Abstract
Data obtained from genetically modified mouse models suggest a detrimental role for p16High senescent cells in physiological aging and age-related pathologies. Our recent analysis of aging mice revealed a continuous and noticeable accumulation of liver sinusoid endothelial cells (LSECs) expressing numerous senescence markers, including p16. At early stage, senescent LSECs show an enhanced ability to clear macromolecular waste and toxins including oxidized LDL (oxLDL). Later in life, however, the efficiency of this important detoxifying function rapidly declines potentially due to increased endothelial thickness and senescence-induced silencing of scavenger receptors and endocytosis genes. This inability to detoxify toxins and macromolecular waste, which can be further exacerbated by increased intestinal leakiness with age, might be an important contributing factor to animal death. Here, we propose how LSEC senescence could serve as an endogenous clock that ultimately controls longevity and outline some of the possible approaches to extend the lifespan.
Keywords: aging, liver sinusoid endothelial cells, senescencee, lifespan
The accumulation of senescent cells has been shown detrimental in many contexts [1–5]. In turn, the analysis of senescence in vascular endothelial cells is one of the main directions in the field of vascular aging as it plays a key role in the initiation, progression, and advancement of cardio-vascular diseases [3, 6–9]. While attenuating senescence has already been shown to ameliorate several pathological conditions, more recent work suggested that elimination of senescent cells could be advantageous for health and lifespan [4, 10–12]. Removing certain senescent cells that can be robustly replaced without conferring changes on organ structure or function is clearly beneficial. However, accumulating data suggest that there are functionally important senescent cell types that might not be efficiently replaced under physiological conditions, especially in old organisms. For example, age-induced senescence has been recently described in hypothalamic stem cells [13], while we found a significant build-up of senescence in liver sinusoid endothelial cells (LSECs).
LSECs are fenestrated endothelial cells that line the hepatic sinusoids. These cells have several important physiological roles, including facilitating the bidirectional transfer of substrates between the blood and hepatocytes, endocytosing circulating proteins, regulating immunotolerance, and maintaining sinusoidal microenvironment [14–24]. LSECs are the main cell type responsible for clearing blood-borne macromolecular waste [14–16], including most viruses [17–19] and lipopolysaccharides (LPS) [20, 21]. Furthermore, LSECs are responsible for the selective uptake of high-density lipoprotein [20, 22], in this way governing cardiovascular risk and all-cause mortality [23, 24]. Among the many toxins that are removed by LSECs, oxidized low density lipoprotein (oxLDL) [25, 26] is of specific interest as a major atherogenic substance [27–29]. Other toxic agents endocytosed by LSECs include Advanced Glycation End products (AGEs) — heterogenous metabolic by-products formed by non-enzymatic irreversible protein glycosylation/glycoxidation and that are resistant to proteolysis [30–32]. The accumulation of AGEs in tissues is harmful, observed in several pathological conditions [33–35], and thought to result from chronic hyperglycemia and increased oxidative and carbonyl stress [36–38]. The removal of both oxLDL and AGEs, however, is an inefficient process and their build up, as seen in several pathological conditions or after a depletion of LSECs [39], could rapidly overwhelm the LSEC clearing capacity. This in turn could cause further oxidative stress and has significant negative impact both in liver sinusoids and extrahepatic vascular beds [25, 40–42].
There are substantial age-induced changes in the structure and function of LSECs which in turn impact liver functions contributing to hepatic insulin resistance but also have a systemic risk of cardio-metabolic diseases [29, 30, 43–45]. Age-induced morphological changes in LSECs have been described in several species including humans [46], rats [47], baboons [48], and wild-type [30] and genetic mouse models of premature aging [49]. These morphological changes are characterized by pseudocapillarization, which includes defenestration (a reduction in the number and size of fenestrations), endothelial thickening, and basal lamina and collagen deposition [14, 50, 51]. Age-induced pseudocapillarization is a sequential process, starting with defenestration at a relatively young age and progressing towards LSEC thickening and fibrosis later in life (Figure 1) [30]. In turn, finding approaches to reverse defenestration and pseudocapillarization have being actively and successfully pursued in several laboratories as a strategy to ameliorate some age-related diseases [52–55].
Figure 1.
