Abstract
Introduction:
The Diabetes Prevention Program intensive lifestyle change program effectively reduces the risk of progression from prediabetes to type 2 diabetes but is underutilized. An implementation study used formative research to increase Diabetes Prevention Program referrals at a primary care clinic.
Study design:
A pragmatic, cluster randomized, mixed methods study.
Setting/participants:
Clusters were teams of primary care clinicians from two primary care clinics. The three intervention clusters had eight to 11 clinicians and the three control clusters had seven to 20 clinicians.
Intervention:
Implementation activities occurred from December 2017 to February 2019. Activities included targeted clinician education, a prediabetes clinician champion, and a custom electronic health record report identifying patients with prediabetes.
Main outcome measures:
The primary outcome was referral of patients with prediabetes to the institutional Diabetes Prevention Program. Study data, including patient demographic and clinical variables, came from the electronic health record. Interviews with clinicians evaluated the implementation strategies. Generalized estimating equation analyses that accounted for multiple levels of correlation and interview content analysis occurred in 2019.
Results:
Study clinicians cared for 2,992 patients with a prediabetes diagnosis or hemoglobin A1c (HbA1c) indicative of prediabetes (5.7%–6.4%). Clinicians in the intervention clusters referred 6.9% (87/1,262) of patients with prediabetes to the Diabetes Prevention Program and those in the control clusters referred 1.5% (26/1,730). When adjusting for patient age, sex, race, HbA1c value, HbA1c test location, and insurance type, intervention clinicians had 3.85 (95% CI=0.40–36.78) greater odds of referring a patient with prediabetes to the Diabetes Prevention Program. The 11 interviewed intervention clinicians had mixed opinions about the utility of the interventions, reporting as most helpful the prediabetes clinic champion (n=7, 64%) and educational presentations (n=6, 55%).
Conclusions:
Intervention clinicians were more likely to make Diabetes Prevention Program referrals; however, the study lacked power to achieve statistical significance. Clinician interviews suggested that intervention components that triggered Diabetes Prevention Program referrals varied among clinicians.
INTRODUCTION
Prediabetes, a condition of impaired blood glucose regulation, affects about one in three American adults.1 People with prediabetes are at increased risk of progressing to type 2 diabetes mellitus (T2DM) and developing microvascular and macrovascular disease.2 Effective lifestyle interventions reduce the risk of progression to T2DM in people with prediabetes by promoting healthy dietary patterns and improvement in physical activity leading to weight loss in participants.3 The National Diabetes Prevention Program (DPP) provides a framework for T2DM prevention efforts in the U.S.4 The DPP is a 12-month intensive lifestyle change program that meets as a small group to provide a structured core curriculum and individualized case management under the guidance of a trained diabetes prevention lifestyle coach. The landmark DPP trial showed that successful completion of the intensive lifestyle change program reduced the risk of progression to T2DM by 58% at 3 years5 with a lasting risk reduction of 27% at 15 years.6
Utilization of the DPP is low—estimates suggest that <1%–2.4% of patients who qualify for the program enroll.7–9 A variety of potential barriers to DPP utilization exist, including clinician perceptions and awareness,10,11 geographic access,12 health insurance coverage,11 patient identification,13 and patient acceptance of an intensive lifestyle change program.9 DPP utilization was similarly low in the healthcare system hosting this implementation study with clinician referral to the DPP nonexistent.14 The authors identified an opportunity to increase DPP utilization by intervening with clinicians because clinician referral has been associated with increased likelihood of patient DPP participation.9
This implementation study used elements of the Tailored Interventions for Chronic Diseases framework15 and the results of formative evaluations.14,16 Surveys completed by clinicians in the academic primary care clinic revealed that they believed prediabetes to be an important health issue, were knowledgeable about its diagnosis, but had limited awareness of DPPs.14 Review of electronic health record (EHR) data found high diabetes screening coverage per U.S. Preventive Services Task Force (USPSTF) guidelines (75.9%) and moderate documentation of the prediabetes diagnosis (50.7%).14 Focus groups of patients with prediabetes revealed that patients held mixed perceptions regarding the health risk associated with prediabetes, valued the opinions of their primary care clinicians, desired more detailed health behavior guidance, and were open to using the DPP, but had concerns about the cost and time commitment.16 The results of the formative evaluation guided tailored interventions with the intent to increase clinician referrals of patients with prediabetes to the DPP.
METHODS
This implementation study used a pragmatic cluster quasi-randomized study design with clusters defined by clinician teams. The sample size and study duration in this pilot were limited by study funding and sample size estimates were not calculated. The study included data from two primary care clinics within the healthcare system for a period of 13 months. Using Excel’s random sequence function, KLR randomized five clinician teams at a family medicine clinic, which was co-located with the DPP, to three intervention and two control clusters. A sixth cluster from an internal medicine primary care clinic within the same health system was added to the control groups to assess for study contamination and the effect of geographic proximity to the DPP. Neither the research team nor the clinicians were blinded regarding their status. The study occurred from December 2017 through February 2019. The primary outcome was referral of patients with prediabetes to the DPP. Secondary outcomes were diabetes screening coverage per USPSTF guidelines (age 40–70 years and BMI ≥25 kg/m2)17 and prediabetes diagnosis documentation. Guided interviews evaluated physician perception of the implementation process and utility of interventions.
