Abstract
With an increase in biodiesel demand, a large surplus of glycerol is expected, and there is interest regarding the usage of glycerol as a value-added product. One such idea is to use glycerol as a “green solvent” to replace petroleum-based organic solvents. Glycerol is nontoxic to humans, and its vapor pressure is sufficiently high for the chemical reaction to be performed at high temperatures under ambient atmospheric pressures. Its dielectric constant is between those of water and organic solvents, and it dissolves widely varying materials, spanning between salts and organic molecules. Metal nanoparticles have been known to be synthesized in glycerol within limited experimental conditions, including high temperatures, alkaline pH conditions, and the irradiance of ultraviolet light. Herein, we report that silver nanoparticles have been formed in glycerol under completely green conditions (e.g., room temperature, neutral pH conditions, and without irradiance of ultraviolet light). We suggest that aldehydes and free radicals are generated in glycerol, which is operating as reducing species.
1. Introduction
Recently, with an increase in biodiesel demand worldwide, the production of glycerol, which is a byproduct of biodiesel, has also increased over the last decade.1 Because biodiesel has more environmental and energy-efficient advantages than conventional fossil fuels, biodiesel production is expected to grow steadily in the future2,3 and leads to an increased surplus of glycerol with a subsequent reduction of its market price.4,5 Many scientists and engineers have focused on the economical mass consumption of oversupplied glycerol and have researched various new chemical applications for glycerol, such as its conversion into valuable chemicals (e.g., ethylene glycol, ethanol, or citric acid), hydrogen gas production, or additives for a variety of products (e.g., cosmetics, foods, or pharmaceuticals).4−6 In 2007, Wolfson et al. added another application of glycerol for mass consumption using glycerol as a “green solvent”.7
Since the movement of “green chemistry” began in the late 1990s, the search for environmentally benign green solvents to replace petroleum-based organic solvents has been one of the major issues in the green chemistry community.8 Because glycerol is not only nontoxic, renewable, and biodegradable but also able to dissolve a wide range of compounds spanning from inorganic salts, acids, and bases to organic compounds that have poor solubility in water; glycerol could be a suitable candidate as a green solvent. Additionally, because glycerol has a high boiling point and low vapor pressure, the chemical reaction can be performed in a glycerol solvent at high temperatures under atmospheric pressure for an extended period. The first chemical reaction in a glycerol solvent was demonstrated by Wolfson et al., in which they showed that several catalytic and noncatalytic reactions could be successfully performed in a glycerol solvent. Since then, other groups have successfully developed a wide variety of chemical reactions.9,10 At the same time, the synthesis of metal nanoparticles in a glycerol solvent has reemerged as a potential green pathway.
Sinha et al. published the first report concerning the synthesis of metal particles in a glycerol solvent in 2002, several years before the emergence of glycerol as a green solvent.11 In that paper, the authors synthesized micron-sized copper particles in glycerol near its boiling point (230 °C). After the first demonstration in 2002, other metal particles (e.g., gold, silver, and platinum) were also synthesized in the same medium at high temperatures of above 100 °C.12−16 In these reactions, glycerol is generally known to serve as both a solvent and a reducing agent. Although a few papers have described a detailed mechanism of how glycerol reduces metal ions at high temperatures, resulting in the formation of metal particles, previous research has indicated that the reduction process is carried out by an aldehyde (e.g., glyceraldehyde) converted from alcohol (e.g., glycerol) at high temperatures.17−20 The reduction process of metal ions by glycerol is possible not only at high temperatures but also at room temperature. In 2010, Sarkar et al. found that silver nanoparticles could be synthesized in a glycerol solvent even at room temperature without adding any reducing agents when sodium hydroxide (NaOH) was added.21 In this study, based on previous research papers, the authors examined a plausible mechanism for the reduction of silver ions and subsequent formation of silver nanoparticles at room temperature under highly alkaline conditions but did not discover any direct evidence.
To date, many research papers have demonstrated the synthesis of metal nanoparticles in glycerol, and most of the reactions were performed at high temperatures or under alkaline conditions.22−28 Three mechanisms can generally explain the reduction of metal ions in glycerol: catalytic reduction,21,29 reduction by aldehydes,18,21 or reduction by alkoxides.28 Under alkaline conditions, silver ions easily interact with hydroxide ions and form silver oxide (Ag2O) particles.30 Silver ions could be catalytically reduced on the surface of silver oxide particles preformed in the alkaline solution at room temperature.29 Metal ions have already been demonstrated to be reduced by aldehydes (e.g., Tollens reaction), and aldehyde could be formed through the dehydration process of glycerol in heated or alkaline glycerol.19,20 Another mechanism is the reduction by alkoxides that could be formed from alcohols, aldehydes, or ketones by hydroxide ions. According to this mechanism, glycerol does not need to be converted to aldehydes because alkoxides generated from alcohols can reduce metal ions directly.28 These three mechanisms provide reasonable explanations for the synthesis of metal nanoparticles in glycerol but cannot explain some experimental results, as shown herein. For example, all mechanisms suggested above emphasize alkaline conditions due to hydroxide ions that are essential for the synthesis of metal nanoparticles. According to our experiments, however, an alkaline condition is not critical, and silver nanoparticles can be synthesized in glycerol even in neutral conditions, which is a completely benign process compared to previously reported ones. The silver nanoparticles prepared at the neutral pH condition could be more acceptable for medical and pharmaceutical applications.31 In this report, we propose a novel mechanism for the synthesis of silver nanoparticles in glycerol and discuss it with direct evidence.
2. Results and Discussion
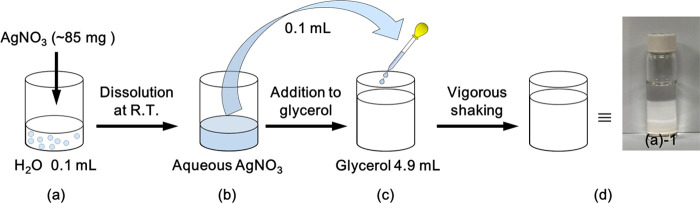
Most of the papers published to date deal with the synthesis of metal nanoparticles in a glycerol solvent only at high temperatures or in alkaline conditions. Therefore, the synthetic mechanism for the structural change of glycerol into molecules that can act as reducing agents has focused mainly on the role of heating or hydroxide ions. Recently, we found that silver nanoparticles are formed in a glycerol solvent at ambient pH (5–7) and temperatures (20–25 °C) without any extra additives. Figure 1 is a representative of our findings, in which approximately 85 mg of silver nitrate was dissolved in 0.1 mL of water (Scheme 2a) and then 0.1 mL of silver nitrate aqueous solution was added to 4.9 mL of glycerol solvent (Scheme 2b,d). The color of the solution was initially transparent ((a)-1 in Scheme 2d and Figure 1a); however, it began to change to orange ((a)-2 in Figure 1a and the black solid line in Figure 1b), after several hours. This orange color and spectral profile showing the maximum near 400 nm are traditional characteristics of spherical silver nanoparticles with several tens of nanometers in diameter. In this case, because extra additives, such as sodium hydroxide or stabilizers, were not added, and the reaction was performed at room temperature, it can be said with certainty that silver nanoparticles were synthesized in the glycerol solvent by mechanisms different from those generally accepted. As the reaction time progressed, the color of the solution gradually changed. After approximately 27 h, the color of the solution changed to grayish orange ((a)-3 in Figure 1a), concomitant with spectral broadening, as shown as the red line in Figure 1b. Finally, the orange color disappeared, the color of the solution turned gray after approximately 46 h ((a)-4 in Figure 1a), and their spectral width broadened (blue line in Figure 1b), which means that nanoparticles grew inhomogeneously and the glycerol solution contained silver nanoparticles of various sizes. This result was confirmed by an electron micrograph (Figure 2a) and its energy-dispersive spectrum (Figure 2b), which shows that although the sizes of nanoparticles varied (average diameter 38 ± 13 nm), silver nanoparticles had certainly formed in the glycerol solvent without any extra additives.