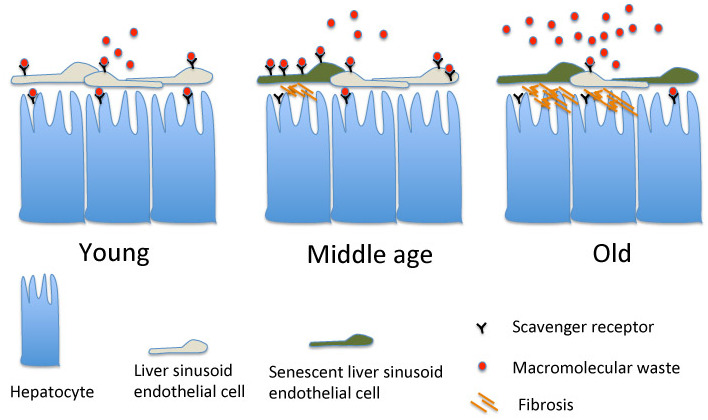
LSEC senescence throughout animal lifespan. The accumulation of p16High senescent LSECs starts gradually and is characterized by an increased expression of scavenger receptors (SRs) and endocytic activity in middle (1-year) age animals. This increase well-compensates for the loss of clearing functions by hepatocytes potentially due to LSEC defenestration. Later in life, however, the expression of SRs and LSEC endocytic activity are significantly reduced resulting in build up of blood-born macromolecular waste. This in turn contributes negatively to lifespan.
Pseudocapillarization is accompanied by changes in the expression of multiple genes, of which some are associated with senescence. Accumulated p16 senescence marker expression, elevated mitochondrial oxidative stress and increased expression of inflammatory genes resembling the senescent secretome have been recently reported in LSECs [30, 47]. Furthermore, LSECs in old animals are considered to be in a moderate pro- inflammatory state [30, 47, 51]. We further extended this analysis and found that numerous markers of senescence are continuously increased in mouse LSECs with age (Figure 1) [39]. These findings unambiguously confirm that LSECs undergo aging-induced senescence, which in turn could be a part of the pseudocapillarization process. The way in which senescence and pseudocapillarization are interconnected however warrants further analysis.
Both LSECs [14, 21] and hepatocytes [56] express numerous receptors that are essential for removing different macromolecules from the bloodstream. Any deregulation to this process could trigger compensatory mechanisms [57]. If access between the blood content and hepatocytes is blocked, as occurs during age-induced LSEC defenestration [30, 47, 58], this could trigger a compensatory upregulation in SR expression on the LSEC surface. This effect would in turn result in enhanced oxLDL and AGE intake, further inducing oxidative stress and eventually senescence.
In our own analyses of middle-aged mice (1 year old), we observed a substantial increase in SR expression on p16High senescent LSECs, further supporting this mechanism as a starting point for LSEC senescence [39]. Increased SR expression on early senescent cells fuels a further intake of toxic substances, thus creating a positive feedback loop to drive mitochondrial oxidative stress and deeper senescence. Once senescence progresses, it triggers heterochromatin silencing, which in turn can suppress the expression of numerous SRs and endocytosis genes to reduce endocytic LSEC activity both in vivo and in vitro [31, 32]. A downregulation in LSEC endocytic activity was previously observed in old rats and mice when using FSA/BSA and AGEs as substrates [30–32]. A “traffic jam hypothesis” was put forward to explain this downregulation: here, the decreased LSEC endocytic capacity in old animals is due, at least in part, to increased endothelial thickness, which in turn slows down the transport of internalized ligands to endo/lysosomal compartments [32]. Our recent results argue that reduced LSEC endocytic activity with older age could also be a consequence of transcriptional suppression that is common to senescent cells due to widespread heterochromatin silencing (Figures 1, 2) [59]. In fact, some SRs are indeed downregulated in older animals [39, 47, 51]. It would be important, however, to investigate the level of expression of SRs and endocytosis genes in very old animals (for example in 2.5-3 year-old mice) where the mechanism of senescence-induced transcriptional silencing could be especially relevant. Ultimately, the inability of LSECs to clear numerous dangerous substances from the blood could be a significant contributing factor to various age-related pathologies [58]. Furthermore, an accumulation of macromolecular waste and toxins, which is further fueled by increased intestinal permeability with age [60, 61], could create a “death cross” (Figure 2) leading to the loss of the animal once the level of clearance drops below the threshold required for survival.
Figure 2.
