Abstract
In recent years, the consumption of polyphenols has been increasing, largely due to its beneficial effects on health. They are present in a wide variety of foods, but their extraction and characterization are complicated since they are mostly in complex matrices. For this reason, the use of selective, sensitive, and versatile analytical techniques such as liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS) is necessary. In this review, the most relevant studies of the last years regarding the analysis of polyphenols in different matrices by comprehensive LC–MS/MS are discussed. Relevant steps such as extraction, sample purification, and chromatographic analysis methods are emphasized. In particular, the following methodological aspects are discussed: (a) the proper selection of the extraction technique, (b) the extraction and elution solvents, (c) the purification step, (d) the selection of both stationary and mobile phases for the chromatographic separation of compounds, and (e) the different conditions for mass spectrometry. Overall, this review presents the data from the most recent studies, in a comprehensive way, thus providing and simplifying the information of the great variety of works that exist in the literature on this wide topic.
Keywords: LC–MS/MS, electrospray ionization, analytical methods, anthocyanins, flavonols, phenolic compounds
1. Introduction
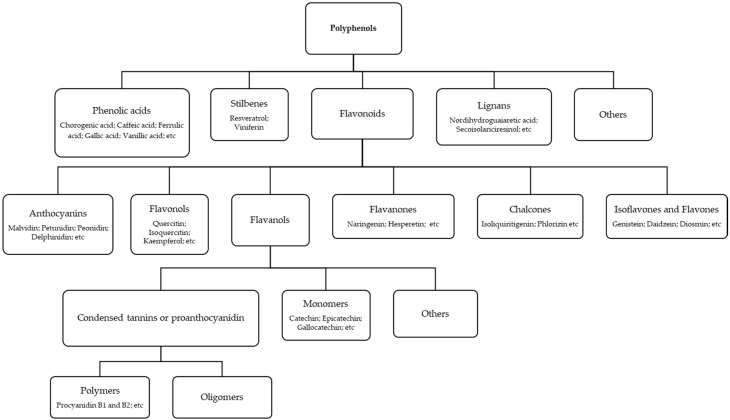
Polyphenols are plant secondary metabolites that are found in a wide variety of foods [1,2,3]. These natural compounds constitute a group of molecules that are divided according to their chemical structure [2,4,5], although they can also be classified by their source of origin, natural distribution or biological function. In particular, according to their chemical structure, they can be classified into different groups, as function of the number of phenol rings contained and the structural elements that bind these rings [2], as can be seen in the Figure 1.
Figure 1.
Polyphenols classification based on the number of phenol rings and their structural elements.
The most common classification of polyphenols include five main classes, namely phenolic acids, stilbenes, flavonoids, lignans, and others [5,6,7]. In nature, the most abundant group of phenolic compounds are flavonoids; this is because the phenolic compounds in plants are mainly synthesized through the phenylpropanoid pathway [5,8]. Flavonoids are characterized by a phenyl benzo(c) pyrone-derived structure consisting of two benzene rings linked to a heterocyclic pyran or pyrone [9,10]. In general, they are found in a glycosylated form although they may also occur in their free form (aglycones) or polymerized [10,11]. The flavonoids are divided into anthocyanins, flavonols, flavanones, chalcones, isoflavones, flavones, and flavan-3-ols according to the degree of hydroxylation and the degree of polymerization [12]. Flavonoids can be found in vegetables (red onions, celery), cereal (buckwheat, beans), fruits and fruit by-products (apples, grapes, cherries, red wine, cherry tomatoes), spices and herbs (rosemary, oregano) [10].
Phenolic acids are derivatives of benzoic acid and cinnamic acid characterized by a high antioxidant activity, and constitute about one-third of the phenolic compounds in the human diet [5,13]. They are mainly found in strawberries, grape juice, pomegranate juice, pear, apple, lemon, and peach, among others. On the other hand, a minority group of polyphenols is represented by the stilbenes. These compounds are present in low quantities in the human diet and are characterized by a 1,2-diphenylethylene backbone. They can be found in grapes, berries, peanuts, or red wine [14]. The last group of polyphenols is the lignans that are formed from two units of a phenylpropane derivative. Overall, there are two major classes of lignans, namely the dibenzylbutane lignans and the furofuran lignans. Lignans can be found in rye, wheat, onions, citrus fruits, etc.
In recent years, numerous studies have shown that the consumption of polyphenols in the diet provides numerous health benefits. This is largely due to the antioxidant properties that help to prevent various diseases associated with oxidative stress [1,15,16]. Studies like those of Scalbert et al. [17] and Seo et al. [3] demonstrated that the antioxidant activity of plant polyphenols can retard the development of diseases such as cancer and cardiovascular and neurodegenerative diseases [3,18].
Besides the health implications, there is a growing interest in the use of new natural additives in food industry [19,20,21]. It is well known that oxidative reactions are the main non-microbial cause of food quality deterioration [22]. However, consumers are concerned about the diet–health relationship, and demand healthy and natural foods, forcing manufacturers to limit the use of synthetic antioxidants in food formulation. Thus, the use of polyphenol-rich extracts as synthetic additives replacers was an important strategy for food manufacturers [23,24,25].
However, the extraction and characterization of phenolic compounds in plant matrices are complex, since the phenolic compounds can be found in simple or highly polymerized structures, which can also form complexes with various other plant-matrix components. In this regard, many polyphenols are often associated with sugar moieties [2]. Thus, the use of different methods of extraction combined with proper solvents characterized by different polarities are strongly required to recover them [26]. According to Naczk and Shahidi [27], the extraction of phenolic compounds in plants is influenced by several factors. For example, some phenolic compounds are very photosensitive, as a result, rapid extraction methods are necessary to avoid the degradation of them [28]. Liquid–liquid extraction (LLE) and solid–liquid extraction (SLE) followed by a stage of concentration and purification are the most widely used methods to make a selective extraction of phenolic compounds from various matrices [2,3,29,30,31,32].
On the other hand, the most used technique for the quantification of polyphenols is UV spectroscopy due to its simplicity and low cost. However, this technique only gives an estimation of the total phenolic content and it does not separate the compounds individually. Nowadays, the liquid chromatography with diode array detector (LC–DAD) is employed for the individually separation and quantification of phenolic compounds. Nevertheless, the main limitation that presents this detector is that the compound identification is only by retention time and UV spectra. Thus, standards need to be used to correctly identify the compounds. Additionally, it may present other limitations like low detection and quantification limits in complex samples. To overcome this problem, in recent years, the use of liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS) has been increasing in order to characterize the polyphenol-rich extracts. In addition, LC–MS/MS was able to achieve noise reduction and sensitivity improvements by exploiting multiple reaction monitoring (MRM) scan mode [1,28,29,33,34,35,36,37,38]. Besides, in the last years, high-resolution LC–MS and LC–MS/MS approaches coupled with multivariate statistics have been widely used to realize the so-called "metabolomic profiling" of plant foods for human nutrition. These metabolomics-based techniques (both targeted and untargeted) require minimal sample preparation and can offer a better overview regarding the polyphenol composition of a matrix under investigation, thus evaluating its bioactivity and nutraceutical potential [39,40,41]. With this in mind, the main objective of the present review is to explore the different extraction techniques, purification, separation and identification, and quantification of polyphenols by liquid chromatography-tandem mass spectrometry (LC–MS/MS). Additionally, the authors present the data from the most recent studies, in a comprehensive way, providing complete information about the main analytical parameters.
2. Extraction and Clean-Up Procedures
For the purpose of obtaining the good recoveries and low detection and quantification limits in the analysis of polyphenols by LC–MS/MS, the extraction and clean up stages are very important. Although these compounds have been studied extensively, there is still no common technique for their isolation.
2.1. Extraction
Extraction is an important step in the isolation and identification of phenolic compounds. The liquid–liquid extraction (LLE) or solid–liquid extraction (SLE) are the most commonly used and simplest extraction techniques for the isolation of phenolic compounds. Several researchers in the literature focus on the extraction and analysis of polyphenols in different plant materials such as wine, tea, oil, herbs, and fruits among others (Table 1).
Table 1.
Different conditions for the polyphenol extraction and purification.
| Matrix | Analyte | Extraction (Solvent Extraction) and Purification (Cartridge) | Recovery (%) | LOD (mg/L) | LOQ (mg/L) | Ref |
|---|---|---|---|---|---|---|
| Mutamba (Guazuma ulmifolia Lam.) fruit | Phenolic acids (n = 10) | SLE (1 g + 15 mL methanol/acetone/water (7/7/6, v/v/v), 30 min US, RT) × 3 times | - | - | - | [50] |
| Flavanols (n = 3) | ||||||
| Flavonols (n = 6) | ||||||
| Flavanones (n = 1) | ||||||
| Flavones (n = 1) | ||||||
| Procyanidins (n = 2) | ||||||
| Meiguihua oral solution | Phenolic acids (n = 2) | LLE (dilution 1:100 in methanol) | 92.68–101.45 | 1.09–6.54 | 13.14–3269 | [33] |
| Flavonols (n = 8) | 92.30–102.80 | 0.11–0.87 | 0.44–5.54 | |||
| Wheat pasta chia flour | Phenolic acids (n = 13) | SLE (5 g + 20 mL solvent mixture of acetone/water (4:1), 1 h shaking, RT, darkness) × 2 times | 0.31–0.95 | 0.09–0.28 | [48] | |
| Extra-virgin olive oils, olive fruits and pomaces | Phenolic acids (n = 3) | LLE or SLE (2.5 g + 5 mL ethanol/water, 80/20, v/v, 10 min US at 21 °C) | 10.0–30.0 | 3.0–10–0 | [36] | |
| Flavonols (n = 2) | 10.0 | 3.0 | ||||
| Flavones (n = 1) | 10.0 | 3.0 | ||||
| Flavanones (n = 1) | 10.0 | 4.0 | ||||
| Artemisia campestris | Phenolic acids (n = 3) | SLE (1 g + 10 mL ethanol/water 8/2 v/v, 30 min US at RT) | [51] | |||
| Flavonols (n = 2) | ||||||
| Flavones (n = 1) | ||||||
| Residual brewing yeast | Phenolic acids (n = 6) | MSPD (0. 10 g + 10 mg TiO2 nanoparticles (NPs) and 0.1g diatomaceous earth); mixed 2 min in mortar, add 2 mL ethanol/water 60/40 v/v, 1 min vortex | [1] | |||
| Flavonols (n = 3) | ||||||
| Flavanones (n = 1) | ||||||
| Flavones (n = 1) | ||||||
| Achyrocline satureioides | Phenolic acids (n = 1) | SLE (methanol) and SPE C-18 | - | - | - | [10] |
| Flavones (n = 1) | ||||||
| Flavonols (n = 3) | ||||||
| Fragaria ananassa cv. Camarosa fruits | Anthocyanins (n = 6) | SLE (10 g + 10 mL methanol/formic acid (97/3, v/v), 30 s US, RT and 16 h, orbital shaking, RT) × 2 times and SPE (Oasis MCX cartridges eluted with 15 mL methanol) | - | 0.14 | 0.48 | [32] |
| Flavonols (n = 1) | 0.4 | 1.5 | ||||
| Sweet lupin seed | Phenolic acids (n = 4) | SLE (2 g + 10 mL methanol/water 80/20 v/v, 10 s vortex, 2 h orbital shaking) × 2 times | 97.61–100.76 | 0.001–0.035 | 0.004–0.119 | [49] |
| Isoflavones (n = 1) | 97.89 | 0.004 | 0.013 | |||
| Flavones (n = 1) | 97.72 | 0.030 | 0.100 | |||
| Flavanonol (n = 1) | 104.38 | 0.019 | 0.065 | |||
| Lucerne, goldenrod, phacelia, buckwheat, licorice, and lavender | Phenolic acids (n = 13) | SLE (2.5 g + 10- or 20-mL methanol, automatic shaker 5 h at 900 rpm) and SPE (C18, 6 mL, 500 mg, eluted with 6 mL methanol) | 58.9–95.5 | - | 0.0004–0.02 | [31] |
| Flavonols (n = 3) | 72.6–79.3 | - | 0.0004–0.0008 | |||
| Isoflavones (n = 2) | 56.3–79.5 | - | 0.0004 | |||
| Flavanones (n = 10) | 49.1–95.2 | - | 0.0004–0.0008 | |||
