Abstract
Motor abnormalities in schizophrenia spectrum disorders (SSD) have increasingly attracted scientific interest in the past years. However, the neural mechanisms underlying parkinsonism in SSD are unclear. The present multimodal magnetic resonance imaging (MRI) study examined SSD patients with and without parkinsonism, as defined by a Simpson and Angus Scale (SAS) total score of ≥4 (SAS group, n = 22) or <4 (non-SAS group, n = 22). Parallel independent component analysis (p-ICA) was used to examine the covarying components among gray matter volume maps computed from structural MRI (sMRI) and fractional amplitude of low-frequency fluctuations (fALFF) maps computed from resting-state functional MRI (rs-fMRI) patient data. We found a significant correlation (P = .020, false discovery rate [FDR] corrected) between an sMRI component and an rs-fMRI component, which also significantly differed between the SAS and non-SAS group (P = .042, z = −2.04). The rs-fMRI component comprised the cortical sensorimotor network, and the sMRI component included predominantly a frontothalamic/cerebellar network. Across the patient sample, correlations adjusted for the Positive and Negative Syndrome Scale (PANSS) total scores showed a significant relationship between tremor score and loadings of the cortical sensorimotor network, as well as between glabella-salivation score, frontothalamic/cerebellar and cortical sensorimotor network loadings. These data provide novel insights into neural mechanisms of parkinsonism in SSD. Aberrant bottom-up modulation of cortical motor regions may account for these specific motor symptoms, at least in patients with SSD.
Keywords: schizophrenia, parkinsonism, MRI, motor abnormalities
Introduction
Over the last 2 decades, research on motor abnormalities in schizophrenia spectrum disorders (SSD) has witnessed a renaissance, with the majority of neuroimaging studies investigating neurological soft signs (NSS), tardive dyskinesia (TD), and catatonia.1–7 In addition to these phenomena, a substantial proportion of both antipsychotic-naïve (median prevalence of 17%) and antipsychotic-treated (prevalence varies from 15% to 30%) SSD patients, independent of medication effects, experience parkinsonism, characterized by rigidity, tremor, bradykinesia, and occasionally positive glabellar tap sign or increased salivation.8,9 Using a cutoff of ≥4 on the total Simpson and Angus Scale (SAS),10 recent studies established a clinically meaningful and scientifically relevant distinction between SSD patients with and without parkinsonism.8,9,11 However, the neuroimaging evidence on parkinsonism is scarce.12
In this study, we employed 2 distinct neuroimaging modalities to specifically investigate function-structure interrelationships which are related to parkinsonism.3,13 Thus, in addition to gray matter volume (GMV), we incorporated resting-state functional magnetic resonance imaging (rs-fMRI) and particularly the fractional amplitude of low-frequency fluctuations (fALFF), as fALFF captures the relative magnitude of blood-oxygenation-level-dependent (BOLD) signal changes on intrinsic neural activity (INA) in specific brain regions. While previous studies have already shown that INA is abnormal in catatonia,14,15 we expected that the study of SSD patients with and without parkinsonism would provide further insight into an abnormal local function within selective networks responsible for motor excitation/inhibition and psychomotor organization.16
This study had 2 major objectives: First, conducting a categorical approach, we predicted a difference in both modality-specific (ie, GMV or INA) and transmodal (ie, GMV and INA) systems comprising frontoparietal and frontostriatal networks between SSD patients with and without parkinsonism. Second, acknowledging the dimensional nature of motor symptoms in SSD,4 we expected that distinct dimensions of parkinsonism—ie, hypokinesia, tremor, rigor, glabella/salivation—will be significantly associated with abnormal brain structure and function that can be revealed by transmodal components in distinct cortico-subcortical networks, as involved in motor excitation/inhibition and psychomotor organization and speed.
Methods
Participants
We examined a total of 87 right-handed17 patients meeting Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition-Text Revision (DSM-IV-TR)18 criteria for schizophrenia (n = 84) and schizoaffective disorder (n = 3).1,3 The inclusion and exclusion criteria are listed in the Supplementary Material. The local Ethics Committee (Medical Faculty at Heidelberg University, Germany) approved the study. Written informed consent was obtained from all SSD patients after all aims and procedures of the study had been fully explained.
Clinical Assessment
All patients were recruited and examined within 1 week after partial remission of psychotic symptoms. The duration between psychometric testing, motor assessment, and MRI examination was less than 3 days. At the time of examination, none of the SSD patients had taken benzodiazepines or anticholinergic medication, and all patients were on a stable antipsychotic medication for at least 2 weeks (for details on antipsychotic medication see Supplementary Material). Daily doses of antipsychotic medication were converted to olanzapine (OLZ) equivalents, according to the classical mean dose method.19 For a detailed assessment of parkinsonism, we used the SAS10 (for details on SAS domains, see Supplementary Material). The SAS criteria, according to Cuesta and colleagues,8 were used to identify a clear cutoff to distinguish subjects with (SAS total score ≥ 4) and without (SAS < 4) parkinsonism (SAS and non-SAS group). We excluded 43 SSD individuals from the original patient group (87−43 = 44) to introduce 2 well-balanced (in terms of age, gender, education, and OLZ) groups of SSD patients with (n = 22) and without (n = 22) parkinsonism (categorical approach) (table 1). The patient groups were carefully matched with respect to gender and education because (1) previous gender-specific studies on motor symptoms in SSD showed contradictory results, (2) patients with higher education can recruit alternative brain networks to compensate for aberrant motor functioning, (3) educational status might influence motor performance in Parkinson’s disease (PD), and (4) education-associated brain structure and function are more resilient to illness-related abnormalities, respectively.20–23 In the next step, we followed a correlative approach, assuming dimensional symptom expression as well as a neurobiological continuum in SSD patients with various degrees of parkinsonism (n = 44).24
Table 1.
