Abstract
Pathogenesis of Alzheimer’s disease (AD), the most common type of dementia, involves misfolding and aggregation of the extracellular amyloid-β (Aβ) protein where the intermediate oligomers, formed during the aggregation progression cascade, are considered the prime toxic species. Here, we identify an active peptide fragment from a medicinal plant-derived (Aristolochia indica) fibrinolytic enzyme having anti-amyloidogenic effects against Aβ fibrillation and toxicity. Liquid chromatography with tandem mass spectrometry (LC-MS/MS), followed by computational analysis of the peptide pool generated by proteolytic digestion of the enzyme, identifies two peptide sequences with predictive high-propensity binding to Aβ42. Microscopic visualizations in conjunction with biochemical and biophysical assessments suggest that the synthetic version of one of the peptides (termed here Pactive, GFLLHQK) arrests Aβ molecules in off-pathway oligomers that can no longer participate in the cytotoxic fibrillation pathway. In contrast, the other peptide (termed P1) aggravates the fibrillation process. Further investigations confirm the strong binding affinity of Pactive with both Aβ42 monomers and toxic oligomers by biolayer interferometric assays. We have also shown that, mechanistically, Pactive binding induces conformational alterations in the Aβ molecule along with modification of Aβ hydrophobicity, one of the key players in aggregation. Importantly, the biostability of Pactive in human blood serum and its nontoxic nature make it a promising therapeutic candidate against Alzheimer’s, for which no disease-modifying treatments are available to date.
Introduction
Aggregation of amyloid-β (Aβ) resulting in accumulation of plaques and consequent neurodegeneration in the cerebral cortex of brain are the neuropathological hallmarks of Alzheimer’s disease (AD).1 Following primary nucleation, Aβ aggregation proceeds via formation of intermediate oligomers and protofibrils, which then intertwine into mature fibrils and eventually form plaques.2 Elucidation of the aggregation pathway highlighting intermediate oligomers as the major cytotoxic agents and drivers of neurodegeneration3 has shifted the research focus toward the development of agents that will inhibit the neurotoxic effects of these species. Hence, treatment of AD requires a multifunctional molecule that will not only serve as an anti-aggregation and fibril destabilization agent but also attenuate oligomer toxicity.
Aristolochia indica, a medicinal plant of the Indian subcontinent is used for the treatment of various diseases, with reports indicating its potential against certain amyloids.4 Here, we isolated a new enzyme from the roots of the plant having potential Aβ aggregation inhibition as well as disaggregation properties. Interestingly, the efficacy of the inactivated enzyme eliminated any possible role of its proteolytic activity behind the anti-amyloid property. Instead, its ability to modify Aβ hydrophobicity implicated the involvement of specific interacting portions of the enzyme. Moreover, the enzyme size (13.6 kDa) poses limitation to its use as an inhibitor molecule. Compared to larger sized proteins, smaller peptides are currently being considered possible drug molecules against AD due to their relatively easier delivery along with other advantages of increased stability and lower chance of interaction with the immune system.5 Thus, a rational approach would be to bring down the study to the peptide level.
Repeated liquid chromatography with tandem mass spectrometry (LC-MS/MS) analysis followed by computational screening of the fragments generated by proteolytic digestion of the enzyme identified two effective sequences with predicted high-propensity binding to Aβ42. Synthetic version of one of the peptides (Pactive) displayed remarkable anti-amyloidogenicity against Aβ, sequestering toxic oligomers and fibrils in nontoxic off-pathway forms, while the other peptide (P1) augmented the aggregation process.
Further investigation to elucidate the mechanism revealed Pactive-induced modifications of hydrophobicity along with conformational alterations of Aβ. We have also shown that Pactive is not only nontoxic but also reasonably stable in human blood serum (HBS), suggesting that the peptide can be a potential candidate for designing therapeutics against AD.
Results
Anti-Amyloid Property of the Purified Enzyme against Aβ40
Purification of the fibrinolytic enzyme from the roots of A. indica involved chromatographic separation, followed by size-exclusion high-pressure liquid chromatography (SE-HPLC), which was confirmed with fibrinogen zymography (Figure S1). The enzyme-mediated aggregation inhibition of Aβ40 was assessed. Atomic force microscopy (AFM) imaging revealed that, while the untreated Aβ40 solution formed an extensive network of long branched fibrils within 7 days (Figure 1A), co-incubation of Aβ40 with the enzyme under similar aggregation conditions resulted in nonfibrillating multimeric species accompanying a few short unbranched fibrils (Figure 1B). Importantly, formation of the fibrillar network completely ceased, even after incubation for an extended time (up to 30 days; Figure S2). Transmission electron microscopy (TEM) imaging of the untreated and treated samples further supplemented the AFM observations (Figure S3).
Figure 1.
Modulation of Aβ40 aggregation. (A–D) AFM visualizations of Aβ40 aggregation inhibition by active/inactive enzymes and EDPs. While untreated Aβ (A) reveals extensive fibrillation, treatments with inhibitors (B–D) show fibrillation inhibition and formation of oligomers. (E–H) Fibril destabilization efficiency of the inhibitors. (E) Untreated preformed fibril revealing enhanced aggregation. (F–H) Treated preformed fibrils reveal degradation of fibrillar networks to oligomers. All experiments are performed under aggregation conditions for 7 days.
Next, the fibril destabilization potential of the enzyme was monitored by treating preformed aggregates, which resulted in loss of pre-existing fibrils and conversion to multimeric species within 7 days (Figure 1E,F). Untreated Aβ, under aggregation conditions, eventually gave rise to plaques (after 30 days), whereas treatment (for 30 days) in the presence of the enzyme revealed similar multimeric species with a few broken fibrils, suggesting that the Aβ structures formed upon enzyme treatment are stable oligomeric forms (Figure S2).
Relevance of the Proteolytic Activity of the Enzyme in Its Anti-Amyloid Property
To evaluate the relevance of the enzymatic activity of the fibrinolytic protein, inhibition and destabilization experiments were performed with the inactivated (thermal denaturation) enzyme. Inactivity was confirmed with zymography, an electrophoretic method for measuring proteolysis.6
Proteolytic activity of the inactivated enzyme was compared with that of the active enzyme in a sodium dodecyl sulfate (SDS) gel impregnated with a substrate, fibrinogen. While the active enzyme, by virtue of its proteolytic activity, showed clear bands (due to proteolysis of fibrinogen) against a blue background (post-Coomassie staining), when SDS was replaced with Titron X-100 and the gel was subjected to reaction buffer, that band was missing for the inactive one, confirming the loss of proteolytic activity after denaturation (Figure S1).
Interestingly, after inactivation, the enzyme retained comparable fibrillation inhibition as well as fibril degradation properties (Figures 1C,G and S2). Clearly, the results indicated that the aggregation modification potency of the enzyme is not a property associated with its proteolytic activity but involves the interaction of Aβ with a certain segment of the enzyme molecule, which is equally accessible in both the active and inactive forms.