Figure 1.
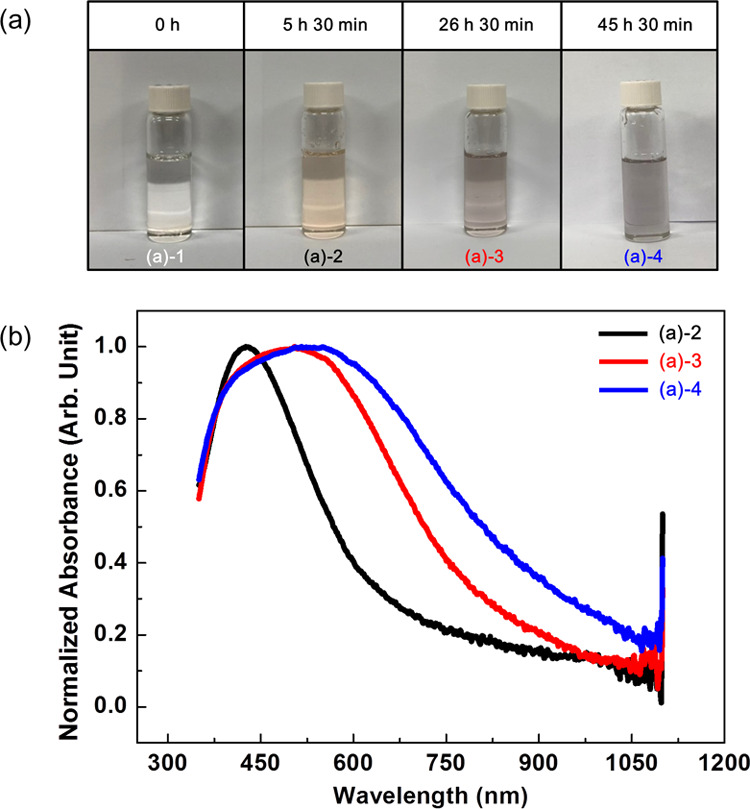
Chronological sample photos (a) and corresponding UV–vis spectra (b). The color of the glycerol solution containing silver ions gradually changed to light orange and finally to gray. Concomitant with these color changes, the width of spectra broadens. (Photograph courtesy of Tianhao Liu. Copyright 2020.).
Scheme 2. Scheme of Synthesis of Silver Nanoparticles with Silver Nitrate and Glycerol under Natural Conditions.
Figure 2.
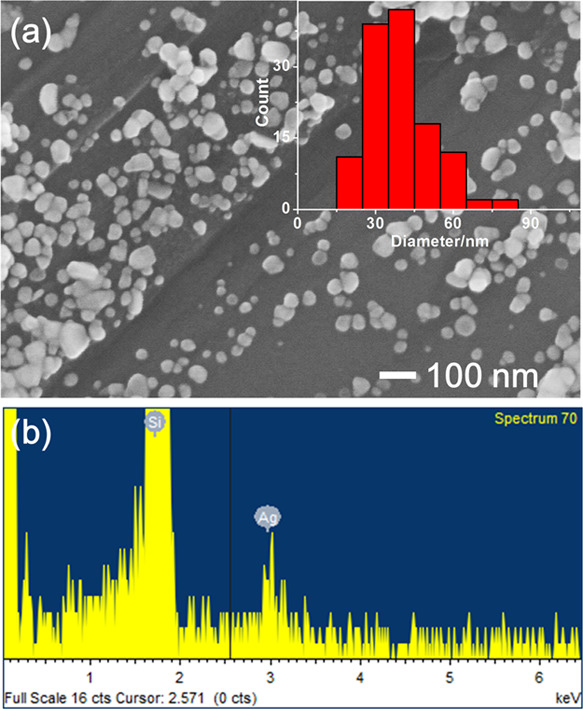
Electron micrograph (a) and its energy-dispersive spectrum (b) of silver nanoparticles prepared in glycerol without the stabilizer, poly(vinylpyrrolidone).
In this experiment, a small amount of water did not affect the nanoparticle formation. As demonstrated as the black line (∼70 h after dissolution of silver nitrate in glycerol) in Supporting Figure 1, silver nanoparticles were also synthesized in a pure glycerol solvent without adding water. In this case, however, the dissolution rate of silver nitrate was extremely slow. It took about a few hundred hours to fully dissolve 85 mg of silver nitrate in 5 mL of glycerol completely. Due to the slow dissolution of silver nitrate in glycerol, the increase in the concentration of silver ions and the production of seeds were also slow. According to the nucleation theory on the synthesis of nanoparticles, the slow rate of seed formation is expected to lead to broad size distribution of nanoparticles, and this expectation is observed in the broad spectrum, represented as a blue line (∼170 h after dissolution of silver nitrate in glycerol) in Supporting Figure 1a and transmission electron micrograph in Supporting Figure 1b.
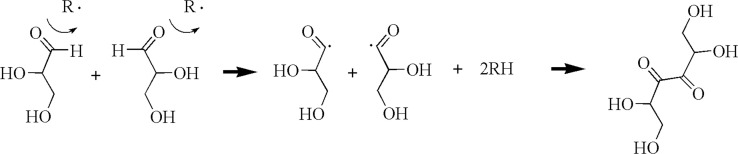
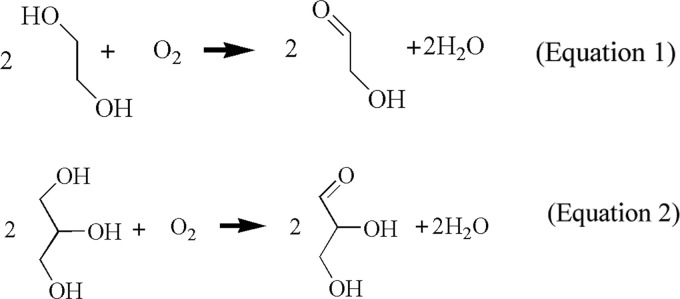
So far, we discussed the synthesis of silver nanoparticles in a glycerol solvent. In this reaction, glycerol must play an important vital role as a reducing agent at room temperature in neutral pH conditions. Our aim was to determine the mechanism for this reaction and how glycerol reduces silver ions at room temperature and in neutral pH conditions. We obtained some clues for the reduction mechanism of glycerol suggested in a previous paper,16 which states that ethylene glycol can be converted to glycolaldehyde by oxygen (chemical equation 1) and the converted glycolaldehyde reduces the silver ions. We think that a similar conversion and reduction can occur in a glycerol solvent (chemical equation 2). Similar to ethylene glycol, glycerol may also be converted to glyceraldehyde, which can reduce the silver ions, as verified in Supporting Figure 2 in which silver nanoparticles are formed in the glyceraldehyde aqueous solution. Alkoxides generated from glycerol could be another candidate, but their contribution effect on the reduction of silver ions should be very weak because silver nanoparticles could be synthesized even in weak acidic conditions (Supporting Figure 3). Therefore, we can assume that glyceraldehydes are the main reducing species and try to verify our assumption using infrared spectroscopy.
