Abstract
Perceptual inference depends on an optimal integration of current sensory evidence with prior beliefs about the environment. Alterations of this process have been related to the emergence of positive symptoms in schizophrenia. However, it has remained unclear whether delusions and hallucinations arise from an increased or decreased weighting of prior beliefs relative to sensory evidence. To investigate the relation of this prior-to-likelihood ratio to positive symptoms in schizophrenia, we devised a novel experimental paradigm which gradually manipulates perceptually ambiguous visual stimuli by disambiguating stimulus information. As a proxy for likelihood precision, we assessed the sensitivity of individual participants to sensory evidence. As a surrogate for the precision of prior beliefs in perceptual stability, we measured phase duration in ambiguity. Relative to healthy controls, patients with schizophrenia showed a stronger increment in congruent perceptual states for increasing levels of disambiguating stimulus evidence. Sensitivity to sensory evidence correlated positively with the individual patients’ severity of perceptual anomalies and hallucinations. Moreover, the severity of such experiences correlated negatively with phase duration. Our results indicate that perceptual anomalies and hallucinations are associated with a shift of perceptual inference toward sensory evidence and away from prior beliefs. This reduced prior-to-likelihood ratio in sensory processing may contribute to the phenomenon of aberrant salience, which has been suggested to give rise to the false inferences underlying psychotic experiences.
Keywords: psychosis, Bayesian perceptual inference, predictive coding, bistable perception
Introduction
When perceiving our surroundings, we are confined to inherently noisy and ambiguous sensory representations of the environment. However, conscious experience usually provides us with an unequivocal impression of our world. According to Bayesian theories,1–3 our brain bridges this gap by actively employing beliefs to interpret sensory information and forms a hypothesis (or posterior probability distribution, figure 1A) about the cause of current sensory data.4 Along this line of thought, conscious experience represents a controlled hallucination, that is concurrently being shaped by internally generated beliefs (prior distributions) and constrained by external sensory information (the likelihood distribution).5
Fig. 1.
The prior-to-likelihood ratio in Bayesian perceptual inference. Perceptual inference depends on the ratio of prior and likelihood precision. (A) Here, we depict a reference scenario with optimal precision estimates (Gaussian distributions, variance in white, mean of the posterior in black). (B) Changes in these estimates of precision may lead to alterations in perception. In case of an overestimation of prior precision and/or underestimation of likelihood precision, the posterior is shifted toward the prior. (C) By analogy, an overestimation of likelihood precision and/or underestimation of prior precision is associated with a shift of the posterior toward the likelihood.
Alterations in the relative weighting (or precision6) of prior and likelihood may lead to false (or dysfunctional) inferences7–9: If prior precision is overestimated relative to the likelihood (increased prior-to-likelihood ratio, figure 1B), inference will be driven too strongly by prior beliefs and violations of prior beliefs by sensory data (ie, prediction errors) will be overly attenuated. In contrast, a decreased prior-to-likelihood ratio (figure 2C) will lead to a stronger weighting of the sensory data, thus instigating aberrant prediction errors.
Fig. 2.
Behavioral experiment. (A) In the main experiment, we measured the individual participants’ sensitivity to disambiguating stimulus evidence as a proxy for the prior-to-likelihood ratio. To visualize relevant variables, the lower panel displays typical perceptual responses in an ambiguous block and the corresponding partially disambiguated block. (B) To probe potential differences in stereovision, we determined individual stereo-disparity thresholds in an independent stereoacuity test.
Previous work has discussed both increases and decreases of the prior-to-likelihood ratio in relation to cognitive and perceptual anomalies in psychosis-prone individuals and patients with schizophrenia (Scz, for review, see10 and11). Interestingly, delusions have often been related to a decreased prior-to-likelihood ratio,8,12–16 whereas studies on hallucinations have pointed to an increased prior-to-likelihood ratio.17–22 As it seems unlikely that delusions and hallucinations, 2 frequently co-occurring symptom domains, should be due to opposing alterations in inference, it was recently proposed that these apparently contradictory findings may be reconciled within the framework of hierarchical predictive coding1,2,23: The prior-to-likelihood ratio may indeed be generally reduced at low levels, eg, in early sensory areas, leading to aberrant salience of sensory stimuli and the emergence of delusions.24,25 In contrast, higher-level priors may become overly precise in an attempt to compensate for aberrant salience and contribute to the emergence of hallucinations.10,11,26
In the present study, we tested the hypothesis that psychotic experiences in Scz are related to a decreased prior-to-likelihood ratio at low hierarchical levels. We asked whether the precision of the likelihood mapping between the causes of sensations and the sensory consequences was elevated in Scz relative to healthy controls. This precision is often referred to as sensory precision, where an elevated precision is sometimes attributed to a failure of sensory attenuation. Moreover, we tested whether such a stronger weighting of sensory evidence is associated with the experience of delusions, hallucinations, or both.
