Abstract
Amyloid-β (Aβ) accumulation is one of the main pathological hallmarks of Alzheimer’s disease (AD). Porphyromonas gingivalis (P. gingivalis), the pathogen of chronic periodontitis, could cause Aβ accumulation and was identified in the brain of AD patients. Salvianolic Acid B (SalB) has been proven to have the neuroprotective effect. Whether SalB could protect against P. gingivalis-induced cognitive impairment is still unknown. In this study, a P. gingivalis-infected mouse model was employed to study the neuroprotective role of SalB. The results showed that SalB (20 and 40 mg/kg) treatment for 4 weeks could shorten the escape latency and improve the percentage of spontaneous alternation in the P. gingivalis-infected mice. SalB inhibited the levels of reactive oxygen species and malondialdehyde, while increased the levels of antioxidative enzymes (superoxide dismutase and glutathione peroxidase). SalB decreased the levels of IL-1β and IL-6, increased the mRNA levels of bdnf and ngf in the brain of P. gingivalis-infected mice. In addition, SalB obviously decreased the level of Aβ. SalB elevated the protein expression of ADAM10, while downregulated BACE1 and PS1. SalB increased the protein expression of LRP1, while decreased RAGE. In conclusion, SalB could improve cognitive impairment by inhibiting neuroinflammation and decreasing Aβ level in P. gingivalis-infected mice.
Keywords: Salvianolic Acid B, Porphyromonas gingivalis, neuroinflammation, Aβ metabolism
INTRODUCTION
Alzheimer’s disease (AD) is an age-related chronic neurodegenerative disease. In clinical, AD is characterized by gradual decline in memory and cognitive ability [1]. Excessive accumulation of amyloid-β (Aβ) and abnormally hyperphosphorylated tau protein are the main pathologies of AD [2]. The Aβ hypothesis is considered as one of the main reason for AD. Aβ accumulation induces neural oxidative stress, neuroinflammation and apoptosis, which lead to synaptic injury, neuronal death and cognitive impairment [3]. Chronic periodontitis (CP), which is caused by Porphyromonas gingivalis (P. gingivalis), has been identified as a risk factor for AD [4, 5]. Recently, in AD patients, infectious agents have been found in the brain, which cause neuroinflammation [6, 7]. Aβ seems to be an antimicrobial peptide against infectious microorganisms [8]. P. gingivalis lipopolysaccharide (LPS) has been found in AD patients’ brains [9]. Treatments targeting Aβ are considered to slow the progression of AD. However, all of the clinical trials were reported failed [10]. The main reason is that drug treatment is too late during AD course. Currently, there are no drugs that could prevent or delay AD progression. Thus, finding effective therapeutic drugs targeting P. gingivalis is a possible way for AD.
Salvia miltiorrhiza is a common Chinese medicinal herb in China. It contains some potential active ingredients, including Salvianolic acid B (SalB; Figure 1A). SalB is extracted from the roots of Salvia miltiorrhiza Bunge [11]. SalB have some well-known pharmacological actions, such as anti-oxidative stress, anti-inflammation [12–14]. Previous studies have indicated that SalB could protect against cardiovascular diseases [15–17]. In addition, SalB also possesses neuroprotective effect against AD. SalB can inhibit Aβ aggregation and fibril formation [18]. Besides, SalB can decrease Aβ level by inhibiting BACE1 activity [19, 20]. However, whether SalB could improve the cognitive impairment in P. gingivalis-infected mice is unclear.
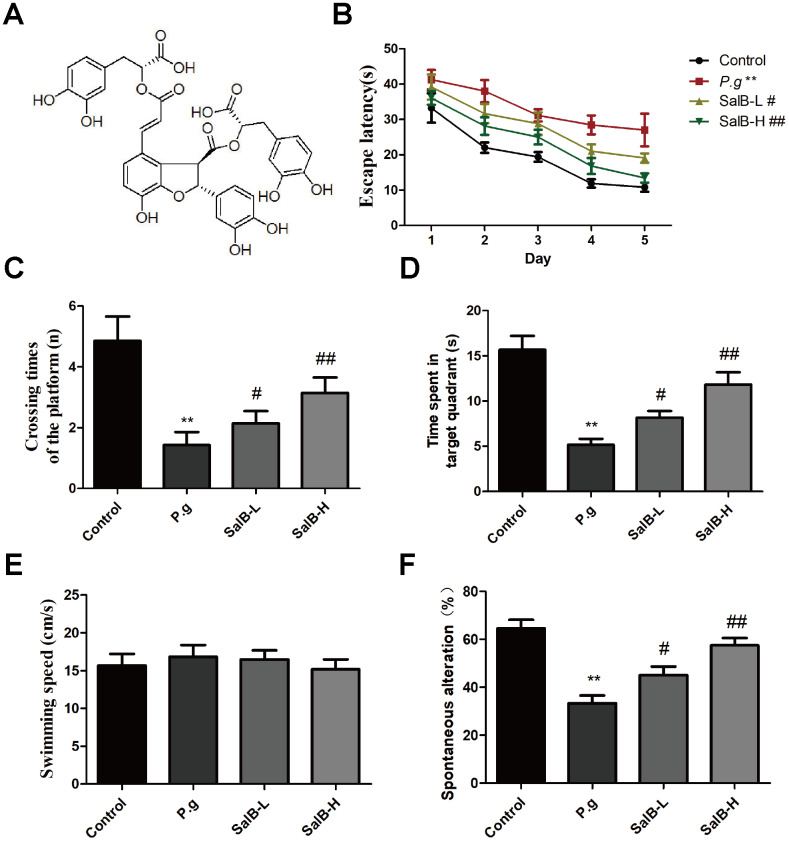
Figure 1.
