Abstract
The study aimed to assess the prevalence of pain, severe pain, and pain in four or more regions associated with physical activity and sedentary behavior, as well as other associated factors in severely obese adults (Body Mass Index ≥ 35 kg/m2). Baseline data from the DieTBra Trial were analyzed. The outcome variables were pain (yes/no) and pain in four or more sites (yes/no), as identified by the Brazilian version of the Nordic Musculoskeletal Questionnaire, along with the presence of severe pain (yes/no), identified based on the Numerical Pain Rating Scale (≥8). The main independent variables were moderate to vigorous physical activity (MVPA), light physical activity, and sedentary behavior, assessed by triaxial accelerometry. The variables were analyzed using multiple hierarchical Poisson regression. In 150 individuals (men, 14.67%; and women, 85.33%), with a mean age of 39.6 ± 0.7 years, there was a high prevalence of pain (89.33%), severe pain (69.33%), and pain in four or more regions (53.33%). The associated factors were shorter MVPA time with pain (p = 0.010); arthritis/arthrosis (p = 0.007) and the use of muscle relaxants (p = 0.026) with severe pain; and economic class C (p = 0.033), and economic class D (p = 0.003), along with arthritis and arthrosis (p = 0.025) with pain in four or more sites. There were no significant associations between sedentary behavior and any of the three outcomes analyzed. These findings indicate that, in severely obese individuals, shorter MVPA time is associated with a higher prevalence of pain. Future studies on physical activity intervention may contribute to the reduction in the prevalence and severity of pain in adults with severe obesity.
Keywords: musculoskeletal pain, morbid obesity, arthritis, muscle relaxants, central, sedentary lifestyle
1. Introduction
The worldwide prevalence of obesity (body mass index, BMI ≥ 30 kg/m2) and severe obesity (BMI ≥ 35 kg/m2) has increased considerably in the last 40 years [1,2], with a higher prevalence in women [3]. A high BMI, as is seen in obese and severely obese individuals, represents a risk factor for many chronic health problems, including musculoskeletal diseases (e.g., osteoarthritis, low back pain, osteoporosis, gout, and fibromyalgia), which are considered to be the second leading cause of years spent with disability [3].
Obese adults have a high prevalence of pain, in general, and severe pain, particularly in a variety of areas such as the lower back, knee, feet, soft tissues, bones, and joints [4,5,6]. In addition, obese individuals with musculoskeletal pain have lower levels of physical activity [4,7]. The relationship between obesity and musculoskeletal pain is multifactorial, and many studies investigating pain-associated factors have been conducted in adults and the elderly [8,9,10,11,12]. The available data on pain in the obese show that low back pain is associated with reduced sleep, poor quality of life, nocturia, divorce [4], smoking, time spent sedentary, and low physical activity [13]. In obese adults, pain in more than two sites was more frequent in women and individuals in the higher BMI ranges. In addition, low levels of physical activity and sedentary behavior are considered to be potential predictors for both obesity and higher pain reporting [3,13]. The coexistence of pain and obesity often leads to a vicious cycle of pain [14], which may be influenced by physical activity [7].
There are a few studies in the literature regarding severe obesity and their associated factors such as physical activity practice and sedentary behavior [4,7,13,15]. Therefore, it is important to explore whether there are associations among severe obesity, pain, and physical activity/sedentary behavior. Such studies may provide a better understanding of these conditions, thus enabling more effective pain prevention and treatment alternatives. In this context, the aim of this study was to assess the prevalence of pain, severe pain, and widespread localization of pain in four or more sites associated with physical activity and sedentary behavior in severely obese adults, as well as to investigate any potential impact of sociodemographic status, lifestyle, and food intake, along with clinical and anthropometric variables.
2. Methods
2.1. Study Design
This study presents the baseline data of the clinical trial “DieTBra Trial,” registered on the ClinicalTrials.gov platform (NCT02463435) [16,17,18]. The data collection was conducted between June 2015 and September 2016. This study was approved by the Research Ethics Committee (CEP/HC/UFG) under protocol number 747.792/2014; all subjects signed the Informed Consent Form. The DieTBra Trial evaluates several outcomes [19,20,21,22].
2.2. Participants
Adults aged between 18 and 65 years, of both genders, who were severely obese (with BMI ≥ 35 kg/m2) were included in the study, after being referred to our outpatient clinic by the primary care department of the Brazilian Public Healthcare System (SUS—Sistema Único de Saúde). Pregnant women, nursing mothers, people with disabilities who were unable to walk, who had bariatric surgery, or who had been receiving nutritional treatment or dietary regulation for weight loss in the previous 2 years were excluded, as were those who were currently taking antiobesity or anti-inflammatory medication, or were diagnosed with chronic obstructive pulmonary disease or cancer.
2.3. Measures
2.3.1. Independent Variables
Sociodemographic Data, Lifestyle, and Food Consumption
Sociodemographic, lifestyle, and food consumption data were collected using a standardized questionnaire that was previously tested. The sociodemographic variables were: gender, age, marital status (living with or without a partner), occupation (formal, informal, self-employed, retired, and housewife), head of household’s level of education, and economic class based on the Economic Classification Criterion for Brazil of the Brazilian Association of Research Companies—ABEP (sum of household items plus education of the head of household) [23].
Lifestyle variables were: smoking (if the patient smokes or has ever smoked a cigarette/pipe/cigar at any time), binge drinking (referring to the consumption of five or more units of any kind of beverage containing alcohol in a single occasion for males and four or more units for females) assessed by the simplified version of the study entitled Gender, Alcohol and Culture: an International Study—GENACIS [24,25]. Physical activity and sedentary behavior were assessed using the accelerometer Triaxial ActiGraph wGT3X (ActiGraph, Pensacola, FL, USA). Accelerometers were used on the nondominant wrist (above the ulnar styloid process), and subjects were instructed to wear them 24 h a day, including while bathing and sleeping, for 6 consecutive days. The accelerometer sampling frequency was set to 30 Hz, and the data collection interval was set to a 1 min Epoch. The accelerometers were configured, and the respective data were accessed using the software ActiLife 6.11.7. The output data were processed using the R GGIR package (http://cran.r-project.org). The outcome measure used in this study was moderate to vigorous physical activity (MVPA), which was analyzed using a 5-min bout. Nonbouted light physical activity (LPA) represented more routine activities, while a 5-min MVPA bout represented more structured activities [26,27]. The sedentary time (<50 mg, without bouts) was measured in min per day [28]. The variables MVPA, LPA, and sedentary behavior (SB) were categorized using the median values. The cutoffs for classifications were: for the SB time: ˂1182.15 min/day as lower SB and ≥1182.15 min/day as higher SB; for LPA: ˂164.89 min/day as lower LPA time and ≥164.89 min/day as higher LPA; and for MVPA: ˂8.48 min/day as lower MVPA and ≥8.48 min/day as higher MVPA.
The food consumption variables were restricted to the consumption of fruits and vegetables. Daily intake of boiled vegetables (such as squash, okra, chayote, cauliflower, and broccoli) and fresh fruits was assessed using the Food Frequency Questionnaire (FFQ) [29].
Clinical Variables
The clinical variables assessed were: anxiety, depression, arthritis/arthrosis, type 2 diabetes, fall experienced in the last 12 months, bone fractures, medications taken, and biochemical tests of blood samples. For anxiety and depression, the Hospital Anxiety and Depression Scale was applied [30]. For the other variables, the following questions were asked: “Have you ever had a fracture in your life?”; “Have you had a fall in the last 12 months?”; “Indicate if your doctor has already told you that you have any of the following conditions: arthritis/arthrosis/joint problems?”. The diagnosis of type 2 diabetes was based on the criteria of the American Diabetes Association, which includes concentrations of fasting glucose ≥126 mg/dL, glycated hemoglobin ≥6.5%, and/or use of hypoglycemic agents [31].
