Abstract
Dysfunction of sensorimotor predictive processing is thought to underlie abnormalities in self-monitoring producing passivity symptoms in psychosis. Experimentally induced sensorimotor conflict can produce a failure in bodily self-monitoring (presence hallucination [PH]), yet it is unclear how this is related to auditory self-monitoring and psychosis symptoms. Here we show that the induction of sensorimotor conflict in early psychosis patients induces PH and impacts auditory-verbal self-monitoring. Participants manipulated a haptic robotic system inducing a bodily sensorimotor conflict. In experiment 1, the PH was measured. In experiment 2, an auditory-verbal self-monitoring task was performed during the conflict. Fifty-one participants (31 early psychosis patients, 20 matched controls) participated in the experiments. The PH was present in all participants. Psychosis patients with passivity experiences (PE+) had reduced accuracy in auditory-verbal self-other discrimination during sensorimotor stimulation, but only when sensorimotor stimulation involved a spatiotemporal conflict (F(2, 44) = 6.68, P = .002). These results show a strong link between robotically controlled alterations in sensorimotor processing and auditory misattribution in psychosis and provide evidence for the role of sensorimotor processes in altered self-monitoring in psychosis.
Keywords: early psychosis, source monitoring, sensorimotor conflict, predictive processing, sense of agency
Introduction
Psychosis is characterized by abnormal mental states including hallucinations and delusions and a deterioration of cognitive and emotion processing.1 Moreover, people with psychosis often report strange and compelling feelings that other people or external forces are influencing them.2 They may experience external forces as having a direct and uncontrollable influence on their inner experience, leading to intriguing symptoms such as auditory-verbal hallucinations (AVHs) in the form of voices commenting the person’s thoughts, delusional ideas that thoughts are inserted or stolen from their minds, or sensations of external control over their bodies and actions. These symptoms, termed “Passivity Experiences (PE)” and formerly known as Schneiderian first-rank symptoms, reflect a diminished demarcation of self-other boundaries and misattributions of self-generated thoughts and actions to external sources.3–5 It has been suggested that these deficiencies are due to abnormal sensorimotor prediction mechanisms, causing erroneous self-monitoring.6–9 The putative mechanism for self-monitoring is based upon the comparison of predictions regarding sensory outcomes of self-generated actions (ie, efference copy) with afferent sensory signals.10,11 When predictions and afferent sensory signals match, actions are self-attributed and the sensory signal is attenuated.12–15 If a discrepancy is found, sensory signals are less attenuated, indicating a probable external origin.5,16 Several studies have shown aberrant sensorimotor prediction and reduction of normal sensory attenuation for self-generated actions in schizophrenia,17–24 providing evidence linking deficient sensorimotor prediction with self-other discrimination symptoms of psychosis.6,7,25 While the neurobiological basis for AVH is still debated,25–28 it has been suggested that AVH may arise from misattribution of inner speech to an external source due to defective efference copy mechanisms.28–31 Studies have found correlational evidence for deficits in self-monitoring of auditory-verbal content in schizophrenia29,32,33 as well as a decreased sensorimotor prediction in patients with AVH.17,34,35
However, this previous evidence is correlational, showing that deficient sensorimotor predictions in schizophrenia often correlate with positive symptom severity. Thus, more causal evidence employing an experimentally generated sensorimotor conflict to induce a psychosis-like mental state able to impair auditory-verbal processing in an online fashion is still lacking. Here, we used a custom robotic device36 to investigate this issue. By directly manipulating sensorimotor contingencies, the device induces a mental state characterized by an illusory feeling that a person is standing behind them (ie, presence pallucination [PH]).37 Here, standing and blindfolded participants control the “lead” robot38 by moving their arm. Their movements are sent to a “follow” robot, which applies tactile feedback in real time to the participants’ backs. In order to manipulate sensorimotor contingency, in the asynchronous condition, a 500-ms delay is introduced between the participants’ movements and the haptic feedback. We previously showed that, in healthy individuals, this asynchronous condition induces the feeling that a person is standing behind them, ie, PH.37 Here we investigated whether the PH effects could be reproduced in psychosis patients with (PE+) and without (PE−) passivity symptoms, and critically, whether the same manipulation would also induce deficits in self-monitoring of auditory-verbal stimuli. To this aim, healthy participants and psychosis patients were presented during the sensorimotor stimulation with prerecorded words, spoken either by themselves or by another person, and were asked to discriminate between the two.30 Thus, in the present study, we directly tested (1) whether robotic induction of a sensorimotor conflict, able to induce a mental state characterized by PH in healthy participants, induces the same effect in psychosis patients (PH experiment) and (2) whether it impacts self-monitoring for auditory-verbal information, as is thought to occur for AVH in psychosis (self-monitoring experiment). Thus, contrary to previous investigations, our design allows for online and active induction of self-monitoring errors through task-irrelevant sensorimotor conflicts.
