 h on HCT116 cells in cell cycle distribution. (B) Annexin-FITC/PI flow cytometry results of HCT116 cells in 12/24 h under the exposure of 5-FU-NCM up to the concentration of 5 μM.
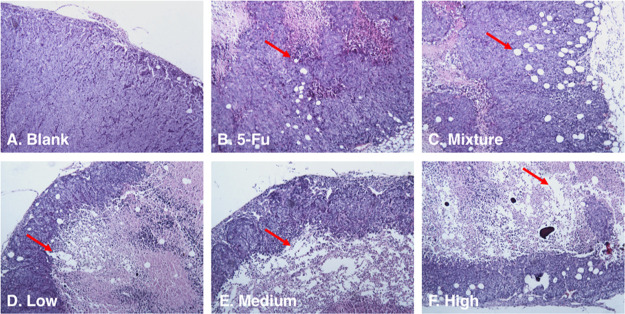
To determine the 5-FU-NCM co-crystal being an anti-proliferative effect in vivo, we used a xenograft model in which 0.1 mL of solution containing 5 × 105 viable HCT116 cells were implanted subcutaneously in the right flank of all the nude mice. After 21 days of continuous daily injections into the left hypogastrium of the following, three groups received 0.006, 0.012, and 0.018 mmol/kg/d of 5-FU-NCM, respectively; one group was treated with the mixture (0.036 mmol/kg/d 5-FU and 0.012 mmol/kg/d NCM); one group received 5-FU alone (0.036 mmol/kg/d), and all the animals were euthanized and subjected to histopathological analysis and measurement of tumor weight. A dose-dependent decrease in the tumor weight is observed in 5-FU-NCM-treated compared to the control group (Table 2). Histopathological analysis of paraffin-embedded, hematoxylin and eosin (H & E)-stained section demonstrates a higher number of necrotic cells in the co-crystal group compared to the 5-FU only, 5-FU and NCM mixture (but not co-crystallized), and control groups (Figure 3). These results indicate that the 5-FU-NCM co-crystals maintained the efficacy of the API in vivo, while showing a stronger anti-tumor effect compared to API alone.
Table 2. Inhibitory Effect of 5-FU-NCM on HCT116 Tumor Massa.
| group | dose (mmol/kg) | W̅ (g) | TGI (%) | |
|---|---|---|---|---|
| blank | 0.83 ± 0.19 | |||
| 5-FU | 0.036 | 0.65 ± 0.10 | 21.8 | |
| mixture (5-FU/NCM) | 0.036/0.012 | 0.65 ± 0.21 | 22.1 | |
| low | 0.006 | 0.62 ± 0.15 | 25.6 | |
| 5-FU-NCM | medium | 0.012 | 0.59 ± 0.13 | 28.5 |
| High | 0.018 | 0.50 ± 0.10 | 39.2 | |
Tumor growth inhibition (TGI %)
was determined by the formula: 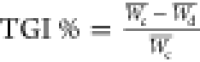 , where
, where 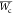 = average tumor growth weight of blank
group and
= average tumor growth weight of blank
group and 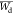 = average tumor growth weight of treated
group. W̅ = average tumor growth.
= average tumor growth weight of treated
group. W̅ = average tumor growth.
Figure 3.
Histology (H & E ×100) results of the tumor are compared. (a) Blank control group. (b) Positive control group (0.036 mmol/kg/d 5-FU). (c) Mixture group, the group treated with the compounds 5-FU and NCM in a non-co-crystallized form at a 3:1 molar ratio (0.036 mmol/kg/d 5-FU + 0.012 mmol/kg/d NCM). (d–f) Groups treated with 5-FU-NCM at different concentrations [(d) 0.006 mmol/kg/d; (e) 0.012 mmol/kg/d; (f) 0.018 mmol/kg/d].