The drug products analyzed included analgesics, nonsteroidal anti-inflammatory drugs (NSAIDs), statins, and muscle relaxants. These were of continuous use and were classified according to the Anatomical Therapeutic Chemical Classification System (ATC codes) according to their mechanisms of action [32].
Blood was collected after 12 h of fasting for biochemical tests to quantify uric acid (mg/dL), C-reactive protein (CRP; mg/dL), vitamin D (ng/mL), glucose (ng/mL), and glycated hemoglobin (%). The reference values were: male uric acid 2.5–7.0 mg/dL and female uric acid 1.5–6 mg/dL [33]; CRP reagent >6 mg/L. Vitamin D deficiency was considered if the serum concentration of vitamin D was below 20 ng/mL, insufficiency if it was between 21 and 29 ng/mL, and sufficiency if it was over 30 ng/mL [34].
Anthropometry
The information on body weight and height was used to calculate the BMI (BMI = weight (kg)/height (m)2). Body weight was measured on a digital platform scale capable of weighing up to 200 kg within 100 g of accuracy (Welmy, USA). For height measurements, we used a stadiometer attached to a digital scale, which provides accuracy within 0.1 cm. The calculation and classification of BMI followed the World Health Organization recommendations [35]: severe obesity: 35–39.9 kg/m2; morbid obesity 40–49.9 kg/m2; and super obesity ≥50.0 kg/m2.
2.3.2. Outcome Variables
Musculoskeletal Pain
The Nordic Musculoskeletal Questionnaire was applied to measure the musculoskeletal pain [36,37,38]. This instrument has been validated and adapted to the Brazilian population for the purpose of estimating the prevalence of pain [39]. Outcomes based on pain symptoms were reported for nine anatomical regions over the previous 7 days: neck, shoulders, elbows, upper back, lower back, wrist/hands, hip/thighs, knees, and ankles/feet [36,37,40]. Three outcomes were considered: pain (yes/no), severe pain (yes/no), and pain in four or more sites (yes/no). Severe pain was assessed using the Numerical Pain Rating Scale [41], which classifies a score of eight or higher as severe pain. Severe pain was classified according to the criteria of Boonstra et al. (2016), which considers: no pain (score = 0), mild pain (≤5), moderate pain (6 and 7), and severe pain (≥8) [42].
2.4. Statistical Analysis
The database was built using the program EPI DATA® version 3.1; two people entered and verified the information. For the analyses, the statistical package Stata version 13.0 (Stata Corp LP, College Station, TX, USA) was used. Statistical significance was established using a cutoff value of p < 0.05.
The Kolmogorov–Smirnov test was used to assess normality for physical activity and sedentary behavior variables. For normally distributed variables (sedentary behavior and LPA), the Student’s t-test was used; for the variables that were not normally distributed, the Mann-Whitney U test was used for comparison between MVPA and presence of pain in general, presence of severe pain, and pain in four or more regions.
Descriptive analyses are presented in absolute numbers (n) and relative frequencies (%), along with the mean and standard deviation. The Chi-square test (χ2) or Fisher’s exact test were used in the bivariate analysis. Poisson regression was used to calculate the prevalence ratio and 95% confidence interval, whereas the p-value was obtained using the Wald test. Variables with p-value < 0.20 in the bivariate analysis were included in multiple hierarchical Poisson regression analyses, with robust variance based on a hierarchical model [43]. The independent variables in this hierarchical analysis were grouped into three categories: (I) demographic data (gender, education, and economic class; (II) diet and exercise (fruit and vegetable consumption and MVPA [min/day]); and (III) clinical characteristics (falls in the last 12 months, fracture, anxiety, depression, arthritis/arthrosis, use of analgesics, and muscle relaxant use). In the multivariate analysis, variables without statistical power were excluded (n < 10 in all of the strata) [44].
3. Results
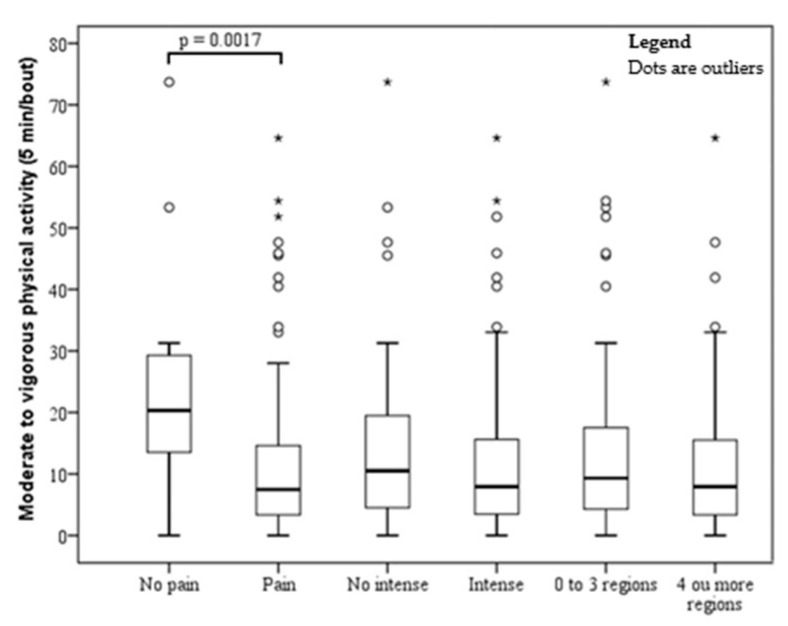
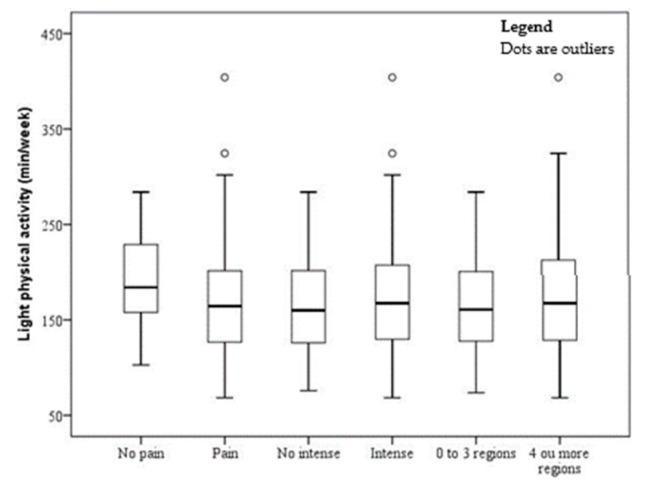
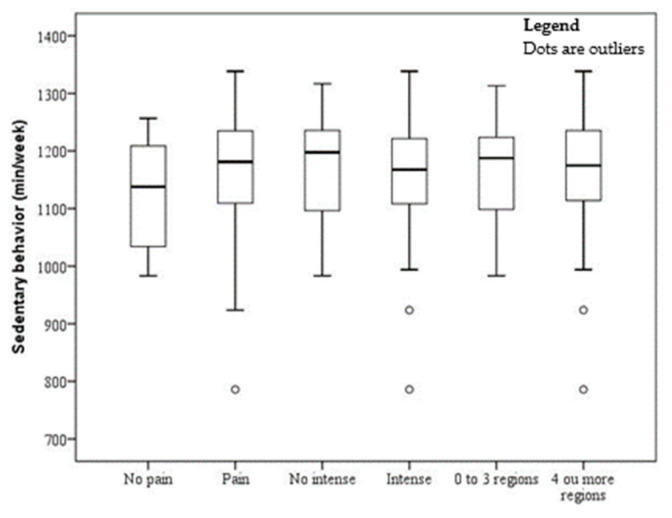
The sample comprised 150 subjects (men, 14.67% and women, 85.33%), with a mean age of 39.57 ± 0.72 years and a mean BMI of 46.12 ± 0.53 kg/m2. The prevalence of musculoskeletal pain, severe pain, and pain in four or more sites were 89.33%, 69.33%, and 53.33%, respectively. The mean time of sedentary behavior was 1167.65 ± 89.82 (min/day), LPA was 170.55 ± 55.54 (min/day), and MVPA was 12.74 ± 13.70 (min/day). The associations of MVPA, LPA, and sedentary behavior with pain, severe pain, and number of painful sites are shown in Figure 1, Figure 2 and Figure 3. There was a significant association between pain and MVPA (p = 0.0017), indicating that individuals with shorter MVPA have more pain (Figure 1). There was no significant association between pain and LPA (Figure 2) and between pain and sedentary behavior (Figure 3).