Further, 8-anilinonaphthalene-1-sulfonic acid (ANS) binding studies with Aβ in the presence of the enzyme suggested enzyme-induced hydrophobic modifications of the Aβ molecule. Upon successive increase in concentration of Aβ alone, ANS fluorescence was enhanced, indicating accessibility of its hydrophobic cores. However, addition of increasing concentrations of the enzyme to Aβ reduced ANS fluorescence intensities along with a blue shift in maxima (Figure S4). This result further substantiated the idea of involvement of specific interactions, possibly hydrophobic.
The above observations along with the size barrier of the enzyme prompted us to identify the specific interacting stretches within the enzyme responsible for the anti-amyloid property. To this end, the inactivated enzyme was subjected to tryptic digestion followed by separation by gel filtration of generated peptides (enzyme-derived peptides, abbreviated as EDPs) within the range of 1.5 kDa (as confirmed by electronspray ionization (ESI), Figure S1). Interestingly, the EDPs exhibited equal efficiency in fibrillation inhibition and disaggregation (Figures 1D,H and S2), which further supported the previous postulation that the interaction of Aβ with specific portions of the enzyme is likely crucial for its anti-amyloidogenic property.
Modification of β-Content and Amyloid Nature
Thioflavin T (ThT) binding assay was performed to compare the aggregation states of the treated samples with respect to the untreated aggregates. Confocal imaging of ThT-stained samples identified the presence of β-sheet rich structures in the control while treatment revealed structures with much lowered ThT binding efficiencies, as indicated by the reduced fluorescence intensities in treated samples (Figure 2A–H).
Figure 2.

ThT binding of treated and untreated Aβ40. Confocal images of ThT-stained (A) untreated and (B–D) treated Aβ40 monomers after 7 days of incubation under aggregation-inducing conditions revealing fibrils with strong ThT fluorescence in the untreated control while reduced ThT binding in the treated samples. (E) Staining of preformed aggregates reveals ThT-bound fibrils (control). (F–H) Treatments of preformed aggregates indicate broken fibrils with reduced intensity of ThT stain post-treatment (7 days). (I) Aβ40 fibrillation inhibition by the candidates was monitored by ThT fluorescence. (J) Destabilization of preformed aggregates in the presence of inhibitors. The presence of fibrils was measured by comparing the ThT fluorescence at any given time (up to 30 days). Error bars represent the mean ± standard deviation (SD) of three independent experiments.
Association of ThT with β-sheet rich structures results in enhanced fluorescence,7 which was observed for the untreated aggregate within 24 h. With increased incubation (up to 30 days), there was a gradual increase in the fluorescence intensity, indicating strong fibrillation. Conversely, kinetics of ThT binding for Aβ when followed in the presence of either the active/inactivated enzyme or the EDPs confirmed that the rate of aggregation was dramatically slower than that in their absence (Figure 2I). Similarly, treatment of preformed aggregates showed degradation of fibrils to structures with lower ThT binding capacity (Figure 2J).
Subsequently, Congo red (CR)7staining was performed with both untreated and treated (with active/inactivated enzyme and EDPs) Aβ samples to identify their amyloid-like characteristics. Confocal imaging of untreated Aβ40 samples revealed CR-positive structures, confirming their amyloid character, whereas treated samples showed much reduced intensity of CR staining compared to untreated controls, indicating lower amyloidogenicity of the treated samples (Figure S5).
Inhibitors Reduce Oligomer-Induced Cytotoxicity
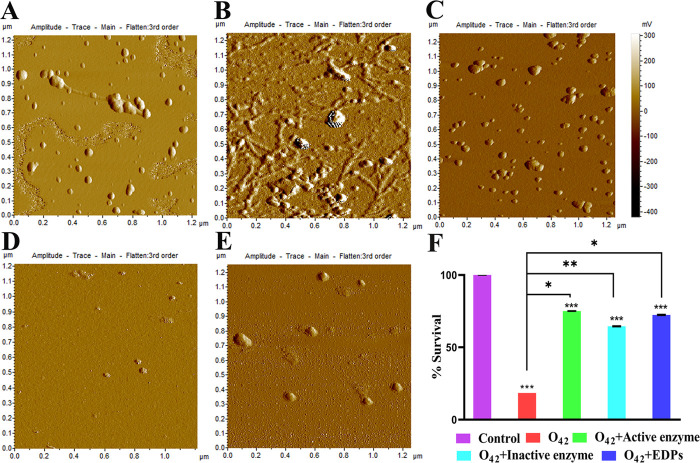
The role of oligomers in neurodegeneration3 prompted us to evaluate the potency of the active/inactive enzyme and EDPs to prevent fibrillation of preformed Aβ40 and Aβ42 oligomers. While untreated Aβ40 oligomers revealed fibrillation within 6 h under aggregation conditions, no significant fibrillation was observed in treated samples even after 24 h (Figure S6). Similarly, untreated Aβ42 oligomers also formed extensive fibrillar networks within 48 h, while treated samples revealed mainly multimeric species by AFM imaging (Figure 3A–E).
Figure 3.
Modification of Aβ42 oligomer-induced toxicity. (A) Control oligomers. (B) Untreated oligomers showing fibrillation. (C–E) Treated oligomers – active enzyme, inactive enzyme, and EDPs, respectively—incubated under aggregation conditions for 24 h. (F) Cell viability and toxicity assay in the absence or presence of the inhibitors. Inhibitor treatment could successfully ameliorate oligomer toxicity and increase cell viability. Error bars represent the mean ± standard deviation (SD). Symbols indicate statistically significant differences compared with control and Aβ42 treatment (*p < 0.05, **p < 0.01, ***p < 0.001).
Additionally, the ability of the candidates (active/inactive enzyme and EDPs) to ameliorate Aβ42 oligomer-induced cytotoxicity was evaluated on SHSY-5Y human neuroblastoma cells with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay. Cell treatment with Aβ42 oligomers alone resulted in ∼76% reduction in survivability (only ∼25% viable cells), whereas treatment with the active/inactive enzyme or EDPs improved cell health, as suggested by increased survivability (Figure 3F).
LC-MS/MS-Guided Identification of the Active Peptide Fragment
The EDPs were subjected to repeated LC-MS/MS followed by analysis of fragments generated by trypsin and chymotrypsin digestion with available database (Mascot Matrix Science Server8), leading to the identification of eight common peptides from which those with <1 kDa molecular weight (four fragments) were considered for further evaluation. The binding propensity of these four fragments to the Aβ42 monomer (PDB ID: 1IYT,9 model 1) was evaluated using the online web server PepSite 2.2.20.10 This prediction helps to identify peptide binding regions on proteins, when provided with the peptide sequence as the “query” and the protein structure as the “target.” Of the four sequences, the peptides (P1 and Pactive) that showed binding to Aβ42 with highly significant p-values (provided by the web server) were considered for further evaluation (Table S1).
