Abstract
The emergence of antimicrobial resistance in Gram-negative bacteria poses a huge health challenge. The therapeutic use of polymyxins (i.e., colistin and polymyxin B) is commonplace due to high efficacy and limiting treatment options for multidrug-resistant Gram-negative bacterial infections. Nephrotoxicity and neurotoxicity are the major dose-limiting factors that limit the therapeutic window of polymyxins; nephrotoxicity is a complication in up to ~60% of patients. The emergence of polymyxin-resistant strains or polymyxin heteroresistance is also a limiting factor. These caveats have catalyzed the search for polymyxin combinations that synergistically kill polymyxin-susceptible and resistant organisms and/or minimize the unwanted side effects. Curcumin—an FDA-approved natural product—exerts many pharmacological activities. Recent studies showed that polymyxins–curcumin combinations showed a synergistically inhibitory effect on the growth of bacteria (e.g., Gram-positive and Gram-negative bacteria) in vitro. Moreover, curcumin co-administration ameliorated colistin-induced nephrotoxicity and neurotoxicity by inhibiting oxidative stress, mitochondrial dysfunction, inflammation and apoptosis. In this review, we summarize the current knowledge-base of polymyxins–curcumin combination therapy and discuss the underlying mechanisms. For the clinical translation of this combination to become a reality, further research is required to develop novel polymyxins–curcumin formulations with optimized pharmacokinetics and dosage regimens.
Keywords: polymyxin B, colistin, curcumin, oxidative stress, mitochondrial dysfunction
1. Introduction
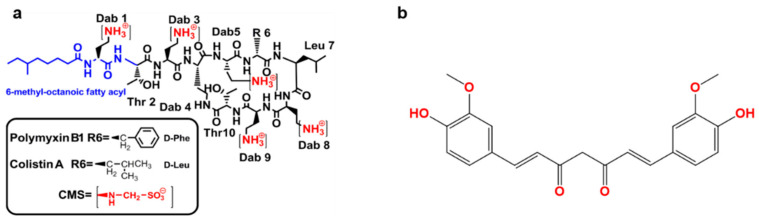
The World Health Organization [1] has highlighted antimicrobial resistance as a major global health concern [1]. As no new classes of antibiotics will be available for Gram-negative ‘superbugs’ in the near future, we have to develop novel polymyxin combination therapies [2]. Polymyxins were firstly discovered in 1947 from different species of Bacillus polymyxa [3]. There are five members in the polymyxins family, i.e., polymyxin A, B, C, D and E (also named colistin). Out of these, only polymyxins B and colistin are used in clinical practice, as agents against multi-drug resistant (MDR) Gram-negative pathogens, in particular Pseudomonas aeruginosa (P. aeruginosa) Acinetobacter baumannii (A. baumannii) and Klebsiella pneumoniae (K. pneumoniae). They differs by only one amino acid (Figure 1a) [4,5,6,7,8,9,10,11].
Figure 1.
The structure of polymyxin B and colistin (a) and curcumin (b). CMS—colistin methanesulfonate; Thr—threonine; Leu—leucine; Phe—phenylalanine; Dab—α,γ- diaminobutyric acid.
Unlike polymyxin B, which is available in the clinic as the sulfate salt, colistin is used in the clinic as an inactive pro-drug, colistin methanesulfonate (CMS) (Figure 1a) [12,13]. Polymyxin B is available for intravenous, oral and topical use and CMS for parenteral use; both can be delivered by inhalation [14]. Plasmid carried mobilized colistin resistance (mcr) gene-mediated polymyxin resistance has been increasingly reported in MDR Gram-negatives worldwide. Polymyxin resistance implies a total lack of antibiotics against Gram-negative ‘superbugs’, without the availability of novel antibiotics in the near future, the development of superior polymyxin combinations to combat a ‘post-antibiotic era’ is paramount [15,16]. This has brought the effectiveness of polymyxin monotherapy into question. Unfortunately, only increasing the dose of polymyxins to overcome resistance is not an option due to toxic side effects (i.e., nephrotoxicity and neurotoxicity) [17]. Previous clinical observations showed that the rates of nephrotoxicity occurred in approximately 60% after patients received colistin or polymyxin B therapy [18]. Based on the narrow therapeutic indices of polymyxins, a key strategy for overcoming resistance and concomitantly ablating unwanted side effects is combining polymyxins with other agents. Although polymyxin-antibiotic combinations exhibit well-confirmed synergistic effects against bacterial growth in vitro [19,20]. The clinical findings from randomized, controlled prospective trials have shown that these combinations have no increased benefits compared to their respective monotherapies [19,20]. Moreover, some polymyxin-antibiotic combinations may increase the risk of renal failure (e.g., colistin–vancomycin combination) [20].
Secondary metabolite natural products have been at the forefront of drug candidates for the treatment of cancer, infection and neurodegenerative diseases for decades [21]. Natural products have been the source of most of our antibacterial armamentarium and efforts over the last 30 years [22]. Recent studies have shown that combining polymyxins with curcumin has many pharmacological and toxicological benefits over monotherapy with each compound per se including: (1), the in vitro synergy against various strains of MDR bacteria [23], including polymyxin resistant isolates [24]; (2), protection against polymyxin-induced nephron- and neuro-toxicity [25,26,27]; (3), curcumin supplementation improves recovery from the bacterial infection or LPS—induced inflammatory response or sepsis [28,29,30,31,32,33]. Importantly, human clinical trials indicated that curcumin displays good safety and tolerability [34,35,36]; oral administration of curcumin at eight grams per day over three months or a single oral dose of 12 grams has no marked adverse effect [37,38].
In combination with curcumin, polymyxins hold great promise as combination therapy for infections caused by MDR Gram-negative pathogens. In this review, we summarize the current understanding of the underlying molecular mechanisms on nephro- and neuroprotection and synergistic effects on bacterial growth of this novel combination.
2. Curcumin
Curcumin, also known as diferuloylmethane, E100 or natural yellow 3 (International Union of Pure and Applied Chemistry (IUPAC) name is (1E, 6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, Figure 1b), is a natural polyphenol extracted from the rhizome of Curcuma longa [39]. Curcumin displays many beneficial pharmacological actives, such as anti-inflammatory, anti-oxidant, antitumor and notably, antimicrobial activities [24,40,41,42,43]. The direct antibacterial activities of curcumin have been widely studied [44,45]. It is purported that the anti-inflammation and antioxidant abilities may contribute to the indirect antibacterial activities of curcumin by modulating the interaction of host cells with bacteria or via increasing the potential loading dose of a combination antibiotic drug by inhibiting the unwanted adverse effects.
2.1. Antibacterial Activity of Curcumin
Curcumin exhibits antibacterial activities against both Gram-negative and Gram-positive bacteria, including MDR and polymyxin-resistant isolates [44,45]. Curcumin has been shown to disrupt filamenting temperature-sensitive mutant Z (FtsZ) protofilament activity that orchestrates bacterial cell division [46]. In contrast to its action on mammalian cells, in bacteria, curcumin induces the production of reduced reactive oxygen species (ROS), including superoxide anions (O2•−), hydroxyl radicals (•OH) and hydrogen peroxide (H2O2), which kills bacteria by damaging proteins, lipids and DNA [47,48,49]. ROS-mediated phototoxicity also contributes to the antibacterial activities of curcumin [50]. Curcumin inhibits the expression of biofilm initiation genes and quorum sensing (QS) genes, and downregulates the virulence factors including the production of acyl-homoserine lactone (HSL), pyocyanin biosynthesis and elastase/protease activity [51,52]. A study from Mun et al., showed that the minimum inhibitory concentration (MIC) values of curcumin against 10 strains of Staphylococcus aureus (S. aureus) ranged from 125 to 250 μg/mL [53]. Moreover, curcumin displays significant antibacterial activity against the stomach ulcer-causing pathogen Helicobacter pylori (H. pylori) with MIC values ranging from 5 to 50 μg/mL against 65 clinical isolates [54]. Another study with a crude aqueous rhizome extract of Curcuma longa showed MIC value of 4 to 16 μg/mL against strains of S. epidermis ATCC 12228, S. aureus ATCC 25923, K. pneumonia ATCC 10031 and E. coli ATCC 25922 [55]. The rhizome extract of Curcuma longa includes primarily curcumin and other derivative compounds such as curdione, isocurcumenol, curcumenol, curzerene, β-elemene, germacrone and curcumol [56]. Notwithstanding its direct antibacterial activities, curcumin displays potent synergistic effects when combined with antibiotics (e.g., oxacillin, ampicillin, polymyxin B and norfloxacin) [23,53].
The therapeutic potential of curcumin is limited owing to its poor oral bioavailability and insufficient solubility in aqueous solvents. Therefore, oral curcumin often present poor absorption, fast metabolism and quick systemic elimination in animal experiment and human clinical trial [44,45,52]. Researchers have attempted to solve these problems by developing new drug delivery methods such as liposomes, solid dispersion, microemulsion, micelles, nanogels and dendrimers [52]. For example, the poly (lactic-co-glycolic acid) (PLGA) polymeric nanocapsules for the delivery of curcumin can enhance its solubility (increase by ~1500-fold, compared to free curcumin) and antibacterial activity (MIC values decrease by ~2-fold, compared to free curcumin) [57]. In another example, curcumin–β-cyclodextrin nanoparticle complex formation exhibited a potent bactericidal activity by increasing the production of ROS and inhibiting electron transport; polyelectrolyte-coated monolithic nanoparticle formation exhibited a potent bacteriostatic effect by increasing membrane depolarization and reducing ATP concentrations [58].
2.2. Effect of Curcumin on Bacteria or Its Toxin-Induced Inflammatory Response
The curcumin structure has functional groups that contribute to its ability to scavenge ROS including phenyl rings, carbon–carbon double bonds and β-diketone structures [59]. Curcumin also directly targets a plethora of pathways that play important roles in the inflammatory response, oxidative stress and cell death, including cyclooxygenase 2 (COX-2), lipoxygenase, protein kinase B (PKB, also named Akt), toll-like receptor (TLR)-4, nuclear factor erythroid 2-related factor 2 (Nrf2), glycogen synthase kinase (GSK)-3β, phosphorylase-3 kinase, focal adhesion kinase, glutathione, xanthine oxidase, pp60 src tyrosine kinase and ubiquitin isopeptidase [60,61,62,63]. These signals and/or pathways also play critical roles in response to bacterial infection or its pathogenic factors (e.g., α-hemolysin and lipopolysaccharide [LPS])-mediated pathology [29,64,65,66]. For example, curcumin has an inhibitory effect on NF-κB activation, the release of interleukin (IL)-8 and matrix metalloproteinase-3 and metalloproteinase-9 activity induced by H. pylori infection in the stomach of mice or cell culture with a dose-dependent manner [67,68]. Curcumin can also inhibit LPS-induced activation of NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome and the secretion of IL-1β and high mobility group box 1 (HMGB1) [69,70,71].