Because the co-crystal molecule appears to enhance the efficacy of API in vivo and in vitro, it is possible that the physical and chemical properties of API have been altered. Thus, we explored whether the co-crystal could prolong the metabolic cycle and improve the bioavailability. The pharmacokinetic parameters were obtained by the drug analysis system DAS 2.0, and the drug–time curve was plotted (Figure 4 and Table 3). The Cmax and the AUC of co-crystals are found to be increased, which suggests that 5-FU-NCM has increased the absorption and bioavailability of API compared to 5-FU only. These results could explain the enhanced inhibitory property of the co-crystal in vivo.
Figure 4.
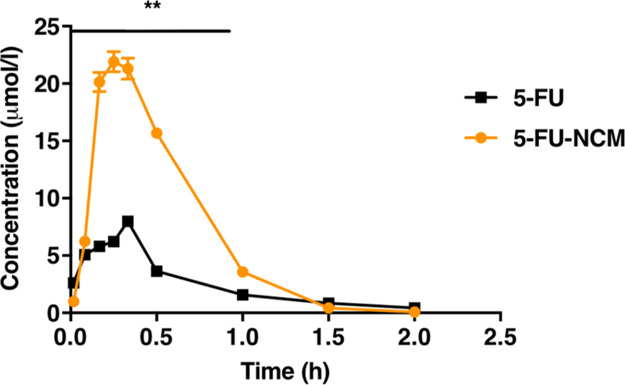
Average plasma concentrations versus time of 5-FU and 5-FU-NCM after oral administration in rats. The initial dose given to SD rats was based on their body weight at the rate of 0.06 mmol/kg. Blood samples were collected at 1, 5, 10, 15, 20, 30, 60, 90, and 120 min after administration. x̅ ± SD, n = 8, ** p < 0.01, two-way ANOVA.
Table 3. Major Pharmacokinetic Parameters of 5-FU-NCMa.
| parameters | unit | 5-FU | 5-FU-NCM |
|---|---|---|---|
| t1/2α | h | 0.064 ± 0.013 | 0.197 ± 0.05 |
| t1/2β | h | 0.302 ± 0.062 | 0.229 ± 0.041 |
| V1/F | L/kg | 2.783 ± 0.915 | 0.974 ± 0.442 |
| CL/F | L/h/kg | 11.845 ± 0.239 | 4.27 ± 0.165 |
| AUC (0 – t) | μmol/L h | 4.928 ± 0.076 | 14.017 ± 0.520 |
| AUC (0 – ∞) | μmol/L h | 5.067 ± 0.104 | 14.071 ± 0.528 |
| Tmax | h | 0.333 | 0.250 |
| Cmax | μmol/L | 7.993 ± 0.375 | 21.910 ± 0.872 |
Different parameters were measured as listed (x̅ ± s, n = 8).
It is possible that increasing the bioavailability while maintaining the plasma concentration at a higher level could also result in increased toxicity. An acute toxicity study was performed to evaluate the toxicity property of the 5-FU-NCM co-crystal. The median lethal dose (LD50) of 5-FU is 1.002 mmol/kg and the co-crystal LD50 is 0.50 mmol/kg. Because the co-crystal is composed of 5-FU and NCM in a 3:1 ratio, the LD50 equivalent to 5-FU is 1.5 mmol/kg, which is 1.5 times that of the API in the fully dissociated state. This decreased LD50 of 5-FU-NCM suggests reduced toxicity of the co-crystal compared to API (Tables 4 and 5).
Table 4. Acute Toxicity Test Result of 5-FUa.
| group | dose (mg/kg)/(mmol/kg) | Ni | Nd | R (%) | lg dose | probit (P + 5) |
|---|---|---|---|---|---|---|
| 1 | 158.5/1.22 | 10 | 9 | 90 | 2.200 | 6.28 |
| 2 | 133.4/1.03 | 10 | 7 | 70 | 2.125 | 5.520 |
| 3 | 122.2/0.86 | 10 | 1 | 10 | 2.050 | 3.720 |
The LD50 was 1.00 mmol/kg; 95% confidence interval is 0.38 ≤ LD50 ≤ 2.63 mmol/kg. R = Nd/Ni. Ni represents the initial animal number, while Nd represents the number of dead animals.