We developed a novel experimental paradigm based on bistable perception, ie, the spontaneous alternation between 2 perceptual states that occurs when sensory information is ambiguous.27 Predictive coding posits that the dynamics of bistability reflect the 2 components of the prior-to-likelihood ratio28,29: The current perceptual state represents the best hypothesis (ie, the prior) about the cause of sensory information (ie, the likelihood). Due to ambiguity, neither of the 2 mutually exclusive perceptual hypotheses can fully account for the sensory data. Hence, a prediction error accumulates and eventually leads to a perceptual transition.
Here, we induced the phenomenon of graded ambiguity by parametrically manipulating the available sensory evidence for the 2 alternative perceptual hypotheses of an ambiguous Lissajous figure (see figure 2A and Supplementary Video 1). When a perceptual hypothesis is congruent to disambiguating stimulus evidence, prediction errors should be reduced and perceptual transitions to the incongruent perceptual states less likely. Incongruence, in turn, should lead to enhanced prediction errors and increased probability of a transition to the congruent perceptual state. In sum, the probability of perceptual states congruent with disambiguating stimulus evidence should vary with the individual participants’ sensitivity to sensory evidence. Thus, it serves as a proxy for the prior-to-likelihood ratio.
We studied the sensitivity to disambiguating stimulus evidence in patients with paranoid Scz and a matched control group. Under the assumption of a decreased prior-to-likelihood ratio in psychosis, we expected an increased sensitivity to disambiguating stimulus evidence in patients with Scz. We furthermore hypothesized a positive correlation of sensitivity to disambiguating stimulus evidence with the severity of delusions and hallucinations.
Methods
Participants
We excluded 1 control due to impaired stereovision, 3 controls due to elevated scores for Cardiff Anomalous Perception Scale (CAPS) and Peters Delusion Inventory (PDI) (threshold/scores ≥ 3 SDs above the group’s mean), 1 control due to reduced frequency of congruent perceptual states (frequency ≤ 3 SDs below the mean computed across groups in any of the conditions D1–D7), and 1 patient who did not complete the experiment. The final sample was matched for gender, age, and handedness (see table 1) and consisted of 23 patients (International Classification of Diseases 10: F20.0, 18 male, age = 37.13 ± 2.42) recruited from in- and out-patient services at Charité Universitétsmedizin Berlin and 23 control participants (17 male, age = 33.57 ± 1.74 y). All participants had (corrected-to-)normal vision, were naive to the purpose of the study, and gave informed, written consent prior to the experiment authorized by the Charité Ethics Committee.
Table 1.
Sample Characteristics
| Group | N | Female | Smoking | Stat | Age | ED | CAPS | PDI | PANSS: P | N | G | DOI | CPZe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 33.6 | 77 | 6.7 | 22 | NA | NA | NA | NA | NA | ||||
| Controls | 23 | 6 | 10 | SD | 8.4 | 40 | 9.2 | 28 | NA | NA | NA | NA | NA |
| Mean | 37.1 | 75 | 65.0 | 139 | 18.4 | 19.4 | 33 | 15 | 190 | ||||
| Patients | 23 | 5 | 15 | SD | 11.6 | 44 | 50.1 | 80 | 6.3 | 8.2 | 10 | 12 | 172 |
Note: Patients with Scz scored higher than controls on the PDI (patients: 138.83 ± 16.64 SEM, controls: 21.87 ± 5.75, Welch 2-sample t-test: T(27) = 6.64, P = 3.81 × 10−7) and CAPS (patients: 64.96 10.45, controls: CAPS of 6.65 1.91, T(23) = 5.49, P = 1.32 × 10−5). One patient received a typical antipsychotic, 18 patients were prescribed an atypical antipsychotic, and 4 were without medication.
Questionnaires and Clinical Rating
Participants completed the 40-item PDI30 to quantify delusional ideation13,14,17,31–33 and the 32-item CAPS34 to measure perceptual anomalies. Reported scores reflect sums over questionnaire subscales. We assessed clinical symptom severity using the Positive and Negative Syndrome Scale (PANSS).35
Behavioral Experiments
Apparatus.
We presented all stimuli using a mirror stereoscope placed in front of a 98PDF-CRT-Monitor (60 Hz, 1042 × 768 pixels, 59.50 cm viewing distance, 30.28 pixels per degree visual angle; °) using Psychtoolbox 336 and Matlab R2007b (MathWorks).
Main Experiment.