Methods
Participants
Thirty-one individuals in the early phase of psychosis having met criteria for a psychotic episode according to the Comprehensive Assessment of At-Risk Mental States (CAARMS) criteria (mean age = 26.4 SD = 4.6 years; 9 women) were recruited among the Treatment and early Intervention in Psychosis Program (TIPP), a 3-year program launched in 2004 in Lausanne (CHUV, Switzerland). Twenty age-matched control subjects (mean age = 25.95, SD = 4.7 years; 5 women) from the control cohort of the TIPP program were also recruited. Exclusion criteria for the study were history of neurological illness or trauma, nonpsychiatric visual or auditory disorders, diagnosis of psychosis related to intoxication or organic brain disease, intelligence quotient <70, age below 18 and above 35 at the TIPP inclusion, or antipsychotic medication more than 6 months before the inclusion; 90.3% of the subjects were right-handed. The participant group included all participants who were available and willing to participate in this study and met the inclusion criteria from the TIPP cohort at the time of the study. Controls were assessed and selected with the Diagnostic Interview for Genetic Studies. Major mood, psychotic, or substance-use disorder and having a first-degree relative with a psychotic disorder were exclusion criteria for controls. All were clinically stable during testing and gave an informed written consent. The study was approved by the Ethical Committee of Clinical Research of the University of Lausanne, Switzerland, and was run in accordance with the ethical guidelines of the ethical committee and the Declaration of Helsinki.
The patients underwent an in-depth clinical assessment by a trained psychiatrist the same day as the behavioral experiment. Symptom severity and classification were assessed in the patient group using the Positive and Negative Syndrome Scale (PANSS) for schizophrenia with a mean of the total score of 52.52 (SD = 13.67) (13.90 [SD = 4.96] on the positive subscale, 13.72 [SD = 4.71] on the negative subscale, and 24.62 [SD = 7.2] on the general psychopathology subscale). Passivity symptoms were assessed using specific items of the Scale for the Assessment of Positive Symptoms (SAPS)39 (item 2: voices commenting; item 3: voices conversing; item 15: delusions of being controlled; item 18: though insertion; item 19: thought withdrawal). Patients were considered PE + (N = 19) if they had presented at least one of these five PE during the psychotic episodes35,40; 12 patients never presented passivity symptoms and were thus included in the PE− group. The two groups were demographically similar in terms of age (P = .94) and level of education (P = .84). However, although there were no differences between the two groups in the global clinical features (total PANSS scores) (P = .41) and treatment duration (P = .24), there was an expected significant difference in the positive PANSS subscore (related to PE) (P = .02) and the chlorpromazine equivalent medication (used to treat passivity symptoms) (P = .01) (see table 1 & supplementary material for full clinical details).
Table 1.
Demographic and Clinical Information for all Participant Groups
| Patients with Passivity Experiences PE + (N = 19) | Patients Without Passivity Experiences PE− (N = 12) | Controls (N = 20) | Comparison Between All Groups (P Value) | Comparison Between PE + and PE− Groups (P Value) | |
|---|---|---|---|---|---|
| Gender M/F (male %) | 13/6 (68.4%) | 9/3 (75%) | 15/5 (75%) | 0.87 | 0.35 |
| Handedness (right handed %) | 18/1 (94.7%) | 10/2 (83.3%) | 16/3 (80%) | 0.53 | 0.16 |
| Mean age (year) (SD) | 26.3 (±4.84) | 26.5 (±4.56) | 25.95 (±4.7) | 0.94 | 0.45 |
| Year of education (SD) | 12.5 (±2.71) | 13.09 (±2.02) | 15.6 (±2.72) | <0.01* | 0.28 |
| GAF score (SD) | 61.2 (±9.32) | 60.2 (±5.61) | 84.05 (±4.41) | <0.01* | 0.38 |
| Chlorpromazine equivalent- mg (SD) | 399.9 (±54.06) | 195.25 (±62.97) | — | — | 0.01* |
| Treatment duration years (SD) | 1.7 (±1.68) | 1.3 (±1.11) | — | — | 0.24 |
| PANSS /210 | 52.1 (±11.04) | 53.2 (±17.58) | — | — | 0.41 |
| Positive subscale mean /49 (SD) | 15.3 (±5.03) | 11.7 (±4.1) | — | — | 0.02* |
| Negative subscale mean /49 (SD) | 13.1 (±4.04) | 15.1 (±5.58) | — | — | 0.13 |
| General psychopathology subscale mean /112 (SD) | 23.6 (±4.34) | 26.4 (±10.43) | — | — | 0.15 |
| SAPS item rating /6 (SD) | |||||
| 2: voices commenting | 3.53 (±0.63) | 0 | — | — | — |
| 3: voices conversing | 2.95 (±0.69) | 0 | — | — | — |
| 15: delusions of being controlled | 1.68 (±0.56) | 0 | — | — | — |
| 18: though insertion | 3.6 (±0.52) | 0 | — | — | — |
| 19: thought withdrawal | 1 (±0.44) | 0 | — | — | — |
*Significant difference of the P value.