3. Synergistic Antibacterial Effects of the Polymyxin in Combination with Curcumin
The putative primary mechanism of action of polymyxins is the disruption of the Gram-negative outer membrane through an initial electrostatic interaction that results in cation displacement (Ca2+ and Mg2+) and subsequent binding to the lipid A component of LPS, leading to leakage of the cytoplasmic content and ultimately causing cell death [72]. As a secondary killing effect, polymyxins induce ROS [73]. The inhibition of bacterial respiration has also been associated with the bacterial killing action of polymyxins [74].
Polymyxin resistance is conferred by mcr-mediated structural modifications of LPS that act to reduce the negative charge of the outer membrane, which in turn repels the polymyxin molecule [75]. The two main LPS modifications conferring polymyxin resistance are the addition of phosphoethanolamine (PetN) and 4-amino-4-deoxy-L-arabinose (AraN) to the lipid A [76]. Recently, plasmid-mediated colistin resistance was reported in Enterobacteriaceae due to the PetN transferase mcr-1 [15].
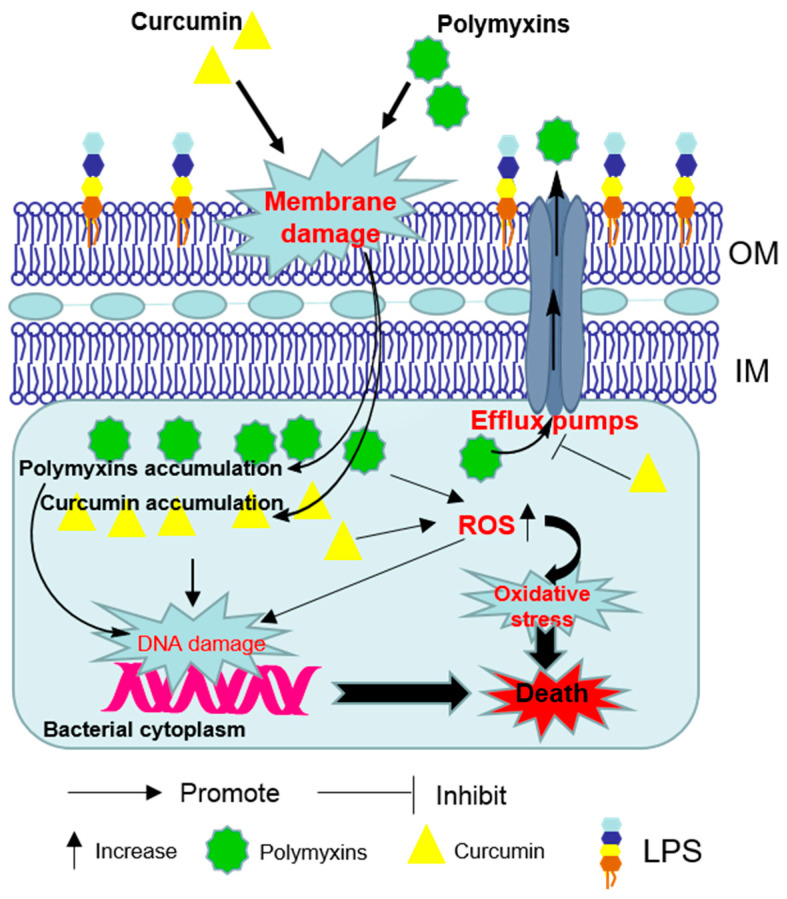
In vitro time-kill curves for polymyxin B in combination with curcumin showed a marked synergetic effect against antibiotic-susceptible and—resistant Gram-positive (Enterococci, S. aureus and streptococci) and Gram-negative (A. baumannii, E. coli, P. aeruginosa and S. maltophilia) bacterial isolates associated isolated from traumatic wound infections [23]. The synergistic effect may result from the ability of polymyxin to permeabilize the outer membrane which facilitates the access of greater concentrations of curcumin to its intracellular targets (Figure 2) [24]. Curcumin may also overcome the polymyxin-resistance by inhibiting the activities of efflux pumps where located in outer membrane (OM) or inner membranes (IM) [24].
Figure 2.
Schematic diagram depicting the synergistic bacterial killing mechanism of the polymyxins–curcumin combination. Polymyxins permeabilize the Gram-negative outer membrane and thereby promote curcumin enter the bacterial cell. Bacterial cell death finally ensues via reactive oxygen species (ROS) production, oxidative stress and DNA damage. Curcumin may also overcome the polymyxin-resistance by disturbing the activities of efflux pumps. OM—outer membrane; IM—inner membranes; LPS—lipopolysaccharide.
4. Polymyxin-Induced Nephrotoxicity and Protective Effect of Curcumin
Nephrotoxicity is the major dose-limiting factor of polymyxins in clinical practice and it occurs in up to 60% of patients after intravenous administration [18]. Clinical manifestations include a decrease in creatinine (CRE) clearance, as well as proteinuria, oliguria (low output of urine) and cylindruria (presence of casts in the urine) [18,77,78]. Pathologic characteristics of colistin or polymyxin B-induced nephrotoxicity were included tubular dilation and tubular epithelial cell vacuolation and necrosis, tubular casts and inflammatory cell infiltration [79,80,81]. In clinical practice, serum CRE and blood urea nitrogen (BUN) are usually employed as the biomarkers of renal function. However, their use as the biomarkers has some limitations in polymyxin therapy, such as dependence on nutrition, age, sex and body mass and is likely to reflect already advanced damage. Studies in animal models showed that kidney injury molecule 1 (KIM-1), Cystatin C and α-glutathione S-transferase may be more reliable markers than CRE or BUN to monitor renal function during polymyxin therapy [82,83].
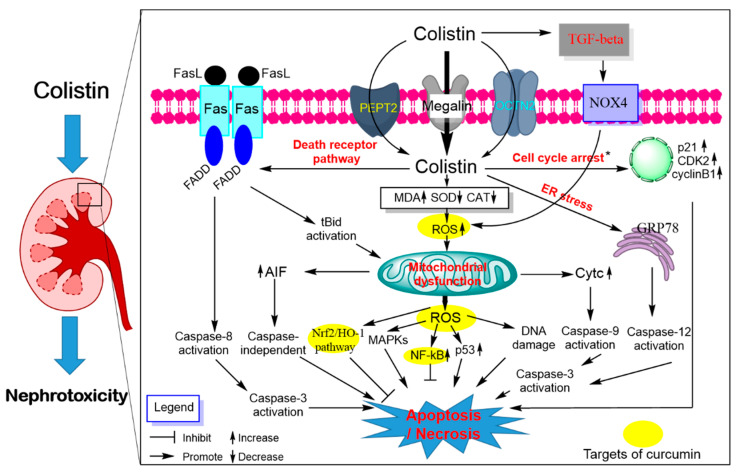
Our understanding of the molecular mechanisms underlying polymyxin-induced nephrotoxicity have significantly advanced in the past 15 years owing to the pioneering work of the Li group from Monash University, Australia [84,85,86,87,88,89,90,91,92,93,94,95,96]. Their recent studies have revealed significant renal accumulation of polymyxins using immunostaining, fluorescence microscopy, mass spectrometry imaging and X-ray fluorescence microscopy (XFM) [84,85,86,87]. Predominant accumulation of polymyxin B was distinct in the renal cortex, in particular the renal proximal tubular cells, but much less in the distal tubular cells [84,85]. Moreover, polymyxins substantially accumulate in proximal tubular cells via receptor-mediated endocytosis mainly by megalin [97,98], human peptide transporter 2 (PEPT2, syn. SLC15A2) [91,96] and the carnitine/organic cation transporter 2 (OCTN2, syn. SLC22A5) [99] (Figure 3). Inhibitors of these receptors were shown to block the uptake of polymyxins and then attenuate colistin or polymyxin B- induced nephrotoxicity in mice [91,97,100].
Figure 3.
Pathways involved in polymyxin-induced nephrotoxicity and the potential nephro-protective mechanism of curcumin. Colistin extensively accumulates in kidney tubular cells via receptor-mediated uptake, megalin, peptide transporter 2 (PEPT2) and the carnitine/organic cation transporter 2 (OCTN2). Intracellular colistin accumulation in kidney tubular cells induces the production of ROS, mitochondrial dysfunction and triggers a series of signaling cascades (e.g., cell cycle arrest, p53, nuclear factor kappa B [NF-κB] and mitogen-activated protein kinase [MAPK], nuclear factor erythroid 2-related factor 2 [Nrf2]/heme oxygenase-1 [HO-1] pathways). The activation of the transforming growth factor (TGF)-β/nicotinamide adenine dinucleotide 3-phosphate oxidase-4 (NOX-4) pathway contributes to oxidative stress by promoting ROS production. All three major apoptosis pathways (e.g., mitochondrial, death receptor and endoplasmic reticulum pathways) participated in colistin-induced nephrotoxicity. Curcumin treatment improved colistin-induced nephrotoxicity by targeting the NF-kB mediated inflammatory response, oxidative stress and upregulating the antioxidant Nrf2/HO-1 pathways.