The main experiment (figure 2A) assessed the modulation of perceptual states by levels of disambiguating stimulus evidence. In 3 runs (10.52 min each), participants viewed 7 pairs of ambiguous and partially disambiguated versions of a rotating discontinuous Lissajous figure (see Supplementary Video 1) presented in blocks of 40.08 s each, separated by 5 s of fixation. We randomly placed 300 dots (0.05°) on the stimulus waveform (2.05° × 2.05°) defined by the perpendicular intersection of 2 sinusoids [ and ) with A = 3, B = 6, and increasing from 0 to at 6.80 s per revolution and 6 revolutions per block]. We relocated the dots at a probability of 0.02 per frame. Stimuli were surrounded by rectangular fusion frames and presented on the background of random-dot noise (700 dots of 0.05°, 1.98°/s speed, changes in motion direction at 1 Hz). We displayed a fixation cross in the center of the visible screen (0.10°).
During ambiguous blocks, we presented identical Lissajous figures to the 2 eyes. Participants indicated changes in the perceived direction of rotation by pressing the left (rotation of the front surface to the left, right index finger), right (rotation to the right, right ring finger), or down (unclear direction of rotation, right middle finger) arrow key on a standard USB keyboard.
The indicated direction of rotation in an ambiguous block determined the time-points of changes in sensory evidence in the upcoming disambiguated block. To add additional sensory evidence (graded disambiguation) to the Lissajous figure, we shifted a proportion of the stimulus dots by a of 0.02 in the corresponding direction between monocular channels. Crucially, we varied the amount of disambiguating stimulus evidence across 7 conditions (D1: 1.25%, D2: 3.75%, D3: 8.75%, D4: 16.25%, D5: 26.25%, D6: 50.00%, and D7: 100.00% of dots disambiguated). Each condition appeared once per run and in random order. Participants reported changes in the perceived direction of rotation as well as unclear perceptual states.
Stereoacuity.
We assessed stereo-disparity thresholds in an independent stereoacuity test (similar to37, figure 2B). To this end, we presented a number of 5000 dots (each at 0.15°) within a square of 11 × 11°. We attached a stereo-disparity signal to dots lying on a Landolt C, ie, a circle (1.37° radius, 2.06° width) with a 90° gap located at the left, top, right, or bottom. Following 5 s of fixation and 1 s of stimulus presentation, participants reported the location of the gap in the Landolt C by pressing the up-, down-, left-, or right-arrow key (response interval = 2 s). Fixation crosses (0.10°) were presented in the center of visible screen.
Participants performed 2 runs of 40 trials each. At each trial, we determined the amount of presented stereo disparity based on the response from the previous trial by a 2-up-1-down staircase procedure (correct response: decrease in the available stereo disparity by 1 step; incorrect response: increase by 2 steps, initial step size: 0.001°, reduction to 0.0005° after first reversal). The initial stereo disparity was 0.0045° in run 1 and 0.0005° in run 2.
Analyses
Main Experiment.
For the main experiment, we based our analyses on perceptual transitions reported by the participants. Because perceptual transitions occur at overlapping configurations of the Lissajous figure,29,38–41 we corrected the timing of each perceptual transition to the time of the overlap preceding the corresponding button press. This decomposed the perceptual time course into a sequence of discrete perceptual states (leftward, rightward, and unclear rotation of the front surface, 3.40 s inter-overlap interval).
As variable-of-interest (see figure 2A), we computed the proportion of congruent perceptual states (ie, perceptual states perceived in congruence with the disambiguating stimulus evidence) for all parametric levels of disambiguation (D1–D7). This variable served as a proxy for the prior-to-likelihood balance during graded ambiguity. In addition, we determined individual perceptual stability in terms of average phase duration (ie, time spent between 2 perceptual transitions). As potential confounds, we computed the probability of unclear perceptual states for all conditions (ambiguity and D1–D7) separately and absolute perceptual bias42 (ie, the absolute difference between the probability of both perceptual states and chance level) in ambiguous blocks. Within participants, we averaged all dependent variables across runs.
We performed group-level statistics using mixed ANOVA (within-subject factor: levels of disambiguating stimulus evidence D1–D7; between-subject factor: diagnostic group). Given heteroscedasticity between groups for congruent perceptual states (Levene test: P = .043), we used a linear mixed-effects (nlme R-package) model. The diagnostic group and disambiguating stimulus evidence defined fixed effects. Individual participants defined random effects. Weights were adjusted to account for unequal variance between groups.
We further fitted a set of functions [linear: ; exponential: ; sigmoid: ] to the proportion of congruent perceptual states across conditions D1–D7. After identifying the exponential fit by means of the highest adjusted R2, we compared individual growth rates as surrogates for the sensitivity to sensory evidence between groups. Because the number of free parameters (ie, complexity) in these models was fixed, the measure of accuracy can be treated as model evidence (ie, we performed a simple form of model comparison). Due to non-normality (Kolmogorov-Smirnov test: P < .0001), we used bootstrapping (R-dabestr43) to estimate confidence intervals (CI) for between-group differences in growth rates (see Supplementary Materials 1 for analyses of the linear fit) and perceptual bias.
