Abstract
Graphitic carbon nitride (g-C3N4)-supported V2O5 catalysts were prepared by the impregnation pyrolysis method, and their physicochemical properties were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared (FT-IR), Raman, X-ray photoelectron spectroscopy (XPS), UV–vis, TGA, N2 adsorption, and H2-TPR. These catalysts exhibit extremely high activity and selectivity in the aerobic oxidation of ethyl lactate to ethyl pyruvate. The excellent catalytic performance derives from the high surface-chemisorbed oxygen species. Low calcination temperature and interaction with g-C3N4 are conducive to increasing the surface-chemisorbed oxygen species of V2O5. The optimal catalyst 13V2O5/g-C3N4 gives 96.2% conversion of ethyl lactate with 85.6% selectivity toward ethyl pyruvate in 4 h at 130 °C and 1 atm oxygen, which is significantly superior to those of previously reported V-containing catalysts. This catalyst is also stable and reusable, and the ethyl pyruvate yield is reduced by less than 10% after four runs without any regeneration treatment.
1. Introduction
Significant changes in the social consumption of carbon sources can be expected in the near future due to the rapid depletion of fossil carbon sources.1 As a regenerable carbon source for commodity chemicals, biomass has attracted more and more intense attention.2,3 High-value chemicals can be prepared using the so-called “platform molecules” derived from biomass as feedstocks.4 Lactic acid (or lactate) is one such platform molecule, which is readily obtained from biomass fermentation.4 Direct oxidation of ethyl lactate to ethyl pyruvate is especially important, since it is a promising route to produce ethyl pyruvate, a key intermediate in the pharmaceuticals, agrochemicals, food additive sectors, and cosmetics.5−7 Ethyl pyruvate itself can be used as a highly effective active ingredient in flavors, fragrances, and air fresheners because of its special aroma. Ethyl pyruvate is also an important raw material for synthetic resins and plastics. Thus, the demand for the artificial synthesis of ethyl pyruvate is extremely high.
The aerobic oxidation of ethyl lactate to ethyl pyruvate has been widely studied in recent years owing to its friendly environment and high atom economy. However, challenges still remain in obtaining high yield of ethyl pyruvate due to overoxidation of lactate as well as decomposition of pyruvates (usually by decarbonylation or decarboxylation). It was reported that these side reactions can be repressed in liquid-phase systems,7 in which noble-metal catalysts are often required for oxygen activation under a mild reaction condition.8 Pb (or Te)-doped Pd–metal alloy on activated carbon and bimetallic Pb–Pt on carbon materials were once used for pyruvate production.9,10 High yields can be obtained using an excess of NaOH or LiOH. Despite that, developing simple, environmentally benign, inexpensive, and recyclable catalysts is still indispensable for the commercial replacement of fossil-based carbon sources by biobased ones.
Recently, activated carbon supported Cu, Fe, Co, and V oxides have been explored for the aerobic oxidation of ethyl lactate to ethyl pyruvate.11 Vanadium oxide is superior to other metal oxides. Supported vanadia was found to be more active when adding pyridine-type additives, through complexation of this additive to the substrate, followed by the oxidation on V active site.11 Based on that, vanadium–nitrogen-doped carbon nanosheets were prepared, which can convert ethyl lactate with oxygen into ethyl pyruvate under mild conditions, although auxiliary reagent needed to be added to suppress the occurrence of side reactions.12 The pyridinic N-oxide species in the catalyst was found to act synergistically with vanadium active sites in converting ethyl lactate with oxygen.12 Mesoporous vanadia–titania was also efficient and recyclable in the conversion of ethyl lactate to ethyl pyruvate.13 The V4+–O–Ti bonds were thought to play a key role in the reaction.
Graphitic carbon nitride (g-C3N4) is a kind of polymeric material comprising s-triazine or s-heptazine units. Its nitrogen-rich property provides abundant anchoring sites, which may facilitate the dispersion and stability of metal oxide species.14,15 Thus, we prepared vanadia supported on g-C3N4 as an efficient catalyst in the aerobic oxidation of ethyl lactate to ethyl pyruvate. Low calcination temperature of vanadia and unique interaction between vanadia and g-C3N4 can greatly enhance the activity of the V species, leading to excellent yield of ethyl pyruvate. Moreover, V2O5/g-C3N4 catalysts exhibited good stability in terms of ethyl lactate conversion and ethyl pyruvate yield. The reasons for the remarkable performance of the V2O5/g-C3N4 catalyst were also elucidated.
2. Results and Discussion
2.1. Structure of Catalysts
Figure 1 exhibits the X-ray diffraction (XRD) patterns of V2O5/g-C3N4 with different V2O5 contents. All of the samples exhibit the characteristic peaks at 2θ of 13.2 and 27.7°, attributed to the (100) and (002) faces of g-C3N4. The diffraction patterns corresponding to V2O5 are also found for the samples, whose intensity increases with the V2O5 content. However, the peak intensities are all very weak, manifesting the tiny grain sizes. The XRD patterns of V2O5 and V2O5/TiO2 calcined at 300 and 550 °C are shown in Figure S1 (Supporting Information). It is obvious that the catalysts obtained at 550 °C have more intense peaks as compared with those calcined at 300 °C because the aggregation and crystallization of vanadia species increase with the calcination temperature.
Figure 1.
XRD patterns of (a) g-C3N4, (b) 5V2O5/g-C3N4, (c) 10V2O5/g-C3N4, (d) 13V2O5/g-C3N4, (e) 15V2O5/g-C3N4, (f) 20V2O5/g-C3N4, and (g) 25V2O5/g-C3N4.
