Abstract
Reduction in the adsorption of cellulase onto lignin has been thought to be the common reason for the improvement of enzymatic hydrolysis of lignocellulose (EHLC) by a nonionic surfactant (NIS). Few research studies have focused on the relationship between lignocellulosic features and NIS for improving EHLC. This study investigated the impact of Tween20 on the enzymatic hydrolysis and enzyme adsorption of acid-treated and alkali-treated sugarcane bagasse (SCB), cypress, and Pterocarpus soyauxii (PS) with and without being ground. After addition of Tween20, the adsorption of cellulase onto unground and ground alkali-treated SCB increased, and the unground acid-treated SCB exhibited little change in adsorption cellulase, while other unground and ground, treated samples showed decreased cellulase adsorption. Tween20 could improve the enzymatic hydrolysis of acid-treated SCB, while it had little influence on the enzymatic hydrolysis of other treated materials. After being ground, both cellulase adsorption and enzymatic hydrolysis of treated lignocelluloses increased, and Tween20 could enhance the enzymatic hydrolysis of acid-treated materials while hardly affected the enzymatic hydrolysis of alkali-treated materials. This indicated that the promotion effect of Tween20 on enzymatic hydrolysis of treated lignocellulose could not be mainly ascribed to the hindrance of Tween20 to cellulase adsorption on lignin but was related to the lignocellulosic features such as hemicellulose removal and surface morphology changes.
1. Introduction
Bioconversion of lignocellulosic biomass into fermentable sugars is the common process for the production of biofuels or bioproducts. It can not only recycle low-value materials such as crop straw, wood chips, etc. to avoid environmental pollution due to their casual abandonment and combustion but also produce high-value-added products like biofuels, which can substitute fossil fuels to reduce greenhouse gas emissions and guard the national energy safety.1,2 Lignocellulose is mainly composed of cellulose, hemicellulose, and lignin, which are linked with each other via covalent and noncovalent bonds to endow lignocellulose with recalcitrance to microbial and enzymatic attack.2,3 A pretreatment, that destroys the compact structure of lignocellulose to facilitate the access of enzyme to holocellulose is the prerequisite step for obtaining efficient enzymatic hydrolysis of lignocellulose (EHLC).4−6 The addition of promoters has been a supplemental approach to further improve the enzymatic digestibility of lignocellulose.
A nonionic surfactant (NIS), one of the promoters having significant ability to enhance EHLC at low loadings, has been receiving more and more attention.7−10 However, the mechanism of NIS in enhancing EHLC has been controversial. Initially, it was pointed out that the NIS aided cellulase to desorb from cellulose to make more free cellulase to participate in cellulosic hydrolysis.11−13 Disrupting the substrate structure as well as stabilizing and stimulating enzyme activity were further thought as the positive influences of NIS on enzymatic hydrolysis of cellulose.14,15 Nevertheless, Eriksson et al. found that NIS hardly played any role as a substrate disrupter, an enzyme stabilizer, and an effector and explained that NIS improved EHLC by reducing the unproductive adsorption of the enzyme onto lignin.16 This has become the mainstream viewpoint,17−20 but there have been still different theories on NIS improving EHLC. Rocha-Martín et al. observed that PEG4000 could promote the enzymatic hydrolysis efficiency of microcrystalline cellulose more significantly than that of steam explosion-treated crop straw and pointed out that the improvement effect of NIS on glucose release during enzymatic hydrolysis of pretreated lignocellulose had hardly any relation with lignin.21 Seo et al. studied the influence of Tween20 on enzymatic hydrolysis of pine wood chips with different lignin contents and concluded that the crucial role of Tween20 in enhancing the enzymatic hydrolysis efficiency was to increase the effective accessible surface of cellulose to cellulase.22 Our previous study also showed that NIS benefitted the adsorption of cellulase onto sugarcane bagasse (SCB) samples containing different lignin contents and their purified lignin,23 which was obviously different from the viewpoint of Eriksson et al.16 that NIS blocked the adsorption of cellulase onto lignin. We proposed that NIS could promote the access of cellulase to cellulose via reducing the solid–liquid interfacial tension, and lignin might not play a key role in adsorbing cellulase but hinder the contact between cellulase and cellulose.23,24 Several researchers also claimed that intensifying the accessibility of cellulose to cellulase was more important than lignin removal to facilitate EHLC.25−27 In addition, some studies indicated that the impact of NIS on cellulosic hydrolysis would depend on the types of substrates and pretreatment methods.18,28,29
Our previous findings were obtained with SCB containing various lignin contents as materials,23 which might have limitations in concluding that the increase of cellulose accessibility was the reason for the improvement of cellulosic hydrolysis by NIS. To further support the previous conclusion, in this study, solid wastes of herbaceous and woody plants were selected as the materials and treated with diluted sulfuric acid (H2SO4) and sodium hydroxide (NaOH) to investigate the effect of lignocellulosic features on NIS impacting EHLC. It also preliminarily showed that blocking the adsorption of cellulase onto lignin could not be thought as the main reason for the improvement of EHLC by NIS.