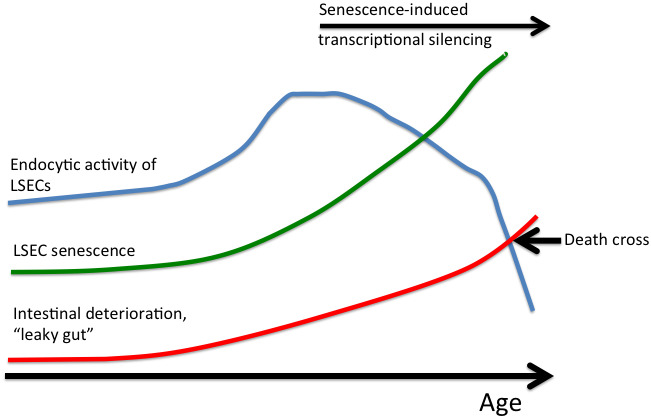
LSEC senescence as an endogenous aging clock. Senescent LSECs loose the ability to clear numerous dangerous substances from blood resulting in an age-induced accumulation of macromolecular waste and toxins. This in turn is further exacerbated by increased intestinal permeability, which induces further increase in the level of endogenous toxins. Once the level of clearance drops below the threshold required for survival (“death cross”), the animal dies.
Based on proposed model, several approaches could be considered to target senescent LSECs in order to ameliorate some age-related diseases and potentially to extend lifespan.
Delaying LSEC senescence
A list of drugs that might efficiently block and/or delay senescence is still in the making. However, possible candidates could come from the ongoing analysis of compounds that reduce or reverse LSEC defenestration, as both events seem to be interconnected in vivo. A recent study indicated that by targeting the nitric oxide pathway and inducing actin remodeling, it is possible to attenuate defenestration in old mice [52]. In another study, activating AMPK signaling and autophagy with either acute fasting (which increases the diameter of fenestrations) or chronic caloric restriction over a lifetime reversed the age-related loss of fenestrations [62]. In a similar manner, metformin, which acts on the AMPK nutrient-sensing pathway [55, 63], increased fenestration porosity in old mice and improved insulin sensitivity [53]. Finally, there have been reports that resveratrol, which acts on the sirtuin-mediated nutrient-sensing pathway [64], increases fenestrations in a Werner Syndrome mouse, which is a model for premature aging [65]. While many of the above-mentioned compounds could potentially attenuate LSEC senescence in vivo, to achieve their maximal effect, these compounds should be taken continuously which somewhat diminishes their overall utility.
Reversing transcriptional silencing of SRs
It is not fully understood how enhancer chromatin, epigenetic marks, transcription factor recruitment, and the organizational principles of transcription factor networks drive the senescence program. Recent studies showed that the senescence program is predominantly encoded at the enhancer level [66, 67] and that the enhancer landscape is dynamically reshaped at each step of the senescence transition [68]. A deeper understanding of the underlying mechanisms might help identify ways in which enhancer regulation could be manipulated to overcome potential SR and endocytosis gene transcriptional silencing in senescent LSECs, which express enhanced levels of heterochromatic marks such as macroH2A and Histone H9Me2 [39]. In turn, reversing the transcriptional suppression of several SRs and endocytosis genes in the later stages of LSEC senescence could contribute positively to lifespan.
Reprogramming senescent LSECs
Another approach to target and rejuvenate LSECs could lie in senescence reprogramming [67, 69]. This approach represents a broader direction that is not restricted just to reversing transcriptional silencing of SRs but to changes of entire transcriptional network to a younger non-senescent state. Pioneer transcription factors (that directly bind condensed chromatin) are critical in establishing new cell fate competence: they grant long-term chromatin access to non-pioneer factors and help determine cell identity by opening and licensing the enhancer landscape [68, 70, 71]. In addition, DNA methylation could play an important role in establishment of senescence as recently was shown for a DNMT1-dependent downregulation of BRCA1/ZNF350/RBBP8 repressor complex in the course of oncogene-induced senescence [72]. Thus, efforts to identify different factors and the signaling pathways that they control could be critical in understanding the feasibility of rejuvenating senescent LSECs as a therapeutic strategy.
Removing and replacing senescent LSECs
Removing and replacing senescent LSECs is perhaps the most challenging approach of all those described here. LSEC removal will instigate an immediate fibrotic response [39], which must be suppressed before any active replacement mechanism takes place. Furthermore, replacement of senescent LSECs seem to be challenging and could be divided into (i) stimulating the proliferation of remaining LSECs (perhaps via the hyperactivation of VEGF signaling) [73] or (ii) repopulating damaged sites with hepatic and extrahepatic LSEC progenitors [74]. The latter approach however requires further investigation, as the nature of LSEC progenitors is highly debated.
Preventing an age-induced “leaky gut”
Although not directly related to targeting senescent LSECs, finding ways to ameliorate intestinal health to improve health and extend the lifespan seem justified [61, 75]. An increasingly leaky intestine that ultimately increases the toxic load with age will counteract any benefits of blocking LSEC senescence. While the approaches to pharmacologically address this problem are still incomplete, some dietary recommendations could be considered. For example, there is emerging evidence that heavy alcohol use, stress and even the Western pattern diet, which is low in fiber and high in sugar and saturated fats, might initiate intestinal deterioration [75, 77]. As such, balancing your diet and controlling your gut flora could not only improve your intestinal health but also positively contribute to the efforts of reducing the senescent LSEC load.