SalB improves memory deficits in P. gingivalis-infected mice. (A) The molecular structure of SalB; (B) Escape latency of Morris Water Maze test; (C) Crossing times of the platform; (D) Time spend in the target quadrant; (E) Swimming speed; (F) Percentage of spontaneous alternation of Y-maze. Experimental values were expressed as mean ± SEM (n = 15 per group). *P < 0.05, **P < 0.01 vs. Control; #P < 0.05, ##P < 0.01 vs. P.g.
In this study, a new AD model, P. gingivalis-infected mouse model, was employed to investigate the neuroprotective role of SalB. P. gingivalis infection can cause Aβ aggregation and neuroinflammation in the brain, which is similar to AD pathology. Different doses (20 and 40 mg/kg) of SalB were used to treat the mice. We revealed that SalB could obviously protect against P. gingivalis-induced memory deficits by inhibiting neuroinflammation and decreasing Aβ level.
RESULTS
SalB improves memory deficits in P. gingivalis-infected mice
In order to know whether SalB could improve the cognitive impairment in P. gingivalis-infected mice, we firstly tested the effect of SalB on cognitive deficits in P. gingivalis-infected mice by behavioral tests. As shown in the Morris water maze test (Supplementary Figure 1, Figure 1B–1E), the escape latency was obviously increased in P. gingivalis-infected mice, when compared with control group. However, SalB (20 and 40 mg/kg) significantly shortened the time spent in finding the platform. On the 7th day, we removed the platform to estimate the spatial working memory. The P. gingivalis-infected mice had less crossing times and spent shorter time in the target quadrant than control group. The SalB groups increased crossing times and shortened time spent in the target quadrant. The average swimming speed was similar among all groups (Figure 1E). The Y-maze spontaneous alternation test was also used to detect the neuroprotective effect of SalB (Figure 1F). The spontaneous alternation index was impaired in P. gingivalis-infected mice. SalB could improve the spontaneous alternation in P. gingivalis-infected mice. The data suggested that SalB had neuroprotective effect against memory impairment in P. gingivalis-infected mice.
SalB ameliorates oxidative stress and neuroinflammation in P. gingivalis-infected mice
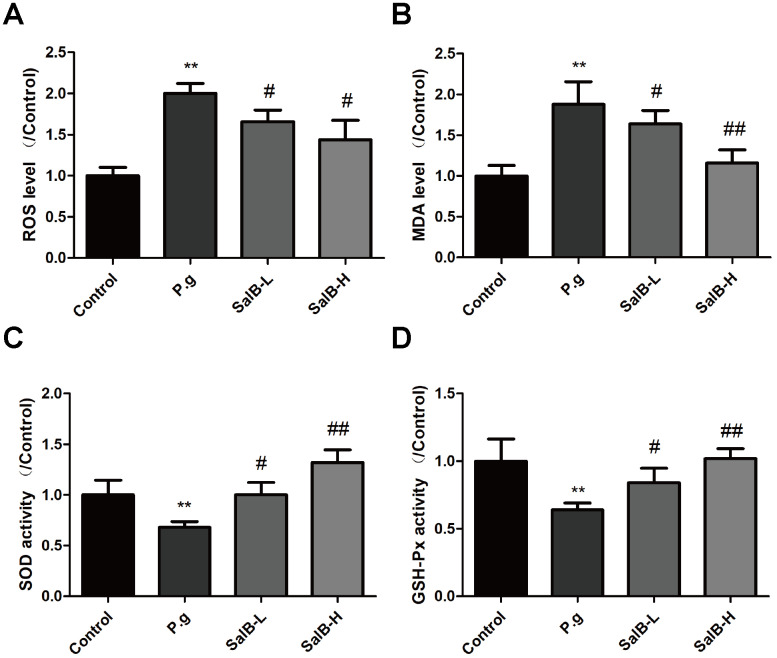
We further investigated the protection against oxidative stress and anti-neuroinflammation effects of SalB. As shown in Figure 2, increased levels of oxidative stress indicators, ROS and MDA, as well as suppressed activities of antioxidant enzymes, SOD and GSH-Px, were observed in the hippocampus of P. gingivalis-infected mice. SalB significantly decreased the levels of ROS and MDA. Moreover, SalB significantly increased the activities of SOD and GSH-Px. In addition, inflammatory factors (IL-1β and IL-6) were also elevated in the hippocampus of P. gingivalis-infected mice, compared with control group (Figure 3). SalB significantly decreased the levels of IL-1β and IL-6. These results indicated that the neuroprotective effects of SalB could be related to its protection against oxidative stress and anti-neuroinflammation effects.
