Abstract
Background
Extended elevations of non-HDL cholesterol (non-HDL-C) across a lifespan are associated with increased risk of cardiovascular disease (CVD). However, optimal testing intervals to identify individuals with high lipid-related CVD risk are unknown.
Objectives
We determined the extent to which lipid levels in young adulthood predict future lipid trajectories and associated long-term CVD risk.
Methods
A sample of 2516 Framingham Offspring participants age 25–40 free of CVD and diabetes had their non-HDL-C progression modeled over 8 study examinations (mean follow-up 32.6 years) using group-based methods. CVD risk based on 25–30 years of follow up was evaluated using Kaplan-Meier analyses for those with mean non-HDL-C≥160 mg/dl (“high”) and <130 mg/dl (“low”) at the first two examinations. Levels of non-HDL-C for participants on lipid treatment were adjusted by non-parametric algorithm.
Results
Lipid levels trajectories were generally stable over the 30-year life-course; mean non-HDL-C measured in young adulthood were highly predictive of levels later in life. Individuals could be reliably assigned to high and low non-HDL-C groups based on two measurements collected between age 25–40. Overall, 80% of those with non-HDL-C≥160 mg/dl at the first two exams remained in the “high” group on subsequent 25-year testing whereas 88% of those with non-HDL-C <130 mg/dl remained below 160 mg/dl. Those with high non-HDL-C in young adulthood had a 22.6% risk of CVD in the next 25 years as compared with a 6.4% risk in those with low non-HDL-C.
Conclusions
Most adults with elevated non-HDL-C early in life continue to have high non-HDL-C over their life course, leading to significantly increased risk of cardiovascular disease. Our results demonstrate that early lipid monitoring before age 40 would identify a majority of those with high likelihood for lifetime elevated lipid levels who also have a high long-term risk for CVD. This information could facilitate informed patient-provider discussion about the potential benefits of preventive lipid lowering efforts during the early midlife period.
Keywords: non-HDL cholesterol, lipids, cardiovascular disease prevention
Condensed Abstract
Extended elevations of non-HDL cholesterol during midlife are associated with increased risk of cardiovascular disease. However, optimal testing intervals to identify individuals with high lipid-related CVD risk are unknown. We determined the extent to which lipid levels in young adulthood predict future lipid trajectories and associated long-term CVD risk using data from Framingham Heart Study Offspring Cohort. We have shown that most adults with elevated non-HDL-C early in life continue to have high non-HDL-C over their midlives, leading to significantly increased risk of cardiovascular disease during this period.
Introduction
Current American College of Cardiology/American Heart Association (ACC/AHA) lipid guidelines suggest that selection for primary prevention of cardiovascular disease (CVD) should be based principally on the patient’s individual 10 year predicted risk of a CVD event over the next decade. (1) This recommendation is based on a wealth of data supporting the concept that the benefit of lipid-lowering is greater in those with higher baseline cardiovascular risk than in those with lower cardiovascular risk. However, age is the major determinants of cardiovascular risk and therefore, few young patients are eligible for primary prevention until after age 60 despite perhaps having very high risk lipid profiles. (2) This likely represents a missed opportunity for prevention for two reasons. First, just under half of all cardiovascular events occur before age 60. Second, much of the atherosclerotic disease that produces events in those over 60 began and matured in those under 60. (3) Yet how to identify potential candidates for LDL lowering therapy to prevent premature cardiovascular events remains a challenge.
The causal exposure model of atherosclerosis (4) posits that cardiovascular risk is based on the extent and severity of atherosclerosis, which, in turn, is, to a major degree, a direct function of years of exposure to the major causes of atherosclerosis- hyperlipidemia, hypertension, diabetes and smoking. The preventive strategy this model suggests is to identify individuals, who have risks for CVD early in life and intervene. (5)
To identify the optimal point where primary prevention through cholesterol lowering might start, it is critical to establish to what extent cholesterol levels before age 40 determine the future trajectories of lipid health in later life. Accordingly, we studied the life-course experience of participants enrolled at age 30 in the Framingham Offspring Cohort and followed for over 30 years to determine how reliably elevations of cholesterol in early adulthood point to persistent elevations in midlife as well as the risk of premature cardiovascular events over that period. We also determined how likely it is that individuals with low levels of cholesterol early in their adult life course will develop high levels later in life and what was their level of risk for CVD events was during this period.