Figure 2 displays the FT-IR spectra of V2O5/g-C3N4 catalysts together with V2O5 (300 °C) and g-C3N4. All of the characteristic vibration modes of g-C3N4 are clearly seen in these spectra, indicating that the basic structure of g-C3N4 still remains after vanadia loading. The strong absorption band located at 1250–1700 cm–1 belongs to the typical skeletal stretching and bending vibrations of the s-triazine or tri-s-trizaine. The sharp peak at 808 cm–1 is ascribed to the breathing vibrations of the triazine.16 The stretching vibration modes of the N–H components or the O–H bands in the range of 3100–3300 cm–1 are accompanied by absorption of uncondensed amino groups and H2O molecules. A significant shift of this band can also be seen, which may be due to the interaction between g-C3N4 and vanadia species. It is worth mentioning that all of the supported catalysts contain a weak but noticeable peak at 1015 cm–1, which is attributed to the stretching modes of V=O bonds.17,18
Figure 2.
FT-IR spectra of (a) 5V2O5/g-C3N4, (b) 10V2O5/g-C3N4, (c) 13V2O5/g-C3N4, (d) 15V2O5/g-C3N4, (e) 20V2O5/g-C3N4, (f) 25V2O5/g-C3N4, (g) g-C3N4, and (h) V2O5 (300 °C).
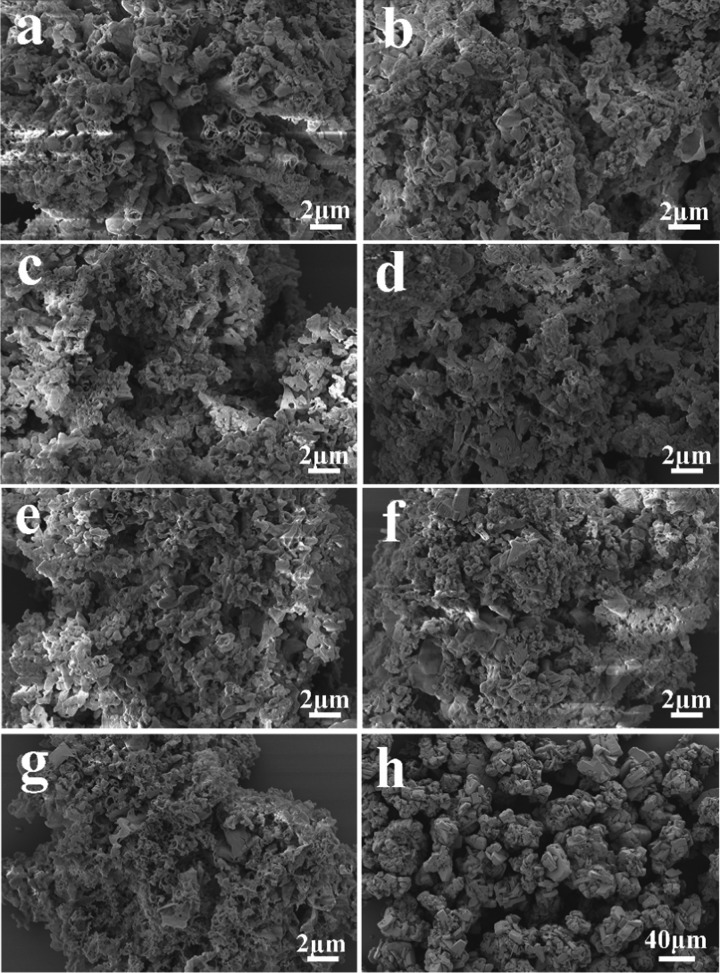
The scanning electron microscopy (SEM) images of the catalysts are illustrated in Figure 3. It can be noticed that g-C3N4 is mainly composed of small particles and flakes, and the pore structure is closely arranged. No clear differences in morphology were found after V2O5 loading, signifying that V2O5 did not destroy the g-C3N4 structure, which is in accordance with the results of XRD and FT-IR.
Figure 3.
SEM images of (a) g-C3N4, (b) 5V2O5/g-C3N4, (c) 10V2O5/g-C3N4, (d) 13V2O5/g-C3N4, (e) 15V2O5/g-C3N4, (f) 20V2O5/g-C3N4, (g) 25V2O5/g-C3N4, and (h) V2O5 (300 °C).
The N2 adsorption–desorption isotherms of g-C3N4 and V2O5/g-C3N4 are recorded and shown in Figure S2 (Supporting Information). All display similar type III isotherm with type H3 hysteresis loops, revealing that all materials have interparticle voids. The hysteresis loop of g-C3N4 starts at the relative pressure of ∼0.8 and shifts to lower values with the increasing of V2O5, suggesting that V2O5 loading widens the pore distribution of g-C3N4. The specific surface areas and pore volumes are shown in Table 1. The loading of V2O5 has little impact on the specific surface area but has a great influence on the pore volume, which can be ascribed to the formation of small particles of V2O5 in the pores of g-C3N4, thus making the pore size and pore volume smaller. However, the crystallinity of the V2O5 particles is poor, which does not block the pore channels of g-C3N4.
Table 1. Textural Properties of g-C3N4 and V2O5/g-C3N4.
| sample | SBET (m2/g) | Vtotal (cm3/g) | pore size (nm) | V2O5 content (%)a |
|---|---|---|---|---|
| 5V2O5/g-C3N4 | 47 | 0.20 | 16.8 | 4.5 |
| 10V2O5/g-C3N4 | 45 | 0.18 | 16.0 | 9.7 |
| 13V2O5/g-C3N4 | 41 | 0.17 | 18.1 | 15.3 |
| 15V2O5/g-C3N4 | 46 | 0.17 | 15.2 | 22.1 |
| 20V2O5/g-C3N4 | 43 | 0.19 | 18.0 | 23.5 |
| 25V2O5/g-C3N4 | 39 | 0.16 | 16.6 | 27.3 |
| g-C3N4 | 49 | 0.33 | 26.8 | |
| V2O5 (300 °C) | 15 | 0.10 |
Weight percentage of V2O5 obtained by TGA.