Polymyxin treatment can lead to DNA damage and apoptotic cell death in renal tubular cells in the cultured cell model or in vivo animal model [93,101,102,103] (Figure 3). Our group has been pioneering this area in reporting a series of studies showing that polymyxin B or colistin-induced apoptosis involved the death receptor (upregulation of Fas, FasL and Fas-associated death domain [FADD]), mitochondrial (downregulation of B-cell lymphoma 2 [Bcl-2] and upregulation of cytochrome C [CytC] and bcl-2-like protein 4 [Bax] and endoplasmic reticulum (upregulation of activating transcription factor 6 [ATF6], glucose-regulated protein 78 [GRP78], caspase-12 and growth arrest and DNA damage-inducible gene 153 [GADD153/CHOP]) pathways in cultured renal tubular cells [101] and the kidney tissue of mouse [93]. Polymyxin B or colistin treatment can induce loss of mitochondrial membrane potential, morphology changes and the generation of ROS mediated oxidative damage in a concentration-dependent manner [93,101]. Significant Elevated protein expression levels of the p53, cyclin-dependent kinase 2 (CDK2) and phosphorylated Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) and autophagy were detected in kidney tissues of the colistin-treated mice [93]. The lipid peroxidation marker malondialdehyde (MDA), nitrative stress-related nitric oxide (NO) and inducible nitric oxide synthase activities and inflammation response were significantly increased in the kidney tissues from colistin-treated mice [80,104]. Additionally, the activation of transforming growth factor (TGF)-β/nicotinamide adenine dinucleotide 3-phosphate oxidase-4 (NOX-4) pathway contributes to the production of ROS in kidneys of mice; inhibition of TGF-β expression markedly attenuated colistin-induced renal damage in mice, indicating that TGF-β/NOX-4 pathway may play a critical role in colistin-induced nephrotoxicity [105].
Curcumin has been shown to protect against colistin-induced nephrotoxicity via inhibition of oxidative stress, inflammation and apoptosis. A previous study reported that curcumin supplementation (e.g., at 200 mg/kg/day for 3 days) markedly protects against glycerol-induced acute kidney injury by inhibiting oxidative damage and activating Nrf2 pathway in rats [106]. A similar molecular mechanism has also been detected in the protection of curcumin against gentamicin-induced nephrotoxicity in rats [107]. Consistent with these findings, curcumin supplementation (e.g., at 200 mg/kg/day for 6 days) can also markedly improve colistin-induced nephrotoxicity via the inhibition of oxidative stress, apoptosis, NO signaling and inflammatory response (i.e., decreases the tumor necrosis factor-α [TNF-α], interleukin-6 [IL-6] in the kidney tissue) in a rat model [27]. Our recent data reveal that curcumin after oral administration at 50 and 200 mg/kg/day for 7 days can be detected and accumulated in multiple organs of mice, including kidney tissues [25]. It was shown that curcumin can promote Nrf2 translocation into nuclei through modification of cysteine sulfhydryl groups in Kelch-like ECH-associated protein 1 (Keap1), a principal negative regulator of Nrf2 in the cytoplasm. The binding of Nrf2 and antioxidant responsive element (ARE) in nuclei activated the transcription activity of ARE that mediates the expression of antioxidant gene such as heme oxygenase-1 (HO-1), CAT and SOD [108]. Indeed, curcumin can directly activate Nrf2/ARE pathway and protects against oxidative stress-mediated apoptotic cell death [109]. Therefore, the activation of Nrf2/HO-1 may contribute to the protective effect of curcumin against colistin–oxidativedamage in the kidney tissues. Besides, it has been reported that curcumin supplementation can protect some drugs (e.g., cisplatin, glycerol and doxorubicin) or toxins (e.g., potassium dichromate, maleate and arsenic)-induced nephrotoxicity by inhibiting oxidative stress, ER stress, inflammatory response, NO pathway, ferroptosis, p53 pathway, MAPK, AMP-activated protein kinase (AMPK) pathways, Akt pathway or activation of autophagy [106,110,111,112,113,114,115,116]. The previous study showed that the antioxidant ascorbic acid can protect against colistin-induced nephrotoxicity and cellular apoptosis. Meanwhile, ascorbic acid altered the pharmacokinetics of colistin in a rat model, the total body clearance of colistin decreased from 3.78 ± 0.36 mL/min/kg (colistin alone group) to 2.46 ± 0.57 mL/min/kg (ascorbic acid + colistin co-treatment group), and the half-life of plasma colistin concentration increased from 1.20 ± 0.23 (colistin alone group) h to 3.91 ± 0.42 h (ascorbic acid + colistin co-treatment group) [94].
5. Colistin-Induced Neurotoxicity and Protective Effects of Curcumin
Neurotoxicity caused by colistin has been observed in a dose-dependent manner that may be associated with colistin accumulation in the nerve tissues [25]. In contrast to nephrotoxicity, the incidence of neurotoxicity associated with the use of polymyxins is less commonly reported in the literature, despite being a significant clinical complication [117]. Patients who received intravenous polymyxins have been reported to present with potential neurological symptoms including dizziness, vertigo, visual disturbances, hallucinations, confusion, seizures, ataxia, facial and peripheral paresthesias [117,118,119]. Mild neurotoxicity symptoms (e.g., paresthesias) is more frequent than others, especially in elderly patients, but are often ignored due to the lack of effective assessment protocols [117,118,119,120]. An early study reported that paresthesias and ataxia as the major neurotoxicity symptoms occur about in the 29% of patients that received intravenous administration of CMS at the dose of over 5 mg/kg/day (range from 5.7 to 8.0 mg/kg/day) [121]. A recent study indicated that intravenous administration of high doses of polymyxin B (range from 3 to 6 mg/kg/day) may increase the clinical outcome, but the neurotoxicity events also increased in patients [122].
Investigation of polymyxin uptake into the central nervous system (CNS) and neuronal cells is a critical aspect of understanding the neurotoxicity. The blood–brain barrier (BBB) is the “first barrier” that mediates the uptake of the drug into neuronal cells in the brain tissues, and it only allows small molecules that both have the low molecular mass (<450 Da) and high lipid solubility to pass [123]. The tight junctions of the interendothelial domains limit the passage of large hydrophilic molecules cross the BBB and this is considered as the main reason underlying the low BBB penetration of polymyxins (molecular mass of colistin and polymyxin B are ~1155 Da and ~1, 203, respectively) [124]. This may also explain the higher incidence in peripheral nervous system (PNS)- than CNS- mediated polymyxin neurotoxicity in clinic [117,119,125]. It is negligible in the brain uptake of colistin when healthy mice were received a single intravenous dose (5 mg/kg) or subcutaneous dose (40 mg/kg) [124,126,127,128]. However, Wang et al., showed that the continuous administration of intravenous colistin sulfate at 15 mg/kg/day for 7 days to mice significantly increased the colistin concentration in brain tissues without BBB damage [129]. Consistently, our group showed that colistin could be accumulated in nerve tissues including the brain, cerebellum and sciatic nerve with the abnormal neurobehavioral and electrophysiology changes when mice were intraperitoneally injected with colistin sulfate at 18 mg/kg/day for 7 days [25,120,130,131,132]. It has also been demonstrated that the intravenous administration of LPS can increase the BBB transport of colistin in mice, indicating that systemic inflammation (e.g., CNS infections and sepsis) may increase the colistin concentrations in the brain tissues [124,126,127,128]. Our in vitro studies have shown that the absorption of colistin into mouse primary cortical neuronal cells is in a concentration-dependent manner [133]. As above mentioned, megalin, PEPT2, and OCTN2 can mediate the uptake of colistin into kidney cells [91,97,99,134]. Notably, the expressions of megalin, PEPT2, and OCTN2 are also detected in neuronal cells [135,136,137], and as such may mediate polymyxin uptake into neurons.
Due to its high oxygen use and high content of polyunsaturated fatty acids, the CNS was well known to be particularly vulnerable to oxidative damage [138]. Mitochondria play a critical role in maintaining basic cellular functions, including cellular energy metabolism, ATP production and triggering cellular apoptosis and autophagy [138]. We have shown that mitochondria in the cerebrum and sciatic nerve tissues showed abnormal ultrastructure (e.g., disruption of cristae and swelling) and functions when mice were intravenously injected with colistin at 15 mg/kg/day for 7 days [131,139]. Mitochondrial dysfunction was also detected in primary chick neuronal cells when they were treated with colistin at 4.15 and 8.3 μg/mL for 24 h [140]. Excessive ROS from mitochondria can lead to lipids, proteins and DNA damage and ultimately results in cell death [141]. Our recent study showed that colistin treatment (200 μM for 24 h) significantly induced the increase in the levels of intracellular ROS with a decrease in the levels of glutathione (GSH) levels and the activity of the antioxidant enzymes SOD and CAT in the N2a neuronal cells [142]. These data lend substantial evidence that oxidative stress and mitochondria dysfunction play critical roles in colistin-induced neurotoxicity. Moreover, Nrf2 is suggested as the “housekeeping” gene in response to oxidative stress. Nrf2 activation can transcriptionally activate the expression of several phase II enzymes, including HO-1, SOD and CAT [92]. Not surprisingly, the upregulation of Nrf2/HO-1 pathway in N2a cells and the cerebral cortices of mice treated with colistin were detected [131,132,133,142]. These evidences suggested that the activation of Nrf2/HO-1 plays a protective role in polymyxins-induced neurotoxicity.
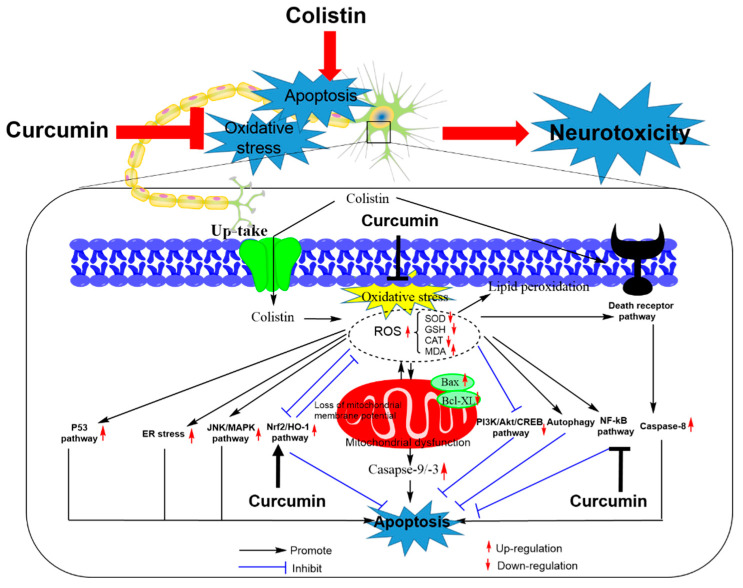
It has been reported that the main pathways of colistin-induced apoptosis in neuronal cells involve the intrinsic mitochondrial and extrinsic death receptor pathways [142,143,144,145]. Colistin treatment can cause an increase in the Bax/Bcl-2 ratio and cytochrome C (CytC) release, in cascade to triggering the activations of caspases-3 and -9 and inducing apoptotic death in N2a and PC12 neuronal cells [142,143,144]. Similar in kidney cells, colistin treatment of PC12 cells also exhibited the increased expression of Fas and Fas-L, followed to activate caspase-8 through the FADD [145]. Notably, the pan-caspase inhibitor Z-VAD-FMK can partially block colistin-induced apoptotic death in N2a neuronal cells, highlighting the role of caspases activation in intrinsic and extrinsic apoptotic pathways [142]. In a mouse model, the marked increase of GRP78, an ER stress marker, was detected in the cerebral cortical tissues, indicated the ER stress may also contribute to colistin-induced neurotoxicity [132]. Our recent studies showed that colistin treatment (200 μM) of N2a neuronal cells downregulated the expression of mammalian target of rapamycin (mTOR) and phospho (p)-p70S6 kinase (p70s6k) and upregulates unc-51 like autophagy activating kinase 1 (ULK1) expression, suggesting that mTOR inhibition-mediated autophagy is triggered in the process of colistin-induced apoptosis in neuronal cells [128]. In addition, colistin-induced neuronal cell death also involves p53, MAPK and PI3 K/Akt/c-AMP response element-binding (CREB) pathways, and their roles are shown in Figure 4 [26,27,78,81,132,142,146,147,148,149,150].
