Summary
An ultra-rapid method for electrophoresing proteins on SDS PAGE, transfer of proteins to nitrocellulose membranes and immunoblotting is described here. Electrophoresis of the autoantigens La and Ro60, as well as molecular weight standards on a 4–20% gradient gel was performed in about 10 minutes using heated (70–75°C) normal Laemmli running buffer. Electrophoretic transfer of these proteins was achieved in 7 minutes using a semi-dry transfer method. Finally, immunoblotting of La and Ro60 was carried out in 30 minutes. Thus, the entire process of electrophoresis, electrotransfer and immunoblotting could be carried out in one hour.
Keywords: SDS-PAGE, Western blotting, Nitrocellulose, Molecular weight standards
1. Introduction
Proteins are first separated on the basis of size in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (1,2). Proteins separated on SDS PAGE can visualized by either staining with various protein stains or by immunoblotting. Electrophoresis of proteins on a mini-gel takes at least 2 hours to complete to ensure ideal separation without “smiling” artifacts. However, Haeberle demonstrated with a special gel and a special buffer that was heated to 70 ˚C, that it is possible to electrophorese proteins in 5 minutes (3).
For immunoblotting, the separated proteins are transferred to nitrocellulose or polyvinylidene difluoride membranes. The transfer to membranes has been achieved by (a) simple diffusion (4); vacuum-assisted solvent flow (5); and (c) electrophoretic elution (6). Electrophoretic transfer of proteins, resolved by SDS-PAGE, to nitrocellulose is a fundamental step prior to detection of specific proteins with specific antibodies (7–9). The protein transfer procedure normally takes about 2–4 hours at about 70 volts or an overnight transfer at 30 volts. High molecular weight proteins are often stubbornly resistant to transfer (7) in spite of prolonged runs and this problem is accentuated when higher percentage gels are used. Prolonged electrotransfer (16–20 h) at high current density coupled with inclusion of 0.01% sodium dodecyl sulfate, to enhance protein elution, has been used to efficiently transfer high-molecular weight proteins (9,10).
Trans-blot turbo system employs a semi-dry method of transfer of proteins from gel to membranes. Proteins of varying sizes (10 −250 kD) can be transferred to membranes in 3 to 7 minutes very efficiently (9). We have shown that both low and high molecular weight proteins can be transferred very efficiently to nitrocellulose membranes in a very short time using heated transfer buffer without methanol (10,11).
Traditional immunoblotting normally takes about four to five hours, with 1 hour for blocking, 2 hours for incubation with primary antibody, 1 hour with secondary antibody, 30 min for washing between primary and secondary antibody incubation and finally development with substrate.
Here we show that the entire process of electrophoresis of autoantigens Ro60 and La (actual gel running), western transfer and immunoblotting with specific autoantibodies can be carried out in one hour (Fig. 1,2), a process normally carried out in a period of up to 2 days.
Figure 1:

Ultrafast SDS PAGE electrophoresis and electrotransfer of proteins to nitrocellulose membrane. Prestained high molecular weight proteins were electrophoresed on a 4–20% gradient gel in 10 min and transferred to nitrocellulose membrane in 7 min. Lanes 1–4 show pre-stained high molecular weight protein standards.