The thermographic (TG) analysis was conducted and revealed in Figure S3 (Supporting Information). g-C3N4 is quite stable at the temperature below 500 °C, with only a slight loss of weight caused by the desorption of H2O and/or CO2 at the temperature of 20–200 °C. It is completely decomposed at 670 °C. When V2O5 is loaded on g-C3N4, the thermal stability of g-C3N4 decreases obviously. The V2O5-supported catalysts begin to decompose at about 450 °C. Since no weight loss was detected for V2O5 even when the temperature reached 800 °C, the exact V2O5 content in the V2O5/g-C3N4 catalysts can be measured by the remaining weight after TG experiments. These results are also shown in Table 1, which are quite close to the contents added.
Raman spectroscopy is used to analyze the structural features of vanadium species, and the results are illustrated in Figures 4 and S4 (Supporting Information). It was reported that the bulk V2O5 can be identified according to the bands at 143, 195, 282, 405, 480, 527, 698, and 995 cm–1.19 These bands can be identified on V2O5 (300 °C) and V2O5 (550 °C) as well as V2O5/g-C3N4 and V2O5/TiO2, indicating the formation of tiny V2O5 crystals on both the supports. The intensities of these peaks are weaker for V2O5/TiO2, showing better dispersion of vanadia species on TiO2 than on g-C3N4. Peaks at 513 and 637 cm–1 are attributed to the anatase phase of TiO2.13,20,21 All of these are consistent with the XRD results.
Figure 4.
Raman spectra of (a) 13V2O5/TiO2 (300 °C) and (b) 13V2O5/g-C3N4.
2.2. Catalytic Performance
The activities of g-C3N4-supported V2O5 catalysts for the aerobic oxidation of ethyl lactate to ethyl pyruvate are evaluated and the results are summarized in Table 2. The control experiment revealed that the catalytic activity of g-C3N4 is low and the conversion of ethyl lactate is only 1.5%. When V2O5 was loaded, the catalytic activity improved significantly. The conversion of ethyl lactate increases dramatically with the V2O5 contents and then slowly decreases. This can be ascribed to the growth of V2O5 crystalline, resulting in a decrease of the surface V species. It is worth noting that 13V2O5/g-C3N4 shows the best activity, with the conversion of ethyl lactate reaching 96.2% within 4 h, which is significantly higher than the catalytic activity of the previously reported unsupported V2O5 catalyst.13 In all cases, the selectivity does not reach 100% due to the occurrence of side reactions such as oxidative dehydrogenation, hydrolysis, and decarboxylation, leading to the formation of byproducts including acetic acid, ethanol, and acetaldehyde. This is exactly the same as the earlier reported result.13,22
Table 2. Summary of the Activities for Aerobic Oxidation of Ethyl Lactate to Ethyl Pyruvate.
| catalyst | conversion (%)a | selectivity (%)a | yield (%)a | TOF (h–1)b |
|---|---|---|---|---|
| 5V2O5/g-C3N4 | 64.7 (27.0) | 87.3 (86.3) | 56.5 (23.3) | 33.9 |
| 10V2O5/g-C3N4 | 79.0 (42.6) | 87.4 (87.4) | 69.1 (37.2) | 27.1 |
| 13V2O5/g-C3N4 | 96.2 (67.4) | 85.6 (88.1) | 82.2 (59.4) | 33.2 |
| 15V2O5/g-C3N4 | 87.3 (60.3) | 86.5 (86.5) | 75.5 (52.2) | 25.3 |
| 20V2O5/g-C3N4 | 87.6 (61.4) | 87.6 (86.6) | 76.8 (53.2) | 19.4 |
| 25V2O5/g-C3N4 | 73.4 (50.4) | 87.9 (87.9) | 64.5 (44.3) | 12.9 |
| V2O5 (300 °C) | 94.0 (29.8) | 91.3 (91.7) | 85.8 (27.3) | 2.0 |
| V2O5 (550 °C) | 17.3 (7.1) | 82.1 (82.5) | 14.2 (5.9) | 0.4 |
| 13V2O5/TiO2 (300 °C) | 99.8 (49.8) | 60.2 (82.2) | 60.1 (40.9) | 22.9 |
| 13V2O5/TiO2 (550 °C) | 98.7 (20.9) | 67.4 (84.5) | 66.5 (17.7) | 9.9 |
| g-C3N4 | 1.5 | 78.2 | 1.2 | |
| 13V2O5/g-C3N4c | 96.0 (64.4) | 83.8 (84.5) | 80.5 (54.4) | 30.4 |
The values outside and inside the bracket are the data obtained at 4 and 2.5 h, respectively. Conditions: 10 mmol ethyl lactate, 2 mL diethyl succinate, 50 mg catalyst, 130 °C, and 1 atm O2.
Calculated as moles of ethyl pyruvate formed per moles of V per hour at 2.5 h.
Reaction conditions are magnified 5 times.