 |
1 |
According to chemical equation 2, the concentration of glyceraldehyde is proportional to the amount of dissolved oxygen. To confirm the presence of glyceraldehyde generated by oxygen in glycerol, we prepared a total of three types of glycerols that were manufactured in different years and prepared in different ways. Old and new glycerols, manufactured in 2018 and 2019 years, respectively (based on the vendor-provided specifications), were used without any treatment, and the other glycerol was prepared by purging new glycerol (manufactured in 2019) with air for about 2 weeks. Because old glycerol and new glycerol purged with air would have had more opportunities to be exposed to oxygen, the amount of dissolved oxygen concomitant with the concentration of glyceraldehyde could be higher than that in the untreated new glycerol. IR spectra for the three samples were obtained using the attenuated total reflection (ATR) method, and their spectra were compared, as shown in Figure 3. The three spectra do not show the characteristic peaks of the carbonyl group at approximately 1730 cm–1 and are almost identical except for the band that appears at 1650 cm–1, as represented by arrows in Figure 3. A closer look at the three spectra at approximately 1650 cm–1 reveals that this peak appears not only in the purged new glycerol (blue line in Figure 3) but also in the other glycerols (black and red lines in Figure 3), although the strength of each spectrum is different. The IR band appearing near 1650 cm–1 can be attributed to the hydrogen-bonded carbonyl stretching mode. Generally, the stretching mode of carbonyl groups in aldehyde appears at 1720–1740 cm–1. However, if the bond strength of C=O becomes weak through the loss of the double bond character by a hydrogen bond or hybridization, the vibrational frequency can be shifted to a lower energy region, resulting in the appearance of a carbonyl band at approximately 1650 cm–1. Because, in general, glyceraldehyde can form intramolecular hydrogen bonding followed by a dimeric hemiacetal,32−34 the stretching band of C=O could appear at approximately 1650 cm–1. Because the concentration of glyceraldehyde, however, should be low not enough to form dimers, and the portion of dimers would not be high, this assignment could be excluded. This peak could also arise from O–H scissoring of water molecules contained in the glycerol because glycerol is so hygroscopic that glycerol can effectively absorb water molecules from the atmosphere to some extent.35 Because the amount of water contained in glycerol equilibrates with the humidity of the atmosphere, the intensity of the peak appearing at approximately 1650 cm–1 in each spectrum should vary according to atmospheric conditions. Nevertheless, the intensity near 1650 cm–1 on the blue line (the new glycerol purged with air) is exceptionally strong compared with the other two spectra, probably because a large amount of water was produced and contained in this glycerol, according to chemical equation 2. Because the amount of water in glycerol can be estimated from its density,36 we measured the density of the three glycerols and compared the amount of contained water as a weight-to-weight percent. As a result, we found that the glycerol purged with air contained extraordinarily larger amounts of water (19% w/w), 19 times more than that in the other two glycerols (both 1% w/w). The strong IR peak appearing at 1650 cm–1 and high water concentration in the purged new glycerol indicate that glycerol reacted with dissolved oxygen and produced glyceraldehyde and water, as described in chemical equation 2. From the density measurements, we also found that the amount of water content in the purged new glycerol was not maintained and decreased as time progressed. This result is probably because the concentration of water in the glycerol equilibrated with the atmospheric water concentration. This equilibrium between glycerol and the atmosphere could also be the reason that the peak intensities at around 1650 cm–1 of old (red line) and new (black line) glycerols have similar values. Although a considerable amount of glyceraldehyde and water was produced in old glycerol, its water concentration could be similar to that of the new glycerol due to the equilibration of water.
Figure 3.
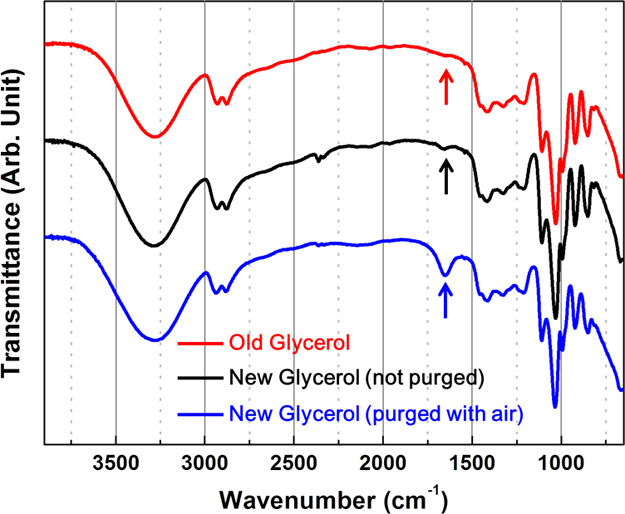
IR spectra of three kinds of glycerols (old glycerol produced in 2018, new glycerol produced in 2019, and new glycerol purged with air for about 2 weeks).
Glyceraldehyde in glycerol might be detectable using a more sensitive technique. Of the various analytical methods, traditional colorimetric methods are as sensitive and straightforward as IR spectroscopy and we adopted the colorimetric method, especially the “Schiff method,” to reconfirm the presence of glyceraldehyde in glycerol. In this method, an especially designed molecule, a fuchsin-sulfite reagent named “Schiff reagent” is mixed with the analyzed target solution, and the presence of the aldehyde is determined by the appearance of magenta color in the mixed solution. To investigate the presence of aldehydes produced by dissolved oxygen in glycerol, 1 mL of Schiff solution was mixed with 2 mL of old and new glycerols at room temperature (20–23 °C), allowing ambient light. The color of the mixed solution was investigated after the required periods (15 min or 1 h). Pictures 4(a)-1 and 4(a)-2 of Figure 4a were taken 15 min after mixing glycerol and the Schiff reagent.
Figure 4.