Figure 2.
SalB ameliorates oxidative stress in P. gingivalis-infected mice. The levels of ROS (A), MDA (B) and the enzymes activities of SOD (C), GSH-Px (D) were detected in the hippocampus of P. gingivalis-infected mice. Experimental values were expressed as mean ± SEM (n = 6 per group). *P < 0.05, **P < 0.01 vs. Control; #P < 0.05, ##P < 0.01 vs. P.g.
Figure 3.
SalB ameliorates neuroinflammation in P. gingivalis-infected mice. The inflammatory factors levels of IL-1β (A) and IL-6 (B) in the hippocampus of P. gingivalis-infected mice. Experimental values were expressed as mean ± SEM (n = 6 per group). *P < 0.05, **P < 0.01 vs. Control; #P < 0.05, ##P < 0.01 vs. P.g.
SalB improves the expressions of neurotrophic factors in P. gingivalis-infected mice
We also tested the neurotrophic factors (BDNF and NGF) to evaluate the nervous system function of P. gingivalis-infected mice. As shown in Figure 4, the mRNA levels of bdnf and ngf were decreased in the hippocampus of P. gingivalis-infected mice. After treatment with SalB, the mRNA levels of bdnf and ngf were increased. These data suggested that SalB could increase the gene expressions of bdnf and ngf.
Figure 4.
SalB improves neurotrophic factors in P. gingivalis-infected mice. The mRNA levels of bdnf (A) and ngf (B) were detected in the hippocampus of P. gingivalis-infected mice. Experimental values were expressed as mean ± SEM (n = 6 per group). *P < 0.05, **P < 0.01 vs. Control; #P < 0.05, ##P < 0.01 vs. P.g
SalB decreases Aβ metabolism in P. gingivalis-infected mice
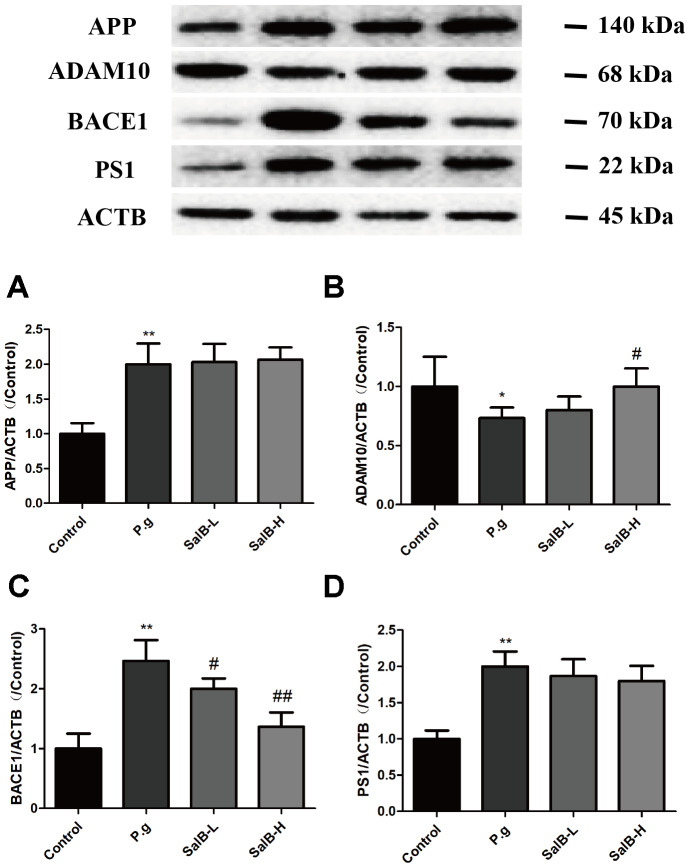
In order to illustrate whether the neuroprotective effect of SalB was related to Aβ, we tested the Aβ level in the hippocampus of P. gingivalis-infected mice (Figure 5). Both Aβ 1-40 and Aβ 1-42 were obviously increased in the hippocampus of of P. gingivalis-infected mice. SalB effectively decreased the Aβ 1-40 and Aβ 1-42 levels. We further studied the related mechanisms. As shown in Figure 6, SalB could elevate the protein expression of ADAM10, while downregulate the protein expressions of BACE1 and PS1. These data indicated that SalB could inhibit Aβ generation, which was in accord with previous study. In addition, Aβ transportation-related proteins were also detected. SalB increased the protein expression of LRP1 and decreased the protein expression of RAGE (Figure 7). These data suggested that Aβ generation and transportation mechanisms were also involved in the neuroprotective effects of SalB.