Figure 4.
Schematic diagram depicting the major apoptosis pathways involved in colistin-induced neurotoxicity and the potential targets of curcumin. Colistin-induced apoptosis involves oxidative stress, the activation of death receptor and mitochondrial pathways by upregulating the Bax/Bcl2 ratio, inducing the loss of membrane potential and production of reactive oxygen species [ROS] and activating caspases-3, -8 and -9. Colistin-induced apoptosis also involved the activation of p53, nuclear factor kappa B (NF-κB), ER stress, c-Jun N-terminal kinases (JNKs)/MAPK pathways and the inhibition of the PI3 K/Akt/CREB pathway. The activation of autophagy and Nrf2/HO-1 pathways inhibits colistin-induced apoptosis. Curcumin ameliorates colistin-induced apoptosis and neurotoxicity by targeting the Nrf2/HO-1, NF-κB and oxidative stress pathways.
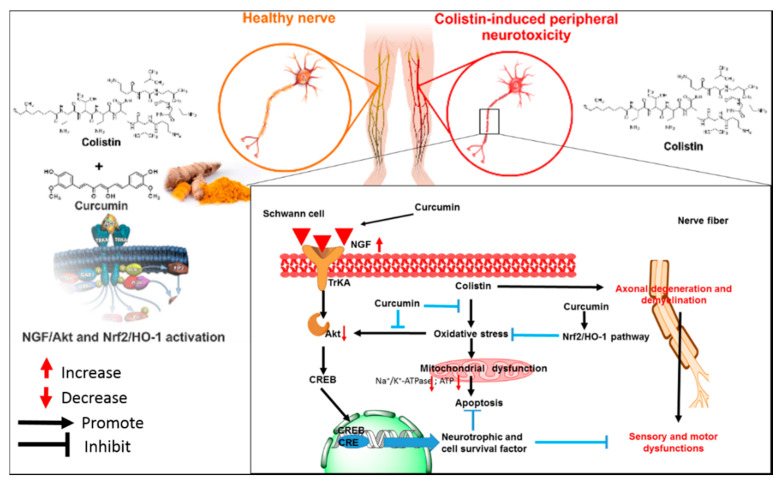
Curcumin supplementation effectively protects against colistin treatment-induced neurotoxicity in vitro (e.g., mouse primary and N2a neuronal cells) and animal models (e.g., mice and rats) (Figure 4) [25,26,27]. Curcumin indicated the potential application as a neuroprotective agent with an ability to cross the BBB [40,151,152]. Our in vitro data showed that curcumin could effectively improve colistin-induced oxidative stress and apoptotic cell death in N2a neuronal cells by up-regulating the activities of the intracellular SOD, CAT and GSH levels, and promoting the activation of Nrf2/HO-1 pathway [26]. Curcumin treatment also downregulated the expression of NF-κB and NF-κB-regulated genes that are involved in the process of inflammatory response and apoptotic death in N2a cells [26]. In a rat model, oral administration of curcumin at the dose of 200 mg/kg/day for 6 days significantly improved colistin-induced inflammatory response, oxidative stress damage and apoptosis in the brain tissues (e.g., cerebral cortex and cerebellar cortex tissues) [27]. Our study revealed that oral administration of curcumin at the dose of 200 mg/kg/day for 7 days significantly ameliorated colistin-induced sciatic nerve damage by inhibiting oxidative stress, apoptosis and upregulating the nerve growth factor (NGF)/Akt and Nrf2/HO-1 pathways (Figure 5) [25]. Mechanically, NGF treatment promotes cell survival of nerve cells via the activation of Akt pathway [25]. The activation of Akt caused by NGF stimulation significantly attenuated colistin-induced apoptotic death and the inhibition of axon growth in N2a and PC12 neuronal cells, which involved the activation of Nrf2/ARE pathway [132,150,153]. Nerve growth factor plays a decisive role in promoting the growth and survival of peripheral sensory and sympathetic nerve cells [150]. Our recent study demonstrated that NGF administration could significantly inhibit colistin-induced peripheral neurotoxicity in mice [154]. Consistently, NGF treatment per se can inhibit colistin-induced oxidative stress and apoptosis in PC12 cells [150]. Thus, the protective effect of curcumin on colistin-induced peripheral neurotoxicity is partly attributed to the activation of NGF/Akt pathway.
Figure 5.
Schematic diagram depicting the putative action of curcumin against colistin-induced peripheral neurotoxicity. Oral administration of curcumin improves colistin-induced dysfunctional motor and sensory symptoms by inhibition of oxidative stress and activation of the NGF/Akt and Nrf2/HO-1 pathways in the sciatic nerve tissues of mice. The figure is a revision from Dai. et al., [26] and [154].
6. Conclusions
During the past two decades, parenteral polymyxin B and colistin have been increasingly used for the treatment of problematic Gram-negative pathogens. Notwithstanding, neurotoxicity and nephrotoxicity remain the major dose-limiting factors hampering the clinical utility of these essential last-line antibiotics. Increasing reports of polymyxin resistance in the nosocomial setting and widespread environmental polymyxin resistance due to the plasmid mcr-1 has rattled the cage for the long-term therapeutic utility of polymyxins. Polymyxin combination treatments with FDA approved drugs and natural products is an emerging strategy for overcoming the aforementioned unwanted side-effects and bacterial resistance. Combining polymyxins with the existing drugs is prudent, in the view that FDA approved drugs have known pharmacokinetic and safety profiles; therefore, combination therapies could be fast-tracked into clinical practice. Curcumin is an FDA- approved natural product exhibiting many pharmacological antioxidant and healing activities. An increasing body of literature suggests that polymyxins–curcumin combination therapy has a synergistic inhibitory effect on the bacterial growth and lowers polymyxin-induced nephrotoxicity and neurotoxicity via inhibition of oxidative stress, mitochondrial dysfunction, inflammation and apoptosis. Future clinical trials of polymyxins–curcumin combination therapy are certainly warranted, albeit there is currently a dearth of data on optimal dosage regimens and pharmacokinetics for the combination, both of which require further studies.
Author Contributions
Writing—original draft preparation, C.D.; writing—review and editing, C.D., T.V., X.X., Y.W., J.S., G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Key Projects in the Chinese National Science and Technology Pillar Program during the 12th Five-year Plan Period (2015BAD11B03). T.V. was supported by the Australian National Health and Medical Research Council (NHMRC).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.The World Health Organization . Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. WHO; Geneva, Switzerland: 2017. [Google Scholar]
- 2.Tyers M., Wright G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019;17:141–155. doi: 10.1038/s41579-018-0141-x. [DOI] [PubMed] [Google Scholar]
- 3.Koyama Y., Kurosasa A., Tsuchiya A., Takakuta K. A new antibiotic “colistin” produced by spore-forming soil bacteria. J. Antibiot. 1950;3:457–458. [Google Scholar]
- 4.Braine T. Race against time to develop new antibiotics. Bull. World Health Organ. 2011;89:88–89. doi: 10.2471/BLT.11.030211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zowawi H.M., Harris P.N., Roberts M.J., Tambyah P.A., Schembri M.A., Pezzani M.D., Williamson D.A., Paterson D.L. The emerging threat of multidrug-resistant gram-negative bacteria in urology. Nat. Rev. Urol. 2015;12:570–584. doi: 10.1038/nrurol.2015.199. [DOI] [PubMed] [Google Scholar]
- 6.Demiraslan H., Cevahir F., Berk E., Metan G., Cetin M., Alp E. Is surveillance for colonization of carbapenem-resistant gram-negative bacteria important in adult bone marrow transplantation units? Am. J. Infect. Control. 2017;45:735–739. doi: 10.1016/j.ajic.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Karlowsky J.A., Lob S.H., Kazmierczak K.M., Hawser S.P., Magnet S., Young K., Motyl M.R., Sahm D.F. In vitro activity of imipenem/relebactam against gram-negative eskape pathogens isolated in 17 european countries: 2015 smart surveillance programme. J. Antimicrob. Chemother. 2018;73:1872–1879. doi: 10.1093/jac/dky107. [DOI] [PubMed] [Google Scholar]
- 8.Adams-Sapper S., Gayoso A., Riley L.W. Stress-adaptive responses associated with high-level carbapenem resistance in kpc-producing klebsiella pneumoniae. J. Pathog. 2018;2018:11. doi: 10.1155/2018/3028290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Exner M., Bhattacharya S., Christiansen B., Gebel J., Goroncy-Bermes P., Hartemann P., Heeg P., Ilschner C., Kramer A., Larson E., et al. Antibiotic resistance: What is so special about multidrug-resistant gram-negative bacteria? GMS Hyg. Infect. Control. 2017;12:Doc05. doi: 10.3205/dgkh000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira V.D., Rubio F.G., Almeida M.T., Nogueira M.C., Pignatari A.C. Trends of 9416 multidrug-resistant gram-negative bacteria. Rev. Assoc. Med. Bras. 2015;61:244–249. doi: 10.1590/1806-9282.61.03.244. [DOI] [PubMed] [Google Scholar]
- 11.Perez F., Adachi J., Bonomo R.A. Antibiotic-resistant gram-negative bacterial infections in patients with cancer. Clin. Infect. Dis. 2014;59:S335–S339. doi: 10.1093/cid/ciu612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nation R.L., Velkov T., Li J. Colistin and polymyxin b: Peas in a pod, or chalk and cheese? Clin. Infect. Dis. 2014;59:88–94. doi: 10.1093/cid/ciu213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nation R.L., Garonzik S.M., Thamlikitkul V., Giamarellos-Bourboulis E.J., Forrest A., Paterson D.L., Li J., Silveira F.P. Dosing guidance for intravenous colistin in critically-ill patients. Clin. Infect. Dis. 2017;64:565–571. doi: 10.1093/cid/ciw839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J., Nation R.L., Turnidge J.D., Milne R.W., Coulthard K., Rayner C.R., Paterson D.L. Colistin: The re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect. Dis. 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y.Y., Wang Y., Walsh T.R., Yi L.X., Zhang R., Spencer J., Doi Y., Tian G., Dong B., Huang X., et al. Emergence of plasmid-mediated colistin resistance mechanism mcr-1 in animals and human beings in china: A microbiological and molecular biological study. Lancet Infect. Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 16.Napier B.A., Band V., Burd E.M., Weiss D.S. Colistin heteroresistance in enterobacter cloacae is associated with cross-resistance to the host antimicrobial lysozyme. Antimicrob. Agents Chemother. 2014;58:5594–5597. doi: 10.1128/AAC.02432-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garonzik S.M., Li J., Thamlikitkul V., Paterson D.L., Shoham S., Jacob J., Silveira F.P., Forrest A., Nation R.L. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 2011;55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartzell J.D., Neff R., Ake J., Howard R., Olson S., Paolino K., Vishnepolsky M., Weintrob A., Wortmann G. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin. Infect. Dis. 2009;48:1724–1728. doi: 10.1086/599225. [DOI] [PubMed] [Google Scholar]