2. Results and Discussion
2.1. Effect of Acid and Alkaline Pretreatment on Chemical Features of Lignocellulose
2.1.1. Compositional Changes
The current pretreatment methods can produce two kinds of treated lignocellulose. One is mainly composed of lignin and cellulose, and the other chiefly consists of cellulose and hemicellulose.5 Acid pretreatment degrading hemicellulose and alkaline pretreatment removing lignin were used in this study. From Table 1, the relative xylan (representing hemicellulose) contents in raw cypress and Pterocarpus soyauxii (PS) are 6.5 and 15.3%, respectively, which are less than that in raw SCB, while the relative lignin contents in raw cypress and PS are 11.0 and 22.7%, respectively, which are more than that in raw SCB. This corresponds to the previous reports that the herbaceous biomass (SCB) contains more hemicellulose but less lignin than the woody biomass (cypress and PS).6,30 After pretreatment, the relative xylan contents in the three acid-treated materials are much lower than those in the raw materials, and the alkaline pretreatment shows gradually decreased capability to remove lignin from SCB, PS, and cypress. Lignin usually contains three principal phenylpropane units (syringyl (S), guaiacyl (G), and p-hydroxyphenyl (H)). It had been pointed out that the lignin in softwood (cypress), which is only composed of G and H units, makes softwood more recalcitrant than herbaceous biomass (SCB) and hardwood (PS) for being treated with alkaline chemicals.30 The lignin containing S unit seems more vulnerable to alkaline pretreatment.31 It had been reported that NIS had a slight influence on the enzymatic hydrolysis of lignocellulose with lignin removal.16,32,33 Compared with alkali-treated SCB, the relatively higher retention of lignin in alkali-treated cypress and PS might give more interesting results for the involvement of NIS in the enzymatic hydrolysis process.
Table 1. Components of Raw and Treated Materialsa.
| materials | solid recovery (%) | glucan (%) | xylan (%) | lignin (%) |
|---|---|---|---|---|
| raw sugarcane bagasse (SCB) | 41.9 ± 0.3 | 22.8 ± 0.2 | 23.6 ± 0.2 | |
| alkali-treated SCB | 67.3 ± 0.1 | 37.9 ± 1.0 | 16.8 ± 0.0 | 6.6 ± 0.9 |
| acid-treated SCB | 64.0 ± 0.3 | 38.9 ± 0.6 | 2.8 ± 0.0 | 20.3 ± 0.0 |
| raw cypress | 38.3 ± 0.1 | 16.3 ± 0.0 | 34.6 ± 0.3 | |
| alkali-treated cypress | 87.8 ± 0.0 | 36.6 ± 0.3 | 14.0 ± 0.0 | 29.1 ± 2.1 |
| acid-treated cypress | 74.4 ± 0.1 | 36.1 ± 1.2 | 4.5 ± 0.2 | 32.5 ± 0.0 |
| raw P. soyauxii (PS) | 40.0 ± 0.1 | 7.5 ± 0.1 | 46.3 ± 0.5 | |
| alkali-treated PS | 75.2 ± 0.0 | 36.9 ± 1.6 | 6.2 ± 0.3 | 28.0 ± 0.7 |
| acid-treated PS | 78.4 ± 0.2 | 35.3 ± 0.0 | 1.8 ± 0.0 | 39.5 ± 0.0 |
Solid recovery was used to calculate the components’ contents of treated materials relative to those of the raw materials.
2.1.2. Chemical Group Changes Based on Fourier Transform Infrared (FTIR) Data
The changes in the chemical groups of lignocellulose after pretreatment were detected by FTIR from 400 to 4000 cm–1 (Figure S1). Raw SCB and cypress had similar spectrogram patterns as those reported in the literature.34,35 Compared with the raw materials, some peaks disappeared and some new peaks appeared after pretreatment (Figure S1). The most divergent peaks of raw and treated SCB, cypress, and PS from 800 to 1900 cm–1 are presented in Figure 1, and the peak assignment is shown in Table S1. The peak divergence of the three materials is indicated with arrows in Figure 1. The spectrogram patterns of raw and treated SCB were obviously different from those of raw and treated cypress and PS. As for the raw materials, the peaks at 853, 864, 873, 1204, 1348, 1377, 1441, and 1504 cm–1 appeared only in SCB (Figure 1a). At around 1042 cm–1 belonging to the C–O ether bond of the G unit, the raw SCB had one peak, while there were two peaks for raw cypress and PS. The peaks in the range from 1599 to 1659 cm–1 were mainly ascribed to the chemical groups of lignin. The same small peaks appeared in raw SCB and cypress from 1632 to 1659 cm–1, while the peak at 1605 cm–1 was present only in raw SCB and the peaks at 1599 and 1620 cm–1 existed only in raw PS. The peaks at 1693 and 1713 cm–1 caused by C=O of conjugated aldehyde and carboxyl groups in raw SCB were more obvious than those in raw cypress and PS. The peak belonging to C=O of unconjugated ketone, carbonyl, and ester groups in hemicellulose and lignin shifted from 1738 cm–1 for raw cypress and PS to 1730 cm–1 for raw SCB and disappeared after acid or alkaline pretreatment, which corresponded to the hemicellulose degradation during acid pretreatment and lignin removal in the alkaline pretreatment process (Table 1). The same phenomenon was also observed for acid-treated or alkali-treated SCB in other research studies.34,36 As for acid-treated samples, the peaks at 1092, 1123, 1204, and 1695 cm–1 appeared solely in SCB and those at 816, 858, 1711, and 1722 cm–1 were found only in cypress (Figure 1b). The peaks at 1227, 1269, 1425, and 1454 cm–1 for acid-treated cypress and PS were sharper than those for acid-treated SCB. The peaks at 1323 and 1331 cm–1 for acid-treated cypress formed a wide peak for acid-treated SCB and PS. All of the different peaks preferably indicated the different chemical groups of lignin for these three acid-treated lignocelluloses (Table S1). After alkaline pretreatment, the peaks at 1032 and 1059 cm–1 mainly assigned to the chemical groups of lignin for cypress and PS formed a wide peak for SCB in addition to a wide peak for SCB in the range from 1229 to 1265 cm–1 due to lignin removal (Figure 1c). The peaks at 914, 1236, and 1244 cm–1 existed only in alkali-treated PS, which indicated that acetyl group still existed in lignin of PS after alkaline pretreatment (Table S1). The small peaks at 1360 and 1504 cm–1 appearing in alkali-treated SCB and PS were absent for alkali-treated cypress, which was probably caused by the S unit of lignin (Table S1). The peak at 1391 cm–1 present in alkali-treated SCB and cypress was absent in alkali-treated PS, which might be from hemicellulose (Table S1). In other words, the raw and treated SCB, cypress, and PS had significantly different compositional contents and chemical groups.