Figure 5.
SalB decreases Aβ levels in P. gingivalis-infected mice. The levels of Aβ1-40 (A) and Aβ1-42 (B) were detected in the hippocampus of P. gingivalis-infected mice. Experimental values were expressed as mean ± SEM (n = 6 per group). *P < 0.05, **P < 0.01 vs. Control; #P < 0.05, ##P < 0.01 vs. P.g.
Figure 6.
SalB inhibits Aβ generation in P. gingivalis-infected mice. Western blot results of APP (A), ADAM10 (B), BACE1 (C) and PS1 (D) were detected in the hippocampus of P. gingivalis-infected mice. Experimental values were expressed as mean ± SEM (n = 3 per group). *P < 0.05, **P < 0.01 vs. Control; #P < 0.05, ##P < 0.01 vs. P.g.
Figure 7.
SalB improves Aβ transportation in P. gingivalis-infected mice. Western blot results of LRP1 (A) and RAGE (B) were detected in the hippocampus of P. gingivalis-infected mice. Experimental values were expressed as mean ± SEM (n = 3 per group). *P < 0.05, **P < 0.01 vs. Control; #P < 0.05, ##P < 0.01 vs. P.g.
DISCUSSION
In this study, we used a new AD animal model, P. gingivalis-infected mouse model, to study the neuroprotective effect of SalB. P. gingivalis can be found AD patients’ brains and cause Aβ aggregation and neuroinflammation. Behavioral tests (Morris Water Maze test and Y-maze test) suggested that SalB (20 and 40 mg/kg) could ameliorate the memory impairment in P. gingivalis-infected mice. Moreover, SalB could protect neuron against oxidative stress and increase the mRNA levels of neurotrophic factors. In addition, SalB could ameliorate neuroinflammation, inhibit Aβ generation and promote Aβ transportation.
P. gingivalis is mainly found in gingival and periodontal infections [21]. Recent studies have proven that patients with P. gingivalis infection could develop into AD or hasten the progression of AD [6, 22]. Periodontitis allows the bacteria entering the whole body through the bloodstream. AD is clinically characterized by the impairment of learning and memory. A great deal of studies showed that Aβ plays the central role in AD development [23–25]. P. gingivalis can cause Aβ accumulation, which initiates the cascade reaction in AD process. In this study, P. gingivalis-infected mice were employed to mimic AD pathology. To study the neuroprotective effect of SalB, two behavioral tests, including Morris water maze and Y-maze tests, were used to evaluate the learning and memory ability. Results indicated that SalB treatment protected against memory impairment in the P. gingivalis-infected mice.
SalB, an active compound from the roots of Salvia miltiorrhiza Bunge, has been revealed to neuroprotective effects [26, 27], which is mainly related to the anti-Aβ fiber and antioxidant effects [18, 28, 29]. Aβ accumulation in the brain can induce a series of pathological changes, including oxidative stress. Oxidative stress can cause apoptosis of neuron and induce dysfunction of nervous system [30]. ROS generation and increased level of MDA in the brain are the most important indicators of oxidative stress [31, 32]. In addition, antioxidant enzymes, such as SOD and GSH-Px, can be impaired by Aβ fiber [33]. Consistently, an increased oxidative stress statement and a decrease of neurotrophic factors were observed in the brain of P. gingivalis-infected mice. As expected, SalB ameliorated the Aβ-induced oxidative stress and increased the levels of neurotrophic factors in the brain of P. gingivalis-infected mice. These findings suggested that of the antioxidant capacity of SalB might be involved in the neuroprotective effect.