The identified sequences were chemically synthesized based on solid-phase peptide synthesis and purified with reverse-phase high-pressure liquid chromatography (RP-HPLC). Purity of the peptides was confirmed with mass spectrometry and the absence of trace amounts of trifluoroacetic acid (TFA) used to cleave from the solid resin support was verified with 19F NMR spectroscopy (Figure S7).
Evaluation of the Anti-Amyloidogenic Property of the Identified Peptides
Aggregation inhibition and disaggregation experiments were performed with the two synthetic peptides (P1 and Pactive) to assess their ability to interfere with Aβ40 aggregation. AFM visualization revealed potent aggregation inhibition by Pactive along with its ability to remodel pre-existing fibrils into small oligomers, which was further supplemented by ThT binding assay that revealed reduced fluorescence in the presence of Pactive, indicating reduction in β load (Figure 4A–C). In contrast, P1 aggravated the aggregation tendency, with enhanced fibrillation, as visualized by AFM and even higher ThT fluorescence in P1-treated samples compared to the control (Figure S8).
Figure 4.
Effect of Pactiveon Aβ40 aggregation. (A) Inhibition of Aβ40 aggregation and (B) destabilization of preformed aggregates by Pactive. (C) ThT binding assay comparing the aggregation states of Aβ40 in the absence and presence of Pactive. (D) Circular dichroism (CD) spectra indicating changes in the secondary structure before and after treatment. All experiments are performed under aggregation conditions for 7 days. (E) Secondary structure prediction is shown using the “CD Analysis and Plotting Tool” web server (CAPITO). (F) ANS binding assay with Aβ40 in the presence of different concentrations of Pactive.
Further studies were done with only Pactive to evaluate its activity against Aβ42, the more aggregation prone species, which again revealed its equally efficient Aβ42 fibrillation inhibition property along with successful degradation of existing fibril networks (Figure 5A).
Figure 5.
Pactive interaction and modification of Aβ42 aggregation. (A) Aβ42 aggregation inhibition and fibril destabilization by Pactive (48 h). From left to right: untreated control showing fibrillation; fibrillation inhibition by Pactive; and degradation of preformed fibrils by Pactive. (B, C) Biolayer interferometry (BLI) interaction assay of Pactive with (B) Aβ42 monomers and (C) Aβ42 oligomers.
Pactive-Mediated Alterations in the Aβ Secondary Structure
Pactive-mediated changes in the secondary structure of Aβ40 were analyzed with CD spectroscopy. Aβ aggregation was preceded by the appearance of β-sheet rich structures, and CAPITO analysis11 of the raw CD spectra clearly indicated a sharp increase in β content in the aggregated sample compared to the initial secondary structure (t = 0) of Aβ40. However, Pactive treatment resulted in species with a completely different secondary structure with significantly reduced β content (Figure 4D,E). Alterations in the secondary structure of Aβ aggregates, monitored in the presence of the enzyme (active/inactive) or EDPs, also indicated a similar decrease in β content (Figure S9).
Modification of Aβ Surface Hydrophobicity due to Pactive Binding
Hydrophobicity along with aromatic packing is known to drive the process of aggregation of Aβ.12 Hydrophobic modification in the Aβ molecule due to Pactive interactions was followed with ANS binding assay. The interaction of extrinsic probe ANS with solvent-exposed hydrophobic clusters of proteins resulted in enhanced fluorescence intensities.13 Addition of gradually increasing concentrations of Pactive to Aβ revealed that ANS failed to bind the hydrophobic patches as reflected by a gradual reduction in fluorescence with increasing Pactive concentrations (Figure 4F). Reduced exposure of hydrophobic clusters in Aβ following Pactive interactions indicated either masking of hydrophobic cores by the peptide binding or conformational alterations in Aβ that made the hydrophobic patches less accessible.
Pactive–Aβ42 Interactions and Conformational Alterations
Bioaffinities of Pactive with Aβ42 monomers and oligomers were followed in real time with biolayer interferometry (BLI) with immobilized Pactive. Aβ42 (monomers and oligomers) binding constants to Pactive were determined over a range of concentrations at 37 °C, which indicated that Pactive could bind to both monomers and oligomers with reasonably strong affinity (monomers: Kd ∼ 1.11 × 10–7 M; oligomers: Kd ∼ 1.88 × 10–5 M).
Increasing concentration of the monomer showed an exponential increase in binding to Pactive, with its binding toward saturation, but the binding equilibrium showed a tendency to dominate toward association rather than complete dissociation, indicating stable complex formation (Figure 5B). On the other hand, oligomer and Pactive binding kinetics revealed a dose-dependent induction of a negative BLI signal. This may occur due to the binding-induced conformational changes in Aβ oligomers and due to the binding of larger oligomers to BLI sensors, which overwhelm the positive wavelength shift (in nm), resulting in a negative value14 (Figure 5C).
The interaction between the Aβ42 monomer and Pactive was also followed by NMR. The one-dimensional (1D) NMR spectrum of the Aβ42–Pactive complex clearly showed notable differences in peak distribution in amino regions compared to both the constituent apo forms, indicating global rearrangement of Aβ42 conformation upon complex formation with the peptide (Figure 6A). Further, docking followed by MD simulation with the Aβ42–Pactive complex (structures used for docking: Aβ42, PDB ID: 1IYT,9 model 1; Pactive, PEP-FOLD315-generated structure; Figure S10) also supported the same. Throughout the 0.4 μs MD trajectory of the complex, Pactive-bound Aβ42 underwent significant conformational changes, which led to conformational alteration of the single tyrosine at position 10 (Y10) of the Aβ molecule, making it feasible to be exposed toward the solvent surface from the preferred orientation of fibrillation (Figure 6B). Thus, NMR interaction studies (along with MD simulation) confirmed Pactive-mediated global conformational rearrangement of Aβ monomers upon complex formation.
Figure 6.
Pactive-mediated conformational alteration of Aβ42 monomers and oligomers. (A) 1D NMR spectra of N–H regions of the free Aβ42 monomer, Pactive, and the complex revealing chemical shifts due to complex formation. (B) Mapping MD simulation trajectory of the complex from 0.4 μs (Pactive, cyan in line representation) where the transition of the Aβ42 monomer from the initial point to 0.4 μs is shown in different colors, corresponding to their representative conformations indicated by arrows. (C, D) Differential scanning calorimetric (DSC) thermograms of (C) control Aβ42 oligomers and (D) Pactive-treated Aβ42 oligomers, confirming the formation of conformationally altered species. The Tm and ΔH values are provided in the respective insets.