Study Population
Interventions targeted primary care clinicians, including faculty physicians, resident physicians, advanced nurse practitioners, and a physician assistant at a large academic family medicine clinic. All clinicians actively caring for adult patients were included. The family medicine clinic was co-located in the same building as the DPP and the internal medicine clinic was in a separate location 2.3 miles distant. Patient inclusion criteria were age ≥18 years, primary care practitioner at the family medicine or internal medicine primary care clinic, and actively in care (at least one visit to a study clinic within 3 years of February 2019). Patients were classified as having prediabetes if their most recent HbA1c was 5.7%–6.4% and they did not have an ICD-10 code indicating a diagnosis of diabetes. The analysis also included patients with an ICD-10 prediabetes code in recognition that prediabetes is a dynamic condition and patients may have had an earlier HbA1c indicative of prediabetes whereas their most recent HbA1c result was normal.
Measures
Implementation activities occurred from December 2017 to February 2019. Figure 1 details the timing of the various interventions. As an initial step, an electronic DPP referral was created in the existing enterprise-wide EHR. Clinician survey18 and DPP implementation efforts at other institutions have found that electronic referrals increase DPP utilization.19,20 This referral was available to all clinicians within the health system and diabetes education staff presented the referral process and described diabetes education services to both intervention and control clinic clinicians at ―all provider‖ meetings prior to study onset. Electronic referrals were screened by a referral coordinator for patient DPP eligibility and eligible patients were called and invited to an informational session on the DPP. The study had three interventions intended to increase clinician DPP referrals: clinician education, a champion, and patient identification. These interventions were selected based on formative data that revealed under-documentation of prediabetes diagnosis and limited clinician knowledge about the DPP,14 their feasibility of implementation in the clinic setting, and evidence of effectiveness from other settings. To increase clinician DPP awareness and knowledge, intervention clinicians received prediabetes and DPP education between April and June 2018 via a routine clinic meeting, a dedicated 1-hour educational lunch session, and a follow-up e-mail that contained the materials from the educational lunch session. A prediabetes care champion (JWK, an active clinician) raised awareness, shared knowledge and created enthusiasm about the DPP via targeted conversations with clinician colleagues. A diabetes quality improvement initiative in Colorado found that primary care practices with change champions were better able to implement diabetes care improvements.21 A daily EHR report of patients meeting prediabetes HbA1c criteria or diabetes screening criteria (by USPSTF) who had a next-day appointment with a clinician in the intervention cluster was e-mailed to intervention clinicians the evening prior to the patient’s appointment. The report was e-mailed because the EHR used by the healthcare system did not support the creation of a prediabetes patient registry or the flagging of patients with prediabetes.
Figure 1.
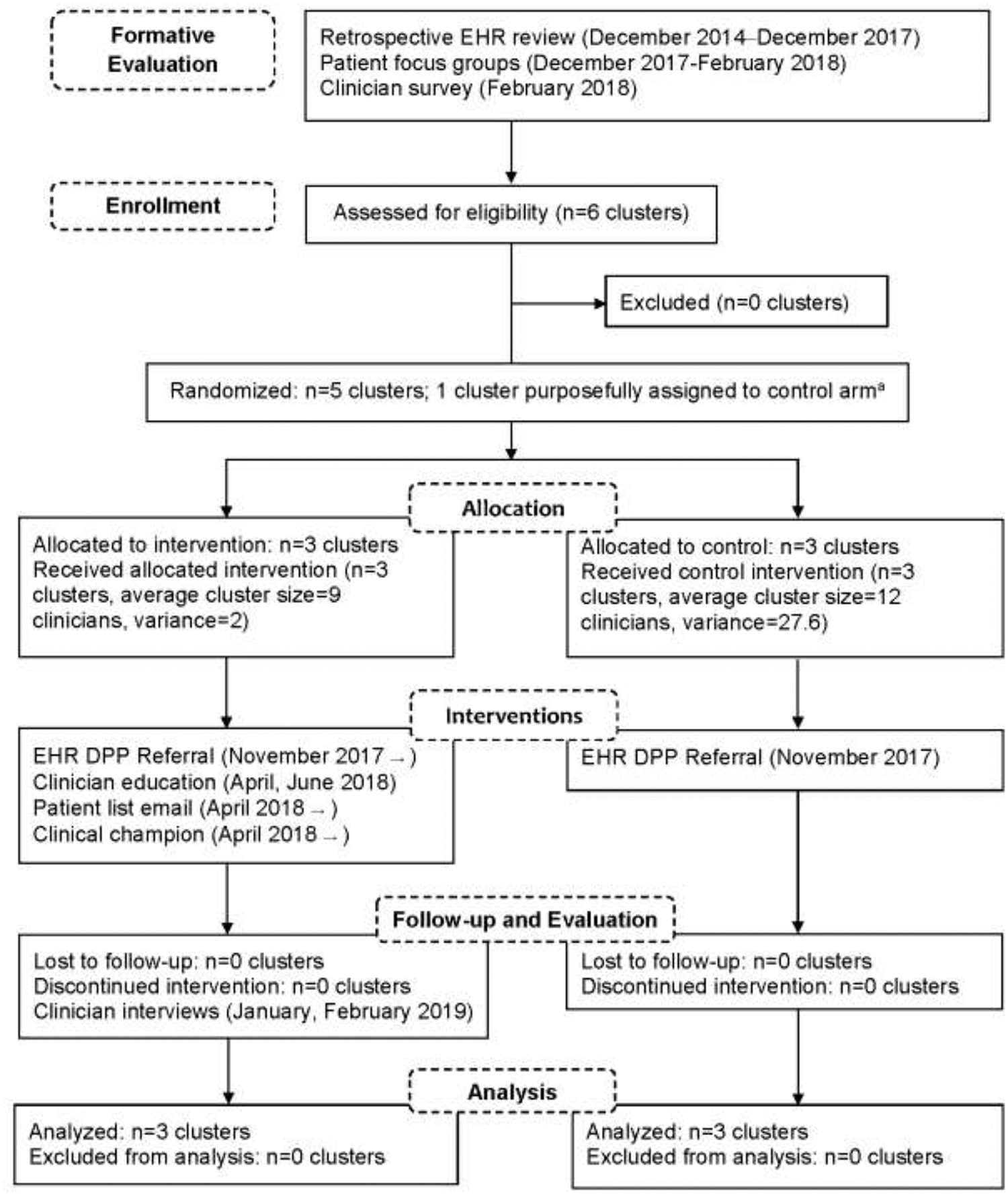
Flow diagram and implementation activities for cluster randomized study to increase Diabetes Prevention Program referrals in academic primary care clinics, 2017‒2019.
aA primary care clinic geographically distinct from the other clusters was purposefully assigned to the control arm to minimize study contamination.
DPP, Diabetes Prevention Program; EHR, electronic health record.
Statistical Analysis
