Abstract
Deficits in cognitive function are a major characteristic of schizophrenia. Many functional magnetic resonance imaging (fMRI) studies examine brain correlates of cognitive function in adults with schizophrenia, showing altered implication of associative areas such as the prefrontal cortex and temporal cortex. fMRI studies also examine brain representation of cognitive function in adolescents with early onset schizophrenia and those at risk of the disorder, yet results are often inconsistent. We compile and analyze data from eligible fMRI studies using quantitative meta-analyses to reveal concordant brain activity associated with adolescent relatives of patients with schizophrenia and those with early onset schizophrenia. Results show similar functional hubs of brain activity (eg, precuneus) yet in opposite hemispheres and clusters in ventrolateral rather than dorsolateral prefrontal cortices. Other areas of altered implication include the middle temporal gyrus, insula, and cerebellum. We discuss the findings in reference to the protracted maturation of the prefrontal cortex and possible effects due to the medication status of the two groups.
Keywords: fMRI, meta-analyses, adolescence/early onset schizophrenia, schizophrenia relatives
Schizophrenia is a severe mental illness marked by cognitive and psychotic symptoms, as well as debilitating negative symptoms. The disorder leads to significant difficulties in daily functioning, employability, and social relations.1 Cognitive symptoms in schizophrenia have been linked with altered anatomical and functional brain indices, mainly in frontal and temporal cortices. Specifically, functional neuroimaging research has examined brain areas of adults with schizophrenia showing increased and decreased implication in prefrontal brain regions when solving cognitive tasks.2–6 The majority of individuals at risk of developing schizophrenia do so during early adulthood, at around 18 to 25 years of age, and there is evidence of prodromal signs that occur prior to illness onset in childhood and adolescence.7 Target groups for identifying prodromal signs are adolescent relatives of individuals with schizophrenia and youth with early onset schizophrenia. Results from adult functional magnetic resonance imaging (fMRI) meta-analyses of working memory and executive function tasks of unaffected relatives compared to controls also show both hypo- and hyperactivation in prefrontal cortices.8,9 Critically, the prefrontal cortex has protracted developmental patterns spanning into young adulthood.10 Therefore, abnormalities in brain responses of youth at risk of schizophrenia may show altered implication in this region. To investigate this hypothesis, we use quantitative fMRI meta-analyses of brain responses to cognitive tasks of youth with relatives affected by schizophrenia and youth with early onset schizophrenia.
Although biological markers differentiating patients with schizophrenia from normal controls and other patients have been elusive,1 schizophrenia is associated with specific brain characteristics. Patients with schizophrenia have been shown to exhibit enlarged ventricles, reduced brain volume in various regions, including the frontal, parietal, occipital, insula, and anterior cingulate cortices, as well as the hippocampus.11–14 Cortical white matter abnormalities and connectivity problems are common in those at risk for schizophrenia,15–19 yet few have extensively studied the course of the illness.20 A meta-analysis has also shown that individuals at risk of developing psychosis showed gyral reductions in frontal and temporal regions, as well as reductions in anterior cingulate, hippocampal/parahippocampal regions, and the left precuneus.21 Moreover, those who transitioned to psychosis showed gray matter reductions in inferior frontal and superior temporal gyri.22 Similarly, those who transition compared with those who do not may show volumetric decreases in the cingulate, insular, prefrontal and temporal cortices, and the cerebellum.12,23
Meta-analyses of functional correlates of cognitive abilities in adult patients with schizophrenia have examined convergence across studies. The first meta-analysis was published in 2005 by Glahn and colleagues, who evaluated 12 studies that recorded brain responses to working memory processes in individuals with and without schizophrenia. Results showed both increased and decreased implications of the prefrontal cortex in persons with schizophrenia compared to control participants.4 A subsequent meta-analysis with 41 studies on executive function replicated this finding.5 A more recent meta-analysis examined gray matter volume and resting state across studies and reported reductions in the left insula and right inferior temporal gyral gray matter volume in relatives with schizophrenia compared to controls,14 supporting an earlier structural and functional meta-analysis on patients with first-episode psychosis.21 Taken together, these meta-analyses implicate a set of areas associated with working memory processing and executive function, including the temporal, cingulate, and insular cortices.4,5 These brain areas are associated with the executive (ie, working memory) networks, also found in large quantitative meta-analyses of healthy adult participants performing cognitive tasks.24,25
Importantly, cognitive abilities such as working memory improve gradually over childhood and adolescence.26,27 Although we could assume that younger age groups possess similar brain systems as adults, empirical data are in partial support. Specifically, younger, healthy, typically developing individuals show conjunction with adults in posterior parts of the cortex (eg, parietal areas), and disjunction in prefrontal parts of the cortex (eg, dorsolateral prefrontal areas28). Lack of prefrontal concordance across studies using children and adolescents may be associated with increased variability in activity location; for instance, different prefrontal locations and hemispheric asymmetry are identified by original studies.28 Thus, if the prefrontal cortex is a key brain marker for adult cognitive symptoms in schizophrenia, it remains to be investigated whether it is a brain marker related to prodromal signs of schizophrenia in younger age groups at risk of schizophrenia, such as relatives of patients with schizophrenia.
Prodromal signs of the disorder may also be observed in relatives of patients with schizophrenia. A meta-analysis comparing adults with relatives with schizophrenia and healthy controls showed hyperactivation within the right frontal, and left parietal regions, yet hypoactivation within the bilateral frontal, left temporal, and right parietal cortex was also reported.8 fMRI studies have examined the brain correlates on cognitive abilities in youth with early onset schizophrenia and youth with a relative, usually a parent, with schizophrenia.29–33 For example, some studies identify working memory deficits in association with the dorsolateral cortex,32 specifically in adolescents with schizophrenic parents.29 Other studies show no behavioral difference in the performance between adolescents with schizophrenic parents and controls yet schizophrenic relatives did show increased, rather than reduced, dorsolateral prefrontal as well as striatal activity during correct vs. incorrect memory performance.30 Adolescents with early onset schizophrenia show reduced working memory performance and reduced ventrolateral prefrontal activity,34 whereas other studies show reduced activity in posterior brain regions, but not in prefrontal areas.33,35
Since no single study is definitive, quantitative fMRI meta-analyses can identify overarching concordance across studies. Notably, the majority of quantitative meta-analyses in the literature use the activation likelihood estimation (ALE) method in software provided by GingerALE.36 However, recent recommendations of the software developers suggest that a minimum number of 17 to 20 experiments are needed for sufficient power.37 Although past studies with schizophrenia did not adhere to this recommendation, we decided to analyze our data with an alternative method of quantitative meta-analyses that uses foci as well as effect-size to estimate concordance. Specifically, effect-size signed differential mapping (ES-SDM)38 adopts features of ALE and incorporates statistical values (t-statistic) of peak coordinates to increase statistical power. Moreover, to evaluate the robustness and replicability of suprathreshold clusters, ES-SDM offers options for a jackknife sensitivity procedure. Therefore, we used ES-SDM to examine brain responses to cognitive tasks in youth with schizophrenic relatives and youth with early onset schizophrenia.