Differential scanning calorimetric (DSC) studies were performed with control and Pactive-treated Aβ42 oligomers to compare Pactive-induced subtle structural reorganization. The DSC thermogram of the toxic control oligomers showed two endothermic peaks at 55.26 and 72.17 °C (Figure 6C). The occurrence of two peaks reflects the presence of a mixture of two populations with different heat capacities. In contrast, treatment of these oligomers with Pactive for 24 h resulted in a thermally homogeneous single population with Tm = 68.61 °C (Figure 6D). Clearly, the species formed upon treatment are distinctly different from the toxic oligomers, as indicated by the different Tm values and heat capacities, confirming that structural reorganization indeed occurs.
Biostability of Pactive against Degradation by Human Serum Enzymes
Although peptides offer a powerful tool for drug designing, the major impediments in the process are related to their high susceptibility to serum enzymes that compromises their therapeutic efficiency.16 The stability of Pactive against degradation in HBS was evaluated at 37 °C with NMR spectroscopy. Incubation of Pactive with HBS confirmed no visible degradation up to 24 h, as indicated by no significant change in the backbone N–H region spectra (Figure 7A). Precisely, conformational alterations were observed from 0 to 2 h, as suggested by the peak shift, but the connectivity pattern between amino acids remained the same along with their intensity with respect to the same number of scans used for data acquisition. Moreover, Pactive was also found to be nontoxic toward SHSY-5Y cells up to a concentration of 1 mM (Figure 7B) where cell viability upon treatment is comparable to that of the control.
Figure 7.
Biostability of Pactive in human blood serum. (A) 1D NMR spectra (N–H region) of Pactive in human blood serum assessed over a period of 24 h incubated at 37 °C showing no significant change in the backbone or degradation, confirming its stability. (B) MTT cell viability assay with different concentrations of Pactive showing no significant cytotoxicity up to 1 mM. Error bars represent the mean ± standard deviation (SD). Symbols indicate statistically significant differences compared with control (0 μM Pactive) (*p < 0.05, **p < 0.01, ***p < 0.001).
Discussion
AD currently afflicts the largest percentage of elderly population with the only available treatment being symptomatic interventions with drugs that barely help to palliate the symptoms without solving the underlying problems.17 Furthermore, it has been known that amyloid plaques including fibrils begin to form even before disease symptoms start to develop.17a Therefore, agents that can prevent Aβ aggregation and destabilize existing fibrils would be considered attractive therapeutics for AD.
Our study reported effective anti-amyloidogenic potency of a heptameric peptide (Pactive) identified from a fibrinolytic enzyme isolated from a medicinal plant A. indica. The enzyme was found efficient both as an inhibitor and a destabilization agent against Aβ aggregation. Importantly, though oligomers are known to exert major cytotoxicity,3 enzyme treatment could successfully abate cytotoxic effects of these oligomers in SHSY-5Y cells, suggesting that the enzyme-arrested, nonfibrillating oligomeric forms are much less toxic compared to the cytotoxicity exerted by the on-pathway oligomeric intermediates of Aβ fibrillation.
The study established that Pactive arrests Aβ in an oligomeric form (off-pathway) distinctly different from the cytotoxic, on-pathway oligomers. The characterizations of Pactive and its activity assays clearly demonstrated its strong bioaffinities and consequent structural reorganizations of both Aβ42 monomers and toxic oligomers. It can be concluded that Pactive-induced conformational alterations along with modulation of Aβ hydrophobicity (so that the hydrophobic patches are less accessible) suppress the aggregation propensity and toxicity of Aβ. Importantly, we have shown that Pactive remains stable in HBS for a reasonably long period.
Conclusions
In summary, the serum-stable heptapeptide (Pactive) presented here is capable of sequestering Aβ in an off-pathway oligomeric form that can no longer participate in the toxic fibrillation cascade. In the recent era where peptide-based drug designing is being considered a viable alternative, this novel peptide can be a potential lead molecule for drug formulation against Alzheimer’s. Further relevant studies on animal models can find the fitness of the heptameric peptide for its use for clinical purposes.
Experimental Section
Extraction and Isolation of the Enzyme
Roots (100 mg/mL) were kept overnight in sodium phosphate buffer (pH 7.5) at 4 °C. Roots were cut, macerated, and centrifuged. The supernatant served as the extract.
Isolation of the fibrinolytic enzyme was achieved with substrate affinity chromatography (fibrinogen-coupled Sepharose CL-6B, Sigma-Aldrich) followed by sodium dodecyl sulfate poly-acrylamide gel electrophoresis (SDS-PAGE). Further purification involved size-exclusion HPLC (SE-HPLC, Waters) with a Waters Protein-Pak 300 column pre-equilibrated with 10 mM sodium phosphate buffer containing 0.1 M NaCl operated at 0.8 mL/min. HPLC was performed on a Waters 600 HPLC system with a Waters 2487 dual λ absorbance detector. A calibration curve was constructed using marker proteins, and a linear dependence of log Mw (molecular weight) versus retention time was observed (R2 = 0.9755, where R2 is the regression coefficient). The approximate molecular weight of the enzyme was calculated from the corresponding retention time that revealed the highest (major) peak corresponding to 13.6 kDa (Figure S1, indicated by the area within dashed lines). Purification was further confirmed with fibrinogen zymography.
Enzyme Inactivation by Thermal Denaturation
Inactivation of the proteolytic activity of the enzyme was done by heat denaturation at 100 °C for 2 h in a water bath and verified with fibrinogen zymography.
Tryptic Digestion of the Inactivated Enzyme
The inactivated enzyme was digested overnight at 37 °C with trypsin and chymotrypsin (enzyme: trypsin/chymotrypsin—50:1 w/w) followed by separation with a Sephadex G-10 gel filtration column (GE Healthcare Life Sciences). The peptide pool was characterized by Q-TOF ESI XENO XS mass spectrometry to identify the molecular weights of the digested separated fragments (abbreviated here as EDPs, enzyme-digested peptides) as well as to identify any residual undigested enzyme.
Zymography
Zymography was performed with the purified active and inactivated enzyme in 10% PAGE copolymerized with fibrinogen (10 mg/mL). After electrophoresis, SDS was removed by incubating the gel in 2.5% Triton X-100 for 1 h at 25 °C under gentle shaking, followed by overnight incubation in 50 mM Tris–HCl (pH 7.5), 0.02 mM CaCl2, at 37 °C. The gels were stained with Coomassie blue and destained, which revealed sites of proteolysis as white bands against a dark blue background.6
LC-MS/MS Analysis of EDPs
Three different sets of EDPs were analyzed using a Thermo Fisher Scientific LTQ-Orbitrap mass spectrometer with an integrated Easy nLC-1000 operating at a 40 min acetonitrile/water elution gradient conducted at 300 nL/min. MS/MS data were searched with Mascot Matrix Science Server8 using the following parameters—type of search: MS/MS ion search; database: SwissProt; taxonomy: all entries; enzyme: trypsin/chymotrypsin/TrypChymo; mass value: monoisotopic; protein mass: unrestricted; peptide mass tolerance: ±10 ppm; fragment mass tolerance: ±0.5 Da; max missed cleavage: 1/2.