Figure 1.
FTIR spectra of raw and treated lignocelluloses in the range of 800–1900 cm–1: (a) raw samples, (b) acid-treated samples, and (c) alkali-treated samples.
2.2. Effect of Tween20 on Enzymatic Hydrolysis of Treated Lignocelluloses
2.2.1. Effect of Tween20 on Enzymatic Hydrolysis of Unground Treated Lignocelluloses
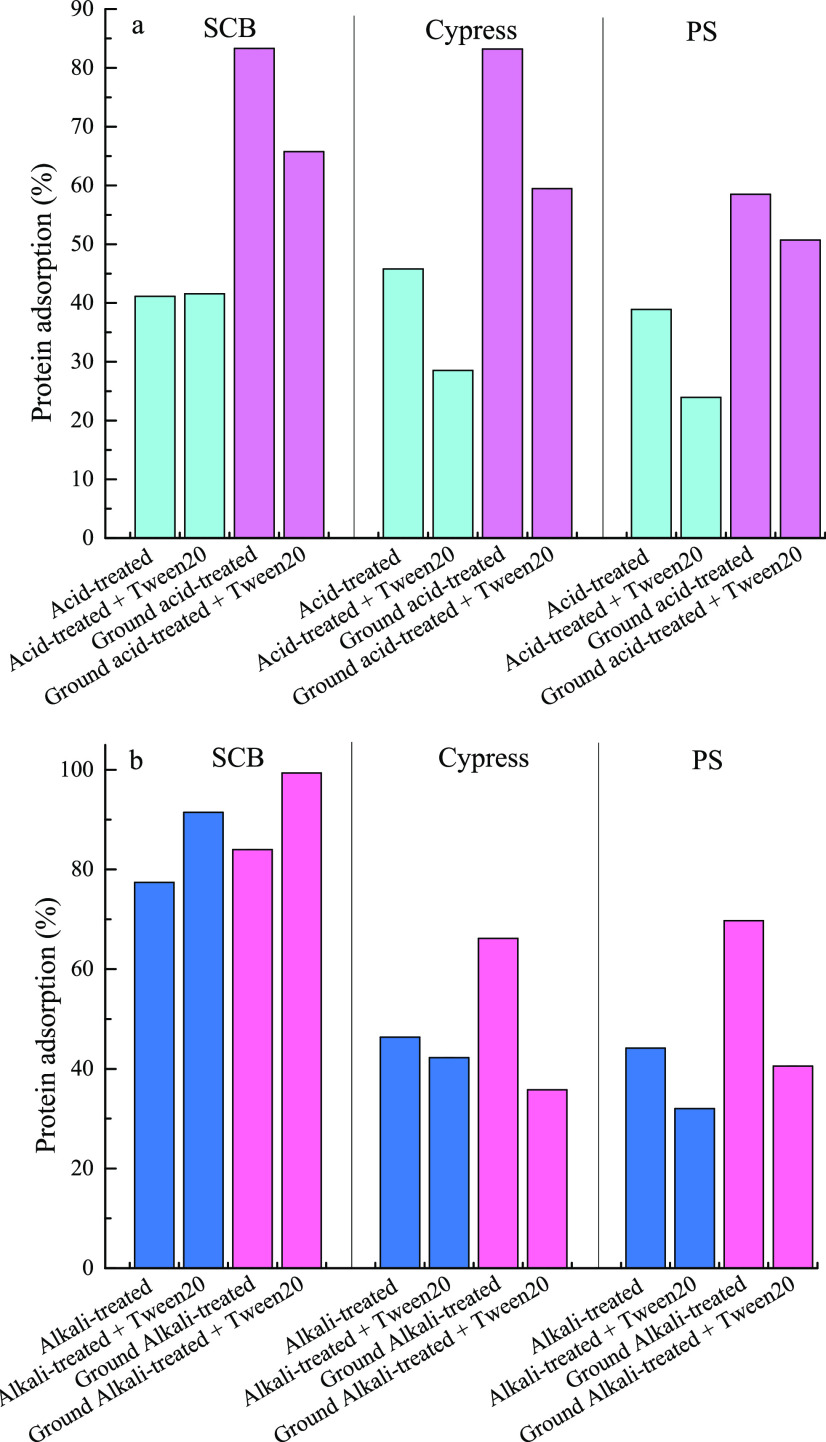
There have been several mechanisms to explain the improvement effect of NIS on EHLC, such as facilitating the desorption of the enzyme from a substrate,11 as an enzyme effector and stabilizer,14,15 as a substrate disrupter,14 blocking the adsorption of the enzyme onto lignin,16,33 reducing enzyme deactivation by shear force and at the air–liquid interface,37−39 and so on. Among them, hindering the adsorption of the enzyme onto lignin has become the common mechanism. Kim et al. contrarily pointed out that the enhancement effect of NIS on EHLC had hardly any relation with lignin but depended on the substrate type.28 Our previous research also found the opposite result that NIS enhanced the adsorption of cellulase onto SCB samples containing different lignin contents and their purified lignin.23 Because of only one kind of substrate used in the previous study, the reported results might be special for those treated SCB samples. This work prepared acid- and alkali-treated herbaceous biomass (SCB), softwood (cypress), and hardwood (PS) as materials to investigate the effect of Tween20 on EHLC (Figure 2). The alkali-treated materials containing higher relative xylan contents and lower relative lignin contents had higher enzymatic hydrolysis efficiencies than the acid-treated materials (Table 1 and Figure 2), which meant that lignin removal was more important than xylan removal to enhance cellulosic hydrolysis. Tween20 could significantly improve the conversion of glucan and xylan in acid-treated SCB to glucose and xylose, respectively, but had negligible influence on the enzymatic hydrolysis of other treated materials (Figure 2). It seemed that the impact of Tween20 on EHLC was highly related to the substrate type but not to lignin. From Figure 3, it could be found that Tween20 had little impact on the enzyme adsorption of acid-treated SCB, while it reduced the adsorption of enzyme onto acid-treated and alkali-treated cypress and PS. The enzyme adsorption of alkali-treated SCB increased after Tween20 addition, which corresponded to the previous results that Tweens improved the enzyme adsorption of alkali-treated SCB.23 This indicated that the impact of Tween20 on the adsorption behavior of enzyme onto lignocelluloses first had a relation with the substrate type and then the pretreatment type. SCB, cypress, and PS belong to different types of lignocelluloses that have different compositional contents and chemical groups, and those undergoing various pretreatment methods generate various samples with special components and chemical groups (Table 1 and Figure 1). From Figure 4, it can be observed that acid-treated and alkali-treated cypress and PS had a tighter surface structure than acid-treated and alkali-treated SCB, which might hinder the access of cellulase to cellulose. It is inferred that the effect of the compact surface structure on blocking the contact of cellulase with cellulose eliminates the promotion effect of Tween20 on EHLC.