Identifying the full repertoire of senescent cells in vivo is critical in understanding how their removal might affect a healthy lifespan. There is no doubt that eliminating some senescent cells is beneficial for healthy aging and overall lifespan [78–80]. However, there are abundant p16High senescent cell types in the aging organism that are structurally and functionally important while their removal could have detrimental consequences. Specifically, we recently showed that senescent LSECs are not replaced by non-senescent neighbors, but instead their removal activates another type of regenerative response — fibrosis [39]. As such, non-selective senescent cell removal should be considered with great caution as it could have a serious negative health impact in older organisms. This problem however could be partially solved by using drugs that selectively remove defined senescent cell types. While such selective elimination could be beneficial in age-related diseases, this approach as a life-extension strategy has its limitations as non-removed senescent cells will continue to accumulate with age, ultimately debilitating the organism. We recently found that LSECs undergo a noticeable aging-induced senescence in mice and this provides significant advantages in terms of their targeting due to a relatively easy accessibility and high endocytic activity. We thus propose that delaying senescence, reprogramming or replacing senescent LSECs could represent a powerful tool to retard aging.
Footnotes
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Katsuumi G, Shimizu I, Yoshida Y, Minamino T. Vascular senescence in cardiovascular and metabolic diseases. Front Cardiovasc Med. 2018; 5:18. 10.3389/fcvm.2018.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan RG, Ives SJ, Lesniewski LA, Cawthon RM, Andtbacka RH, Noyes RD, Richardson RS, Donato AJ. Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. Am J Physiol Heart Circ Physiol. 2013; 305:H251–58. 10.1152/ajpheart.00197.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossman MJ, Kaplon RE, Hill SD, McNamara MN, Santos-Parker JR, Pierce GL, Seals DR, Donato AJ. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am J Physiol Heart Circ Physiol. 2017; 313:H890–95. 10.1152/ajpheart.00416.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016; 15:973–77. 10.1111/acel.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018; 123:849–67. 10.1161/CIRCRESAHA.118.311378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graves SI, Baker DJ. Implicating endothelial cell senescence to dysfunction in the ageing and diseased brain. Basic Clin Pharmacol Toxicol. 2020. [Epub ahead of print]. 10.1111/bcpt.13403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia G, Aroor AR, Jia C, Sowers JR. Endothelial cell senescence in aging-related vascular dysfunction. Biochim Biophys Acta Mol Basis Dis. 2019; 1865:1802–09. 10.1016/j.bbadis.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 8.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002; 105:1541–44. 10.1161/01.cir.0000013836.85741.17 [DOI] [PubMed] [Google Scholar]
- 9.Yamazaki Y, Baker DJ, Tachibana M, Liu CC, van Deursen JM, Brott TG, Bu G, Kanekiyo T. Vascular cell senescence contributes to blood-brain barrier breakdown. Stroke. 2016; 47:1068–77. 10.1161/STROKEAHA.115.010835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016; 530:184–89. 10.1038/nature16932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, Elisseeff JH. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017; 23:775–81. 10.1038/nm.4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, et al. The achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015; 14:644–58. 10.1111/acel.12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao YZ, Yang M, Xiao Y, Guo Q, Huang Y, Li CJ, Cai D, Luo XH. Reducing hypothalamic stem cell senescence protects against aging-associated physiological decline. Cell Metab. 2020; 31:534–48.e5. 10.1016/j.cmet.2020.01.002 [DOI] [PubMed] [Google Scholar]
- 14.Sørensen KK, Simon-Santamaria J, McCuskey RS, Smedsrød B. Liver sinusoidal endothelial cells. Compr Physiol. 2015; 5:1751–74. 10.1002/cphy.c140078 [DOI] [PubMed] [Google Scholar]