Demographics and Clinical Scores for Schizophrenia Spectrum Disorders Patients Divided Into SAS and SAS Groups
| Variable | non-SAS (n = 22)mean ± SD | SAS (n = 22)mean ± SD | t a | df | Significance |
|---|---|---|---|---|---|
| Age | 38.64 ± 12.96 | 40.41 ± 11.38 | −0.482 | 42 | .632 |
| Gender (m/f)b | 14/8 | 14/8 | 1 | 1 | |
| Education (y) | 13.64 ± 2.80 | 13.36 ± 3.63 | 0.279 | 42 | .782 |
| Olanzapine equivalents | 15.81 ± 10.34 | 19.17 ± 9.56 | −1.118 | 42 | .27 |
| Duration of illness (y) | 11.36 ± 11.37 | 15.50 ± 10.87 | −1.233 | 42 | .224 |
| PANSS | |||||
| Positive | 14.22 ± 5.96 | 14.27 ± 6.42 | −0.024 | 42 | .981 |
| Negative | 15.00 ± 6.28 | 18.41 ± 7.82 | −1.594 | 42 | .118 |
| Global | 30.68 ± 6.45 | 35.09 ± 7.71 | −2.056 | 42 | .046* |
| Total | 59.91 ± 14.23 | 67.77 ± 15.51 | −1.752 | 42 | .087 |
| SAS | |||||
| Hypokinesia | 0.32 ± 0.48 | 0.91 ± 0.29 | −4.947 | 42 | <.001* |
| Rigidity | 0.14 ± 0.47 | 2.60 ± 2.42 | −4.665 | 42 | <.001* |
| Tremor | 0.36 ± 0.49 | 0.82 ± 0.79 | −2.28 | 42 | .028* |
| Glabella-salivation | 0.45 ± 0.21 | 1.22 ± 0.97 | −5.567 | 42 | <.001* |
| Total | 0.77 ± 0.81 | 5.50 ± 2.17 | −9.543 | 42 | <.0001* |
| NCRS | |||||
| Motor | 0.27 ± 0.55 | 1.27 ± 1.60 | −2.758 | 42 | .009* |
| Affective | 1.68 ± 1.91 | 1.73 ± 1.51 | −0.087 | 42 | .931 |
| Behavioral | 0.32 ± 0.71 | 1.00 ± 1.34 | −2.098 | 42 | .042* |
| Total | 2.14 ± 2.39 | 3.91 ± 3.55 | −1.938 | 42 | .059 |
| AIMS | |||||
| Total | 0.86 ± 2.33 | 1.91 ± 3.66 | −1.129 | 42 | .265 |
Note: SD, standard deviation; df, degrees of freedom; PANSS, The Positive and Negative Syndrome Scale; SAS, Simpson and Angus Scale; NCRS, Northoff Catatonia Rating Scale; AIMS, Abnormal involuntary movement scale.
aThe t values were obtained using a 2-tailed independent samples t-test.
bThe P-values for distribution of gender were obtained by chi-square test.
Statistically significant (P < .05) results are marked with an asterisk.
MRI Data Acquisition
MRI scans were acquired at the Central Institute of Mental Health, Mannheim, Germany on a 3.0 Tesla Magnetom TIM Trio MR scanner (Siemens Medical Systems) equipped with a 32-channel multi-array head-coil. Technical details on MRI sequences are provided as supplementary information.
MRI Data Analysis
Data Preprocessing
Voxel-based morphometry (VBM) of T1-weighted sMRI images was employed using CAT12 (http://dbm.neuro.uni-jena.de/cat/), which is an extension to SPM12 (http://www.fil.ion.ucl.ac.uk/spm/). The process included (a) segmentation of images into gray matter, white matter, and cerebrospinal fluid; (b) normalization using the DARTEL approach25; and (c) smoothing the gray matter probability values using an 8-mm full-width half-maximum (FWHM) isotropic Gaussian kernel. The fALFF method was applied to rs-fMRI images using the Data Processing Assistant for rs-fMRI (DPARSF)26 (for details on the pipeline, see Supplementary Material).
MRI Data Fusion
Parallel ICA27,28 on sMRI and rs-fMRI data was applied using the Fusion ICA Toolbox (FIT; http://mialab.mrn.org/software/fit) in MATLAB 9.0.0 (R2016a). The number of components for each modality was estimated using both the minimum description length (MDL) and the Akaike information criterion (AIC), as described by Calhoun and colleagues.29 Four components were identified for each modality. ICASSO30 was run 100 times to assess the consistency of the components, and the most central run was selected to ensure replicability and stability. For component visualization, the source matrix was converted back to a 3D image, scaled to unit standard deviations (z), and thresholded at |z| > 2. Maps from the 2 components described in results section were overlaid onto a Montreal Neurological Institute (MNI) normalized anatomical template. Anatomical denominations and stereotaxic coordinates were derived from clusters above a threshold of z = 3.5 by linking the ICA output images (ie, the chosen components of interest) to the Talairach Daemon database (http://www.talairach.org/daemon.html).
Statistical Analyses
The procedure was similar to that described by Meda and colleagues31. First, Pearson’s correlations (2-tailed) were used to explore the relationships between the loading coefficients of sMRI and rs-fMRI independent components (ICs). Significant correlations between sMRI and rs-fMRI ICs were further investigated by comparing the correlation coefficients of each IC across groups. The resulting correlation values were compared using Fisher’s z-transformation to reveal significant differences between matched SAS and non-SAS groups. Between-group analyses were adjusted for the Positive and Negative Syndrome Scale (PANSS) total scores. Second, 2-tailed Pearson’s correlation was used to investigate the relationship between SAS scores and the loading coefficients which showed significant between-group differences. Third, SAS scores of SSD patients (n = 44) and their sMRI and rs-fMRI loading coefficients were each computed using partial Pearson’s correlation, 2-tailed, again adjusted for PANSS total score. Finally, the abovementioned data analyses, we rerun on the whole sample (n = 85) of SSD patients with and without parkinsonism using similar parameters (see Supplementary Material for details and results). As nominal significance threshold, a P-value <.05 was chosen, followed by correction for multiple comparisons using the false discovery rate (FDR).