In Supplementary Materials 2, we provide post hoc simulation analyses to illustrate the relation of our psychophysical approach to the predictive coding model of bistable perception.29
Stereo Disparity.
We determined stereo-disparity thresholds by computing the average of presented stereo disparity at trials following the third reversal of each run and averaged across runs. Due to non-normality (Kolmogorov-Smirnov test: P < .0001), we probed a potential between-group difference by bootstrapping CIs.
Correlative Analyses.
Finally, we asked whether individual questionnaire scores (PDI and CAPS; Bonferroni-corrected) correlated with the sensitivity to sensory evidence and average phase duration. In addition, we tested correlations with the PANSS subitems P1 (delusions) and P3 (hallucinations). Control analyses probed potential correlations to perceptual bias, unclear perceptual states, stereoacuity, as well as negative and general PANSS subscales (see Supplementary Materials 1 for median split analyses of CAPS/P3 and complete correlograms). Due to non-normality (Kolmogorov-Smirnov tests P < .0001 for all variables), we computed standard Spearman correlations. To correct for potential confounds that may influence performance in the Lissajous task and/or the severity of psychotic experiences, we assessed partial correlation coefficients. Such factors comprised stereoacuity (due to its potential influence on graded ambiguity, see above), the participants’ age (due to its impact on bistable perception44), as well as the duration of illness and chlorpromazine equivalents as measures of disease severity. To ascertain specificity for the dimensions of psychotic experience, we also included scores on the alternative questionnaire (for correlations with PDI/CAPS), the respective alternative PANSS subitems (for correlations with P1/P3) and PANSS subscales (general and negative).
Results
Main Experiment
The nlme R-package model indicated a main effect of disambiguating stimulus evidence on the fraction of congruent perceptual states [F(6) = 15.16, P = ], but no main effect of group [F(1) = 0.02, P = .88]. Importantly, we observed a significant interaction between diagnostic group and disambiguating stimulus evidence [F(6) = 2.52, P = .02, see figure 3A]. Mixed ANOVA yielded qualitatively identical results.
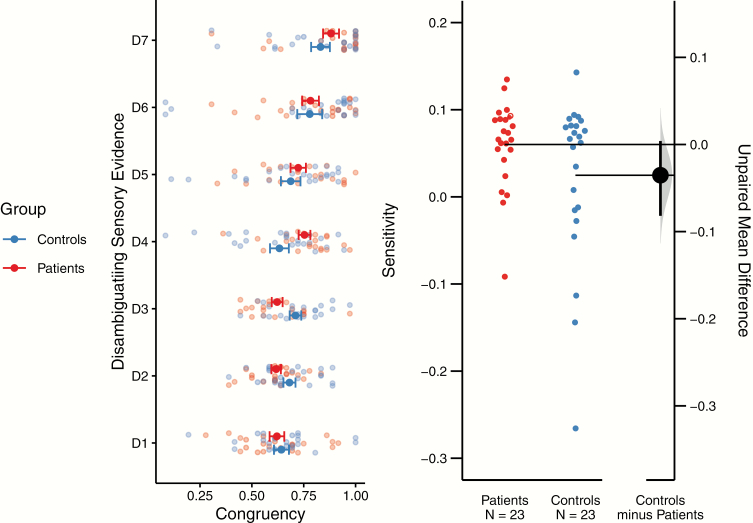
Fig. 3.
Sensitivity to disambiguating stimulus evidence. We depict the fraction of congruency between perceptual states and sensory evidence across the levels of disambiguating stimulus evidence (D1–D7, left panel). Error bars represent the respective standard error of the mean. The nlme model yielded a main effect of disambiguating stimulus evidence [F(6) = 15.16, P = 6.44 × 10−15], and a significant interaction between the diagnostic group and the disambiguating stimulus evidence [F(6) = 2.52, P = .02]. The left panel shows the implicit interaction between levels of disambiguating stimulus evidence and diagnostic group: At low levels of disambiguation (D1–D3), controls exhibit a marginally higher proportion of congruent perceptual states. This is reversed for higher levels of disambiguating stimulus evidence (D4–D7), where patients show a greater proportion of congruency. We used the growth rate of individual exponential fits to the fraction of congruent perceptual states to express the individual sensitivities to disambiguating stimulus evidence during graded ambiguity (right panel; horizontal lines point to sample means; vertical line spans over the 95% CI). Bootstrapping revealed a borderline-significant between-group difference (estimated 95% CI = 0.004 to −0.08).
The change in the fraction of congruent perceptual states across D1–D7 was best fit by an exponential function (adjusted R2 = 0.39 0.10, best fit in 70% of Scz patients and 65% of controls) as compared with linear (adjusted R2 = 0.38 0.10) and sigmoid (adjusted R2 = 0.10 0.10) functions. Sensitivity to additional sensory evidence as expressed by the growth rate of the exponential function was equal to 0.06 0.01 in patients and 0.02 0.02 in controls. Bootstrapping revealed a borderline significant difference between patients and controls (95% CI = 0.004 to −0.08, see figure 3B). Analysis of the linear fit yielded qualitatively identical results (see Supplementary Materials 1).