- 15.DeLeve LD, Maretti-Mira AC. Liver sinusoidal endothelial cell: an update. Semin Liver Dis. 2017; 37:377–87. 10.1055/s-0037-1617455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poisson J, Lemoinne S, Boulanger C, Durand F, Moreau R, Valla D, Rautou PE. Liver sinusoidal endothelial cells: physiology and role in liver diseases. J Hepatol. 2017; 66:212–27. 10.1016/j.jhep.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 17.Ganesan LP, Mohanty S, Kim J, Clark KR, Robinson JM, Anderson CL. Rapid and efficient clearance of blood-borne virus by liver sinusoidal endothelium. PLoS Pathog. 2011; 7:e1002281. 10.1371/journal.ppat.1002281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knolle PA, Thimme R. Hepatic immune regulation and its involvement in viral hepatitis infection. Gastroenterology. 2014; 146:1193–207. 10.1053/j.gastro.2013.12.036 [DOI] [PubMed] [Google Scholar]
- 19.Klenerman P, Ramamurthy N. Liver sinusoidal endothelial cells: an antiviral “defendothelium”. Gastroenterology. 2015; 148:288–91. 10.1053/j.gastro.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 20.Yao Z, Mates JM, Cheplowitz AM, Hammer LP, Maiseyeu A, Phillips GS, Wewers MD, Rajaram MV, Robinson JM, Anderson CL, Ganesan LP. Blood-borne lipopolysaccharide is rapidly eliminated by liver sinusoidal endothelial cells via high-density lipoprotein. J Immunol. 2016; 197:2390–99. 10.4049/jimmunol.1600702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sørensen KK, McCourt P, Berg T, Crossley C, Le Couteur D, Wake K, Smedsrød B. The scavenger endothelial cell: a new player in homeostasis and immunity. Am J Physiol Regul Integr Comp Physiol. 2012; 303:R1217–30. 10.1152/ajpregu.00686.2011 [DOI] [PubMed] [Google Scholar]
- 22.Ganesan LP, Mates JM, Cheplowitz AM, Avila CL, Zimmerer JM, Yao Z, Maiseyeu A, Rajaram MV, Robinson JM, Anderson CL. Scavenger receptor B1, the HDL receptor, is expressed abundantly in liver sinusoidal endothelial cells. Sci Rep. 2016; 6:20646. 10.1038/srep20646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krieger M. Charting the fate of the “good cholesterol”: identification and characterization of the high-density lipoprotein receptor SR-BI. Annu Rev Biochem. 1999; 68:523–58. 10.1146/annurev.biochem.68.1.523 [DOI] [PubMed] [Google Scholar]
- 24.Bowe B, Xie Y, Xian H, Balasubramanian S, Zayed MA, Al-Aly Z. High density lipoprotein cholesterol and the risk of all-cause mortality among U.S. Veterans. Clin J Am Soc Nephrol. 2016; 11:1784–93. 10.2215/CJN.00730116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oteiza A, Li R, McCuskey RS, Smedsrød B, Sørensen KK. Effects of oxidized low-density lipoproteins on the hepatic microvasculature. Am J Physiol Gastrointest Liver Physiol. 2011; 301:G684–93. 10.1152/ajpgi.00347.2010 [DOI] [PubMed] [Google Scholar]
- 26.Li R, Oteiza A, Sørensen KK, McCourt P, Olsen R, Smedsrød B, Svistounov D. Role of liver sinusoidal endothelial cells and stabilins in elimination of oxidized low-density lipoproteins. Am J Physiol Gastrointest Liver Physiol. 2011; 300:G71–81. 10.1152/ajpgi.00215.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh ST, Park H, Yoon HJ, Yang SY. Long-Term Treatment of Native LDL Induces Senescence of Cultured Human Endothelial Cells. Oxid Med Cell Longev. 2017; 2017:6487825. 10.1155/2017/6487825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao S, Liu J. Association between circulating oxidized low-density lipoprotein and atherosclerotic cardiovascular disease. Chronic Dis Transl Med. 2017; 3:89–94. 10.1016/j.cdtm.2017.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fraser R, Cogger VC, Dobbs B, Jamieson H, Warren A, Hilmer SN, Le Couteur DG. The liver sieve and atherosclerosis. Pathology. 2012; 44:181–86. 10.1097/PAT.0b013e328351bcc8 [DOI] [PubMed] [Google Scholar]