To examine the relationship between parkinsonism and catatonia in the whole sample (n = 44) and the SAS group (n = 22), we run a partial correlation (2-tailed) between an individual’s SAS and Northoff Catatonia Rating Scale (NCRS) total scores, while controlling for age, gender, OLZ, and PANSS-N score. Further, we run a partial correlation (2-tailed) between an individual’s SAS total and PANSS-N scores, while controlling for age, gender, OLZ, and NCRS total score to determine the relationship between parkinsonism and negative symptoms in the whole sample (n = 44) and SAS group (n = 22). A nominal significance threshold of P < .05 was defined, followed by Bonferroni correction for multiple comparisons (corrected P = .01). Finally, we also included an additional analysis comparing SSD patients (SAS and non-SAS groups) and 22 healthy controls (HC) matched for age (mean = 39.81 y; SD = 11.79; F(2, 63) = 0.12; P = .88), gender (14 males, 8 females), education (mean = 14.48 y; SD = 1.28; F(2, 63) = 0.98; P = .38), and handedness (see Supplementary Material for details and results).
Results
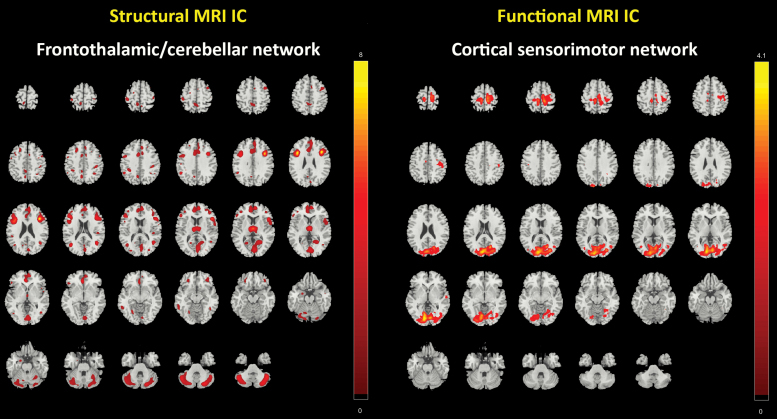
Results showed significant correlations between 5 sMRI and rs-fMRI IC pairs (table 2). These significant IC pairs were examined group-wise (supplementary table 1), and their correlation coefficients were compared using Fisher’s z transformation. A significant difference was observed in 1 structural and 1 functional IC, P = .042, z = −2.04 (figures 1 and 2). The sMRI component predominantly comprised the middle frontal gyrus (MFG), inferior frontal gyrus (IFG), anterior cingulate gyrus, thalamus, precentral gyrus, and the cerebellar structures (tonsil, uvula, tuber, and culmen) (supplementary table 2), correspondingly labeled as frontothalamic/cerebellar network. The rs-fMRI component predominantly comprised the lingual gyrus and the cuneus (supplementary table 4), correspondingly labeled as the cortical sensorimotor network. For other 4 IC pairs, no significant difference was observed, all P-values > .25, all z-scores < −1.14 (see supplementary figure 1, supplementary tables 1 and 5). Furthermore, we reanalyzed the ICs’ mixing coefficients, while adjusting for the PANSS negative score to control for the influence of negative symptoms. A significant difference was still observed between the frontothalamic/cerebellar network and the cortical sensorimotor network, P = .045, z = −2.01.
Table 2.
Pearson’s Correlation Between Structural and Functional magnetic resonance imaging (MRI) independent components (ICs) Mixing Coefficients for all Participants (n = 44)
| Resting-State fMRI ICs | ||||
|---|---|---|---|---|
| Cortical Sensorimotor Network | IC2 | IC3 | IC4 | |
| Structural MRI ICs | ||||
| IC1 | ||||
| Correlation | −0.074 | −0.204 | 0.061 | 0.084 |
| Significance | 0.635 | 0.184 | 0.695 | 0.588 |
| IC2 | ||||
| Correlation | 0.224 | 0.055 | 0.229 | 0.328* |
| Significance | 0.143 | 0.723 | 0.135 | 0.030 |
| Frontothalamic/ cerebellar network | ||||
| Correlation | 0.349* | 0.015 | 0.148 | 0.562** |
| Significance | 0.020 | 0.922 | 0.338 | <0.001 |
| IC4 | ||||
| Correlation | 0.205 | 0.007 | 0.319* | 0.344* |
| Significance | 0.182 | 0.964 | 0.035 | 0.022 |
*P < .05; **P < .01.
Fig. 1.
Spatial patterns of structural magnetic resonance imaging (sMRI) and resting-state functional MRI (rs-fMRI) networks thresholded at z > 2 which showed significant differences between non-Simpson and Angus Scale (SAS) group and SAS group.
Fig. 2.
Scatter plots showing the relationship between the structural frontothalamic/cerebellar network and the functional cortical sensorimotor network in (a) non-Simpson and Angus Scale (SAS) group (n = 22) and (b) SAS group (n = 22). Using Fisher’s z transformation, a significant difference was observed between the correlation coefficients of 2 groups, P = .042, z = −2.04.
Subsequently, the frontothalamic/cerebellar network showed a significant correlation with the glabella-salivation score, P < .001 (figure 3 and table 3). The cortical sensorimotor network showed significant correlations with the tremor score, P = .035, and the glabella-salivation score, P = .020 (figure 3 and table 3). We found no significant association between SAS scores and OLZ equivalents in the study sample (supplementary table 8).