| Commercial herbal dietary supplements | Flavanones (n = 6) | SLE (0.2–0.7 g + 5 mL methanol, 30 min US mL) × 3 times | 64.6–76.8 | 0.00016–0.00025 | 0.0005–0.0008 | [38] |
| Isoflavones (n = 2) | 72.4–81.9 | 0.00022–0.00025 | 0.0007–0.0008 | |||
| Flavonols (n = 2) | 74.6–80.3 | 0.00022–0.00033 | 0.0007–0.001 | |||
| Brown seaweed | Phenolic acids (n = 2) | SLE (5 g + methanol/water 60/40 v/v; under nitrogen atmosphere for 2 h; 40 °C, 100 rpm shaker incubator) and SPE (C18) eluted with 15 mL methanol with 0.1% HCl | 99.3–104.2 | 0.26–0.73 | 0.77–2.50 | [2] |
| Flavonols (n = 2) | 97.2–98.4 | 0.51–0.57 | 1.79–1.82 | |||
| Anthocyanins (n = 1) | 97.7 | 0.34 | 1.14 | |||
| Fruits from Firmiana Simplex (L.) | Phenolic acids (n = 2) | SLE (500 g + 2 L methanol at RT) × 4 times | - | - | - | [52] |
| Flavanols (n = 2) | - | - | - | |||
| Flavones (n = 1) | - | - | - | |||
| Lignans (n = 1) | - | - | - | |||
| Red grapes | Anthocyanins (n = 3) | SLE (0.8 g+ 1 mL methanol (1% formic acid)/water) 60/40, v/v; 72 °C, 100 min 500 rpm | - | 0.003–0.006 | 0.010–0.021 | [34] |
| Phenolic acids (n = 3) | - | 0.002–0.0040 | 0.006–0.135 | |||
| Flavonols (n = 4) | - | 0.003–0.342 | 0.010–1.140 | |||
| Connarus perrottetti var. angustifolius, Cecropia obtusa, Cecropia palmata, and Mansoa alliacea | Phenolic acids (n = 4) | SLE (0.2 g + 70% hydroethanolic, butanol or ethyl acetate, 4 h, US, RT) | 97.6–104.7 | 0.3–0.7 | 0.8–1.0 | [28] |
| Flavonols (n = 2) | 88.2–94.6 | 0.4–0.6 | 0.8–2.4 | |||
| Flavanols (n = 1) | 83.8 | 1.7 | 2.8 | |||
| Lablab purpureus (L.) sweet pods | Anthocyanins (n = 5) | SLE (0.1% HCl in methanol/water; 35/65 v/v) | - | - | - | [53] |
| Syringa vulgaris L. flowers and fruits | Oleuropein | SLE (0.020 g lyophilized sample + 5 mL methanol; stirred 4 h, 200 rpm at RT) × 3 times | 101.0 | 0.0021 | 0.0068 | [37] |
| Acteoside | 97.4 | 0.0008 | 0.0024 | |||
| Rutin | 94.9 | 0.0003 | 0.001 | |||
| Tea | Flavanols (n = 12) | SLE (1 g sample dried + 100 mL hot water, 3 min, mild stirring) × 5 times | 65–115 | 0.12–223.70 | 0.40–745.60 | [54] |
| (pg/injection) | (pg/injection) | |||||
| Euphorbia supina | Phenolic acids (n = 3) | SLE (10 g sample lyophilized + 200 mL ethyl acetate, 20 h, 80 °C) | 79.6–102.8 | 0.030–0.142 | 0.102–0.473 | [30] |
| Flavonols (n = 2) | SPE (silica gel (3 × 1.7 cm i.d.), eluted with 25 mL methanol/dichloromethane 1/5 v/v) | 76.1–100.0 | 0.028–0.037 | 0.094.0.125 | ||
| Black rice wine | Phenolic acids (n = 8) | LLE (5 mL sample pH 2.0 + 5 mL ethyl acetate, 1 min, vortex) and SPE (Oasis HLB (200 mg, 6 mL), eluted with 8 mL methanol with 0.1% of HCl) | 74–103.0 | 0.008–0.003 | 0.027–0.100 | [29] |
| Flavonols (n = 3) | 63.0–81.0 | 0.008–0.024 | 0.027–0.080 | |||
| Anthocyanins (n = 4) | 62.0–70.0 | 0.010–0.020 | 0.030–0.060 | |||
| Scutellaria baicalensis | Flavones (n = 9) | SLE (10 g lyophilized sample + 200 mL methanol, 24 h, 50 °C) and SPE (silica gel, eluted with 50 mL methanol/dichloromethane, 1/5, v/v) | 82.3–107.7 | 0.007–0.044 | 0.021–0.133 | [3] |
| Flavanones (n = 5) | 80.1–99.0 | 0.11–0.76 | 0.025–0.145 | |||
| Phenolic acids (n = 2) | 104.3–101.7 | 82.3–101.7 | 0.004–0.010 | |||
| Flavonols (n = 1) | 87.6 | 0.017 | 0.052 |
US: ultrasound extraction; RT: room temperature; SLE: solid–liquid extraction; LLE: liquid–liquid extraction; MSPD: matrix solid-phase dispersion; SPE: solid-phase extraction.
Solvent extractions consist of a direct extraction of polyphenolic compounds in samples (previously ground, dried, or lyophilized) by soaking the samples with the extraction solvent [42]. Polyphenol extraction in samples takes place by stirring (vortexes, orbital shaker, automatic shaker, or ultrasonic bath) during a determinate time at controlled temperature.
The efficiency of extraction process can vary in function of process conditions [27,43,44]. Phenolic compounds extraction is influenced by several factors, such as chemical nature of phenolic compound, extraction method, sample particle size, extraction solvent, pH, and temperature, among others [43,44]. Many authors have studied the influence of these factors on the efficacy of the extraction process [45].
The most important factor is the choice of the correct extraction technique. Wang et al. [29] compared the liquid–liquid extraction (LLE) and the solid-phase extraction (SPE) for the extraction of 15 polyphenols (eight phenolic acids, three flavonols, and four anthocyanins) in rice wine. In this work, the authors concluded that LLE is more effective for phenolic acids and flavonoids, whereas for anthocyanin extraction by SPE is better. Similarly, Bajckacz [31] compared solid–liquid extraction with the QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) method for the extraction of flavonoids and phenolic acids in plant material. QuEChERS is a novel extraction method created to avoid the use of large solvent volumes and to reduce the purification times [46]. However, worse results were obtained with this method than with traditional SPE extraction.
In general, as can be seen in Table 1, the most common extraction solvents for polyphenol extraction are methanol, acidified methanol, or combinations of methanol–water. The choice of the solvent is also vital for an optimal extraction. In fact, in 2018 Bajkacz et al. [31] observed how the polyphenols content varied as a function of solvents and extraction times used. These authors studied the flavonoid and phenolic acids content in different plant materials (lucerne, goldenrod, phacelia, buckwheat, licorice, and lavender) by solid–liquid extraction using water, ethanol, methanol, or combination of them as extraction solvents. In this study a considerable increase of the mean content of extracted polyphenol can be observed when methanol or ethanol were used instead of water. For example, in licorice plants an increase in the mean of polyphenols from 207 to 5566 ng/g was observed when water or methanol, respectively, was used. Methanol was the best extraction solvent followed by ethanol or combinations of them. Nevertheless, in food industry ethanol is preferred due to the methanol toxicity [31,43]. The chemical nature of the matrix constituents and the polarity of the extraction solvent influence the phenolic compound solubility. In the case of highly polar phenolic compounds, the extraction with pure organic solvents is not effective. Consequently, the addition of solvents with higher polarities are necessary to increase the overall polarity of the solvent mixture [47]. Phenolic acids or highly glycosylated flavonoids require mixtures of organic solvents with water, for example, 75% aqueous acetone [48], 80% aqueous ethanol [36], or 80% aqueous methanol [49].
The pH is another important factor that influences the extraction of phenolic compounds. It depends on the nature of the compounds to be extracted and the sample. In general, it is necessary to use low pH in the solvent extraction in order to prevent the oxidation of phenolic compounds. Acidification of the solvent increases the ability to extract phenolic compound. This fact is due to the addition of acid control charge, which greatly influences polyphenol extraction. Table 1 shows several works using methanol acidified with formic acid or hydrochloric acid for the extraction.
Polyphenols extraction is also affected by contact time and liquid–solid or liquid–liquid ratio [43]. In the literature the extraction time of polyphenols is very variable ranging from a few minutes to several hours (Table 1). Bajkacz et al. [31] studied the influence of two extraction times (2 or 5 h) over the content of polyphenols in plant material extracts observing an increase in polyphenols content with longer extraction times. Extraction cycles are usually repeated various times and the obtained extracts are further mixed to increase the extraction efficiency. Bajkacz et al. [31] also compared the efficacy between a single extraction or various extraction cycles. They observed that various extraction cycles improve the extraction efficiency of polyphenol compounds from plant materials compared to one extraction cycle with the same solvent [31]. However, an excessive increase in the extraction time may cause degradation of polyphenols mainly due to oxidation [44,55].
Finally, another relevant parameter is the temperature. It is known that high temperatures improve extraction efficiency since heat increases the permeability of cells, the diffusion coefficients, the solubility, and mass transfer rate of the compounds studied. It also modifies the solvent properties making it less viscous, leading to an increase of polyphenol transference to the solvent [44,56]. In 2018 Carres et al. [57] selected five different temperatures between 25 and 85 °C to study the effect of temperature over the polyphenols extraction yield in red grapes. Generally, they observed how an increase in temperature meant an increase in the yield of the extraction process. However, in the case of anthocyanins, the yield was increased until 70 °C. At higher temperatures (85 °C) the performance dropped slightly in comparison with the other extraction temperatures, probably due to the thermosensitivity of anthocyanins (Table 1). In the same manner temperatures below 40 °C were not effective for polyphenol extraction [58,59,60].
All extraction methods involve a stirring stage that can be mechanical stirring, vortex, or ultrasound treatment. The latter one is considered the most effective method to isolate polyphenols [61]. This fact is due to the ability of ultrasound treatment to damage cell walls, allowing the release of intracellular compounds and increased the solute/solvent contact [62]. Moreover, nowadays, improvements in ultrasound technology grant the opportunity to extract bioactive compounds with economic advantages [62]. Adjé et al. [63] evaluated the efficiency of agitating mode (ultrasound-assisted procedure or mechanical stirring) for anthocyanin, flavonols, and phenolic acid extractions from Delonix regia tree flowers. The results obtained showed that total polyphenol content was similar with both stirring modes or slightly higher for mechanical stirring. However, the ultrasound procedure shortened maceration time up to three times. This is important considering that shorter extraction times may avoid compound degradation. In the same way, Altemimi et al. [64] showed this for spinach extracts, demonstrating that the content in total polyphenols was four times higher with ultrasound compared to conventional agitation. This aspect is related to the fact that ultrasound-assisted extraction involves the formation of cavitation bubbles, which assist the release of the vegetable content, thus increasing the mass transfer [65].
2.2. Clean-Up
Purification, fractionation, and concentration of the sample are of great importance for polyphenol analysis [44]. Generally, the solvent extraction implies the co-extraction of other non-phenolic substances, such as sugars, glycosides, organic acids, fats, alkaloids, terpenoids, waxes, and pigments [56,66,67]. Hence, one additional step of clean-up prior to liquid chromatography analysis is necessary, with the aim of removing these substances and avoiding possible interferences.
The extraction in solid phase (SPE) and liquid–liquid extraction (LLE) are the most employed techniques in clean-up procedures [46,68]. In liquid–liquid extraction the use of non-polar solvents contributes to avoiding lipid interferences of the matrix in LC–MS/MS analysis. Most authors use solvents such as hexane, chloroform, dichloromethane, or petroleum ether for defatting the samples [46,56]. However, since the LLE technique requires large amounts of solvents in this process, nowadays, SPE is used as an alternative for the purification of polyphenols [46].