- 30.Ito Y, Sørensen KK, Bethea NW, Svistounov D, McCuskey MK, Smedsrød BH, McCuskey RS. Age-related changes in the hepatic microcirculation in mice. Exp Gerontol. 2007; 42:789–97. 10.1016/j.exger.2007.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Svistounov D, Oteiza A, Zykova SN, Sørensen KK, McCourt P, McLachlan AJ, McCuskey RS, Smedsrød B. Hepatic disposal of advanced glycation end products during maturation and aging. Exp Gerontol. 2013; 48:549–56. 10.1016/j.exger.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon-Santamaria J, Malovic I, Warren A, Oteiza A, Le Couteur D, Smedsrød B, McCourt P, Sørensen KK. Age-related changes in scavenger receptor-mediated endocytosis in rat liver sinusoidal endothelial cells. J Gerontol A Biol Sci Med Sci. 2010; 65:951–60. 10.1093/gerona/glq108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira EN, Silvares RR, Flores EE, Rodrigues KL, Ramos IP, da Silva IJ, Machado MP, Miranda RA, Pazos-Moura CC, Gonçalves-de-Albuquerque CF, Faria-Neto HC, Tibiriça E, Daliry A. Hepatic microvascular dysfunction and increased advanced glycation end products are components of non-alcoholic fatty liver disease. PLoS One. 2017; 12:e0179654. 10.1371/journal.pone.0179654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fishman SL, Sonmez H, Basman C, Singh V, Poretsky L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: a review. Mol Med. 2018; 24:59. 10.1186/s10020-018-0060-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sayej WN, Knight Iii PR, Guo WA, Mullan B, Ohtake PJ, Davidson BA, Khan A, Baker RD, Baker SS. Advanced glycation end products induce obesity and hepatosteatosis in CD-1 wild-type mice. Biomed Res Int. 2016; 2016:7867852. 10.1155/2016/7867852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999; 48:1–9. 10.2337/diabetes.48.1.1 [DOI] [PubMed] [Google Scholar]
- 37.Rhee SY, Kim YS. The role of advanced glycation end products in diabetic vascular complications. Diabetes Metab J. 2018; 42:188–95. 10.4093/dmj.2017.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamagishi S, Imaizumi T. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des. 2005; 11:2279–99. 10.2174/1381612054367300 [DOI] [PubMed] [Google Scholar]
- 39.Grosse L, Wagner N, Emelyanov A, Molina C, Lacas-Gervais S, Wagner KD, Bulavin DV. Defined p16 High senescent cell types are indispensable for mouse healthspan. Cell Metab. 2020; S1550-4131:30241–42. 10.1016/j.cmet.2020.05.002 [DOI] [PubMed] [Google Scholar]
- 40.Schledzewski K, Géraud C, Arnold B, Wang S, Gröne HJ, Kempf T, Wollert KC, Straub BK, Schirmacher P, Demory A, Schönhaber H, Gratchev A, Dietz L, et al. Deficiency of liver sinusoidal scavenger receptors stabilin-1 and -2 in mice causes glomerulofibrotic nephropathy via impaired hepatic clearance of noxious blood factors. J Clin Invest. 2011; 121:703–14. 10.1172/JCI44740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansen B, Svistounov D, Olsen R, Nagai R, Horiuchi S, Smedsrød B. Advanced glycation end products impair the scavenger function of rat hepatic sinusoidal endothelial cells. Diabetologia. 2002; 45:1379–88. 10.1007/s00125-002-0912-8 [DOI] [PubMed] [Google Scholar]
- 42.Hyogo H, Yamagishi S. Advanced glycation end products (AGEs) and their involvement in liver disease. Curr Pharm Des. 2008; 14:969–72. 10.2174/138161208784139701 [DOI] [PubMed] [Google Scholar]
- 43.Mohamad M, Mitchell SJ, Wu LE, White MY, Cordwell SJ, Mach J, Solon-Biet SM, Boyer D, Nines D, Das A, Catherine Li SY, Warren A, Hilmer SN, et al. Ultrastructure of the liver microcirculation influences hepatic and systemic insulin activity and provides a mechanism for age-related insulin resistance. Aging Cell. 2016; 15:706–15. 10.1111/acel.12481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LE Couteur DG, Cogger VC, McCuskey RS, DE Cabo R, Smedsrød B, Sorensen KK, Warren A, Fraser R. Age-related changes in the liver sinusoidal endothelium: a mechanism for dyslipidemia. Ann N Y Acad Sci. 2007; 1114:79–87. 10.1196/annals.1396.003 [DOI] [PubMed] [Google Scholar]
- 45.Le Couteur DG, Fraser R, Cogger VC, McLean AJ. Hepatic pseudocapillarisation and atherosclerosis in ageing. Lancet. 2002; 359:1612–15. 10.1016/S0140-6736(02)08524-0 [DOI] [PubMed] [Google Scholar]
- 46.McLean AJ, Cogger VC, Chong GC, Warren A, Markus AM, Dahlstrom JE, Le Couteur DG. Age-related pseudocapillarization of the human liver. J Pathol. 2003; 200:112–17. 10.1002/path.1328 [DOI] [PubMed] [Google Scholar]