Fig. 3.
Scatter plots showing the relationship between (a) frontothalamic/cerebellar resting-state network and glabella-salivation score, (b) cortical sensorimotor structural network and tremor score, and (c) cortical sensorimotor structural network and glabella-salivation score.
Table 3.
Pearson’s Correlation Between the Mixing Coefficients of Independent Components (ICs) for all Participants (n = 44) and the Simpson and Angus Scale (SAS) Scores
| SAS Scores | ||||
|---|---|---|---|---|
| Hypokinesia | Rigidity | Tremor | Glabella- salivation | |
| Frontothalamic/cerebellar network | ||||
| Correlation | −.139 | −.173 | −.220 | −.487** |
| Significance | .367 | .262 | .151 | .001 |
| Cortical sensorimotor network | ||||
| Correlation | −.266 | −.222 | −.319* | −.351* |
| Significance | .081 | .147 | .035 | .020 |
*P < .05; **P < .01.
In the combined sample of SSD patients with and without parkinsonism (n = 44), we found no significant association between SAS and NCRS total scores (r = .108, P = .506). In SSD patients with parkinsonism (n = 22), there was no significant association between SAS and NCRS total scores (r: −.006, P = .98). In the combined sample of SSD patients with and without parkinsonism (n = 44), we found no significant association between SAS total and PANSS-N scores (r = .219, P = .176). In SSD patients with parkinsonism (n = 22), there was no significant association between SAS total and PANSS-N scores (r = −.067, P = .791).
Discussion
This is the first multimodal MRI study that aimed at studying the associations between brain structure and resting-state neural activity in SSD patients with and without parkinsonism. Four main findings emerged: First, correlational coefficients of interrelated frontothalamic/cerebellar sMRI component and a cortical sensorimotor rs-fMRI component differed significantly between SAS and non-SAS groups. Second, the tremor score was associated with the loadings of the cortical sensorimotor rs-fMRI component. Third, the glabella-salivation score was associated with loadings of the frontothalamic/cerebellar sMRI and cortical sensorimotor rs-fMRI component. Fourth, an interrelated frontotemporal/cerebellar sMRI component and temporo-cerebellar rs-fMRI component differed between SAS and HC groups.
Group Differences
We detected frontothalamic/cerebellar and cortical sensorimotor networks that represent interrelated GMV-INA components that differ between SSD patients with and without parkinsonism. Our findings are consistent with the prior suggestion of aberrant “bottom-up modulation” (alterations in the thalamocortical loop) between subcortical (basal ganglia and thalamic motor nuclei) and cortical/motor regions (premotor cortex and M1) in PD.7,32 Yet, unlike PD, we did not observe associations between distinctly “striatal” networks and parkinsonism. Nonetheless, our results are in line with previous MRI studies in SSD patients that showed a significant relationship between abnormal involuntary movements (mainly TD) and structural alterations of the frontal gyrus, temporal lobe, and pons, respectively.33–37 We essentially expand these studies by showing that SSD patients with significant GMV reduction in regions comprising the MFG and IFG, anterior cingulate cortex (ACC), and M1 as well as the thalamus and cerebellar structures tend to be those who exhibit parkinsonism. These results are important for 4 reasons: First, neurodegenerative conditions in the MFG and IFG, which are part of the prefrontal cortex, might lead to aberrant response inhibition, motor planning and imagery, decision making, and disordered cognitive processing, particularly executive function, in SSD patients.38 Second, structural alterations in ACC or other limbic structures can lead to aberrant ascending non-dopaminergic projections to cortical regions and hence, to the development of apathy, depression and psychomotor slowing, up to freezing of gait, ie, to characteristic symptoms of parkinsonism.39,40 Furthermore, the dorsal part of ACC41 modulates motor behavior by acting as a major interface between sensorimotor and cognitive networks.42 Third, with regard to the symptom triad in parkinsonism, the interaction between cognitive and motor regions in terms of the modulation of motor behavior by cognitive functioning is particularly relevant.42–44 Fourth, the cerebellum is crucial for adjustment of time and force of movements or muscle commands.44,45 Dysfunction of neuronal loops originating from cerebellar structures, which are modulated by thalamus nuclei, might give rise to deficits in the smooth control and online updating of bodily movements leading to ataxia and tremor.44–48 Such motor disorders have already been found in SSD patients and persons with psychosis risk syndrome.46–48 Our results are also in line with Walther and colleagues,49 who showed a significant correlation between daily activity level (eg, hypokinesia) and baseline perfusion in the cingulate motor area, right M1, and bilateral dorsolateral prefrontal cortex.
Our results are relevant because the abovementioned GMV network changes are closely associated with aberrant INA in the cortical sensorimotor rs-fMRI component. Structural changes of the thalamic nuclei and the cerebellum might contribute to disturbed bottom-up projections into cortical regions such as the lingual gyrus and the cuneus. A recent MRI study found reduced volume in the cuneus and lingual gyrus in patients with TD.50 The authors suggested that structural alterations of visual and frontoparietal regions are characteristic for schizophrenia.50,51 The involvement of occipital regions might be related to aberrant modulation of visual stimuli, which can lead to disordered spatial motor coordination. SSD patients might not be able to use their movements purposefully and have serious problems when moving their body in space leading to stiff or even stooped shuffling gait and bumpy movements.
Finally, our results of aberrant interrelated frontotemporal/cerebellar sMRI component and temporo-cerebellar rs-fMRI component in SAS patients compared with HC revealed the cerebro-cerebellar dysfunction underlying parkinsonism in SSD patients. Specifically, aberrant network-level interaction between temporal and cerebellar regions might lead to disinhibition and disturbed bottom-up modulation of frontal regions and the development of parkinsonism in SSD. Because we found no difference between non-SAS and HC groups, our findings suggest interrelated structural and functional abnormalities, which define SSD patients with but not those without parkinsonism.