Inhibiting Aβ generation is an effective method to prevent Aβ accumulation. Aβ peptides are generated by APP excision. APP is a transmembrane protein, which can be cleaved by α- (ADAM10), β- (BACE1) and γ-secretase (PS1). The cleavage site of ADAM10 can prevent Aβ generation [34, 35]. When APP is mainly cleaved by BACE1, Aβ generation is increased. Thus, activation of ADAM10 and inhibition of BACE1 is an effective path to reduce Aβ accumulation. Previous studies have reported that SalB could inhibit BACE1 activity in vitro [19, 20]. In this study, we further studied this in vivo. Consistently, SalB decreased the level of Aβ. SalB decreased the protein expressions of BACE1 and PS1 and increased the protein expression of ADAM10 in the brain of P. gingivalis-infected mice. Aβ can also be transported across the BBB to the outside of brain. LRP1 is the main transporter for Aβ efflux at the BBB, while RAGE can transport Aβ from the circulation into the brain [36–38]. Thus, activating LRP1 and inhibiting RAGE can prevent Aβ enter the brain. In this study, SalB effectively increased the protein expression of LRP1 and decreased the protein expression of RAGE in the brain of P. gingivalis-infected mice. These results indicated that SalB could improve cognitive impairment via regulating Aβ metabolism.
In summary, this study provided some evidences that SalB could improve cognitive impairment via regulating neuroinflammation and Aβ metabolism in P. gingivalis-infected mice. SalB might be developed as an anti-AD drug. However, further studies are still needed. We will further study the mechanisms of P. gingivalis-induced Aβ accumulation. In addition, the key molecule of the target in SalB’s effect is still needed to identify.
MATERIALS AND METHODS
Materials
Salvianolic acid B (SalB, purity > 99%) was purchased from the Chinese National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). 2’,7’-dichlo-rofluorescin diacetate (DCFH-DA) were obtained from Invitrogen (Carlsbad, CA, USA). Chemical kits used for the detection of malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) were purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The RNeasy kit was purchased from Qiagen (Hilden, Germany). SuperScript III Reverse Transcriptase was purchased from Invitrogen (Carlsbad, CA, USA). ELISA kits used for the detection of IL-1β, IL-6, Aβ1-40 and Aβ1-42 were purchased from Invitrogen. The blots were probed with the following antibodies: anti-Low density lipoprotein receptor-related protein 1 (LRP1, Abcam); anti-receptor for advanced glycation endproducts (RAGE, Abcam); anti-ACTB (Sigma, Aldrich); secondary antibody horseradish peroxidase- (HRP-) conjugated goat anti-rabbit IgG (Cell Signaling Technology). The Western blot chemiluminescent horseradish peroxidase substrate was purchased from Millipore (USA). All other reagents and chemicals used in the study were of analytical grade.
Animal and treatment
Twelve-month-old male C57BL/6J mice were obtained from the Model Research Centre of Shandong University and were kept in a specific-pathogen-free environment according to protocols approved by the Animal Care and Use Committee of Shandong University. The mice were kept under controlled temperature (22 ± 2 °C) and humidity (55 - 70 %) conditions. A 12-hour light/12- hour dark cycle was maintained with food and water available ad libitum. The mice were kept in the cage for one week to adapt to the environment, a week prior to the experiment.
These mice were infected with P. gingivalis according to previous method [22]. Briefly, mice were infected with P. gingivalis (1×108 CFU/mouse) every 3 days by intraperitoneally injection for 3 weeks. The mice were randomly assigned to four treatment groups (n = 15 per group): vehicle control group (0.9 % saline), P. gingivalis-infected group (P.g), low-dose SalB group (P. gingivalis-infected mice, SalB 20 mg/kg/d), high-dose SalB group (P. gingivalis-infected mice, SalB 40 mg/kg/d). Mice were treated with saline and SalB, respectively, by gavage, once per day for four weeks.
Morris water maze test
The Morris water maze test was used to detect spatial learning and memory ability. The procedure that was followed has been mentioned in previous studies [39]. In brief, the mice were subjected to the navigation test for five consecutive days. For each daily trial, there were four sequential training trials. The escape latency was recorded.
Y-maze test
The Y-maze test was used to evaluate the working memory performance. The procedure has been reported previously [40]. Initially, the mice were placed in one arm of the maze. Subsequently, the number of time each mouse entered the other arm in a 5 min interval was recorded. After behavior tests, all mice were anesthetized with chloral hydrate (intraperitoneal injection) and sacrificed by cervical dislocation. Then, the hippocampus tissues of brain were separated on the ice and frozen in the -80 °C refrigerator for further tests.
Reactive oxygen species (ROS) level
The tissues of the mice hippocampus were homogenized in cold saline. The ROS level was measured by the DCFH-DA fluorescent dye method. Measure fluorescence (Ex/Em = 485/535 nm) in a microplate reader with a fluorescence microscope. The intracellular ROS level obtained was noted.
Malondialdehyde (MDA) level, superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities
The tissues of the mice hippocampus were homogenized in cold saline. The homogenate was centrifuged, and the supernatant was collected to detect the level of MDA and the activities of SOD and GSH-Px using kits (Nianjing Jiancheng Bioengineering Institute, Nanjing, China). The procedures mentioned in the manufacturer’s instructions were followed.