Figure 1.
Boxplot of time spent on moderate to vigorous physical activity by obese patients without and with pain, intensity, and four or more painful sites. *°outliers
Figure 2.
Boxplot of time spent on light physical activity by obese patients without and with pain, intensity, and four or more painful sites. °outliers
Figure 3.
Boxplot of time spent in sedentary behavior by obese patients without and with pain, intensity, and four or more painful sites. °outliers
The characteristics of the population studied in terms of pain, severe pain, and the number of painful sites are presented in Table 1 and Table 2. Bivariate analysis indicated that pain was associated with shorter MVPA time and muscle relaxant use; severe pain with fracture, arthritis/arthrosis, and muscle relaxant use; and pain in four or more sites with females, low economic class, anxiety, depression, and arthritis/arthrosis (Table 1 and Table 2).
Table 1.
Prevalence and bivariate analysis of the association of pain, severe pain, and number of pain sites with the variables of socio-demographic, status, food intake, and lifestyle in severely obese adults (n = 150).
| Variable | Pain | Severe Pain | Four or More Painful Sites | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Prevalence n (%) | PR (95% CI) | p | Prevalence n (%) | PR (95% CI) | p | Prevalence n (%) | PR (95% CI) | p | |
| Gender | 0.158 | 0.901 | 0.036 | ||||||
| Male | 17 (77.27) | 1 | 15 (68.18) | 1 | 6 (27.27) | 1 | |||
| Female | 117 (91.41) | 1.18 (0.94–1.49) | 89 (69.53) | 1.02 (0.75–1.39) | 74 (57.81) | 2.12 (1.05–4.27) | |||
| Age (years) | 0.406 * | 0.687 | 0.864 | ||||||
| 18–39 | 69 (90.79) | 1 | 51 (67.11) | 1 | 39 (51.32) | 1 | |||
| 40–49 | 48 (90.57) | 0.99 (0.89–1.12) | 37 (69.81) | 1.04 (0.82–1.32) | 29 (54.72) | 1.07 (0.77–1.48) | |||
| 50 or more | 17 (80.95) | 0.89 (0.72–1.11) | 16 (76.19) | 1.14 (0.85–1.51) | 12 (57.14) | 1.11 (0.72–1.71) | |||
| Occupation | 0.968 | 0.936 | 0.678 | ||||||
| Formal worker | 45 (90.00) | 1.01 (0.89–1.16) | 34 (68.00) | 0.96 (0.74–1.24) | 26 (52.00) | 1.04 (0.71–1.52) | |||
| Informal worker/self-employed | 46 (88.46) | 1 | 37 (71.15) | 1 | 26 (50.00) | 1 | |||
| Retired/housewife and others 1 | 43 (89.58) | 1.01 (0.88–1.16) | 33 (68.75) | 0.97 (0.75–1.25) | 28 (58.33) | 1.17 (0.81–1.68) | |||
| Lives with partner | 0.552 | 0.748 | 0.204 | ||||||
| No | 48 (87.27) | 0.96 (0.85–1.09) | 39 (70.91) | 1.04 (0.83–1.29) | 33 (60.00) | 1.21 (0.90–1.63) | |||
| Yes | 86 (90.53) | 1 | 65 (68.42) | 1 | 47 (49.47) | 1 | |||
| Schooling (years) | 0.598 | 0.081 | 0.744 | ||||||
| ≤10 | 66 (88.00) | 0.97 (0.87–1.08) | 57 (76.00) | 1.21 (0.97–1.51) | 41 (54.67) | 1.05 (0.78–1.42) | |||
| ≥11 | 68 (90.67) | 1 | 47 (62.67) | 1 | 39 (52.00) | 1 | |||
| Economic class | 0.303 * | 0.079 * | 0.004 | ||||||
| A-B | 28 (82.35) | 1 | 19 (55.88) | 1 | 10 (29.41) | 1 | |||
| C | 83 (90.22) | 1.09 (0.92–1.30) | 65 (70.65) | 1.26 (0.91–1.75) | 52 (56.52) | 1.92 (1.11–3.34) | |||
| D | 23 (95.83) | 1.16 (0.97–1.39) | 20 (83.33) | 1.49 (1.05–2.11) | 18 (75.00) | 2.55 (1.44–4.52) | |||
| Smoker | 0.267 * | 0.232 | 0.508 | ||||||
| No | 88 (87.13) | 1 | 67 (66.34) | 1 | 52 (51.49) | 1 | |||
| Yes | 46 (93.88) | 1.08 (0.97–1.20) | 37 (75.51) | 1.14 (0.92–1.41) | 28 (57.14) | 1.11 (0.82–1.51) | |||
| Alcohol consumption | 0.450 | 0.497 | 0.981 | ||||||
| No | 97 (90.65) | 1.05 (0.92–1.21) | 76 (71.03) | 1.09 (0.85–1.40) | 57 (53.27) | 0.99 (0.71–1.39) | |||
| Yes | 37 (86.05) | 1 | 28 (65.12) | 1 | 23 (53.49) | 1 | |||
| Fruit consumption (daily) | 0.173 | 0.774 | 0.528 | ||||||
| No | 101 (91.82) | 1.11 (0.95–1.30) | 77 (70.00) | 1.04 (0.81–1.33) | 57 (51.82) | 0.90 (0.65–1.24) | |||
| Yes | 33 (82.50) | 1 | 27 (67.50) | 1 | 23 (57.50) | 1 | |||
| Vegetable consumption (daily) | 1.000 * | 0.867 | 0.420 | ||||||
| No | 97 (88.99) | 1 | 76 (69.72) | 1 | 56 (51.38) | 1 | |||
| Yes | 37 (90.24) | 1.01 (0.90–1.14) | 28 (68.29) | 0.98 (0.77–1.25) | 24 (58.54) | 1.14 (0.83–1.56) | |||
| Sedentary time (min/day) | 0.399 | 0.432 | 0.680 | ||||||
| <Median (1182.15) | 63 (88.73) | 0.95 (0.86–1.06) | 52 (73.24) | 1.09 (0.88–1.35) | 40 (56.34) | 1.06 (0.79–1.44) | |||
| ≥Median (1182.15) | 65 (92.86) | 1 | 47 (67.14) | 1 | 37 (52.86) | 1 | |||
| LPA (min/day) | 0.752 | 0.498 | 0.551 | ||||||
| <Median (164.89) | 65 (91.55) | 1 | 48 (67.61) | 1 | 37 (52.11) | 1 | |||
| ≥Median (164.89) | 63 (90.00) | 0.98 (0.88–1.09) | 51 (72.86) | 1.08 (0.87–1.34) | 40 (57.14) | 1.10 (0.81–1.48) | |||
| MVPA (min/day) | 0.009 * | 0.432 | 0.680 | ||||||
| <Median (8.48) | 69 (97.18) | 1.15 (1.03–1.29) | 52 (73.24) | 1.09 (0.88–1.35) | 40 (56.34) | 1.06 (0.79–1.44) | |||
| ≥Median (8.48) | 59 (84.29) | 1 | 47 (67.14) | 1 | 37 (52.86) | 1 | |||
1 Other includes: student, rural worker, unemployed, laid off, receiving pension, and sick-pay recipient. CI: confidence interval; LPA: light physical activity; MVPA: moderate to vigorous physical activity; PR: adjusted prevalence ratio. Wald test was used to calculate all “p” values, except when frequencies were below five, in which case, Fisher’s exact test * was used. p < 0.05 was considered statistically significant (bold mark). Variables with p < 0.20 were further analyzed by multiple hierarchical Poisson regression.