The analysis used clinical data extracted from the EHR in March 2019. These data included patient demographics, treatment dates, laboratory values, prediabetes and diabetes diagnoses by ICD-10 classification, and DPP referrals. The analysis used an intention-to-treat approach with patient data analyzed by assigned primary care provider and not the treating clinician. Descriptive statistics of patient demographic and clinical variables were calculated. To account for clustering at three levels (clinician, team, clinic), generalized estimating equations22 were used to fit unadjusted and adjusted population-average models for the odds of DPP referral. The adjusted model used prespecified variables considered to influence DPP referral. The generalized estimating equation–type analyses utilized small sample corrections to SE estimates and degrees of freedom to ensure the validity of statistical inference.23–25 Two-sided p-values of <0.05 were considered significant. All statistical analyses were conducted in SAS, version 9.4 in 2019.
Intervention arm clinicians were invited to participate in a one-on-one guided interview at the end of the study. After obtaining consent, a trained interviewer (ART) interviewed clinicians in January and February 2019, taking notes during the interview in addition to audio recordings. The interview included a Likert scale paper survey about the utility of various intervention components, a semi-structured interview focusing on implementation effectiveness, and chart-stimulated recall26 to assess clinical decision making in prediabetes care. Chart-stimulated recall used recent patient encounters to stimulate discussion. Interviews occurred in a private space, such as the clinician’s office, and lasted about 45 minutes. Clinicians received $75 compensation for completing the interview. Two independent coders (ART, KLR) conducted thematic analysis of interview transcripts using QDA Miner Lite, version 2.0 to describe and organize interview themes in 2019.
This study was approved by the University of Kentucky IRB (#42484).
RESULTS
During the 13-month study, study clinicians cared for 2,992 unique patients with an HbA1c in the prediabetes range. Demographic and clinical characteristics were similar (Table 1) between intervention and control groups, although those in the intervention group on average were younger (56.7 vs 58.9 years), had a greater BMI (33.4 vs 32.8 kg/m2), and were less likely to have insurance coverage of the DPP (54.8% vs 65.6%).
Table 1.
Characteristics of Patients With Prediabetes by Clinician Intervention Status and Clinic Location
| Variable | Intervention FM (N=1262) | No intervention FM (N=744) | No intervention GIM (N=986) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| DPP referral | 87 (6.9) | 20 (2.7) | 6 (0.6) |
| Age, mean (SD, range) | 56.70 (0.38) (19.0–95.0) | 56.78 (0.51) (19.0–92.0) | 60.42 (0.44) (20.0–95.0) |
| BMI, mean (SD, range) | 33.36 (0.24) (15.5–75.5) | 33.23 (0.31) (15.5–86.2) | 32.39 (0.25) (16.5–80.4) |
| Sex | |||
| Male | 594 (47.1) | 313 (42.1) | 586 (59.4) |
| Female | 668 (52.9) | 431 (57.9) | 400 (40.6) |
| Race | |||
| White | 883 (70.0) | 538 (72.3) | 767 (77.8) |
| Black | 305 (24.2) | 172 (23.1) | 134 (13.6) |
| Other | 74 (5.9) | 34 (4.6) | 85 (8.6) |
| HbA1c value (mean, SD, range) | 5.82 (0.01) (4.3–8.8) | 5.78 (0.01) (4.3–7.1) | 5.80 (0.01) (3.9–6.9) |
| Prediabetes diagnosis, | 40 (3.2) | 36 (4.8) | 69 (7.0) |
| normal HbA1c | |||
| HbA1c location | |||
| Lab | 1,036 (84.8) | 615 (86.9) | 740 (80.7) |
| POC | 186 (15.2) | 93 (13.1) | 177 (19.3) |
| Insurance | |||
| Medicare | 327 (25.9) | 212 (28.5) | 351 (35.6) |
| Employera | 365 (28.9) | 219 (29.4) | 353 (35.8) |
| Other private | 366 (29.0) | 201 (27.0) | 200 (20.3) |
| Medicaid | 141 (11.2) | 80 (10.8) | 64 (6.5) |
| None | 63 (5.0) | 32 (4.3) | 18 (1.8) |
| Insurance pays for DPP | 692 (54.8) | 431 (57.9) | 704 (71.4) |
| Documented prediabetes diagnosis | 780 (61.8) | 515 (69.2) | 623 (63.2) |
Health insurance plan offered by university employer where study conducted.
