Abstract
BACKGROUND
Bariatric surgery results in weight loss and health improvements in adults and adolescents. However, whether outcomes differ according to the age of the patient at the time of surgery is unclear.
METHODS
We evaluated the health effects of Roux-en-Y gastric bypass in a cohort of adolescents (161 patients enrolled from 2006 through 2012) and a cohort of adults (396 patients enrolled from 2006 through 2009). The two cohorts were participants in two related but independent studies. Linear mixed and Poisson mixed models were used to compare outcomes with regard to weight and coexisting conditions between the cohorts 5 years after surgery. The rates of death and subsequent abdominal operations and selected micronutrient levels (up to 2 years after surgery) were also compared between the cohorts.
RESULTS
There was no significant difference in percent weight change between adolescents (−26%; 95% confidence interval [CI], −29 to −23) and adults (−29%; 95% CI, −31 to −27) 5 years after surgery (P = 0.08). After surgery, adolescents were significantly more likely than adults to have remission of type 2 diabetes (86% vs. 53%; risk ratio, 1.27; 95% CI, 1.03 to 1.57) and of hypertension (68% vs. 41%; risk ratio, 1.51; 95% CI, 1.21 to 1.88). Three adolescents (1.9%) and seven adults (1.8%) died in the 5 years after surgery. The rate of abdominal reoperations was significantly higher among adolescents than among adults (19 vs. 10 reoperations per 500 person-years, P = 0.003). More adolescents than adults had low ferritin levels (72 of 132 patients [48%] vs. 54 of 179 patients [29%], P = 0.004).
CONCLUSIONS
Adolescents and adults who underwent gastric bypass had marked weight loss that was similar in magnitude 5 years after surgery. Adolescents had remission of diabetes and hypertension more often than adults. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases; ClinicalTrials.gov number, NCT00474318.)
BARIATRIC SURGERY, WHICH IS Effective in treating severe obesity in adults, is most commonly performed in the fourth or fifth decade of life. The cumulative effect of sustained obesity from adolescence through mid-life increases the likelihood of complications and death related to diabetes and cardiovascular disease.1,2 Some evidence also suggests that there are cumulative effects of remaining severely obese (i.e., having a body-mass index [BMI, the weight in kilograms divided by the square of the height in meters] of ≥35) from adolescence into adulthood, such that severely obese adults seeking bariatric surgery will be more likely to present with diabetes, hypertension, respiratory conditions, kidney dysfunction, walking limitations, and venous edema in the legs and feet than adults seeking surgery who did not report severe obesity during adolescence.3 In this analysis, we examined outcomes of Roux-en-Y gastric bypass in a cohort of adolescents with severe obesity and compared them with outcomes in a cohort of adults who had sustained obesity that began during their adolescent years. We hypothesized that surgical intervention for severe obesity during adolescence would be associated with a greater likelihood of remission of coexisting conditions than the same operation performed in adults who had been obese since adolescence.
METHODS
STUDY DESIGN AND PARTICIPANTS
The Teen–Longitudinal Assessment of Bariatric Surgery (Teen–LABS) study and the LABS study (ClinicalTrials.gov number, NCT00465829) were designed similarly as prospective, multicenter, observational studies of consecutive cases of bariatric surgery.4–7 The Teen–LABS study incorporated the design features and data collection forms of the LABS study in order to facilitate valid comparisons between the two cohorts. The Teen–LABS study enrolled adolescents (19 years of age or younger) at five clinical centers from 2006 through 2012. The LABS study enrolled patients 18 years of age or older who were undergoing any first-time bariatric surgical procedure at one of 10 clinical centers from 2006 through 2009. Adult study participants completed a weight-history questionnaire8 in which they characterized their body weight at age 18, which permitted the selection of adults whose BMI was 30 or more at age 18 for the current analysis. Comparisons were limited to adult participants 25 to 50 years of age at the time of surgery who had gastric bypass surgery as their primary bariatric operation, since this was the predominant bariatric operation in adolescents when the study was designed. The steering committee, which consisted of the principal investigator at each site, the data coordinating center, and the project scientist from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), designed and implemented the study. The first author drafted the manuscript and all the authors participated in critical reviews, editing, and the decision to submit the manuscript for publication. The study statisticians analyzed the data and vouch for integrity and completeness of the data and analysis and for the fidelity of the study to the protocol, available with the full text of this article at NEJM.org. The protocol and the data and safety monitoring plan were approved by the institutional review board at each participating institution and by the data and safety monitoring board for each study.
DATA COLLECTION
Research methods and data collection were described previously.4–6 Research visits for both studies occurred at baseline (within 30 days before surgery), at 6 months after surgery, and annually up to 5 years after surgery. Data collected by each consortium were maintained in a central database by their respective data coordinating centers.