Structure/Function Interrelationship Underlying Parkinsonism
First, we found that the tremor score was associated with the loading score of the cortical sensorimotor rs-fMRI component. A possible explanation for this finding is that aberrant INA in the sensorimotor network (involving cuneus und lingual gyrus) leads to impaired visual-motor integration and finally contribute to the development of tremor in SSD patients.52,53
Second, the glabella-salivation score was associated with the interrelated frontothalamic/cerebellar sMRI component and a cortical sensorimotor rs-fMRI component. Remarkably, the glabellar tap belongs to the frontal release signs (also known as primitive reflexes), which can be found early after birth but should disappear in the course of further brain development.54 Therefore, the origin of glabellar sign may not be attributed to the antipsychotic effect, but to the underlying SSD itself. In SSD patients, frontal release signs might get disinhibited by alterations of the frontal lobe.55 In this study, GMV alterations of the thalamus and cerebellum may have led to disturbed bottom-up modulation of cortical regions and reappearance of glabella tap reflex. Hypersalivation (or drooling) may be caused by increased saliva production and swallowing dysfunction (mainly due to bradykinesia of the oropharyngeal phase) in SSD and PD patients.56 This finding has been shown in the studies of PD patients (especially during the “off” period).57 On one side, saliva production is modulated by dopamine and can occur in the cases of hypodopaminergic states and/or clozapine treatment.57 In the study sample, only 10 patients were receiving clozapine, which might have caused sialorrhea. The other patients were receiving other antipsychotic drugs causing rather different anticholinergic effects (dry mouth, tachycardia, constipation, etc.). On the other side, the premotor cortex, M1, basal ganglia, pedunculopontine nuclei, and the cerebellum are involved in the oropharyngeal phase of swallowing.53 Our data suggest that GMV alterations in frontothalamic/cerebellar and aberrant INA in the sensorimotor network might to some extent lead to swallowing dysfunction in SSD patients. Therefore, hypersalivation might be caused by disease-related autonomous reduction of spontaneous swallowing and not by antipsychotics. This said, the two symptoms are more likely to be disease-related phenomena of the autonomic nervous system.58
To sum up, SSD can present with a variety of overlapping motor signs and symptoms. However, it is unclear whether they share the same, similar, or very different pathomechanism. Understanding the pathogenesis of different motor symptoms can support the development of new treatment strategies. In this regard, 2 recent multimodal fusion studies in SSD patients have shown distinct structure-/function associations with NSS13 and catatonia,3 respectively. Research into parkinsonism in SSD may also lead to the identification of regions with a specific receptor profile to develop effective medication against parkinsonism. The present study provides novel insights into complex co-altered neural patterns in fronto-thalamo-cingulate-cerebellar circuit underlying parkinsonism. In comparison to the identified regions underlying NSS and catatonia, parkinsonism is more likely related to aberrant “vertical” (bottom-up)7 modulation of cortico-subcortical motor circuits. Last but not least, our results are also in line with the widespread theory of PD that postulates a neurodegeneration of dopaminergic neurons in the substantia nigra pars compacta leading to a depletion of dopaminergic neurons in the striatum (posterior putamen) and further aberrant bottom-up modulation of cortical motor areas (M1, premotor area, and supplementary motor area [SMA]).59–62 For instance, a recent MRI study conducted by Kübel and colleagues showed that limb kinetic apraxia is associated with an intrinsic disruption of the left SMA function in patients with PD.63 Overall, these mechanisms contribute to cardinal motor abnormalities of PD such as rigidity and bradykinesia.59–62
Strengths and Limitations
The main strength of this study is the multimodal fusion analysis of rs-fMRI and sMRI data in a moderate sample of SSD patients with and without parkinsonism. However, this study is not without limitations. First, our study did not include antipsychotic-naïve SSD patients. Such patients do exhibit parkinsonism as well, as suggested by previous research,9 so that medication effects cannot account for motor symptoms in these individuals. In the present sample, we did not find any significant correlation between current antipsychotic dosage and SAS total score in our sample (P = .79, r = .04). Our groups were balanced for OLZ, so that the different severity of parkinsonism between the groups is rather independent of medication. It is possible that neural mechanisms of parkinsonism may vary in antipsychotic-naïve persons, yet there are no robust data available suggesting that this may indeed be the case. Second, the present data do not necessarily imply a causal relationship between structural and functional alterations and the presence or absence of parkinsonism. Parkinsonism levels may vary over time, and given the lack of longitudinal data, it is unknown whether neural structure and function could vary along with such clinical changes. Third, we could not accurately record the entire history of antipsychotic medication in the present patient sample. Cumulative antipsychotic dosage is particularly important for the investigation of long-term effects, eg, TD, which usually develops after several months of antipsychotic treatment. Therefore, the current daily dosage may not be a reliable reflection of the lifelong cumulative effects of antipsychotics on sensorimotor networks. However, in this study, only 1 patient received first-generation antipsychotic and the vast majority of patients were treated with second-generation antipsychotics that are less frequently associated with acute extrapyramidal symptoms (EPS). Recent studies showed that the prevalence estimates of motor abnormalities associated with antipsychotic medications are not influenced by treatment duration.64,65 Nonetheless, future studies should consider the dimensional nature of motor behavior and dysfunction in SSD in order to differentiate between medication-induced and genuine motor symptoms.11 Fourth, we acknowledge that there are different assessments of motor phenomena in neurological and psychiatric disorders (the St. Hans Rating Scale [HS],66 the Drug-Induced Extrapyramidal Symptoms Scale [DIEPSS],67 the Extrapyramidal Symptoms Rating Scale [ESRS],68 the Schedule for the Assessment of Drug-Induced Movement Disorders [SADIMoD],69 the Unified Parkinson’s Disease Rating Scale [UPDRS],70 and the Cambridge Neurological Inventory [CNI]71), and the SAS scale may potentially overestimate rigor because it provides a total of 6 items examining this specific symptom.8,9 However, Simpson and colleagues10 were able to show that all 10 items are better in separating specific patient groups (placebo vs 1 mg haloperidol) than the 6 rigidity items alone. We also acknowledge that the SAS was initially developed and validated to rate the severity of drug-induced parkinsonism. In this study, we specifically chose the SAS scale to assess spontaneous parkinsonism to facilitate comparisons with other studies that examined this condition, particularly in schizophrenia patients.8,9
Future studies could benefit from wearable devices that could provide more objective measures of parkinsonism.71–73 Furthermore, all SSD patients with a total SAS score ≥ 4 in this study had abnormalities in more than 1 subdomain. Four patients showed dysfunction in 2 subdomains, 11 patients in 3 subdomains, and 7 patients in all subdomains. This distribution suggests that our sample included SSD patients that featured a relatively wide parkinsonism symptom spectrum. Finally, we are aware of the relatively high prevalence of tremor in this patient group. We screened all study participants for the presence of manifest medical conditions potentially affecting central nervous system function, as well as for manifest neurological disorders, including essential tremor. Nevertheless, we cannot fully rule out the possibility of comorbid neurological diseases in prodromal stages, especially disorders that could present with clinically subthreshold tremor.