Mixed ANOVA did not yield a main effect of group or disambiguating stimulus evidence nor a between-factor interaction for the proportion of unclear perceptual states (patients: 0.01 0.001; controls: 0.004 0.001) or phase duration (patients: 21.25 0.35 s; controls: 21.56 0.36 s; see Supplementary Materials 1). Furthermore, we did not observe a significant between-group difference with regard to perceptual biases in ambiguity (patients: 0.09 0.02, controls: 0.10 0.02, 95% CI = −0.06 to 0.04).
Stereoacuity
Stereo-disparity thresholds amounted to 0.003 0.001° in patients and 0.003 0.001° in controls with no significant between-group difference (95% CI = –0.002 to 0.001).
Correlative Analyses
Within patients, sensitivity to disambiguating stimulus evidence correlated positively with the CAPS (R = 0.51, P = .02; figure 4). This was corroborated by the respective partial correlation (R = 0.55, P = .03, see above). Similarly, there was a significant correlation of the sensitivity parameter to PANSS subitem P3 (standard correlation: R = 0.52, P = .01; partial correlation: R = 0.52, P = .04). We did not observe a significant association between sensitivity to disambiguating stimulus evidence and PDI (standard correlation: R = 0.36, P = .19; partial correlation: R = −0.35, P = .19) or P1 (standard correlation: R = 0.35, P = .11; partial correlation: R = 0.07, P = .78). Analyses of the linear fit yielded qualitatively identical results.
Fig. 4.
Individual symptom severity. Here, we depict the individual patients’ symptom severity with regard to perceptual anomalies (CAPS, top) and hallucination (P3, bottom) against the sensitivity to stimulus evidence (left) and phase duration (right) alongside regression lines (black) and 95% CI (light gray).
Furthermore, we observed a significant negative correlation of average perceptual phase duration with the CAPS (standard correlation: R = −0.54, P = .01; partial correlation: R = −0.64, P = .01) and a trendwise correlation to P3 (standard correlation: R = −0.39, P = .07; partial correlation: R = −0.46, P = .07). We did not find a significant association of phase duration to PDI or P1 in standard (PDI: R = −0.21, P = .68; P1: R = −0.26, P = .23) or partial correlations (PDI: R = −0.35, P = .19; P1: R = −0.21, P = .44).
Confirmatory analyses indicated a significant positive correlation of the sensitivity parameter to the positive and general PANSS subscale (“Positive”: R = 0.5, P = .02; “General”: R = 0.52, P = .01; “Negative”: R = 0.11, P = .61). Interestingly, there were no significant correlations between sensory precision and negative symptoms or signs. CAPS and PDI were highly correlated in patients (R = 0.76, P = 2.81 × 10−5) and showed a trend for controls (R = 0.35, P = .1).
Neither of the 2 questionnaire scores (PDI/CAPS) and PANSS subitems (P1/P3) correlated with perceptual biases, fraction of unclear perceptual states, stereo-disparity thresholds, duration of illness, or chlorpromazine equivalents. Within controls, we did not find any significant correlation between questionnaire scores and the aforementioned variables (see Supplementary Materials 1 for additional correlation analyses and correlograms).
Discussion
In this study, we asked whether the experience of psychotic symptoms is associated with an increased impact of sensory evidence on perceptual inference relative to prior predictions (ie, a reduced prior-to-likelihood ratio at sensory processing levels).
Firstly, Scz patients showed an increased proportion of disambiguation-congruent perceptual states at high levels of stimulus information (D4–D7). At low levels (D1–D3), this proportion was similar between groups or even appeared to be reduced in patients (D3). This interaction thus speaks against a global increase in sensitivity to sensory evidence in Scz. Rather, it may suggest that patients show a greater benefit (or gain) at increasing levels of stimulus information. Indeed, due to this nonlinearity, these findings defy a simple explanation. Supplementary Materials 2 provides post hoc simulations of this interaction from a predictive coding model of bistable perception.28,29
Secondly, we found that the severity of perceptual anomalies and hallucinations correlated positively with the sensitivity to disambiguating stimulus evidence and negatively with average phase duration in Scz. Predictive coding models of bistable perception28,29 relate enhanced sensory sensitivity to a shift of precision estimates toward stimulus representations (ie, the likelihood). In turn, such models assume that shorter phase durations signal a shift of precision estimates away from implicit predictions about perceptual stability (see29 and Supplementary Materials 2). Through this lens, the two behavioral results, therefore, suggest that hallucinations are related to a decreased prior-to-likelihood ratio at sensory processing levels. At the same time, they contradict the hypothesis that a global shift toward prior precision (ie, an increased prior-to-likelihood ratio) underlies the experience of hallucinations.