Analysis of the three sets of EDPs identified four peptide sequences with molecular weight <1 kDa. Upon computational screening, only two sequences of the four were predicted to bind Aβ42 (PDB: 1IYT,9 model 1) with high propensity, as indicated by p-values from the PepSite 2.2.2010 web server (Table S1).
Solid-Phase Synthesis and RP-HPLC Purification of the Identified Peptides (P1 and Pactive)
The identified peptides were manually synthesized in the solid phase18 on a 0.05 mmol scale using the Rink amide MBHA resin (mesh size: 0.58) and Fmoc-protected amino acids. Peptides were cleaved from the resin using a cocktail of trifluoroacetic acid (TFA) (81%), phenol (5%), thioanisole (5%), 1,2-ethanedithiol (2.5%), dimethyl sulfide (2.5%), and water (3%), followed by precipitation with chilled ether and washing four times with the same. The precipitate was then dried overnight and dissolved in water.
The crude peptides were then purified using a C-18 (Thermo Fisher) reverse-phase HPLC (RP-HPLC) column with a 60 min linear gradient of water/acetonitrile containing 0.1% TFA conducted at 1 mL/min. Pure peptides were then lyophilized and stored at −20 °C until further use. The molecular weight and purity of the synthesized peptides were verified by matrix-assisted laser desorption ionization (MALDI) mass spectroscopy with a 4800 MALDI TOF/TOF analyzer (Applied Biosystems MDA SCIEX) and 19F NMR spectroscopy.
For further experiments, the HPLC-purified lyophilized peptides were re-dissolved in desired buffer (PBS) and evaluated for their efficiency in disaggregating and inhibiting Aβ aggregation.
Aβ40 and Aβ42 Aggregation Inhibition and Destabilization Assay
Aβ peptides were dissolved in hexafluoroisopropanol (HFIP) to 1.0 mg/mL (Aβ40) and 5.0 mg/mL (Aβ42). Thereafter, the solutions were lyophilized by vacuum-freeze-drying and finally stored at −20 °C. Immediately prior to use, the HFIP-treated Aβ was re-dissolved in dimethyl sulfoxide (DMSO) to make the final concentration of 5 mM and sonicated for 10 min to remove any pre-existing aggregates.19 For fibrillation, the Aβ40 solution was diluted with phosphate-buffered saline (PBS) (pH 7.5) containing 0.2% SDS leading to a final Aβ40 concentration of 100 μM and incubated at 37 °C for 7 days.19 Aβ42 fibrils were formed by incubating Aβ42 (100 μM) in PBS at 37 °C for 48 h under shaking conditions.20
For inhibition experiments, monomeric Aβ (100 μM) was incubated in the presence of active/inactive enzyme or EDPs (2.5 μg/mL) or the identified peptides (Aβ40/peptide = 10:1; Aβ42/peptide = 2:1) under abovementioned aggregation conditions for different time periods. For disaggregation assay, preformed fibrils (10 μM) were treated with the active/inactive enzyme or EDPs (2.5 μg/mL) or the identified peptides (Aβ40/peptide = 10:1; Aβ42/peptide = 2:1) under similar aggregation conditions for different time periods.
Aβ40 and Aβ42 Oligomerization
Aβ40 oligomers were obtained by incubating the monomers under predescribed aggregation conditions at a final concentration of 100 μM for 6 h.19 Aβ42 oligomers (100 μM) were formed by diluting the peptide solution in PBS and 0.2% SDS. The samples were incubated overnight at 37 °C, followed by a further dilution with PBS. The diluted sample was re-incubated for another 24 h.21
Atomic Force Microscopy (AFM)
Imaging was done with acoustic AC (AAC) mode AFM using a Pico plus 5500 inverted light microscope atomic force microscope (ILM AFM) (Agilent Technologies) with a piezoscanner having a maximum range of 9 μm. Microfabricated silicon cantilevers of 225 μm length with a nominal spring force constant of 21–98 N/m were used from Nano sensors. The cantilever oscillation frequency was tuned into the resonance frequency (150–300 kHz). The images (256 by 256 pixels) were captured with a scan size ranging between 0.8 and 9 μm at the scan speed rate of 1 lines/S. The images were processed using PicoView 1.12 software (Agilent Technologies).
ThT Binding Assay
Assay mixtures containing treated/untreated Aβ40 (5 μM) were added to ThT (20 μM) and detected immediately (ex: 450 nm; em: 480–600 nm; ex/em slit width: 10 nm). The fluorescence intensity of the solution without Aβ40 was subtracted as background from each reading with Aβ40.
Circular Dichroism (CD) Spectroscopy
Far-UV CD (range: 195–240 nm) spectra of treated–untreated Aβ40 solutions in PBS (pH 7.5 at room temperature) were recorded with a J-815 spectrometer (JASCO, Japan) using a quartz cell with 1 mm path length at room temperature with 1 nm bandwidth, at a scanning speed of 100 nm/min. The spectra of solutions without Aβ were subtracted as background from the CD signals with Aβ to identify Aβ-specific changes. All spectra are average of three consecutive scans.
The raw spectra were analyzed using CAPITO11 (CD Analysis and Plotting Tool), a web server-based tool for analyzing and plotting CD data that allows reliable estimation of secondary structure content.
Cell Cytotoxicity and Viability Assay
SHSY-5Y human neuroblastoma cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (GIBCO) supplemented with 10% heat-inactivated FBS (GIBCO), 1% Penstrap (GIBCO), and 0.2% sodium bicarbonate (Sigma-Aldrich) at 37 °C under 5% CO2. Cells were grown till they reached 70–80% confluency followed by trypsinization with 0.25% trypsin (GIBCO).
For cell viability assays, cells were seeded into 96-well plates at a density of 104/well in DMEM–serum media and allowed to grow. Cells were treated with 5 μM Aβ42 oligomers in the absence or presence of the inhibitors at a final concentration of 2.5 μg/mL in DMEM–serum-deprived media for 4 h followed by serum replenishment for 44 h. Cytotoxicity was assessed after 48 h by monitoring the reduction of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 0.5 mg/mL, Invitrogen) reagent for 4 h at 570 nm.
To assess the cytotoxicity of Pactive, MTT cell viability assay was performed on SHSY-5Y cells. The cells were treated with different concentrations of Pactive (up to 1 mM) in serum-deprived media for 24 h followed by assessment of cell viability. Average of three replicates was used for each treatment as well as the control. Experiments were repeated in triplicate and survivability was expressed as percentage of control/untreated cells.
ANS Binding Assay
Aβ40 with or without the enzyme or Pactive at different concentrations were mixed with 8-anilinonaphthalene-1-sulfonic acid (ANS, 20 μM, ex: 360 nm; em: 400–700 nm, ex/em slit: 10 nm, Sigma-Aldrich). The fluorescence intensity of the solution without Aβ was subtracted as background from each reading with Aβ.