Conclusion
This study provides novel neuromechanistic insights on parkinsonism in SSD, emphasizing the importance of interrelated structure/function-alterations in neural systems subserving distinct components of coordinated motor behavior. The observed multiple network-level effects reflect parkinsonism in SSD as a multidimensional clinical construct in which subcortical deficits, particularly in thalamus and cerebellum, could lead to alteration in bottom-up (“vertical”) modulation of sensorimotor cortical regions.
Supplementary Material
Acknowledgments
We are grateful to all the participants and their families for their time and interest in this study. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
This work was supported by the German Research Foundation (DFG) (grant number DFG HI 1928/2-1 to D.H. and WO 1883/6-1 to R.C.W.) and German Federal Ministry of Education and Research (BMBF, grant number 01GQ1102 to H.T.). The DFG and BMBF had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
References
- 1. Hirjak D, Kubera KM, Northoff G, Fritze S, Bertolino AL, Topor CE, Schmitgen MM, Wolf RC. Cortical contributions to distinct symptom dimensions of catatonia. Schizophr Bull. 2019;45(6):1184–1194. doi: 10.1093/schbul/sby192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hirjak D, Kubera KM, Wolf RC, Northoff G. Going back to Kahlbaum’s psychomotor (and GABAergic) origins – is catatonia more than just a motor and dopaminergic syndrome? Schizophr Bull. July 30, 2019;sbz074. doi: 10.1093/schbul/sby192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hirjak D, Rashidi M, Kubera KM, et al. Multimodal magnetic resonance imaging data fusion reveals distinct patterns of abnormal brain structure and function in catatonia. Schizophr Bull. 2020;46(1):202–210. doi: 10.1093/schbul/sby192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hirjak D, Thomann PA, Kubera KM, Wolf ND, Sambataro F, Wolf RC. Motor dysfunction within the schizophrenia-spectrum: a dimensional step towards an underappreciated domain. Schizophr Res. 2015;169(1–3):217–233. [DOI] [PubMed] [Google Scholar]
- 5. Walther S, Stegmayer K, Wilson JE, Heckers S. Structure and neural mechanisms of catatonia. Lancet Psychiatry. 2019;6(7):610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rogers JP, Pollak TA, Blackman G, David AS. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6(7):620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25(5):555–577; discussion 578–604. [DOI] [PubMed] [Google Scholar]
- 8. Cuesta MJ, Sánchez-Torres AM, de Jalón EG, et al. Spontaneous parkinsonism is associated with cognitive impairment in antipsychotic-naive patients with first-episode psychosis: a 6-month follow-up study. Schizophr Bull. 2014;40(5):1164–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peralta V, Basterra V, Campos MS, de Jalón EG, Moreno-Izco L, Cuesta MJ. Characterization of spontaneous Parkinsonism in drug-naive patients with nonaffective psychotic disorders. Eur Arch Psychiatry Clin Neurosci. 2012;262(2):131–138. [DOI] [PubMed] [Google Scholar]
- 10. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. [DOI] [PubMed] [Google Scholar]
- 11. Peralta V, de Jalón EG, Campos MS, Cuesta MJ. Phenomenological differences between spontaneous and drug-related extrapyramidal syndromes in patients with schizophrenia-spectrum disorders. J Clin Psychopharmacol. 2013;33(3):438–440. [DOI] [PubMed] [Google Scholar]
- 12. Molina V, Lubeiro A, Blanco J, et al. Parkinsonism is associated to fronto-caudate disconnectivity and cognition in schizophrenia. Psychiatry Res Neuroimaging. 2018;277:1–6. [DOI] [PubMed] [Google Scholar]
- 13. Hirjak D, Rashidi M, Fritze S, et al. Patterns of co-altered brain structure and function underlying neurological soft signs in schizophrenia spectrum disorders. Hum Brain Mapp. 2019;40(17):5029–5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walther S, Schäppi L, Federspiel A, et al. Resting-state hyperperfusion of the supplementary motor area in catatonia. Schizophr Bull. 2017;43(5):972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Viher PV. Aberrant hyperconnectivity in the motor system at rest is linked to motor abnormalities in schizophrenia spectrum disorders. Schizophr Bull. 2017;43(5):982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Di X, Kim EH, Huang CC, Tsai SJ, Lin CP, Biswal BB. The influence of the amplitude of low-frequency fluctuations on resting-state functional connectivity. Front Hum Neurosci. 2013;7:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. [DOI] [PubMed] [Google Scholar]
- 18. Sass H, Wittchen HU, Zaudig M, Houben I.. Diagnostisches und Statistisches Manual Psychischer Störungen DSM-IV-TR: Textrevision. Auflage: Hogrefe Verlag; 2003 [Google Scholar]
- 19. Leucht S, Samara M, Heres S, et al. Dose equivalents for second-generation antipsychotic drugs: the classical mean dose method. Schizophr Bull. 2015;41(6):1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chan RC, Xie W, Geng FL, et al. Clinical utility and lifespan profiling of neurological soft signs in schizophrenia spectrum disorders. Schizophr Bull. 2016;42(3):560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hindle JV, Martyr A, Clare L. Cognitive reserve in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2014;20(1):1–7. [DOI] [PubMed] [Google Scholar]