Based on past literature, we expect altered brain responses mainly in posterior brain regions in youth affected by schizophrenia and reduced implication of the prefrontal cortex in (a) early onset schizophrenia and (b) relatives of schizophrenia patients. The coordinate results that we provide may serve as a topographical atlas for target brain regions in future neuroimaging investigations and theoretically inform psychological models of schizophrenia.
Methods
Study Selection
The literature was searched to identify fMRI articles that focused on developing individuals with schizophrenia or relatives of those with schizophrenia. Our search focused on broad terms associated with fMRI, schizophrenia, and youth/adolescent, rather than terms such as “early onset schizophrenia” in an effort to capture as many published articles as possible. We used both terms “youth” and “adolescents” to improve the likelihood of assessing as many published articles as possible. Specifically, the first search was performed by entering keywords “schizophrenia” AND “fMRI” AND “youth” into the web of knowledge database (http://www.webofknowledge.com) and Pubmed (https://www.ncbi.nlm.nih.gov/pubmed/), while the second search was performed by entering the keywords “schizophrenia” AND “fMRI” AND “adolescents.” Searches were performed in October 2019. The search yielded a total of 193 articles, which were screened for eligibility. We only considered published studies and associated published supplementary material. To identify eligible articles, we relied on a standard protocol39 and removed studies that: (1) did not report fMRI foci in standard stereotactic coordinate space (either Talairach or Montreal Neurological Institute, MNI); (2) did not use whole-brain analysis; (3) used contrasts examining adults or nonhumans; and (4) used contrasts that did not include individuals with early onset schizophrenia or relatives of individuals with schizophrenia. The total number of eligible studies was 16, which reported between-group whole-brain results in MNI or Talairach space. Activations (hyperactivity) of schizophrenia-related individuals were extracted from “schizophrenia” minus “control” contrasts, whereas deactivations (hypoactivity) of schizophrenia-related individuals were extracted from “control” minus “schizophrenia.” See figure 1 for the flowchart of article selection for the meta-analyses.
Fig. 1.
Prisma flowchart showing steps taken for identifying articles included in the meta-analyses (template by Moher et al., 2009).40
Software and Analysis
ES-SDM meta-analysis software from the Seed-based d Mapping project (http://www.sdmproject.com) was used to perform the meta-analyses.38 Based on ALE, this analysis combines statistical parametric t-maps and peak coordinates of clusters from multiple studies to increase statistical power.38 Effect-size brain maps and variances are derived from reported t-statistics. The full width at half maximum (FWHM) in SDM was set at the default (20 mm) to control for false positives.38 To optimally balance sensitivity and specificity, resulting statistical maps were thresholded at P = .005 to control for family-wise error rate.38 Jackknife sensitivity analyses were performed to assess the replicability of suprathreshold clusters. ES-SDM jackknife analyses reiterate the meta-analyses as many times as the number of studies and remove one study at a time outputting a percentage that signifies the strength of replicability. For illustration, ES-SDM values were overlaid onto an anatomical template normalized to MNI space using Mango image viewer software (http://rii.uthscsa.edu/mango/mango.html).
Results
Descriptive Results
Sixteen articles were eligible for inclusion in the meta-analyses. Table 1 summarizes participant demographics, task type, group type, and contrast type. Data from a total of 269 participants were included in the meta-analyses; 8 articles reported contrasts from early onset schizophrenia (118 participants; 86 males; mean age = 15.85; SD = 2.01), and 8 articles reported contrasts from schizophrenia relatives (151 participants; 85 males; mean age = 15.20; SD = 1.98).
Table 1.
Information on Source Datasets Included in the Meta-analyses
| Article | N | Male | Hand (R) | Mean (SD); Range | Task Type | Foci | Group Type | Contrast Typea |
|---|---|---|---|---|---|---|---|---|
| Bakshi et al., 201193 | 19 | 12 | NA | 14.3(3.1); 8–20 | n-back | 2 | SCZ-Rel | + |
| 2 | SCZ-Rel | + | ||||||
| Barbour et al., 201294 | 19 | 14 | NA | 14.71; 10–20 | Rating pictures | 1 | SCZ-Rel | + |
| 2 | SCZ-Rel | − | ||||||
| Bittner et al., 201534 | 17 | 11 | 13 | 17.9; 15.1–19.9 | Delayed discrimination | 7 | EOS | + |