Biolayer Interferometry
Interaction studies were performed with Pactive and Aβ42 monomers and oligomers in real time with biolayer interferometry22 with an Octet RED 96e (Pall ForteBio, CA). Experiments were performed at 37 °C in PBS with a constant agitation at 1000 rpm. Aβ42 monomers and oligomers at different dilutions in PBS were loaded onto 96-well black microtiter plates. For biotinylation, Pactive was incubated with 10-fold excess biotin (Thermo Fisher Scientific) in PBS for 1 h at room temperature. Excess biotin was removed and the biotinylated Pactive was then used for the experiments.
Streptavidin biosensors (Pall ForteBio, CA) were hydrated in PBS for 10 min and then loaded with biotinylated Pactive (100 μg/mL), which were then transferred to fresh PBS for baseline measurements. These preloaded sensors were then associated with Aβ42 monomers or oligomers for 5 min and finally moved back to PBS for dissociation for 10 min. The Kd values of interaction were determined by full local fitting of the binding curves for each ligand dilution and fitted by applying a 2:1 interaction model using ForteBio Data Analysis HT software (CA), version 11.1.0.25.
Differential Scanning Calorimetry
Calorimetric measurements were performed with Aβ42 oligomers as control and compared with Pactive-treated oligomers (24 h) on a MicroCal VP-differential scanning calorimeter (MicroCal, Malvern Instruments, U.K.). DSC scans were conducted between 25 and 95 °C with a constant scan rate of 1.5 °C/min. Instrumental baselines were obtained with PBS, and buffer scans were repeated until reproducible. On cooling, the sample cells were rinsed and loaded with control or Pactive-treated Aβ42 oligomers. Excess heat capacities as a function of temperature were measured and the DSC thermograms of excess heat capacity versus temperature were analyzed with Origin 7.0 software. The area under the experimental heat capacity (Cp°) curves was used to determine the calorimetric transition enthalpy (ΔHcal) given by the equation
where T is the absolute scale temperature in kelvin. This calorimetrically determined enthalpy is model-dependent and thus unrelated to the nature of the transition. The temperature at which excess heat capacity is at a maximum defines the transition temperature (Tm).
Biostability of Pactive against Degradation by Human Serum Enzymes
The stability of Pactive against degradation in HBS was assessed over a time period of 24 h with NMR spectroscopy with the same number of scans to monitor peak intensity and correlated with peptide concentration over time. HBS was centrifuged to remove the lipid components, and the supernatant was incubated with Pactive (1 mM) at 37 °C. Stability was analyzed by comparing the 1D NMR spectra at different time points (0, 2, 5, 12, 16, and 24 h).
NMR Spectroscopy
All NMR spectra were acquired with a BRUKER 600 MHz equipped with a 5 mm QCI room-temperature probe at 298 K using the standard BRUKER pulse program (zg30). Concentrations used for both free Aβ42 and Pactive were 300 and 600 μM, respectively, as stock concentrations. To monitor the peak shift or conformational changes, Aβ42 was titrated with Pactive at 1:0.1 ratio (concentrations for free Aβ and peptide: 140 and 250 μM, respectively, at the experimental level) on the assumption of formation of a 1:1 complex. A series of 1D spectra for both free Aβ42 and Pactive as well as the complex were acquired for 128 scans. Standardization was done with TSP.
19F NMR spectra of the HPLC-purified peptides were recorded with a JEOL 400 MHz at 298 K. Externally added TFA served as the standard for comparison.
Docking and MD Simulation
Docking studies were done with Aβ42 monomers (PDB ID: 1IYT,9 model 1) and the heptameric peptide Pactive to identify the binding energy and generate a conformation for MD simulation. The structure of Pactive for docking studies was generated with PEP-FOLD3,15 a de novo approach that helps in peptide structure prediction from the amino acid sequence. The Haddock 2.223 web server was used for docking studies.
Molecular dynamics simulation studies were performed with the free Aβ42 and Aβ42–Pactive complex at pH 7.4, temperature 37 °C, and pressure 1 bar with the AMBER99sb-ildn force field24 using the GROMACS 5.1.5 software package.25 To correlate with the physiological conditions, the pH of all complexes was adjusted by protonating the required residues on the basis of pKa values, obtained from the PROPKA framework of PDB: 2PQR web server (http://nbcr-222.ucsd.edu/pdb2pqr_2.0.0/). Solvation was done by the TIP3P water model followed by neutralizing the overall charge with 137 mM NaCl within a cubic box with cutoff distances of 1 nm between the complex surfaces and the edges of the box. A 1.2 nm cutoff was used for nonbonded interactions. The particle-mesh Ewald method was used for long-range force calculations. The energy-minimized systems were equilibrated in an NVT followed by an NPT condition by restraining positions for 1 ns. The final simulation was carried out for up to 0.4 μs without position restraints and trajectories were stored every 50 ps interval. Data corresponding to all trajectories were analyzed by different GROMACS tools.
All-atom principal component analysis (PCA) was done by calculating the covariance matrix of the atomic fluctuations. Diagonalization of this matrix gives a set of eigenvectors and eigenvalues, which describe different modes of fluctuations of the protein. The eigenvectors corresponding to the largest eigenvalues, i.e., “principal components”, were analyzed by g_covar and g_anaeig tools provided by GROMACS based on the 0.4 μs trajectory. Pymol (The PyMOL Molecular Graphics System, Version 1.2r3pre, Schrödinger, LLC) was used for visualization of the molecular models.
General Procedures
All fluorescence measurements were done using a Hitachi F-7000 fluorescence spectrometer equipped with a thermostatic cell holder and magnetic stirrer. Transmission electron microscopy (TEM) imaging was done using a TECHNAI G2 transmission electron microscope system with an accelerating voltage of 120 kV. Confocal imaging was done with an Olympus Fluoview FV10i confocal microscope.
Statistical Analysis
All reported values are represented as the mean ± standard deviation (SD) of at least three independent experiments. Statistical analysis to compare mean values was performed using one-way analysis of variance (ANOVA) (n = 9) (*p < 0.05, **p < 0.01, ***p < 0.001).
Acknowledgments
We gratefully acknowledge the help from Dr. Debasish Bhattacharyya (retired professor), who initiated research on Aristolochia indica at CSIR-IICB. We thank the technical staff of the Central Instrument Facility (CIF) of CSIR-IICB, with special thanks to T. Muruganandan for AFM, Dr. E. Padmanaban and S. Kundu for NMR, K. S. Kumar for BLI, and Prof. S. Adhya (CSIR-IICB) for providing the cell culture facility. The SHSY-5Y cell line was received as a kind gift from Dr. S. N. Bhattacharyya (CSIR-IICB). We sincerely thank Prof. Siddhartha Roy, Bose Institute, for help with peptide synthesis. This work was supported by a SERB, India and CSIR-IICB, Kolkata, India. R.B. and S.B. acknowledge CSIR and UGC, respectively, for awarding fellowships, and B.P. acknowledges financial support from DBT-RA.