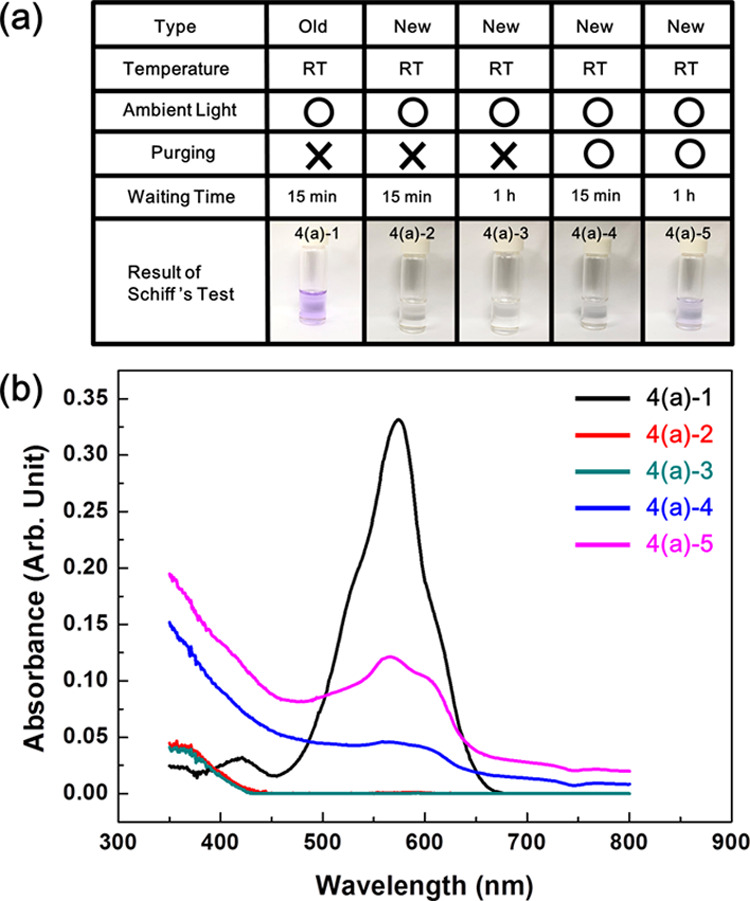
Schiff’s test results of various kinds of glycerol (a) and their UV–vis spectra (b). Violet color means that aldehydes are contained in the test solution. Only old glycerol manufactured in 2018 (4(a)-1) shows the violet color. The color of new glycerol manufactured in 2019 is colorless and transparent, but if it is purged with air, its color changes to violet. (Photograph courtesy of Tianhao Liu. Copyright 2020.).
Interestingly, the color of glycerol had changed to magenta only in old glycerol, but the color of new glycerol had not changed, which means that aldehyde existed only in old glycerol and not in new glycerol. Although the waiting time was prolonged to 1 h, the color change was not observed (4(a)-3 in Figure 4a) in new glycerol. The color change can be observed more sensitively using a UV–vis spectrophotometer (Figure 4b). Contrary to strong absorbance of the black solid line, which is the absorption spectrum corresponding to 4(a)-1 in Figure 4a, the absorption spectra of 4(a)-2 and even 4(a)-3 do not show any absorbance at wavelengths above 450 nm, as represented as the red and green solid lines in Figure 4b. With these experimental results, it was successfully predicted that some glycerol could be converted to glyceraldehyde by oxygen dissolved in old glycerol, while such conversion rarely occurs in new glycerol due to the low amount of dissolved oxygen.
To further confirm the oxygen effect, new glycerol was purged with air using a bubble stone for about 40 h, and the Schiff test was conducted again. Because purging with air increased the amount of dissolved oxygen, resulting in the generation of glyceraldehyde in new glycerol, it was expected that the aldehyde could be detected (i.e., magenta color appearing in new glycerol purged with air). As expected, the color of new glycerol purged with air had changed to magenta, as shown in 4(a)-4 of Figure 4a, which was taken 15 min after mixing. Although the magenta color is not clear in 4(a)-4, an absorption peak distinctively appeared at wavelengths between 450 and 650 nm (blue solid line in Figure 4b) in the UV–vis spectrum. Additionally, when the waiting time was prolonged to 1 h after mixing, the color of the solution becomes more clear (4(a)-5 of Figure 4a), concomitant with the increase in the absorption peak (magenta solid line in Figure 4b). To the best of our knowledge, this is the first evidence that revealed that glyceraldehydes are produced from glycerol by dissolved oxygen.
The experimental results obtained above state that old glycerol contains glyceraldehyde converted from glycerol by dissolved oxygen, but the newly produced glycerol does not contain glyceraldehyde. If glyceraldehyde is the only species that contributes to the formation of silver nanoparticles by reducing silver ions, nanoparticles would be produced only in old glycerol but not in new glycerol. In reality, however, silver nanoparticles were synthesized in both old and new glycerols, which means that silver ions were reduced by not only glyceraldehyde but also other species. Referring to the previous papers related to the synthesis of metal nanoparticles in glycerol,37,38 we thought that some free radicals would be produced and might affect nanoparticle synthesis.
To investigate the formation of free radicals in glycerol, we adopted the colorimetric method, in which relatively stable radical molecules (usually a certain color) are added as indicators to glycerol. If there are free radicals formed in glycerol, they react with the indicators (radical molecules) followed by a structural change of the indicators, finally resulting in a color change. Herein, 2,2-diphenyl-1-picrylhydrazyl (DPPH), which is a violet solid compound at ambient conditions, was used as the indicator. DPPH dissolved in glycerol, but its dissolution rate was very slow. It took approximately 20 h with vigorous stirring to prepare 0.1 mM DPPH solution in glycerol. Therefore, DPPH was first dissolved in ethanol to a concentration of 0.1 mM, and this solution was mixed with old and new glycerols in a 1:1 ratio (v/v), respectively. Initially, the color of the solution was violet, as shown in pictures 5(a)-1 and 5(a)-4 in Figure 5a, regardless of the manufacture date of glycerol. The mixed solutions were placed on the laboratory bench for the required time (30 min or 15 h) for the free radicals to be scavenged by the indicators at room temperature (∼20 °C) without protection from ambient light. The color of the mixed solutions was investigated after 30 min (5(a)-2 and 5(a)-5) and 15 h (5(a)-3 and 5(a)-6). When compared with the initial color of the solution (5(a)-1 and 5(a)-4), the color of the old glycerol solution had hardly changed and was still violet (5(a)-2 and 5(a)-3). The color of the new glycerol solution, however, had already begun to change to pink rose (5(a)-5) after 30 min and turned to yellow (5(a)-6) after 15 h. It should be noted that the ethanol did not affect to the scavenging efficiency, as represented in Supporting Figure 4, in which the color of the ethanol was still violet even after 15 h. For quantitative analysis, the UV–vis spectra were taken (Supporting Figure 5), and the scavenging efficiency was calculated for each sample using eq 3.
| 3 |
where A0 or A1 is the area in between 500 and 550 nm in the UV–vis absorption spectrum of the mixed solution in the initial (5(a)-1 or 5(a)-4) stage or final (5(a)-2,3 and 5(a)-5,6) stage after waiting for the required times after the addition of DPPH, respectively. As represented in Figure 5b, the scavenging efficiency of new glycerol (82.3% for 30 min and 92.0% for 15 h) is higher than that of old glycerol (18.4% for 30 min and 58.0% for 15 h) by 4.5 times in 30 min and 1.6 times in 15 h. This experimental result demonstrates that the concentration of free radicals in old glycerol is lower than that in new glycerol. Interestingly, this result shows the opposite trend from that of this Schiff’s test in that old glycerol with a high concentration of aldehydes contains a low concentration of radicals and new glycerol with a low concentration of aldehydes contains a high concentration of radicals. To reconfirm these experimental results, we repeated the same experiment with various glycerols manufactured on different dates and found that all of the tested glycerols show the same trend (Supporting Figure 6). This suggests that free radicals are generated in both old and new glycerols, but the glyceraldehydes present in old glycerols could play the role of a radical scavenger. Referring to ref (38), in which glyceraldehyde radicals form dimer products, we can suggest the following chemical reaction (Scheme 1) for the scavenging effect of glyceraldehydes, where the free radicals snatch the hydrogen radicals from the glyceraldehydes and two glyceraldehyde radicals form a dimer.