| 2 | EOS | − | ||||||
| 11 | EOS | − | ||||||
| 3 | EOS | − | ||||||
| Diwadkar et al., 2011a29 | 19 | 12 | NA | 14.16(2.85); 8–19 | n-back | 6 | SCZ-Rel | − |
| Diwadkar et al., 2011ba31 | 18 | 5 | NA | 14(3.1); 8–19 | Visual memory | 3 | SCZ-Rel | − |
| Diwadkar et al., 201230 | 19 | 12 | NA | 14.3; 8–19 | Visual memory | 2 | SCZ-Rel | + |
| Hart et al., 201395 | 21 | 11 | 19 | 14.4(2.56); 9–18 | Oddball | 4 | SCZ-Rel | + |
| 1 | SCZ-Rel | + | ||||||
| 14 | SCZ-Rel | + | ||||||
| 10 | SCZ-Rel | − | ||||||
| 2 | SCZ-Rel | − | ||||||
| 1 | SCZ-Rel | − | ||||||
| 7 | SCZ-Rel | − | ||||||
| Kyriakopoulos et al., 201296 | 25 | 14 | 24 | 16.1(1.5); 12–19 | n-back | 13 | EOS | + |
| Pauly et al., 200833 | 12 | 12 | All | 17.5(0.7) | Visual memory | 3 | EOS | + |
| 8 | EOS | + | ||||||
| 1 | EOS | + | ||||||
| 7 | EOS | − | ||||||
| 1 | EOS | − | ||||||
| 5 | EOS | − | ||||||
| Rajarethinam et al., 201197 | 15 | 7 | All | 15.9(3.1) | Comprehension | 5 | SCZ-Rel | − |
| Seiferth et al., 200998 | 12 | 12 | All | 17.8(1.4) | Discrimination | 1 | EOS | + |
| 4 | EOS | + | ||||||
| 1 | EOS | + | ||||||
| 3 | EOS | − | ||||||
| 18 | EOS | − | ||||||
| 3 | EOS | − | ||||||
| 3 | EOS | − | ||||||
| Thermenos et al., 200799 | 21 | 12 | 18 | 19.9(4); 13–28 | Elaborative encoding | 2 | SCZ-Rel | + |
| Urben et al., 2016100 | 6 | 5 | All | 16.5(1.05) | Verbal fluency | 15 | EOS | + |
| Wagshal et al., 2014101 | 10 | 5 | All | 12.6(2.32); 8–16 | Weather prediction | 6 | EOS | + |
| 5 | EOS | + | ||||||
| 17 | EOS | + | ||||||
| 9 | EOS | − | ||||||
| 6 | EOS | − | ||||||
| 1 | EOS | − | ||||||
| White et al., 2011a35 | 14 | 12 | NA | 13.4(2.6) | Sternberg | 1 | EOS | − |
| White et al., 2011b102 | 22 | 15 | 17 | 15(2.8) | Sternberg | 10 | EOS | + |
| 11 | EOS | + | ||||||
| 4 | EOS | + | ||||||
| 4 | EOS | + | ||||||
| 6 | EOS | + | ||||||
| 3 | EOS | − |
Note: n = sample size; R = Right-handed; NA = not available; SCZ-Rel = Relative or relative of Schizophrenia patient; EOS = Early onset schizophrenia; aschizophrenia minus healthy controls group (+), healthy controls group minus schizophrenia (−).
ALE Maps
ES-SDM38 adopts features of ALE and incorporates statistical values (t-statistic) of peak coordinates to estimate significant clusters. ALE maps illustrated on figure 2 show suprathreshold clusters associated with adolescents with early onset schizophrenia, with all coordinates listed on table 2. Specifically, analysis on adolescents with early onset schizophrenia revealed reliable hyperactivity compared to controls within the left precuneus (Brodmann area [BA] 23), left inferior frontal gyrus (BA 45), and right cerebellum, wheras hypoactivity included the left fusiform gyrus (BA 19) extending to the cerebellum and right precuneus (BA 5).
Fig. 2.
2-D and 3-D images showing suprathreshold clusters associated with hyper- and hypo-activation of adolescents with early onset schizophrenia.
Table 2.
Significant Regions of Activation for (A) EOS > HC and (B) EOS < HC (Thresholded at P < .005; FWHM 20 mm)
| Region | BAa | x | y | z | SDM-Z | P | Voxelsb | Jackknife |
|---|---|---|---|---|---|---|---|---|
| (A) EOS > HC | ||||||||
| L Precuneus | 23 | −4 | −60 | 28 | 2.599 | 1.217e-4 | 433 | 100c |
| L Inferior frontal gyrus | 45 | −52 | 38 | 12 | 2.169 | 1.035e-3 | 290 | 57 |
| R Cerebellum | 10 | −80 | −22 | 2.482 | 2.270e-4 | 147 | 86c | |
| (B) EOS < HC | ||||||||
| L Fusiform gyrus /cerebellum | 19 | −38 | −68 | −12 | −1.614 | 4.577e-4 | 709 | 77c |
| R Precuneus | 5 | 4 | −50 | 46 | −1.462 | 1.076e-3 | 164 | 77c |
Note: EOS = early onset schizophrenia patients; HC = healthy controls; BA = Brodmann area; apeak coordinates with overlapping BA areas (in brackets); b2 mm × 2 mm × 2 mm; SDM-Z = signed differential mapping z-score; Jackknife replicability is represented as percentage; L = Left; R = Right; foci represented in MNI space; cregions greater than 75% replicability.
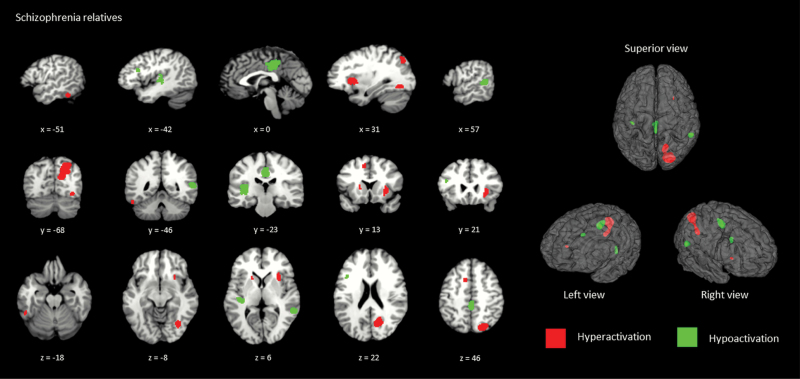
ALE maps illustrated on figure 3 show suprathreshold clusters associated with adolescents who had a parent diagnosed with schizophrenia, with all coordinates listed on table 3. The meta-analysis on adolescents who had a parent with schizophrenia showed concordance (ie, agreement across studies) based on hyperactivations in the right precuneus (BA 7/19) and right superior longitudinal fasciculus III extending to the insula. Concordance based on hypo-activations was observed in the right cingulate (BA 23), left insula (BA 13/48), and right middle temporal gyrus (BA 22).
Fig. 3.
2-D and 3-D images showing suprathreshold clusters associated with hyper- and hypo-activation of relatives with schizophrenia compared to controls.
Table 3.