These findings align with the “canonical” predictive coding account of Scz,10 which assumes that psychotic symptoms arise due to a relative shift of inference away from priors and toward sensory evidence.8 Along these lines, our results reverberate with the association of Scz to a reduced susceptibility to visual illusions,16 impaired smooth pursuit,45 and reduced sensory attenuation during force matching.15,46 While our findings speak for a decrease as opposed to an increase in the prior-to-likelihood ratio, they cannot distinguish between a decrease in prior precision alone, an increase in likelihood precision alone or a combination of the two. Moreover, our results are compatible with alternative algorithms of dynamic belief updating such as circular inference47,60 and alternative implementational frameworks of bistable perception such as mutual inhibition and adaption models.48 In this context, differences in the excitation-inhibition balance49 may lead to weaker inhibition between competing neuronal populations, which could explain why hallucinations correlated with individual characteristics of bistable perception.
Importantly, our results seem to contradict the association of hallucinations to overly precise priors.19,21,22 However, this apparent discrepancy may be resolved by a differential modulation of the prior-to-likelihood ratio across levels of the predictive coding hierarchy: Our paradigm targeted the interaction of prior and likelihood at sensory levels. A reduced prior-to-likelihood ratio may elicit the aberrant salience of sensory events.24,25 This may drive higher levels into an overly strong weighting of priors and entail enhanced top-down influences on perception.11 Finally, such a compensatory mechanism may trigger hallucinations,21 thereby explaining away5 aberrant salience at sensory levels.
Albeit strongly correlated with perceptual anomalies and hallucinations, our current findings did not reveal an association of delusional ideation to either sensitivity to sensory evidence or perceptual stability. This discrepancy to previous work14 may result from differences between the experimental paradigms (Schmack et al.14 stabilized perceptual states through intermittent presentation,50 while we used a continuous stimulus). Speculatively, intermittent paradigms may boost perceptual priors and thus be more sensitive toward the relation of perceptual stability and delusions. In turn, manipulating sensory evidence through graded ambiguity may be more apt to detect associations to perceptual abnormalities. To resolve this discrepancy, future work should combine the novel paradigm of graded ambiguity with both intermittent presentation of bistable stimuli13,14 and manipulations of higher-level beliefs.33,51–53
In contrast to our findings, previous research has revealed deficits in binocular depth perception in Scz.54–57 Our stereoacuity assessment was analogous to the established Random-Dot test,37,55 but estimated perceptual thresholds in a psychophysical staircase. This yielded values in the range commonly reported for stereoacuity.55 In addition, our study did not show a global reduction in perceptual performance in Scz patients relative to controls. It thus seems less likely that low-level deficits (eg, reduced stereoacuity, contrast sensitivity,55 or motion intergration58) can account for the current findings. Finally, perceptual biases (eg, when perceiving facial expressions59) are frequently reported in Scz. In the context of bistable perception, global differences in the probabilities of perceptual alternatives are a common phenomenon.42 Importantly, this study did not reveal any significant effect of bias, which is thus unlikely to contribute to our results.
In sum, this study associates the experience of psychotic symptoms with an altered integration of prior beliefs and sensory evidence. Our results relate perceptual anomalies and hallucinations to a reduction of the prior-to-likelihood ratio in perception. This provides empirical evidence for the view that predictive processing deficits contribute to the emergence of psychotic symptoms and will enable novel approaches to the pathophysiological mechanisms of psychosis.
Supplementary Material
Acknowledgment
We thank Dr Rick Adams for a very valuable discussion on a previous version of the manuscript. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
V.W. and H.S. were supported by the Berlin Institute of Health (BIH). A.H. and P.S. were supported by Deutsche Forschergemeinschaft (DFG; Funding Identification: HE 2597/19-1 and STE 1430/8-1). A.E. was supported by the Einstein Center for Neurosciences (ECN) Berlin.