Initial diagnosis of the patient based on their first-episode symptoms revealed that at the time of the experiment, 19 patients satisfied the ICD-10 criteria for schizophrenia, 2 for schizoaffective disorders, 6 for acute and transient psychotic disorders, 3 for severe depressive episode with psychotic symptoms, and 1 for bipolar affective disorder.
Procedure
Presence Hallucination Experiment.
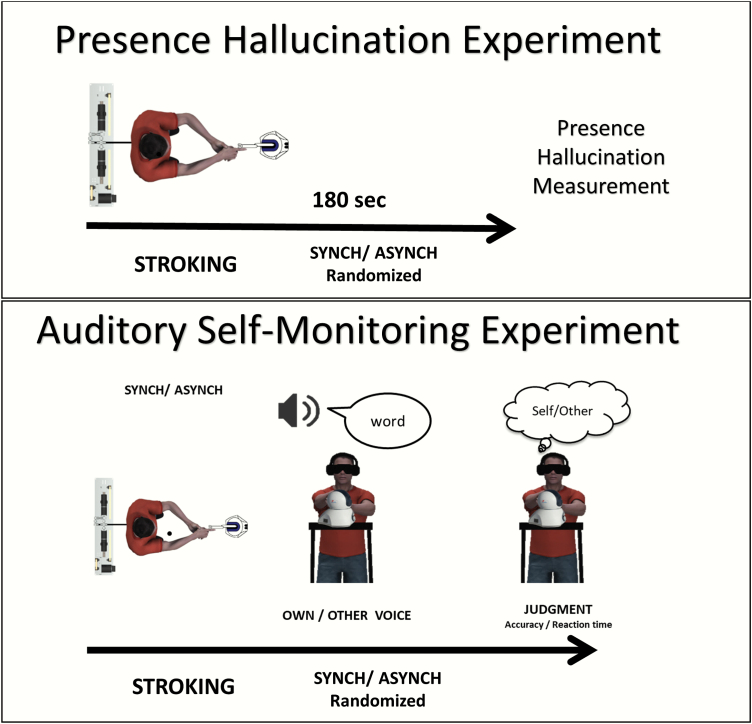
The procedure of the PH experiment was identical to that previously used in healthy participants.37 While standing and blindfolded, participants manipulated the robot for 3 minutes and were presented with synchronous or asynchronous haptic feedback. At the end of each session, participant filled an 8-item questionnaire regarding the sensations elicited by the manipulation (see figure 1, top), including 3 experimental questions aimed at assessing illusory states (see Q2, Q6, and Q8 in supplementary material, and 5 control questions [Q1, Q3, Q4, Q5, Q7]), added to control for spurious effects, such as general susceptibility and response biases. Synchronous and asynchronous conditions were presented in a randomized order.
Fig. 1.
Experimental design of the presence hallucination and the self-monitoring experiments. Top figure. Presence hallucination (PH) experimental design. Participants moved the master robot of the robotic system while receiving haptic feedback on their back from the “follow” robot. Critically, the tactile feedback could be either temporally synchronous to their movements or asynchronous through the introduction of a 500-ms delay. After 180 s of stroking, participants responded to the PH questionnaire. This was repeated for both synchronous and asynchronous conditions, in a randomized order.
Bottom figure. Auditory misattribution experimental design. Participants moved the master component of the robotic system while receiving haptic feedback on their back from the “follow” component. Again, the tactile feedback could be either temporally synchronous to their movements or asynchronous. After 60 s of stroking, participants heard words either in their own voice or in the voice of another person and were required to judge if the word was in their own voice or that of another person. Each block included 25 words and was repeated 4 times.
Auditory Misattribution Experiment.