STATISTICAL ANALYSIS
The definitions of prevalence, remission, and incidence of coexisting conditions that were used for these analyses have been published previously5 and are included in the Supplementary Appendix, available at NEJM.org, along with a detailed description of the statistical methods that we used. We evaluated weight change, coexisting conditions, and micronutrient outcomes using linear mixed and Poisson mixed models with robust error variance. Least-squares means estimates and 95% confidence intervals were generated. These models assessed missing data values by the maximum-likelihood method, under the assumption of data missing at random. Sensitivity analyses were performed to evaluate this assumption. Values that are accompanied by a P value, a 95% confidence interval, or both are modeled estimates; numbers and percentages alone are observed data. Rates of intraabdominal operation and death were calculated separately as the number of operations or deaths occurring up to 5 years after surgery, divided by person-years of observation. Poisson regression, with the logarithm of person-years as an offset variable, was used to calculate incidence rates and 95% confidence intervals (expressed per 500 person-years).
RESULTS
PARTICIPANTS
A total of 242 adolescents were enrolled in the Teen–LABS study, 161 (67%) of whom underwent gastric bypass surgery and were included in the current analysis; 2458 adults were enrolled in the LABS study, 1738 (71%) of whom underwent gastric bypass surgery. Of the 1738 adult participants, 396 reported a history of obesity dating back to age 18 (or earlier) and were selected for the comparison adult cohort for the current analysis (Fig. S1 in the Supplementary Appendix). Unadjusted baseline demographic features of the two cohorts were similar with the exception of BMI, which was higher in adolescents than in adults (54±10 vs. 51±8, P<0.001) (Table S1 in the Supplementary Appendix). Baseline demographic features adjusted for differences in BMI between adolescents and adults are shown in Table 1.
Table 1.
Adjusted Clinical Variables at Baseline and 5-Year Follow-up, According to Study Group.*
| Characteristic | Adolescents (N = 161) | Adults (N = 396) | ||
|---|---|---|---|---|
| At Baseline | At 5 Years | At Baseline | At 5 Years | |
| Age | ||||
| No. with data | 161 | 141 | 396 | 301 |
| Range — yr | 13 to 19 | 18 to 25 | 25 to 50 | 30 to 56 |
| Mean (95% CI) — yr | 17.0 (16.8 to 17.2) | 22.1 (21.8 to 22.4) | 37.9 (37.2 to 38.6)† | 43.2 (42.4 to 44.0)† |
| Female sex — % | 78 | 79 | 76 | 81 |
| BMI | ||||
| No. with data | 161 | 136 | 396 | 277 |
| Mean (95% CI) | 50 (47 to 52) | 37 (35 to 40) | 50 (48 to 52) | 36 (34 to 38) |
| Weight | ||||
| No. with data | 161 | 140 | 396 | 294 |
| Mean (95% CI) — kg | 147 (139 to 154) | 111 (103 to 118) | 148 (142 to 154) | 108 (102 to 114) |
| Change from baseline weight | ||||
| No. with data | — | 140 | — | 294 |
| Mean change (95% CI) — % | — | −26 (−29 to −23) | — | −29 (−31 to −27) |
| Systolic blood pressure | ||||
| No. with data | 158 | 135 | 394 | 251 |
| Mean (95% CI) — mm Hg | 127 (123 to 131) | 121 (117 to 125) | 129 (126 to 133) | 124 (121 to 127) |
| Diastolic blood pressure | ||||
| No. with data | 158 | 135 | 394 | 251 |
| Mean (95% CI) — mm Hg | 75 (72 to 78) | 73 (70 to 76) | 81 (79 to 83)† | 77 (75 to 79)† |
| Glycated hemoglobin | ||||
| No. with data | 156 | 121 | 380 | 196 |
| Mean (95% CI) — % | 5.2 (4.9 to 5.5) | 5.1 (4.8 to 5.3) | 5.7 (5.5 to 5.9)† | 5.2 (5.0 to 5.4) |
| HDL cholesterol | ||||
| No. with data | 160 | 124 | 384 | 197 |
| Mean (95% CI) — mg/dl | 39 (35 to 43) | 56 (51 to 60) | 43 (39 to 46)† | 59 (55 to 62) |
| Non-HDL cholesterol | ||||
| No. with data | 160 | 124 | 384 | 197 |
| Mean (95% CI) — mg/dl | 117 (107 to 127) | 97 (88 to 107) | 140 (132 to 147)† | 112 (105 to 120)† |
| LDL cholesterol | ||||
| No. with data | 160 | 124 | 384 | 197 |
| Mean (95% CI) — mg/dl | 89 (81 to 98) | 80 (72 to 89) | 108 (101 to 115)† | 92 (85 to 99)† |
| Triglyceride | ||||
| No. with data | 160 | 124 | 384 | 197 |
| Mean (95% CI) — mg/dl | 121 (105 to 139) | 73 (63 to 84) | 143 (128 to 159)† | 93 (83 to 104)† |
To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglyceride to millimoles per liter, multiply by 0.01129. CI denotes confidence interval, HDL high-density lipoprotein, and LDL low-density lipoprotein.