In SPE, the target compounds are retained in a sorbent and then are eluted with an adequate solvent (methanol, ethanol, ethyl acetate). The SPE process is rapid, economical, and simple [69] and allows the purification and concentration of polyphenols at once. In liquid samples SPE is used as an extraction technique more than a clean-up step. In the last few years, as shown in Table 1, different SPE cartridges were used to remove interfering compounds from several extracts, such as C18 [2,31], Oasis MCX [32], HLB [29], or silica gel [3,30] as stationary phases. Prior to the loading of the sample into the cartridges, it is necessary to precondition them. The most used conditioning solvents are water, methanol, and their combinations [2,29,31]. Bajkacz et al. [31] conditioned the C18 SPE columns (500 mg sorbent mass) with 6 mL of methanol and 6 mL of acidified water, while Rajauria [2] conditioned C18 SPE (10 g sorbent mass) with 60 mL of methanol and 60 mL of water. Similarly, Wang [29] also conditioned both, C18 (500 mg sorbent mass) and Oasis HLB (200 mg sorbent mass) with 2 mL of methanol, followed by 2 mL of water. After loading the sample into the cartridge, the co-extracted substances such as sugars, acids, and other polar compounds were eluted from the SPE columns with acidic water [2] or water [29]. Thereby, in order to remove the more hydrophilic compounds Martinechi et al. [35] used a mixed of methanol:water (20:80, v/v). Finally, to elute phenolic compounds, it is common to use organic compounds such as methanol [31,32] or acidified methanol [2,29] (Table 1). Other authors use methanol combined with water [35] or dichloromethane [3,30]. In 2014, Wang et al. [29] studied the influence of two purification sorbents (C18 silica and Oasis HLB), as well as, the influence of different elution solvents (methanol and acidified methanol) and different elution volumes (2, 4, 6, 8, and 10 mL). Regarding retention of polyphenols, Oasis HLB sorbent (from two monomers divinylbenzene and N-vinylpyrrolidone) was more effective for both polar and nonpolar compounds. While, acidified methanol turned out to be the most suitable extraction solvent since it improved the extraction, especially in the case of anthocyanins. This is because acidic environments help the dissolution of anthocyanins [29,70].
Another alternative to traditional solid-phase extraction (SPE) is the matrix solid-phase dispersion assisted extraction (MSPD). This methodology is rapid and simple, consumes less solvent, and generates few residues. Gómez-Mejía et al. [1] in 2019 used the MSPD technique to extract and purify several polyphenols from residual brewing yeast. After extraction, polyphenols were identified and quantified by liquid chromatography coupled to a triple quadrupole analyzer (LC–MS/MS). Nevertheless, to obtain good results it was convenient to optimize several parameters such as extraction solvent, amount of sample, and stirring mode. Thus, Gomez-Mejía evaluated the selectivity and efficiency of methanol, ethanol, and ethanol-water 20:80 (v/v) and 60:40 (v/v), two different amounts of samples (0.05 and 0.10 g) and stirring mode (ultrasonic bath or vortex shaking). Among the studied solvents ethanol-water mixtures and pure methanol gave better results. A reduction of sample amount showed a decrease of the major compounds (gallic acid and naringin) and the non-detection of rutin and quercetin. In general, the best results were obtained when vortex-assisted stirring mode was used. The ultrasound bath produced reductions between 55% and 85%.
Thus, the most important parameters in extraction and clean-up procedures must be carefully selected to ensure correct extraction of phenolic compounds for a reliable identification and quantification.
3. Chromatographic and Mass Spectrometry Conditions
The total content of polyphenols is determined by spectrophotometric techniques that are fast, easy, and cheap, however these techniques are not able to identify phenolic compounds individually. Due to the need to identify them individually, it is required to replace these traditional methods by chromatographic analysis that provide more specific and detailed information [46,71].
Liquid chromatography (LC) is the most used technique for achieving the separation, identification, and quantification of polyphenolic compounds in different matrices. However, to date there is still no single chromatographic method capable of separating the different types of phenolic compounds. Depending on each group of compounds it is necessary to optimize the stationary phase, mobile phase, gradient elution, temperature, and flow rate. In addition, other factors such as stereochemistry, molecular weight, polarity, and degree of polymerization of polyphenols have to be taken into consideration since they affect the retention of the compounds [72,73,74,75].
Usually, separation of phenolic compounds by LC is carried out in the reverse phase (RP) mode with columns, generally packed with particles of silica bonded with alkyl chains (C8 or C18) and various mobile phases as can be seen in Table 2 [46,71,75]. In the scientific literature (Table 2), the column length varied from 10 to 250 mm in length and the internal diameter varied between 2.0 and 4.6 mm. Baranowska and Bajkacz [38] evaluated the efficacy of C8 and C18 columns and different composition of mobile phase for polyphenol determination in nine commercial herbal dietary supplements. In this study, the authors concluded that C18 was found to be more suitable as it showed better separation of analytes with satisfactory peak shapes as compared to C8.
Table 2.
Chromatographic and mass spectrometer conditions used in polyphenol analysis.
| Analyte | Matrix | Chromatographic Conditions | Mass Spectrometer Conditions | Ref. |
|---|---|---|---|---|
| Quinic acid; danshensu; caftaric acid; caffeic acid hexoside; salvianolic acid; fertaric acid; caffeic acid; ferulic acid; salviaflaside; rosmarinic acid; salvianolic acid c; methylrosmarinate; methylquercetin | Wheat pasta chia flour | Column: Luna C18 (250 × 4.6 mm, 5 μm) | Capillary voltage: 4500 V | [48] |
| Column temperature: 35 °C | Nebulizer gas: 4.0 bar | |||
| Mobile phase: 0.5% formic acid in water and 0.5% formic acid in methanol (v/v) | Drying gas: 8.0 L/min and 180 °C | |||
| Flow rate: 0.4 mL/min | Nebulizer gas: nitrogen | |||
| Injection volume: 40 µL | Collision gas: argon | |||
| Gallic acid juglanin; quercetin-3-O-sophoroside; ellagic acid; quercitrin; sophoraflavonoloside; hyperoside; astragalin; isoquercitrin; avicularin | Meiguihua oral solution | Column: Hypersil Gold C18 (100 × 2.1 mm, 1.9 μm) | Capillary voltage: −4500 V | [33] |
| Column temperature: 30 °C | Declustering potential: −10 V | |||
| Mobile phase: 0.1% formic acid in water and acetonitrile | Nebulizer gas: 60 Curtain gas: 35 | |||
| Flow rate: 0.3 mL/min | Auxiliary gas: 50 | |||
| Injection volume: 2 µL | Turbo gas temperature: 450 °C | |||
| P-hydroxybenzoic acid; caffeic acid; chlorogenic acid; ellagic acid; ferulic acid; gallic acid; gentisic acid; p-coumaric acid; luteolin; protocatechuic acid; catechin; epicatechin; epigallocatechin; kaempferol; astragalin; nicotiflorin; cynaroside; naringenin; procyanidin dimer b1 y b2; procyanidin trimer c1; quercetin; hyperoside; isorhamnetin; rutin; vanillin | Mutamba (Guazuma ulmifolia Lam.) fruit | Column: Shimpack XR-ODS III column (150 × 2 mm, 2.2 μm) | Capillary voltage: 3.5 kV | [50] |
| Column temperature: 40 °C | Heat block temperature: 300 °C | |||
| Mobile phase: 0.1% formic acid in water and methanol | Desolvation line temperature: 250 °C | |||
| Flow rate: 0.4 mL/min | Nebulizer and drying gas: nitrogen | |||
| Injection volume: 10 µL | Drying flow: 20 L/min | |||
| Nebulizing flow: 3 L/min | ||||
| Collision induced dissociation gas: argon at 224 kPa | ||||
| Protocatechuic acid; 5-O-Caffeoylquinic acid; Quinic acid methyl ester; 3-O-Caffeoylquinic acid; Caffeic acid; 4-O-Feruloylquinic acid; Quercetin-O-glucoside; Rutin; 3,4-Dicaffeoylquinic acid; 4,5-Dicaffeoylquinic acid; 4′,7′-Dimethoxy luteolin | Artemisia campestris | Column: Lichrocart RP-18 column (250 × 4 mm, 5 μm) | Collision gas: argon at 10−4 mbar | [51] |
| Column temperature: 35 °C | Nebulizer and drying gas: nitrogen | |||
| Mobile phase: Formic acid aqueous solution (0.5% v/v) and acetonitrile | ||||
| Flow rate: 0.3 mL/min | ||||
| Injection volume: 10 µL | ||||
| Caffeic, chlorogenic, p-coumaric, 3,4-dihydroxibenzoic, trans-ferulic and gallic acids, kaempferol, myricetin, naringin; quercetin; rutin | Residual brewing yeast | Column: C18 Fusion-RP (150 × 3 mm, 4 µm) | Nebulizer and drying gas: nitrogen | [1] |
| Column temperature: room temperature | Flow nebulizer gas: 1.5 L·min−1 | |||
| Mobile phase: 0.2% formic acid aqueous solution and methanol | Flow drying gas: 15.0 L·min−1 | |||
| Flow rate: 0.50 mL/min | Collision gas: argon at 230 kPa | |||
| Injection volume: 20 µL | Ionization voltage: −4.5 kV | |||
| Verbascoside; Isoverbascoside; Forsythoside A; Leucosceptoside A; Plantainoside C; Purpureaside D; Martynoside | Aloysia polystachya | Column: Ascentis Express C18 (100 × 2.1 mm, 2.7 µm) | Capillary voltage: −4000 V | [35] |
| Column temperature: 30 °C | Drying gas: nitrogen | |||
| Mobile phase: 0.1% formic acid in water and 0.1% formic acid in acetonitrile | Drying gas temperature: 350 °C | |||
| Flow rate: 0.2 mL/min | Flow drying gas: 9 L/min | |||
| Injection volume: 10 µL | Nebulizing gas: 25 psi | |||
| Dicaffeoylquinic acid isomer A and B; iIsoquercitrin; quercetin; luteolin; 3-O-methylquercetin | Achyrocline satureioides | Column: Luna C18 (150 × 4.6 mm, 5 µm) | Capillary voltage: 4000 V | [10] |
| Column temperature: 40 °C | Nebulizer: 40 psi | |||
| Mobile phase: 10 mM formic acid in ultra-pure water and methanol | Dry gas flow: 9.0 L/min at temperature 365 °C | |||
| Flow rate: 0.3 mL/min | Drying and nebulizing gas: nitrogen | |||
| Injection volume: 5 µL | ||||
| Cyanidin-3-glucoside; pelargonidin-3-glucoside; pelargonidin-3 rutinoside; pelargonidin-acetylglucoside; pelargonidin-succinyl-arabinoside; pelargonidin-malonylrhamnoside; quercetin-rhamnoside | Fragaria ananassa var. Camarosa fruits | Column: Kromasil C18 (250 × 4.6 mm, 5 μm) | - | [32] |
| Column temperature: 40 °C | ||||
| Mobile phase: water/acetonitrile/formic acid (87/3/10 v/v/v) and water/acetonitrile/formic acid (40/50/10 v/v/v) | ||||
| Flow rate: 0.8 mL/min | ||||