Word recordings (50 in participant’s voice and the same 50 words in a gender matched voice) were randomly grouped in 4 blocks of 25 words. Blindfolded participants freely manipulated the robot with their left hand throughout the experimental session (figure 1, bottom). These movements were replicated in real time to the participants’ backs by the “follow” robot. After 1 minute moving the lead robot while they received synchronous or asynchronous (500 ms delay) haptic feedback, and while still stroking their backs, participants heard the prerecorded words and were required to report if the word was spoken in their own voice (Self condition) or in another person’s voice (Other condition). An interstimulus interval of 0.5, 0.6, or 0.7 seconds was presented between words, and the next word was presented only after the participant’s response. Reaction time and accuracy were measured for each trial. The synchrony of sensorimotor feedback was pseudo-randomized between subjects. There were 4 blocks in total (2 synchronous and 2 asynchronous). Thus, all participants heard the same words in both their own voice and another’s voice and under both synchronous and asynchronous sensorimotor conditions.
Statistical Analysis
Presence Hallucination Experiment.
Ordinal responses to the PH questionnaire were analyzed using linear mixed effects models,37 with synchrony (synchronous/ asynchronous), group (Control/PE+/PE−) and question type (experimental vs control) as fixed effects, and subjects and questions as random effects. This was followed by nonparametric Mann-Withney-Wilcoxon (MWW) tests on the PH experimental questions.
Auditory Misattribution Experiment.
We used signal detection theory41 to analyze the responses to the auditory misattribution task. Responses were categorized as hits, misses, false alarms, and correct rejections for synchronous and asynchronous conditions; d′ measures of perceptual sensitivity, as well as beta measures for subjective criterion changes, were then calculated for each condition separately and analyzed using a Synchrony(Synchronous/Asynchronous) × Group(Control/PE+/PE−) Analysis of Variance (ANOVA), with synchrony as a within-subject variable and Group as a between-subject factor.
Results
Presence Hallucination Experiment
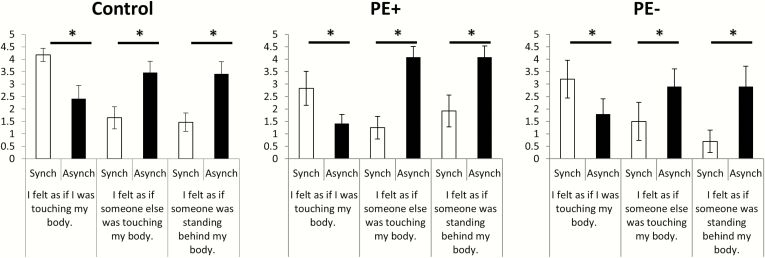
As expected, the mixed model ANOVA indicated a significant interaction between synchrony and question type (F(1, 808) = 5.62, P = .01), showing that experimental questions measuring the PH were more modulated by synchrony than the control questions. A main effect of synchrony (F(1, 808) = 5.82, P = .01) was also found, showing overall higher ratings in the synchronous when compared with asynchronous condition; no other effects or interactions were significant (all F < .75) (figure 2). Contrary to our expectations, no differences in the PH were found between the PE + and control groups. The analysis of experimental questions indicated that participants experienced the touch in the synchronous condition to be self-generated (Q2, P = .003) and experienced the asynchronous touch as originating from another person (Q6, P = .0005). Critically, participants reported a higher PH in the asynchronous condition than in the synchronous condition (Q8, P = .000007). No other effects reached significance (all P > .15).
Fig. 2.
Induction of a presence hallucination by sensorimotor conflict. Subjective ratings indicated that the sensorimotor conflict in the asynchronous vs synchronous feedback condition induced a feeling of a presence in all experimental groups (left plots, controls; middle plot PE + patients; right plot, PE− patients), extending previous findings in healthy participants to psychosis patients. Contrarily, synchronous stimulation induced a feeling of self-touch.