P<0.001 for the comparison between the two groups.
Through the 5-year study period, 96% (154 of 161) of the adolescent cohort and 96% (379 of 396) of the adult cohort remained as active participants; of the 784 possible postoperative research visits in the adolescent cohort and 1957 in the adult cohort, participants completed 698 (89%) and 1623 (83%) of the visits, respectively. At the 5-year visit, some data (body weight, at a minimum) were collected for 81% of all participants.
ANTHROPOMETRIC CHANGES
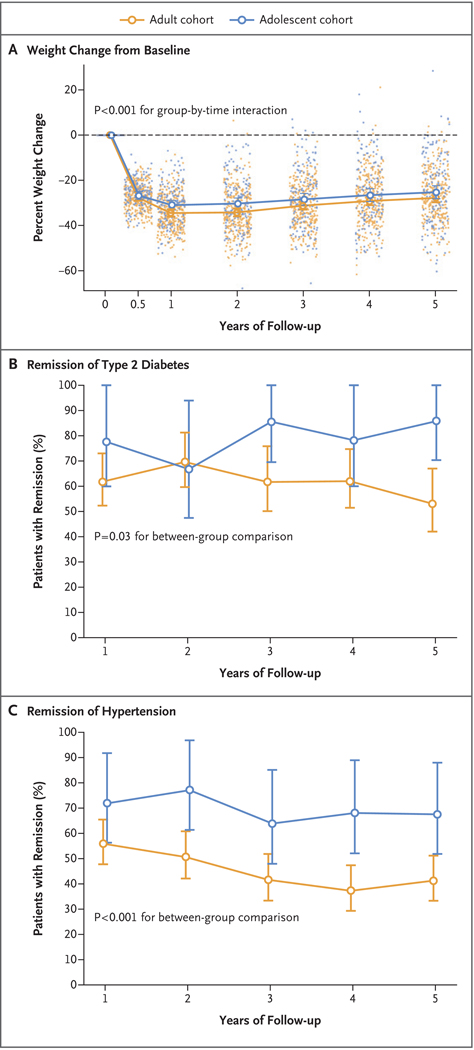
In adjusted analyses, there was no significant difference in the mean percent weight change between adolescents (−26%; 95% confidence interval [CI], −29 to −23) and adults (−29%; 95% CI, −31 to −27) 5 years after surgery (P = 0.08) (Table 1 and Fig. 1A). Five years after surgery, 60% (95% CI, 51 to 72) of adolescents and 76% (95% CI, 71 to 81) of adults maintained a weight reduction of 20% or more (P = 0.02). Conversely, 4% (95% CI, 2 to 9) of adolescents and 1% (95% CI, 0.4 to 2) of adults maintained a weight reduction of less than 5% (P = 0.005); 4% of adolescents (6 of 140 with available data) and 1% of adults (4 of 294 with available data) exceeded their baseline weight 5 years after surgery.
Figure 1. Weight Change and Remission of Diabetes and Hypertension during the 5-Year Period after Gastric Bypass Surgery.
Line graphs represent modeled mean percent changes in weight from baseline to 5 years for gastric bypass surgery in adult and adolescent cohorts, and dots represent observed values from individual participants (Panel A). Also shown is the modeled remission of type 2 diabetes (Panel B) and hypertension (Panel C) at each study visit during the 5 years after gastric bypass surgery in the two cohorts. I bars in all panels represent 95% confidence intervals.
CHANGES IN RISKS AND COMPLICATIONS OF OBESITY
Unadjusted estimates of important clinical variables are shown in Table 1. Improvements in non–high-density lipoprotein (HDL) cholesterol, triglyceride, and HDL cholesterol levels were observed over the 5-year period in both adolescent and adult cohorts, but adjusted comparisons of the mean changes in the adolescent and adult cohorts did not detect any significant differences between the groups (Table S2 in the Supplementary Appendix).