| Caffeic acid; chlorogenic acid; p-Coumaric acid; ferulic acid; rutin; quercetin; luteolin; naringenin; genistein | Olea europaea L. | Column: Kinetex biphenyl (10 × 2.1 mm, 5 μm) | Nebulizer gas: nitrogen | [36] |
| Column temperature: 35 °C | Capillary voltage: 4000 V | |||
| Mobile phase: 0.1% formic acid in water and 0.1% formic acid in methanol | Inlet pressure: 30 psi and temperature 270 °C | |||
| Flow rate: 0.5 mL/min | ||||
| Injection volume: 5 µL | ||||
| Protocatechuic acid; caffeic acid; vitexin; ferulic acid; taxifolin; trans-cinnamic acid; genistein | Sweet lupin seed | Column: Kinetex XB-C 18 (250 × 4.6 mm, 5 μm) | Nebulizing gas: nitrogen at 45 psi, 300 °C, and 5 L/min | [49] |
| Column temperature: 25 °C | Capillary voltage: 3.5 kV | |||
| Flow rate: 0.5 mL/min | Nozzle voltage: −500 V | |||
| Mobile phase: 0.05% formic acid in water and acetonitrile | Sheath gas: nitrogen at 11 L/min and 250 °C | |||
| Injection volume: 20 µL | ||||
| Hesperetin; quercetin; naringenin; benzoic acid; naringin; narirutin; hesperidin; caffeic acid; neohesperidin; pinocembrin; taxifolin; fisetin; glabridin; eriocitrin; eriodictyol; formononetin; liquiritin; liquiritigenin; 3-hydroxybenzoic acid; 3,4-dihydroxybenzoic acid; 3-(4-hydroxyphenyl)propionic acid; 4-hydroxybenzoic acid; 3,4-dihydroxy-phenylacetichippuric acid; α-hydroxyhippuric acid; 3-hydroxyphenylacetic acid; p-coumaric acid; ferulic acid; and 4-hydroxy-3-methoxyphenylacetic acid | Plant materials | Column: Zorbax Eclipse XDB-C18 column (50 × 2.1 mm, 1.8 μm) | Capillary voltage: −4500 V | [31] |
| Column temperature: 30 °C | Temperature: 500 °C | |||
| Mobile phase: 0.1% v/v formic acid in water and acetonitrile | Nebulizer gas: 60 psi | |||
| Flow rate: 0.5 mL/min | Turbo-gas: 50 psi | |||
| Injection volume: 5 µL | Collision activated dissociation gas: 4 psi | |||
| Curtain gas: 20 psi | ||||
| Eriocitrin; taxifolin; naringin; hesperidin; neohesperidin; fisetin; eriodictyol; naringenin; hesperetin; kaempferol; chrysin; glabridin | Commercial herbal dietary supplements | Fusion-RP XDB-C18 (50 × 2.0 mm, 4 μm) | - | [38] |
| Column temperature: 30 °C | ||||
| Mobile phase: 0.1% formic acid in water and acetonitrile | ||||
| Flow rate: 0.3 mL/min | ||||
| Injection volume: 2 µL | ||||
| Phloroglucinol; gallic acid; cyanidin 3-glucoside; chlorogenic acid, rutin; quercetin | Brown seaweed | Column: Atlantis C18 (250 × 4.6 mm, 5 µm) | Capillary voltage: 4000 V | [2] |
| Column temperature: 25 ℃ | Gas nebulizer: nitrogen | |||
| Mobile phase: 0.25% aqueous acetic acid and acetonitrile/water (80/20 v/v) | Pressure gas: 50 psi | |||
| Flow rate: 1.0 mL/min | Flow rate: 10 L/min | |||
| Injection volume: 10 µL | Drying temperature: 350 °C | |||
| Gallic acid; catechin; caffeic acid; rutin; ferulic acid; quercitrin; resveratrol | Connarus perrottetti var. angustifolius, Cecropia obtusa, Cecropia palmata, and Mansoa alliacea | Column: C18 (250 × 4.6 mm, 5 μm) | Capillary voltage: ±2.4 kV | [28] |
| Column temperature: 21 °C | Gas flow: 11 L/min | |||
| Mobile phase: orthophosphoric acid solution (0.1%, w/w) and acetonitrile | Nebulizer: 30 psi | |||
| Flow rate: 0.8 mL/min | Gas temperature: 250 °C | |||
| Drying gas: nitrogen | ||||
| Cis- and trans- resveratrol-3-O-galloylglucoside; methyl-(S)-flavogallonate; quercetin-7-O-di-glucoside; quercetin-7-O-galloyl-glucoside; naringenin-40-methoxy-7-pyranoside; 5,6-dihydroxy-30,40,7-tri-methoxy flavone; terminalin; corilagin derivative; oleanane type triterpenoids | Terminalia brownii (Fresen) | Column: Varian LC–18 (250 × 4.6 mm; 5 µm) | Spray voltage: 5000 V | [76] |
| Column temperature: 30 °C | Capillary temperature: 280 °C | |||
| Mobile phase: acetonitrile and water containing 0.005% formic acid, acetonitrile, and glacial acetic acid | Sheathing gas: nitrogen at 40 U | |||
| Flow rate: 0.5 mL/min | Collision gas: helium at 0.8 mTorr | |||
| Injection volume: 5 µL | ||||
| Gallocatechin; epigallocatechin; catechin; epicatechin; epigallocatechin gallate; gallocatechin gallate; epicatechin gallate; catechin gallate; theaflavin; theaflavin-3-gallate | Tea | Column: Capcellpak C18 MGIII (100 × 2.0 mm, 3 µm) | Nebulizer gas flow: 60 mL/min. | [54] |
| Column temperature: 30 °C | Cone temperature: 200 °C | |||
| Mobile phase: 0.1% aqueous formic acid and methanol | Cone gas flow: 20 mL/min | |||
| Flow rate: 0.3 mL/ min | Heated probe temperature: 300 °C. | |||
| Injection volume: 2 µL | ||||
| Oleuropein; acteoside; rutin | Syringa vulgaris L. flowers and fruits | Column: Zorbax SB-C18 (150 × 3.0 mm, 3.5 μm) | Capillary voltage: 3500 V | [37] |
| Column temperature: 25 °C | Nebulizing and drying gas: nitrogen | |||
| 0.1% (v/v) formic acid and methanol | Nebulizing gas pressure: 45 psi | |||
| Flow rate: 0.7 mL/min | Drying gas flow and temperature: 10 L/min and 300 °C | |||
| Fragmentor voltage: 170 V | ||||
| Nozzle voltage: 500 V | ||||
| Sheath gas flow and temperature: 10 L/min and 300 °C | ||||
| Gallic acid; protocatechuic acid; p-Hydroxybenzoic acid; vanillic acid; caffeic acid; syringic acid; p-coumaric acid; ferulic acid; rutin; quercetin-3-O-glucoside; quercetin; cyanidin-3,5-O-diglucoside; cyanidin-3-O-glucoside; cyanidin-3-O-rutinoside; eonidin-3-O-glucoside | Black rice wine | Column: SHIM-PACK XR-ODS (75 × 3.0 mm, 2.2 μm) | Ion spray voltage: 4400 and –4400 V | [29] |
| Column temperature: 30 °C | Curtain gas (CUR): nitrogen | |||
| Mobile phase: 50% aqueous acetonitrile (v/v) with 0.2% formic acid and water with 0.2% formic acid | Nebulizer gas: air at 50 psi | |||
| Flow rate: 0.3 mL/min | Heater gas: air at 50 psi | |||
| Injection volume: 20 µL | ||||
| Apigenin; Baicalein; chrysin; p-coumaric acid; dihydroxytetramethoxy-flavone; dihydroxytrimethoxy-flavanone; eriodictyol; luteolin; naringenin; norwogonin; oroxylin a; pentahydroxyflavanone; pinocembrin; quercetin; scutellarein; sinapic acid; verbascoside; wogonin | Column: Zorbax Stable Bond Analytical SB-C18 column (250 × 4.6 mm, 5 μm) | Nebulizing and drying gas: nitrogen at 45 psi | [3] | |
| Column temperature: 35 °C | Electron spray voltage: 5.2 kV | |||
| Mobile phase: 0.1% aqueous formic acid and methanol | Source temperature at 500 °C | |||
| Flow rate: 0.5 mL/min | ||||
| Injection volume: 10 µL |
To have more reproducible elution times and greater resolution of the peaks, column temperature is generally controlled. The used temperature values normally varied between 25 and 40 °C (Table 2). Higher temperatures also contribute to reducing the pressure of the column when high flow rates are applied and decrease the analysis time [46].
In columns with non-modified alkyl chains, such as the C18 columns, the phenolic compounds are eluted according to their polarity. Generally, the phenolic compounds separation is carried out by gradient elution using binary systems comprising an aqueous component and a less polar organic solvent such as methanol or acetonitrile. Furthermore, with the aim to control pH in order to control the charge of the molecule, acids such as formic [1,33,77], acetic [2,54], or phosphoric [28] are normally incorporated in low percentages, between 0.005% and 0.5% (v/v) in the aqueous phase or even in both phases. Despite not being frequent, because silica-based columns can be irreversibly damaged at very low pH, some authors use higher percentages of acid (10%) [32]. Additionally, because the phosphoric acid is non-volatile, its use in mass spectrometer detection is not recommended. Acid pH between 2 and 4, contribute to avoiding phenolic compounds dissociation, help with defining peaks and improving the ionization efficiency for mass characterization [71,78]. Tong et al. [79] optimized different concentrations of acetic or formic acid in two mobile phases (water-methanol or water-acetonitrile) and also various gradient programs to determine several polyphenols in Citrus paradisi cv. Changshanhuyu peel. These authors found that the addition of 0.4% formic acid in the aqueous phase improved the polyphenol determination.
The selection of the flow rates and injection volume usually varies depending on the chosen column. As can be seen in Table 2, for polyphenols identification by LC–MS/MS the flow rate ranged between 0.2 and 0.8 mL/min and the injection volume from 2 to 40 μL.
Diode array detection (DAD) is the more used detector to quantify and identify polyphenols since it is cheap and robust. However, the identification and quantification of polyphenols is really complex largely due to the complexity of the plant material samples and the low concentrations in which they can be found. Although many standards are available, it is difficult to choose the correct standards, and the researchers must know in advance the components that the samples contain, to make a good selection of the standards. Additionally, the DAD identification is by retention time and by UV-vis spectrum. The polyphenols are linked to sugars that are not UV-active and hence will not affect the spectrum, which complicates correct polyphenol identification. Considering these difficulties, in many cases, it is necessary to use a more sensitive and selective detector such as a mass spectrometer to a LC system (LC–MS) or to a tandem mass spectrometer (LC–MS/MS). In some cases, the use of single quadrupole mass spectrometer is not selective enough for target compounds. In these cases, the use of a tandem mass spectrometer is necessary. Tandem mass spectrometers consist of three quadrupoles in which the first (Q1) and third quadrupole (Q3) are mass filters and the second quadrupole (Q2) acts as a collision cell. Thus, in comparison with a single quadrupole mass spectrometer, the presence of three quadrupoles make the spectrometer more selective, reduce signal-to-noise (S/N), present a wider linear range of quantitation, better accuracy, and reproducibility. Additionally, the identification of analytes is more real since it is able to use the multiple reaction monitoring (MRM).
Despite the differences and advantages reported for tandem mass spectrometers, multiple types of mass spectrometers can be used for polyphenol analysis, such as quadrupole (single or triple) [49,50], ion trap mass spectrometer [80,81,82], time-of flight or quadrupole-time-of-flight [10,79,80,83], and Orbitrap [33] among others. In the consulted literature there are studies demonstrating efficacy in polyphenols detection and quantification with different mass analyzers (Table 3).
Table 3.