Since higher calcination temperature (550 °C) is usually used in the preparation of V2O5-based catalysts in the previous work, the effect of calcination temperature on the catalytic activity of V2O5 is also studied. As demonstrated in Table 2, the activity of V2O5 calcined at 300 °C is much higher than the one calcined at 550 °C, although it is still less active than 13V2O5/g-C3N4. The TiO2-supported vanadia catalyst calcined at 300 °C is also found to be more active as compared with the one calcined at 550 °C. The above results indicate that a low calcination temperature may account for the excellent activity of 13V2O5/g-C3N4, higher than that of the similar vanadium–nitrogen-doped carbon nanosheets prepared at 400 °C.12
It was reported that TiO2 was a good support for V2O5 because of its strong interaction with V species.20 Therefore, the catalytic activity of the TiO2-supported V2O5 catalyst in the oxidation of ethyl lactate was investigated. Similarly, 13V2O5/TiO2 had superior activity than that of the unsupported V2O5 calcined at the same temperature, although it contained less V2O5. It seemed that dispersing V2O5 on the support favored the activity since more V species can be exposed on the surface. However, 13V2O5/TiO2 was less active than 13V2O5/g-C3N4 with the same V2O5 content. Since V2O5 was better dispersed on TiO2 as compared with g-C3N4, the above difference in activity may have originated from the different effects of the supports on V species.
2.3. UV–vis Studies
The interaction between g-C3N4 and vanadium species can be identified by means of UV–vis diffuse reflectance spectroscopy. As seen from Figure 5, two intense peaks at around 260 nm and 368 nm can be observed on the spectrum of g-C3N4. The absorption edge shows a significant red shift after V2O5 is loaded. This phenomenon was found previously in studies of metal-doped g-C3N4, in which a red shift was due to the interaction of N species in g-C3N4 with metal or metal-based complexes.23−25 Absorption edge energy (Eg) is obtained by the Kubelka–Munk equation [hνF(R∞)]2 = A(hν – Eg).26 The results show that the Eg value gradually decreased from 2.85 to 2.68 eV as the vanadium content increased from 0 to 13% (Figure S5, Supporting Information). When the vanadium content exceeds 13%, the Eg value remains substantially unchanged. This band gap reduction is thought to enhance the electron transport capability, accelerating the formation of reduced V species. The reducing species is considered to be an important agent for forming superoxide radicals, which can increase the oxidation activity.27 The above result is consistent with the catalytic behaviors of V2O5/g-C3N4 in the aerobic oxidation of ethyl lactate.
Figure 5.
UV–vis absorption spectra of (a) g-C3N4, (b) 5V2O5/g-C3N4, (c) 10V2O5/g-C3N4, (d) 13V2O5/g-C3N4, (e) 15V2O5/g-C3N4, (f) 20V2O5/g-C3N4, and (g) 25V2O5/g-C3N4.
2.4. Temperature-Programmed Reduction (TPR) and X-ray Photoelectron Spectroscopy (XPS)
The H2-TPR experiment is performed to study the redox ability of V species. The results are shown in Figure 6. As we know, the lower reduction temperature means the stronger ability to provide active oxygen, i.e., a better redox ability.28 The reduction temperature of V2O5 calcined at 300 °C is lower than that calcined at 550 °C, which means that the former has a better redox ability. A similar result can be obtained for the 13V2O5/TiO2 catalyst. It can also be seen that the reduction temperatures of 13V2O5/TiO2 and 13V2O5/g-C3N4 are significantly lower than that of vanadia particles, suggesting that the support has a great influence on the reduction of the V species, which may be due to the good dispersion of vanadia. Since 13V2O5/g-C3N4 would decompose at the temperature higher than 500 °C, its TPR profiles are deconvoluted and illustrated in Figure 6B. The first peak is considered as the reduction of the highly dispersed V species and the corresponding peak temperature is significantly lower than that of 13V2O5/TiO2, which demonstrates that V species supported on g-C3N4 have better redox capacity than those supported on TiO2.
Figure 6.
(A) H2-TPR profiles of (a) V2O5 (550 °C), (b) 13V2O5/TiO2 (300 °C), (c) 13V2O5/TiO2 (550 °C), (d) 13V2O5/g-C3N4, and (e) V2O5 (300 °C). (B) Deconvolution curve of 13V2O5/g-C3N4.
The XPS spectra of various V2O5 catalysts are recorded. The deconvoluted spectra in the O (1s) and V (2p) region are illustrated in Figure S6 (Supporting Information). The spectrum of O1s can be fitted into two peaks. The high-intensity peak at 530.8 eV is ascribed to the lattice oxygen (Olatt), while the low-intensity one at 532.5 eV is assigned to the chemisorbed oxygen (Oads).29,30 A higher surface Oads/Olatt ratio can be obtained with catalysts calcined at low temperatures compared with those calcined at high temperatures (Table 3). In addition, the supported catalysts have a higher Oads/Olatt ratio than the unsupported counterparts. It is worth noting that 13V2O5/g-C3N4 has the highest surface Oads/Olatt ratio among these catalysts. Previous studies have shown that the surface-adsorbed oxygen (Oads), which is located at surface defects, is easier to reduce.31−33 Other literature has also shown that the surface-adsorbed oxygen is the most active species in aerobic oxidation, which promotes the exchange of gaseous oxygen with the surface-adsorbed ones.34−37 Since a high surface Oads/Olat ratio means the facile activation of oxygen molecules on the catalyst, the above results are quite consistent with the catalytic activities.