- 47.Maeso-Díaz R, Ortega-Ribera M, Fernández-Iglesias A, Hide D, Muñoz L, Hessheimer AJ, Vila S, Francés R, Fondevila C, Albillos A, Peralta C, Bosch J, Tacke F, et al. Effects of aging on liver microcirculatory function and sinusoidal phenotype. Aging Cell. 2018; 17:e12829. 10.1111/acel.12829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cogger VC, Warren A, Fraser R, Ngu M, McLean AJ, Le Couteur DG. Hepatic sinusoidal pseudocapillarization with aging in the non-human primate. Exp Gerontol. 2003; 38:1101–07. 10.1016/j.exger.2003.07.002 [DOI] [PubMed] [Google Scholar]
- 49.Cogger VC, Svistounov D, Warren A, Zykova S, Melvin RG, Solon-Biet SM, O’Reilly JN, McMahon AC, Ballard JW, De Cabo R, Le Couteur DG, Lebel M. Liver aging and pseudocapillarization in a werner syndrome mouse model. J Gerontol A Biol Sci Med Sci. 2014; 69:1076–86. 10.1093/gerona/glt169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Couteur DG, Cogger VC, Markus AM, Harvey PJ, Yin ZL, Ansselin AD, McLean AJ. Pseudocapillarization and associated energy limitation in the aged rat liver. Hepatology. 2001; 33:537–43. 10.1053/jhep.2001.22754 [DOI] [PubMed] [Google Scholar]
- 51.Hunt NJ, Kang SW, Lockwood GP, Le Couteur DG, Cogger VC. Hallmarks of aging in the liver. Comput Struct Biotechnol J. 2019; 17:1151–61. 10.1016/j.csbj.2019.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunt NJ, Lockwood GP, Warren A, Mao H, McCourt PA, Le Couteur DG, Cogger VC. Manipulating fenestrations in young and old liver sinusoidal endothelial cells. Am J Physiol Gastrointest Liver Physiol. 2019; 316:G144–54. 10.1152/ajpgi.00179.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hunt NJ, Lockwood GP, Kang SW, Pulpitel T, Clark X, Mao H, McCourt PA, Cooney GJ, Wali JA, Le Couteur FH, Le Couteur DG, Cogger VC. The effects of metformin on age-related changes in the liver sinusoidal endothelial cell. J Gerontol A Biol Sci Med Sci. 2020; 75:278–85. 10.1093/gerona/glz153 [DOI] [PubMed] [Google Scholar]
- 54.Xie G, Wang X, Wang L, Wang L, Atkinson RD, Kanel GC, Gaarde WA, Deleve LD. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology. 2012; 142:918–27.e6. 10.1053/j.gastro.2011.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hunt NJ, McCourt PA, Le Couteur DG, Cogger VC. Novel targets for delaying aging: the importance of the liver and advances in drug delivery. Adv Drug Deliv Rev. 2018; 135:39–49. 10.1016/j.addr.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 56.Terpstra V, van Amersfoort ES, van Velzen AG, Kuiper J, van Berkel TJ. Hepatic and extrahepatic scavenger receptors: function in relation to disease. Arterioscler Thromb Vasc Biol. 2000; 20:1860–72. 10.1161/01.atv.20.8.1860 [DOI] [PubMed] [Google Scholar]
- 57.Duryee MJ, Freeman TL, Willis MS, Hunter CD, Hamilton BC 3rd, Suzuki H, Tuma DJ, Klassen LW, Thiele GM. Scavenger receptors on sinusoidal liver endothelial cells are involved in the uptake of aldehyde-modified proteins. Mol Pharmacol. 2005; 68:1423–30. 10.1124/mol.105.016121 [DOI] [PubMed] [Google Scholar]
- 58.Hilmer SN, Cogger VC, Fraser R, McLean AJ, Sullivan D, Le Couteur DG. Age-related changes in the hepatic sinusoidal endothelium impede lipoprotein transfer in the rat. Hepatology. 2005; 42:1349–54. 10.1002/hep.20937 [DOI] [PubMed] [Google Scholar]
- 59.Criscione SW, Teo YV, Neretti N. The chromatin landscape of cellular senescence. Trends Genet. 2016; 32:751–61. 10.1016/j.tig.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fukui H. Gut-liver axis in liver cirrhosis: how to manage leaky gut and endotoxemia. World J Hepatol. 2015; 7:425–42. 10.4254/wjh.v7.i3.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qi Y, Goel R, Kim S, Richards EM, Carter CS, Pepine CJ, Raizada MK, Buford TW. Intestinal permeability biomarker zonulin is elevated in healthy aging. J Am Med Dir Assoc. 2017; 18:810.e1–e4. 10.1016/j.jamda.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jamieson HA, Hilmer SN, Cogger VC, Warren A, Cheluvappa R, Abernethy DR, Everitt AV, Fraser R, de Cabo R, Le Couteur DG. Caloric restriction reduces age-related pseudocapillarization of the hepatic sinusoid. Exp Gerontol. 2007; 42:374–78. 