Ionization mode, collision energy, and multiple reaction monitoring (MRM) transitions used in the polyphenol determination.
| Analyte | Analyzer/Ionization Mode | Precursor Ion (m/z) | Product Ion (m/z) | Ref |
|---|---|---|---|---|
| 3-(3,4-Dihydroxyphenyl)propionic acid | QqQ/ESI (−) | 181 | 137 | [34] |
| 3-(3-Hydroxyphenyl)propionic acid | QqQ/ESI (−) | 165 | 121 | [34] |
| 3-(4-hydroxy)phenylpropionic acid | QqQ/ESI (−) | 165 | 121 | [34] |
| 3-(4-hydroxyphenyl)propionic acid | QqQ/ESI (−) | 164.9 | 120.5 | [31] |
| 3,4-Dicaffeoylquinic acid | QqQ/ESI (−) | 515 | 353, 235,191, 179, 173, 135 | [51] |
| 3,4-Dihydroxybenzoic acid | QqQ/ESI (−) | 152.9 | 108.9 | [31] |
| 3,4-Dihydroxy-phenylacetic acid | QqQ/ESI (−) | 166.9 | 122.7 | [31] |
| 3-Hydroxybenzoic acid | QqQ/ESI (−) | 137 | 93 | [31,34] |
| 3-Hydroxyphenylacetic acid | QqQ/ESI (−) | 150.9 | 107.0 | [31] |
| 3-Methoxyphenylacetic acid | QqQ/ESI (−) | 180.8 | 136.8 | [31] |
| 3-O-Caffeoylquinic acid | QqQ/ESI (−) | 353 | 191, 173, 85 | [51] |
| 3-O-methylquercetin | QTOF/ESI (−) | 315 | 151, 271 | [10] |
| 4,5-Dicaffeoylquinic acid | QqQ/ESI (−) | 515 | 353, 191, 179, 173, 135 | [51] |
| 4′,7′-Dimethoxy luteolin | QqQ/ESI (−) | 313 | 298, 283, 255, 163, 117 | [51] |
| 4-Hydroxybenzoic acid | QqQ/ESI (−) | 136.9 | 93.0 | [31] |
| 4-O-Feruloylquinic acid | QqQ/ESI (−) | 367 | 191, 173, 134, 93, 87 | [51] |
| Qtrap/ESI (−) | 193, 191, 173 | [52] | ||
| 5-(3,4-Dihydroxyphenyl)-γ-valerolactone | QqQ/ESI (−) | 207 | 85 | [34] |
| 5-(3,4-Dihydroxyphenyl)-γ-valerolactone glucuronide | QqQ/ESI (−) | 383 | 207 | [34] |
| 5,6,7,30,40-Pentahydroxyflavanon | Q-Trap/ESI (−) | 479 | 303, 285, 181, 167, 135 | [3] |
| 5-O-Caffeoylquinic acid | QqQ/ESI (−) | 353 | 191, 179, 173 | [51] |
| 5-O-p-Coumaroylquinic acid | Qtrap/ESI (−) | 337 | 191,173 | [52] |
| 7-O-glucoronide | Q-Trap/ESI (−) | 480 | 303, 285, 181, 167, 136 | [3] |
| Acteoside | QqQ/ESI (−) | 623.2 | 160.9 | [37] |
| Apigenin-7-O-β-apiofuranosyl-6,8-di-C– β-glucopyranoside | QqQ/ESI (−) | 725 | 635, 605, 593, 575, 503 | [49] |
| Aromadendrin-6-C-β-D-glucopyranosyl-7-O-[β-D-apiofuranosyl-(1→2)]-O-β-D-glucopyranoside | QqQ/ESI (−) | 743 | 653, 623, 581, 563 | [49] |
| Astragalin | QqQ/ESI (−) | 447.09 | 284.0 | [33,50] |
| Avicularin | QqQ/ESI (−) | 433.08 | 301.0 | [33] |
| Benzoic acid | QqQ/ESI (−) | 121 | 77 | [31,34] |
| Caffeic acid | QqQ/ESI (−) | 179 | 135 | [1,2,29,31,34,36,50,84] |
| 135, 107, 89 | [51] | |||
| Catechin | QqQ/ESI (−) | 289.1 | 109.20 | [50] |
| 245.1 | [1] | |||
| 203 | [34] | |||
| Catechin glucuronide | QqQ/ESI (−) | 465 | 289 | [34] |
| Catechin | QqQ/ESI (−) | 289.1 | 245.1 | [84] |
| Chlorogenic acid | QqQ/ESI (−) | 353.1 | 191.1 | [1,2,50] |
| 79, 191 | [36] | |||
| Chrysin | QqQ/ESI (−) | 252.9 | 143.0 | [31] |
| Qtrap/ESI (−) | [38] | |||
| Cinnamic acid glucoside | QqQ/ESI (−) | 309 | 291, 247, 180, 128 | [49] |
| Coumarin glycoside ester | Qtrap/ESI (−) | 351 | 307, 145 | [52] |
| Cyanidin-3,5-O-diglucoside | QqQ/ESI (+) | 611.4 | 287.2 | [29] |
| Cyanidin-3-O-glucoside | Qtrap/ESI (+) | 449.2 | 287.2 | [32] |
| QqQ/ESI (+) | [29] | |||
| Cyanidin-3-O-rutinoside | QqQ/ESI (+) | 595.4 | 287.2 | [29] |
| Cynaroside | QqQ/ESI (−) | 446.90 | 285.10 | [50] |
| Dicaffeoylquinic acid | QTOF/ESI (−) | 515 | 353, 191, 179 | [10] |
| QqQ/ESI (−) | 249, 179, 135 | [49] | ||
| Dihydrocaffeic acid glucuronide | QqQ/ESI (−) | 357 | 181 | [34] |
| Dihydroferulic acid glucuronide | QqQ/ESI (−) | 371 | 195 | [34] |
| Dihydro-p-coumaric acid derivative | Qtrap/ESI (−) | 415 | 385, 165 | [52] |
| Dihydroxybenzoic acid | QqQ/ESI (−) | 153.0 | 109.0 | [1] |
| Ellagic acid | QqQ/ESI (−) | 301 | 145 | [33,50] |
| Epicatechin | QqQ/ESI (−) | 289.1 | 109.2 | [50] |
| Epicatechin derivative | Qtrap/ESI (−) | 397 | 365, 289, 207, 151 | [52] |
| Epicatechin glucuronide | QqQ/ESI (−) | 465 | 289 | [34] |
| Epicatechin | QqQ/ESI (−) | 289 | 203 | [34] |
| Epigallocatechin | QqQ/ESI (−) | 305.1 | 125.0 | [50] |
| Qtrap/ESI (−) | 305 | 305, 273, 179 | [52] | |
| Eriocitrin | QqQ/ESI (−) | 595.2 | 286.9 | [31] |
| Qtrap/ESI (−) | [38] | |||
| Eriodictyol | QqQ/ESI (−) | 287.0 | 150.7 | [31] |
| Qtrap/ESI (−) | [38] | |||
| Ferulic acid | QqQ/ESI (−) | 193 | 134 | [31,34,36,50,84] |
| 177.9 | [29] | |||
| 149 | [2] | |||
| Ferulic acid glucoside | QqQ/ESI (-) | 355 | 193, 178, 134 | [49] |
| Fisetin | QqQ/ESI (−) | 284.9 | 134.8 | [31] |
| Qtrap/ESI (−) | [38] | |||
| Formononetin | QqQ/ESI (−) | 266.9 | 251.8 | [31] |
| Gallic acid | QqQ/ESI (−) | 169 | 125 | [1,2,29,33,34,50,84] |
| Qtrap/ESI (−) | [52] | |||
| 169, 125, 97 | [30] | |||
| Gallic acid glycoside | Qtrap/ESI (−) | 331 | 169 | [52] |
| Genistein | QqQ/ESI (−) | 269 | 269, 195, 133 | [49] |
| Gentisic acid | QqQ/ESI (−) | 153.10 | 109.30 | [50] |
| Glabridin | QqQ/ESI (−) | 323.2 | 201.3 | [31] |
| Qtrap/ESI (−) | [38] | |||
| Hesperetin | QqQ/ESI (−) | 300.9 | 163.7 | [31] |
| Qtrap/ESI (−) | [38] | |||
| Hesperidin | QqQ/ESI (−) | 609.0 | 301 | [1,31] |
| Qtrap/ESI (−) | [38] | |||
| Hippuric acid | QqQ/ESI (−) | 178 | 134 | [31,34] |
| Homovanillic | QqQ/ESI (−) | 181 | 163 | [34] |
| Hyperoside | QqQ/ESI (−) | 463.1 | 300.0 | [33,50] |
| Isoquercitrin | QqQ/ESI (−) | 463 | 300.0 | [33] |
| QTOF/ESI (-) | 301, 151 | [10] | ||
| Isorhamnetin | QqQ/ESI (−) | 315.10 | 300.10 | [50] |
| Juglanin | QqQ/ESI (−) | 417.08 | 284.0 | [33] |
| Kaempferol | QqQ/ESI (−) | 285 | 93.1 | [50] |
| 93.4 | [1] | |||
| 239 | [34] | |||
| Qtrap/ESI (-) | 150.7 | [38] | ||
| Q-trap/ (+) | 287 | 287, 258, 165, 153, 121 | [30] | |
| Kaempferol 3-O-hexoside | Q-trap/ (-) | 447 | 447, 285, 255 | [30] |
| Kaempferol 3-O-pentoside | Q-trap/ (+) | 419 | 419, 309, 287, 155 | [30] |
| Liquiritigenin | QqQ/ESI (−) | 255.1 | 118.7 | [31] |
| Liquiritin | QqQ/ESI (−) | 417.2 | 255.0 | [31] |
| Luteolin | QqQ/ESI (−) | 285.10 | 133.20 | [50] |
| QTOF/ESI (-) | 285 | 217, 151 | [10] | |
| Methylcatechin | QqQ/ESI (−) | 303 | 137 | [34] |
| Methylcatechin glucuronide | QqQ/ESI (−) | 479 | 303 | [34] |
| Methylepicatechin glucuronide | QqQ/ESI (−) | 479 | 303 | [34] |
| Methylgallate | Qtrap/ESI (−) | 183 | 169,125 | [52] |
| Methylgallic acid | QqQ/ESI (−) | 183 | 168 | [34] |
| Myricetin | QqQ/ESI (−) | 316.9 | 179 | [2] |
| 317.0 | 151.0 | [1] | ||
| Naringenin | QqQ/ESI (−) | 271 | 151 | [31,36,50] |
| Qtrap/ESI (−) | 270.9 | 118.7 | [38] | |
| Naringin | Qtrap/ESI (−) | 579.2 | 270.9 | [38] |
| QqQ/ESI (−) | 579.0 | 271.1 | [1,31] | |
| Narirutin | QqQ/ESI (−) | 579.3 | 270.9 | [31] |
| Neohesperidin | QqQ/ESI (−) | 609.0 | 300.8 | [31] |
| Qtrap/ESI (−) | [38] | |||
| Nicotiflorin | QqQ/ESI (−) | 593.00 | 285.00 | [50] |
| Nodakenin | Q-trap/ESI(+) | 409 | 409, 391, 353, 389, 247, 229, 203, 185 | [30] |
| Oleuropein | QqQ/ESI (−) | 539.2 | 275.1 | [37] |
| p-Coumaric acid | QqQ/ESI (−) | 163 | 119 | [1,29,31,34,36,50] |
| p-coumaric acid glucoside | QqQ/ESI (−) | 325 | 163, 119 | [49] |
| Pelargonidin-3 rutinoside | Qtrap/ESI (+) | 579.2 | 433.1, 271.1 | [32] |
| Pelargonidin-3-glucoside | Qtrap/ESI (+) | 433.2 | 271.6 | [32] |
| Pelargonidin-acetylglucoside | Qtrap/ESI (+) | 475.2 | 271.2 | [32] |
| Pelargonidin-malonylrhamnoside | Qtrap/ESI (+) | 503.2 | 271.1 | [32] |
| Pelargonidin-succinyl-arabinoside or | Qtrap/ESI (+) | 503.2 | 271.1 | [32] |
| Pentahydroxyflavanone | Q-Trap/(-) | 303 | 257, 219, 167, 141, 129, 113 | [3] |
| Pentahydroxyflavone | Q-Trap/(+) | 303 | 303, 285, 257, 247, 235, 229, 179, 165, 153, 149, 137, 127 | [3] |
| Peonidin-3-O-glucoside | QqQ/ESI (+) | 463.0 | 301.2 | [29] |
| Phenylpropionic acid | QqQ/ESI (−) | 149 | 105 | [34] |
| Phloroglucinol | QqQ/ESI (−) | 125 | 57 | [34] |
| 97 | [2] | |||
| p-Hydroxybenzoic acid | QqQ/ESI (−) | 137 | 93 | [29,50] |
| Pinocembrin | QqQ/ESI (−) | 254.8 | 150.7 | [31] |
| Pinoresinol rhamnoside | Qtrap/ESI (−) | 503 | 357 | [52] |
| Procyanidin dimer | QqQ/ESI (−) | 557 | 425 | [34] |
| Procyanidin dimer B1and B2 | QqQ/ESI (−) | 577.10 | 407.20 | [50] |
| Procyanidin trimer C1 | QqQ/ESI (−) | 865.00 | 289.00 | [50] |
| Protocatechuic acid | QqQ/ESI (−) | 153 | 109. | [29,34,50] |
| 141, 109 | [51] | |||
| 135, 109 | [49] | |||
| Q-trap/ (-) | 153, 109, 108 | [30] | ||
| Quercetin | QqQ/ESI (−) | 301 | 151 | [1,29,31,34,36,50] |
| 179 | [2] | |||
| QTOF/ESI (-) | 151, 179, 121 | [10] | ||
| Q-trap/ (+) | 301, 273, 179, 153 | [30] | ||
| Quercetin 3-O-hexoside | Q-trap/ (−) | 463 | 463, 301, 300, 283, 271, 255, 151 | [30] |
| Quercetin 3-O-pentoside | Q-trap/ (−) | 433 | 433, 300, 273, 271, 255, 179, 151 | [30] |
| Quercetin derivative | Qtrap/ESI (−) | 657 | 493, 327, 301, 255 | [52] |
| Quercetin-3-O-glucoside | QqQ/ESI (−) | 463.1 | 300.7 | [29] |
| Quercetin-3-O-sophoroside | QqQ/ESI (−) | 625.2 | 299.8 | [33] |
| Quercetin-O-glucoside | QqQ/ESI (−) | 463 | 301, 179, 151 | [51] |
| Quercitrin | Qtrap/ESI (+) | 447.0 | 301.0 | [32,33,84] |
| QqQ/ESI (−) | ||||
| Quinic acid butyl ester | Qtrap/ESI (−) | 247 | 247, 191 | [52] |
| Quinic acid derivative | QqQ/ESI (−) | 405 | 191, 111 | [49] |
| Quinic acid methyl ester | QqQ/ESI (−) | 205 | 143, 129, 114 | [51] |
| Resveratrol | QqQ/ESI (−) | 227.1 | 143.1 | [1,34] |
| Rutin | QqQ/ESI (−) | 609.0 | 300.1 | [1,29,31,36,37,50,51,84] |
| Sinapoyl hexoside | Q-Trap/(−) | 385 | 223, 205, 190, 179, 175, 163 | [3] |
| Sophoraflavonoloside | QqQ/ESI (−) | 609.20 | 284.0 | [33] |
| Syringic acid | QqQ/ESI (−) | 197.0 | 181.9 | [29] |
| Taxifolin | QqQ/ESI (−) | 303.2 | 284.7 | [31] |
| Qtrap/ESI (−) | [38] | |||
| trans-Ferulic acid | QqQ/ESI (−) | 193.2 | 134.0 | [1] |
| Tricin | Qtrap/ESI (−) | 329 | 329, 189, 137 | [52] |
| Tricin O-(syringyl alcohol) ether O-hexoside | Qtrap/ESI (−) | 659 | 497, 329 | [52] |
| Valeric acid | QqQ/ESI (−) | 225 | 163 | [34] |
| Vanillic acid | QqQ/ESI (−) | 167 | 108 | [29,34] |
| Vanillin | QqQ/ESI (−) | 151.10 | 136.20 | [50] |
| Vicenin | QqQ/ESI (−) | 593 | 503, 473, 383, 353, 297 | [49] |
| α-Hydroxyhippuric acid | QqQ/ESI (−) | 193.9 | 72.8 | [31] |
ESI: electrospray ionization; Q-trap: quadrupole ion trap; QqQ: triple quadrupole mass spectrometer; Q-TOF: quadrupole time of flight.