Figure 5.
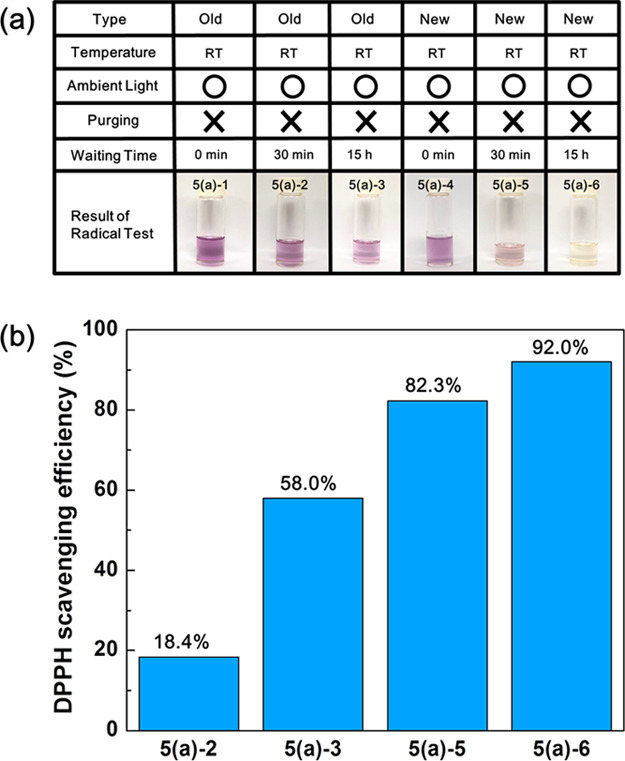
(a) DPPH test result of old (5(a)-1, 2, and 3) and new (5(a)-4, 5, and 6) glycerols produced in 2018 and 2019, respectively, at room temperature and under ambient light. In this test, when the solution contains radicals, the color of the solution changes from violet to yellow, and the “DPPH-scavenging efficiency” is increased in (b). (Photograph courtesy of Tianhao Liu. Copyright 2020.).
Scheme 1. Schematic Chemical Reaction Representing the Scavenging Effect of Glyceraldehyde.
The energy source for generating free radicals in glycerol, especially in new glycerol, was also considered. Ultraviolet or visible light mixed in the ambient light was considered as the first candidate. Because we did not cover the vial containing glycerol, glycerol was exposed to visible light and could have been exposed to ultraviolet light. We conducted a DPPH test for the same glycerol under different conditions to confirm the light effect, where one was placed directly under an LED light (6(a)-2) and the other one was covered with aluminum foil to block the ambient light (6(a)-3). The temperature of the two glycerols was adjusted to 25 °C and stabilized in a water bath for 15 h. The scavenging efficiencies of the glycerol irradiated by LED light (6(a)-2) and the glycerol (6(a)-3) protected from ambient light were 79.3 and 71.4%, respectively. These values indicate that light affects the DPPH-scavenging efficiency but only slightly, signifying that light irradiance is not the primary driving force for generating free radicals in glycerol. Another candidate for the generation of free radicals in glycerol is temperature. When the same experiment was performed at −22 °C, the scavenging efficiency sharply dropped to 34.2% for the glycerol irradiated by the LED light and to 23.6% for the nonirradiated glycerol. From this result, it can be concluded that the scavenging efficiency of DPPH is largely dependent on temperature more than light irradiance (Figure 6).
Figure 6.
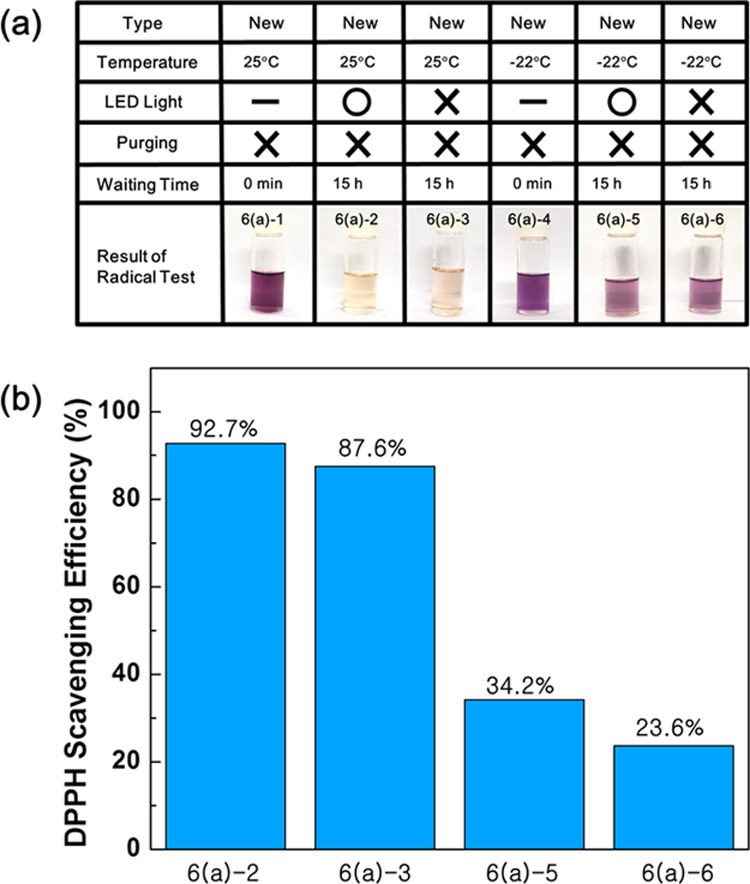
(a) DPPH test results and (b) their scavenging efficiencies of new glycerols to investigate temperature and light effect for the generation of radicals. (Photograph courtesy of Tianhao Liu. Copyright 2020.).