Methods
Study Sample
Participants from the Framingham Offspring cohort have been previously described. (6) Our study population included women and men age 25 to 40 who attended examination 1 (1971–1975) or 2 (1979–1983) of the Offspring cohort1. Individuals with baseline assessment at first examination were followed up to examination 8 (2005–2008) and those with baseline assessment at examination 2 were followed up to exam 9 (2011–2014). Participants with prevalent cardiovascular disease or diabetes mellitus at either of the first two examinations were excluded from all analyses. Patients with non-missing records at baseline and at least one follow-up examination for non-HDL C and for lipid treatment were included. Of 2847 participants aged 25 to 40 at baseline, 2516 individuals were analyzed (88 patients excluded due to prior CVD event, 30 due to diabetes mellitus, 52 have missing records at baseline and 161 patients did not have follow-up records for non-HDL C and lipid treatment). Because participants with LDL-C≥190 mg/dL are already recommended for lipid-lowering treatment by most current cholesterol guidelines (1), analyses investigating clinical impact of elevated cholesterol in the early adulthood, excluded these participants (158 individuals).
Definitions of cardiovascular disease events (myocardial infarction, angina, coronary insufficiency, stroke, transient ischemic attack, intermittent claudication or congestive heart failure), their collection and adjudication in the Framingham Heart Study have been described previously. (6) Body Mass Index (BMI) was calculated as weight (kilograms) divided by the square of height (meters). Blood was obtained after a 12-hour fast. Plasma cholesterol and triglyceride concentrations were measured by enzymatic methods and HDL C after precipitation of apoB lipoproteins with dextran-sulfate magnesium reagent. Non-HDL C was calculated as total cholesterol minus HDL C. Systolic and diastolic blood pressure (SBP, DBP) were recorded as the average of two physician measurements during the examination. Hypertension was defined as a systolic/diastolic blood pressure ≥140/90 mmHg or current use of antihypertensive medication. Diabetes mellitus (DM) was defined as fasting blood glucose ≥ 126 mg/dl or receiving glucose-lowering treatment. Dyslipidemia was defined as non-HDL C ≥ 160 mg/dl or the use of lipid-lowering medications. All participants in the Framingham Heart Study provided informed consent. This analysis protocol was approved by the McGill University Health Center Institutional Review Board and all participants gave written informed consent.
Data analysis
Baseline characteristics of the study cohort were expressed as means and standard deviation or number and percentages for continuous and categorical variables, respectively. Data for non-HDL C outcomes were visually inspected for normality before analyses. Levels of non-HDL C for participants on lipid treatment were adjusted by non-parametric algorithm to approximate what the values would have been in the absence of treatment. (7, 8) The algorithm follows a logic similar to that adopted by the Kaplan-Meier estimator used to estimate survival in the presence of right censoring. The robustness of our findings was further assessed in sensitivity analyses which considered adjustment for lipid lowering treatment which assumes LDL-C reduction of 40% and thus increases the on-treatment LDL-C level by 1/(1–0.40)=1.67. Additional analyses were performed among individuals who had non-missing records at all follow-up exams, and further, among participants who were not on lipid lowering medications on follow-up examination cycles.
Lipid level progression over time was analyzed using group-based modelling for normally distributed data (9, 10) (SAS Proc Traj). This method, also known as trajectory analysis, identifies clusters of individuals based on changes in their non-HDL C through the entire follow-up. We chose this data-driven approach to determine to how cholesterol levels change over time and to what extent levels in early adulthood inform us about future levels and trajectories. The model assumes a number of subgroups exist in the population, which are characterized based on their longitudinal outcome patterns. Each individual is then assigned to one of the groups. In this method, model selected subpopulations are determined through the semi-parametric method and should not be considered actual categories, but rather an approximation of an underlying continuous process. (11) In this study, the group-based model assigned individuals to one of the three categories of low, medium and high non-HDL C based on their longitudinal trajectory of non-HDL C over eight exams. (12, 13)
Because trajectory analysis revealed strong associations between cholesterol levels in early and later adulthood, we sought to translate these results to clinical practice. For this purpose, we defined the “high” non-HDL C group as those whose mean non-HDL C level at their first two examinations is greater or equal to 160 mg/dl (approximately the 10th percentile of the high trajectory group) and the low lipid group as those whose first two-examination mean was less than 130 mg/dl (the 88th percentile of the low trajectory group). We opted for cholesterol levels that are familiar in clinical practice (“round numbers”) but they also happened to correspond to levels that separate our high and low trajectories. Then, we looked at the distributions of mean non-HDL C, over the subsequent examinations (from the third through the eighth).