Glossary
Abbreviations
- Aβ
amyloid β
- AD
Alzheimer’s disease
- EDPs
enzyme-derived peptides
- ThT
Thioflavin T
- CR
Congo red
- ANS
8-anilinonaphthalene-1-sulfonic acid
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- TEM
transmission electron microscopy
- CD
circular dichroism
- BLI
biolayer interferometry
- DSC
differential scanning calorimetry
- LC-MS/MS
liquid chromatography with tandem mass spectroscopy
- SE-HPLC
size-exclusion high-pressure liquid chromatography
- HBS
human blood serum
- ILM AFM
inverted light microscope atomic force microscopy
- AAC
acoustic AC
- NMR
nuclear magnetic resonance
- RP-HPLC
reverse-phase high-pressure liquid chromatography
- PCA
principal component analysis
- ESI
electronspray ionization
- TFA
trifluoroacetic acid
- HFIP
1,1,1,3,3,3-hexafluoro-2-propanol
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01730.
Sequence of the identified peptides along with the p-values; purification of the enzyme; anti-amyloidogenic property of the candidates; purity of the synthetic peptides; effect of P1 on Aβ aggregation; docked structure; HPLC profiles and mass spectrometric data; 19F NMR spectra; TEM; AFM and confocal images; CD spectra; fluorescence data (PDF)
Author Contributions
J.S. and R.B. conceived the project. R.B. and S.B. designed and performed the experiments with consultation with B.P. R.B., S.B., and J.S. analyzed the data and wrote the paper. R.B. and S.B. contributed equally. All authors have given approval to the final version of the manuscript.
Author Contributions
§ R.B. and S.B. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- a Alzheimer A.; Stelzmann R. A.; Schnitzlein H. N.; Murtagh F. R. An English translation of Alzheimer’s 1907 paper, ″Uber eine eigenartige Erkankung der Hirnrinde″. Clin. Anat. 1995, 8, 429–431. 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]; b Ross C. A.; Poirier M. A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10, S10–S17. 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]; c Irvine G. B.; El-Agnaf O. M.; Shankar G. M.; Walsh D. M. Protein aggregation in the brain: the molecular basis for Alzheimer’s and Parkinson’s diseases. Mol. Med. 2008, 14, 451–464. 10.2119/2007-00100.Irvine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Culyba E. K.; Powers E. T.; Kelly J. W. Amyloid-beta forms fibrils by nucleated conformational conversion of oligomers. Nat. Chem. Biol. 2011, 7, 602–609. 10.1038/nchembio.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Lesne S.; Koh M. T.; Kotilinek L.; Kayed R.; Glabe C. G.; Yang A.; Gallagher M.; Ashe K. H. A specific amyloid-beta protein assembly in the brain impairs memory. Nature 2006, 440, 352–357. 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]; b Sakono M.; Zako T. Amyloid oligomers: formation and toxicity of Abeta oligomers. FEBS J. 2010, 277, 1348–1358. 10.1111/j.1742-4658.2010.07568.x. [DOI] [PubMed] [Google Scholar]; c Benilova I.; Karran E.; De Strooper B. The toxic Abeta oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat. Neurosci. 2012, 15, 349–357. 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee P.; Bhattacharyya D. An Enzyme from Aristolochia indica Destabilizes Fibrin-beta Amyloid Co-Aggregate: Implication in Cerebrovascular Diseases. PLoS One 2015, 10, e0141986 10.1371/journal.pone.0141986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ladner R. C.; Sato A. K.; Gorzelany J.; de Souza M. Phage display-derived peptides as therapeutic alternatives to antibodies. Drug Discovery Today 2004, 9, 525–529. 10.1016/S1359-6446(04)03104-6. [DOI] [PubMed] [Google Scholar]; b Berthoumieu O.; Faller P.; Doig A. J.; Derreumaux P. Inhibitors of amyloid beta peptide aggregation: Toward new drugs against Alzheimer’s disease. Alzheimer’s Dementia 2015, 11, P352–P353. 10.1016/j.jalz.2015.06.235. [DOI] [Google Scholar]; c Oller-Salvia B.; Sanchez-Navarro M.; Giralt E.; Teixido M. Blood-brain barrier shuttle peptides: an emerging paradigm for brain delivery. Chem. Soc. Rev. 2016, 45, 4690–4707. 10.1039/C6CS00076B. [DOI] [PubMed] [Google Scholar]; d Baig M. H.; Ahmad K.; Rabbani G.; Choi I. Use of Peptides for the Management of Alzheimer’s Disease: Diagnosis and Inhibition. Front. Aging Neurosci. 2018, 10, 21 10.3389/fnagi.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Baig M. H.; Ahmad K.; Saeed M.; Alharbi A. M.; Barreto G. E.; Ashraf G. M.; Choi I. Peptide based therapeutics and their use for the treatment of neurodegenerative and other diseases. Biomed. Pharmacother. 2018, 103, 574–581. 10.1016/j.biopha.2018.04.025. [DOI] [PubMed] [Google Scholar]; f Ribarič S. Peptides as Potential Therapeutics for Alzheimer’s Disease. Molecules 2018, 23, 283 10.3390/molecules23020283. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Rajasekhar K.; Madhu C.; Govindaraju T. Natural Tripeptide-Based Inhibitor of Multifaceted Amyloid beta Toxicity. ACS Chem. Neurosci. 2016, 7, 1300–1310. 10.1021/acschemneuro.6b00175. [DOI] [PubMed] [Google Scholar]; h Paul A.; Kumar S.; Kalita S.; Kumar Ghosh A.; Mondal A.; Mandal B. A Peptide Based Pro-drug Disrupts Alzheimer’s Amyloid into Non-toxic Species and Reduces Aβ Induced Toxicity In Vitro. Int. J. Pept. Res. Ther. 2017, 24, 201–211. 10.1007/s10989-017-9602-8. [DOI] [Google Scholar]; i Lu J.; Cao Q.; Wang C.; Zheng J.; Luo F.; Xie J.; Li Y.; Ma X.; He L.; Eisenberg D.; Nowick J.; Jiang L.; Li D. Structure-Based Peptide Inhibitor Design of Amyloid-beta Aggregation. Front. Mol. Neurosci. 2019, 12, 54 10.3389/fnmol.2019.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber T. M.; Balkwill F. R. Zymography: a single-step staining method for quantitation of proteolytic activity on substrate gels. Anal. Biochem. 1997, 249, 24–28. 10.1006/abio.1997.2170. [DOI] [PubMed] [Google Scholar]
- Li H.; Rahimi F.; Sinha S.; Maiti P.; Bitan G.; Murakami K.. Amyloids and Protein Aggregation—Analytical Methods. Encycl. Anal. Chem. 2009. 10.1002/9780470027318.a9038 [DOI] [Google Scholar]
- Perkins D. N.; Pappin D. J.; Creasy D. M.; Cottrell J. S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 1999, 20, 3551–3567. . [DOI] [PubMed] [Google Scholar]