Table 3. XPS Results of V2O5, 13V2O5/g-C3N4, and 13V2O5/TiO2.
| V 2p3/2 peaks |
O 1s peaks |
|||||
|---|---|---|---|---|---|---|
| catalysts | V4+ (eV) | V5+ (eV) | V4+/V5+ | Oads (eV) | Olat (eV) | Oads/Olat |
| V2O5 (300 °C) | 516.8 | 517.9 | 0.85 | 532.1 | 530.8 | 0.73 |
| V2O5 (550 °C) | 516.4 | 517.5 | 0.38 | 532.6 | 530.3 | 0.49 |
| 13V2O5/g-C3N4 | 516.1 | 517.2 | 1.76 | 532.6 | 530.8 | 1.84 |
| 13V2O5/TiO2 (300 °C) | 517.8 | 518.9 | 1.11 | 532.5 | 531.0 | 1.13 |
| 13V2O5/TiO2 (550 °C) | 516.5 | 517.6 | 0.57 | 531.4 | 530.4 | 0.58 |
For the V2p spectra, two peaks at ∼524.5 and ∼517.0 eV were detected, corresponding to the V 2p1/2 and V 2p3/2 levels, respectively. The latter one can be deconvoluted into two peaks with binding energies of 517.5 and 516.8 eV, assigned to the V5+ and V4+ states in vanadium oxide, respectively.38,39 The V4+ species were reported to play a crucial role in the catalytic oxidation, as more V4+ species meant more defect sites with unsaturated chemical bonds, resulting in increased surface-chemisorbed oxygen species.34 As we can see from Table 3, the ratio of V4+/V5+ has the same regularity with Oads/Olat in all of the catalysts, which further proves the above conclusion.
2.5. Reusability of the Catalyst
The reusability of the 13V2O5/g-C3N4 catalyst in the aerobic oxidation of ethyl lactate to ethyl pyruvate was also investigated. After reacting at 2.5 h, the catalyst was filtered out and then dried for repeatability testing. The results are listed in Figure 7. The activity of the catalyst decreased very slowly in succession. The selectivity to ethyl pyruvate reduced a bit after the first run and remained unchanged after the second and third circles. All in all, the ethyl pyruvate yield reduced only by less than 10% after being reused four times. The recycling of 13V2O5/g-C3N4 after 4 h of oxidation of ethyl lactate was also conducted, and the results are illustrated in Figure S7 (Supporting Information). Similar results can be obtained, showing that 13V2O5/g-C3N4 was quite stable in liquid-phase reactions.
Figure 7.
Recycling of 13V2O5/g-C3N4 in the aerobic oxidation of ethyl lactate at 2.5 h.
Table 4 presents a summary of the catalytic activities of the significant systems published before for the aerobic oxidation of ethyl lactate to ethyl pyruvate. A high yield of ethyl pyruvate can be obtained over our present recyclable catalyst under mild conditions without the addition of any auxiliary reagent or precious metal, which exhibits a promising application in the industry. To exclude the possibility of active material leaching, a hot filtration experiment is also carried out, and the results are shown in Figure S8 (Supporting Information). The oxidation was conducted for the first 2.5 h catalyzed by 13V2O5/g-C3N4 and then go on for the next 2.5 h without 13V2O5/g-C3N4. The results show that there is no significant increase in the yield without a catalyst, indicating that the reaction is predominantly heterogeneous.
Table 4. Summary of the Activities for Aerobic Oxidation of Ethyl Lactate to Ethyl Pyruvate over Various Catalysts.
| catalyst | temperature (°C) | catalyst/lactate (%) | reaction time (h) | conversion (%) | selectivity (%) | TOFa (h–1) | ref |
|---|---|---|---|---|---|---|---|
| 13V2O5/g-C3N4 | 130 | 4.2 | 4 | 96.2 | 85.6 | 29 | present work |
| CoOx/C | 130 | 4.2 | 4 | 1 | 64 | 1.3 | (11) |
| CuOx/C | 130 | 4.2 | 4 | 2.2 | 20 | 0.8 | (11) |
| FeOx/C | 130 | 4.2 | 4 | 2.4 | 47 | 2.0 | (11) |
| Ru/C | 130 | 4.2 | 4 | 9 | 99 | 9.0 | (11) |
| Pt/C | 130 | 4.2 | 4 | 4 | 97 | 45 | (11) |
| VOx/C | 130 | 4.2 | 4 | 34 | 84 | 56 | (11) |
| V-NCNs | 130 | 4.2 | 9 | 99 | 86.6 | 62 | (12) |
| VCl3 | 130 | 5 | 4 | 63.9 | 12.3 | 0.5 | (13) |
| VOSO4 | 130 | 5 | 4 | 63.4 | 32.9 | 1.4 | (13) |
| VO(acac)2 | 130 | 5 | 4 | 79.9 | 64.1 | 5.7 | (13) |
| NH3VO3 | 130 | 5 | 4 | 64.3 | 72.6 | 2.3 | (13) |
| V2O5 | 130 | 5 | 4 | 13.9 | 61.8 | 0.3 | (13) |
| VCl3@VTN | 130 | 5 | 4 | 20.8 | 85.1 | 61 | (13) |
| VOSO4@VTN | 130 | 5 | 4 | 25.6 | 71.3 | 86 | (13) |
| VO(acac)2@VTN | 130 | 5 | 4 | 28.8 | 78.5 | 97 | (13) |
| NH4VO3@VTN | 130 | 5 | 4 | 34.6 | 89.4 | 118 | (13) |
| TiO2 | 130 | 1 | 6 | 50 | 75 | 4.3 | (40) |
| VOx/P4VP | 130 | 4.2 | 8 | 84 | 90 | 70 | (11) |
Calculated as moles of ethyl pyruvate formed per moles of the total metal per hour.
3. Conclusions
Ethyl lactate can be converted to ethyl pyruvate effectively by aerobic oxidation over V2O5/g-C3N4 catalysts without any auxiliary reagent. The superior catalytic performance comes from abundant defect sites with unsaturated chemical bonds on the vanadia surface, derived from the low calcination temperature of catalysts and the unique interaction between g-C3N4 and supported V2O5, resulting in an increase in the surface-chemisorbed oxygen species. These chemisorbed oxygen are more easily reduced, leading to enhanced oxidation activity. The V2O5/g-C3N4 catalyst is also very stable, which can be easily separated and reused multiple times.