- 19.Ni W.T., Shao X.D., Di X.Z., Cui J.C., Wang R., Liu Y.N. In vitro synergy of polymyxins with other antibiotics for acinetobacter Baumannii: A systematic review and meta-analysis. Int. J. Antimicrob. Agents. 2015;45:8–18. doi: 10.1016/j.ijantimicag.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 20.O’Hara J.A., Ambe L.A., Casella L.G., Townsend B.M., Pelletier M.R., Ernst R.K., Shanks R.M.Q., Doi Y. Activities of vancomycin-containing regimens against colistin-resistant acinetobacter Baumannii clinical strains. Antimicrob. Agents Chemother. 2013;57:2103–2108. doi: 10.1128/AAC.02501-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salam A.M., Quave C.L. Opportunities for plant natural products in infection control. Curr. Opin. Microbiol. 2018;45:189–194. doi: 10.1016/j.mib.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silver L.L. Natural products as a source of drug leads to overcome drug resistance. Future Microbiol. 2015;10:1711–1718. doi: 10.2217/fmb.15.67. [DOI] [PubMed] [Google Scholar]
- 23.Betts J.W., Sharili A.S., La Ragione R.M., Wareham D.W. In vitro antibacterial activity of curcumin-polymyxin b combinations against multidrug-resistant bacteria associated with traumatic wound infections. J. Nat. Prod. 2016;79:1702–1706. doi: 10.1021/acs.jnatprod.6b00286. [DOI] [PubMed] [Google Scholar]
- 24.Kaur A., Sharma P., Capalash N. Curcumin alleviates persistence of acinetobacter Baumannii against colistin. Sci. Rep. 2018;8:11029. doi: 10.1038/s41598-018-29291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai C., Xiao X., Zhang Y., Xiang B., Hoyer D., Shen J., Velkov T., Tang S. Curcumin attenuates colistin-induced peripheral neurotoxicity in mice. ACS Infect. Dis. 2020;6:715–724. doi: 10.1021/acsinfecdis.9b00341. [DOI] [PubMed] [Google Scholar]
- 26.Dai C., Ciccotosto G.D., Cappai R., Tang S., Li D., Xie S., Xiao X., Velkov T. Curcumin attenuates colistin-induced neurotoxicity in n2a cells via anti-inflammatory activity, suppression of oxidative stress, and apoptosis. Mol. Neurobiol. 2018;55:421–434. doi: 10.1007/s12035-016-0276-6. [DOI] [PubMed] [Google Scholar]
- 27.Edrees N.E., Galal A.A.A., Monaem A.R.A., Beheiry R.R., Metwally M.M.M. Curcumin alleviates colistin-induced nephrotoxicity and neurotoxicity in rats via attenuation of oxidative stress, inflammation and apoptosis. Chem. Biol. Interact. 2018;294:56–64. doi: 10.1016/j.cbi.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Lin X.P., Xue C., Zhang J.M., Wu W.J., Chen X.Y., Zeng Y.M. Curcumin inhibits lipopolysaccharide-induced mucin 5ac hypersecretion and airway inflammation via nuclear factor erythroid 2-related factor 2. Chin. Med. J. 2018;131:1686–1693. doi: 10.4103/0366-6999.235863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lobo de Sa F.D., Butkevych E., Nattramilarasu P.K., Fromm A., Mousavi S., Moos V., Golz J.C., Stingl K., Kittler S., Seinige D., et al. Curcumin mitigates immune-induced epithelial barrier dysfunction by campylobacter Jejuni. Int. J. Mol. Sci. 2019;20:4830. doi: 10.3390/ijms20194830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos A.M., Lopes T., Oleastro M., Gato I.V., Floch P., Benejat L., Chaves P., Pereira T., Seixas E., Machado J., et al. Curcumin inhibits gastric inflammation induced by helicobacter pylori infection in a mouse model. Nutrients. 2015;7:306–320. doi: 10.3390/nu7010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaefers M.M., Breshears L.M., Anderson M.J., Lin Y.C., Grill A.E., Panyam J., Southern P.J., Schlievert P.M., Peterson M.L. Epithelial proinflammatory response and curcumin-mediated protection from Staphylococcal toxic shock syndrome toxin-1. PLoS ONE. 2012;7:e32813. doi: 10.1371/journal.pone.0032813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou J., Miao H., Li X., Hu Y., Sun H., Hou Y. Curcumin inhibits placental inflammation to ameliorate lps-induced adverse pregnancy outcomes in mice via upregulation of phosphorylated akt. Inflamm. Res. 2017;66:177–185. doi: 10.1007/s00011-016-1004-4. [DOI] [PubMed] [Google Scholar]
- 33.Kumari A., Dash D., Singh R. Curcumin inhibits Lipopolysaccharide (lps)-induced endotoxemia and airway inflammation through modulation of sequential release of inflammatory mediators (tnf-alpha and tgf-beta1) in murine model. Inflammopharmacology. 2017;25:329–341. doi: 10.1007/s10787-017-0334-3. [DOI] [PubMed] [Google Scholar]
- 34.Sharma R.A., McLelland H.R., Hill K.A., Ireson C.R., Euden S.A., Manson M.M., Pirmohamed M., Marnett L.J., Gescher A.J., Steward W.P. Pharmacodynamic and pharmacokinetic study of oral curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- 35.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 36.Gupta S.C., Patchva S., Aggarwal B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng A.L., Hsu C.H., Lin J.K., Hsu M.M., Ho Y.F., Shen T.S., Ko J.Y., Lin J.T., Lin B.R., Ming-Shiang W., et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 38.Lao C.D., Ruffin M.T.T., Normolle D., Heath D.D., Murray S.I., Bailey J.M., Boggs M.E., Crowell J., Rock C.L., Brenner D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Malo D., Villaron-Casares C.A., Alarcon-Jimenez J., Miranda M., Diaz-Llopis M., Romero F.J., Villar V.M. Curcumin as a therapeutic option in retinal diseases. Antioxidants. 2020;9:48. doi: 10.3390/antiox9010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosh S., Banerjee S., Sil P.C. The beneficial role of curcumin on inflammation; diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015;83:111–124. doi: 10.1016/j.fct.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 41.Aggarwal B.B., Surh Y.-J., Shishodia S. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. Volume 595 Springer; New York, NY, USA: 2007. [Google Scholar]
- 42.He Y., Yue Y., Zheng X., Zhang K., Chen S.H., Du Z.Y. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules. 2015;20:9183–9213. doi: 10.3390/molecules20059183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gunes H., Gulen D., Mutlu R., Gumus A., Tas T., Topkaya A.E. Antibacterial effects of curcumin: An in vitro minimum inhibitory concentration study. Toxicol. Ind. Health. 2016;32:246–250. doi: 10.1177/0748233713498458. [DOI] [PubMed] [Google Scholar]
- 44.Poonam T., Madhuri S., Himani K., Anita K., Kasturi M., One Z.D.J.P. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS ONE. 2015;10:e0121313. doi: 10.1371/journal.pone.0121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Praditya D., Kirchhoff L., Bruning J., Rachmawati H., Steinmann J., Steinmann E. Anti-infective properties of the golden spice curcumin. Front. Microbiol. 2019;10:912. doi: 10.3389/fmicb.2019.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rai D., Singh J.K., Roy N., Panda D. Curcumin inhibits ftsz assembly: An attractive mechanism for its antibacterial activity. Biochem. J. 2008;410:147–155. doi: 10.1042/BJ20070891. [DOI] [PubMed] [Google Scholar]
- 47.Ezraty B., Gennaris A., Barras F., Collet J.F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017;15:385–396. doi: 10.1038/nrmicro.2017.26. [DOI] [PubMed] [Google Scholar]
- 48.Van Acker H., Coenye T. The role of reactive oxygen species in antibiotic-mediated killing of bacteria. Trends Microbiol. 2017;25:456–466. doi: 10.1016/j.tim.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Gao Y., Wu J., Li Z., Zhang X., Lu N., Xue C., Leung A.W., Xu C., Tang Q. Curcumin-mediated photodynamic inactivation (pdi) against dh5alpha contaminated in oysters and cellular toxicological evaluation of pdi-treated oysters. Photodiagn. Photodyn. 2019;26:244–251. doi: 10.1016/j.pdpdt.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 50.Freitas M.A., Pereira A.H., Pinto J.G., Casas A., Ferreira-Strixino J. Bacterial viability after antimicrobial photodynamic therapy with curcumin on multiresistant staphylococcus aureus. Future Microbiol. 2019;14:739–748. doi: 10.2217/fmb-2019-0042. [DOI] [PubMed] [Google Scholar]
- 51.Rudrappa T., Bais H.P. Curcumin, a known phenolic from curcuma longa, attenuates the virulence of pseudomonas aeruginosa pao1 in whole plant and animal pathogenicity models. J. Agric. Food Chem. 2008;56:1955–1962. doi: 10.1021/jf072591j. [DOI] [PubMed] [Google Scholar]