DPP, Diabetes Prevention Program; FM, Family Medicine Clinic; GIM, General Internal Medicine clinic; POC, point of care test.
Clinicians in the intervention arm referred 6.9% (87/1,262) of patients with prediabetes to the DPP and those in the control arm referred 1.5% (26/1,730). In an unadjusted model, clinicians in the intervention group had 3.82 greater odds (95% CI=0.51, 28.80) of referring a patient with prediabetes to the DPP as compared with clinicians in the control group. When adjusting for patient age, sex, race, HbA1c value, HbA1c test location, and insurance payment for DPP, intervention clinicians had 3.85 (95% CI=0.40, 36.87) greater odds of referring a patient with prediabetes to the DPP. Patient characteristics associated with increased odds of DPP referral appear in Table 2 and included female sex (OR=1.63, 95% CI=0.98, 2.72), BMI (OR=1.15 per 5-unit increase, 95% CI=1.07, 1.23), and HbA1c value (OR=1.82 per 0.5-unit increase, 95% CI=1.08, 3.07). Patients receiving point-of-care (POC) HbA1c testing had 1.65 greater odds of receiving a DPP referral than those with laboratory-based HbA1c testing (95% CI=1.05, 2.58).
Table 2.
Univariate and Multivariate Analysis of Odds of Diabetes Prevention Program Referral by Patient Characteristics and Control Group
| Variable | All | FM control | GIM control | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Intervention | 3.82 (0.51, 28.80) | 0.14a | 2.51 (0.37, 17.17) | 0.23b | 12.14 (0.79, 187.77) | 0.059c |
| Age: 10-year increase | 0.89 (0.81, 0.99) | 0.037 | 0.90 (0.79, 1.02) | 0.072 | 0.90 (0.74, 1.10) | 0.16 |
| BMI: 5-unit increase | 1.15 (1.07, 1.23) | 0.005 | 1.15 (1.07, 1.24) | 0.009 | 1.16 (1.04, 1.28) | 0.023 |
| Female vs male | 1.63 (0.98, 2.72) | 0.057 | 1.57 (0.91, 2.68) | 0.077 | 1.53 (0.62, 3.78) | 0.18 |
| Race: Black vs white | 0.58 (0.32, 1.04) | 0.062 | 0.59 (0.32, 1.09) | 0.073 | 0.63 (0.30, 1.34) | 0.12 |
| Race: Others vs white | 0.61 (0.15, 2.51) | 0.39 | 0.40 (0.04, 3.59) | 0.27 | 0.73 (0.052, 10.05) | 0.65 |
| HbA1c location: POC vs lab | 1.65 (1.05, 2.58) | 0.037 | 1.59 (0.91, 2.78) | 0.078 | 1.80 (0.75, 4.34) | 0.10 |
| HbA1c: 0.5-unit increase | 1.82 (1.08, 3.07) | 0.033 | 1.84 (0.90, 3.73) | 0.072 | 1.83 (0.61, 5.43) | 0.14 |
| Insurance pay for DPP: yes vs no Adjusted modeld | 1.49 (0.96, 2.33) | 0.067 | 1.52 (0.89, 2.61) | 0.088 | 1.69 (0.87, 3.30) | 0.077 |
| Intervention | 3.85 (0.40, 36.87) | 0.17 | 2.48 (0.25, 24.43) | 0.29 | 13.62 (0.55, 335.65) | 0.073 |
Notes: Boldface indicates statistical significance (p<0.05).
2,992 observations are used in this analysis. Intraclass correlation coefficient (ICC) is 0.10 for outcomes from subjects having the same provider. The estimate is 0.02 for outcomes from subjects having the same team but different provider.
2,006 observations are used in the analysis restricted to the FM clinic. The ICC is 0.11 for outcomes from subjects having the same provider. The estimate is 0.005 for outcomes from subjects having the same team but different provider.
2,248 observations are used in the analysis restricted to the intervention clusters and the GIM clinic control cluster. The ICC is 0.12 for outcomes from subjects having the same provider. The estimate is 0.01 for outcomes from subjects having the same team but different provider.