Table 2.
Prevalence and bivariate analysis of the association of pain, severe pain, and number of pain sites with the clinical and anthropometric variables in severely obese adults (n = 150).
| Variable | Pain | Severe Pain | Four or More Painful Sites | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Prevalence n (%) | PR (95% CI) | p | Prevalence n (%) | PR (95% CI) | p | Prevalence n (%) | PR (95% CI) | p | |
| Fall in the last 12 months | 0.354 * | 0.155 | 0.219 | ||||||
| No | 84 (86.60) | 1 | 64 (65.98) | 1 | 51 (52.58) | 1 | |||
| Yes | 34 (94.44) | 1.09 (0.97–1.22) | 28 (77.78) | 1.18 (0.94–1.48) | 23 (63.89) | 1.22 (0.89–1.66) | |||
| Fracture | 0.554 * | 0.028 | 0.249 | ||||||
| No | 83 (87.37) | 1 | 61 (64.21) | 1 | 50 (52.63) | 1 | |||
| Yes | 35 (92.11) | 1.05 (0.93–1.19) | 31 (81.58) | 1.27 (1.03–1.57) | 24 (63.16) | 1.2 (0.88–1.64) | |||
| Anxiety | 0.188 | 0.060 | 0.006 | ||||||
| No | 34 (82.93) | 1 | 23 (56.10) | 1 | 13 (31.71) | 1 | |||
| Yes | 100 (91.74) | 1.11 (0.95–1.29) | 81 (74.31) | 1.32 (0.99–1.78) | 67 (61.47) | 1.94 (1.21–3.12) | |||
| Depression | 0.279 | 0.080 | 0.002 | ||||||
| No | 47 (85.45) | 1 | 33 (60.00) | 1 | 19 (34.55) | 1 | |||
| Yes | 87 (91.58) | 1.07 (0.95–1.21) | 71 (74.74) | 1.25 (0.97–1.59) | 61 (64.21) | 1.86 (1.25–2.76) | |||
| Arthritis/arthrosis | 0.524 * | 0.002 * | 0.007 | ||||||
| No | 104 (88.14) | 1 | 75 (63.56) | 1 | 57 (48.31) | 1 | |||
| Yes | 30 (93.75) | 1.06 (0.95–1.19) | 29 (90.63) | 1.43 (1.19–1.70) | 23 (71.88) | 1.49 (1.12–1.98) | |||
| Type 2 diabetes | 0.828 | 0.610 | 0.501 | ||||||
| No | 80 (88.89) | 1 | 61 (67.78) | 1 | 46 (51.11) | 1 | |||
| Yes | 54 (90.00) | 1.01 (0.90–1.13) | 43 (71.67) | 1.06 (0.85–1.31) | 34 (56.67) | 1.11 (0.82–1.50) | |||
| Use of analgesics | 0.615 | 0.932 | 0.113 | ||||||
| No | 76 (90.48) | 1 | 58 (69.05) | 1 | 40 (47.62) | 1 | |||
| Yes | 58 (87.88) | 0.97 (0.87–1.09) | 46 (69.70) | 1.01 (0.81–1.25) | 40 (60.61) | 1.27 (0.94–1.71) | |||
| Use of anti-inflammatory agents | 0.362 * | 0.754 | 0.597 | ||||||
| No | 101 (87.83) | 1 | 79 (68.70) | 1 | 60 (52.17) | 1 | |||
| Yes | 33 (94.29) | 1.07 (0.97–1.19) | 25 (71.43) | 1.04 (0.81–1.33) | 20 (57.14) | 1.10 (0.78–1.53) | |||
| Use of statin | - | 0.724 * | 1.000* | ||||||
| No | 124 (88.57) | - | 96 (68.57) | 1 | 75 (53.57) | 1 | |||
| Yes | 10 (100.00) | - | 8 (80.00) | 1.17 (0.84–1.62) | 5 (50.00) | 0.93 (0.49–1.77) | |||
| Use of muscle relaxant | 0.016 * | 0.037 | 0.291 | ||||||
| No | 56 (82.35) | 1 | 41 (60.29) | 1 | 33 (48.53) | 1 | |||
| Yes | 78 (95.12) | 1.16 (1.02–1.30) | 63 (76.83) | 1.27 (1.02–1.60) | 47 (57.32) | 1.18 (0.87–1.61) | |||
| Uric acid (mg/dL) | 0.430 * | 0.640 | 0.344 | ||||||
| Normal | 118 (90.08) | 1.07 (0.87–1.31) | 90 (68.70) | 0.93 (0.69–1.25) | 72 (54.96) | 1.31 (0.75–2.27) | |||
| High | 16 (84.21) | 1 | 14 (73.68) | 1 | 8 (42.11) | 1 | |||
| C-reactive protein (mg/dL) | 0.418 | 0.963 | 0.885 | ||||||
| Nonreactive | 98 (90.74) | 1.06 (0.92–1.22) | 75 (69.44) | 1.01 (0.79–1.28) | 58 (53.70) | 1.03 (0.73–1.44) | |||
| Reactive | 36 (85.71) | 1 | 29 (69.05) | 1 | 22 (52.38) | 1 | |||
| Vitamin D (ng/mL) | 0.450 * | 0.467 | 0.594 | ||||||
| Deficiency | 28 (96.55) | 1 | 17 (58.62) | 1 | 14 (48.28) | 1 | |||
| Insufficiency | 45 (88.24) | 0.91 (0.81–1.03) | 37 (72.55) | 1.24 (0.87–1.76) | 30 (58.82) | 1.22 (0.78–1.90) | |||
| Sufficiency | 61 (87.14) | 0.90 (0.81–1.01) | 50 (71.43) | 1.22 (0.87–1.71) | 36 (51.43) | 1.07 (0.68–1.65) | |||
| Degree of obesity (kg/m2) | 0.668 * | 0.539 | 0.585 | ||||||
| 35–39.9 | 23 (92.00) | 1 | 15 (60.00) | 1 | 12 (48.00) | 1 | |||
| 40–49.9 | 76 (90.48) | 0.98 (0.86–1.13) | 61 (72.62) | 1.21 (0.85–1.71) | 48 (57.14) | 1.19 (0.76–1.87) | |||
| ≥50 | 35 (85.37) | 0.93 (0.78–1.10) | 28 (68.29) | 1.14 (0.77–1.67) | 20 (48.78) | 1.02 (0.61–1.70) | |||
CI: confidence interval; PR: adjusted prevalence ratio. Wald test was used to calculate all “p” values, except when frequencies were below five, in which case, the Fisher’s exact test was used (indicated by *). p < 0.05 was considered statistically significant (bold mark). Variables with p < 0.20 were further analyzed by multiple hierarchical Poisson regression.