4. Experimental Section
4.1. Reagent and Materials
Urea, anatase titanium (99.8%, 5–10 nm), ethyl lactate (≥99.0%), diethyl succinate (99%), ethyl pyruvate (99%), and NH4VO3 were sourced from Aladdin and used as received.
4.2. Catalyst Preparation
g-C3N4 was synthesized by the self-condensation of urea. In a typical synthesis, 10 g of urea in a covered ceramic crucible was heated under an airflow at 550 °C for 2 h at a heating rate of 2 °C/min. The resulting product was ground into powder, and 0.5 g of it was dispersed in 15 mL of deionized water, followed by the addition of a calculated amount of NH4VO3, stirring at 80 °C for 1 h, drying, and calcining in a muffle furnace for 3 h at 300 °C. The obtained product was designated as xV2O5/g-C3N4, where x represented the weight percentage of V2O5. V2O5 was synthesized by the thermal decomposition of NH4VO3 for comparison. V2O5/TiO2 is prepared by the same method as V2O5/g-C3N4, except that the g-C3N4 support is replaced with anatase-type titanium oxide.
4.3. Characterization of Catalysts
X-ray diffraction (XRD) was carried out on a Persee XD-2 X-ray diffractometer using a Cu Kα radiation (λ = 0.15418 nm) at 40 kV and 30 mA with a range of 10–80°. Fourier transform infrared spectra (FT-IR) were obtained by a Nicolet iS10 spectrometer. Scanning electron microscopy (SEM) measurements were conducted on a field-emission scanning electron microscope (FESEM, Ultra 55, Zeiss, Germany) with an acceleration voltage of 20.0 kV. X-ray photoelectron spectra (XPS) were obtained with a Mg Kα radiation (hν = 1253.6 eV) on a Perkin-Elmer PHI 5000C spectrometer. All binding energy values were referenced to the C1s peak at 284.6 eV. UV–vis diffuse reflectance spectroscopy was performed on a Shimadzu UV-2450 UV spectrometer with BaSO4 as a reference calibration. The Brunauer–Emmett–Teller (BET) surface area and pore volume of the catalysts were calculated from N2 adsorption isotherms at liquid N2 temperature using a Micromeritics ASAP 2000 instrument. Thermogravimetric (TG) analysis was conducted on a thermal analyzer SETSYS-1750 (SETARAM Instrumentation, France) under the inflow of air with a ramp rate of 10 °C/min from 20 to 900 °C. Raman spectroscopy was measured on a Horiba JY XploRA Raman spectrometer using a 532 nm line as an excitation source with a measurement range of 100–1200 nm. The H2-TPR was performed in a Micromeritics AutoChem II apparatus under 10% H2/Ar flow (40 mL/min). The experiments were conducted on 150 mg of catalyst from room temperature to 800 °C at a ramping rate of 10 °C/min.
4.4. Catalytic Testing
The aerobic oxidation of ethyl lactate to ethyl pyruvate was carried out in a round-bottom flask with a reflux condenser under magnetic stirring. Typically, 50 mg of catalyst was added into the mixture of 10 mmol ethyl lactate and 2 mL of diethyl succinate. The reaction was conducted at 130 °C with an O2 stream of 20 mL/min. The products were analyzed by a GC9560 gas chromatograph equipped with a SE-30 capillary column (30 m × 0.25 mm × 0.3 μm) using biphenyl as an external standard.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (91645201 and 21273043), the National Key R&D Program of China (2017YFB0602204), and the Science & Technology Commission of Shanghai Municipality (19DZ2270100).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01822.
XRD patterns of the catalysts; N2 adsorption–desorption isotherms of the catalysts; TG thermograms of the catalysts; Raman spectra of the catalysts; Eg value corresponding to different catalysts in UV diffuse reflectance spectroscopy; XPS spectra of V2p and O1s in the catalysts; recycling experiment of 13V2O5/g-C3N4; and leaching experiment of 13V2O5/g-C3N4 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- He M.; Sun Y.; Han B. Green carbon science: Scientific basis for integrating carbon resource processing, utilization, and recycling. Angew. Chem., Int. Ed. 2013, 52, 9620–9633. 10.1002/anie.201209384. [DOI] [PubMed] [Google Scholar]
- Corma A.; Iborra S.; Velty A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. 10.1021/cr050989d. [DOI] [PubMed] [Google Scholar]
- Vennestrøm P. N. R.; Osmundsen C. M.; Christensen C. H.; Taarning E. Beyond petrochemicals: The renewable chemicals industry. Angew. Chem., Int. Ed. 2011, 50, 10502–10509. 10.1002/anie.201102117. [DOI] [PubMed] [Google Scholar]
- Beerthuis R.; Rothenberg G.; Shiju N. R. Catalytic routes towards acrylic acid, adipic acid and ε-caprolactam starting from biorenewables. Green Chem. 2015, 17, 1341–1361. 10.1039/C4GC02076F. [DOI] [Google Scholar]