Adjusted model includes age, BMI, sex, race, HbA1c value, HbA1c location, and insurance payment for DPP. 2,829, 1,916, and 2,125 observations are used for all locations, FM, and GIM, respectively.
FM, Family Medicine Clinic; GIM, General Internal Medicine; POC, point of care test.
Intervention effect size varied depending on the control group used for analysis (Table 2). Analysis restricted to family medicine clinic intervention and control clusters showed lower adjusted odds of DPP referral (OR=2.48, 95% CI=0.25, 24.43) than the intervention and internal medicine control group (OR=13.62, 95% CI=0.55, 333.65).
Appendix Table 1 shows the results for diabetes screening coverage and clinical documentation of prediabetes by intervention. Diabetes screening coverage per USPSTF guidelines was greater in the intervention group (65.5% vs 56.8%, p=0.42); however, there was no difference between groups when restricting the analysis to the family medicine clinic intervention and control clusters (66.3% and 66.4%, p=0.90). Clinical documentation of prediabetes by ICD-10 code was also greater in the intervention group (57.4% vs 51.2%, p=0.52). When restricted to the family medicine clinic, the difference decreased (57.5% vs 55.6%, p=0.84).
Limited data on DPP enrollment came from three cohorts that started the program between May 2018 and April 2019. Of the 28 enrolled patients, 15 were referred by intervention clinicians, three by control clinicians, six by self-referral, and four from other sources.
For the implementation evaluation, 11 clinicians (47.8%) participated in guided interviews. Participating clinicians referred 0%–67% of their patients with prediabetes to the DPP. Table 4 shows responses to survey questions regarding the utility of various implementation interventions. Respondents found most helpful the electronic DDP referral (n=11, 100%; includes agree and somewhat agree), prediabetes clinic champion (n=7, 64%), and educational presentations (n=6, 55%). Fewer clinicians felt the same prediabetes management education materials sent by e-mail were useful (n=2, 18%) or that e-mailed lists of patients with prediabetes changed their clinical practice (n=3, 27%).
The chart-stimulated recall portion of the interview revealed several factors that influenced the likelihood of DPP referral. The role of patient motivation differed by clinician, with some (n=3) reporting referral of more motivated patients whereas others (n=3) referred the less motivated ones. Similarly, insurance coverage of DPP services played a role for some providers but not for others. Clinician perception of their patients’ ability to participate in the DPP also influenced referrals, particularly patient access, resources, literacy, age, and comorbidities. Clinicians reported not offering DPP if there were more immediate needs to address, if their patient stated they were making lifestyle changes, or if they had previously declined DPP referral. Clinicians recommended several systems-level supports to improve prediabetes care: a standing order for HbA1c screening, automated addition of prediabetes diagnosis to the problem list based on the HbA1c result, and a prediabetes care plan EHR template. Clinicians desired further education on DPP services, program location, and insurance coverage.
DISCUSSION
Clinicians receiving the bundle of prediabetes care interventions, namely a clinic champion, targeted education, and e-mailed patient lists, were more likely to refer their patients with prediabetes to the DPP. This pilot study did not have the power to demonstrate a statistically significant intervention response; however, the effect size (OR=3.8) and clinician interviews suggested that the targeted interventions increased DPP referrals.
There is likely a DPP referral ceiling effect due to patient and system factors. The referral of 6.9% of eligible patients in the intervention group was likely tempered by the 10%–20% of patients with prediabetes receiving metformin therapy14 and the 45.2% of eligible patients who lacked insurance coverage of the DPP. Other patients offered DPP referral by their clinician may have declined for a variety of reasons, which were not measured in this study. An optimal referral proportion is not clear and will vary between patient populations.
There is little literature discussing DPP referral rates in other settings. Eligible patients reported a DPP referral frequency of 4.2% in the National Health Information Survey of 2016.8 A project between Federally Qualified Health Centers and the YMCA in the Bronx found that implementing an EHR referral plus clinician education increased referral frequency.20 The authors did not report denominators, preventing an understanding of the absolute increase in referral frequency. A collaborative effort between the American Medical Association and the YMCA to increase DPP referrals reported that clinics that used registries to identify eligible patients made more DPP referrals than practices using POC methods.27 This study also lacked denominator data on the number of patients eligible for referral, and referral rates were not described.
Intervention components were low-cost and sustainable, although the pilot nature of this study precluded formal costing analysis. Implementation costs were limited to staff time, primarily EHR report programming and the development and delivery of educational materials, which were discrete activities. These interventions are easily replicated in other settings. Of note, the existence of the institutional DPP predated this implementation study; other organizations considering prediabetes care initiatives without an institutional DPP would need to balance costs and benefits of starting a DPP versus partnering with a community-based DPP provider such as the YMCA. The institution where the pilot took place is transitioning to a new EHR platform that better supports population health efforts, and the authors plan to use this functionality to identify eligible patients and encourage clinician DPP referrals.
The subanalysis by control group location (family medicine or internal medicine) showed a larger intervention effect when the control group was restricted to the remotely located internal medicine clinic. There are several likely explanations for this finding. The control group clinicians in the family medicine clinic may have had greater awareness of the DPP because of its co-location in the same building. Study activities targeting intervention clinicians, particularly the clinic champion efforts, may have spilled over into the control clinicians in the family medicine clinic and mitigated the intervention effect. Additionally, clinician characteristics related to the likelihood of making a DPP referral may have varied between the two control group locations. The adjusted analysis controlled for patient characteristics associated with DPP referral making patient differences a less likely cause.