At baseline, the prevalence of diabetes was 14% (95% CI, 9 to 20) among adolescents and 31% (95% CI, 27 to 36) among adults. The prevalence of diabetes declined in both groups by year 1; 5 years after surgery, the prevalence was 2% (95% CI, 1 to 7) among adolescents and 12% (95% CI, 9 to 17) among adults (Table 2, and Table S3 and Figs. S2 and S3 in the Supplementary Appendix). Before the surgery, 88% of adolescents and 79% of adults received medications for diabetes, but the percentage decreased to zero among adolescents and 26% among adults by year 5 (P<0.001) (Table 2). Remission of diabetes differed significantly between the two cohorts. Among patients with diabetes at baseline, 86% (95% CI, 70 to 100) of adolescents and 53% (95% CI, 42 to 67) of adults no longer met the criterion for diabetes (i.e., they had a glycated hemoglobin level of <6.5% without receiving diabetes medication) 5 years after surgery (Fig. 1B and Table 2). In adjusted analyses, adolescents were 27% more likely than adults to have remission of diabetes after the surgery (relative risk, 1.27; 95% CI, 1.03 to 1.57; P = 0.03) (Fig. 2). In addition, adolescents were more likely than adults to achieve glycemic control (glycated hemoglobin level of <6.5%) irrespective of medication use postoperatively (P = 0.04) (Table 2). In modeled estimates, the incidence of diabetes was less than 1% at the 5-year postoperative visit among both adolescents and adults.
Table 2.
Prevalence, Remission, and Incidence of Coexisting Conditions, According to Study Group.*
| Coexisting Condition | Adolescents (N = 161) | Adults (N = 396) | ||
|---|---|---|---|---|
| At Baseline | At 5 Years | At Baseline | At 5 Years | |
| Diabetes | ||||
| No. with data | 161 | 139 | 388 | 223 |
| Prevalence — % (95% CI) | 14 (9–20) | 2.4 (0.8–6.7) | 31 (27–36)† | 12 (9–17)‡ |
| Remission — % (95% CI) | — | 86 (70–100) | — | 53 (42–67)‡ |
| Incidence — % (95% CI) | — | 0.17 (0.02–1.14) | — | 0.52 (0.18–1.50) |
| Glycemic control with or without medication — % (95% CI)§ | — | 85 (69–100) | — | 77 (66–90)‡ |
| Use of any diabetes medication | ||||
| Prevalence — % (95% CI) | 88 (75–100) | 0 | 79 (71–89) | 26 (18–37)† |
| Observed — no./total no. | 26/28 | 0/17 | 112/137 | 20/77 |
| Use of insulin | ||||
| Prevalence — % (95% CI) | 20 (9–42) | 0 | 22 (14–34) | 4 (1–13)† |
| Observed — no./total no. | 6/28 | 0/17 | 30/137 | 4/77 |
| Hypertension | ||||
| No. with data | 159 | 136 | 385 | 234 |
| Prevalence — % (95% CI) | 30 (22–40) | 15 (9–25) | 61 (56–67)† | 39 (33–46)† |
| Remission — % (95% CI) | — | 68 (52–88) | — | 41 (33–51)† |
| Incidence — % (95% CI) | — | 7 (3–17) | — | 11 (6–20) |
| Hypertension controlled, with or without medication — % (95% CI)¶ | — | 81 (71–94) | — | 78 (72–85)‡ |
| Use of antihypertensive medication | ||||
| Prevalence — % (95% CI) | 57 (45–71) | 11 (5–22) | 68 (61–75) | 33 (27–42)‡ |
| Observed — no./total no. | 37/57 | 6/50 | 181/240 | 57/152 |
| Low HDL cholesterol level | ||||
| No. with data | 160 | 124 | 384 | 197 |
| Prevalence — % (95% CI) | 53 (33–85) | 13 (6–28) | 37 (20–67) | 7 (3–15) |
| Remission — % (95% CI) | — | 78 (68–91) | — | 84 (76–92) |
| Incidence — % (95% CI) | — | 0.12 (0.02–0.65) | — | 0.05 (0.02–0.15) |
| Hypertriglyceridemia | ||||
| No. with data | 160 | 124 | 379 | 187 |
| Prevalence — % (95% CI) | 36 (28–48) | 6 (3–14) | 30 (26–36) | 12 (9–18) |
| Remission — % (95% CI) | — | 81 (68–96) | — | 69 (59–81) |
| Incidence — % (95% CI) | 0 | 2.1 (0.7–6.4)† | ||
Prevalence was calculated as the number of patients who met case criteria for the condition divided by the number of patients whose data could be evaluated and who were eligible to have that condition at baseline or follow-up. Remission was calculated as the percentage of patients without the condition at postoperative time points among those who had the condition at baseline and had data that could be evaluated at follow-up. Incidence was calculated as the percentage of patients with the condition at postoperative time points among those who did not have the condition at baseline.
P<0.001 for the comparison between the two groups.
P<0.05 for the comparison between the two groups.
Glycemic control was defined as a glycated hemoglobin less than 6.5%.
Hypertension control was defined as systolic blood pressure less than 140 mm Hg and diastolic blood pressure less than 90 mm Hg.
Figure 2. Adjusted Risk Ratios for Remission of Multiple Coexisting Conditions of Obesity During the 5-Year Period After Gastric Bypass Surgery.