Significant Regions of Activation for (A) SCZ-Rel > HC and (B) SCZ-Rel < HC (Thresholded at P < .005; FWHM 20 mm)
| Region | BAa | x | y | z | SDM-Z | P | Voxelsb | Jackknife |
|---|---|---|---|---|---|---|---|---|
| (A) SCZ-Rel > HC | ||||||||
| R Precuneus | 7/19 | 16 | −74 | 36 | 1.523 | 2.017e-4 | 887 | 77c |
| R Longitudinal fasciculus /insula | 28 | 16 | 6 | 1.115 | 3.373e-3 | 130 | 55 | |
| (B) SCZ-Rel < HC | ||||||||
| R Posterior cingulate gyrus | 23 | 2 | −24 | 34 | −1.364 | 2.083e-3 | 344 | 77c |
| L Insula | 13(48) | −32 | −24 | 4 | −1.387 | 1.835e-3 | 183 | 77c |
| R Middle temporal gyrus | 22 | 56 | −44 | 8 | −1.428 | 1.403e-3 | 149 | 77c |
Note: SCZ-Rel = adolescent relatives of schizophrenia patients; HC = healthy controls; BA = Brodmann area; apeak coordinates with overlapping BA areas (in brackets); b2 mm × 2mm × 2mm; SDM-Z = signed differential mapping z-score; Jackknife replicability is represented as percentage; L = Left; R = Right; foci represented in MNI space; cregions greater than 75% replicability.
Jackknife Analyses
To test the robustness of significant clusters, we used ES-SDM jackknife sensitivity analyses that reiterate the meta-analyses as many times as the number of studies and remove one study at a time outputting a percentage that signifies the strength of replicability. Results show that all clusters were highly replicable with more than 75% replicability, with the exception of the left inferior frontal gyrus that shows increased implication for adolescents with early onset schizophrenia compared to healthy controls (replicability 57%), and the right longitudinal fasciculus that showed increased implication to adolescents with relatives with schizophrenia compared to healthy controls (replicability 55%).
Discussion
Schizophrenia symptoms consist of disabling abnormalities in cognitive abilities such as deficits in logical sequences and loose associations. Functional neuroimaging studies use event-related cognitive paradigms to evaluate brain correlates of these processes. We investigated concordance across studies that examined brain responses to cognitive abilities in youth with early onset schizophrenia and youth with a parent diagnosed with schizophrenia. We highlight four main findings. First, in the prefrontal cortex, we observe clusters in ventrolateral prefrontal regions, rather than dorsolateral prefrontal regions. Second, the precuneus showed an altered activity compared to control participants for both groups affected by schizophrenia. Third, an altered implication of the cerebellum was observed for youth with early onset schizophrenia, whereas the implication of the insula was altered only for the youth with parents with schizophrenia. Fourth, the posterior cingulate and middle temporal gyrus showed reduced implications for youth with parents with schizophrenia.
The prefrontal cortex is well known for its dysfunction in adults with schizophrenia.2,41,42 Critically, the prefrontal cortex is a large cortical region making 12.51% of the total brain volume.43 The results revealed a cluster within the left inferior frontal gyrus demonstrating increased implication in adolescents with early onset schizophrenia compared to controls, with weak reliability. The inferior frontal gyrus has been ascribed with many cognitive functions such as working memory,28,44 inhibitory control,45–47 negative priming,48 and voluntary risky decision-making.49,50 Thus, according to the hierarchical model of prefrontal function, the inferior frontal gyrus does not underlie a single cognitive process but instead it is common to many processes that have a generic common mental activity requirement for processing a few items in mind.51 Notably, no suprathreshold concordance in the dorsolateral prefrontal cortex is observed for any of the performed meta-analyses. Unlike the inferior frontal gyrus, the dorsolateral prefrontal cortex consists mainly of the middle frontal gyrus, which is associated with processing problems of higher abstraction and complexity51 and is one of the last areas to mature in humans.10 The absence of reduced (or increased) involvement of dorsolateral areas may be related to incomplete maturation of the prefrontal cortex during adolescence. This finding is consistent with past meta-analyses with children and adolescents, which suggests that increased variability in the implication of prefrontal regions results in discordant findings for these age groups.28,52 This may be attributed to the fact that adolescent participants in studies on early onset schizophrenic were medicated. There is some evidence to suggest that antipsychotic medication may impact prefrontal cortical activity and cortical thickness, with some differences between first- and second-generation antipsychotics.53,54 Hence, the differential activation observed between the medicated and unmedicated groups may be at least partly artefactual.
Differences in activation between groups affected by schizophrenia and control groups were observed within the insula and cerebellum. Although less widely discussed, the insula and the cerebellum play a key role in cognitive abilities such as working memory in children28 and adults.24 In the current findings, hemispheric asymmetry in the cerebellum and insula was observed for adolescents with schizophrenia and adolescents with relatives with schizophrenia, respectively. Specifically, the insula shows reduced implication in the left hemisphere and increased implication in the right hemisphere for adolescents with relatives with schizophrenia. Anatomically, the insula is located deep in the gyri that connect the frontal and temporal regions. Functionally, in addition to interoceptive feeling,55 the insula has been associated with various mental functions such as working memory,44 different types of inhibition,47 emotional valence,56 and reward anticipation,57 to which the insula has its own topographic map.58 Perhaps the altered insula implication in adolescent relatives of schizophrenia may relate to the differences in motivational aspects of cognitive processes.
The cerebellum has been associated with motor coordination and temporal sequencing of cognitive information,28,44 an important aspect of cognitive function that requires visuomotor sequencing of various actions. Meta-analyses of adult studies addressing the role of the cerebellum in schizophrenia have discussed its dysfunction in tasks of working memory and emotion.59,60 We observed increased implication of the right cerebellum and a decreased implication of the left cerebellum for youth with early onset schizophrenia. The fact that the cerebellum is observed in the early onset group rather than the youth with relatives with schizophrenia may mark it as a target area for future studies of this disorder. Notably, laterality differences in both the insula and cerebellum serve as reference points for future research.