References
- 1. Lee TS, Mumford D. Hierarchical Bayesian inference in the visual cortex. J Opt Soc Am A Opt Image Sci Vis. 2003;20(7):1434–1448. [DOI] [PubMed] [Google Scholar]
- 2. Friston K. A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci. 2005;360(1456):815–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clark A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav Brain Sci. 2013;36(3):181–204. [DOI] [PubMed] [Google Scholar]
- 4. Hohwy J. Attention and conscious perception in the hypothesis testing brain. Front Psychol. 2012;3:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clark A. Surfing Uncertainty: Prediction, Action and The Embodied Mind. Oxford, UK: Oxford University Press; 2016:424. [Google Scholar]
- 6. Mathys C, Daunizeau J, Friston KJ, Stephan KE. A Bayesian foundation for individual learning under uncertainty. Front Hum Neurosci. 2011;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci. 2009;10(1):48–58. [DOI] [PubMed] [Google Scholar]
- 8. Adams RA, Stephan KE, Brown HR, Frith CD, Friston KJ. The computational anatomy of psychosis. Front Psychiatry. 2013;4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stephan KE, Mathys C. Computational approaches to psychiatry. Curr Opin Neurobiol. 2014;25:85–92. [DOI] [PubMed] [Google Scholar]
- 10. Sterzer P, Adams RA, Fletcher P, et al. The predictive coding account of psychosis. Biol Psychiatry. 2018;84(9):634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corlett PR, Horga G, Fletcher PC, Alderson-Day B, Schmack K, Powers AR 3rd. Hallucinations and Strong Priors. Trends Cogn Sci. 2019;23(2):114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jardri R, Hugdahl K, Hughes M, et al. Are hallucinations due to an imbalance between excitatory and inhibitory influences on the brain? Schizophr Bull. 2016;42(5):1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmack K, Gòmez-Carrillo de Castro A, Rothkirch M, et al. Delusions and the role of beliefs in perceptual inference. J Neurosci. 2013;33(34):13701–13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmack K, Schnack A, Priller J, Sterzer P. Perceptual instability in schizophrenia: probing predictive coding accounts of delusions with ambiguous stimuli. Schizophr Res Cogn. 2015;2(2):72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Teufel C, Kingdon A, Ingram JN, Wolpert DM, Fletcher PC. Deficits in sensory prediction are related to delusional ideation in healthy individuals. Neuropsychologia. 2010;48(14):4169–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Notredame CE, Pins D, Deneve S, Jardri R. What visual illusions teach us about schizophrenia. Front Integr Neurosci. 2014;8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teufel C, Subramaniam N, Dobler V, et al. Shift toward prior knowledge confers a perceptual advantage in early psychosis and psychosis-prone healthy individuals. Proc Natl Acad Sci U S A. 2015;112(43):13401–13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davies DJ, Teufel C, Fletcher PC. Anomalous perceptions and beliefs are associated with shifts toward different types of prior knowledge in perceptual inference. Schizophr Bull. 2018;44(6):1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Powers AR III, Kelley M, Corlett PR. Hallucinations as top-down effects on perception. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(5):393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alderson-Day B, Lima CF, Evans S, et al. Distinct processing of ambiguous speech in people with non-clinical auditory verbal hallucinations. Brain. 2017;140(9):2475–2489. [DOI] [PubMed] [Google Scholar]
- 21. Powers AR, Mathys C, Corlett PR. Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science. 2017;357(6351):596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cassidy CM, Balsam PD, Weinstein JJ, et al. A perceptual inference mechanism for hallucinations linked to striatal dopamine. Curr Biol. 2018;28(4):503–514.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rao RP, Ballard DH. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat Neurosci. 1999;2(1):79–87. [DOI] [PubMed] [Google Scholar]
- 24. Heinz A. Dopaminergic dysfunction in alcoholism and schizophrenia–psychopathological and behavioral correlates. Eur Psychiatry. 2002;17:9–16. [DOI] [PubMed] [Google Scholar]
- 25. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160(1):13–23. [DOI] [PubMed] [Google Scholar]
- 26. Heinz A., et al. Towards a unifying cognitive, neurophysiological, and computational neuroscience account of schizophrenia. Schizophr Bull. 2018;45(5):1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brascamp J, Sterzer P, Blake R, Knapen T. Multistable perception and the role of the frontoparietal cortex in perceptual inference. Annu Rev Psychol. 2018;69:77–103. [DOI] [PubMed] [Google Scholar]
- 28. Hohwy J, Roepstorff A, Friston K. Predictive coding explains binocular rivalry: an epistemological review. Cognition. 2008;108(3):687–701. [DOI] [PubMed] [Google Scholar]
- 29. Weilnhammer V, Stuke H, Hesselmann G, Sterzer P, Schmack K. A predictive coding account of bistable perception – a model-based fMRI study. PLoS Comput Biol. 2017;13(5):e1005536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peters ER, Joseph SA, Garety PA. Measurement of delusional ideation in the normal population: introducing the PDI (Peters et al. Delusions Inventory). Schizophr Bull. 1999;25(3):553–576. [DOI] [PubMed] [Google Scholar]