Figure 2:
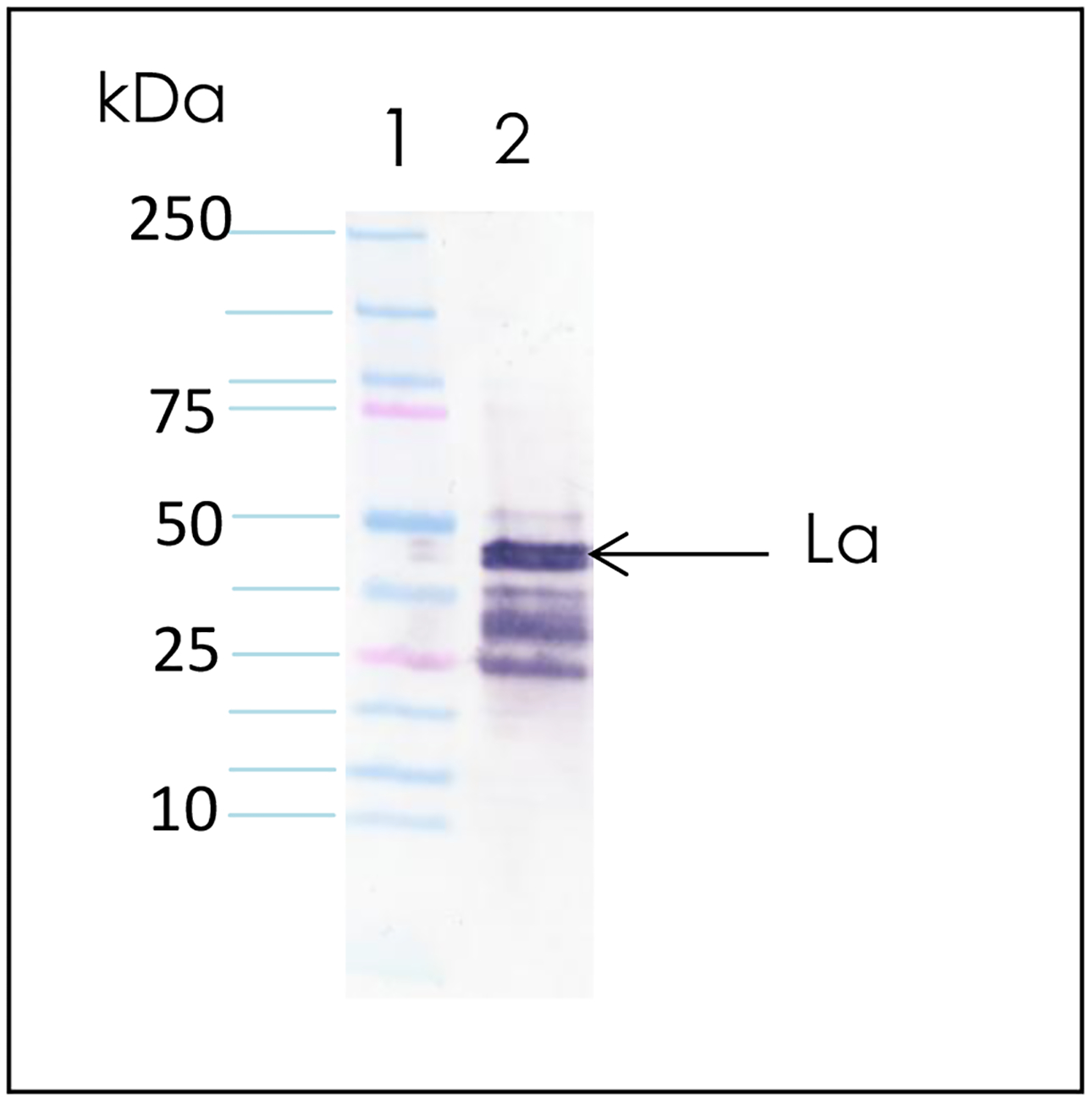
Ultrafast immunoblotting. Autoantigens La and Ro were electrophoresed in 10 min, transferred in 7 min and immunoblotted in 35 min. Lane 1: Prestained molecular weight standards; Lane 2: Bovine La autoantigen
2. Materials
Prepare all solutions using ultrapure water (prepared by purifying deionized water, to attain a sensitivity of 18 M Ω cm at 25°C) and analytical grade reagents. Prepare and store all reagents at room temperature (unless indicated otherwise). Diligently follow all waste disposal regulations when disposing waste materials. We do not add sodium azide to our reagents.
10% SDS-PAGE precast gels (10-well).
SDS lysis buffer (5x): 0.3 M Tris-HCl (pH 6.8), 10% SDS, 25% β-mercaptoethanol, 0.1% bromophenol blue, 45% glycerol. Leave one aliquot at 4°C for current use and store remaining aliquots at −20 ˚C (see Note 1).
SDS-PAGE running buffer: 10x running buffer from BioRad (see Note 2).
Phosphate buffered saline (PBS), pH 7.4.
Purified bovine Ro60 (SS-A) (12,13) and La (SS-B) autoantigen (14) were from Immunovision, Springdale, AK, USA.