Auditory Misattribution Experiment
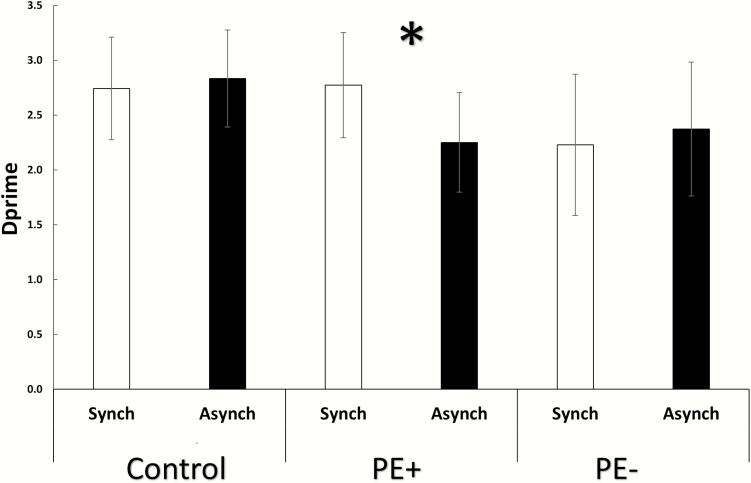
Overall accuracy rates were high (M = 84.99, 95% CI = +/− 0.05) and did not differ between the groups (F(2, 44) = 1.28, P = .28), indicating that all participants were able to perform the self-recognition task with good accuracy. The ANOVA on d′ scores revealed a significant interaction of Group and Synchrony (F(2, 44) = 6.68, P = .002, partial η 2 = 0.23). Post hoc analysis indicated that this was driven by reduced d′ for the PE + group in the asynchronous condition (M = 2.25, 95% CI = +/− 0.45) compared to the synchronous condition (M = 2.77, 95% CI = +/− 0.47, P = .0003). Thus, the asynchronous stimulation decreased the PE + patients’ ability to discriminate their own voice from another’s voice. This difference in d′ for the PE + group in the asynchronous condition resulted from both a reduction in the hit rate (0.85–0.79) and a rise in the rate of false alarms (0.1–0.13). This indicates a reduction of auditory self-discrimination for both self and other voices. There was no significant main effect of Group (F(2, 44) = 0.95, P = .39), nor any main effect of Synchrony (F(1, 44) = 1.1, P = 0.28; figure 3), indicating that this decrease was specific to the PE + group during the asynchronous condition. This difference in sensitivity could not be explained as a consequence of a subjective change in the criterion, as the ANOVA run on beta values showed no main effect of Group (F(2, 44) = 0.57, P = .56), no main effect of Synchrony (F(1, 44) = 0.59, P = .44), nor a significant interaction (F(2, 44) = 0.24, P = .78). Although not a primary outcome, reaction times were also recorded and analyzed (see supplementary material and supplementary figure S1for further analysis).
Fig. 3.
Induction of auditory-verbal self-monitoring deficits by sensorimotor conflict. d′ scores in self-monitoring for synchronous and asynchronous feedback conditions for all experimental groups are shown. Sensorimotor conflicts induced a decrease of self-other discrimination for auditory-verbal stimuli, but only for the PE + group. Note that overall discrimination was not different between patients and controls.
Discussion
Here we show that robotically induced sensorimotor conflicts can experimentally generate the PH, extending previous findings from healthy participants37 to early psychosis patients. Critically, the same sensorimotor conflict induced a decrease in self-monitoring during the auditory-verbal task, only observed in PE + patients, who present symptoms of external control such as AVH and other passivity experiences.
These findings indicate that experimentally induced, task-irrelevant sensorimotor conflict can cause source-monitoring errors for auditory-verbal stimuli in PE + patients, supporting cognitive models of schizophrenia which state that deficits in self-related processing are related to deficient sensorimotor prediction.5,23,42 While influential previous research has shown that schizophrenia patients have reduced self-monitoring and sensory attenuation for self-generated actions,17,19,20,31 this is, to the best of our knowledge, the first study to show experimentally a causal link between a task-irrelevant sensorimotor conflict and the induction of an online auditory-verbal misattribution. Interestingly, this effect is in line with a decreased ability for self-other discrimination, as discrimination errors were present both for one’s own voice and for the voice of another person. Importantly, this effect was not due to changes in the response criterion or to general differences in performance between the psychosis and healthy individuals, and was not observed in the experimental control condition. This work has some limitations that should be mentioned. Although the PH was found in all participants, no differences were found in the subjective experience of the PH between groups. This may stem from reduced sensitivity of subjective, explicit reports of the PH vs more objective measures of its effects.
Previous work has shown that the brain network related to PHs is comprised of temporoparietal, insular, and frontoparietal nodes, which are key regions in a network of multisensory and sensorimotor areas underlying bodily self-consciousness.43 This was revealed using lesion mapping in patients with PHs caused by neurological disease.37 In particular, the temporoparietal junction is a multisensory region critical for self-location and first-person perspective, and neurologically or electrophysiologically induced alterations of this area have been shown to induce altered states of self-consciousness such as the so-called out-of-body experiences and PH.44,45 The insular cortex is considered a fundamental cortical hub integrating interoceptive and exteroceptive stimuli45–49 and is involved in bodily self-representation49,50 and source monitoring.51 Insular lesions have been linked to distortions in bodily experience, such as somatoparaphrenia50 and heautoscopy.52 The insular cortex is also altered in schizophrenia51,53 and is related to auditory hallucinations.26,54 Although not tested directly in the present study, future studies may reveal that the regions causing PHs in neurological patients are also of relevance for PH in psychiatric patients as well as self-monitoring deficits and related symptoms, including AVHs in schizophrenia.