Figure 2.

Effect of Tween20 on the enzymatic hydrolysis of acid-treated and alkali-treated lignocelluloses with and without being ground: (a) glucan conversion of acid-treated lignocelluloses, (b) xylan conversion of acid-treated lignocelluloses, (c) glucan conversion of alkali-treated lignocelluloses, and (d) xylan conversion of alkali-treated lignocelluloses.
Figure 3.
Effect of Tween20 on the adsorption of enzyme protein onto acid-treated (a) and alkali-treated (b) lignocelluloses with and without being ground.
Figure 4.
SEM images of raw and treated lignocelluloses.
2.2.2. Effect of Tween20 on Enzymatic Hydrolysis of Ground, Treated Lignocelluloses
To prove the above inference, all of the treated materials were milled into smaller particles, which would have a slight influence on the chemical groups and compositional contents. After being ground, more pores and cracks appeared on the treated materials (Figure 4), and the enzyme adsorption of acid-treated and alkali-treated materials increased (Figure 3), which indicated that the grinding step further disrupted the physical structure of treated lignocelluloses to facilitate the access of cellulase to cellulose. The efficiencies of enzymatic hydrolysis of ground acid-treated and alkali-treated lignocelluloses were improved, which hinted that the increment of enzyme adsorption was beneficial for enzymatic hydrolysis. After the addition of Tween20, the efficiencies of enzymatic hydrolysis of the ground acid-treated materials were enhanced (Figure 2a), while the enzyme adsorption of the ground acid-treated materials was weakened, as well as that of unground acid-treated materials (Figure 3a). The impacts of Tween20 on the enzymatic hydrolysis and enzyme adsorption of ground alkali-treated lignocelluloses were similar to those of unground alkali-treated lignocelluloses (Figures 2c and 3b). This meant that more release of free enzyme would probably not be the main reason for Tween20 improving cellulosic hydrolysis, but the physical barrier should be the key factor to affect the action of Tween20 on enzymatic hydrolysis. In addition, compared with acid-treated cypress and PS, alkali-treated cypress and PS had larger xylan contents and smaller lignin amounts, which were still more than 30% of relative contents. The grinding step made the surface of treated cypress and PS rougher and led to the formation of cracks (Figure 4) and crystallinity reduction (Figure 5). Tween20 could enhance the enzymatic hydrolysis of ground acid-treated cypress and PS, while it still had no influence on the enzymatic hydrolysis of ground alkali-treated cypress and PS. This indicated that xylan removal might have a relation with the promotion effect of Tween20 on EHLC after the physical structure of lignocellulose was significantly changed. Although alkali-treated SCB contained relatively higher xylan content than alkali-treated cypress and PS, its xylan was degraded more significantly than the xylans of alkali-treated cypress and PS in the enzymatic hydrolysis process (Figure 2d).
Figure 5.
Crystallinity index of raw and treated lignocelluloses.