Variables with p-values less than 0.20 were included in the multivariate analysis. For pain in general, these included gender, fruit consumption, MVPA, anxiety, and use of muscle relaxant; for severe pain, schooling, economic class, fall in the last 12 months, fracture, anxiety, depression, arthritis/arthrosis, and use of muscle relaxant; and for pain in four or more sites, economic class, anxiety, depression, arthritis/arthrosis, use of analgesics, and muscle relaxant use.
Following the multiple analysis, presence of pain was still associated with lower MVPA (p = 0.010), severe pain was associated with the presence of arthritis/arthrosis (p = 0.007) and the use of muscle relaxants (p = 0.026), and the presence of pain in four or more sites was associated with low economic class (C (p = 0.033) and D (p = 0.003)) and arthritis/arthrosis (p = 0.025) (Table 3).
Table 3.
Multiple analysis of the association of pain, severe pain, and four or more painful sites with the independent variables in severely obese adults.
| Variable | Pain | Severe Pain | Four or More Painful Sites | Variable | Pain | |
|---|---|---|---|---|---|---|
| PR (95% CI) | p | PR (95% CI) | p | PR (95% CI) | p | |
| First Level | ||||||
| Gender * | – | – | – | – | ||
| Male | 1 | – | – | |||
| Female | 1.21 (0.95–1.54) | 0.127 | – | – | ||
| Schooling (years) | – | – | – | – | ||
| ≤10 | – | 1.09 (0.87–1.36) | 0.478 | – | ||
| ≥11 | – | 1 | – | |||
| Economic class | – | – | ||||
| A–B | – | 1 | 1 | |||
| C | – | 1.15 (0.83–1.59) | 0.417 | 1.71 (1.05–2.81) | 0.033 a | |
| D | – | 1.29 (0.89–1.88) | 0.176 | 2.15 (1.30–3.58) | 0.003 a | |
| Second Level | ||||||
| Fruit Consumption (daily) | – | – | – | – | ||
| No | 1.13 (0.97–1.31) | 0.109 | – | – | ||
| Yes | 1 | – | – | |||
| MVPA (min/day) (median) | – | – | – | – | ||
| <Median (8.48) | 1.15 (1.03–1.28) | 0.010 a | – | – | ||
| ≥Median (8.48) | 1 | – | – | |||
| Third Level | ||||||
| Fall in the last 12 months | – | – | – | – | ||
| No | – | 1 | – | |||
| Yes | – | 1.06 (0.84–1.33) | 0.621 | – | ||
| Fracture | – | – | – | – | ||
| No | – | 1 | – | |||
| Yes | – | 1.23 (0.99–1.54) | 0.068 | – | ||
| Anxiety | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 1.07 (0.93–1.23) | 0.337 | 1.17 (0.84–1.62) | 0.358 | 1.45 (0.86–2.45) | 0.159 |
| Depression | – | – | ||||
| No | – | 1 | 1 | |||
| Yes | – | 1.03 (0.79–1.36) | 0.810 | 1.42 (0.94–2.15) | 0.096 | |
| Arthritis/arthrosis | – | – | ||||
| No | – | 1 | 1 | |||
| Yes | – | 1.31 (1.08–1.59) | 0.007 a | 1.38 (1.04–1.82) | 0.025 a | |
| Use of analgesics | – | – | – | – | ||
| No | – | – | 1 | |||
| Yes | – | – | 1.30 (0.99–1.71) | 0.059 | ||
| Use of muscle relaxant | – | – | ||||
| No | 1 | 1 | – | |||
| Yes | 1.07 (0.96–1.19) | 0.211 | 1.31 (1.03–1.66) | 0.026 a | – |
CI: confidence interval; PR: prevalence ratio. * The gender variable was excluded from the multiple analysis, only for pain in four or more sites. Wald test was used to calculate all p-values. a p < 0.05 was considered statistically significant (bold mark).
4. Discussion
To the best of our knowledge, this is the first study to investigate the prevalence and associated factors of pain, severe pain, and pain in four or more regions associated with physical activity and sedentary behavior in adults with severe obesity. Among the contributions of this article, we highlight the following results in severely obese adults: high prevalence of pain, severe pain, and pain in four or more regions; low MVPA time and elevated sedentary behavior time; an association between MVPA and higher prevalence of musculoskeletal pain; severe pain associated with arthritis/arthrosis and the use of muscle relaxants; and pain in four or more sites associated with low economic class and arthritis/arthrosis. These results are important in the field of clinical research and could contribute in the clinical contexts related to the treatment of severe obesity and pain, as well as pain prevention in severely obese adults to provide a greater incentive for participating in physical activity; this is an easily addressable risk factor that could benefit severely obese individuals.
Our findings regarding the high prevalence of pain, severe pain, and pain in four or more regions in adults with severe obesity are consistent with other studies in the same patient populations [4,5,13,15,45,46]. In Brazil, the prevalence of pain in adults with BMI ≥ 35 kg/m2 ranges from 66.7% to 90.1% [46,47]. The prevalence of pain in several body regions has also been reported in many studies [4,5,48]. Our results indicate that pain in adults with severe obesity cannot be neglected, and its treatment should occur concurrently with obesity treatment.
In the present study, individuals with a shorter MVPA time and a longer duration of sedentary behavior experienced more pain. Despite the differences in the origins and pathogeneses in most individuals, the pain is characterized by deficient physical function, mobility limitations, depression, anxiety, and sleeping disorders. Physical activity promotes an improvement in all these parameters, and any level of physical activity is preferable to sedentary behavior [49]. Obesity can contribute to musculoskeletal pain and, consequently, it also contributes to an insufficient amount of MVPA along with increased sedentary behavior [14,50,51]. Although no studies regarding adults with obesity were found, in studies on nonobese adults and the elderly, chronic musculoskeletal pain and a higher number of painful sites were associated with low MVPA [52,53,54]. The interdisciplinary treatment of patients with severe obesity is critical, especially those in pain. Patients with severe obesity who underwent treatment through the Interdisciplinary Multimodal Pain Rehabilitation program showed improvement in physical and mental conditions and maintained these benefits at a 12-month follow-up [55]. Improvement in the physical activity in the severely obese enables better adherence to the practice of physical activity that is often not achieved due to difficulty in staying active and motivated [52,53,54].
Severe pain and pain in four or more sites were associated with arthritis/arthrosis. These findings are consistent with previous studies that have shown that obese adults have a high prevalence of arthritis, which is often associated with severe pain intensity and widespread pain in several regions [56,57,58]. Weight loss treatment has been reported to have positive effects on individuals with arthritis and obesity [58]. In addition, individuals with rheumatoid arthritis should be encouraged to exercise as part of their routine care [59]. Therefore, educational interventions involving self-management should be performed to reduce pain and improve physical function and quality of life. Further, physical training should be included in the treatment of obese adults with arthritis [57,59]. Physical training for individuals with severe obesity can involve a fitness program that includes a warm-up, stretching, and resistance training phase [60].
There was an association between severe pain and muscle relaxant use. Muscle relaxants provide clinically significant relief from acute pain, particularly in the lower back [61], and should be used temporarily for pain relief [62]. The efficacy of muscle relaxants for chronic pain is unknown [61]. Although we have not found evidence for the cause of the association between severe pain and the use of muscle relaxants, we believe that the high prevalence of pain and the use of this medication in higher quantities than recommended may have contributed to this result [62]. However, this is an important finding and serves as a warning for consistent use of muscle relaxants and possible adverse effects.