To conclude, this work presents a novel link between sensorimotor conflicts and self-monitoring in psychosis. We show not only that sensorimotor conflicts can induce a sensation of an external agent (PH) but also that such conflicts propagate to other sensory and cognitive processes causing self-monitoring errors in the auditory-verbal domain. Using a novel approach combining robotics, behavioral paradigms, and psychophysics, we show for the first time that psychotic-like symptoms can be induced by sensorimotor conflicts in patients and thus relate to processes known to alter the representation of the bodily self. These findings open the way for novel explorations of the mechanisms for different levels of self-disorder in psychosis.
Funding
This work was supported by Bertarelli Foundation, the Swiss National Science Foundation, and the European Science Foundation grants to O.B. R. S. was supported by the National Center of Competence in Research (NCCR) “SYNAPSY—The Synaptic Bases of Mental Diseases” financed by the Swiss National Science Foundation (grant no. 51AU40_125759) and an Israel Science Foundation (grant No. 1169/17). A.G. was financially supported by Swiss National Science Foundation grant (grant No. 29631).
Supplementary Material
Acknowledgments
Author Contributions: Dr. Salomon & Dr. Progin had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Salomon, Progin, Blanke, Conus, Griffa, Serino, Rognini, Marchesotti. Acquisition of data: Progin, Salomon, Blanke, Conus, Griffa, Do, Hagmann, Serino, Rognini, Marchesotti. Analysis and interpretation of data: Salomon, Blanke, Progin, Conus, Griffa, Do, Hagmann, Serino, Rognini. Drafting of the manuscript: Salomon, Blanke, Progin, Conus, Griffa, Hagmann, Serino, Rognini. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Salomon, Blanke, Progin, Griffa. Obtained funding: Blanke, Conus, Hagmann, Do. Administrative, technical, or material support: All authors. Study supervision: Blanke, Conus, Do, Hagmann. Conflict of Interest Disclosures: None reported. Additional Contributions: We thank all the patients and control subjects for their participation in this study.
References
- 1. Bleuler E. The basic symptoms of schizophrenia. In: Rapaport D, ed. Organization and Pathology of Thought: Selected Sources. New York, NY: Columbia University Press; 1951:581–649. [Google Scholar]
- 2. Sass LA, Parnas J. Schizophrenia, consciousness, and the self. Schizophr Bull. 2003;29(3):427–444. [DOI] [PubMed] [Google Scholar]
- 3. Sass LA, Parnas J. Phenomenology of self-disturbances in schizophrenia: some research findings and directions. Philosophy, Psychiatry, & Psychology. 2001;8(4):347–356. [Google Scholar]
- 4. Parnas J. Self and schizophrenia: a phenomenological perspective. In: Kircher T, David A, eds The Self In Neuroscience and Psychiatry. Cambridge, UK: Cambridge University Press; 2003:217–241. [Google Scholar]
- 5. Frith CD, Blakemore SJ, Wolpert DM. Abnormalities in the awareness and control of action. Philos Trans R Soc Lond B Biol Sci. 2000;355(1404):1771–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frith CD, Done DJ. Experiences of alien control in schizophrenia reflect a disorder in the central monitoring of action. Psychol Med. 1989;19(2):359–363. [DOI] [PubMed] [Google Scholar]
- 7. Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci. 2009;10(1):48–58. [DOI] [PubMed] [Google Scholar]
- 8. Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull. 1978;4(4):636–640. [DOI] [PubMed] [Google Scholar]
- 9. Corlett PR, Murray GK, Honey GD, et al. . Disrupted prediction-error signal in psychosis: evidence for an associative account of delusions. Brain. 2007;130(pt 9):2387–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blakemore SJ, Goodbody SJ, Wolpert DM. Predicting the consequences of our own actions: the role of sensorimotor context estimation. J Neurosci. 1998;18(18):7511–7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269(5232):1880–1882. [DOI] [PubMed] [Google Scholar]
- 12. Bays PM, Flanagan JR, Wolpert DM. Attenuation of self-generated tactile sensations is predictive, not postdictive. PLoS Biol. 2006;4(2):e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Elk M, Salomon R, Kannape O, Blanke O. Suppression of the N1 auditory evoked potential for sounds generated by the upper and lower limbs. Biol Psychol. 2014;102: 108–117. [DOI] [PubMed] [Google Scholar]