The prediabetes care interventions had little impact on diabetes screening and prediabetes diagnosis documentation. This may be explained by the fact that diabetes screening was already common in the family medicine clinic prior to this study.14 Although targeted education reviewed diabetes screening guidelines, increasing diabetes screening was not the primary focus. Similarly, although documentation of the prediabetes diagnosis is needed to place the electronic referral for the DPP, the implementation interventions did not emphasize this step in the prediabetes care cascade.
The positive influence of POC HbA1c screening on DPP referral is a new finding. Formative work showed an association between POC HbA1c testing and metformin prescription for patients with prediabetes.14 POC HbA1c has been shown to aid T2DM management resulting in more frequent treatment adjustments and better HbA1c control,28,29 but its utility in prediabetes care has not previously been reported. Two possible explanations for this association are: (1) clinicians using POC testing have different clinical practices or resources that make them more likely to refer patients to the DPP and (2) POC testing facilitates a face-to-face conversation and development of a treatment plan with the patient, resulting in increased DPP utilization. The latter explanation seems to underlie the benefit of POC HbA1c testing in T2DM management and may explain the increased likelihood of a DPP referral; further study will help elucidate its effect on prediabetes care.
Clinicians had mixed impressions on the utility of the various components of the intervention. All clinicians felt that the electronic DPP referral made it more likely that they would refer a patient. Of note, this electronic referral was available to all clinicians, not only those in the intervention, so other intervention components were necessary to increase DPP referrals. Clinicians generally felt that the prediabetes care champion (JWK) positively influenced their prediabetes care. All clinicians, control and intervention, at the family medicine clinic had some awareness of the study and the prediabetes care champion; only those clinicians working at the geographically distinct primary care internal medicine clinic had no interaction with the clinic champion. Although clinicians perceived the educational presentations and prediabetes patient lists as less helpful, these were more clearly restricted to the intervention clinicians. There was significant heterogeneity in DPP referral frequency within the intervention clinicians (not reported), and less-appreciated interventions, like the e-mailed prediabetes patient list, may have impacted DPP referral rates if they strongly influenced the clinicians referring the most patients.
Limitations
The pragmatic nature of the study raises some important limitations of the findings. There were few clusters because of the pilot nature of the study, which reduced study power and resulted in wide CIs. However, the statistical analysis considered the multiple levels of intraclass correlation to produce robust estimates of effect size. Missing diagnosis data in the EHR may have led to misclassification of diabetic patients with HbA1c results in the prediabetes range as eligible for DPP referral, which could decrease the apparent intervention effect. Incomplete demographic data prevented a comparison of clinician characteristics between groups; however, the cluster randomization design minimized selection bias. There was some contamination of the family medicine control group with certain intervention components—particularly the clinician champion. To counter this anticipated contamination, the study included an internal medicine primary care clinic as an additional control group and subanalyses suggested the location of the control group mediated the intervention effect. This pilot study occurred within an academic healthcare setting which may limit its generalizability to other locations.
The primary outcome of DPP referral did not assess whether patients enrolled and completed the DPP. DPP cohorts that started during or shortly after the study period showed proportionally more enrollees from intervention arm referrals (15 of 87) than control arm referrals (three of 26).
Caution is needed in interpreting these enrollment figures as referred patients may defer planned enrollment until a cohort is created that meets at a time convenient to the participant. The reported number of enrolled patients likely under-reports the total eventual enrollment of patients referred during the study period. Given the year-long duration of the DPP and the short time frame of this pilot study, assessing DPP completion was not feasible.
Using a package of interventions can obscure the relative effectiveness of each component. Post-intervention clinician interviews and case-stimulated recall helped to understand prediabetes clinical decisions and the potential effects of the intervention components.
CONCLUSIONS
The package of interventions influenced clinician behavior related to prediabetes care. The implementation evaluation suggested that different intervention components resonated with different clinicians, which supports using a multipronged implementation approach. Importantly, these results inform future interventions for increasing uptake of the evidence-based DPP.
Supplementary Material
Figure 2.