Pain in four or more regions was associated with low economic class. This finding is consistent with previous research on adults, with and without obesity [63,64,65,66,67,68]. Studies have shown that low economic class and financial concerns are associated with severe pain and widespread pain in several body regions [63,64,65,66,67,68]. In contrast, those with higher incomes tend to have a healthier life, live longer, and suffer less from illness and disability [69,70,71].
The strengths of this investigation are the inclusion of individuals with severe obesity and the rigorous controls used at all stages of the research. The research team was well trained, and the rules and procedures were strictly applied. Another relevant aspect of this study is the assessment of pain and the analysis of several variables that had not been investigated in previous studies. The possible limitations of this study are related to memory bias associated with self-reporting as a result of using the Nordic Musculoskeletal Questionnaire and the Numerical Pain Rating Scale. However, to minimize the impact of the bias, we opted to use the pain scores reported during the previous 7 days. Other limitations were the nonuse of the cutoff points established in the literature for determining the level of physical activity and sedentary behavior due to the characteristics of the population assessed (low levels of physical activity and prolonged sedentary behavior). These last two limitations were mitigated by using the median to establish the cutoff point. Another limitation of this study is the low number of men in our study sample. However, it should be noted that women seek treatment for obesity and bariatric surgery more frequently than men both in Brazil and in the United States [72]. In addition, this is a study on severely obese individuals whose prevalence in Brazil is low but growing. Thus, the sample size is representative of this population, and these data are critical in stimulating the development of clinical treatments and preventing other comorbidities and pain in these individuals.
5. Conclusions
This study provides evidence of the high prevalence of pain, severe pain, and pain in four or more regions in severely obese adults. In addition, this study is the first to demonstrate the factors associated with pain and their characteristics in severely obese individuals. We emphasize the importance of moderate to vigorous physical activity, which can contribute to the reduction of symptoms of pain, arthritis/arthrosis, and the use of muscle relaxants. Several physical activity modalities are available at a low cost for participants and are affordable for all social classes. Moderate to vigorous physical activity alone is important for both pain and obesity treatment. Future studies on physical activity intervention may contribute to the reduction in the prevalence and severity of pain in adults with severe obesity. It is crucial to establish intervention protocols to treat the pain in this group of individuals.
Acknowledgments
The authors also thank the management of the Clinical Research Unit (UPC/HC/UFG) for making the physical infrastructure available. Goiano Federal Institute for partial supporting. To participants and researchers who contributed to the study. To the Department of Epidemiology of the Universidade Federal de Pelotas and Pedro Rodrigues Curi Hallal for the partnership with the accelerometers. To the Department of Chronic-Degenerative Diseases of the Municipal Health Department (SMS) of Goiânia.
Author Contributions
Conceptualization, C.R.M. and E.A.S.; conceived the study, performed analysis, and drafted the manuscript, C.R.M., M.N., A.P.d.S.R., and E.A.S.; funding acquisition and principal investigator of DieTBra Trial, E.A.S.; and carried out critical analysis of the paper, P.V.d.O.V. and M.d.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for the research [number 201310267000003] and scholarship for CRM was provided by the Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG) (www.fapeg.go.gov.br).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Jaacks L.M., Vandevijvere S., Pan A., McGowan C.J., Wallace C., Imamura F., Mozaffarian D., Swinburn B., Ezzati M. The obesity transition: Stages of the global epidemic. Lancet Diabetes Endocrinol. 2019;7:231–240. doi: 10.1016/S2213-8587(19)30026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaboration N.R.F. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19· 2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborators G.O. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacLellan G.A., Dunlevy C., O’Malley E., Blake C., Breen C., Gaynor K., Wallace N., Yoder R., Casey D., Mehegan J., et al. Musculoskeletal pain profile of obese individuals attending a multidisciplinary weight management service. Pain. 2017;158:1342–1353. doi: 10.1097/j.pain.0000000000000918. [DOI] [PubMed] [Google Scholar]
- 5.Calenzani G., Santos F.F.D., Wittmer V.L., Freitas G.K.F., Paro F.M. Prevalence of musculoskeletal symptoms in obese patients candidates for bariatric surgery and its impact on health related quality of life. Arch. Endocrinol. Metab. 2017;61:319–325. doi: 10.1590/2359-3997000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Son K.M., Kang S.H., Seo Y.I., Kim H.A. Association of body composition with disease activity and disability in rheumatoid arthritis. Korean J. Intern. Med. 2020 doi: 10.3904/kjim.2019.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herzog W. Reflections on obesity, exercise, and musculoskeletal health. J. Sport Health Sci. 2020;9:108–109. doi: 10.1016/j.jshs.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceballos A.G.D.C.D., Santos G.B. Factors associated with musculoskeletal pain among teachers: Sociodemographics aspects, general health and well-being at work. Rev. Bras. Epidemiol. 2015;18:702–715. doi: 10.1590/1980-5497201500030015. [DOI] [PubMed] [Google Scholar]
- 9.Fujii T., Matsudaira K. Prevalence of low back pain and factors associated with chronic disabling back pain in Japan. Eur. Spine J. 2013;22:432–438. doi: 10.1007/s00586-012-2439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genebra C.V.D.S., Maciel N.M., Bento T.P.F., Simeão S.F.A.P., De Vitta A. Prevalence and factors associated with neck pain: A population-based study. Braz. J. Phys. Ther. 2017;21:274–280. doi: 10.1016/j.bjpt.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malchaire J., Cock N., Vergracht S. Review of the factors associated with musculoskeletal problems in epidemiological studies. Int. Arch. Occup. Environ. Health. 2001;74:79–90. doi: 10.1007/s004200000212. [DOI] [PubMed] [Google Scholar]
- 12.Park S.J., Yoon D.M., Yoon K.B., Moon J.A., Kim S.H. Factors associated with higher reported pain levels in patients with chronic musculoskeletal pain: A cross-sectional, correlational analysis. PLoS ONE. 2016;11:e0163132. doi: 10.1371/journal.pone.0163132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smuck M., Kao M.-C.J., Brar N., Martinez-Ith A., Choi J., Tomkins-Lane C.C. Does physical activity influence the relationship between low back pain and obesity? Spine J. 2014;14:209–216. doi: 10.1016/j.spinee.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Okifuji A., Hare B.D. The association between chronic pain and obesity. J. Pain Res. 2015;8:399. doi: 10.2147/JPR.S55598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magnusson K., Østerås N., Mowinckel P., Natvig B., Hagen K. No strong temporal relationship between obesity and multisite pain–results from a population-based 20-year follow-up study. Eur. J. Pain. 2014;18:120–127. doi: 10.1002/j.1532-2149.2013.00338.x. [DOI] [PubMed] [Google Scholar]
- 16.Santos A., Rodrigues A.P.S., Rosa L.P.S., Sarrafzadegan N., Silveira E.A. Cardiometabolic risk factors and Framingham Risk Score in severely obese patients: Baseline data from DieTBra trial. Nutr. Metab. Cardiovasc. Dis. 2020;30:474–482. doi: 10.1016/j.numecd.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues A.P.D.S., Rosa L.P.S., da Silva H.D., Silveira-Lacerda E.D.P., Silveira E.A. The Single Nucleotide Polymorphism PPARG2 Pro12Ala Affects Body Mass Index, Fat Mass, and Blood Pressure in Severely Obese Patients. J. Obes. 2018;2018:2743081. doi: 10.1155/2018/2743081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silveira E.A., de Souza Rosa L.P., de Carvalho Santos A.S.E.A., de Souza Cardoso C.K., Noll M. Type 2 Diabetes Mellitus in Class II and III Obesity: Prevalence, Associated Factors, and Correlation between Glycemic Parameters and Body Mass Index. Int. J. Environ. Res. Public Health. 2020;17:3930. doi: 10.3390/ijerph17113930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos A., Rodrigues A., Rosa L.P.S., Noll M., Silveira E.A. Traditional Brazilian Diet and Olive Oil Reduce Cardiometabolic Risk Factors in Severely Obese Individuals: A Randomized Trial. Nutrients. 2020;12:1413. doi: 10.3390/nu12051413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigues A.P.S., Rosa L.P.S., Silveira E.A. PPARG2 Pro12Ala polymorphism influences body composition changes in severely obese patients consuming extra virgin olive oil: A randomized clinical trial. Nutr. Metab. 2018;15:52. doi: 10.1186/s12986-018-0289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silveira E.A., Souza J.D., Rodrigues A.P.R., Lima R.M., Cardoso C.K.S., Oliveira C. Effects of Extra Virgin Olive Oil (EVOO) and the Traditional Brazilian Diet on Sarcopenia in Severe Obesity: A Randomized Clinical Trial. Nutrients. 2020;12:1498. doi: 10.3390/nu12051498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardoso C.K.S., Santos A., Rosa L.P.S., Mendonça C.R., Vitorino P.V.O., Peixoto M., Silveira E.A. Effect of Extra Virgin Olive Oil and Traditional Brazilian Diet on the Bone Health Parameters of Severely Obese Adults: A Randomized Controlled Trial. Nutrients. 2020;12:403. doi: 10.3390/nu12020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Associação Brasileira de Empresas de Pesquisa Critério de classificação econômica Brasil. [(accessed on 22 June 2020)];2008 Available online: http://www.abep.org/criterio-brasil.