- 22. Kotagal V, Bohnen NI, Müller ML, et al. Educational attainment and motor burden in Parkinson’s disease. Mov Disord. 2015;30(8):1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Souza Cde O, Voos MC, Francato DV, Chien HF, Barbosa ER. Influence of educational status on executive function and functional balance in individuals with Parkinson disease. Cogn Behav Neurol. 2013;26(1):6–13. [DOI] [PubMed] [Google Scholar]
- 24. Dazzan P, Morgan KD, Orr KG, et al. The structural brain correlates of neurological soft signs in AESOP first-episode psychoses study. Brain. 2004;127(pt 1):143–153. [DOI] [PubMed] [Google Scholar]
- 25. Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. [DOI] [PubMed] [Google Scholar]
- 26. Chao-Gan Y, Yu-Feng Z. DPARSF: a MATLAB toolbox for “Pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu J, Kiehl KA, Pearlson G, Perrone-Bizzozero NI, Eichele T, Calhoun VD. Genetic determinants of target and novelty-related event-related potentials in the auditory oddball response. Neuroimage. 2009;46(3):809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu J, Pearlson G, Windemuth A, Ruano G, Perrone-Bizzozero NI, Calhoun V. Combining fMRI and SNP data to investigate connections between brain function and genetics using parallel ICA. Hum Brain Mapp. 2009;30(1):241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14(3):140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Himberg J, Hyvärinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22(3):1214–1222. [DOI] [PubMed] [Google Scholar]
- 31. Meda SA, Jagannathan K, Gelernter J, et al. A pilot multivariate parallel ICA study to investigate differential linkage between neural networks and genetic profiles in schizophrenia. Neuroimage. 2010;53(3):1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Schipper LJ, Hafkemeijer A, van der Grond J, Marinus J, Henselmans JML, van Hilten JJ. Altered whole-brain and network-based functional connectivity in Parkinson’s disease. Front Neurol. 2018;9:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buckley P, O’Callaghan E, Mulvany F, et al. Basal ganglia T2 relaxation times in schizophrenia: a quantitative magnetic resonance imaging study in relation to tardive dyskinesia. Psychiatry Res. 1995;61(2):95–102. [DOI] [PubMed] [Google Scholar]
- 34. Waddington JL, O’Callaghan E, Buckley P, et al. Tardive dyskinesia in schizophrenia. Relationship to minor physical anomalies, frontal lobe dysfunction and cerebral structure on magnetic resonance imaging. Br J Psychiatry. 1995;167(1):41–44. [DOI] [PubMed] [Google Scholar]
- 35. Li CT, Chou KH, Su TP, et al. Gray matter abnormalities in schizophrenia patients with tardive dyskinesia: a magnetic resonance imaging voxel-based morphometry study. PLoS One. 2013;8(8):e71034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bai YM, Chou KH, Lin CP, et al. White matter abnormalities in schizophrenia patients with tardive dyskinesia: a diffusion tensor image study. Schizophr Res. 2009;109(1–3):167–181. [DOI] [PubMed] [Google Scholar]
- 37. Granholm E, Bartzokis G, Asarnow RF, Marder SR. Preliminary associations between motor procedural learning, basal ganglia T2 relaxation times, and tardive dyskinesia in schizophrenia. Psychiatry Res. 1993;50(1):33–44. [DOI] [PubMed] [Google Scholar]
- 38. Talati A, Hirsch J. Functional specialization within the medial frontal gyrus for perceptual go/no-go decisions based on “what,” “when,” and “where” related information: an fMRI study. J Cogn Neurosci. 2005;17(7):981–993. [DOI] [PubMed] [Google Scholar]
- 39. Prange S, Metereau E, Maillet A, et al. Early limbic microstructural alterations in apathy and depression in de novo Parkinson’s disease. Mov Disord. 2019;34(11):1644–1654. [DOI] [PubMed] [Google Scholar]
- 40. Mi TM, Mei SS, Liang PP, et al. Altered resting-state brain activity in Parkinson’s disease patients with freezing of gait. Sci Rep. 2017;7(1):16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37(2):579–588. [DOI] [PubMed] [Google Scholar]
- 42. Asemi A, Ramaseshan K, Burgess A, Diwadkar VA, Bressler SL. Dorsal anterior cingulate cortex modulates supplementary motor area in coordinated unimanual motor behavior. Front Hum Neurosci. 2015;9:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Diwadkar VA, Asemi A, Burgess A, Chowdury A, Bressler SL. Potentiation of motor sub-networks for motor control but not working memory: interaction of dACC and SMA revealed by resting-state directed functional connectivity. PLoS One. 2017;12(3):e0172531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16(3):367–378. [DOI] [PubMed] [Google Scholar]
- 45. Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bernard JA, Dean DJ, Kent JS, et al. Cerebellar networks in individuals at ultra high-risk of psychosis: impact on postural sway and symptom severity. Hum Brain Mapp. 2014;35(8):4064–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bernard JA, Mittal VA. Cerebellar-motor dysfunction in schizophrenia and psychosis-risk: the importance of regional cerebellar analysis approaches. Front Psychiatry. 2014;5:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mittal VA, Bernard JA, Northoff G. What can different motor circuits tell us about psychosis? An RDoC perspective. Schizophr Bull. 2017;43(5):949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Walther S, Federspiel A, Horn H, et al. Resting state cerebral blood flow and objective motor activity reveal basal ganglia dysfunction in schizophrenia. Psychiatry Res. 2011;192(2):117–124. [DOI] [PubMed] [Google Scholar]