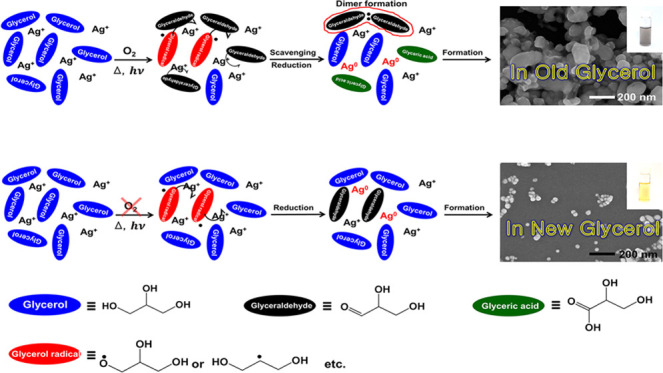
Based on the experimental results so far, glycerol can be divided into two types depending on the chemical species it contains. Because the newly produced glycerol has not been exposed to oxygen as much, it contains a small amount of aldehydes and a relatively more substantial amount of free radicals. Contrary to this, old glycerols would have had more chances to come in contact with oxygen and contain a large amount of aldehydes and a relatively small amount of free radicals. Because both glyceraldehydes and free radicals have reducing power, silver ions would be reduced primarily by glyceraldehyde in old glycerol and reduced mainly by free radicals in new glycerols. These different pathways for the synthesis of silver nanoparticles in old and new glycerols could lead to different morphological properties (e.g., particle size and size distribution) of the synthesized silver nanoparticles.
Figure 7 illustrates the UV–vis spectra and scanning electron micrographs of the silver nanoparticles produced in old and new glycerols. Interestingly, as shown in Figure 7, the morphologies of the silver nanoparticles synthesized in new (Figure 7b) and old (Figure 7c) glycerols are much different. When the silver nanoparticles were synthesized in new glycerol, the color of the solution was yellow (the inset of Figure 7b), which is the representative color of spherical silver nanoparticles with about 20 nm diameter. These results are also confirmed in the UV–vis spectrum (black solid line in Figure 7a) and the scanning electron micrograph (Figure 7b), in which the average diameter is 24 ± 11 nm. When the silver nanoparticles were produced in old glycerol, the color of the solution was gray (the inset of Figure 7c), which is quite different from the yellow solution in the inset of Figure 7b. Usually, the gray color represents that the particle size is large and inhomogeneous, as reflected in the broad UV–Vis spectrum (blue line in Figure 7a) and scanning electron micrograph of Figure 7c, in which the particle size is 94 ± 36 nm. In this experiment, the concentration of poly(vinylpyrrolidone) (PVP) in both old and new glycerols was 40% (w/v). Small amounts of PVP dissolve faster in glycerol, but the produced silver nanoparticles can be more easily aggregated (Supporting Figure 7). The reaction yields in old and new glycerols were 29 and 38%, respectively.
Figure 7.
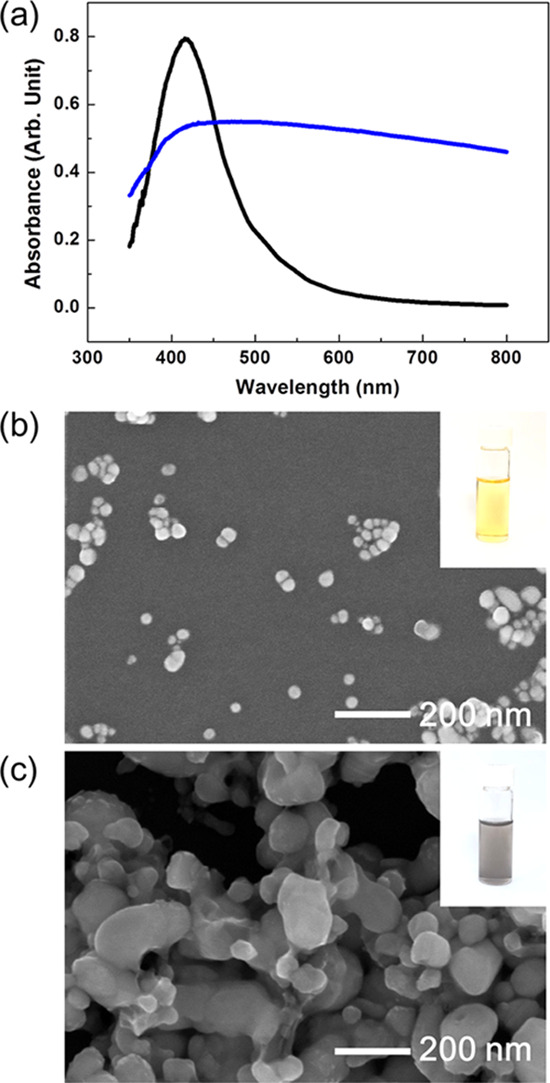
Silver nanoparticles with relatively homogeneous size distribution are synthesized by radicals, as represented by the black line in (a) and the electron micrograph (b). However, when the silver nanoparticles are formed by aldehydes, their size distribution is wide, as shown by the blue line in (a) and the electron micrograph (c). (Photograph courtesy of Tianhao Liu. Copyright 2020.).
More research is needed to determine the causes of the different sizes and uniformities of the nanoparticles in various types of glycerol, but we could suggest some explanation using the nucleation and growth theory of nanoparticles. According to the nucleation and growth theory of nanoparticles, the period for seed formation is an important factor determining the nanoparticle size and size distribution. When the seeds are rapidly formed during a short period, the size of the nanoparticle becomes small and the size distribution becomes narrow. Because the radical species is more reactive than aldehydes for the reduction process, seeds are formed in new glycerol more rapidly than in old glycerol. Rapid seed formation in the new glycerol could lead to the formation of small nanoparticles and narrow size distribution.
3. Conclusions
In this paper, we reported the unprecedented synthesis of silver nanoparticles in glycerol in which no extra energy and chemical additives are needed, and all chemical reactions are performed at room temperature in neutral pH conditions. Many research papers have reported the synthesis of silver nanoparticles in glycerol, but these studies were conducted under extreme conditions (e.g., high temperatures, ultraviolet light, or alkaline pH conditions). Because this is the first example demonstrating the synthesis of silver nanoparticles in glycerol under normal conditions, the synthesis mechanism should be different from those generally accepted. Moreover, it was found as a result of a mechanistic study that two different chemical species reduce silver ions in glycerol. One species is glyceraldehydes converted from glycerol by dissolved oxygen, and the other one is free radicals generated from glycerol primarily by ambient thermal energy. The concentration of free radicals is dependent on the amount of aldehydes because aldehydes could play the role of scavengers of free radicals. Notably, the nanoparticle size and size distribution are dependent on reducing chemical species. When silver ions are reduced mainly by aldehydes, large nanoparticles are formed and their size distribution is wide; however, when reduced by free radicals, small nanoparticles with a narrow size distribution are obtained. According to our results, small silver nanoparticles with a narrow size distribution can be synthesized in glycerol, but the glycerol should not contain aldehydes (e.g., glyceraldehydes). If we can effectively remove aldehydes from glycerol that has been exposed to air for a long time, small nanoparticles with a narrow size distribution are expected, even in old glycerol produced long ago.