Quantitative PCR
The tissues of the mice hippocampus were homogenized. The total RNA was isolated using an RNeasy kit and reverse-transcribed using SuperScript III Reverse Transcriptase. The gene expression was determined relative to a calibrator and normalized to the housekeeping gene β-actin using the standard curve method. Forward and reverse primers were as follows: Nerve growth factor (Ngf): For, 5′-CAAGGACGCAG CTTTCTATACTG-3′, Rev, 5′-CTTCAGGGACAGAG TCTCCTTCT-3′; Brain-derived neurotrophic factor (Bdnf): For, 5′-TACTTCGGTTGCATGAAGGCG-3′, Rev, 5′-GTCAGACCTCTCGAACCTGCC-3′; β-actin: For, 5′-AGAGCTACGAGCTGCCTGAC-3′, Rev, 5′-AGCACTGTGTTGGCGTACAG-3′.
ELISA
The tissues of the mice hippocampus were homogenized in cold saline. The homogenate was centrifuged, and the supernatant was collected to detect the levels of IL-1β, IL-6, Aβ1-40 and Aβ1-42 by using an ELISA kit (Invitrogen) according to the manufacturer’s instruction.
Western blot analysis
The tissues of the mice hippocampus were homogenized and lysed in the sample buffer. The protein was subjected to SDS-polyacrylamide gel electrophoresis (PAGE) analysis and transferred to PVDF membranes. The membranes were incubated with anti-LRP1, anti-RAGE, anti-ACTB. Then the membrane was incubated with horseradish peroxidase-conjugated anti-rabbit. Finally, the bands on the membrane were visualized.
Statistical analysis
Analysis of the data was performed using GraphPad Prism (V6, California, USA). The results were expressed as the mean ± SD. All statistical analyses were evaluated using Student’s t-test and ANOVA in SPSS 19.0 statistical software (IBM, Endicott, NY). The differences were considered as statistically significant at p < 0.05.
Supplementary Material
Footnotes
AUTHOR CONTRIBUTIONS: Q.S. designed the study. J.L., Y.W. and J.S. conducted the experiments and drafted the manuscript. Q.S. supervised all aspects of the study.
CONFLICTS OF INTEREST: The authors declare that there is no conflicts of interest.
REFERENCES
- 1.Bischof GN, Jacobs HI. Subthreshold amyloid and its biological and clinical meaning: long way ahead. Neurology. 2019; 93:72–79. 10.1212/WNL.0000000000007747 [DOI] [PubMed] [Google Scholar]
- 2.Ji C, Tang M, Johnson GV. Assessing the degradation of tau in primary neurons: the role of autophagy. Methods Cell Biol. 2017; 141:229–44. 10.1016/bs.mcb.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 3.Yang T, Dang Y, Ostaszewski B, Mengel D, Steffen V, Rabe C, Bittner T, Walsh DM, Selkoe DJ. Target engagement in an alzheimer trial: Crenezumab lowers amyloid β oligomers in cerebrospinal fluid. Ann Neurol. 2019; 86:215–224. 10.1002/ana.25513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamer AR, Pirraglia E, Tsui W, Rusinek H, Vallabhajosula S, Mosconi L, Yi L, McHugh P, Craig RG, Svetcov S, Linker R, Shi C, Glodzik L, et al. Periodontal disease associates with higher brain amyloid load in normal elderly. Neurobiol Aging. 2015; 36:627–33. 10.1016/j.neurobiolaging.2014.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, Nguyen M, Haditsch U, Raha D, Griffin C, Holsinger LJ, Arastu-Kapur S, Kaba S, et al. Porphyromonas gingivalis in alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019; 5:eaau3333. 10.1126/sciadv.aau3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panza F, Lozupone M, Solfrizzi V, Watling M, Imbimbo BP. Time to test antibacterial therapy in alzheimer’s disease. Brain. 2019; 142:2905–29. 10.1093/brain/awz244 [DOI] [PubMed] [Google Scholar]
- 7.Schnaider L, Arnon ZA, Gazit E. Reevaluating the microbial infection link to alzheimer’s disease. J Alzheimers Dis. 2020; 73:59–62. 10.3233/JAD-190765 [DOI] [PubMed] [Google Scholar]
- 8.Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, Lefkowitz A, McColl G, Goldstein LE, Tanzi RE, Moir RD. Amyloid-β peptide protects against microbial infection in mouse and worm models of alzheimer’s disease. Sci Transl Med. 2016; 8:340ra72. 10.1126/scitranslmed.aaf1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poole S, Singhrao SK, Kesavalu L, Curtis MA, Crean S. Determining the presence of periodontopathic virulence factors in short-term postmortem alzheimer’s disease brain tissue. J Alzheimers Dis. 2013; 36:665–77. 10.3233/JAD-121918 [DOI] [PubMed] [Google Scholar]