2.3. Proposed Mechanism of Tween20 in Improving Enzymatic Hydrolysis of Lignocelluloses
It had been observed that lignin extracted with the combination of ball milling and organic solvents (partial original lignin, namely, milled wood lignin, MWL), enzymes and organic solvents (partial original lignin, namely, cellulase enzyme lignin, CEL), or ball milling and enzymes (whole original lignin, namely, milled wood enzyme lignin, MWEL) did have the ability to adsorb cellulase.23,40,41 It was also reported that alkaline lignin could hardly influence the cellulosic hydrolysis42 and lignin extracted from maleic-acid-treated lignocellulose could improve the enzymatic hydrolysis.43 This meant that lignin originated from lignocellulose treated with different pretreatment methods would show different abilities to impact enzymatic hydrolysis of lignocellulose. Furthermore, the properties of lignin retained in the pretreated lignocellulose should be different from those of lignin extracted with different chemical reagents. To completely extract the whole original lignin from pretreated lignocellulose or in situ investigation would be more authentic to discover the mechanism of lignin influencing cellulosic hydrolysis. In our previous work,23 whole lignin was purified from alkali-treated SCB to be applied for exploring the influence of NIS on the adsorption of cellulase onto lignin. It was found that NIS enhanced the cellulase adsorption onto lignin, which was different from the results on NIS reducing the adsorption of cellulase onto MWL and CEL.8,16,44 The improvement of NIS on the whole lignin adsorbing cellulase should be closer to reflect the real mechanism due to the completeness of lignin. Furthermore, in this work, the treated lignocelluloses were used as samples to study the influence of Tween20 on enzymatic hydrolysis and enzyme adsorption. It was found that Tween20 could make more cellulase dissociate from lignocellulosic substrates, but it could not be concluded that hindering the adsorption of cellulase onto lignin was the main reason for Tween20 improving enzymatic hydrolysis because no increase in enzymatic hydrolysis efficiency was observed for unground, treated cypress and PS in the presence of Tween20 (Figure 2). It was previously found that the relative lignin content in treated lignocelluloses of more than 15% mainly acted as a barrier to the pathway of cellulase accessing cellulose to influence EHLC.24 Combined with our previous findings,23,24 the improvement effect of NIS on EHLC could not be mainly ascribed to the reason that NIS blocks the unproductive adsorption of cellulase onto lignin but was related to the features of the substrate like the compact surface structure and xylan removal. The physical barrier of pretreated lignocellulose might be mainly responsible for affecting the improvement effect of NIS on enzymatic hydrolysis of lignocellulose. Detecting the microstructural changes would be an effective way to further disclose the real mechanism of NIS in affecting EHLC in future works.
3. Conclusions
The improvement effect of a nonionic surfactant on enzymatic hydrolysis of lignocellulose cannot be mainly attributed to the hindrance of the nonionic surfactant on the unproductive cellulase adsorption onto lignin. The compact surface structure and hemicellulose removal can influence the enhancement effect of the nonionic surfactant on enzymatic hydrolysis of lignocellulose. This means that better cellulose accessibility might be the reason for the improvement of enzymatic hydrolysis of lignocellulose by nonionic surfactant. Further work needs to be designed to reveal the way the nonionic surfactant facilitates the access of cellulase to cellulose.
4. Experimental Section
4.1. Materials
SCB provided by Pingxiang Fenghao Alcohol Co. Ltd. (Guangxi, China) was ground, and the particles of 0.25–0.85 mm in diameter were sieved out. The sawdust of cypress and P. soyauxii (PS) were purchased from https://www.taobao.com/. All of the materials were washed with tap water until the wastewater was clear. Then, they were dried in an oven at 60 °C to a constant weight and stored in a desiccator at room temperature. The enzyme containing 191.7 FPU cellulase/g powder assayed by Ghose’s procedure45 was bought from Imperial Jade Bio-Technology, Ltd. (Ningxia, China). Other chemical reagents were analytically pure.
4.2. Pretreatment
All of the materials were separately subjected to acid and alkaline pretreatments. The acid pretreatment was conducted with 8% (v/v) H2SO4 at a solid–liquid ratio of 1:10 and 121 °C for 1 h. The alkaline pretreatment was performed with 2% (m/v) NaOH at a solid–liquid ratio of 1:10 and 80 °C for 2 h. All of the treated solid residues were washed with deionized water until a neutral pH was achieved and then oven-dried at 60 °C to a constant weight. Parts of dried treated materials were ground for 2 h with a planetary ball mill (PMQW, Nanjing Chishun Science and Technology Co., Ltd., China). All of the samples were stored in a desiccator at room temperature.
4.3. Enzymatic Hydrolysis
Enzymatic hydrolysis with and without the addition of 0.5% (v/v) Tween20 was carried out under the conditions of a solid–liquid ratio of 1:10, 20 FPU cellulase/g cellulose, 150 rpm, and 50 °C for 72 h.