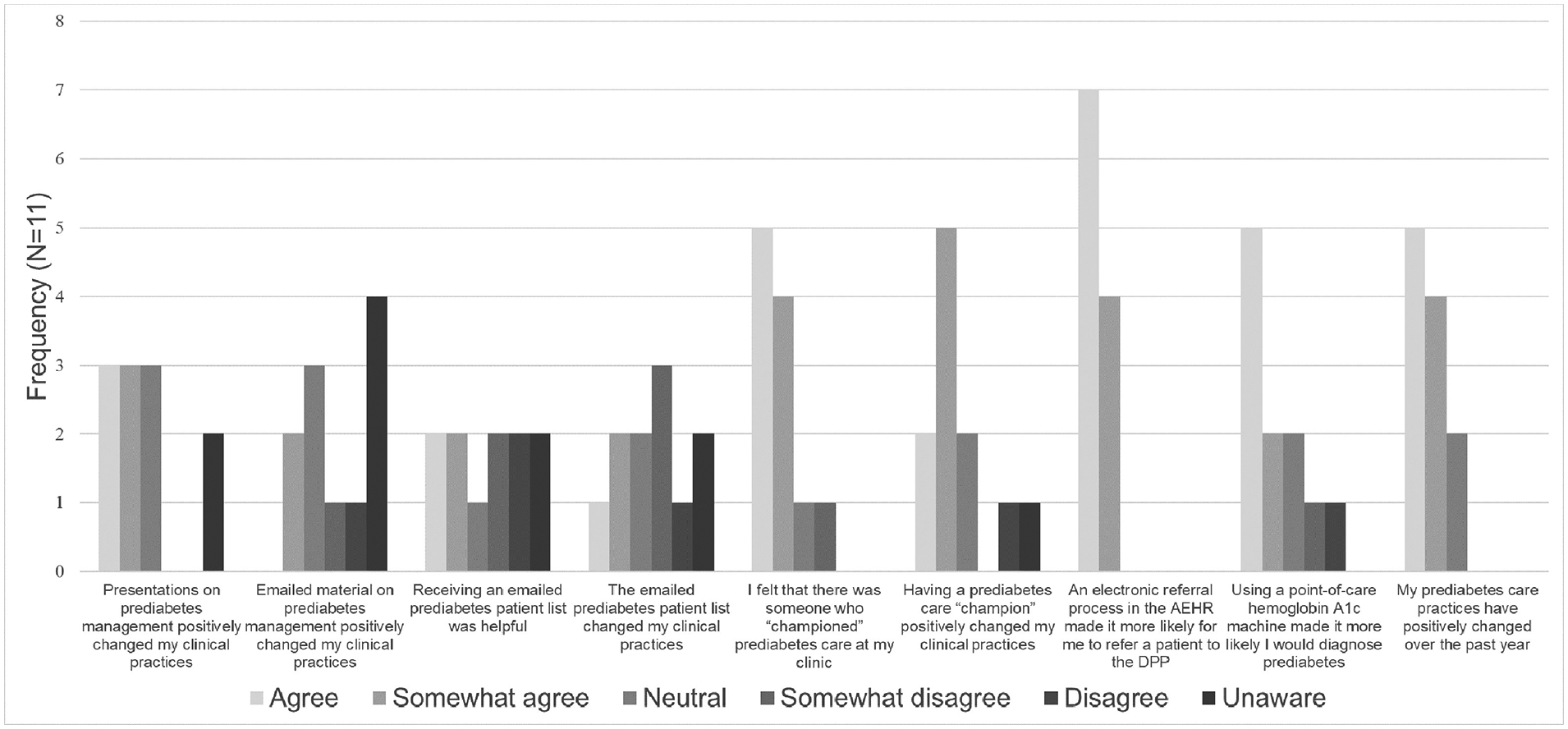
Intervention clinician survey responses on utility of implementation strategies to promote Diabetes Prevention Program referral.
AEHR, Allscripts electronic health record; DPP, Diabetes Prevention Program.
ACKNOWLEDGMENTS
The research presented in this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This publication was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through Grant UL1TR001998.
JWK, KLR, LBH, JLF, and RC conceived of and designed the study. JWK, KLR, ART, and LBH participated in data collection. ZH and PW conducted the statistical analyses. KLR and ART conducted the qualitative analysis. All authors drafted and critically reviewed the manuscript.
Elements of this manuscript were presented at the 2019 American Diabetes Association Scientific Sessions in San Francisco, CA.
JWK, KLR, ART, LBH, received intramural grant support from the University of Kentucky College of Medicine. The University of Kentucky College of Medicine had no role in the study design; data collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures were reported by the authors of this paper.