- 50. Yu T, Li Y, Fan F, et al. Decreased gray matter volume of cuneus and lingual gyrus in schizophrenia patients with tardive dyskinesia is associated with abnormal involuntary movement. Sci Rep. 2018;8(1):12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gupta CN, Calhoun VD, Rachakonda S, et al. Patterns of gray matter abnormalities in schizophrenia based on an international mega-analysis. Schizophr Bull. 2015;41(5):1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Benito-Leon J, Sanz-Morales E, Melero H,et al. Graph theory analysis of resting-state functional magnetic resonance imaging in essential tremor. Human Brain Map. 2019;40(16): 4686–4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Benito-León J, Serrano JI, Louis ED, et al. Essential tremor severity and anatomical changes in brain areas controlling movement sequencing. Ann Clin Transl Neurol. 2019;6(1):83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hyde TM, Goldberg TE, Egan MF, Lener MC, Weinberger DR. Frontal release signs and cognition in people with schizophrenia, their siblings and healthy controls. Br J Psychiatry. 2007;191:120–125. [DOI] [PubMed] [Google Scholar]
- 55. Schott JM, Rossor MN. The grasp and other primitive reflexes. J Neurol Neurosurg Psychiatry. 2003;74(5):558–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Praharaj SK, Arora M, Gandotra S. Clozapine-induced sialorrhea: pathophysiology and management strategies. Psychopharmacology (Berl). 2006;185(3):265–273. [DOI] [PubMed] [Google Scholar]
- 57. Srivanitchapoom P, Pandey S, Hallett M. Drooling in Parkinson’s disease: a review. Parkinsonism Relat Disord. 2014;20(11):1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zorn SH, Jones SB, Ward KM, Liston DR. Clozapine is a potent and selective muscarinic M4 receptor agonist. Eur J Pharmacol. 1994;269(3):R1–R2. [DOI] [PubMed] [Google Scholar]
- 59. Foffani G, Obeso JA. A cortical pathogenic theory of Parkinson’s disease. Neuron. 2018;99(6):1116–1128. [DOI] [PubMed] [Google Scholar]
- 60. Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. [DOI] [PubMed] [Google Scholar]
- 61. Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K. Stanley Fahn Lecture 2005: the staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord. 2006;21(12):2042–2051. [DOI] [PubMed] [Google Scholar]
- 62. Del Tredici K, Braak H. Review: Sporadic Parkinson’s disease: development and distribution of α-synuclein pathology. Neuropathol Appl Neurobiol. 2016;42(1):33–50. [DOI] [PubMed] [Google Scholar]
- 63. Kübel S, Stegmayer K, Vanbellingen T, Walther S, Bohlhalter S. Deficient supplementary motor area at rest: neural basis of limb kinetic deficits in Parkinson’s disease. Hum Brain Mapp. 2018;39(9):3691–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Parksepp M, Ljubajev Ü, Täht K, Janno S. Prevalence of neuroleptic-induced movement disorders: an 8-year follow-up study in chronic schizophrenia inpatients. Nord J Psychiatry. 2016;70(7):498–502. [DOI] [PubMed] [Google Scholar]
- 65. Martino D, Karnik V, Osland S, Barnes TRE, Pringsheim TM. Movement disorders associated with antipsychotic medication in people with schizophrenia: an overview of cochrane reviews and meta-analysis. Can J Psychiatry. 2018;706743718777392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gerlach J, Korsgaard S, Clemmesen P, et al. The St. Hans Rating Scale for extrapyramidal syndromes: reliability and validity. Acta Psychiatr Scand. 1993;87(4):244–252. [DOI] [PubMed] [Google Scholar]
- 67. Kim JH, Jung HY, Kang UG, et al. Metric characteristics of the drug-induced extrapyramidal symptoms scale (DIEPSS): a practical combined rating scale for drug-induced movement disorders. Mov Disord. 2002;17(6):1354–1359. [DOI] [PubMed] [Google Scholar]
- 68. Chouinard G, Margolese HC. Manual for the Extrapyramidal Symptom Rating Scale (ESRS). Schizophr Res. 2005;76(2–3): 247–265. [DOI] [PubMed] [Google Scholar]
- 69. Loonen AJ, Doorschot CH, van Hemert DA, Oostelbos MC, Sijben AE. The schedule for the assessment of drug-induced movement disorders (SADIMoD): inter-rater reliability and construct validity. Int J Neuropsychopharmacol. 2001;4(4):347–360. [DOI] [PubMed] [Google Scholar]
- 70. Goetz CG, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord. 2007;22(1):41–47. [DOI] [PubMed] [Google Scholar]
- 71. Chen EY, Shapleske J, Luque R, et al. The Cambridge Neurological Inventory: a clinical instrument for assessment of soft neurological signs in psychiatric patients. Psychiatry Res. 1995;56(2):183–204. [DOI] [PubMed] [Google Scholar]
- 72. Loonen AJ, van Praag HM. Measuring movement disorders in antipsychotic drug trials: the need to define a new standard. J Clin Psychopharmacol. 2007;27(5):423–430. [DOI] [PubMed] [Google Scholar]
- 73. van Harten PN, Walther S, Kent JS, Sponheim SR, Mittal VA. The clinical and prognostic value of motor abnormalities in psychosis, and the importance of instrumental assessment. Neurosci Biobehav Rev. 2017;80:476–487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.