- 52.Moghadamtousi S.Z., Kadir H.A., Hassandarvish P., Tajik H., Abubakar S., Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed. Res. Int. 2014;2014:186864. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mun S.H., Joung D.K., Kim Y.S., Kang O.H., Kim S.B., Seo Y.S., Kim Y.C., Lee D.S., Shin D.W., Kweon K.T., et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine. 2013;20:714–718. doi: 10.1016/j.phymed.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 54.De R., Kundu P., Swarnakar S., Ramamurthy T., Chowdhury A., Nair G.B., Mukhopadhyay A.K. Antimicrobial activity of curcumin against helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother. 2009;53:1592–1597. doi: 10.1128/AAC.01242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niamsa N., Sittiwet C. Antimicrobial activity of curcuma longa aqueous extract. J. Pharmacol. Toxicol. 2009;4:174–177. [Google Scholar]
- 56.Chen C., Long L., Zhang F., Chen Q., Chen C., Yu X., Liu Q., Bao J., Long Z. Antifungal activity, main active components and mechanism of curcuma longa extract against Fusarium graminearum. PLoS ONE. 2018;13:e0194284. doi: 10.1371/journal.pone.0194284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu W.F., Wang J.N., Li Z., Wei B., Jin J., Gao L., Li H.D., Li J., Chen H.Y., Meng X.M. 7-hydroxycoumarin protects against cisplatin-induced acute kidney injury by inhibiting necroptosis and promoting sox9-mediated tubular epithelial cell proliferation. Phytomedicine. 2020;69:153202. doi: 10.1016/j.phymed.2020.153202. [DOI] [PubMed] [Google Scholar]
- 58.Shlar I., Droby S., Rodov V. Modes of antibacterial action of curcumin under dark and light conditions: A toxicoproteomics approach. J. Proteom. 2017;160:8–20. doi: 10.1016/j.jprot.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 59.Tan B.L., Norhaizan M.E. Curcumin combination chemotherapy: The implication and efficacy in cancer. Molecules. 2019;24:2527. doi: 10.3390/molecules24142527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aggarwal B.B., Harikumar K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma R.A., Gescher A.J., Steward W.P. Curcumin: The story so far. Eur. J. Cancer. 2005;41:1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 62.Aggarwal B.B., Kumar A., Bharti A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 63.Zhou H., Beevers C.S., Huang S. The targets of curcumin. Curr. Drug Targets. 2011;12:332–347. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu F., Diao R., Liu J., Kang Y., Wang X., Shi L. Curcumin attenuates Staphylococcus aureus-induced acute lung injury. Clin. Respir. J. 2015;9:87–97. doi: 10.1111/crj.12113. [DOI] [PubMed] [Google Scholar]
- 65.Zhang J., Zheng Y., Luo Y., Du Y., Zhang X., Fu J. Curcumin inhibits lps-induced neuroinflammation by promoting microglial m2 polarization via trem2/ tlr4/ nf-kappab pathways in bv2 cells. Mol. Immunol. 2019;116:29–37. doi: 10.1016/j.molimm.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 66.Bai X., Oberley-Deegan R.E., Bai A., Ovrutsky A.R., Kinney W.H., Weaver M., Zhang G., Honda J.R., Chan E.D. Curcumin enhances human macrophage control of mycobacterium tuberculosis infection. Respirology. 2016;21:951–957. doi: 10.1111/resp.12762. [DOI] [PubMed] [Google Scholar]
- 67.Foryst-Ludwig A., Neumann M., Schneider-Brachert W., Naumann M. Curcumin blocks nf-kappab and the motogenic response in helicobacter pylori-infected epithelial cells. Biochem. Biophys. Res. Commun. 2004;316:1065–1072. doi: 10.1016/j.bbrc.2004.02.158. [DOI] [PubMed] [Google Scholar]
- 68.Kundu P., De R., Pal I., Mukhopadhyay A.K., Saha D.R., Swarnakar S. Curcumin alleviates matrix metalloproteinase-3 and -9 activities during eradication of Helicobacter pylori infection in cultured cells and mice. PLoS ONE. 2011;6:e16306. doi: 10.1371/journal.pone.0016306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yin H.P., Guo Q., Li X., Tang T.T., Li C.L., Wang H.X., Sun Y.X., Feng Q., Ma C.H., Gao C.J., et al. Curcumin suppresses il-1 beta secretion and prevents inflammation through inhibition of the nlrp3 inflammasome. J. Immunol. 2018;200:2835–2846. doi: 10.4049/jimmunol.1701495. [DOI] [PubMed] [Google Scholar]
- 70.Kim D.C., Lee W., Bae J.S. Vascular anti-inflammatory effects of curcumin on hmgb1-mediated responses in vitro. Inflamm. Res. 2011;60:1161–1168. doi: 10.1007/s00011-011-0381-y. [DOI] [PubMed] [Google Scholar]
- 71.Karlstetter M., Lippe E., Walczak Y., Moehle C., Aslanidis A., Mirza M., Langmann T. Curcumin is a potent modulator of microglial gene expression and migration. J. Neuroinflamm. 2011;8:125. doi: 10.1186/1742-2094-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poirel L., Jayol A., Nordmann P. Polymyxins: Antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin. Microbiol. Rev. 2017;30:557–596. doi: 10.1128/CMR.00064-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sampson T.R., Liu X., Schroeder M.R., Kraft C.S., Burd E.M., Weiss D.S. Rapid killing of acinetobacter Baumannii by polymyxins is mediated by a hydroxyl radical death pathway. Antimicrob. Agents Chemother. 2012;56:5642–5649. doi: 10.1128/AAC.00756-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu Z., Qin W., Lin J., Fang S., Qiu J. Antibacterial mechanisms of polymyxin and bacterial resistance. Biomed. Res. Int. 2015;2015:679109. doi: 10.1155/2015/679109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Needham B.D., Trent M.S. Fortifying the barrier: The impact of lipid a remodelling on bacterial pathogenesis. Nat. Rev. Microbiol. 2013;11:467–481. doi: 10.1038/nrmicro3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olaitan A.O., Morand S., Rolain J.M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014;5:643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kofteridis D.P., Alexopoulou C., Valachis A., Maraki S., Dimopoulou D., Georgopoulos D., Samonis G. Aerosolized plus intravenous colistin versus intravenous colistin alone for the treatment of ventilator-associated pneumonia: A matched case-control study. Clin. Infect. Dis. 2010;51:1238–1244. doi: 10.1086/657242. [DOI] [PubMed] [Google Scholar]
- 78.Gai Z.B., Samodelov S.L., Kullak-Ublick G.A., Visentin M. Molecular mechanisms of colistin-induced nephrotoxicity. Molecules. 2019;24:653. doi: 10.3390/molecules24030653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dezoti-Fonseca C., Watanabe M., Vattimo-Mde F. Role of heme oxygenase-1 in polymyxin b-induced nephrotoxicity in rats. Antimicrob. Agents Chemother. 2012;56:5082–5087. doi: 10.1128/AAC.00925-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dai C., Tang S., Wang Y., Velkov T., Xiao X. Baicalein acts as a nephroprotectant that ameliorates colistin-induced nephrotoxicity by activating the antioxidant defence mechanism of the kidneys and down-regulating the inflammatory response. J. Antimicrob. Chemother. 2017;72:2562–2569. doi: 10.1093/jac/dkx185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wallace S.J., Li J., Nation R.L., Rayner C.R., Taylor D., Middleton D., Milne R.W., Coulthard K., Turnidge J.D. Subacute toxicity of colistin methanesulfonate in rats: Comparison of various intravenous dosage regimens. Antimicrob. Agents Chemother. 2008;52:1159–1161. doi: 10.1128/AAC.01101-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ghlissi Z., Hakim A., Mnif H., Ayadi F.M., Zeghal K., Rebai T., Sahnoun Z. Evaluation of colistin nephrotoxicity administered at different doses in the rat model. Ren. Fail. 2013;35:1130–1135. doi: 10.3109/0886022X.2013.815091. [DOI] [PubMed] [Google Scholar]
- 83.Keirstead N.D., Wagoner M.P., Bentley P., Blais M., Brown C., Cheatham L., Ciaccio P., Dragan Y., Ferguson D., Fikes J., et al. Early prediction of polymyxin-induced nephrotoxicity with next-generation urinary kidney injury biomarkers. Toxicol. Sci. 2014;137:278–291. doi: 10.1093/toxsci/kft247. [DOI] [PubMed] [Google Scholar]
- 84.Azad M.A.K., Roberts K.D., Yu H.D.H., Liu B.Y., Schofield A.V., James S.A., Howard D.L., Nation R.L., Rogers K., de Jonge M.D., et al. Significant accumulation of polymyxin in single renal tubular cells: A medicinal chemistry and triple correlative microscopy approach. Anal. Chem. 2015;87:1590–1595. doi: 10.1021/ac504516k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yun B., Azad M.A.K., Wang J., Nation R.L., Thompson P.E., Roberts K.D., Velkov T., Li J. Imaging the distribution of polymyxins in the kidney. J. Antimicrob. Chemother. 2015;70:827–829. doi: 10.1093/jac/dku441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Velkov T., Yun B., Schneider E.K., Azad M.A., Dolezal O., Morris F.C., Nation R.L., Wang J., Chen K., Yu H.H., et al. A novel chemical biology approach for mapping of polymyxin lipopeptide antibody binding epitopes. ACS Infect. Dis. 2016;13:41–51. doi: 10.1021/acsinfecdis.6b00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Manchandani P., Zhou J., Ledesma K.R., Truong L.D., Chow D.S., Eriksen J.L., Tam V.H. Characterization of polymyxin b biodistribution and disposition in an animal model. Antimicrob. Agents Chemother. 2016;60:1029–1034. doi: 10.1128/AAC.02445-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sivanesan S.S., Azad M.A.K., Schneider E.K., Ahmed M.U., Huang J., Wang J., Li J., Nation R.L., Velkov T. Gelofusine ameliorates colistin-induced nephrotoxicity. Antimicrob. Agents Chemother. 