4. Experimental Section
4.1. Chemicals and Apparatus
Silver nitrate (AgNO3, ≥99.0%, Aldrich), glycerol (C3H8O3, ≥99.0%, Daejung), and poly(vinylpyrrolidone) (PVP) (average MW 40 000, Aldrich) were used without further purification, and highly purified deionized (DI) water (18.2 MΩ cm) produced using QPAK1 (MILLI Pore) was used for all aqueous solutions to prepare the silver nanoparticles. Schiff’s reagent for aldehydes (Sigma) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) (95%, Alfa Aesar) were purchased and used as received to detect aldehydes and free radicals in the glycerol. All apparatuses were treated with a “piranha” solution (acidic solution mixed with concentrated sulfuric acid (H2SO4)) and 40% hydrogen peroxide (H2O2) at a 4:1 ratio (v/v) for at least 2 h and dried in an oven at 70 °C until completely dry.
4.2. Preparation of Silver Nanoparticles in Glycerol
When synthesizing silver nanoparticles in glycerol, silver nitrate typically has not been dissolved directly in glycerol due to its slow dissolution rate in a glycerol solvent. Instead, the silver nitrate (∼85 mg) was first dissolved in 0.1 mL water instead of glycerol (Scheme 2a), and then 0.1 mL of the silver nitrate aqueous solution was added to 4.9 mL of glycerol (Scheme 2b,c). The final mixed solution was transparent, as shown in the picture ((a)-1) in Scheme 2d. When placed on a shelf of the laboratory at room temperature (20–25 °C) for some hours (5–50 h), the color of the solution turns into a visible color, indicating the formation of silver nanoparticles. In this experiment, DI water and glycerol containing PVP in the required concentration were used instead of pure solvents to prepare stabilized silver nanoparticles. The PVP quickly dissolved in the water; however, it took a relatively long time (e.g., 2 h, 5 h, and 3 days for 0.4, 4, and 40% w/v, respectively) for PVP to be completely dissolved in glycerol at room temperature
4.3. Characterization
The formation of silver nanoparticles was confirmed by observing the color of the solutions and their UV–vis spectra obtained by a spectrophotometer (UH5300; Hitachi). The size distribution of the nanoparticles was obtained by measuring their diameters in electron micrographs taken by a scanning electron microscope (FE-SEM, S-4800; Hitachi) or a transmission electron microscope (TEM, JEM-3010; JEOL). Infrared (IR) spectroscopy and the colorimetric method were used for detecting aldehydes in glycerol. IR spectra were obtained for glycerol using an IR spectrometer (6700; Thermo Fisher Scientific) in attenuated total reflection (ATR) mode on zinc selenide with 200 accumulations. In the colorimetric method, Schiff’s reagent (1 mL), which is a transparent solution but its color changes to violet in the presence of aldehydes, was mixed with 2 mL of glycerol and the solution’s color change was monitored. The presence of free radicals in glycerol was also investigated using a colorimetric reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH) (95%, Alfa Aesar), which is a violet powder in ambient conditions. Because DPPH slowly dissolves in glycerol, ethanolic solutions of DPPH (0.1 mM) were first prepared and then added to the glycerol tested by a 1:1 ratio (v/v). The color of the solution changed from violet to yellow under the presence of free radicals.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2017R1E1A1A01075141).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02066.
UV–vis spectra and TEM image of silver nanoparticles prepared in glycerol without the addition of water; UV–vis spectra and a photo of silver nanoparticles prepared in glyceraldehyde aqueous solutions; UV–vis spectra and a photo of silver nanoparticles in glycerol under acidic condition; photos of the ethanol solution containing radical indicators taken at different time periods; UV–vis spectra for old and new glycerols taken before and after the addition of the radical indicator; photos and UV–vis spectra of glycerols after the addition of radical and aldehyde indicators; and UV–vis spectra and photos of silver nanoparticles generated in new glycerol containing different concentrations of PVP (PDF)
Author Contributions
The manuscript was written with the contribution of all authors. All authors have given approval for the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Quispe C. A. G.; Coronado C. J. R.; Carvalho J. A. Jr. Glycerol: production, consumption, prices, characterization and new trends in combustion. Renewable Sustainable Energy Rev. 2013, 27, 475–493. 10.1016/j.rser.2013.06.017. [DOI] [Google Scholar]
- Monisha J.; Harish A.; Sushma R.; Krishna M. T. P.; Blessy B. M.; Ananda S. Biodiesel: a review. Int. J. Eng. Res. Appl. 2013, 3, 902–912. [Google Scholar]
- Pagliaro M.; Ciriminna R.; Kimura H.; Rossi M.; Pina C. D. From glycerol to value-added products. Angew. Chem., Int. Ed. 2007, 46, 4434–4440. 10.1002/anie.200604694. [DOI] [PubMed] [Google Scholar]
- Ciriminna R.; Pina C. D.; Rossi M.; Pagliaro M. Understanding the glycerol market. Eur. J. Lipid Sci. Technol. 2014, 116, 1432–1439. 10.1002/ejlt.201400229. [DOI] [Google Scholar]
- Bagnato G.; Iulianelli A.; Sanna A.; Basile A. Glycerol production and transformation: a critical review with particular emphasis on glycerol reforming reaction for producing hydrogen in conventional and membrane reactors. Membranes 2017, 7, 17 10.3390/membranes7020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya P. D.; Rodrigues A. E. Glycerol reforming for hydrogen production: a review. Chem. Eng. Technol. 2009, 32, 1463–1469. 10.1002/ceat.200900120. [DOI] [Google Scholar]
- Wolfson A.; Dlugy C.; Shotland Y. Glycerol as a green solvent for high product yields and selectivities. Environ. Chem. Lett. 2007, 5, 67–71. 10.1007/s10311-006-0080-z. [DOI] [Google Scholar]
- Clark J. H.; Tavener S. J. Alternative solvents: shades of green. Org. Process Res. Dev. 2007, 11, 149–155. 10.1021/op060160g. [DOI] [Google Scholar]
- Díaz-Álvarez A. E.; Cadierno V. Glycerol: a promising green solvent and reducing agent for metal-catalyzed transfer hydrogenation reactions and nanoparticles formation. Appl. Sci. 2013, 3, 55–69. 10.3390/app3010055. [DOI] [Google Scholar]
- Gu Y.; Jérôme F. Glycerol as a sustainable for green chemistry. Green Chem. 2010, 12, 1127–1138. 10.1039/c001628d. [DOI] [Google Scholar]