BenchMark pre-stained molecular weight standards.
Nitrocellulose membranes.
Transfer buffer: 0.025 mM Tris, 192 mM glycine, 20% methanol.
BioRad TransBlot® Turbo™ transfer system.
BioRad TransBlot® Turbo™ mini nitrocellulose transfer pack
TBS containing 0.05% Tween-20 (TBST).
Blocking solution: 0.5% milk in TBS, pH 7.4. Store at 4°C (see Note 3).
Diluent solution: 0.5% milk in TBS, pH 7.4 containing 0.05% Tween. Store at 4°C (see Note 3).
Mini PROTEAN® 3 System Glass plates.
FB300 power supply.
Corning PC-351 magnetic stirrer.
One StepR Nitro blue tetrazolium (NBT)/5-bromo-4-chloro-3-indolyl phosphate (BCIP) reagent.
3. Methods
All procedures are carried out at room temperature unless otherwise specified.
3.1. SDS-PAGE
Set up commercial (4–20%) gradient gels in electrophoresis chamber for electrophoresis.
Add molecular weight marker and the autoantigens to the wells of the gel.
Transfer the heated buffer very carefully into the electrophoresis chamber without disturbing the samples in the wells (see Notes 6,7).
Add couple of drops of 0.1% bromophenol blue to the running buffer and mix gently.
Electrophorese the sample at top settings of the FB power supply.
The samples can be immediately seen to start migrating from the wells into the gel. The sample dye reaches the bottom of the gradient gel in about 10 min (see Note 8).
3.2. Semi-dry electrophoretic transfer
Set up the sandwich for electro-transfer using the transfer kit according to manufacturer instructions.
Pre-warm all reagents (blocking, diluent, TBST, substrate) in a walk-in 37 ˚C incubation room.
Insert the membrane gel sandwich into a cassette of the BioRad turbo transfer system.
Transfer at 2.5 A (25 V) for 7 minutes (use Turbo Midi setting).
Remove the nitrocellulose membrane from the sandwich and incubate for 10 min with 0.5% milk blocking that has been pre-warmed to 37 ˚C (see Notes 9,10).
Incubate membrane for 10 min with primary antibody diluted in diluent pre-warmed to 37 ˚C (see Note 11,12).
Wash three times with deionized water and three times with prewarmed TBST, all in 3 min.
Incubate with appropriate conjugate for 10 min in diluent pre-warmed to 37˚C (see Note 13).
Wash as in Step 7.
Incubate with pre-warmed substrate (see Note 14).
Scan and save results.
Notes
SDS precipitates at 4°C. Therefore, the lysis buffer needs to be warmed prior to use.
Simple method of preparing running buffer (0.025 M Tris, pH 8.3, 0.192 M glycine, 0.1% SDS): Prepare 10X native buffer (0.25 M Tris, 1.92 M glycine). Weigh 30.3 g Tris and 144 g glycine, mix and make it to 1L with water. Dilute 100 mL of 10x native buffer to 990 mL with water and add 10 mL of 10% SDS. Care should be taken to add SDS solution last, since it makes bubbles.
Add 100 mL of 10x TBS to a 1L graduated cylinder and make it to about 800 mL with water. Transfer 50 g skim milk powder into the cylinder and mix stir until dissolved. Make to 1L with water. Separate 500 mL as the blocking solution. To the remaining 500 mL add 250 μL of Tween-20 (cut end of blue tip to aspirate Tween-20 easily), dissolve and use it as the diluent.
If using l liter of buffer, use at least a 2-liter glass beaker to heat. This is because the SDS bubbles. We used Bio-Rad’s running buffer with SDS already in it. If making own running buffer, add SDS after heating.
Cover beaker with Saran wrap or some kind of cling-wrap. Use extreme caution when heating buffer.
Exercise caution in handling the hot contents. Use thermo gloves to hold the hot beaker and pour carefully.
Transfer hot buffer directly into outer electrophoresis chamber. However, when transferring into inner chamber the hot buffer comes directly in contact with the antigens in the well. Therefore, transfer the buffer gently into chamber along a 10 mL pipette so that the antigens in the wells are not disturbed significantly. Alternatively, the molecular weight marker and other antigens can be added as done normally, after filling up the electrophoresis chamber with buffer.