Table 5. Acute Toxicity Test Result of 5-FU-NCMa.
| group | dose (mg/kg)/(mmol/kg) | Ni | Nd | R (%) | lg dose | probit (P + 5) |
|---|---|---|---|---|---|---|
| 1 | 446.7/0.84 | 10 | 8 | 80 | 2.650 | 5.840 |
| 2 | 266.1/0.50 | 10 | 6 | 60 | 2.425 | 5.250 |
| 3 | 223.9/0.42 | 10 | 4 | 40 | 2.350 | 4.750 |
| 4 | 188.4/0.36 | 10 | 2 | 20 | 2.275 | 4.160 |
The LD50 was 0.50 mmol/kg; 95% confidence interval is 0.29 ≤ LD50 ≤ 0.88 mmol/kg. R = Nd/Ni. Ni represents the initial animal number, while Nd represents the number of dead animals.
Conclusions
In conclusion, our research on the 5-FU-NCM co-crystal indicates that it can exist in a stable state at room temperature. 5-FU-NCM has altered physical and chemical properties; in particular, the solubility of the co-crystal is higher than that of 5-FU alone. Our study also verified that the co-crystal preserves API’s anti-tumor property in both in vitro and in vivo experiments. Because the solubility of the co-crystal has been increased compared to 5-FU itself, this corroborated the observation that we had improved the bioavailability while effectively lowering the acute toxicity. These promising results support the concept that co-crystal molecular constructs may have therapeutic advantages in cancer and other applications. We are actively exploring this exciting synthetic methodology using 5-FU-NCM and other co-crystal compounds.
Experimental Section
General Methods
All chemicals and solvents were of A.R. grade and HPLC grade and were used without further purification. Single-crystal diffraction data were collected at 295 K on an Agilent Xcalibur Eos diffractometer with graphite monochromatized Mo-Kα (λ = 0.71073 Å) radiation in ω-scan mode. Elemental analyses were carried out on a Vario MICRO from Elementar Analysensysteme GmbH. Powder X-ray diffraction (PXRD) patterns were collected at 293 K on a Bruker D8 diffractometer (Cu Kα, λ = 1.54059 Å).
General Solution Method and Grinding Method for the Synthesis of 5-Fluorouracil-nicotinamide (5-FU-NCM) Co-Crystals
The 5-FU-NCM co-crystal was synthesized by adding 5-FU (3 mmol, 390 mg) and NCM (1 mmol, 122 mg) to a mixed distilled water and methanol solution (v/v = 1:9). The mixture was stirred at room temperature for 10 min and then filtered. Colorless crystals suitable for X-ray diffraction were isolated from the filtrate after 7 days. Yield: 88% (based on N). Elemental analysis calcd (%) for C18H17F3N8O8: C, 40.72; H, 3.20; N, 21.12. Found: C, 40.76; H, 3.13; N, 21.15. To further explore the assembling process of the 5-FU-NCM co-crystal, a grinding method was employed with the same reactants. A certain amount of 5-FU and NCM in a 3:1 ratio was mixed well and ground for ca. 30 min in an agate mortar, with a few drops of distilled water added to the grinding mixture. After being dried by standing in air, the PXRD pattern of the above-mentioned grinding mixture has been obtained at room temperature. The formation and purity of the 5-FU-NCM co-crystal were confirmed by comparison of the experimental PXRD pattern with the reference powder diffractogram (calculated on the basis of single crystal data on the 5-FU-NCM co-crystal obtained by conventional solution crystallization, Figure S1), which was carried out by the Mercury program, available free of charge via the internet at http://www.ccdc.cam.ac.uk.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (51302067), the Project of Natural Science Foundation of Heilongjiang Province (no. B2015007), the Scientific and Technological Innovation Talents of Harbin (2016RAQXJ005), the Young Innovation Talents of college in Heilongjiang Province (UNPYSCT-2016074), the projects 2016JCZX51 and 2016JCZX57 of Harbin Medical University Scientific Research Innovation Fund, the Innovation and Entrepreneurship Training Program of Harbin Medical University in 2019, and the Special Funds for the Construction of High Level Universities and Specialty Disciplines (31021180107).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03574.