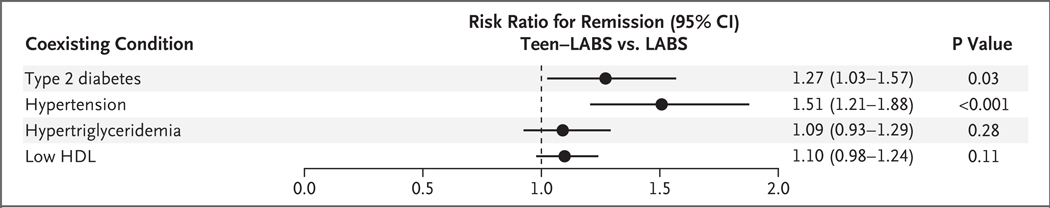
Shown are the risk ratios, estimated from a statistical model, for remission of type 2 diabetes, hypertension, hypertriglyceridemia, and low levels of high-density lipoprotein (HDL) cholesterol among adolescent participants in the Teen–Longitudinal Assessment of Bariatric Surgery (Teen–LABS) study as compared with adults in the LABS study (with adults as the reference group). Definitions for these coexisting conditions are provided in the Supplementary Appendix. Horizontal bars represent 95% confidence intervals.
Hypertension was also more prevalent among adults than among adolescents at baseline and declined in each cohort over the first postoperative year (Table 2, and Figs. S3 and S4 in the Supplementary Appendix). Antihypertensive medications were used before surgery by 57% of adolescents and by 68% of adults; this proportion decreased to 11% of adolescents and 33% of adults by year 5 (P = 0.004) (Table 2). Among patients with hypertension at baseline, 68% (95% CI, 52 to 88) of adolescents and 41% (95% CI, 33 to 51) of adults were in remission (systolic blood pressure of <140 mm Hg and diastolic blood pressure of <90 mm Hg while they were not taking hypertension medications) 5 years after surgery (Table 2 and Fig. 1C). In adjusted analyses, adolescents were 51% more likely than adults to have remission of hypertension (relative risk, 1.51; 95% CI, 1.21 to 1.88; P<0.001) (Fig. 2).
DEATH AND INTRAABDOMINAL PROCEDURES
In the first 5 years after surgery, death occurred in 3 adolescents (1.9%; 2 per 500 person-years; 95% CI, 1 to 6) and 7 adults (1.8%; 2 per 500 person-years; 95% CI, 1 to 5) (Table 3, and Fig. S1 in the Supplementary Appendix). The determination of whether deaths were related to surgery was made by independent clinician reviewers. In the adolescent cohort, one death was attributed to suspected sepsis in a patient with type 1 diabetes who had multiple complications after a hypoglycemic episode 3 years after surgery, and features of the other two deaths in adolescents, both of which occurred 4 years after surgery, were consistent with overdose (acute combined drug toxicity). Of the seven deaths in the adult group, three were related to gastric bypass; all three occurred within 2 weeks after surgery (one death each from bleeding, pulmonary embolus, and fatal arrhythmia). Two deaths were of indeterminate cause (11 months after surgery and 5 years after surgery), one was by suicide (3 years after surgery), and one was due to colon cancer (4 years after surgery).
Table 3.
Death and Abdominal Reoperations 5 Years after Gastric Bypass Surgery.
| Variable | Adolescents (N = 161) | Adults (N = 396) | ||||
|---|---|---|---|---|---|---|
| no. of participants/total no. (%) | no. of events | rate per 500 person-yr (95% CI) | no. of participants/total no. (%) | no. of events | rate per 500 person-yr (95% CI) | |
| Death | 3/161 (1.9) | 3 | 1.96 (0.63–6.08) | 7/396 (1.8) | 7 | 2.26 (1.08–4.74) |
| Intraabdominal operations within 5 years | 32/161 (20) | 46 | 19.5 (12.8–29.8) | 51/310 (16) | 55 | 10.3 (6.8–15.7)* |
| Cholecystectomy | 21/152 (14)† | 21 | 9.4 (5.1–17.3) | 23/210 (11) | 23 | 7.1 (3.9–12.8) |
| Surgery for bowel obstruction | 6/161 (4) | 6 | 2.5 (0.7–8.5) | 6/310 (2) | 7 | 1.4 (0.4–4.8) |
| Repair internal hernia | 4/161 (2) | 6 | 2.0 (0.6–7.4) | 10/310 (3) | 10 | 1.3 (0.4–4.5) |
| Gastrostomy | 2/161 (1) | 3 | 2.0 (0.6–6.1) | 2/310 (1) | 2 | 0.3 (0.05–2.3) |
| Other | 10/161 (6) | 10 | 3.9 (1.5–10.0) | 13/310 (4) | 13 | (0.8–5.3) |
P=0.002 for the comparison between the two groups.
Nine participants underwent cholecystectomy before having gastric bypass surgery.