Using the Haeberle gel electrophoresis system (3), we were able to electrophorese proteins in about 5 min (data not shown).
The membrane can be stained with Fast Green to see effectiveness of protein transfer. However, transferring for 7 min with the BioRad Transblot system has consistently transferred low and high molecular weight protein standards almost completely. This can see directly observed the gel from which the proteins have been transferred. Also, we have stained the gel post-transfer with Coomassie brilliant blue and have observed that most of the proteins have been transferred to membrane.
We used only 0.5% milk, since a report showed that 5% milk blocking can remove antigens from membrane.
We diluted anti-Ro60 human sera 1:100 and the anti-La monoclonal 1:10.
Exercise universal precaution when handling human sera. Treat each serum sample as potentially dangerous.
We typically use Jackson Immunochemicals conjugates at 1:5000 dilution.
With the Ro60 and La antigens the signals could be seen in 30 sec or less.
References
- 1.Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- 2.Srinivas PR (2012) Introduction to protein electrophoresis. Methods Mol Biol 869, 23–8. [DOI] [PubMed] [Google Scholar]
- 3.Haeberle JR. High-temperature sodium dodecyl sulfate polyacrylamide gel electrophoresis. Biotechniques. 1997; 23:638–40. [DOI] [PubMed] [Google Scholar]
- 4.Kurien BT, and Scofield RH (1997) Multiple immunoblots after non-electrophoretic bidirectional transfer of a single SDS-PAGE gel with multiple antigens. J Immunol Methods 205, 91–94. [DOI] [PubMed] [Google Scholar]
- 5.Peferoen M, Huybrechts R, and De Loof A (1982) Vacuum-blotting: a new simple and efficient transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to NC. FEBS Lett. 145, 369–372. [Google Scholar]
- 6.Towbin H, Staehelin T, and Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to NC sheets: procedure and applications. Proc Natl Acad Sci USA 76, 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolt MW, and Mahoney PA (1997) High efficiency blotting of proteins of diverse sizes following sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem. 247, 185–192. [DOI] [PubMed] [Google Scholar]
- 8.MacPhee DJ (2010). Methodological considerations for improving Western blot analysis. J Pharmacol Toxicol Methods 61, 171–177. Review. Methods Mol Biol 536, 181–90. [DOI] [PubMed] [Google Scholar]
- 9.Biorad transblot turbo transfer system. http://www.bio-rad.com/en-us/product/semi-dry-rapid-blotting-systems/trans-blot-turbo-transfer-system.
- 10.Kurien BT, and Scofield RH (2000) Ultrarapid electrophoretic transfer of high and low molecular weight proteins using heat. Methods Mol Biol 536, 181–90. [DOI] [PubMed] [Google Scholar]
- 11.Kurien BT, Scofield RH. Heat-mediated, ultra-rapid electrophoretic transfer of high and low molecular weight proteins to nitrocellulose membranes. J Immunol Methods. 2002; 266, 127–33. [DOI] [PubMed] [Google Scholar]
- 12.Huang SC, Pan Z, Kurien BT, James JA, Harley JB, Scofield RH (1995) Immunization with vesicular stomatitis virus nucleocapsid protein induces autoantibodies to the 60 kD Ro ribonucleoprotein particle. J Investig Med 43:151–158 [PubMed] [Google Scholar]
- 13.Kurien BT, Asfa S, Li C, Dorri Y, Jonsson R, Scofield RH. Induction of oral tolerance in experimental Sjogren’s syndrome autoimmunity. Scand J Immunol. 2005;61:418–25. [DOI] [PubMed] [Google Scholar]
- 14.Tröster H, Bartsch H, Klein R, Metzger TE, Pollak G, Semsei I, Schwemmle M, Pruijn GJ, van Venrooij WJ, Bachmann M. Activation of a murine autoreactive B cell by immunization with human recombinant autoantigen La/SS-B: characterization of the autoepitope. J Autoimmun 1995; 8:825–42. [DOI] [PubMed] [Google Scholar]