2017;61:e00985-17. doi: 10.1128/AAC.00985-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Azad M.A.K., Sivanesan S., Wang J., Chen K., Nation R.L., Thompson P.E., Roberts K.D., Velkov T., Li J. Methionine ameliorates polymyxin-induced nephrotoxicity by attenuating cellular oxidative stress. Antimicrob. Agents Chemother. 2017;62:e01254-17. doi: 10.1128/AAC.01254-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roberts K.D., Azad M.A., Wang J., Horne A.S., Thompson P.E., Nation R.L., Velkov T., Li J. Antimicrobial activity and toxicity of the major lipopeptide components of polymyxin b and colistin: Last-line antibiotics against multidrug-resistant gram-negative bacteria. ACS Infect. Dis. 2015;1:568–575. doi: 10.1021/acsinfecdis.5b00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lu X., Chan T., Xu C., Zhu L., Zhou Q.T., Roberts K.D., Chan H.K., Li J., Zhou F. Human oligopeptide transporter 2 (pept2) mediates cellular uptake of polymyxins. J. Antimicrob. Chemother. 2016;71:403–412. doi: 10.1093/jac/dkv340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dai C., Tang S., Deng S., Zhang S., Zhou Y., Velkov T., Li J., Xiao X. Lycopene attenuates colistin-induced nephrotoxicity in mice via activation of the nrf2/ho-1 pathway. Antimicrob. Agents Chemother. 2015;59:579–585. doi: 10.1128/AAC.03925-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dai C., Li J., Tang S., Li J., Xiao X. Colistin-induced nephrotoxicity in mice involves the mitochondrial, death receptor, and endoplasmic reticulum pathways. Antimicrob. Agents Chemother. 2014;58:4075–4085. doi: 10.1128/AAC.00070-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yousef J.M., Chen G., Hill P.A., Nation R.L., Li J. Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics. J. Antimicrob. Chemother. 2012;67:452–459. doi: 10.1093/jac/dkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yousef J.M., Chen G., Hill P.A., Nation R.L., Li J. Melatonin attenuates colistin-induced nephrotoxicity in rats. Antimicrob. Agents Chemother. 2011;55:4044–4049. doi: 10.1128/AAC.00328-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma Z., Wang J., Nation R.L., Li J., Turnidge J.D., Coulthard K., Milne R.W. Renal disposition of colistin in the isolated perfused rat kidney. Antimicrob. Agents Chemother. 2009;53:2857–2864. doi: 10.1128/AAC.00030-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suzuki T., Yamaguchi H., Ogura J., Kobayashi M., Yamada T., Iseki K. Megalin contributes to kidney accumulation and nephrotoxicity of colistin. Antimicrob. Agents Chemother. 2013;57:6319–6324. doi: 10.1128/AAC.00254-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hori Y., Aoki N., Kuwahara S., Hosojima M., Kaseda R., Goto S., Iida T., De S., Kabasawa H., Kaneko R., et al. Megalin blockade with cilastatin suppresses drug-induced nephrotoxicity. JASN. 2017;28:1783–1791. doi: 10.1681/ASN.2016060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Visentin M., Gai Z., Torozi A., Hiller C., Kullak-Ublick G.A. Colistin is substrate of the carnitine/organic cation transporter 2 (octn2, slc22a5) Drug Metab. Dispos. 2017;45:1240–1244. doi: 10.1124/dmd.117.077248. [DOI] [PubMed] [Google Scholar]
- 100.Li Z.D., Luo J., Jia L.H., Wang X.Y., Xun Z.K., Liu M. Cytochrome c suppresses renal accumulation and nephrotoxicity of polymyxin b. Hum. Exp. Toxicol. 2019;38:193–200. doi: 10.1177/0960327118783543. [DOI] [PubMed] [Google Scholar]
- 101.Azad M.A.K., Akter J., Rogers K.L., Nation R.L., Velkov T., Li J. Major pathways of polymyxin-induced apoptosis in rat kidney proximal tubular cells. Antimicrob. Agents Chemother. 2015;59:2136–2143. doi: 10.1128/AAC.04869-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eadon M.T., Hack B.K., Alexander J.J., Xu C., Dolan M.E., Cunningham P.N. Cell cycle arrest in a model of colistin nephrotoxicity. Physiol. Genom. 2013;45:877–888. doi: 10.1152/physiolgenomics.00076.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yun B., Zhang T., Azad M.A.K., Wang J., Nowell C.J., Kalitsis P., Velkov T., Hudson D.F., Li J. Polymyxin b causes DNA damage in hk-2 cells and mice. Arch. Toxicol. 2018;92:2259–2271. doi: 10.1007/s00204-018-2192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee T.W., Bae E., Kim J.H., Jang H.N., Cho H.S., Chang S.H., Park D.J. The aqueous extract of aged black garlic ameliorates colistin-induced acute kidney injury in rats. Ren. Fail. 2019;41:24–33. doi: 10.1080/0886022X.2018.1561375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jeong B.Y., Park S.R., Cho S., Yu S.L., Lee H.Y., Park C.G., Kang J., Jung D.Y., Park M.H., Hwang W.M., et al. Tgf-beta-mediated nadph oxidase 4-dependent oxidative stress promotes colistin-induced acute kidney injury. J. Antimicrob. Chemother. 2018;73:962–972. doi: 10.1093/jac/dkx479. [DOI] [PubMed] [Google Scholar]
- 106.Wu J., Pan X., Fu H., Zheng Y., Dai Y., Yin Y., Chen Q., Hao Q., Bao D., Hou D. Effect of curcumin on glycerol-induced acute kidney injury in rats. Sci. Rep. 2017;7:10114. doi: 10.1038/s41598-017-10693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.He L., Peng X., Zhu J., Liu G., Chen X., Tang C., Liu H., Liu F., Peng Y. Protective effects of curcumin on acute gentamicin-induced nephrotoxicity in rats. Can. J. Physiol. Pharm. 2015;93:275–282. doi: 10.1139/cjpp-2014-0459. [DOI] [PubMed] [Google Scholar]
- 108.Deck L.M., Hunsaker L.A., Vander Jagt T.A., Whalen L.J., Royer R.E., Vander Jagt D.L. Activation of antioxidant nrf2 signaling by enone analogues of curcumin. Eur. J. Med. Chem. 2018;143:854–865. doi: 10.1016/j.ejmech.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 109.Dai C., Li B., Zhou Y., Li D., Zhang S., Li H., Xiao X., Tang S. Curcumin attenuates quinocetone induced apoptosis and inflammation via the opposite modulation of nrf2/ho-1 and nf-kb pathway in human hepatocyte l02 cells. Food Chem. Toxicol. 2016;95:52–63. doi: 10.1016/j.fct.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 110.Klionsky D.J., Abdelmohsen K., Abe A., Abedin M.J., Abeliovich H., Acevedo Arozena A., Adachi H., Adams C.M., Adams P.D., Adeli K., et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ortega-Dominguez B., Aparicio-Trejo O.E., Garcia-Arroyo F.E., Leon-Contreras J.C., Tapia E., Molina-Jijon E., Hernandez-Pando R., Sanchez-Lozada L.G., Barrera-Oviedo D., Pedraza-Chaverri J. Curcumin prevents cisplatin-induced renal alterations in mitochondrial bioenergetics and dynamic. Food Chem. Toxicol. 2017;107:373–385. doi: 10.1016/j.fct.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 112.Avila-Rojas S.H., Tapia E., Briones-Herrera A., Aparicio-Trejo O.E., Leon-Contreras J.C., Hernandez-Pando R., Pedraza-Chaverri J. Curcumin prevents potassium dichromate (k2cr2o7)-induced renal hypoxia. Food Chem. Toxicol. 2018;121:472–482. doi: 10.1016/j.fct.2018.09.046. [DOI] [PubMed] [Google Scholar]
- 113.Guerrero-Hue M., Garcia-Caballero C., Palomino-Antolin A., Rubio-Navarro A., Vazquez-Carballo C., Herencia C., Martin-Sanchez D., Farre-Alins V., Egea J., Cannata P., et al. Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death. FASEB J. 2019;33:8961–8975. doi: 10.1096/fj.201900077R. [DOI] [PubMed] [Google Scholar]
- 114.Molina-Jijon E., Aparicio-Trejo O.E., Rodriguez-Munoz R., Leon-Contreras J.C., Del Carmen Cardenas-Aguayo M., Medina-Campos O.N., Tapia E., Sanchez-Lozada L.G., Hernandez-Pando R., Reyes J.L., et al. The nephroprotection exerted by curcumin in maleate-induced renal damage is associated with decreased mitochondrial fission and autophagy. Biofactors. 2016;42:686–702. doi: 10.1002/biof.1313. [DOI] [PubMed] [Google Scholar]
- 115.Sankar P., Telang A.G., Kalaivanan R., Karunakaran V., Suresh S., Kesavan M. Oral nanoparticulate curcumin combating arsenic-induced oxidative damage in kidney and brain of rats. Toxicol. Ind. Health. 2016;32:410–421. doi: 10.1177/0748233713498455. [DOI] [PubMed] [Google Scholar]
- 116.Benzer F., Kandemir F.M., Kucukler S., Comakli S., Caglayan C. Chemoprotective effects of curcumin on doxorubicin-induced nephrotoxicity in wistar rats: By modulating inflammatory cytokines, apoptosis, oxidative stress and oxidative DNA damage. Arch. Physiol. Biochem. 2018;124:448–457. doi: 10.1080/13813455.2017.1422766. [DOI] [PubMed] [Google Scholar]
- 117.Falagas M.E., Kasiakou S.K. Toxicity of polymyxins: A systematic review of the evidence from old and recent studies. Crit. Care. 2006;10:R27. doi: 10.1186/cc3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weinstein L., Doan T.L., Smith M.A. Neurotoxicity in patients treated with intravenous polymyxin b: Two case reports. Am. J. Health Syst. Pharm. 2009;66:345–347. doi: 10.2146/ajhp080065. [DOI] [PubMed] [Google Scholar]
- 119.Wahby K., Chopra T., Chandrasekar P. Intravenous and inhalational colistin-induced respiratory failure. Clin. Infect. Dis. 2010;50:e38–e40. doi: 10.1086/650582. [DOI] [PubMed] [Google Scholar]
- 120.Dai C., Tang S., Li J., Wang J., Xiao X. Effects of colistin on the sensory nerve conduction velocity and f-wave in mice. Basic Clin. Pharmacol. Toxicol. 2014;115:577–580. doi: 10.1111/bcpt.12272. [DOI] [PubMed] [Google Scholar]
- 121.Bosso J.A., Liptak C.A., Seilheimer D.K., Harrison G.M. Toxicity of colistin in cystic fibrosis patients. DICP. 1991;25:1168–1170. doi: 10.1177/106002809102501101. [DOI] [PubMed] [Google Scholar]