- Sinha A.; Sharma B. P. Preparation of copper powder by glycerol process. Mater. Res. Bull. 2002, 37, 407–416. 10.1016/S0025-5408(01)00819-4. [DOI] [Google Scholar]
- Sinha A.; Sharma B. P. Preparation of silver powder through glycerol process. Bull. Mater. Sci. 2005, 28, 213–217. 10.1007/BF02711250. [DOI] [Google Scholar]
- Ullah M. H.; Il K.; Ha C.-S. Preparation and optical properties of colloidal silver nanoparticles at a high Ag+ concentration. Mater. Lett. 2006, 60, 1496–1501. 10.1016/j.matlet.2005.11.058. [DOI] [Google Scholar]
- Grace A. N.; Pandian K. One pot synthesis of polymer protected gold nanoparticles and nanoprisms in glycerol. Colloids Surf., A 2006, 290, 138–142. 10.1016/j.colsurfa.2006.05.015. [DOI] [Google Scholar]
- Grace A. N.; Pandian K. One pot synthesis of polymer protected Pt, Pd, Ag and Ru nanoparticles and nanoprisms under reflux and microwave mode of heating in glycerol–a comparative study. Mater. Chem. Phys. 2007, 104, 191–198. 10.1016/j.matchemphys.2007.03.009. [DOI] [Google Scholar]
- Nisaratanaporn E.; Wongsuwan K. Preparation of ultrafine silver powder using glycerol as reducing agent. J. Met. Mater. Miner. 2008, 18, 1–5. [Google Scholar]
- Skrabalak S. E.; Wiley B. J.; Kim M.; Formo E. V.; Xia Y. On the polyol synthesis of silver nanostructures: glycolaldehyde as a reducing agent. Nano Lett. 2008, 8, 2077–2081. 10.1021/nl800910d. [DOI] [PubMed] [Google Scholar]
- Chou K.-S.; Ren C.-Y. Synthesis of nanosized silver particles by chemical reduction method. Mater. Chem. Phys. 2000, 64, 241–246. 10.1016/S0254-0584(00)00223-6. [DOI] [Google Scholar]
- Fievet F.; Lagier J. P.; Blin B.; et al. Homogeneous and heterogeneous nucleations in the polyol process for the preparation of micron and submicron size metal particles. Solid State Ionics 1989, 32–33, 198–205. 10.1016/0167-2738(89)90222-1. [DOI] [Google Scholar]
- Benet W. E.; Lewis G. S.; Yang L. Z.; Hughes D. E. P. The mechanism of the reaction of the Tollens reagent. J. Chem. Res. 2011, 35, 675–677. 10.3184/174751911X13206824040536. [DOI] [Google Scholar]
- Sarkar A.; Kapoor S.; Mukherjee T. Synthesis and characterization of silver nanoparticles in viscous solvents and its transfer into non-polar solvents. Res. Chem. Intermed. 2010, 36, 411–421. 10.1007/s11164-010-0151-4. [DOI] [Google Scholar]
- Preuksarattanawut T.; Asavavisithchai S.; Nisaratanaporn E. Fabrication of silver hollow microspheres by sodium hydroxide in glycerol solution. Mater. Chem. Phys. 2011, 130, 481–486. 10.1016/j.matchemphys.2011.07.013. [DOI] [Google Scholar]
- Nalawade P.; Mukherjee T.; Kapoor S. Green synthesis of gold nanoparticles using glycerol as a reducing agent. Adv. Nanopart. 2013, 2, 78–86. 10.4236/anp.2013.22014. [DOI] [Google Scholar]
- Genç R.; Clergeaud G.; Ortiz M.; O’Sullivan C. K. Green synthesis of gold nanoparticles using glycerol-incorporated nanosized liposomes. Langmuir 2011, 27, 10894–10900. 10.1021/la201771s. [DOI] [PubMed] [Google Scholar]
- Gasparotto L. H. S.; Garcia A. C.; Gomes J. F.; Tremiliosi-Filho G. Electrocatalytic performance of environmentally friendly synthesized gold nanoparticles towards the borohydride electro-oxidation reaction. J. Power Sources 2012, 218, 73–78. 10.1016/j.jpowsour.2012.06.064. [DOI] [Google Scholar]
- Garcia A. C.; Gasparotto L. H. S.; Gomes J. F.; Tremiliosi-Filho G. Straightforward synthesis of carbon-supported Ag nanoparticles and their application for the oxygen reduction reaction. Electrocatalysis 2012, 3, 147–152. 10.1007/s12678-012-0096-z. [DOI] [Google Scholar]
- Kim M.; Son W.-S.; Ahn K. H.; Kim D. S.; Lee H.-S.; Lee Y.-W. Hydrothermal synthesis of metal nanoparticles using glycerol as a reducing agent. J. Supercrit. Fluids 2014, 90, 53–59. 10.1016/j.supflu.2014.02.022. [DOI] [Google Scholar]
- Gomes J. F.; Garcia A. C.; Ferreira E. B.; Pires C.; Oliveira V. L.; Tremiliosi-Filho G.; Gasparotto L. H. S. New insights into the formation mechanism of Ag, Au and AgAu nanoparticles in aqueous alkaline media: alkoxides from alcohols, aldehydes and ketones as universal reducing agents. Phys. Chem. Chem. Phys. 2015, 17, 21683–21693. 10.1039/C5CP02155C. [DOI] [PubMed] [Google Scholar]
- Huang Z. Y.; Mills G.; Hajek B. Spontaneous formation of silver particles in basic 2-propanol. J. Phys. Chem. A. 1993, 97, 11542–11550. 10.1021/j100146a031. [DOI] [Google Scholar]
- Jo J.; Cho S.-P.; Lim J. K. Template synthesis of hollow silver hexapods using hexapod-shaped silver oxide mesoparticles. J. Colloid Interface Sci. 2015, 448, 208–214. 10.1016/j.jcis.2015.02.026. [DOI] [PubMed] [Google Scholar]
- Abdelghany T. M.; Al-Rajhi A. M. H.; Al Abboud M. A.; Alawlaqi M. M.; Magdah A. G.; Helmy E. A. M.; Mabrouk A. S. Recent advances in green synthesis of silver nanoparticles nd their applications: about future directions, a review. BioNanoScience 2018, 8, 5–16. 10.1007/s12668-017-0413-3. [DOI] [Google Scholar]
- Smith B. C. Alcohols-the rest of the story. Spectroscopy 2017, 32, 19–23. [Google Scholar]
- García-Jiménez F.; Zúñiga O. C.; García Y. C.; Cárdenas J.; Cuevas G. Experimental and theoretical study of the products from the spontaneous dimerization of DL- and D-glyceraldehyde. J. Braz. Chem. Soc. 2005, 16, 467–476. 10.1590/S0103-50532005000300022. [DOI] [Google Scholar]
- Yaylayan V. A.; Harty-Majors S.; Ismail A. A. Investigation of DL-glyceraldehyde-dihydroxyacetone interconversion by FTIR spectroscopy. Carbohydr. Res. 1999, 318, 20–25. 10.1016/S0008-6215(99)00077-4. [DOI] [Google Scholar]
- Forney C. F.; Brandl D. G. Control of humidity in small controlled-environment chambers using glycerol-water solutions. HortTechnology 1992, 2, 52–54. 10.21273/HORTTECH.2.1.52. [DOI] [Google Scholar]
- Volk A.; Kähler C. J. Density model for aqueous glycerol solutions. Exp. Fluids 2018, 59, 75 10.1007/s00348-018-2527-y. [DOI] [Google Scholar]
- Mallick K.; Witcomb M. J.; Scurrell M. S. Polymer stabilized silver nanoparticles: a photochemical synthesis route. J. Mater. Sci. 2004, 39, 4459–4463. 10.1023/B:JMSC.0000034138.80116.50. [DOI] [Google Scholar]
- Inwati G. K.; Rao Y.; Singh M. In situ free radical growth mechanism of platinum nanoparticles by microwave irradiation and electrocatalytic properties. Nanoscale Res. Lett. 2016, 11, 458 10.1186/s11671-016-1653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.