4.4. Enzyme Adsorption
Enzyme adsorption was conducted under the conditions of 1% (m/v) solid concentration, pH 4.8, and 50 °C for 90 min and measured with Coomassie brilliant blue G250 according to a previous method.23 The pH of enzyme adsorption must be the same as the enzymatic hydrolysis due to the obvious influence of the pH on the adsorption of the enzyme onto lignocellulosic substrates.46
4.5. Analytic Methods
The compositions of materials were analyzed according to NREL’s procedure.47 After being sprayed with gold, the surface morphologies of the samples were observed with a scanning electron microscope (SEM, S 4800, Hitachi) at an acceleration voltage of 2.0 kV. The Fourier transform infrared (FTIR) spectra of samples from 400 to 4000 cm–1 were obtained using a Tensor-27 (Bruker Optics, Germany) with a spectral resolution of 2 cm–1 and 32 scans. The crystallinity of samples was measured from 5 to 80° at a step size of 0.017° with a wide-angle X-ray diffraction (XRD) instrument (PW3040/60, Philips, Holand) using copper (Cu) as the anode material at 40 kV and 40 mA. The crystallinity index (CrI) was calculated as
| 1 |
where I002 is the diffraction intensity of the lattice plane (002) at around 2θ = 22.5° and Iam is the diffraction intensity of the amorphous area at about 2θ = 18.4°. The sugar concentration after EHLC was detected at 50 °C by a high-performance liquid chromatography (HPLC, Waters 2698) system equipped with a sugar column (SH1011, Shodex). The flow rate of the mobile phase (5 mM H2SO4) was 0.5 mL/min. The conversion efficiencies of glucan to glucose (CEGG) and xylan to xylose (CEXX) were calculated by eqs 2 and 3, respectively,
| 2 |
| 3 |
where Cglu (g/L), Ccello (g/L), and Cxyl (g/L) are the concentrations of glucose, cellobiose, and xylose in the enzymatic hydrolysate, respectively; 0.9, 0.95, and 0.88 are the dehydration coefficients of glucose converted to glucan, cellobiose synthesized to glucan, and xylose polymerized to xylan, respectively; V (L) is the volume of enzymatic hydrolysate; fglu (%) and fxyl (%) are the mass fractions of glucan and xylan in lignocellulose, respectively; and Mligc (g) is the mass of lignocellulose. All of the experiments in this study were performed in duplicate.
Acknowledgments
This work was financially supported by the Guangdong Basic and Applied Basic Research Foundation (2020A1515011012), the Pearl River S&T Nova Program of Guangzhou, China (201806010052), and the National Natural Science Foundation of China (21506216, 51606203, and 51476179).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00526.
FTIR spectra of raw and treated sugarcane bagasse, cypress, and Pterocarpus soyauxii in the range of 400–4000 cm–1 (Figure S1) and FTIR peak assignment (Table S1) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Hassan S. S.; Williams G. A.; Jaiswal A. K. Moving towards the second generation of lignocellulosic biorefineries in the EU: Drivers, challenges, and opportunities. Renewable Sustainable Energy Rev. 2019, 101, 590–599. 10.1016/j.rser.2018.11.041. [DOI] [Google Scholar]
- Zabed H.; Sahu J. N.; Boyce A. N.; Faruq G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renewable Sustainable Energy Rev. 2016, 66, 751–774. 10.1016/j.rser.2016.08.038. [DOI] [Google Scholar]
- Himmel M. E.; Ding S. Y.; Johnson D. K.; Adney W. S.; Nimlos M. R.; Brady J. W.; Foust T. D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- Amoah J.; Kahar P.; Ogino C.; Kondo A. Bioenergy and Biorefinery: feedstock, biotechnological conversion and products. Biotechnol. J. 2019, 14, 1800494 10.1002/biot.201800494. [DOI] [PubMed] [Google Scholar]
- Silveira M. H. L.; Morais A. R. C.; Lopes A. M. D.; Olekszyszen D. N.; Bogel-Lukasik R.; Andreaus J.; Ramos L. P. Current pretreatment technologies for the development of cellulosic ethanol and biorefineries. ChemSusChem 2015, 8, 3366–3390. 10.1002/cssc.201500282. [DOI] [PubMed] [Google Scholar]
- Limayem A.; Ricke S. C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. 10.1016/j.pecs.2012.03.002. [DOI] [Google Scholar]
- Oladi S.; Aita G. M. Interactive effect of enzymes and surfactant on the cellulose digestibility of un-washed and washed dilute ammonia pretreated energy cane bagasse. Biomass Bioenergy 2018, 109, 221–230. 10.1016/j.biombioe.2017.12.005. [DOI] [Google Scholar]
- Agrawal R.; Satlewal A.; Kapoor M.; Mondal S.; Basu B. Investigating the enzyme-lignin binding with surfactants for improved saccharification of pilot scale pretreated wheat straw. Bioresour. Technol. 2017, 224, 411–418. 10.1016/j.biortech.2016.11.026. [DOI] [PubMed] [Google Scholar]
- Jin W. X.; Chen L.; Hu M.; Sun D.; Li A.; Li Y.; Hu Z.; Zhou S. G.; Tu Y. Y.; Xia T.; Wang Y. T.; Xie G. S.; Li Y. B.; Bai B. W.; Peng L. C. Tween-80 is effective for enhancing steam-exploded biomass enzymatic saccharification and ethanol production by specifically lessening cellulase absorption with lignin in common reed. Appl. Energy 2016, 175, 82–90. 10.1016/j.apenergy.2016.04.104. [DOI] [Google Scholar]