Over the 5-year period, 46 intraabdominal procedures were performed in 32 adolescents (20%) and 55 procedures were performed in 51 adults (16%) (Table 3). The crude rate of intraabdominal procedures was 19 (95% CI, 13 to 30) per 500 person-years in adolescents and 10 (95% CI, 7 to 16) per 500 person-years in adults; in adjusted models, the incidence rate ratio between adolescents and adults was 2 (95% CI, 1 to 3; P = 0.003). Cholecystectomy after gastric bypass represented nearly half the procedures in both groups, and rates did not differ between adolescents and adults. The percentage of persons who underwent any subsequent intraabdominal operations in years 1 through 5 after gastric bypass was similar in the two cohorts (30% of adolescents and 27% of adults in year 1; 37% and 32%, respectively, in year 2; 13% and 14% in year 3; 11% and 20% in year 4; and 9% and 7% in year 5).
NUTRITIONAL MEASURES AT 2 YEARS
Micronutrient outcome data were available only through the 2-year time point in both cohorts. Baseline ferritin levels were normal in 98% of adolescents and adults (Table S4 in the Supplementary Appendix). By 2 years, low ferritin levels were found in 48% (95% CI, 37 to 63) of adolescents and in 29% (95% CI, 23 to 36) of adults (P = 0.004) (Table S4 in the Supplementary Appendix). Vitamin B12 values were also normal in more than 99% of participants at baseline; deficiencies were observed in approximately 4% of persons in each cohort by 2 years. At baseline, total 25-hydroxyvitamin D levels were low in 25% (95% CI, 17 to 35) of adolescents and in 36% (95% CI, 27 to 47) of adults, but by 2 years the percentages with low levels increased to 38% (95% CI, 28 to 51) among adolescents and decreased to 24% (95% CI, 18 to 32) among adults (P = 0.02).
DISCUSSION
In this analysis, we found that after 5 years of follow-up, weight loss overall was similar in the cohort of adolescents and the cohort of adults, but there was more variability in the maintenance of weight loss over time among adolescents. Adolescents more often had remission of both type 2 diabetes and hypertension, but abdominal reoperations and short-term nutritional deficiencies were more common among adolescents than among adults. The rate of death was similar in the two groups. Olbers et al.9 previously found similar long-term weight loss outcomes after gastric bypass in adolescents and adults, but neither health outcomes nor adverse events were reported in adults. Thus, our analysis builds on those findings by including 5-year estimates of expected health benefits, deaths, and abdominal reoperations.
Although previous long-term analyses showed substantially decreased cardiovascular10 and all-cause11 mortality among adults who underwent gastric bypass, evidence has also highlighted concerns about increased risks of death from accidental causes, suicide, and poisoning.11 Our study was not designed to address differences in incidence or causes of death, but 5-year all-cause mortality was similar among adolescents and adults (1.9% and 1.8%, respectively) and was similar to the 2.4% among adults at 6 years reported by Adams et al.12 Two of the cause-specific deaths in the adolescent cohort appeared to be related to polysubstance use, a finding that is worrisome given the overall increasing trend of drug overdose deaths in the United States13 and in light of the increased risk of substance- and alcohol-use disorders reported in adults after gastric bypass surgery.14,15 Indeed, despite the small numbers of persons thus far affected by overdose after gastric bypass surgery, these findings may indicate a need for more focused research efforts, patient education, and anticipatory guidance.
Abdominal reoperations were more common among adolescents than among adults, but the cause for this finding was not apparent. Possible factors may include closer monitoring for complications in adolescent patients and the potential for a lower threshold to reoperate for suspected complications in younger patients, which would lead to the capture of more events. Alternatively, differential recall bias cannot be ruled out, given that event data were gathered at each visit in the adolescent cohort, whereas adult data were collected at a single time, at the 5-year annual visit.
Potential nutritional risks among adolescents undergoing gastric bypass have been highlighted previously.16 Specifically, we5,17 and others9 have reported declining ferritin levels over time in adolescents after gastric bypass and have shown that among adolescents, adherence to vitamin and mineral supplementation regimens decreases considerably within the first months after surgery.18 The differences in ferritin and vitamin D levels at 2 years may be related to better adherence to postoperative vitamin and mineral supplementation among adults; thus, over time, the incidence of nutritional deficiencies among adolescents might decrease, if adherence to supplements improves with their emergence into adulthood. In young women, menstruation or pregnancy may also play a contributing role in iron deficiency. Clinical practice guidelines should highlight the vulnerability of adolescents to micronutrient deficiencies after gastric bypass; patients and health care providers should consider strategies to minimize menstrual blood loss,19 in addition to recognizing the need for lifelong micronutrient supplementation and monitoring for adverse effects including anemia, neurologic effects, and osteoporosis.