- 24.Bloomfield K., Gerhard G., Sharon W. Gender, culture and alcohol problems: A multi-national study. Alcohol Alcohol. 2006;41:i3–i7. doi: 10.1093/alcalc/agl070. [DOI] [PubMed] [Google Scholar]
- 25.Kanny D., Naimi T.S., Liu Y., Lu H., Brewer R.D. Annual total binge drinks consumed by US adults, 2015. Am. J. Prev. Med. 2018;54:486–496. doi: 10.1016/j.amepre.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wendt A., da Silva I.C.M., Gonçalves H., Menezes A., Barros F., Wehrmeister F.C. Short-term effect of physical activity on sleep health: A population-based study using accelerometry. J. Sport Health Sci. 2020;2 doi: 10.1016/j.jshs.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hildebrand M., VT V.A.N.H., Hansen B.H., Ekelund U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med. Sci. Sports Exerc. 2014;46:1816–1824. doi: 10.1249/MSS.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 28.da Silva I.C., van Hees V.T., Ramires V.V., Knuth A.G., Bielemann R.M., Ekelund U., Brage S., Hallal P.C. Physical activity levels in three Brazilian birth cohorts as assessed with raw triaxial wrist accelerometry. Int. J. Epidemiol. 2014;43:1959–1968. doi: 10.1093/ije/dyu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson F., Subar A. Nutrition in the Prevention and Treatment of Disease. Academic Press; Burlington, NJ, USA: 2008. Chapter 1—Dietary Assessment Methodology. [Google Scholar]
- 30.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 31.Association A.D. Standards of medical care in diabetes-2017 abridged for primary care providers. Clin. Diabetes. 2017;35:5–26. doi: 10.2337/cd16-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Organization W.H. Guidelines for ATC Classification and DDD Assignment. WHO; Oslo, Norway: 2018. Collaborating centre for drug statistics methodology; p. 3. [Google Scholar]
- 33.Jin M., Yang F., Yang I., Yin Y., Luo J.J., Wang H., Yang X.-F. Uric acid, hyperuricemia and vascular diseases. Front. Biosci. 2012;17:656. doi: 10.2741/3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization . Obesity: Preventing and Managing the Global Epidemic. WHO; Geneva, Switzerland: 2000. [PubMed] [Google Scholar]
- 36.Pinheiro F.A., Tróccoli B.T., Carvalho C.V.D. Validity of the Nordic Musculoskeletal Questionnaire as morbidity measurement tool. Rev. Saude Publica. 2002;36:307–312. doi: 10.1590/S0034-89102002000300008. [DOI] [PubMed] [Google Scholar]
- 37.De Barros E., Alexandre N.M.C. Cross-cultural adaptation of the Nordic musculoskeletal questionnaire. Int. Nurs. Rev. 2003;50:101–108. doi: 10.1046/j.1466-7657.2003.00188.x. [DOI] [PubMed] [Google Scholar]
- 38.Mendonça C.R., Noll M., Silveira E.A. Adaptation and validation of body maps for musculoskeletal pain location in patients with severe obesity. Korean J. Pain. 2018;31:268. doi: 10.3344/kjp.2018.31.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coggon D., Ntani G., Palmer K.T., Felli V.E., Harari R., Barrero L.H., Felknor S.A., Gimeno D., Cattrell A., Vargas-Prada S. Patterns of multisite pain and associations with risk factors. Pain. 2013;154:1769–1777. doi: 10.1016/j.pain.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuorinka I., Jonsson B., Kilbom A., Vinterberg H., Biering-Sørensen F., Andersson G., Jørgensen K. Standardised Nordic questionnaires for the analysis of musculoskeletal symptoms. Appl. Ergon. 1987;18:233–237. doi: 10.1016/0003-6870(87)90010-X. [DOI] [PubMed] [Google Scholar]
- 41.Haefeli M., Elfering A. Pain assessment. Eur. Spine J. 2006;15(Suppl. 1):S17–S24. doi: 10.1007/s00586-005-1044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boonstra A.M., Stewart R.E., Köke A.J.A., Oosterwijk R.F.A., Swaan J.L., Schreurs K.M.G., Schiphorst Preuper H.R. Cut-Off Points for Mild, Moderate, and Severe Pain on the Numeric Rating Scale for Pain in Patients with Chronic Musculoskeletal Pain: Variability and Influence of Sex and Catastrophizing. Front. Psychol. 2016;7:1466. doi: 10.3389/fpsyg.2016.01466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenland S., Daniel R., Pearce N. Outcome modelling strategies in epidemiology: Traditional methods and basic alternatives. Int. J. Epidemiol. 2016;45:565–575. doi: 10.1093/ije/dyw040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peduzzi P., Concato J., Kemper E., Holford T.R., Feinstein A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996;49:1373–1379. doi: 10.1016/S0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 45.Blümel J.E., Arteaga E., Mezones-Holguín E., Zúñiga M.C., Witis S., Vallejo M.S., Tserotas K., Sánchez H., Onatra W., Ojeda E. Obesity is associated with a higher prevalence of musculoskeletal pain in middle-aged women. Gynecol. Endocrinol. 2017;33:378–382. doi: 10.1080/09513590.2016.1269741. [DOI] [PubMed] [Google Scholar]
- 46.Luz E.M.F.D., Magnago T.S.B.D.S., Greco P.B.T., Ongaro J.D., Lanes T.C., Lemos J.C. Prevalence and factors associated with musculoskeletal pain in hospital cleaning workers. Texto Contexto Enferm. 2017;26:26. doi: 10.1590/0104-07072017000870016. [DOI] [Google Scholar]
- 47.Pacca D.M., De-Campos G.C., Zorzi A.R., Chaim E.A., De-Miranda J.B. Prevalence of joint pain and osteoarthritis in obese brazilian population. Arq. Bras. Cir. Dig. 2018;31 doi: 10.1590/0102-672020180001e1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peltonen M., Lindroos A.K., Torgerson J.S. Musculoskeletal pain in the obese: A comparison with a general population and long-term changes after conventional and surgical obesity treatment. Pain. 2003;104:549–557. doi: 10.1016/S0304-3959(03)00091-5. [DOI] [PubMed] [Google Scholar]
- 49.Ambrose K.R., Golightly Y.M. Physical exercise as non-pharmacological treatment of chronic pain: Why and when. Best practice & research. Best Pract. Res. Clin. Rheumatol. 2015;29:120–130. doi: 10.1016/j.berh.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prioreschi A., Brage S., Westgate K., Norris S., Micklesfield L. Cardiorespiratory fitness levels and associations with physical activity and body composition in young South African adults from Soweto. BMC Public Health. 2017;17:301. doi: 10.1186/s12889-017-4212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stefansdottir R., Gudmundsdottir S. Sedentary behavior and musculoskeletal pain: A five-year longitudinal Icelandic study. Public Health. 2017;149(Suppl. C):71–73. doi: 10.1016/j.puhe.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 52.Murata S., Doi T., Sawa R., Nakamura R., Isa T., Ebina A., Kondo Y., Tsuboi Y., Torizawa K., Fukuta A. Association between objectively measured physical activity and the number of chronic musculoskeletal pain sites in community-dwelling older adults. Pain Med. 2018;20:717–723. doi: 10.1093/pm/pny112. [DOI] [PubMed] [Google Scholar]