- Mäki-Arvela P.; Simakova I. L.; Salmi T.; Murzin D. Y. Production of lactic acid/lactates from biomass and their catalytic transformations to commodities. Chem. Rev. 2014, 114, 1909–1971. 10.1021/cr400203v. [DOI] [PubMed] [Google Scholar]
- Xu P.; Qiu J.; Gao C.; Ma C. Biotechnological routes to pyruvate production. J. Biosci. Bioeng. 2008, 105, 169–175. 10.1263/jbb.105.169. [DOI] [PubMed] [Google Scholar]
- Sugiyama S.; Kikumoto T.; Tanaka H.; Nakagawa K.; Sotowa K.-I.; Maehara K.; Himeno Y.; Ninomiya W. Enhancement of catalytic activity on Pd/C and Te–Pd/C during the oxidative dehydrogenation of sodium lactate to pyruvate in an aqueous phase under pressurized oxygen. Catal. Lett. 2009, 131, 129–134. 10.1007/s10562-009-9920-3. [DOI] [Google Scholar]
- Mallat T.; Baiker A. Oxidation of alcohols with molecular oxygen on solid catalysts. Chem. Rev. 2004, 104, 3037–3058. 10.1021/cr0200116. [DOI] [PubMed] [Google Scholar]
- Tsujino T.; Ohigashi S.; Sugiyama S.; Kawashiro K.; Hayashi H. Oxidation of propylene glycol and lactic acid to pyruvic acid in aqueous phase catalyzed by lead-modified palladium-on-carbon and related systems. J. Mol. Catal. 1992, 71, 25–35. 10.1016/0304-5102(92)80005-2. [DOI] [Google Scholar]
- Hayashi H.; Sugiyama S.; Shigemoto N.; Miyaura K.; Tsujino S.; Kawashiro K.; Uemura S. Formation of an intermetallic compound Pd3Te with deactivation of Te/Pd/C catalysts for selective oxidation of sodium lactate to pyruvate in aqueous phase. Catal. Lett. 1993, 19, 369–373. 10.1007/BF00767080. [DOI] [Google Scholar]
- Zhang W.; Ensing B.; Rothenberg G.; Shiju N. R. Designing effective solid catalysts for biomass conversion: aerobic oxidation of ethyl lactate to ethyl pyruvate. Green Chem. 2018, 20, 1866–1873. 10.1039/C8GC00032H. [DOI] [Google Scholar]
- Zhang W.; Oulego P.; Slot T. K.; Rothenberg G.; Shiju N. R. Selective aerobic oxidation of lactate to pyruvate catalyzed by vanadium-nitrogen-doped carbon nanosheets. ChemCatChem 2019, 11, 3381–3387. 10.1002/cctc.201900819. [DOI] [Google Scholar]
- Zhang W.; Innocenti G.; Oulego P.; Gitis V.; Wu H.; Ensing B.; Cavani F.; Rothenberg G.; Shiju N. R. Highly selective oxidation of ethyl lactate to ethyl pyruvate catalyzed by mesoporous vanadia–titania. ACS Catal. 2018, 8, 2365–2374. 10.1021/acscatal.7b03843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z.; Yan F.; Yuan G. Vapor-Phase Selective Oxidation of Toluene Catalyzed by Graphitic Carbon Nitride Supported Vanadium Oxide. Catal. Lett. 2017, 147, 509–516. 10.1007/s10562-016-1924-1. [DOI] [Google Scholar]
- Wang C.; Hu L.; Wang M.; Yue B.; He H. Cerium promoted V-g-C3N4 as highly efficient heterogeneous catalysts for the direct benzene hydroxylation. R. Soc. Open Sci. 2018, 5, 180371 10.1098/rsos.180371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A.; Fischer A.; Goettmann F.; Antonietti M.; Muller J. O.; Schlogl R.; Carlsson J. M. Graphitic carbon nitride materials: variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. 10.1039/b800274f. [DOI] [Google Scholar]
- Zou H.; Xiao G.; Chen K.; Peng X. Noble metal-free V2O5/g-C3N4 composites for selective oxidation of olefins using hydrogen peroxide as an oxidant. Dalton Trans. 2018, 47, 13565–13572. 10.1039/C8DT02765J. [DOI] [PubMed] [Google Scholar]
- Xu J.; Jiang Q.; Chen T.; Wu F.; Li Y. X. Vanadia supported on mesoporous carbon nitride as a highly efficient catalyst for hydroxylation of benzene to phenol. Catal. Sci. Technol. 2015, 5, 1504–1513. 10.1039/C4CY01373E. [DOI] [Google Scholar]
- Khodakov A.; Olthof B.; Bell A. T.; Iglesia E. Structure and catalytic properties of supported vanadium oxides: support effects on oxidative dehydrogenation reactions. J. Catal. 1999, 181, 205–216. 10.1006/jcat.1998.2295. [DOI] [Google Scholar]
- Sivaranjani K.; Verma A.; Gopinath C. S. Molecular oxygen-assisted oxidative dehydrogenation of ethylbenzene to styrene with nanocrystalline Ti1–xVxO2. Green Chem. 2012, 14, 461–471. 10.1039/C1GC15907K. [DOI] [Google Scholar]
- Su J.; Zou X.; Li G. D.; Jiang Y. M.; Cao Y.; Zhao J.; Chen J. S. Room-temperature spontaneous crystallization of porous amorphous titania into a high-surface-area anatase photocatalyst. Chem. Commun. 2013, 49, 8217–8219. 10.1039/c3cc43772h. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Oulego P.; Sharma S. K.; Yang X. L.; Li L. J.; Rothenberg G.; Shiju N. R. Self-exfoliated synthesis of transition metal phosphate nanolayers for selective aerobic oxidation of ethyl lactate to ethyl pyruvate. ACS Catal. 2020, 10, 3958–3967. 10.1021/acscatal.9b04452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Chen X.; Thomas A.; Fu X.; Antonietti M. Metal-containing carbon nitride compounds: a new functional organic–metal hybrid material. Adv. Mater. 2009, 21, 1609–1612. 10.1002/adma.200802627. [DOI] [Google Scholar]