The likelihood of remission of type 2 diabetes after gastric bypass in adults is positively influenced by several factors, including shorter duration of diabetes, lower baseline glycated hemoglobin levels, lower use of glucose-lowering medications, higher baseline C-peptide levels, and greater weight reduction after surgery.20–22 After adjustment for known confounders, we still observed a significantly higher rate of diabetes remission among adolescents. Adolescents with type 2 diabetes commonly present for treatment within 1 year after onset of disease,23 and adolescents with impaired glucose tolerance or diabetes present with higher fasting C-peptide levels and greater insulin secretion in response to oral24 or intravenous25 glucose challenge than adults; all these factors are good prognostic indicators for remission after gastric bypass. The fact that after gastric bypass adolescents with diabetes achieved and maintained greater glycemic control, without medication, than adults suggests that there may be greater opportunity for recovery of islet cell secretory capacity in youth with diabetes — a concept that may drive consideration of surgery relatively soon after diagnosis of diabetes in adolescents with severe obesity. Focused research efforts targeting larger numbers of adolescents with diabetes are needed to better define the factors that are predictive of postsurgical remission and to characterize potential reduction in harm related to late adverse cardiovascular effects of diabetes, including early death.1 Such research efforts are also needed to evaluate potential multigenerational effects in offspring of mothers with diabetes,26 especially in light of the documented intergenerational transmission of health benefits from mothers who have undergone gastrointestinal bypass surgery.27,28
The significantly greater proportion of adolescents than adults with remission of hypertension would appear to provide additional evidence that adolescents have greater plasticity for reversal of complications of obesity than do adults. Obesity-associated changes that are believed to contribute to the development of hypertension are multifactorial29; the better outcomes with respect to hypertension among adolescents may reflect a greater effect of surgery on reversible neurohumoral factors30–32 in youth. Conversely, the lower proportion of adults with remission of hypertension could relate to increased vascular stiffness and histologic remodeling, which are strongly related to both age and duration of hypertension and are conditions that may be less responsive to bariatric surgery.33 It should be noted that the effectiveness of medical or surgical treatment of hypertension caused by primary hyperaldosteronism, thyroid disease, and hypercortisolism is known to decrease with increasing age of the patient.34 Taken together, a reasonable model to explain our findings would be that gastric bypass improves obesity-related, reversible physiological–functional mechanisms that underpin hypertension in youth and adults, whereas less-reversible anatomical and structural changes that occur with aging or disease duration contribute to reduced probability of remission of hypertension in some adults after bariatric surgery.
The strengths of the current study include the prospective enrollment of consecutive patients undergoing gastric bypass surgery across multiple institutions participating in two multicenter studies that used harmonized data collection and standardized methods. Limitations include the observational study design, low or infrequent counts for some outcomes, and lack of nonsurgical controls. In addition, although the adult study sample selected for comparison had a longer duration of obesity than the participants in the adolescent study, there may be unmeasured biases in the adult comparison group for which we cannot fully account, including possible effects of weight cycling over the years, noncontemporaneous environmental exposures, and inaccuracies in recall of adolescent weight. Other limitations included the lack of micronutrient data beyond year 2 in the adult cohort and the fact that other than deaths and abdominal reoperations, we did not have long-term data on surgical or medical complications in the adult group, which limited our ability to compare late adverse effects in these populations. Finally, differences between cohorts over time with regard to missing data are also a limitation. However, statistical techniques that addressed missing data were applied, and sensitivity analyses indicated that the missing-at-random assumption was reasonable.
In conclusion, we have documented similar and durable weight loss after gastric bypass in adolescents and adults, but important differences between these cohorts were observed in specific health outcomes. Longer-term follow-up and further research will be important for refinement of the risks and benefits of bariatric surgery in adolescents.
Supplementary Material
Acknowledgments
The Teen-LABS consortium is supported by cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) through grants to Dr. Inge (UM1DK072493) and Dr. Xie (UM1DK095710).