- 14. Baess P, Widmann A, Roye A, Schröger E, Jacobsen T. Attenuated human auditory middle latency response and evoked 40-Hz response to self-initiated sounds. Eur J Neurosci. 2009;29(7):1514–1521. [DOI] [PubMed] [Google Scholar]
- 15. Krugwasser AR, Harel EV, Salomon R. The boundaries of the self: the sense of agency across different sensorimotor aspects. J Vis. 2019;19(4):14. [DOI] [PubMed] [Google Scholar]
- 16. Salomon R, Lim M, Kannape O, Llobera J, Blanke O. “Self pop-out”: agency enhances self-recognition in visual search. Exp Brain Res. 2013;228(2):173–181. [DOI] [PubMed] [Google Scholar]
- 17. Ford JM, Palzes VA, Roach BJ, Mathalon DH. Did I do that? Abnormal predictive processes in schizophrenia when button pressing to deliver a tone. Schizophrenia bulletin. 2013;40(4):804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ford JM, Roach BJ, Faustman WO, Mathalon DH. Synch before you speak: auditory hallucinations in schizophrenia. Am J Psychiatry. 2007;164(3):458–466. [DOI] [PubMed] [Google Scholar]
- 19. Shergill SS, Samson G, Bays PM, Frith CD, Wolpert DM. Evidence for sensory prediction deficits in schizophrenia. Am J Psychiatry. 2005;162(12):2384–2386. [DOI] [PubMed] [Google Scholar]
- 20. Shergill SS, White TP, Joyce DW, Bays PM, Wolpert DM, Frith CD. Functional magnetic resonance imaging of impaired sensory prediction in schizophrenia. JAMA Psychiatry. 2014;71(1):28–35. [DOI] [PubMed] [Google Scholar]
- 21. Blakemore SJ, Smith J, Steel R, Johnstone CE, Frith CD. The perception of self-produced sensory stimuli in patients with auditory hallucinations and passivity experiences: evidence for a breakdown in self-monitoring. Psychol Med. 2000;30(5):1131–1139. [DOI] [PubMed] [Google Scholar]
- 22. Synofzik M, Thier P, Leube DT, Schlotterbeck P, Lindner A. Misattributions of agency in schizophrenia are based on imprecise predictions about the sensory consequences of one’s actions. Brain. 2010;133(Pt 1):262–271. [DOI] [PubMed] [Google Scholar]
- 23. Lindner A, Thier P, Kircher TT, Haarmeier T, Leube DT. Disorders of agency in schizophrenia correlate with an inability to compensate for the sensory consequences of actions. Curr Biol. 2005;15(12):1119–1124. [DOI] [PubMed] [Google Scholar]
- 24. Malenka RC, Angel RW, Hampton B, Berger PA. Impaired central error-correcting behavior in schizophrenia. Arch Gen Psychiatry. 1982;39(1):101–107. [DOI] [PubMed] [Google Scholar]
- 25. Northoff G, Duncan NW. How do abnormalities in the brain’s spontaneous activity translate into symptoms in schizophrenia? From an overview of resting state activity findings to a proposed spatiotemporal psychopathology. Progress in Neurobiology. 2016;145–146:26–45. [DOI] [PubMed] [Google Scholar]
- 26. Powers AR, Mathys C, Corlett PR. Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science. 2017;357(6351):596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alderson-Day B, McCarthy-Jones S, Fernyhough C. Hearing voices in the resting brain: a review of intrinsic functional connectivity research on auditory verbal hallucinations. Neurosci Biobehav Rev. 2015;55:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johns LC, McGuire PK. Verbal self-monitoring and auditory hallucinations in schizophrenia. Lancet. 1999;353(9151):469–470. [DOI] [PubMed] [Google Scholar]
- 29. McGuire PK, Silbersweig DA, Wright I, et al. . Abnormal monitoring of inner speech: a physiological basis for auditory hallucinations. Lancet. 1995;346(8975):596–600. [DOI] [PubMed] [Google Scholar]
- 30. Allen PP, Johns LC, Fu CH, Broome MR, Vythelingum GN, McGuire PK. Misattribution of external speech in patients with hallucinations and delusions. Schizophr Res. 2004;69(2–3):277–287. [DOI] [PubMed] [Google Scholar]
- 31. Ford JM, Mathalon DH, Heinks T, Kalba S, Faustman WO, Roth WT. Neurophysiological evidence of corollary discharge dysfunction in schizophrenia. Am J Psychiatry. 2001;158(12):2069–2071. [DOI] [PubMed] [Google Scholar]
- 32. Brébion G, Amador X, David A, Malaspina D, Sharif Z, Gorman JM. Positive symptomatology and source-monitoring failure in schizophrenia–an analysis of symptom-specific effects. Psychiatry Res. 2000;95(2):119–131. [DOI] [PubMed] [Google Scholar]