Among tandem mass spectrometers, QqQ-MS presented high selectivity and sensitivity, but it is limited to structural characterization of non-target compounds [83]. Ion trap-MS is a good tool for the identification of unknown compounds, but the co-extracted ions can make correct selection of the diagnostic ions difficult. Finally, a QTOF-MS spectrometer offers accurate mass measurement, permitting better capability of identifying unknown chemicals than QqQ-MS and Ion trap-MS. Therefore, it seems clear that each of these analyzers have certain advantages and disadvantages compared to the others. Despite this, the use of tandem mass spectrometry is the most versatile tool for determining and quantifying polyphenols.
In this regard, Nijat et al. [33] combined the ultra-high performance liquid chromatography coupled to quadrupole-orbitrap high resolution mass spectrometry (UHPLC–Q–orbitrap–HRMS) and high performance liquid chromatography triple-quadrupole linear ion trap mass spectrometry (HPLC–QqQ–LITMS) to detect and quantify polyphenols in Meiguihua oral solution; Jin et al. [83] studied the identification of polyphenols in mulberry cultivars with both TOF/MS and QqQ–MS, while Quatrin et al. [80] reported the characterization and identification of tannins, flavonols, anthocyanins, and matrix-bound polyphenols from jaboticaba fruit peel with two different mass spectrometer analyzers (LC–TRAP–MS/MS and LC–Q–TOF–MS/MS), while the quantification was carried out using HPLC–DAD technique. For quantitative analysis, the use of triple quadrupole mass spectrometers (QqQ) is common, which are capable of performing multiple reaction monitoring (MRM) [84,85]. In fact, MRM allows enhanced sensitivity and selectivity.
On the other hand, although in liquid chromatography–tandem mass spectrometry (LC–MS/MS) other sources of ionization can be used, the electrospray ionization (ESI) is the most employed. To improve the sensitivity and minimize the matrix effects it is necessary optimize several MS/MS parameters such as capillary voltage, declustering potential, collision energy, and dwell times before analysis. It is also necessary to choose the ion mode between positive or negative. There are studies that investigated the presence of flavonoid and glycosides phenolic acids in Ajwa date fruits by LC–ESI–MS–MS in both modes [86]. However, as is shown in Table 3, negative ion mode is the most commonly used mode when analyzing phenolic compounds with the exception for anthocyanins for which both ionization modes have been commonly reported. Finally, a particular mention must be reserved to the so-called high-throughput targeted and untargeted metabolomics-based approaches, which have been widely used in the last years to characterize the different polyphenolic classes in several plant-foods for human nutrition [87,88,89,90]. In this regard, the metabolomic approaches have been very helpful in identifying and quantifying a specific set of metabolites in a sample, with several advantages, such as the absence of a sample purification step, thus contributing to the understanding of several factors affecting the phenolic profile of a sample under investigation.
Therefore, taking into account the great complexity and variety of phenolic compounds that may be present in the same food or plant extract, makes the use of LC–MS/MS essential. Furthermore, as commented above, specific parameters must be selected for each family of phenolic compounds for their detection with mass spectrometry. For this reason, this review presents the data from the most recent studies, in a comprehensive way, providing and simplifying the information of the great variety of works that exist in the literature.
4. Conclusions
Polyphenols are of great interest from the point of view of health and industry. However, their use depends largely on good characterization and quantification of the active compounds present in the extracts and plant material. There is not a common extraction method for all types of polyphenols because of the large number of existing phenolic compounds and the old techniques to quantify their content have serious limitations. Therefore, the development of new techniques that allowed a correct characterization of phenolic compounds became essential. Moreover, extraction, purification, and clean-up stages have a key role for obtaining reliable results. The use of liquid chromatography with tandem mass spectrometry (LC–MS/MS) reported good results with low quantification limits in the polyphenols analysis. However, there are several researchers that used different extraction, chromatographic, and mass spectrometer conditions. Therefore, the present review arises from the need to have the information in an organized and well-structured way, since this is vital when deciding the best technique to use.
As a general conclusion, the LC–MS/MS is the best and most powerful technique for the correct identification and quantification of polyphenols. However, the development of the analytical method depends largely on the matrix to be analyzed as well as on the phenolic compounds it contains. Therefore, the information provided by this review, focused on LC–MS/MS technique, allows the scientific community to have a global vision of the main parameters used by other authors in recent studies, both in extraction and clean-up procedures as well as the chromatographic and mass spectrometer conditions.
Acknowledgments
The authors thank GAIN (Axencia Galega de Innovación) for supporting this review (grant numberIN607A2019/01). Special thanks to Agencia Estatal de Investigación for supporting of Olalla López-Fernández (PTA2017-13615-I). Paulo E. S. Munekata acknowledges postdoctoral fellowship support from the Ministry of Economy and Competitiveness (MINECO, Spain) “Juan de la Cierva” program (FJCI-2016-29486).
Author Contributions
Conceptualization, R.D. and J.M.L.; writing—original draft preparation, O.L.-F. and R.D.; writing—review and editing, R.D., M.P., P.E.S.M., G.R. and J.M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gómez-Mejía E., Rosales-Conrado N., León-González M.E., Madrid Y. Determination of phenolic compounds in residual brewing yeast using matrix solid-phase dispersion extraction assisted by titanium dioxide nanoparticles. J. Chromatogr. A. 2019;1601:255–265. doi: 10.1016/j.chroma.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Rajauria G. Optimization and validation of reverse phase HPLC method for qualitative and quantitative assessment of polyphenols in seaweed. J. Pharm. Biomed. Anal. 2018;148:230–237. doi: 10.1016/j.jpba.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Seo O.N., Kim G.S., Kim Y.H., Park S., Jeong S.W., Lee S.J., Jin J.S., Shin S.C. Determination of polyphenol components of Korean Scutellaria baicalensis Georgi using liquid chromatography-tandem mass spectrometry: Contribution to overall antioxidant activity. J. Funct. Foods. 2013;5:1741–1750. doi: 10.1016/j.jff.2013.07.020. [DOI] [Google Scholar]
- 4.Manach C., Williamson G., Morand C., Scalbert A., Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 5.Belščak-Cvitanović A., Durgo K., Huđek A., Bačun-Družina V., Komes D. Overview of polyphenols and their properties. In: Galanakis C.M., editor. Polyphenols: Properties, Recovery, and Applications. Elsevier; Amsterdam, The Netherlands: 2018. pp. 3–44. [Google Scholar]
- 6.Larrauri M., Zunino M.P., Zygadlo J.A., Grosso N.R., Nepote V. Chemical characterization and antioxidant properties of fractions separated from extract of peanut skin derived from different industrial processes. Ind. Crops Prod. 2016;94:964–971. doi: 10.1016/j.indcrop.2016.09.066. [DOI] [Google Scholar]
- 7.Grosso G., Stepaniak U., Topor-Madry R., Szafraniec K., Pajak A. Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition. 2014;30:1398–1403. doi: 10.1016/j.nut.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollman P.C.H. Evidence for health benefits of plant phenols: Local or systemic effects? J. Sci. Food Agric. 2001;81:842–852. doi: 10.1002/jsfa.900. [DOI] [Google Scholar]
- 9.Morand C., Crespy V., Manach C., Besson C., Demigné C., Rémésy C. Plasma metabolites of quercetin and their antioxidant properties. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998;275:212–219. doi: 10.1152/ajpregu.1998.275.1.R212. [DOI] [PubMed] [Google Scholar]
- 10.Martínez-Busi M., Arredondo F., González D., Echeverry C., Vega-Teijido M.A., Carvalho D., Rodríguez-Haralambides A., Rivera F., Dajas F., Abin-Carriquiry J.A. Purification, structural elucidation, antioxidant capacity and neuroprotective potential of the main polyphenolic compounds contained in Achyrocline satureioides (Lam) D.C. (Compositae) Bioorganic Med. Chem. 2019;27:2579–2591. doi: 10.1016/j.bmc.2019.03.047. [DOI] [PubMed] [Google Scholar]
- 11.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 12.Kumar S., Pandey A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang C.S., Landau J.M., Huang M.T., Newmark H.L. Inhibition of Carcinogenesis by Dietary Polyphenolic Compounds. Annu. Rev. Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 14.Chong J., Poutaraud A., Hugueney P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009;177:143–155. doi: 10.1016/j.plantsci.2009.05.012. [DOI] [Google Scholar]
- 15.Arranz S., Chiva-Blanch G., Valderas-Martínez P., Medina-Remón A., Lamuela-Raventós R.M., Estruch R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients. 2012;4:759–781. doi: 10.3390/nu4070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 17.Scalbert A., Manach C., Morand C., Rémésy C., Jiménez L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 18.Lee C.Y. Challenges in providing credible scientific evidence of health benefits of dietary polyphenols. J. Funct. Foods. 2013;5:524–526. doi: 10.1016/j.jff.2012.10.018. [DOI] [Google Scholar]