- Chen D.; Wang K.; Ren T.; Ding H.; Zhu Y. Synthesis and characterization of the ZnO/mpg-C3N4 heterojunction photocatalyst with enhanced visible light photoactivity. Dalton Trans. 2014, 43, 13105–13114. 10.1039/C4DT01347F. [DOI] [PubMed] [Google Scholar]
- Bautista F. M.; Campelo J. M.; Luna D.; Luque J.; Marinas J. M. Vanadium oxides supported on TiO2-sepiolite and sepiolite: preparation, structural and acid characterization and catalytic behaviour in selective oxidation of toluene. Appl. Catal., A 2007, 325, 336–344. 10.1016/j.apcata.2007.02.033. [DOI] [Google Scholar]
- Gao X.; Wachs I. E. Investigation of surface structures of supported vanadium oxide catalysts by UV–vis-NIR Diffuse Reflectance Spectroscopy. J. Phys. Chem. B 2000, 104, 1261–1268. 10.1021/jp992867t. [DOI] [Google Scholar]
- Cheng K.; Liu J.; Zhang T.; Li J. M.; Zhao Z.; Wei Y. C.; Jiang G. Y.; Duan A. J. Effect of Ce doping of TiO2 support on NH3-SCR activity over V2O5–WO3/CeO2–TiO2 catalyst. J. Environ. Sci. 2014, 26, 2106–2113. 10.1016/j.jes.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Chena Q.-L.; Guo R.-T.; Wang Q.-S.; Pan W.-G.; Yang N.-Z.; Lu C.-Z.; Wang S.-X. The promotion effect of Co doping on the K resistance of Mn/TiO2 catalyst for NH3-SCR of NO. J. Taiwan Inst. Chem. Eng. 2016, 64, 116–123. 10.1016/j.jtice.2016.03.045. [DOI] [Google Scholar]
- Xia Y. S.; Dai H. X.; Jiang H. Y.; Zhang L. Three-dimensional ordered mesoporous cobalt oxides: highly active catalysts for the oxidation of toluene and methanol. Catal. Commun. 2010, 11, 1171–1175. 10.1016/j.catcom.2010.07.005. [DOI] [Google Scholar]
- Hu Z.; Zhou G.; Xu Li.; Yang J.; Zhang B.; Xu X. Preparation of ternary Pd/CeO2-nitrogen doped grapheme composites as recyclable catalysts for solvent-free aerobic oxidation of benzyl alcohol. Appl. Surf. Sci. 2019, 471, 852–861. 10.1016/j.apsusc.2018.12.067. [DOI] [Google Scholar]
- Lee K. J.; Kumar P. A.; Maqbool M. S.; Rao K. N.; Song K. H.; Ha H. P. Ceria added Sb-V2O5/TiO2 catalysts for low temperature NH3 SCR: Physico-chemical properties and catalytic activity. Appl. Catal., B 2013, 142-143, 705–717. 10.1016/j.apcatb.2013.05.071. [DOI] [Google Scholar]
- Kang M.; Park E. D.; Kim J. M.; Yie J. E. Manganese oxide catalysts for NOx reduction with NH3 at low temperatures. Appl. Catal., A 2007, 327, 261–269. 10.1016/j.apcata.2007.05.024. [DOI] [Google Scholar]
- Slot T. K.; Eisenberg D.; van Noordenne D.; Jungbacker P.; Rothenberg G. Cooperative catalysis for selective alcohol oxidation with molecular oxygen. Chemistry 2016, 22, 12307–12311. 10.1002/chem.201602964. [DOI] [PubMed] [Google Scholar]
- Jeong Y. E.; Kumar P. A.; Ha H. P.; Lee K. Effect of hydrothermal aging on NOx reduction performance for Sb–V–CeO2/TiO2 catalyst. Res. Chem. Intermed. 2018, 44, 6803–6829. 10.1007/s11164-018-3523-9. [DOI] [Google Scholar]
- Luo D. M.; Liu S. S.; Liu J. J.; Zhao J. X.; Miao C.; Ren J. Catalytic combustion of toluene over cobalt oxides supported on graphitic carbon nitride (CoOx/g-C3N4) catalyst. Ind. Eng. Chem. Res. 2018, 57, 11920–11928. 10.1021/acs.iecr.8b02625. [DOI] [Google Scholar]
- Xu L.; Wang Z.; Wang J.; Xiao Z.; Huang X.; Liu Z.; Wang S. N-doped nanoporous Co3O4 nanosheets with oxygen vacancies as oxygen evolving electrocatalysts. Nanotechnology 2017, 28, 165402 10.1088/1361-6528/aa6381. [DOI] [PubMed] [Google Scholar]
- Christoskova S. G.; Stojanova M.; Georgieva M.; Mehandzhiey D. Study on the thermal stability of a high Co-oxide used as low-temperature catalyst and oxidant for complete oxidation. Thermochim. Acta 1997, 292, 77–83. 10.1016/S0040-6031(96)03144-9. [DOI] [Google Scholar]
- Grigorieva A. V.; Badalyan S. M.; Goodilin E. A.; Rumyantseva M. N.; Gaskov A. M.; Birkner A.; Tretyakov Y. D. Synthesis, structure, and sensor properties of vanadium pentoxide nanorods. Eur. J. Inorg. Chem. 2010, 2010, 5247–5253. 10.1002/ejic.201000372. [DOI] [Google Scholar]
- Sun Y.; Yang S. B.; Lv L. P.; Lieberwirth I.; Zhang L. C.; Ding C. X.; Chen C. H. A composite film of reduced graphene oxide modified vanadium oxide nanoribbons as a free standing cathode material for rechargeable lithium batteries. J. Power Source 2013, 241, 168–172. 10.1016/j.jpowsour.2013.04.093. [DOI] [Google Scholar]
- Ramos-Fernandez E. V.; Geels N. J.; Shiju N. R.; Rothenberg G. Titania-catalysed oxidative dehydrogenation of ethyl lactate: effective yet selective free-radical oxidation. Green Chem. 2014, 16, 3358–3363. 10.1039/C4GC00191E. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.