- Li K.; Wang X.; Wang J.; Zhang J. Benefits from additives and xylanase during enzymatic hydrolysis of bamboo shoot and mature bamboo. Bioresour. Technol. 2015, 192, 424–431. 10.1016/j.biortech.2015.05.100. [DOI] [PubMed] [Google Scholar]
- Park J. W.; Takahata Y.; Kajiuchi T.; Akehata T. Effects of nonionic surfactant on enzymatic-hydrolysis of used newspaper. Biotechnol. Bioeng. 1992, 39, 117–120. 10.1002/bit.260390117. [DOI] [PubMed] [Google Scholar]
- Ooshima H.; Sakata M.; Harano Y. Enhancement of enzymatic-hydrolysis of cellulose by surfactant. Biotechnol. Bioeng. 1986, 28, 1727–1734. 10.1002/bit.260281117. [DOI] [PubMed] [Google Scholar]
- Castanon M.; Wilke C. R. Effects of the surfactant tween 80 on enzymatic hydrolysis of newspaper. Biotechnol. Bioeng. 1981, 23, 1365–1372. 10.1002/bit.260230615. [DOI] [Google Scholar]
- Kaar W. E.; Holtzapple M. T. Benefits from Tween during enzymic hydrolysis of corn stover. Biotechnol. Bioeng. 1998, 59, 419–427. . [DOI] [PubMed] [Google Scholar]
- Helle S. S.; Duff S. J. B.; Cooper D. G. Effect of surfactants on cellulose hydrolysis. Biotechnol. Bioeng. 1993, 42, 611–617. 10.1002/bit.260420509. [DOI] [PubMed] [Google Scholar]
- Eriksson T.; Börjesson J.; Tjerneld F. Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzyme Microb. Technol. 2002, 31, 353–364. 10.1016/S0141-0229(02)00134-5. [DOI] [Google Scholar]
- Kadhum H. J.; Rajendran K.; Murthy G. S. Optimization of surfactant addition in cellulosic ethanol process using integrated techno-economic and life cycle assessment for bioprocess design. ACS Sustainable Chem. Eng. 2018, 6, 13687–13695. 10.1021/acssuschemeng.8b00387. [DOI] [Google Scholar]
- Chen Y. A.; Zhou Y.; Qin Y. L.; Liu D. H.; Zhao X. B. Evaluation of the action of Tween 20 non-ionic surfactant during enzymatic hydrolysis of lignocellulose: Pretreatment, hydrolysis conditions and lignin structure. Bioresour. Technol. 2018, 269, 329–338. 10.1016/j.biortech.2018.08.119. [DOI] [PubMed] [Google Scholar]
- Jiang F.; Qian C.; Esker A. R.; Roman M. Effect of nonionic surfactants on dispersion and polar interactions in the adsorption of cellulases onto lignin. J. Phys. Chem. B 2017, 121, 9607–9620. 10.1021/acs.jpcb.7b07716. [DOI] [PubMed] [Google Scholar]
- Huang R.; Guo H.; Su R.; Qi W.; He Z. Enhanced cellulase recovery without beta-glucosidase supplementation for cellulosic ethanol production using an engineered strain and surfactant. Biotechnol. Bioeng. 2017, 114, 543–551. 10.1002/bit.26194. [DOI] [PubMed] [Google Scholar]
- Rocha-Martín J.; Martinez-Bernal C.; Perez-Cobas Y.; Reyes-Sosa F. M.; Garcia B. D. Additives enhancing enzymatic hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2017, 244, 48–56. 10.1016/j.biortech.2017.06.132. [DOI] [PubMed] [Google Scholar]
- Seo D. J.; Fujita H.; Sakoda A. Effects of a non-ionic surfactant, Tween 20, on adsorption/desorption of saccharification enzymes onto/from lignocelluloses and saccharification rate. Adsorption 2011, 17, 813–822. 10.1007/s10450-011-9340-8. [DOI] [PubMed] [Google Scholar]
- Wang W.; Zhuang X.; Tan X.; Wang Q.; Chen X.; Yu Q.; Qi W.; Wang Z.; Yuan Z. Dual effect of nonionic surfactants on improving the enzymatic hydrolysis of lignocellulose. Energy Fuels 2018, 32, 5951–5959. 10.1021/acs.energyfuels.8b00225. [DOI] [Google Scholar]
- Wang W.; Tan X.; Yu Q.; Wang Q.; Qi W.; Zhuang X.; Wang Z.; Yuan Z. Effect of stepwise lignin removal on the enzymatic hydrolysis and cellulase adsorption. Ind. Crops Prod. 2018, 122, 16–22. 10.1016/j.indcrop.2018.05.053. [DOI] [Google Scholar]
- Arantes V.; Saddler J. N. Access to cellulose limits the efficiency of enzumatic hydrolysis the role of amorphogenesis. Biotechnol. Biofuels 2010, 3, 4 10.1186/1754-6834-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollin J. A.; Zhu Z. G.; Sathitsuksanoh N.; Zhang Y. H. P. Increasing cellulose accessibility is more important than removing lignin: A comparison of cellulose solvent-based lignocellulose fractionation and soaking in aqueous ammonia. Biotechnol. Bioeng. 2011, 108, 22–30. 10.1002/bit.22919. [DOI] [PubMed] [Google Scholar]
- Wiman M.; Dienes D.; Hansen M. A. T.; van der Meulen T.; Zacchi G.; Liden G. Cellulose accessibility determines the rate of enzymatic hydrolysis of steam-pretreated spruce. Bioresour. Technol. 2012, 126, 208–215. 10.1016/j.biortech.2012.08.082. [DOI] [PubMed] [Google Scholar]
- Kim H. J.; Kim S. B.; Kim C. J. The effects of nonionic surfactants on the pretreatment and enzymatic hydrolysis of recycled newspaper. Biotechnol. Bioprocess Eng. 2007, 12, 147–151. 10.1007/BF03028641. [DOI] [Google Scholar]