(CIF) The crystallographic data of 5-FU-NCM file including chemical structural formula, cell parameters, atom coordinate, space group, literature and so on.
General solution method for the synthesis of 5-fluorouracil-nicotinamide (5-FU-NCM) co-crystals; grinding method for the synthesis of 5-fluorouracil-nicotinamide (5-FU-NCM) co-crystals; X-ray crystallographic measurements; IR spectra; TG analysis curves; luminescent properties; determination of solubility and oil–water partition coefficient; cell inhibitory assay; determination of cell cycle; assessment of apoptosis by Annexin V-FITC/PI staining; in vivo anti-tumor activity; pharmacokinetics in vivo; and additional figures and tables (PDF)
Author Contributions
# Z.Z. and N.Y. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Horvath P.; Aulner N.; Bickle M.; Davies A. M.; Nery E. D.; Ebner D.; Montoya M. C.; Östling P.; Pietiäinen V.; Price L. S.; Shorte S. L.; Turcatti G.; von Schantz C.; Carragher N. O. Screening out irrelevant cell-based models of disease. Nat. Rev. Drug Discovery 2016, 15, 751–769. 10.1038/nrd.2016.175. [DOI] [PubMed] [Google Scholar]
- Lara-Ochoa F.; Espinosa-pérez G. Cocrystals Definitions. Supramol. Chem. 2007, 19, 553–557. 10.1080/10610270701501652. [DOI] [Google Scholar]
- Steed J. W. 21st century developments in the understanding and control of molecular solids. Chem. Commun. 2018, 54, 13175–13182. 10.1039/c8cc08277d. [DOI] [PubMed] [Google Scholar]
- Duggirala N. K.; Perry M. L.; Almarsson Ö.; Zaworotko M. J. Pharmaceutical cocrystals: along the path to improved medicines. Chem. Commun. 2016, 52, 640–655. 10.1039/c5cc08216a. [DOI] [PubMed] [Google Scholar]
- Ross S. A.; Lamprou D. A.; Douroumis D. Engineering and manufacturing of pharmaceutical co-crystals: a review of solvent-free manufacturing technologies. Chem. Commun. 2016, 52, 8772–8786. 10.1039/c6cc01289b. [DOI] [PubMed] [Google Scholar]
- Suresh K.; Mannava M. K. C.; Nangia A. Cocrystals and alloys of nitazoxanide: enhanced pharmacokinetics. Chem. Commun. 2016, 52, 4223–4226. 10.1039/c6cc00975a. [DOI] [PubMed] [Google Scholar]
- Gunnam A.; Suresh K.; Ganduri R.; Nangia A. Crystal engineering of a zwitterionic drug to neutral cocrystals: a general solution for floxacins. Chem. Commun. 2016, 52, 12610–12613. 10.1039/c6cc06627e. [DOI] [PubMed] [Google Scholar]
- Kuminek G.; Rodríguez-Hornedo N.; Siedler S.; Rocha H. V. A.; Cuffini S. L.; Cardoso S. G. How cocrystals of weakly basic drugs and acidic coformers might modulate solubility and stability. Chem. Commun. 2016, 52, 5832–5835. 10.1039/c6cc00898d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogienko A. G.; Svetlana A. M.; Anna A. O.; Andrey A. N.; Andrey S. S.; Maxim S. M.; Alexander S. Y.; Tatyana P. S.; Vladimir V. B.; Elena V. B. Cryosynthesis of co-crystals of poorly water-soluble pharmaceutical compounds and their solid dispersions with polymers. “Meloxicam - succinic acid” system as a case study. Cryst. Growth Des. 2018, 18, 7401–7409. 10.1021/acs.cgd.8b01070. [DOI] [Google Scholar]