Posterior brain regions in the precuneus (BA 19) and posterior cingulate (BA 23) were also found to differ between groups affected by schizophrenia and control groups. The precuneus, within the parietal cortex, is bordered medially by the posterior cingulate cortex and ventrally to the fusiform gyrus. BA 19 extends to the posterior parts of the precuneus and fusiform gyri, and BA 23 covers medial parts of the precuneus and the posterior cingulate cortex. The analysis revealed that BA 23 is increased for adolescents with early onset schizophrenia whereas this region is reduced for adolescents with relatives with schizophrenia. Medial parts of the precuneus and posterior cingulate gyrus have been associated with decision-making in reward processing61 and self-directed thinking associated with the default-mode network in the absence of a task in healthy individuals.62 Notably, these networks are negatively related in healthy adults (ie, as brain areas of one network increase in activity, brain areas of the other network decrease in activity44,63), yet are positively related in healthy children,64 suggesting that there is a transformation in the processing of self-referential thinking between childhood and adulthood,65 with profound lateralization of effective connections.66 Interestingly, the default-mode network in schizophrenia patients and relatives of patients with schizophrenia seems to be affected,67,68 thereby establishing further evidence that the precuneus may be associated with internally driven thoughts in early onset schizophrenia and relatives of patients. An increased functional implication in this region for adolescents with early onset schizophrenia may be driven by medication,53,54 as a side effect of overcompensation, similar perhaps to the insula and inferior frontal gyrus, discussed above.
Posterior parts of the cortex (ie, BA 19 in either hemisphere) are associated with visual-spatial processes as they diverge into either the ventral stream that leads to the temporal lobe or the dorsal stream that leads to the parietal lobe69,70 into the “what” and “where” pathways, respectively.71 BA 19 in the parietal cortex, found in the right hemisphere, shows increased implication for adolescents with relatives with schizophrenia. BA 19 in the fusiform gyrus has reduced implication for adolescents with early onset schizophrenia. Because this area is not in the same hemisphere, it may be less likely for this effect to be attributed to medication. An alternative explanation for this reverse effect could be strategy differences based on the visual-spatial requirements of the task.
The right middle temporal gyrus showed reduced implication for adolescents with relatives with schizophrenia, compared to controls. In the left hemisphere, the middle temporal gyrus is associated with language and semantic processing72–74; however, the function of the right middle temporal gyrus has been associated with processing saccades,75 processing emotions and social cognition,76–78 and moral judgments.79 In schizophrenia, the right middle temporal gyrus is associated with retrospective and prospective self-referential thinking,80 and volumetric studies show gray matter abnormalities in patients with schizophrenia81 and those with first-episode schizophrenia,82 which may explain reduce implication of this region.
Limitations
Brain regions identified in these meta-analyses show convergence across published studies that reported experiments on brain responses to various cognitive tasks in adolescents with and without association to schizophrenia (ie, adolescents with a relative with schizophrenia and diagnosis of early onset schizophrenia compared to healthy controls). Brain activation to cognitive tasks inherently involves varying degrees of attention and task difficulty that was not considered in the current study due to an insufficient number of studies by task type. We also note that we only considered peer-reviewed published studies and recognized that results may be prone to type II errors as meta-analyses are exposed to publication bias. Moreover, as with any fMRI meta-analyses, interpretation of findings is liable to reverse inference because secondary analyses cannot manipulate experimental conditions as in original experiments. Although it is common to observe variability in methodological choices of original experiments, meta-analyses have proven to be a valuable tool for integrating many datasets to establish converging patterns among data.83
Recommendations for Future Studies
fMRI meta-analyses are useful at least in two fundamental ways: to identify overarching patterns of results reported in the literature and to identify methodological and reporting issues in the current literature that can improve for future meta-analyses. We identify several points that we experienced in performing these meta-analyses and make related recommendations for future research. First, it is important for future work to report whole-brain coordinates, at least as supplementary material, because a substantial number of studies report only region of interest analyses and had to be excluded from the meta-analyses. Second, it is useful to include behavioral indices associated with the performance of each group as this would allow future meta-analysis to examine coordinates attributed to dysfunction of specific cognitive processes. Although practically difficult, it would be immensely useful to examine cognitive function before medication onset, which would allow for the investigation of the unmedicated state of the disorder. To date, the meta-analyses performed in the current study and prior studies8,9,84 have been performed using between subjects (eg, group affected by schizophrenia > control group). We recommend future studies to report coordinates of within-group results, if possible as supplementary material as this would allow for performing within-group meta-analyses to identify overarching patterns of concordance. Currently, this option is not possible because most studies report between-subject contrasts. Hence, our recommendation is for future studies to report foci corresponding to within-group contrasts as well as between-group contrasts to allow researchers to compare convergence of brain activation derived from both types of experimental design.85 Overall, more research is needed with youth affected by schizophrenia and we feel optimistic that knowledge gained in this direction would help improve early treatment options for affected individuals.
Conclusions
Schizophrenia affects less than 1% of the population worldwide; however, its social costs are staggering as measured by Disability Adjusted Life Years Lost (DALY).86 Specifically, the cost of schizophrenia to the U.S. economy amounts to about $62 billion per year, and the illness is associated with a reduced life expectancy of about 25 years as a result of various associated problems, including suicide, cancer, or heart disease.87–89 Developing a clearer profile of biomarkers at an earlier point before frank illness onset, and before the neurological picture becomes more complicated due to medication, may facilitate a more accurate differential diagnosis, early intervention, and relapse prevention. Our research demonstrates different patterns when adolescents are relatives to patients with schizophrenia and those who have received a diagnosis for early onset of the disease and are on medication. Specifically, our key findings highlight the altered implication of the parietal cortex in both groups affected by schizophrenia; however, the insula and cerebellum are altered only for adolescents with relatives with schizophrenia and adolescents with early onset schizophrenia, respectively. These findings support the clinical notion that neural expressions of the disease are affected by a multiplicity of factors that may benefit from target investigations of marker brain regions. To date, identifying biomarkers for psychiatric illnesses including schizophrenia has been elusive; however, ongoing research in this field is being conducted.90,91 Schizophrenia is considered to be a heterogeneous disorder90 associated with limited diagnostic reliability.92 These issues will make identification of biomarkers an exceptionally challenging process. However, practically, our results provide a set of stereotaxic coordinates that can serve as a neurofunctional atlas for future region-of-interest studies. Considerably more research is needed in understanding brain correlates that may be aberrant in those at risk of converting, in order to eventually be able to accurately distinguish these participants from healthy controls and those with first-episode schizophrenia.
Funding
This work was in part supported by the Russian Foundation for Basic Research grants (ofi-m 17-29-02518) (Neuroimmunological status and basic cognitive-affective structures of the human brain in norm and in patients with schizophrenia).