- Kumar R.; Wyman C. E. Effect of additives on the digestibility of corn stover solids following pretreatment by leading technologies. Biotechnol. Bioeng. 2009, 102, 1544–1557. 10.1002/bit.22203. [DOI] [PubMed] [Google Scholar]
- Mabee W. E.; McFarlane P. N.; Saddler J. N. Biomass availability for lignocellulosic ethanol production. Biomass Bioenergy 2011, 35, 4519–4529. 10.1016/j.biombioe.2011.06.026. [DOI] [Google Scholar]
- Kim J. S.; Lee Y. Y.; Kim T. H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. 10.1016/j.biortech.2015.08.085. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Ouyang J.; Liu B. T.; Yu H.; Jiang T.; Cai C.; Li X. Comparison of hydrolysis efficiency and enzyme adsorption of three different cellulosic materials in the presence of poly(ethylene glycol). BioEnergy Res. 2013, 6, 1252–1259. 10.1007/s12155-013-9334-3. [DOI] [Google Scholar]
- Kristensen J. B.; Börjesson J.; Bruun M. H.; Tjerneld F.; Jørgensen H. Use of surface active additives in enzymatic hydrolysis of wheat straw lignocellulose. Enzyme Microb. Technol. 2007, 40, 888–895. 10.1016/j.enzmictec.2006.07.014. [DOI] [Google Scholar]
- Jin Y.; Shi Z. J.; Xu G. F.; Yang H. Y.; Yang J. A stepwise pretreatment of sugarcane bagasse by alkaline and hydroxymethyl reagent for bioethanol production. Ind. Crops Prod. 2020, 145, 112136 10.1016/j.indcrop.2020.112136. [DOI] [Google Scholar]
- Moniruzzaman M.; Ono T. Separation and characterization of cellulose fibers from cypress wood treated with ionic liquid prior to laccase treatment. Bioresour. Technol. 2013, 127, 132–137. 10.1016/j.biortech.2012.09.113. [DOI] [PubMed] [Google Scholar]
- Li H. L.; Xiong L.; Chen X. F.; Wang C.; Qi G. X.; Huang C.; Luo M. T.; Chen X. D. Enhanced enzymatic hydrolysis and acetone-butanol-ethanol fermentation of sugarcane bagasse by combined diluted acid with oxidate ammonolysis pretreatment. Bioresour. Technol. 2017, 228, 257–263. 10.1016/j.biortech.2016.12.119. [DOI] [PubMed] [Google Scholar]
- Lou H.; Zeng M.; Hu Q.; Cai C.; Lin X.; Qiu X.; Yang D.; Pang Y. Nonionic surfactants enhanced enzymatic hydrolysis of cellulose by reducing cellulase deactivation caused by shear force and air-liquid interface. Bioresour. Technol. 2018, 249, 1–8. 10.1016/j.biortech.2017.07.066. [DOI] [PubMed] [Google Scholar]
- Bhagia S.; Dhir R.; Kumar R.; Wyman C. E. Deactivation of cellulase at the air-liquid interface is the main cause of incomplete cellulose conversion at low enzyme loadings. Sci. Rep. 2018, 8, 1350 10.1038/s41598-018-19848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M. H.; Zhang A. M.; Liu B. B.; Li W. L.; Xing J. M. Improvement of cellulose conversion caused by the protection of Tween-80 on the adsorbed cellulase. Biochem. Eng. J. 2011, 56, 125–129. 10.1016/j.bej.2011.04.009. [DOI] [Google Scholar]
- Yu Z.; Gwak K.-S.; Treasure T.; Jameel H.; Chang H.; Park S. Effect of lignin chemistry on the enzymatic hydrolysis of woody biomass. ChemSusChem 2014, 7, 1942–1950. 10.1002/cssc.201400042. [DOI] [PubMed] [Google Scholar]
- Rahikainen J. L.; Martin-Sampedro R.; Heikkinen H.; Rovio S.; Marjamaa K.; Tamminen T.; Rojas O. J.; Kruus K. Inhibitory effect of lignin during cellulose bioconversion—The effect of lignin chemistry on non-productive enzyme adsorption. Bioresour. Technol. 2013, 133, 270–278. 10.1016/j.biortech.2013.01.075. [DOI] [PubMed] [Google Scholar]
- Wang W.; Chen X.; Tan X.; Wang Q.; Liu Y.; He M.; Yu Q.; Qi W.; Luo Y.; Zhuang X.; Yuan Z. Feasibility of reusing the black liquor for enzymatic hydrolysis and ethanol fermentation. Bioresour. Technol. 2017, 228, 235–240. 10.1016/j.biortech.2016.12.076. [DOI] [PubMed] [Google Scholar]
- Cai C.; Hirth K.; Gleisner R.; Lou H. M.; Qiu X. Q.; Zhu J. Y. Maleic acid as a dicarboxylic acid hydrotrope for sustainable fractionation of wood at atmospheric pressure and ≤100 °C: mode and utility of lignin esterification. Green Chem. 2020, 22, 1605–1617. 10.1039/C9GC04267A. [DOI] [Google Scholar]
- Fritz C.; Ferrer A.; Salas C.; Jameel H.; Rojas O. J. Interactions between cellulolytic enzymes with native, autohydrolysis, and technical lignins and the effect of a polysorbate amphiphile in reducing nonproductive binding. Biomacromolecules 2015, 16, 3878–3888. 10.1021/acs.biomac.5b01203. [DOI] [PubMed] [Google Scholar]
- Ghose T. K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. 10.1351/pac198759020257. [DOI] [Google Scholar]
- Lou H.; Zhu J. Y.; Lan T. Q.; Lai H.; Qiu X. pH-induced lignin surface modification to reduce nonspecific cellulase binding and enhance enzymatic saccharification of lignocelluloses. ChemSusChem 2013, 6, 919–927. 10.1002/cssc.201200859. [DOI] [PubMed] [Google Scholar]
- Sluiter A.; Hames B.; Ruiz R.; Scarlata C.; Sluiter J.; Templeton D.; Crocker D.. Determination of Structural Carbohydrates and Lignin in Biomass. Technical Report NREL/TP-510-42618; 2008.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.