- 122.John J.F., Falci D.R., Rigatto M.H., Oliveira R.D., Kremer T.G., Zavascki A.P. Severe infusion-related adverse events and renal failure in patients receiving high-dose intravenous polymyxin b. Antimicrob. Agents Chemother. 2018;62:e01617-17. doi: 10.1128/AAC.01617-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hitchcock S.A. Blood-brain barrier permeability considerations for cns-targeted compound library design. Curr. Opin. Chem. Biol. 2008;12:318–323. doi: 10.1016/j.cbpa.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 124.Jin L., Nation R.L., Li J., Nicolazzo J.A. Species-dependent blood-brain barrier disruption of lipopolysaccharide: Amelioration by colistin in vitro and in vivo. Antimicrob. Agents Chemother. 2013;57:4336–4342. doi: 10.1128/AAC.00765-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Loho T., Dharmayanti A. Colistin: An antibiotic and its role in multiresistant gram-negative infections. Acta Med. Indones. 2015;47:157–168. [PubMed] [Google Scholar]
- 126.Jin L., Li J., Nation R.L., Nicolazzo J.A. Effect of systemic infection induced by pseudomonas aeruginosa on the brain uptake of colistin in mice. Antimicrob. Agents Chemother. 2012;56:5240–5246. doi: 10.1128/AAC.00713-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jin L., Li J., Nation R.L., Nicolazzo J.A. Impact of p-glycoprotein inhibition and lipopolysaccharide administration on blood-brain barrier transport of colistin in mice. Antimicrob. Agents Chemother. 2011;55:502–507. doi: 10.1128/AAC.01273-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jin L., Li J., Nation R.L., Nicolazzo J.A. Brain penetration of colistin in mice assessed by a novel high-performance liquid chromatographic technique. Antimicrob. Agents Chemother. 2009;53:4247–4251. doi: 10.1128/AAC.00485-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang J., Yi M., Chen X., Muhammad I., Liu F., Li R., Li J., Li J. Effects of colistin on amino acid neurotransmitters and blood-brain barrier in the mouse brain. Neurotoxicol. Teratol. 2016;55:32–37. doi: 10.1016/j.ntt.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 130.Dai C., Li J., Lin W., Li G., Sun M., Wang F., Li J. Electrophysiology and ultrastructural changes in mouse sciatic nerve associated with colistin sulfate exposure. Toxicol. Mech. Methods. 2012;22:592–596. doi: 10.3109/15376516.2012.704956. [DOI] [PubMed] [Google Scholar]
- 131.Dai C., Li J., Li J. New insight in colistin induced neurotoxicity with the mitochondrial dysfunction in mice central nervous tissues. Exp. Toxicol. Pathol. 2013;65:941–948. doi: 10.1016/j.etp.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 132.Dai C., Ciccotosto G.D., Cappai R., Wang Y., Tang S., Hoyer D., Schneider E.K., Velkov T., Xiao X. Rapamycin confers neuroprotection against colistin-induced oxidative stress, mitochondria dysfunction, and apoptosis through the activation of autophagy and mtor/akt/creb signaling pathways. ACS Chem. Neurosci. 2018;9:824–837. doi: 10.1021/acschemneuro.7b00323. [DOI] [PubMed] [Google Scholar]
- 133.Dai C., Ciccotosto G.D., Cappai R., Wang Y., Tang S., Xiao X., Velkov T. Minocycline attenuates colistin-induced neurotoxicity via suppression of apoptosis, mitochondrial dysfunction and oxidative stress. J. Antimicrob. Chemother. 2017;72:1635–1645. doi: 10.1093/jac/dkx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee S.H., Kim J.S., Ravichandran K., Gil H.W., Song H.Y., Hong S.Y. P-glycoprotein induction ameliorates colistin induced nephrotoxicity in cultured human proximal tubular cells. PLoS ONE. 2015;10:e0136075. doi: 10.1371/journal.pone.0136075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Daniel H., Kottra G. The proton oligopeptide cotransporter family slc15 in physiology and pharmacology. Pflug. Arch. 2004;447:610–618. doi: 10.1007/s00424-003-1101-4. [DOI] [PubMed] [Google Scholar]
- 136.Spuch C., Ortolano S., Navarro C. Lrp-1 and lrp-2 receptors function in the membrane neuron. Trafficking mechanisms and proteolytic processing in Alzheimer’s disease. Front. Physiol. 2012;3:269. doi: 10.3389/fphys.2012.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Courousse T., Bacq A., Belzung C., Guiard B., Balasse L., Louis F., Le Guisquet A.M., Gardier A.M., Schinkel A.H., Giros B., et al. Brain organic cation transporter 2 controls response and vulnerability to stress and gsk3beta signaling. Mol. Psychiatry. 2015;20:889–900. doi: 10.1038/mp.2014.86. [DOI] [PubMed] [Google Scholar]
- 138.Ikonomidou C., Kaindl A.M. Neuronal death and oxidative stress in the developing brain. Antioxid. Redox Signal. 2011;14:1535–1550. doi: 10.1089/ars.2010.3581. [DOI] [PubMed] [Google Scholar]
- 139.Dai C., Tang S., Biao X., Xiao X., Chen C., Li J. Colistin induced peripheral neurotoxicity involves mitochondrial dysfunction and oxidative stress in mice. Mol. Biol. Rep. 2019;46:1963–1972. doi: 10.1007/s11033-019-04646-5. [DOI] [PubMed] [Google Scholar]
- 140.Dai C., Zhang D., Li J., Li J. Effect of colistin exposure on calcium homeostasis and mitochondria functions in chick cortex neurons. Toxicol. Mech. Methods. 2013;23:281–288. doi: 10.3109/15376516.2012.754533. [DOI] [PubMed] [Google Scholar]
- 141.Stowe D.F., Camara A.K. Mitochondrial reactive oxygen species production in excitable cells: Modulators of Mitochondrial and cell function. Antioxid. Redox Signal. 2009;11:1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dai C., Tang S., Velkov T., Xiao X. Colistin-induced apoptosis of neuroblastoma-2a cells involves the generation of reactive oxygen species, mitochondrial dysfunction, and autophagy. Mol. Neurobiol. 2016;53:4685–4700. doi: 10.1007/s12035-015-9396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lu Z., Chen C., Wu Z., Miao Y., Muhammad I., Ding L., Tian E., Hu W., Ni H., Li R., et al. A dual role of p53 in regulating colistin-induced autophagy in pc-12 cells. Front. Pharm. 2017;8:768. doi: 10.3389/fphar.2017.00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ahmed M.U., Velkov T., Lin Y.W., Yun B., Nowell C.J., Zhou F., Zhou Q.T., Chan K., Azad M.A.K., Li J. Potential toxicity of polymyxins in human lung epithelial cells. Antimicrob. Agents Chemother. 2017;61:e02690-16. doi: 10.1128/AAC.02690-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jiang H., Li J., Zhou T., Wang C., Zhang H., Wang H. Colistin-induced apoptosis in pc12 cells: Involvement of the mitochondrial apoptotic and death receptor pathways. Int. J. Mol. Med. 2014;33:1298–1304. doi: 10.3892/ijmm.2014.1684. [DOI] [PubMed] [Google Scholar]
- 146.Dai C., Xiao X., Li J., Ciccotosto G.D., Cappai R., Tang S., Schneider-Futschik E.K., Hoyer D., Velkov T., Shen J. Molecular mechanisms of neurotoxicity induced by polymyxins and chemoprevention. ACS Chem. Neurosci. 2019;10:120–131. doi: 10.1021/acschemneuro.8b00300. [DOI] [PubMed] [Google Scholar]
- 147.Zhang L., Zhao Y., Ding W., Jiang G., Lu Z., Li L., Wang J., Li J., Li J. Autophagy regulates colistin-induced apoptosis in pc-12 cells. Antimicrob. Agents Chemother. 2015;59:2189–2197. doi: 10.1128/AAC.04092-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhao W., Pan X., Li T., Zhang C., Shi N. Lycium barbarum polysaccharides protect against trimethyltin chloride-induced apoptosis via sonic hedgehog and pi3k/akt signaling pathways in mouse neuro-2a cells. Oxid. Med. Cell. Longev. 2016;2016:9826726. doi: 10.1155/2016/9826726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lu Z., Miao Y., Muhammad I., Tian E., Hu W., Wang J., Wang B., Li R., Li J. Colistin-induced autophagy and apoptosis involves the jnk-bcl2-bax signaling pathway and jnk-p53-ros positive feedback loop in pc-12 cells. Chem. Biol. Interact. 2017;277:62–73. doi: 10.1016/j.cbi.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 150.Tian E., Hu W., Miao Y., Muhammad I., Zhang L., Xia C., Ding L., Zhang Q., Li R., Chen C., et al. Preventive effects of nerve growth factor against colistin-induced autophagy and apoptosis in pc12 cells. Toxicol. Mech. Methods. 2018;29:177–186. doi: 10.1080/15376516.2018.1534298. [DOI] [PubMed] [Google Scholar]
- 151.Barta A., Janega P., Babal P., Murar E., Cebova M., Pechanova O. The effect of curcumin on liver fibrosis in the rat model of microsurgical cholestasis. Food Funct. 2015;6:2187–2193. doi: 10.1039/C5FO00176E. [DOI] [PubMed] [Google Scholar]
- 152.Garcia-Nino W.R., Pedraza-Chaverri J. Protective effect of curcumin against heavy metals-induced liver damage. Food Chem. Toxicol. 2014;69:182–201. doi: 10.1016/j.fct.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 153.Lu Z., Jiang G., Chen Y., Wang J., Muhammad I., Zhang L., Wang R., Liu F., Li R., Qian F., et al. Salidroside attenuates colistin-induced neurotoxicity in rsc96 schwann cells through pi3k/akt pathway. Chem. Biol. Interact. 2017;271:67–78. doi: 10.1016/j.cbi.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 154.Dai C., Xiong J., Wang Y., Shen J., Velkov T., Xiao X. Nerve growth factor confers neuroprotection against colistin-induced peripheral neurotoxicity. ACS Infect. Dis. 2020 doi: 10.1021/acsinfecdis.0c00107. [DOI] [PubMed] [Google Scholar]