- 19.Domínguez R., Gullón P., Pateiro M., Munekata P.E.S., Zhang W., Lorenzo J.M. Tomato as potential source of natural additives for meat industry. A review. Antioxidants. 2020;9:73. doi: 10.3390/antiox9010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorenzo J.M.M., Pateiro M., Domínguez R., Barba F.J.F.J., Putnik P., Kovačević D.B.B.D.B., Shpigelman A., Granato D., Franco D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018;106:1095–1104. doi: 10.1016/j.foodres.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Munekata P.E.S., Rocchetti G., Pateiro M., Lucini L., Domínguez R., Lorenzo J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020;31:81–87. doi: 10.1016/j.cofs.2020.03.003. [DOI] [Google Scholar]
- 22.Domínguez R., Pateiro M., Gagaoua M., Barba F.J., Zhang W., Lorenzo J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants. 2019;8:429. doi: 10.3390/antiox8100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pateiro M., Vargas F.C., Chincha A.A.I.A., Sant’Ana A.S., Strozzi I., Rocchetti G., Barba F.J., Domínguez R., Lucini L., do Amaral Sobral P.J., et al. Guarana seed extracts as a useful strategy to extend the shelf life of pork patties: UHPLC-ESI/QTOF phenolic profile and impact on microbial inactivation, lipid and protein oxidation and antioxidant capacity. Food Res. Int. 2018;114:55–63. doi: 10.1016/j.foodres.2018.07.047. [DOI] [PubMed] [Google Scholar]
- 24.Lorenzo J.M., Vargas F.C., Strozzi I., Pateiro M., Furtado M.M., Sant’Ana A.S., Rocchetti G., Barba F.J., Dominguez R., Lucini L., et al. Influence of pitanga leaf extracts on lipid and protein oxidation of pork burger during shelf-life. Food Res. Int. 2018;114:47–54. doi: 10.1016/j.foodres.2018.07.046. [DOI] [PubMed] [Google Scholar]
- 25.Munekata P.E.S., Domínguez R., Campagnol P.C.B., Franco D., Trindade M.A., Lorenzo J.M. Effect of natural antioxidants on physicochemical properties and lipid stability of pork liver pâté manufactured with healthy oils during refrigerated storage. J. Food Sci. Technol. 2017;54:4324–4334. doi: 10.1007/s13197-017-2903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naczk M., Shahidi F. Extraction and analysis of phenolics in food. J. Chromatogr. A. 2004;1054:95–111. doi: 10.1016/S0021-9673(04)01409-8. [DOI] [PubMed] [Google Scholar]
- 27.Naczk M., Shahidi F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006;41:1523–1542. doi: 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Pires F.B., Dolwitsch C.B., Dal Prá V., Faccin H., Monego D.L., de Carvalho L.M., Viana C., Lameira O., Lima F.O., Bressan L., et al. Qualitative and quantitative analysis of the phenolic content of connarus var. Angustifolius, cecropia obtusa, cecropia palmata and mansoa alliacea based on HPLC-DAD and UHPLC-ESI-MS/MS. Braz. J. Pharmacogn. 2017;27:426–433. doi: 10.1016/j.bjp.2017.03.004. [DOI] [Google Scholar]
- 29.Wang Y., Liu Y., Xiao C., Liu L., Hao M., Wang J., Liu X. Simultaneous Determination of 15 Phenolic Constituents of Chinese Black Rice Wine by HPLC-MS/MS with SPE. J. Food Sci. 2014;79:C1100–C1105. doi: 10.1111/1750-3841.12483. [DOI] [PubMed] [Google Scholar]
- 30.Song Y., Jeong S.W., Lee W.S., Park S., Kim Y.H., Kim G.S., Lee S.J., Jin J.S., Kim C.Y., Lee J.E., et al. Determination of polyphenol components of Korean prostrate spurge (Euphorbia supina) by using liquid chromatography—Tandem mass spectrometry: Overall contribution to antioxidant activity. J. Anal. Methods Chem. 2014;2014 doi: 10.1155/2014/418690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bajkacz S., Baranowska I., Buszewski B., Kowalski B., Ligor M. Determination of Flavonoids and Phenolic Acids in Plant Materials Using SLE-SPE-UHPLC-MS/MS Method. Food Anal. Methods. 2018;11:3563–3575. doi: 10.1007/s12161-018-1332-9. [DOI] [Google Scholar]
- 32.Ruiz A., Sanhueza M., Gómez F., Tereucán G., Valenzuela T., García S., Cornejo P., Hermosín-Gutiérrez I. Changes in the content of anthocyanins, flavonols, and antioxidant activity in Fragaria ananassa var. Camarosa fruits under traditional and organic fertilization. J. Sci. Food Agric. 2019;99:2404–2410. doi: 10.1002/jsfa.9447. [DOI] [PubMed] [Google Scholar]
- 33.Nijat D., Abdulla R., Liu G.Y., Luo Y.Q., Aisa H.A. Identification and quantification of Meiguihua oral solution using liquid chromatography combined with hybrid quadrupole-orbitrap and triple quadrupole mass spectrometers. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020;1139:121992. doi: 10.1016/j.jchromb.2020.121992. [DOI] [PubMed] [Google Scholar]
- 34.Iglesias-Carres L., Mas-Capdevila A., Bravo F.I., Aragonès G., Arola-Arnal A., Muguerza B. A comparative study on the bioavailability of phenolic compounds from organic and nonorganic red grapes. Food Chem. 2019;299:125092. doi: 10.1016/j.foodchem.2019.125092. [DOI] [PubMed] [Google Scholar]
- 35.Marchetti L., Pellati F., Graziosi R., Brighenti V., Pinetti D., Bertelli D. Identification and determination of bioactive phenylpropanoid glycosides of Aloysia polystachya (Griseb. et Moldenke) by HPLC-MS. J. Pharm. Biomed. Anal. 2019;166:364–370. doi: 10.1016/j.jpba.2019.01.033. [DOI] [PubMed] [Google Scholar]
- 36.Tamasi G., Baratto M.C., Bonechi C., Byelyakova A., Pardini A., Donati A., Leone G., Consumi M., Lamponi S., Magnani A., et al. Chemical characterization and antioxidant properties of products and by-products from Olea europaea L. Food Sci. Nutr. 2019;7:2907–2920. doi: 10.1002/fsn3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tótha G., Barabás C., Tóth A., Kéry Á., Béni S., Boldizsár I., Varga E., Noszál B. Characterization of antioxidant phenolics in Syringa vulgaris L. flowers and fruits by HPLC-DAD-ESI-MS. Biomed. Chromatogr. 2016;30:923–932. doi: 10.1002/bmc.3630. [DOI] [PubMed] [Google Scholar]
- 38.Baranowska I., Bajkacz S. A new UHPLC-MS/MS method for the determination of flavonoids in supplements and DPPH[rad]-UHPLC-UV method for the evaluation of the radical scavenging activity of flavonoids. Food Chem. 2018;256:333–341. doi: 10.1016/j.foodchem.2018.02.138. [DOI] [PubMed] [Google Scholar]
- 39.Catalán Ú., Barrubés L., Valls R.M., Solà R., Rubió L. In vitro Metabolomic Approaches to Investigating the Potential Biological Effects of Phenolic Compounds: An Update. Genom. Proteom. Bioinform. 2017;15:236–245. doi: 10.1016/j.gpb.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rocchetti G., Bhumireddy S.R., Giuberti G., Mandal R., Lucini L., Wishart D.S. Edible nuts deliver polyphenols and their transformation products to the large intestine: An in vitro fermentation model combining targeted/untargeted metabolomics. Food Res. Int. 2019;116:786–794. doi: 10.1016/j.foodres.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Rocchetti G., Lucini L., Giuberti G., Bhumireddy S.R., Mandal R., Trevisan M., Wishart D.S. Transformation of polyphenols found in pigmented gluten-free flours during in vitro large intestinal fermentation. Food Chem. 2019;298:125068. doi: 10.1016/j.foodchem.2019.125068. [DOI] [PubMed] [Google Scholar]
- 42.Merken H.M., Beecher G.R. Liquid chromatographic method for the separation and quantification of prominent flavonoid aglycones. J. Chromatogr. A. 2000;897:177–184. doi: 10.1016/S0021-9673(00)00826-8. [DOI] [PubMed] [Google Scholar]
- 43.Ignat I., Volf I., Popa V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 44.Hasbay I., Galanakis C.M. Recovery technologies and encapsulation techniques. In: Galanakis C.M., editor. Polyphenols: Properties, Recovery, and Applications. Elsevier; Amsterdam, The Netherlands: 2018. pp. 233–264. [Google Scholar]
- 45.Jokić S., Velić D., Bilić M., Bucić-Kojić A., Planinić M., Tomas S. Modelling of solid-liquid extraction process of total polyphenols from soybeans. Czech J. Food Sci. 2010;28:206–212. doi: 10.17221/200/2009-CJFS. [DOI] [Google Scholar]
- 46.Plaza M., Domínguez-Rodríguez G., Castro-Puyana M., Marina M.L. Polyphenols analysis and related challenges. In: Galanakis C.M., editor. Polyphenols: Properties, Recovery, and Applications. Elsevier; Amsterdam, The Netherlands: 2018. pp. 177–232. [Google Scholar]
- 47.Stalikas C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007;30:3268–3295. doi: 10.1002/jssc.200700261. [DOI] [PubMed] [Google Scholar]
- 48.Pigni N.B., Aranibar C., Lucini Mas A., Aguirre A., Borneo R., Wunderlin D., Baroni M.V. Chemical profile and bioaccessibility of polyphenols from wheat pasta supplemented with partially-deoiled chia flour. LWT. 2020;124:109134. doi: 10.1016/j.lwt.2020.109134. [DOI] [Google Scholar]
- 49.Zhong L., Wu G., Fang Z., Wahlqvist M.L., Hodgson J.M., Clarke M.W., Junaldi E., Johnson S.K. Characterization of polyphenols in Australian sweet lupin (Lupinus angustifolius) seed coat by HPLC-DAD-ESI-MS/MS. Food Res. Int. 2019;116:1153–1162. doi: 10.1016/j.foodres.2018.09.061. [DOI] [PubMed] [Google Scholar]
- 50.Pereira G.A., Arruda H.S., de Morais D.R., Peixoto Araujo N.M., Pastore G.M. Mutamba (Guazuma ulmifolia Lam.) fruit as a novel source of dietary fibre and phenolic compounds. Food Chem. 2020;310:125857. doi: 10.1016/j.foodchem.2019.125857. [DOI] [PubMed] [Google Scholar]
- 51.Bakchiche B., Gherib A., Bronze M.R., Ghareeb M.A. Identification, quantification, and antioxidant activity of hydroalcoholic extract of artemisia campestris from Algeria. Turk. J. Pharm. Sci. 2019;16:234–239. doi: 10.4274/tjps.galenos.2018.99267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghareeb M.A., Mohamed T., Saad A.M., Refahy L.A.G., Sobeh M., Wink M. HPLC-DAD-ESI-MS/MS analysis of fruits from Firmiana simplex (L.) and evaluation of their antioxidant and antigenotoxic properties. J. Pharm. Pharmacol. 2018;70:133–142. doi: 10.1111/jphp.12843. [DOI] [PubMed] [Google Scholar]
- 53.Cui B., Hu Z., Zhang Y., Hu J., Yin W., Feng Y., Xie Q., Chen G. Anthocyanins and flavonols are responsible for purple color of Lablab purpureus (L.) sweet pods. Plant Physiol. Biochem. 2016;103:183–190. doi: 10.1016/j.plaphy.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 54.Tao W., Zhou Z., Zhao B., Wei T. Simultaneous determination of eight catechins and four theaflavins in green, black and oolong tea using new HPLC–MS–MS method. J. Pharm. Biomed. Anal. 2016;131:140–145. doi: 10.1016/j.jpba.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 55.Ajila C.M., Brar S.K., Verma M., Tyagi R.D., Godbout S., Valéro J.R. Extraction and Analysis of Polyphenols: Recent trends. Crit. Rev. Biotechnol. 2011;31:227–249. doi: 10.3109/07388551.2010.513677. [DOI] [PubMed] [Google Scholar]