- 10.Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, Masters CL, Targum S, Bush AI, Murdoch R, Wilson J, Ritchie CW, and PBT2-201-EURO study group. Safety, efficacy, and biomarker findings of PBT2 in targeting abeta as a modifying therapy for alzheimer’s disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008; 7:779–86. 10.1016/S1474-4422(08)70167-4 [DOI] [PubMed] [Google Scholar]
- 11.Zhu Z, Ding L, Qiu WF, Wu HF, Li R. Salvianolic acid B protects the myelin sheath around injured spinal cord axons. Neural Regen Res. 2016; 11:487–92. 10.4103/1673-5374.179068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang P, Guo Y, Dang R, Yang M, Liao D, Li H, Sun Z, Feng Q, Xu P. Salvianolic acid B protects against lipopolysaccharide-induced behavioral deficits and neuroinflammatory response: involvement of autophagy and NLRP3 inflammasome. J Neuroinflammation. 2017; 14:239. 10.1186/s12974-017-1013-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Hu Y, E Q, Zuo J, Yang L, Liu W. Salvianolic acid B inhibits mitochondrial dysfunction by up-regulating mortalin. Sci Rep. 2017; 7:43097. 10.1038/srep43097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B, Sun J, Shi Y, Le G. Salvianolic acid B inhibits high-fat diet-induced inflammation by activating the Nrf2 pathway. J Food Sci. 2017; 82:1953–60. 10.1111/1750-3841.13808 [DOI] [PubMed] [Google Scholar]
- 15.Li D, Wang J, Hou J, Fu J, Liu J, Lin R. Salvianolic acid B induced upregulation of miR-30a protects cardiac myocytes from ischemia/reperfusion injury. BMC Complement Altern Med. 2016; 16:336. 10.1186/s12906-016-1275-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Hu W. Salvianolic acid B recovers cognitive deficits and angiogenesis in a cerebral small vessel disease rat model via the STAT3/VEGF signaling pathway. Mol Med Rep. 2018; 17:3146–51. 10.3892/mmr.2017.8203 [DOI] [PubMed] [Google Scholar]
- 17.Yu J, Chen R, Tan Y, Wu J, Qi J, Zhang M, Gu W. Salvianolic acid B alleviates heart failure by inactivating ERK1/2/GATA4 signaling pathway after pressure overload in mice. PLoS One. 2016; 11:e0166560. 10.1371/journal.pone.0166560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Y, Jia K, Li L, Wang Q, Zhang S, Du J, Du H. Salvianolic acid B attenuates mitochondrial stress against Aβ toxicity in primary cultured mouse neurons. Biochem Biophys Res Commun. 2018; 498:1066–72. 10.1016/j.bbrc.2018.03.119 [DOI] [PubMed] [Google Scholar]
- 19.Durairajan SS, Chirasani VR, Shetty SG, Iyaswamy A, Malampati S, Song J, Liu L, Huang J, Senapati S, Li M. Decrease in the generation of amyloid-β due to salvianolic acid B by modulating BACE1 activity. Curr Alzheimer Res. 2017; 14:1229–37. 10.2174/1567205014666170417103003 [DOI] [PubMed] [Google Scholar]
- 20.Tang Y, Huang D, Zhang MH, Zhang WS, Tang YX, Shi ZX, Deng L, Zhou DH, Lu XY. Salvianolic acid B inhibits Aβ generation by modulating BACE1 activity in SH-SY5Y-APPsw cells. Nutrients. 2016; 8:333. 10.3390/nu8060333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puig-Silla M, Montiel-Company JM, Dasí-Fernández F, Almerich-Silla JM. Prevalence of periodontal pathogens as predictor of the evolution of periodontal status. Odontology. 2017; 105:467–76. 10.1007/s10266-016-0286-x [DOI] [PubMed] [Google Scholar]
- 22.Nie R, Wu Z, Ni J, Zeng F, Yu W, Zhang Y, Kadowaki T, Kashiwazaki H, Teeling JL, Zhou Y. Porphyromonas gingivalis infection induces amyloid-β accumulation in monocytes/macrophages. J Alzheimers Dis. 2019; 72:479–94. 10.3233/JAD-190298 [DOI] [PubMed] [Google Scholar]
- 23.Cataldo AM, Petanceska S, Terio NB, Peterhoff CM, Durham R, Mercken M, Mehta PD, Buxbaum J, Haroutunian V, Nixon RA. Abeta localization in abnormal endosomes: association with earliest abeta elevations in AD and down syndrome. Neurobiol Aging. 2004; 25:1263–72. 10.1016/j.neurobiolaging.2004.02.027 [DOI] [PubMed] [Google Scholar]