- Chappa P.; Arthanareeswari M.; Rajasekhar V.; Archan D.; Subhas G.; Mohamed A. B. Drug–Polymer Co-Crystals of Dapsone and Polyethylene Glycol: An Emerging Subset in Pharmaceutical Co-Crystals. Cryst. Growth Des. 2018, 18, 7590–7598. [Google Scholar]
- Gadade D. D.; Pekamwar S. S. Pharmaceutical Cocrystals: Regulatory and Strategic Aspects, Design and Development. Adv. Pharm. Bull. 2016, 6, 479–494. 10.15171/apb.2016.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. L.; Hickey M. B.; Zaworotko M. J.; Almarsson O. Expanding the scope of crystal form evaluation in pharmaceutical science. J. Pharm. Pharm. Sci. 2006, 9, 317–326. [PubMed] [Google Scholar]
- Hubers S. A.; Brown N. J. Combined Angiotensin Receptor Antagonism and Neprilysin Inhibition. Circulation 2016, 133, 1115–1124. 10.1161/circulationaha.115.018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla G.; Nangia A. Pharmaceutical cocrystals: walking the talk. Chem. Commun. 2016, 52, 8342–8360. 10.1039/c6cc02943d. [DOI] [PubMed] [Google Scholar]
- Vincent M.; Labianca R.; Harper P. Which 5-fluorouracil regimen? the great debate. Anti-Cancer Drugs 1999, 10, 337–354. 10.1097/00001813-199904000-00001. [DOI] [PubMed] [Google Scholar]
- Xu L.-M.; Qu C. Y.; Zhou M.; Chen Y. W.; Chen M. M.; Shen F. Engineering of lipid prodrug-based, hyaluronic acid-decorated nanostructured lipid carriers platform for 5-fluorouracil and cisplatin combination gastric cancer therapy. Int. J. Nanomed. 2015, 10, 3911–3920. 10.2147/ijn.s83211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L. Q.; Sherman E. J.; Riaz N.; Setton J.; Koutcher L.; Zhang Z.; Shi W.; Fury M. G.; Wolden S. L.; Pfister D. G.; Morris L.; Lee N. Efficacy of concurrent cetuximab vs. 5-fluorouracil/carboplatin or high-dose cisplatin with intensity-modulated radiation therapy (IMRT) for locally-advanced head and neck cancer (LAHNSCC). Oral Oncol. 2014, 50, 947–955. 10.1016/j.oraloncology.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled Y.; Levavi H.; Krissi H.; Weill Y.; Sabah G.; Eitan R. The use of Fluorouracil (5-FU) and leucovorin in women with heavily pretreated advanced ovarian carcinoma. Am. J. Clin. Oncol. 2013, 36, 472–474. 10.1097/coc.0b013e3182549399. [DOI] [PubMed] [Google Scholar]
- Batista C. S.; Martins T. L., 5-Fluorouracil (5-Fu) - Using and Side Effects. Fluorouracil: Synthesis, Health Effects and Role in Chemotherapy, 2012. [Google Scholar]
- Li S.; Chen J.-M.; Lu T.-B. Synthon polymorphs of 1: 1 co-crystal of 5-fluorouracil and 4-hydroxybenzoic acid: their relative stability and solvent polarity dependence of grinding outcomes. CrystEngComm 2014, 16, 6450–6458. 10.1039/c4ce00221k. [DOI] [Google Scholar]
- Walocko F. M.; Eber A. E.; Keri J. E.; Al-Harbi M. A.; Nouri K. The role of nicotinamide in acne treatment. Dermatol. Ther. 2017, 30, e12481 10.1111/dth.12481. [DOI] [PubMed] [Google Scholar]
- Longley D. B.; Harkin D. P.; Johnston P. G. 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.