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2. Callicott JH, Bertolino A, Mattay VS, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10(11):1078–1092. [DOI] [PubMed] [Google Scholar]
- 3. Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160(12):2209–2215. [DOI] [PubMed] [Google Scholar]
- 4. Glahn DC, Ragland JD, Abramoff A, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25(1):60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol. 2005;1:321–353. [DOI] [PubMed] [Google Scholar]
- 7. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “Just the Facts”: what we know in 2008. Schizophr Res. 2008;100(1–3):4–19. [DOI] [PubMed] [Google Scholar]
- 8. Goghari VM. Executive functioning-related brain abnormalities associated with the genetic liability for schizophrenia: an activation likelihood estimation meta-analysis. Psychol Med. 2011;41(6):1239–1252. [DOI] [PubMed] [Google Scholar]
- 9. Zhang R, Picchioni M, Allen P, Toulopoulou T. Working memory in unaffected relatives of patients with schizophrenia: a meta-analysis of functional magnetic resonance imaging studies. Schizophr Bull. 2016;42(4):1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U. S. A. 2004;101(21):8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gur RE, Loughead J, Kohler CG, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch Gen Psychiatry. 2007;64(12):1356–1366. [DOI] [PubMed] [Google Scholar]
- 12. Smieskova R, Marmy J, Schmidt A, et al. Do subjects at clinical high risk for psychosis differ from those with a genetic high risk? – a systematic review of structural and functional brain abnormalities. Curr Med Chem. 2013;20(3):467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keshavan M, Tandon R, Boutros N, Nasrallah H. Schizophrenia, “just the facts”: what we know in 2008. Part 3: Neurobiology. Schizophr Res. 2008;106(2–3):89–107. [DOI] [PubMed] [Google Scholar]
- 14. Niu Y, Li Z, Cheng R, Peng B, Liu B, Ma Y. Altered gray matter and brain activity in patients with schizophrenia and their unaffected relatives: a multimodal meta-analysis of voxel-based structural MRI and resting-state fMRI studies. Int J Clin Exp Med. 2017;10(2):1866–1878. [Google Scholar]
- 15. Cabral J, Fernandes HM, Van Hartevelt TJ, James AC, Kringelbach ML, Deco G. Structural connectivity in schizophrenia and its impact on the dynamics of spontaneous functional networks. Chaos. 2013;23(4):046111. [DOI] [PubMed] [Google Scholar]
- 16. Wang C, Ji F, Hong Z, et al. Disrupted salience network functional connectivity and white-matter microstructure in persons at risk for psychosis: findings from the LYRIKS study. Psychol Med. 2016;46(13):2771–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang S, Zhan Y, Zhang Y, et al. Abnormal functional connectivity strength in patients with adolescent-onset schizophrenia: a resting-state fMRI study. Eur Child Adolesc Psychiatry. 2017;26(7):839–845. [DOI] [PubMed] [Google Scholar]
- 18. Wang S, Zhan Y, Zhang Y, et al. Abnormal long- and short-range functional connectivity in adolescent-onset schizophrenia patients: a resting-state fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:445–451. [DOI] [PubMed] [Google Scholar]
- 19. Wang X, Zhang Y, Long Z, et al. Frequency-specific alteration of functional connectivity density in antipsychotic-naive adolescents with early-onset schizophrenia. J Psychiatr Res. 2017;95:68–75. [DOI] [PubMed] [Google Scholar]
- 20. Bois C, Ronan L, Levita L, et al. Cortical surface area differentiates familial high risk individuals who go on to develop schizophrenia. Biol Psychiatry. 2015;78(6):413–420. [DOI] [PubMed] [Google Scholar]
- 21. Radua J, Borgwardt S, Crescini A, et al. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci Biobehav Rev. 2012;36(10):2325–2333. [DOI] [PubMed] [Google Scholar]
- 22. Fusar-Poli P, Borgwardt S, Crescini A, et al. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011;35(5):1175–1185. [DOI] [PubMed] [Google Scholar]
- 23. Bersani FS, Minichino A, Fojanesi M, et al. Cingulate cortex in schizophrenia: its relation with negative symptoms and psychotic onset. A review study. Eur Rev Med Pharmacol Sci. 2014;18(22):3354–3367. [PubMed] [Google Scholar]
- 24. Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25(1):46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rottschy C, Langner R, Dogan I, et al. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage. 2012;60(1):830–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arsalidou M, Im-Bolter N. Why parametric measures are critical for understanding typical and atypical cognitive development. Brain Imaging Behav. 2017;11(4):1214–1224. [DOI] [PubMed] [Google Scholar]
- 27. Arsalidou M, Pascual-Leone J, Johnson J. Misleading cues improve developmental assessment of working memory capacity: the color matching tasks. Cogn Dev. 2010;25(3):262–277. [Google Scholar]
- 28. Yaple Z, Arsalidou M. N-back working memory task: meta-analysis of normative fMRI studies with children. Child Dev. 2018;89(6):2010–2022. [DOI] [PubMed] [Google Scholar]
- 29. Diwadkar VA, Pruitt P, Goradia D, et al. Fronto-parietal hypo-activation during working memory independent of structural abnormalities: conjoint fMRI and sMRI analyses in adolescent offspring of schizophrenia patients. Neuroimage. 2011;58(1):234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Diwadkar VA, Pruitt P, Zhang A, et al. The neural correlates of performance in adolescents at risk for schizophrenia: inefficiently increased cortico-striatal responses measured with fMRI. J Psychiatr Res. 2012;46(1):12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Diwadkar VA, Segel J, Pruitt P, et al. Hypo-activation in the executive core of the sustained attention network in adolescent offspring of schizophrenia patients mediated by premorbid functional deficits. Psychiatry Res Neuroimaging 2011b;192(2):91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li X, Xiao YH, Zhao Q, Leung AW, Cheung EF, Chan RC. The neuroplastic effect of working memory training in healthy volunteers and patients with schizophrenia: implications for cognitive rehabilitation. Neuropsychologia. 2015;75:149–162. [DOI] [PubMed] [Google Scholar]
- 33. Pauly K, Seiferth NY, Kellermann T, et al. Cerebral dysfunctions of emotion–cognition interactions in adolescent-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2008;47(11):1299–1310. [DOI] [PubMed] [Google Scholar]
- 34. Bittner RA, Linden DE, Roebroeck A, et al. The when and where of working memory dysfunction in early-onset schizophrenia – a functional magnetic resonance imaging study. Cereb Cortex. 2015;25(9):2494–2506. [DOI] [PubMed] [Google Scholar]