We thank the members of the LABS consortium for their contributions to the study.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Twig G, Tirosh A, Leiba A, et al. BMI at age 17 years and diabetes mortality in midlife: a nationwide cohort of 2.3 million adolescents. Diabetes Care 2016;39: 1996–2003. [DOI] [PubMed] [Google Scholar]
- 2.Twig G, Yaniv G, Levine H, et al. Body-mass index in 2.3 million adolescents and cardiovascular death in adulthood. N Engl J Med 2016;374:2430–40. [DOI] [PubMed] [Google Scholar]
- 3.Inge TH, King WC, Jenkins TM, et al. The effect of obesity in adolescence on adult health status. Pediatrics 2013;132: 1098–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inge TH, Zeller MH, Jenkins TM, et al. Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA Pediatr 2014; 168:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inge TH, Courcoulas AP, Jenkins TM, et al. Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med 2016;374:113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belle SH, Berk PD, Chapman WH, et al. Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Surg Obes Relat Dis 2013;9:926–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) study. JAMA Surg 2018;153:427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins TM, Buncher CR, Akers R, et al. Validation of a weight history questionnaire to identify adolescent obesity. Obes Surg 2013;23:1404–12. [DOI] [PubMed] [Google Scholar]
- 9.Olbers T, Beamish AJ, Gronowitz E, et al. Laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5-year, Swedish nationwide study. Lancet Diabetes Endocrinol 2017;5:174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwok CS, Pradhan A, Khan MA, et al. Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol 2014;173:20–8. [DOI] [PubMed] [Google Scholar]
- 11.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357:753–61. [DOI] [PubMed] [Google Scholar]
- 12.Adams TD, Davidson LE, Hunt SC. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med 2018; 378:93–6. [DOI] [PubMed] [Google Scholar]
- 13.Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999–2016. NCHS Data Brief 2017; 294:1–8. [PubMed] [Google Scholar]
- 14.King WC, Chen JY, Belle SH, et al. Use of prescribed opioids before and after bariatric surgery: prospective evidence from a U.S. multicenter cohort study. Surg Obes Relat Dis 2017;13:1337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King WC, Chen JY, Courcoulas AP, et al. Alcohol and other substance use after bariatric surgery: prospective evidence from a U.S. multicenter cohort study. Surg Obes Relat Dis 2017;13:1392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rand CS, Macgregor AM. Adolescents having obesity surgery: a 6-year follow-up. South Med J 1994;87:1208–13. [DOI] [PubMed] [Google Scholar]
- 17.Inge TH, Jenkins TM, Xanthakos SA, et al. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): a prospective follow-up analysis. Lancet Diabetes Endocrinol 2017;5: 165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Modi AC, Zeller MH, Xanthakos SA, Jenkins TM, Inge TH. Adherence to vitamin supplementation following adolescent bariatric surgery. Obesity (Silver Spring) 2013; 21(3):E190–E195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillman JB, Miller RJ, Inge TH. Menstrual concerns and intrauterine contraception among adolescent bariatric surgery patients. J Womens Health (Larchmt) 2011;20:533–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purnell JQ, Selzer F, Wahed AS, et al. Type 2 diabetes remission rates after laparoscopic gastric bypass and gastric banding: results of the Longitudinal Assessment of Bariatric Surgery study. Diabetes Care 2016;39:1101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon JB, Chuang LM, Chong K, et al. Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes. Diabetes Care 2013;36:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Still CD, Wood GC, Benotti P, et al. Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a retrospective cohort study. Lancet Diabetes Endocrinol 2014;2:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Copeland KC, Zeitler P, Geffner M, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab 2011;96:159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The RISE Consortium. Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: II. Observations using the oral glucose tolerance test. Diabetes Care 2018;41:1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The RISE Consortium. Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic clamp. Diabetes Care 2018;41:1696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klingensmith GJ, Pyle L, Nadeau KJ, et al. Pregnancy outcomes in youth with type 2 diabetes: the TODAY study experience. Diabetes Care 2016;39:122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith J, Cianflone K, Biron S, et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab 2009;94:4275–83. [DOI] [PubMed] [Google Scholar]
- 28.Guénard F, Deshaies Y, Cianflone K, Kral JG, Marceau P, Vohl MC. Differential methylation in glucoregulatory genes of offspring born before vs. after maternal gastrointestinal bypass surgery. Proc Natl Acad Sci U S A 2013;110:11439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams ST, Salhab M, Hussain ZI, Miller GV, Leveson SH. Obesity-related hypertension and its remission following gastric bypass surgery — a review of the mechanisms and predictive factors. Blood Press 2013;22:131–7. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed AR, Rickards G, Coniglio D, et al. Laparoscopic Roux-en-Y gastric bypass and its early effect on blood pressure. Obes Surg 2009;19:845–9. [DOI] [PubMed] [Google Scholar]
- 31.Bueter M, Ahmed A, Ashrafian H, le Roux CW. Bariatric surgery and hypertension. Surg Obes Relat Dis 2009;5:615–20. [DOI] [PubMed] [Google Scholar]
- 32.Schiavon CA, Bersch-Ferreira AC, Santucci EV, et al. Effects of bariatric surgery in obese patients with hypertension: the GATEWAY randomized trial (Gastric Bypass to Treat Obese Patients With Steady Hypertension). Circulation 2018;137:1132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scuteri A, Najjar SS, Muller DC, et al. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. J Am Coll Cardiol 2004;43: 1388–95. [DOI] [PubMed] [Google Scholar]
- 34.Streeten DH, Anderson GH Jr, Wagner S. Effect of age on response of secondary hypertension to specific treatment. Am J Hypertens 1990;3:360–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.