- 31. Stuke H, Stuke H, Weilnhammer VA, Schmack K. Correction: psychotic experiences and overhasty inferences are related to maladaptive learning. PLoS Comput Biol. 2017;13(2):e1005393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Preti A, Rocchi MB, Sisti D, et al. The psychometric discriminative properties of the Peters et al. Delusions Inventory: a receiver operating characteristic curve analysis. Compr Psychiatry. 2007;48(1):62–69. [DOI] [PubMed] [Google Scholar]
- 33. Schmack K, Rothkirch M, Priller J, Sterzer P. Enhanced predictive signalling in schizophrenia. Hum Brain Mapp. 2017;38(4):1767–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bell V, Halligan PW, Ellis HD. The Cardiff Anomalous Perceptions Scale (CAPS): a new validated measure of anomalous perceptual experience. Schizophr Bull. 2006;32(2):366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 36. Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- 37. Tittes J, Baldwin AS, Hess RF, et al. Assessment of stereovision with digital testing in adults and children with normal and impaired binocularity. Vision Res. 2019;164:69–82. [DOI] [PubMed] [Google Scholar]
- 38. Pastukhov A, Vonau V, Braun J. Believable change: bistable reversals are governed by physical plausibility. J Vis. 2012;12(1):17, 1–16. [DOI] [PubMed] [Google Scholar]
- 39. Weilnhammer VA, Ludwig K, Hesselmann G, Sterzer P. Frontoparietal cortex mediates perceptual transitions in bistable perception. J Neurosci. 2013;33(40):16009–16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weilnhammer VA, Ludwig K, Sterzer P, Hesselmann G. Revisiting the Lissajous figure as a tool to study bistable perception. Vision Res. 2014;98:107–112. [DOI] [PubMed] [Google Scholar]
- 41. Weilnhammer VA, Sterzer P, Hesselmann G. Perceptual stability of the Lissajous figure is modulated by the speed of illusory rotation. PLoS One. 2016;11(8):e0160772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang X, Xu Q, Jiang Y, Wang Y. The interaction of perceptual biases in bistable perception. Sci Rep. 2017;7:42018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ho J, Tumkaya T, Aryal S, Choi H, Claridge-Chang A. Moving beyond P values: everyday data analysis with estimation plots. Nat Methods. 2019;16(7):565–566.. [DOI] [PubMed] [Google Scholar]
- 44. Díaz-Santos M,, et al. Bistable perception in normal aging: perceptual reversibility and its relation to cognition. Neuropsychol Dev Cognition B Aging Neuropsychol Cogn. 2017;24;115–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thakkar KN, Diwadkar VA, Rolfs M. Oculomotor prediction: a window into the psychotic mind. Trends Cogn Sci. 2017;21(5):344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shergill SS, Samson G, Bays PM, Frith CD, Wolpert DM. Evidence for sensory prediction deficits in schizophrenia. Am J Psychiatry. 2005;162(12):2384–2386. [DOI] [PubMed] [Google Scholar]
- 47. Jardri R, Duverne S, Litvinova AS, Denève S. Experimental evidence for circular inference in schizophrenia. Nat Commun. 2017;8:14218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wilson HR. Minimal physiological conditions for binocular rivalry and rivalry memory. Vision Res. 2007;47(21):2741–2750. [DOI] [PubMed] [Google Scholar]
- 49. Lisman J. Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr Opin Neurobiol. 2012;22(3):537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pearson J, Brascamp J. Sensory memory for ambiguous vision. Trends Cogn Sci. 2008;12(9):334–341. [DOI] [PubMed] [Google Scholar]
- 51. Schmack K, Weilnhammer V, Heinzle J, Stephan KE, Sterzer P. Learning what to see in a changing world. Front Hum Neurosci. 2016;10:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kornmeier J, Wörner R, Riedel A, Tebartz van Elst L. A different view on the Necker cube-differences in multistable perception dynamics between Asperger and non-Asperger observers. PLoS One. 2017;12(12):e0189197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weilnhammer VA, Stuke H, Sterzer P, Schmack K. The neural correlates of hierarchical predictions for perceptual decisions. J Neurosci. 2018;38(21):5008–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schechter I, Butler PD, Jalbrzikowski M, Pasternak R, Saperstein AM, Javitt DC. A new dimension of sensory dysfunction: stereopsis deficits in schizophrenia. Biol Psychiatry. 2006;60(11):1282–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kantrowitz JT, Butler PD, Schecter I, Silipo G, Javitt DC. Seeing the world dimly: the impact of early visual deficits on visual experience in schizophrenia. Schizophr Bull. 2009;35(6):1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hui L., et al. Stereopsis deficits in patients with schizophrenia in a Han Chinese population. Sci. Rep. 2017;7:45988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang Z., et al. Impaired binocular depth perception in first-episode drug-naive patients with schizophrenia. Front Psychol. 2018;9:850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Carter O, Bennett D, Nash T, et al. Sensory integration deficits support a dimensional view of psychosis and are not limited to schizophrenia. Transl Psychiatry. 2017;7(5):e1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huang J, Chan RC, Gollan JK, et al. Perceptual bias of patients with schizophrenia in morphed facial expression. Psychiatry Res. 2011;185(1–2):60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Leptourgos P, Notredame C-E, Eck M, Jardri R, Denève S. Circular inference in bistable perception. bioRxiv. 2019;521195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.