- Crescenzi O.; Tomaselli S.; Guerrini R.; Salvadori S.; D’Ursi A. M.; Temussi P. A.; Picone D. Solution structure of the Alzheimer amyloid beta-peptide (1-42) in an apolar microenvironment. Similarity with a virus fusion domain. Eur. J. Biochem. 2002, 269, 5642–5648. 10.1046/j.1432-1033.2002.03271.x. [DOI] [PubMed] [Google Scholar]
- Trabuco L. G.; Lise S.; Petsalaki E.; Russell R. B. PepSite: prediction of peptide-binding sites from protein surfaces. Nucleic Acids Res. 2012, 40, W423–W427. 10.1093/nar/gks398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann C.; Bellstedt P.; Gorlach M. CAPITO--a web server-based analysis and plotting tool for circular dichroism data. Bioinformatics 2013, 29, 1750–1757. 10.1093/bioinformatics/btt278. [DOI] [PubMed] [Google Scholar]
- a Kim W.; Hecht M. H. Generic hydrophobic residues are sufficient to promote aggregation of the Alzheimer’s Abeta42 peptide. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 15824–15829. 10.1073/pnas.0605629103. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cukalevski R.; Boland B.; Frohm B.; Thulin E.; Walsh D.; Linse S. Role of aromatic side chains in amyloid beta-protein aggregation. ACS Chem. Neurosci. 2012, 3, 1008–1016. 10.1021/cn300073s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognesi B.; Kumita J. R.; Barros T. P.; Esbjorner E. K.; Luheshi L. M.; Crowther D. C.; Wilson M. R.; Dobson C. M.; Favrin G.; Yerbury J. J. ANS binding reveals common features of cytotoxic amyloid species. ACS Chem. Biol. 2010, 5, 735–740. 10.1021/cb1001203. [DOI] [PubMed] [Google Scholar]
- Bornhop D. J.; Kammer M. N.; Kussrow A.; Flowers R. A. 2nd; Meiler J. Origin and prediction of free-solution interaction studies performed label-free. Proc. Natl. Acad. Sci. U.S.A. 2016, 113, E1595–E1604. 10.1073/pnas.1515706113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Thevenet P.; Shen Y.; Maupetit J.; Guyon F.; Derreumaux P.; Tuffery P. PEP-FOLD: an updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucleic Acids Res. 2012, 40, W288–W293. 10.1093/nar/gks419. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Shen Y.; Maupetit J.; Derreumaux P.; Tuffery P. Improved PEP-FOLD Approach for Peptide and Miniprotein Structure Prediction. J. Chem. Theory Comput. 2014, 10, 4745–4758. 10.1021/ct500592m. [DOI] [PubMed] [Google Scholar]; c Lamiable A.; Thevenet P.; Rey J.; Vavrusa M.; Derreumaux P.; Tuffery P. PEP-FOLD3: faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res. 2016, 44, W449–W454. 10.1093/nar/gkw329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Powell M. F. Chapter 30. Peptide Stability in Drug Development: in vitro Peptide Degradation in Plasma and Serum. Annu. Rep. Med. Chem. 1993, 28, 285–294. 10.1016/S0065-7743(08)60900-8. [DOI] [Google Scholar]; b Sawyer T. K. CHAPTER 1:Renaissance in Peptide Drug Discovery: The Third Wave, in Peptide-based Drug Discovery: Challenges and New Therapeutics. Drug Discovery 2017, 1–34. 10.1039/9781788011532-00001. [DOI] [Google Scholar]
- a Perrin R. J.; Fagan A. M.; Holtzman D. M. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature 2009, 461, 916–922. 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Galimberti D.; Scarpini E. Disease-modifying treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2011, 4, 203–216. 10.1177/1756285611404470. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Yiannopoulou K. G.; Papageorgiou S. G. Current and future treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2013, 6, 19–33. 10.1177/1756285612461679. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Graham W. V.; Bonito-Oliva A.; Sakmar T. P. Update on Alzheimer’s Disease Therapy and Prevention Strategies. Annu. Rev. Med. 2017, 68, 413–430. 10.1146/annurev-med-042915-103753. [DOI] [PubMed] [Google Scholar]
- Chakraborty M.; Roy S. A peptide-based synthetic transcription factor selectively down-regulates the proto-oncogene CFOS in tumour cells and inhibits proliferation. Chem. Commun. 2016, 53, 376–379. 10.1039/C6CC08086C. [DOI] [PubMed] [Google Scholar]
- Barghorn S.; Nimmrich V.; Striebinger A.; Krantz C.; Keller P.; Janson B.; Bahr M.; Schmidt M.; Bitner R. S.; Harlan J.; Barlow E.; Ebert U.; Hillen H. Globular amyloid beta-peptide oligomer - a homogenous and stable neuropathological protein in Alzheimer’s disease. J. Neurochem. 2005, 95, 834–847. 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- Aran Terol P.; Kumita J. R.; Hook S. C.; Dobson C. M.; Esbjorner E. K. Solvent exposure of Tyr10 as a probe of structural differences between monomeric and aggregated forms of the amyloid-beta peptide. Biochem. Biophys. Res. Commun. 2015, 468, 696–701. 10.1016/j.bbrc.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem S.; Biswas S. C. Tribbles Pseudokinase 3 Induces Both Apoptosis and Autophagy in Amyloid-beta-induced Neuronal Death. J. Biol. Chem. 2017, 292, 2571–2585. 10.1074/jbc.M116.744730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ciccone L.; Fruchart-Gaillard C.; Mourier G.; Savko M.; Nencetti S.; Orlandini E.; Servent D.; Stura E. A.; Shepard W. Copper mediated amyloid-beta binding to Transthyretin. Sci. Rep. 2018, 8, 13744 10.1038/s41598-018-31808-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lessard C. B.; Malnik S. L.; Zhou Y.; Ladd T. B.; Cruz P. E.; Ran Y.; Mahan T. E.; Chakrabaty P.; Holtzman D. M.; Ulrich J. D.; Colonna M.; Golde T. E. High-affinity interactions and signal transduction between Abeta oligomers and TREM2. EMBO Mol. Med. 2018, 10, e9027 10.15252/emmm.201809027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zundert G. C. P.; Rodrigues J.; Trellet M.; Schmitz C.; Kastritis P. L.; Karaca E.; Melquiond A. S. J.; van Dijk M.; de Vries S. J.; Bonvin A. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. J. Mol. Biol. 2016, 428, 720–725. 10.1016/j.jmb.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Lindorff-Larsen K.; Piana S.; Palmo K.; Maragakis P.; Klepeis J. L.; Dror R. O.; Shaw D. E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 2010, 78, 1950–1958. 10.1002/prot.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess B.; Kutzner C.; van der Spoel D.; Lindahl E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447. 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.