- 24.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, Orgogozo JM, and AN1792(QS-21)-201 Study Team. Clinical effects of abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005; 64:1553–62. 10.1212/01.WNL.0000159740.16984.3C [DOI] [PubMed] [Google Scholar]
- 25.Thal DR, Rüb U, Orantes M, Braak H. Phases of a beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002; 58:1791–800. 10.1212/wnl.58.12.1791 [DOI] [PubMed] [Google Scholar]
- 26.Kim DH, Park SJ, Kim JM, Jeon SJ, Kim DH, Cho YW, Son KH, Lee HJ, Moon JH, Cheong JH, Ko KH, Ryu JH. Cognitive dysfunctions induced by a cholinergic blockade and Aβ 25-35 peptide are attenuated by salvianolic acid B. Neuropharmacology. 2011; 61:1432–40. 10.1016/j.neuropharm.2011.08.038 [DOI] [PubMed] [Google Scholar]
- 27.Liu CS, Chen NH, Zhang JT. Protection of PC12 cells from hydrogen peroxide-induced cytotoxicity by salvianolic acid B, a new compound isolated from radix salviae miltiorrhizae. Phytomedicine. 2007; 14:492–97. 10.1016/j.phymed.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 28.Fan Y, Luo Q, Wei J, Lin R, Lin L, Li Y, Chen Z, Lin W, Chen Q. Mechanism of salvianolic acid B neuroprotection against ischemia/reperfusion induced cerebral injury. Brain Res. 2018; 1679:125–33. 10.1016/j.brainres.2017.11.027 [DOI] [PubMed] [Google Scholar]
- 29.Xue L, Wu Z, Ji XP, Gao XQ, Guo YH. Effect and mechanism of salvianolic acid B on the myocardial ischemia-reperfusion injury in rats. Asian Pac J Trop Med. 2014; 7:280–84. 10.1016/S1995-7645(14)60038-9 [DOI] [PubMed] [Google Scholar]
- 30.Tönnies E, Trushina E. Oxidative stress, synaptic dysfunction, and alzheimer’s disease. J Alzheimers Dis. 2017; 57:1105–21. 10.3233/JAD-161088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang T, Sun Q, Chen S. Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in parkinson’s disease and alzheimer’s disease. Prog Neurobiol. 2016; 147:1–19. 10.1016/j.pneurobio.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 32.Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S, Nath C, Tyagi N. Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of alzheimer’s disease: understanding the therapeutics strategies. Mol Neurobiol. 2016; 53:648–61. 10.1007/s12035-014-9053-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Z, Zhong C. Oxidative stress in alzheimer’s disease. Neurosci Bull. 2014; 30:271–81. 10.1007/s12264-013-1423-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003; 37:925–37. 10.1016/s0896-6273(03)00124-7 [DOI] [PubMed] [Google Scholar]
- 35.Shaked GM, Kummer MP, Lu DC, Galvan V, Bredesen DE, Koo EH. Abeta induces cell death by direct interaction with its cognate extracellular domain on APP (APP 597-624). FASEB J. 2006; 20:1254–56. 10.1096/fj.05-5032fje [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai Z, Liu N, Wang C, Qin B, Zhou Y, Xiao M, Chang L, Yan LJ, Zhao B. Role of RAGE in alzheimer’s disease. Cell Mol Neurobiol. 2016; 36:483–95. 10.1007/s10571-015-0233-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo YX, He LY, Zhang M, Wang F, Liu F, Peng WX. 1,25-dihydroxyvitamin D3 regulates expression of LRP1 and RAGE in vitro and in vivo, enhancing Aβ1-40 brain-to-blood efflux and peripheral uptake transport. Neuroscience. 2016; 322:28–38. 10.1016/j.neuroscience.2016.01.041 [DOI] [PubMed] [Google Scholar]
- 38.Ma LY, Fei YL, Wang XY, Wu SD, Du JH, Zhu M, Jin L, Li M, Li HL, Zhai JJ, Ji LP, Ma RR, Liu SF, et al. The research on the relationship of RAGE, LRP-1, and Aβ accumulation in the hippocampus, prefrontal lobe, and amygdala of STZ-induced diabetic rats. J Mol Neurosci. 2017; 62:1–10. 10.1007/s12031-017-0892-2 [DOI] [PubMed] [Google Scholar]
- 39.Anderson EM, Moenk MD, Barbaro L, Clarke DA, Matuszewich L. Effects of pretraining and water temperature on female rats’ performance in the morris water maze. Physiol Behav. 2013; 122:216–21. 10.1016/j.physbeh.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 40.Lainiola M, Procaccini C, Linden AM. mGluR3 knockout mice show a working memory defect and an enhanced response to MK-801 in the T- and y-maze cognitive tests. Behav Brain Res. 2014; 266:94–103. 10.1016/j.bbr.2014.03.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.