REFERENCES
- 1.CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: HHS; 2014. https://d2dstudy.org/wp-content/uploads/2012/04/national-diabetes-report-web.pdf. Accessed March 2, 2020. [Google Scholar]
- 2.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for developing diabetes. Lancet. 2012;379(9833):2279–2290. 10.1016/s0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemmingsen B, Gimenez-Perez G, Mauricio D, Roqué i Figuls M, Metzendorf MI, Richter B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2017; (12):CD003054 10.1002/14651858.cd003054.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albright A The National Diabetes Prevention Program: from research to reality. Diabetes Care Educ Newsl. 2012;33(4):4–7. www.ncbi.nlm.nih.gov/pubmed/26451082. Accessed October 26, 2018. [PMC free article] [PubMed] [Google Scholar]
- 5.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. 10.1056/nejmoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3(11):866–875. 10.1016/s2213-8587(15)00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmittdiel JA, Adams SR, Segal J, et al. Novel use and utility of integrated electronic health records to assess rates of prediabetes recognition and treatment: brief report from an integrated electronic health records pilot study. Diabetes Care. 2014;37(2):565–568. 10.2337/dc13-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venkataramani M, Pollack CE, Yeh H-C, Maruthur NM. Prevalence and correlates of Diabetes Prevention Program referral and participation. Am J Prev Med. 2019;56(3):452–457. 10.1016/j.amepre.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Ali MK, McKeever Bullard K, Imperatore G, et al. Reach and use of diabetes prevention services in the United States, 2016‒2017. JAMA Netw Open. 2019;2(5):e193160 10.1001/jamanetworkopen.2019.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mainous AG, Tanner RJ, Scuderi CB, Porter M, Carek PJ. Prediabetes screening and treatment in diabetes prevention: the impact of physician attitudes. J Am Board Fam Med. 2016;29(6):663–671. 10.3122/jabfm.2016.06.160138. [DOI] [PubMed] [Google Scholar]
- 11.Mensa-Wilmot Y, Bowen SA, Rutledge S, et al. Early results of states’ efforts to support, scale, and sustain the National Diabetes Prevention Program. Prev Chronic Dis. 2017;14:E130 10.5888/pcd14.170478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayapaul-Philip B, Dai S, Kirtland K, Haslam A, Nhim K. Availability of the National Diabetes Prevention Program in United States counties, March 2017. Prev Chronic Dis. 2018;15:E109 10.5888/pcd15.180063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll J, Winters P, Fiscella K, et al. Process evaluation of practice-based Diabetes Prevention Programs: what are the implementation challenges? Diabetes Educ. 2015;41(3):271–279. 10.1177/0145721715572444. [DOI] [PubMed] [Google Scholar]
- 14.Keck JW, Thomas AR, Hieronymus L, Roper KL. Prediabetes knowledge, attitudes, and practices at an academic family medicine practice. J Am Board Fam Med. 2019;32(4):505–512. 10.3122/jabfm.2019.04.180375. [DOI] [PubMed] [Google Scholar]
- 15.Flottorp SA, Oxman AD, Krause J, et al. A checklist for identifying determinants of practice: a systematic review and synthesis of frameworks and taxonomies of factors that prevent or enable improvements in healthcare professional practice. Implement Sci. 2013;8:35 10.1186/1748-5908-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roper KL, Thomas AR, Hieronymus L, Brock A, Keck J. Patient and clinician perceptions of prediabetes: a mixed-methods primary care study. Diabetes Educ. 2019;45(3):302–314. 10.1177/0145721719845347. [DOI] [PubMed] [Google Scholar]
- 17.Siu AL U S Preventive Services Task Force. Screening for abnormal blood glucose and type 2 diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(11):861–868. 10.7326/m15-2345. [DOI] [PubMed] [Google Scholar]
- 18.Nhim K, Khan T, Gruss SM, et al. Primary care providers’ prediabetes screening, testing, and referral behaviors. Am J Prev Med. 2018;55(2):e39–e47. 10.1016/j.amepre.2018.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rehm CD, Marquez ME, Spurrell-Huss E, Hollingsworth N, Parsons AS. Lessons from launching the Diabetes Prevention Program in a large integrated health care delivery system: a case study. Popul Health Manag. 2017;20(4):262–270. 10.1089/pop.2016.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chambers EC, Wylie-Rosett J, Blank AE, et al. Increasing referrals to a YMCA-based diabetes prevention program: effects of electronic referral system modification and provider education in federally qualified health centers. Prev Chronic Dis. 2015;12:E189 10.5888/pcd12.150294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw EK, Howard J, West DR, et al. The role of the champion in primary care change efforts: from the State Networks of Colorado Ambulatory Practices and Partners (SNOCAP). J Am Board Fam Med. 2012;25(5):676–685. 10.3122/jabfm.2012.05.110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- 23.Westgate PM. A readily available improvement over method of moments for intra-cluster correlation estimation in the context of cluster randomized trials and fitting a GEE–type marginal model for binary outcomes. Clin Trials. 2019;16(1):41–51. 10.1177/1740774518803635. [DOI] [PubMed] [Google Scholar]
- 24.Fay MP, Graubard BI. Small-sample adjustments for Wald-type tests using sandwich estimators. Biometrics. 2001;57(4):1198–1206. 10.1111/j.0006-341x.2001.01198.x. [DOI] [PubMed] [Google Scholar]
- 25.Ford WP, Westgate PM. A comparison of bias-corrected empirical covariance estimators with generalized estimating equations in small-sample longitudinal study settings. Stat Med. 2018;37(28):4318–4329. 10.1002/sim.7917. [DOI] [PubMed] [Google Scholar]
- 26.Sinnott C, Kelly MA, Bradley CP. A scoping review of the potential for chart stimulated recall as a clinical research method. BMC Health Serv Res. 2017;17:583 10.1186/s12913-017-2539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holliday CS, Williams J, Salcedo V, Kandula NR. Clinical identification and referral of adults with prediabetes to a diabetes prevention program. Prev Chronic Dis. 2019;16:180540 10.5888/pcd16.180540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cagliero E, Levina EV, Nathan DM. Immediate feedback of HbA1c levels improves glycemic control in type 1 and insulin-treated type 2 diabetic patients. Diabetes Care. 1999;22(11):1785–1789. 10.2337/diacare.22.11.1785. [DOI] [PubMed] [Google Scholar]
- 29.Ferenczi A, Reddy K, Lorber DL. Effect of immediate hemoglobin A1c results on treatment decisions in office practice. Endocr Pract. 2001;7(2):85–88. 10.4158/ep.7.2.85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