- 33. Bentall RP, Baker GA, Havers S. Reality monitoring and psychotic hallucinations. Br J Clin Psychol. 1991;30(3):213–222. [DOI] [PubMed] [Google Scholar]
- 34. Knoblich G, Stottmeister F, Kircher T. Self-monitoring in patients with schizophrenia. Psychol Med. 2004;34(8):1561–1569. [DOI] [PubMed] [Google Scholar]
- 35. Franck N, Farrer C, Georgieff N, et al. . Defective recognition of one’s own actions in patients with schizophrenia. Am J Psychiatry. 2001;158(3):454–459. [DOI] [PubMed] [Google Scholar]
- 36. Hara M, Salomon R, van der Zwaag W, et al. . A novel manipulation method of human body ownership using an fMRI-compatible master-slave system. J Neurosci Methods. 2014;235:25–34. [DOI] [PubMed] [Google Scholar]
- 37. Blanke O, Pozeg P, Hara M, et al. . Neurological and robot-controlled induction of an apparition. Curr Biol. 2014;24(22):2681–2686. [DOI] [PubMed] [Google Scholar]
- 38. Hara M, Rognini G, Evans N, et al. . A novel approach to the manipulation of body-parts ownership using a bilateral master-slave system. In: 2011 IEEE/RSJ International Conference on Intelligent Robots and Systems; 2011:4664–4669. [Google Scholar]
- 39. Andreasen NC. Scale for the Assessment of Positive Symptons:(SAPS). Iowa City, Iowa, United States: University of Iowa; 1984. [Google Scholar]
- 40. Farrer C, Franck N, Frith CD, et al. . Neural correlates of action attribution in schizophrenia. Psychiatry Res. 2004;131(1):31–44. [DOI] [PubMed] [Google Scholar]
- 41. Swets JA. Signal Detection and Recognition in Human Observers: Contemporary Readings. Hoboken, New Jersey, USA: John Wiley and Sons; 1964. [Google Scholar]
- 42. Voss M, Moore J, Hauser M, Gallinat J, Heinz A, Haggard P. Altered awareness of action in schizophrenia: a specific deficit in predicting action consequences. Brain. 2010;133(10):3104–3112. [DOI] [PubMed] [Google Scholar]
- 43. Blanke O, Slater M, Serino A. Behavioral, neural, and computational principles of bodily self-consciousness. Neuron. 2015;88(1):145–166. [DOI] [PubMed] [Google Scholar]
- 44. Arzy S, Seeck M, Ortigue S, Spinelli L, Blanke O. Induction of an illusory shadow person. Nature. 2006;443( 7109):287. [DOI] [PubMed] [Google Scholar]
- 45. Blanke O, Ortigue S, Landis T, Seeck M. Stimulating illusory own-body perceptions. Nature. 2002;419(6904):269–270. [DOI] [PubMed] [Google Scholar]
- 46. Salomon R, Ronchi R, Dönz J, et al. . The insula mediates access to awareness of visual stimuli presented synchronously to the heartbeat. J Neurosci. 2016;36(18):5115–5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Salomon R, Ronchi R, Dönz J, et al. . Insula mediates heartbeat related effects on visual consciousness. Cortex. 2018;101:87–95. [DOI] [PubMed] [Google Scholar]
- 48. Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–195. [DOI] [PubMed] [Google Scholar]
- 49. Park H-D, Bernasconi F, Salomon R, et al. . Neural sources and underlying mechanisms of neural responses to heartbeats, and their role in bodily self-consciousness: an intracranial EEG study. Cerebral Cortex. 2017:28(7):2351–2364. [DOI] [PubMed] [Google Scholar]
- 50. Karnath HO, Baier B. Right insula for our sense of limb ownership and self-awareness of actions. Brain Struct Funct. 2010;214(5–6):411–417. [DOI] [PubMed] [Google Scholar]
- 51. Wylie KP, Tregellas JR. The role of the insula in schizophrenia. Schizophr Res. 2010;123(2–3):93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heydrich L, Blanke O. Distinct illusory own-body perceptions caused by damage to posterior insula and extrastriate cortex. Brain. 2013;136(pt 3):790–803. [DOI] [PubMed] [Google Scholar]
- 53. Crespo-Facorro B, Kim J-J, Andreasen NC, O’Leary DS, Bockholt HJ, Magnotta V. Insular cortex abnormalities in schizophrenia: a structural magnetic resonance imaging study of first-episode patients. Schizophrenia research. 2000;46(1):35–43. [DOI] [PubMed] [Google Scholar]
- 54. Shapleske J, Rossell SL, Chitnis XA, et al. . A computational morphometric MRI study of schizophrenia: effects of hallucinations. Cerebral Cortex. 2002;12(12):1331–1341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.