- 53.Pan F., Byrne K.S., Ramakrishnan R., Ferreira M., Dwyer T., Jones G. Association between musculoskeletal pain at multiple sites and objectively measured physical activity and work capacity: Results from UK Biobank study. J. Sci. Med. Sport. 2019;22:444–449. doi: 10.1016/j.jsams.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 54.Terrier P., Praz C., Le Carré J., Vuistiner P., Léger B., Luthi F. Pain interference with physical functioning is associated with physical activity level in patients with chronic musculoskeletal pain. Ann. Phys. Rehabil Med. 2018;61:e19. doi: 10.1016/j.rehab.2018.05.042. [DOI] [Google Scholar]
- 55.Dong H.J., Larsson B., Rivano Fischer M., Gerdle B. Maintenance of quality of life improvement for patients with chronic pain and obesity after interdisciplinary multimodal pain rehabilitation - A study using the Swedish Quality Registry for Pain Rehabilitation. Eur. J. Pain. 2019;23:1839–1849. doi: 10.1002/ejp.1457. [DOI] [PubMed] [Google Scholar]
- 56.Barbour K.E. Prevalence of severe joint pain among adults with doctor-diagnosed arthritis-United States, 2002–2014. MMWR Morb. Mortal. Wkly. Rep. 2016;65:1052–1056. doi: 10.15585/mmwr.mm6539a2. [DOI] [PubMed] [Google Scholar]
- 57.Barbour K.E., Helmick C.G., Boring M., Brady T.J. Vital signs: Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States, 2013–2015. MMWR Morb. Mortal. Wkly. Rep. 2017;66:246. doi: 10.15585/mmwr.mm6609e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klingberg E., Bilberg A., Björkman S., Hedberg M., Jacobsson L., Forsblad-d’Elia H., Carlsten H., Eliasson B., Larsson I. Weight loss improves disease activity in patients with psoriatic arthritis and obesity: An interventional study. Arthritis Res. Ther. 2019;21:17. doi: 10.1186/s13075-019-1810-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cooney J.K., Law R.-J., Matschke V., Lemmey A.B., Moore J.P., Ahmad Y., Jones J.G., Maddison P., Thom J.M. Benefits of exercise in rheumatoid arthritis. J. Aging Res. 2011:681640. doi: 10.4061/2011/681640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McQueen M.A. Exercise aspects of obesity treatment. Ochsner J. 2009;9:140–143. [PMC free article] [PubMed] [Google Scholar]
- 61.Abdel Shaheed C., Maher C., Williams K., McLachlan A. Efficacy and tolerability of muscle relaxants for low back pain: Systematic review and meta-analysis. Eur. J. Pain. 2017;21:228–237. doi: 10.1002/ejp.907. [DOI] [PubMed] [Google Scholar]
- 62.Witenko C., Moorman-Li R., Motycka C., Duane K., Hincapie-Castillo J., Leonard P., Valaer C. Considerations for the appropriate use of skeletal muscle relaxants for the management of acute low back pain. Pharm. Ther. 2014;39:427. [PMC free article] [PubMed] [Google Scholar]
- 63.Davies K.A., Silman A.J., Macfarlane G.J., Nicholl B.I., Dickens C., Morriss R., Ray D., McBeth J. The association between neighbourhood socio-economic status and the onset of chronic widespread pain: Results from the EPIFUND study. Eur J. Pain. 2009;13:635–640. doi: 10.1016/j.ejpain.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dorner T.E., Stein K.V., Hahne J., Wepner F., Friedrich M., Mittendorfer-Rutz E. How are socio-demographic and psycho-social factors associated with the prevalence and chronicity of severe pain in 14 different body sites? A cross-sectional population-based survey. Wien. Klin. Wochenschr. 2018;130:14–22. doi: 10.1007/s00508-017-1223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hagen K., Zwart J.-A., Svebak S., Bovim G., Stovner L.J. Low socioeconomic status is associated with chronic musculoskeletal complaints among 46,901 adults in Norway. Scand. J. Public Health. 2005;33:268–275. doi: 10.1080/14034940510005699. [DOI] [PubMed] [Google Scholar]
- 66.Kortt M., Baldry J. The association between musculoskeletal disorders and obesity. Aust. Health Rev. 2002;25:207–214. doi: 10.1071/AH020207. [DOI] [PubMed] [Google Scholar]
- 67.Mittendorfer-Rutz E., Dorner T.E. Socio-economic factors associated with the 1-year prevalence of severe pain and pain-related sickness absence in the Austrian population. Wien. Klin. Wochenschr. 2018;130:4–13. doi: 10.1007/s00508-017-1222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rios R., Zautra A.J. Socioeconomic disparities in pain: The role of economic hardship and daily financial worry. Health Psychol. 2011;30:58. doi: 10.1037/a0022025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dalstra J.A., Kunst A.E., Borrell C., Breeze E., Cambois E., Costa G., Geurts J.J., Lahelma E., Van Oyen H., Rasmussen N.K. Socioeconomic differences in the prevalence of common chronic diseases: An overview of eight European countries. Int. J. Epidemiol. 2005;34:316–326. doi: 10.1093/ije/dyh386. [DOI] [PubMed] [Google Scholar]
- 70.Huisman M., Kunst A.E., Bopp M., Borgan J.-K., Borrell C., Costa G., Deboosere P., Gadeyne S., Glickman M., Marinacci C. Educational inequalities in cause-specific mortality in middle-aged and older men and women in eight western European populations. Lancet. 2005;365:493–500. doi: 10.1016/S0140-6736(05)17867-2. [DOI] [PubMed] [Google Scholar]
- 71.Suman A., Bostick G.P., Schaafsma F.G., Anema J.R., Gross D.P. Associations between measures of socio-economic status, beliefs about back pain, and exposure to a mass media campaign to improve back beliefs. BMC Public Health. 2017;17:504. doi: 10.1186/s12889-017-4387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kolotkin R.L., Crosby R.D., Gress R.E., Hunt S.C., Engel S.G., Adams T.D. Health and health-related quality of life: Differences between men and women who seek gastric bypass surgery. Surg. Obes. Relat. Dis. 2008;4:651–659. doi: 10.1016/j.soard.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]