- 35. White T, Hongwanishkul D, Schmidt M. Increased anterior cingulate and temporal lobe activity during visuospatial working memory in children and adolescents with schizophrenia. Schizophr Res. 2011a;125(2–3): 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30(9):2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eickhoff SB, Laird AR, Fox PM, Lancaster JL, Fox PT. Implementation errors in the GingerALE Software: description and recommendations. Hum Brain Mapp. 2017;38(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Radua J, Mataix-Cols D, Phillips ML, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry. 2012;27(8):605–611. [DOI] [PubMed] [Google Scholar]
- 39. Eickhoff SB, Bzdok D. Meta-analyses in basic and clinical neuroscience: state of the art and perspective. In: Ulmer S, Jansen O, (eds.), fMRI. Berlin, Heidelberg:Springer; 2013:77–87. [Google Scholar]
- 40. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 41. Quintana J, Wong T, Ortiz-Portillo E, et al. Prefrontal-posterior parietal networks in schizophrenia: primary dysfunctions and secondary compensations. Biol Psychiatry. 2003;53(1):12–24. [DOI] [PubMed] [Google Scholar]
- 42. Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. Prefrontal activation deficits during episodic memory in schizophrenia. Am J Psychiatry. 2009;166(8):863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McBride T, Arnold SE, Gur RC. A comparative volumetric analysis of the prefrontal cortex in human and baboon MRI. Brain, Behav Evol. 1999;54(3):159–166. [DOI] [PubMed] [Google Scholar]
- 44. Arsalidou M, Pascual-Leone J, Johnson J, Morris D, Taylor MJ. A balancing act of the brain: activations and deactivations driven by cognitive load. Brain Behav. 2013;3(3):273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50(3):1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hampshire A, Sharp DJ. Contrasting network and modular perspectives on inhibitory control. Trends Cogn Sci. 2015;19(8):445–452. [DOI] [PubMed] [Google Scholar]
- 47. Hung Y, Gaillard SL, Yarmak P, Arsalidou M. Dissociations of cognitive inhibition, response inhibition, and emotional interference: voxelwise ALE meta-analyses of fMRI studies. Hum Brain Mapp. 2018;39(10):4065–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yaple Z, Arsalidou M. Negative priming: a meta-analysis of fMRI studies. Exp Brain Res. 2017;235(11):3367–3374. [DOI] [PubMed] [Google Scholar]
- 49. Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA. Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI Study of the Balloon Analog Risk Task (BART). Neuroimage. 2008;42(2):902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yaple Z, Martinez-Saito M, Awasthi B, Feurra M, Shestakova A, Klucharev V. Transcranial alternating current stimulation modulates risky decision making in a frequency-controlled experiment. eNeuro. 2017;4(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Christoff K, Keramatian K, Gordon AM, Smith R, Mädler B. Prefrontal organization of cognitive control according to levels of abstraction. Brain Res. 2009;1286:94–105. [DOI] [PubMed] [Google Scholar]
- 52. Arsalidou M, Pawliw-Levac M, Sadeghi M, Pascual-Leone J. Brain areas associated with numbers and calculations in children: meta-analyses of fMRI studies. Dev Cogn Neurosci. 2018;30:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ansell BR, Dwyer DB, Wood SJ, et al. Divergent effects of first-generation and second-generation antipsychotics on cortical thickness in first-episode psychosis. Psychol Med. 2015;45(3):515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Artigas F. The prefrontal cortex: a target for antipsychotic drugs. Acta Psychiatr Scand. 2010;121(1):11–21. [DOI] [PubMed] [Google Scholar]
- 55. Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neurosci. 2015;16(7):419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Duerden EG, Arsalidou M, Lee M, Taylor MJ. Lateralization of affective processing in the insula. Neuroimage. 2013;78:159–175. [DOI] [PubMed] [Google Scholar]
- 57. Tsurumi K, Kawada R, Yokoyama N, et al. Insular activation during reward anticipation reflects duration of illness in abstinent pathological gamblers. Front Psychol. 2014;5:1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214(5–6):519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bernard JA, Mittal VA. Dysfunctional activation of the cerebellum in schizophrenia: a functional neuroimaging meta-analysis. Clin Psychol Sci. 2015;3(4):545–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mothersill O, Knee-Zaska C, Donohoe G. Emotion and theory of mind in schizophrenia-investigating the role of the cerebellum. Cerebellum. 2016;15(3):357–368. [DOI] [PubMed] [Google Scholar]
- 61. Sescousse G, Caldú X, Segura B, Dreher JC. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37(4):681–696. [DOI] [PubMed] [Google Scholar]
- 62. Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21(3):489–510. [DOI] [PubMed] [Google Scholar]
- 63. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U. S. A. 2005;102(27):9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chai XJ, Ofen N, Gabrieli JD, Whitfield-Gabrieli S. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J Cogn Neurosci. 2014;26(3):501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Arsalidou M, Sharaev MG, Kotova T, Martynova O. Commentary: selective development of anticorrelated networks in the intrinsic functional organization of the human brain. Front Hum Neurosci. 2017;11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ushakov V, Sharaev MG, Kartashov SI, Zavyalova VV, Verkhlyutov VM, Velichkovsky BM. Dynamic causal modeling of hippocampal links within the human default mode network: lateralization and computational stability of effective connections. Front Hum Neurosci. 2016;10:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van Buuren M, Vink M, Kahn RS. Default-mode network dysfunction and self-referential processing in healthy siblings of schizophrenia patients. Schizophr Res. 2012;142(1–3):237–243. [DOI] [PubMed] [Google Scholar]
- 68. Liu J, Corbera S, Wexler BE. Neural activation abnormalities during self-referential processing in schizophrenia: an fMRI study. Psychiatry Res. 2014;222(3):165–171. [DOI] [PubMed] [Google Scholar]
- 69. Mishkin M, Ungerleider LG. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav Brain Res. 1982;6(1):57–77. [DOI] [PubMed] [Google Scholar]