10.1016/j.exger.2006.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013; 4:2192. 10.1038/ncomms3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mohar DS, Malik S. The sirtuin system: the holy grail of resveratrol? J Clin Exp Cardiolog. 2012; 3:216. 10.4172/2155-9880.1000216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Labbé A, Garand C, Cogger VC, Paquet ER, Desbiens M, Le Couteur DG, Lebel M. Resveratrol improves insulin resistance hyperglycemia and hepatosteatosis but not hypertriglyceridemia, inflammation, and life span in a mouse model for werner syndrome. J Gerontol A Biol Sci Med Sci. 2011; 66:264–78. 10.1093/gerona/glq184 [DOI] [PubMed] [Google Scholar]
- 66.Sen P, Lan Y, Li CY, Sidoli S, Donahue G, Dou Z, Frederick B, Chen Q, Luense LJ, Garcia BA, Dang W, Johnson FB, Adams PD, et al. Histone acetyltransferase p300 induces de novo super-enhancers to drive cellular senescence. Mol Cell. 2019; 73:684–98.e8. 10.1016/j.molcel.2019.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tasdemir N, Banito A, Roe JS, Alonso-Curbelo D, Camiolo M, Tschaharganeh DF, Huang CH, Aksoy O, Bolden JE, Chen CC, Fennell M, Thapar V, Chicas A, et al. BRD4 connects enhancer remodeling to senescence immune surveillance. Cancer Discov. 2016; 6:612–29. 10.1158/2159-8290.CD-16-0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martínez-Zamudio RI, Roux PF, de Freitas JA, Robinson L, Doré G, Sun B, Gil J, Herbig U, Bischof O. AP-1 Imprints a Reversible Transcriptional Program of Senescent Cells. Europe PMC. 2019. [Epub ahead of print]. 10.1101/633594 [DOI] [Google Scholar]
- 69.Mosteiro L, Pantoja C, de Martino A, Serrano M. Senescence promotes in vivo reprogramming through p16 INK4a and IL-6. Aging Cell. 2018; 17:e12711. 10.1111/acel.12711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iwafuchi-Doi M. The mechanistic basis for chromatin regulation by pioneer transcription factors. Wiley Interdiscip Rev Syst Biol Med. 2019; 11:e1427. 10.1002/wsbm.1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mayran A, Drouin J. Pioneer transcription factors shape the epigenetic landscape. J Biol Chem. 2018; 293:13795–804. 10.1074/jbc.R117.001232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sati S, Bonev B, Szabo Q, Jost D, Bensadoun P, Serra F, Loubiere V, Papadopoulos GL, Rivera-Mulia JC, Fritsch L, Bouret P, Castillo D, Gelpi JL, et al. 4D genome rewiring during oncogene-induced and replicative senescence. Mol Cell. 2020; 78:522–38.e9. 10.1016/j.molcel.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang L, Wang X, Wang L, Chiu JD, van de Ven G, Gaarde WA, Deleve LD. Hepatic vascular endothelial growth factor regulates recruitment of rat liver sinusoidal endothelial cell progenitor cells. Gastroenterology. 2012; 143:1555–1563.e2. 10.1053/j.gastro.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang L, Wang X, Xie G, Wang L, Hill CK, DeLeve LD. Liver sinusoidal endothelial cell progenitor cells promote liver regeneration in rats. J Clin Invest. 2012; 122:1567–73. 10.1172/JCI58789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Man AL, Bertelli E, Rentini S, Regoli M, Briars G, Marini M, Watson AJ, Nicoletti C. Age-associated modifications of intestinal permeability and innate immunity in human small intestine. Clin Sci (Lond). 2015; 129:515–27. 10.1042/CS20150046 [DOI] [PubMed] [Google Scholar]
- 76.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol. 2014; 14:189. 10.1186/s12876-014-0189-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol. 2013; 11:1075–83. 10.1016/j.cgh.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018; 24:1246–56. 10.1038/s41591-018-0092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016; 354:472–77. 10.1126/science.aaf6659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duong L, Radley-Crabb HG, Gardner JK, Tomay F, Dye DE, Grounds MD, Pixley FJ, Nelson DJ, Jackaman C. Macrophage depletion in elderly mice improves response to tumor immunotherapy, increases anti-tumor T cell activity and reduces treatment-induced cachexia. Front Genet. 2018; 9:526. 10.3389/fgene.2018.00526 [DOI] [PMC free article] [PubMed] [Google Scholar]