- 56.Dai J., Mumper R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iglesias-Carres L., Mas-Capdevila A., Sancho-Pardo L., Bravo F.I., Mulero M., Muguerza B., Arola-Arnal A. Optimized extraction by response surface methodology used for the characterization and quantification of phenolic compounds in whole red grapes (Vitis vinifera) Nutrients. 2018;10:1931. doi: 10.3390/nu10121931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tabaraki R., Nateghi A. Optimization of ultrasonic-assisted extraction of natural antioxidants from rice bran using response surface methodology. Ultrason. Sonochem. 2011;18:1279–1286. doi: 10.1016/j.ultsonch.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Yang L., Jiang J.G., Li W.F., Chen J., Wang D.Y., Zhu L. Optimum extraction process of polyphenols from the bark of Phyllanthus emblica L. based on the response surface methodology. J. Sep. Sci. 2009;32:1437–1444. doi: 10.1002/jssc.200800744. [DOI] [PubMed] [Google Scholar]
- 60.Karacabey E., Mazza G. Optimisation of antioxidant activity of grape cane extracts using response surface methodology. Food Chem. 2010;119:343–348. doi: 10.1016/j.foodchem.2009.06.029. [DOI] [Google Scholar]
- 61.Luque de Castro M.D., Priego-Capote F. Ultrasound-assisted crystallization (sonocrystallization) Ultrason. Sonochem. 2007;14:717–724. doi: 10.1016/j.ultsonch.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 62.Al Khawli F., Pateiro M., Domínguez R., Lorenzo J.M., Gullón P., Kousoulaki K., Ferrer E., Berrada H., Barba F.J. Innovative green technologies of intensification for valorization of seafood and their by-products. Mar. Drugs. 2019;17:689. doi: 10.3390/md17120689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adjé F., Lozano Y.F., Lozano P., Adima A., Chemat F., Gaydou E.M. Optimization of anthocyanin, flavonol and phenolic acid extractions from Delonix regia tree flowers using ultrasound-assisted water extraction. Ind. Crops Prod. 2010;32:439–444. doi: 10.1016/j.indcrop.2010.06.011. [DOI] [Google Scholar]
- 64.Altemimi A., Choudhary R., Watson D.G., Lightfoot D.A. Effects of ultrasonic treatments on the polyphenol and antioxidant content of spinach extracts. Ultrason. Sonochem. 2015;24:247–255. doi: 10.1016/j.ultsonch.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 65.Prá V.D., Dolwitsch C.B., Lima F.O., de Carvalho C.A., Viana C., do Nascimento P.C., da Rosa M.B. Ultrasound-assisted extraction and biological activities of extracts of Brassica oleracea var. capitata. Food Technol. Biotechnol. 2015;53:102–109. doi: 10.17113/ftb.53.01.15.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Azwanida N. Citation: Azwanida NN (2015) A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants. 2015;4:196. [Google Scholar]
- 67.Shi J., Nawaz H., Pohorly J., Mittal G., Kakuda Y., Jiang Y. Extraction of polyphenolics from plant material for functional foods—Engineering and technology. Food Rev. Int. 2005;21:139–166. doi: 10.1081/FRI-200040606. [DOI] [Google Scholar]
- 68.Castañeda-Ovando A., de Pacheco-Hernández M.L., Páez-Hernández M.E., Rodríguez J.A., Galán-Vidal C.A. Chemical Studies of Anthocyanins: A Review. Volume 113. Elsevier; Amsterdam, The Netherlands: 2009. pp. 859–871. [Google Scholar]
- 69.Lucci P., Saurina J., Núñez O. Trends in LC-MS and LC-HRMS analysis and characterization of polyphenols in food. TrAC Trends Anal. Chem. 2017;88:1–24. doi: 10.1016/j.trac.2016.12.006. [DOI] [Google Scholar]
- 70.Valls J., Millán S., Martí M.P., Borràs E., Arola L. Advanced separation methods of food anthocyanins, isoflavones and flavanols. J. Chromatogr. A. 2009;1216:7143–7172. doi: 10.1016/j.chroma.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 71.Carrasco-Pancorbo A., Cerretani L., Bendini A., Segura-Carretero A., Gallina-Toschi T., Fernández-Gutiérrez A. Analytical determination of polyphenols in olive oils. J. Sep. Sci. 2005;28:837–858. doi: 10.1002/jssc.200500032. [DOI] [PubMed] [Google Scholar]
- 72.Alonso-Carrillo N., de los Aguilar-Santamaría M.Á., Vernon-Carter E.J., Jiménez-Alvarado R., Cruz-Sosa F., Román-Guerrero A. Extraction of phenolic compounds from Satureja macrostema using microwave-ultrasound assisted and reflux methods and evaluation of their antioxidant activity and cytotoxicity. Ind. Crop. Prod. 2017;103:213–221. doi: 10.1016/j.indcrop.2017.04.002. [DOI] [Google Scholar]
- 73.M’hiri N., Ioannou I., Mihoubi Boudhrioua N., Ghoul M. Effect of different operating conditions on the extraction of phenolic compounds in orange peel. Food Bioprod. Process. 2015;96:161–170. doi: 10.1016/j.fbp.2015.07.010. [DOI] [Google Scholar]
- 74.Montero L., Herrero M., Prodanov M., Ibáñez E., Cifuentes A. Characterization of grape seed procyanidins by comprehensive two-dimensional hydrophilic interaction× reversed phase liquid chromatography coupled to diode array detection and tandem mass spectrometry. Anal. Bioanal. Chem. 2013;405:4627–4638. doi: 10.1007/s00216-012-6567-5. [DOI] [PubMed] [Google Scholar]
- 75.Pyrzynska K., Sentkowska A. Recent Developments in the HPLC Separation of Phenolic Food Compounds. Crit. Rev. Anal. Chem. 2015;45:41–51. doi: 10.1080/10408347.2013.870027. [DOI] [Google Scholar]
- 76.Salih E.Y.A., Fyhrquist P., Abdalla A.M.A., Abdelgadir A.Y., Kanninen M., Sipi M., Luukkanen O., Fahmi M.K.M., Elamin M.H., Ali H.A. LC-MS/MS tandem mass spectrometry for analysis of phenolic compounds and pentacyclic triterpenes in antifungal extracts of Terminalia brownii (Fresen) Antibiotics. 2017;6:37. doi: 10.3390/antibiotics6040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pereira A.M., Fernández-Tajes J., Gaspar M.B., Méndez J. Identification of the wedge clam Donax trunculus by a simple PCR technique. Food Control. 2012;23:268–270. doi: 10.1016/j.foodcont.2011.05.020. [DOI] [Google Scholar]
- 78.Hughey C.A., Wilcox B., Minardi C.S., Takehara C.W., Sundararaman M., Were L.M. Capillary liquid chromatography-mass spectrometry for the rapid identification and quantification of almond flavonoids. J. Chromatogr. A. 2008;1192:259–265. doi: 10.1016/j.chroma.2008.03.079. [DOI] [PubMed] [Google Scholar]
- 79.Tong C., Peng M., Tong R., Ma R., Guo K., Shi S. Use of an online extraction liquid chromatography quadrupole time-of-flight tandem mass spectrometry method for the characterization of polyphenols in Citrus paradisi cv. Changshanhuyu peel. J. Chromatogr. A. 2018;1533:87–93. doi: 10.1016/j.chroma.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 80.Quatrin A., Pauletto R., Maurer L.H., Minuzzi N., Nichelle S.M., Carvalho J.F.C., Maróstica M.R., Rodrigues E., Bochi V.C., Emanuelli T. Characterization and quantification of tannins, flavonols, anthocyanins and matrix-bound polyphenols from jaboticaba fruit peel: A comparison between Myrciaria trunciflora and M. jaboticaba. J. Food Compos. Anal. 2019;78:59–74. doi: 10.1016/j.jfca.2019.01.018. [DOI] [Google Scholar]
- 81.Hilary S., Tomás-Barberán F.A., Martinez-Blazquez J.A., Kizhakkayil J., Souka U., Al-Hammadi S., Habib H., Ibrahim W., Platat C. Polyphenol characterisation of Phoenix dactylifera L. (date) seeds using HPLC-mass spectrometry and its bioaccessibility using simulated in-vitro digestion/Caco-2 culture model. Food Chem. 2020;311:125969. doi: 10.1016/j.foodchem.2019.125969. [DOI] [PubMed] [Google Scholar]
- 82.Mannino G., Perrone A., Campobenedetto C., Schittone A., Margherita Bertea C., Gentile C. Phytochemical profile and antioxidative properties of Plinia trunciflora fruits: A new source of nutraceuticals. Food Chem. 2020;307:125515. doi: 10.1016/j.foodchem.2019.125515. [DOI] [PubMed] [Google Scholar]
- 83.Jin Q., Yang J., Ma L., Wen D., Chen F., Li J. Identification of polyphenols in mulberry (genus Morus) cultivars by liquid chromatography with time-of-flight mass spectrometer. J. Food Compos. Anal. 2017;63:55–64. doi: 10.1016/j.jfca.2017.07.005. [DOI] [Google Scholar]
- 84.Liu S., Xu Q., Li X., Wang Y., Zhu J., Ning C., Chang X., Meng X. Effects of high hydrostatic pressure on physicochemical properties, enzymes activity, and antioxidant capacities of anthocyanins extracts of wild Lonicera caerulea berry. Innov. Food Sci. Emerg. Technol. 2016;36:48–58. doi: 10.1016/j.ifset.2016.06.001. [DOI] [Google Scholar]
- 85.Ivanova-Petropulos V., Hermosín-Gutiérrez I., Boros B., Stefova M., Stafilov T., Vojnoski B., Dörnyei Á., Kilár F. Phenolic compounds and antioxidant activity of Macedonian red wines. J. Food Compos. Anal. 2015;41:1–14. doi: 10.1016/j.jfca.2015.01.002. [DOI] [Google Scholar]
- 86.Nematallah K.A., Ayoub N.A., Abdelsattar E., Meselhy M.R., Elmazar M.M., El-Khatib A.H., Linscheid M.W., Hathout R.M., Godugu K., Adel A., et al. Polyphenols LC-MS2 profile of Ajwa date fruit (Phoenix dactylifera L.) and their microemulsion: Potential impact on hepatic fibrosis. J. Funct. Foods. 2018;49:401–411. doi: 10.1016/j.jff.2018.08.032. [DOI] [Google Scholar]
- 87.Rocchetti G., Lucini L., Rodriguez J.M.L., Barba F.J., Giuberti G. Gluten-free flours from cereals, pseudocereals and legumes: Phenolic fingerprints and in vitro antioxidant properties. Food Chem. 2019;271:157–164. doi: 10.1016/j.foodchem.2018.07.176. [DOI] [PubMed] [Google Scholar]
- 88.Rocchetti G., Giuberti G., Busconi M., Marocco A., Trevisan M., Lucini L. Pigmented sorghum polyphenols as potential inhibitors of starch digestibility: An in vitro study combining starch digestion and untargeted metabolomics. Food Chem. 2020;312:126077. doi: 10.1016/j.foodchem.2019.126077. [DOI] [PubMed] [Google Scholar]
- 89.Rocchetti G., Senizza B., Giuberti G., Montesano D., Trevisan M., Lucini L. Metabolomic Study to Evaluate the Transformations of Extra-Virgin Olive Oil’s Antioxidant Phytochemicals during In Vitro Gastrointestinal Digestion. Antioxidants. 2020;9:302. doi: 10.3390/antiox9040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cuadrado-Silva C., Pozo-Bayón M., Osorio C. Targeted Metabolomic Analysis of Polyphenols with Antioxidant Activity in Sour Guava (Psidium friedrichsthalianum Nied.) Fruit. Molecules. 2016;22:11. doi: 10.3390/molecules22010011. [DOI] [PMC free article] [PubMed] [Google Scholar]