- 70. Zeki S, Marini L. Three cortical stages of colour processing in the human brain. Brain. 1998;121 (pt 9):1669–1685. [DOI] [PubMed] [Google Scholar]
- 71. Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. J Neurosci. 1994;14(11, pt 1):6336–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat. 2000;197(pt 3):335–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Binder JR, Frost JA, Hammeke TA, et al. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 2000;10(5):512–528. [DOI] [PubMed] [Google Scholar]
- 74. Wright TM, Pelphrey KA, Allison T, McKeown MJ, McCarthy G. Polysensory interactions along lateral temporal regions evoked by audiovisual speech. Cereb Cortex. 2003;13(10):1034–1043. [DOI] [PubMed] [Google Scholar]
- 75. Zhou Y, Fan L, Qiu C, Jiang T. Prefrontal cortex and the dysconnectivity hypothesis of schizophrenia. Neurosci Bull. 2015;31(2):207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Arsalidou M, Morris D, Taylor MJ. Converging evidence for the advantage of dynamic facial expressions. Brain Topogr. 2011;24(2):149–163. [DOI] [PubMed] [Google Scholar]
- 77. Müller VI, Höhner Y, Eickhoff SB. Influence of task instructions and stimuli on the neural network of face processing: an ALE meta-analysis. Cortex. 2018;103:240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Alcalá-López D, Smallwood J, Jefferies E, et al. Computing the social brain connectome across systems and states. Cereb Cortex. 2017;28(7):2207–2232. [DOI] [PubMed] [Google Scholar]
- 79. Bryant DJ, Deardeuff K, Zoccoli E, Nam CS. The neural correlates of moral thinking: a meta-analysis. IJCNE. 2016;3(2):28–39. [Google Scholar]
- 80. Fornara GA, Papagno C, Berlingeri M. A neuroanatomical account of mental time travelling in schizophrenia: a meta-analysis of functional and structural neuroimaging data. Neurosci Biobehav Rev. 2017;80:211–222. [DOI] [PubMed] [Google Scholar]
- 81. Wilke M, Kaufmann C, Grabner A, Pütz B, Wetter TC, Auer DP. Gray matter-changes and correlates of disease severity in schizophrenia: a statistical parametric mapping study. Neuroimage. 2001;13(5):814–824. [DOI] [PubMed] [Google Scholar]
- 82. Kuroki N, Shenton ME, Salisbury DF, et al. Middle and inferior temporal gyrus gray matter volume abnormalities in first-episode schizophrenia: an MRI study. Am J Psychiatry. 2006;163(12):2103–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U. S. A. 2016;113(28): 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhang R, Picchioni M, Allen P, Toulopoulou T. Working memory in unaffected relatives of patients with schizophrenia: a meta-analysis of functional magnetic resonance imaging studies. Schizophr Bull. 2016;42(4):1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Müller VI, Cieslik EC, Laird AR, et al. Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev. 2018;84:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. [DOI] [PubMed] [Google Scholar]
- 87. McFarlane WR. Prevention of the first episode of psychosis. Psychiatr Clin North Am. 2011;34(1):95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Parks J, Svendsen D, Singer P, Foti ME, Mauer B.. Morbidity and Mortality In People With Serious Mental Illness. Alexandria, VA: National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council; 2006: 1–87. [Google Scholar]
- 89. Wu EQ, Birnbaum HG, Shi L, et al. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry. 2005;66(9):1122–1129. [DOI] [PubMed] [Google Scholar]
- 90. Lai CY, Scarr E, Udawela M, Everall I, Chen WJ, Dean B. Biomarkers in schizophrenia: A focus on blood based diagnostics and theranostics. World J Psychiatry. 2016;6(1):102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Martins-de-Souza D. Biomarkers for psychiatric disorders: where are we standing? Dis Markers. 2013;35(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Freedman R, Lewis DA, Michels R, et al. The initial field trials of DSM-5: new blooms and old thorns. Am J Psychiatry. 2013;170(1):1–5. [DOI] [PubMed] [Google Scholar]
- 93. Bakshi N, Pruitt P, Radwan J, et al. Inefficiently increased anterior cingulate modulation of cortical systems during working memory in young offspring of schizophrenia patients. J Psychiatr Res. 2011;45(8):1067–1076. [DOI] [PubMed] [Google Scholar]
- 94. Barbour T, Pruitt P, Diwadkar VA. fMRI responses to emotional faces in children and adolescents at genetic risk for psychiatric illness share some of the features of depression. J Affect Disord. 2012;136(3):276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hart SJ, Bizzell J, McMahon MA, Gu H, Perkins DO, Belger A. Altered fronto-limbic activity in children and adolescents with familial high risk for schizophrenia. Psychiatry Res. 2013;212(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kyriakopoulos M, Dima D, Roiser JP, Corrigall R, Barker GJ, Frangou S. Abnormal functional activation and connectivity in the working memory network in early-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2012;51(9):911–20.e2. [DOI] [PubMed] [Google Scholar]
- 97. Rajarethinam R, Venkatesh BK, Peethala R, Phan KL, Keshavan M. Reduced activation of superior temporal gyrus during auditory comprehension in young offspring of patients with schizophrenia. Schizophr Res. 2011;130(1–3):101–105. [DOI] [PubMed] [Google Scholar]
- 98. Seiferth NY, Pauly K, Kellermann T, et al. Neuronal correlates of facial emotion discrimination in early onset schizophrenia. Neuropsychopharmacology. 2009;34(2): 477–487. [DOI] [PubMed] [Google Scholar]
- 99. Thermenos HW, Seidman LJ, Poldrack RA, et al. Elaborative verbal encoding and altered anterior parahippocampal activation in adolescents and young adults at genetic risk for schizophrenia using FMRI. Biol Psychiatry. 2007;61(4):564–574. [DOI] [PubMed] [Google Scholar]
- 100. Urben S, Jaugey L, Fornari E, et al. Comparisons of verbal fluency brain correlates between adults and adolescents suffering from schizophrenia spectrum disorders: a pilot study. Eur J Psychiatry. 2016;30(4):249–257. [Google Scholar]
- 101. Wagshal D, Knowlton BJ, Suthana NA, et al. Evidence for corticostriatal dysfunction during cognitive skill learning in adolescent siblings of patients with childhood-onset schizophrenia. Schizophr Bull. 2014;40(5):1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. White T, Schmidt M, Kim DI, Calhoun VD. Disrupted functional brain connectivity during verbal working memory in children and adolescents with schizophrenia. Cereb Cortex. 2011;21(3):510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]