We further investigated the impact of lipid levels on future cardiovascular events by estimating CVD incidence from exam 2 (Kaplan-Meier rates) over the full follow-up (through year 2014) in participants who would not be selected for treatment in the current guidelines (further excluding those with LDL-C≥190 mg/dl). As an additional sensitivity check, the analyses were repeated for the non-HDL C categories computed from the first three exams. Furthermore, we applied Cox proportional hazards model adjusted for standard cardiovascular risk factors (age, sex, SBP, hypertension treatment and smoking status) at second exam to estimate the increase in relative risk of CVD between the “high” and “low” non-HDL C groups. Finally, we estimated the potential benefit from statin treatment in the “high” non-HDL-C group using the approach described by Thanassoulis et al. (14) This approach multiplies the 25-year CVD event rate by expected risk reduction assuming maintained LDL-C lowering of 40%. All analyses were performed on SAS v.9.4 (SAS Institute, Cary, NC) and R software v.3.2.5 (R Foundation, Vienna).
Results
Subject characteristics
The baseline characteristics of the study cohort are reported in Table 1. The average age of participants at baseline was 31.4 ± 4.1 and 47.3% of the sample were men. At the last follow-up examination, the mean age of participants was 64.0 years. At baseline, 26.8% subjects were dyslipidemic (non-HDL C ≥ 160 mg/dl) and 11.3% had hypertension. Mean total and non-HDL C levels were 190.1 ± 35.2 and 138.9 ± 38.1, respectively.
Table1:
Baseline characteristics of participants. Participants with prevalent CVD or diabetes at baseline excluded from the analyses.
| Characteristics | Non-HDL3 < 130 mg/dL (N=990) | Non-HDL 130–160 mg/dL (N=781) | Non-HDL ≥ 160 mg/dL (N=745) | Total Analytical Sample (N=2516) |
|---|---|---|---|---|
| Age at baseline (years) | 30.8 ± 3.9 | 31.2 ± 4.1 | 32.4 ± 4.1 | 31.4 ± 4.1 |
| Age at follow-up (years) | 63.5 ± 4.1 | 63.8 ± 4.3 | 65.0 ± 4.4 | 64.0 ± 4.3 |
| Men (%) | 29.1 | 49.0 | 69.5 | 47.3 |
| Average follow-up (years)* | 28.3 ± 7.0 | 27.4 ± 8.1 | 24.9 ± 9.3 | 27.0 ± 8.2 |
| Body Mass Index (kg/m2) | 23.2 ± 3.6 | 24.7 ± 4.3 | 26.4 ± 4.2 | 24.6 ± 4.2 |
| Dyslipidemia (%) | 0 | 8.6 | 81.6 | 26.8 |
| Hypertension (%) | 6.2 | 10.4 | 19.1 | 11.3 |
| Diabetes (%) | 0.0 | 0.0 | 0.0 | 0.0 |
| Treatment for Blood Pressure (%) | 0.5 | 1.3 | 1.9 | 1.2 |
| Treatment for Hypercholesterolemia (%) | 0 | 0 | 0.1 | 0.0 |
| Systolic Blood Pressure (mmHg) | 114.4 ± 12.3 | 117.8 ± 13.7 | 121.8 ± 13.7 | 117.7 ± 13.5 |
| Diastolic Blood Pressure (mmHg) | 73.6 ± 8.8 | 76.0 ± 9.8 | 79.6 ± 10.0 | 76.1 ± 9.8 |
| Total Cholesterol (mg/dL) | 162.1 ± 20.4 | 190.2 ± 18.2 | 227.2 ± 29.2 | 190.1 ± 35.2 |
| HDL Cholesterol (mg/dL) | 57.0 ± 14.7 | 50.3 ± 13.9 | 44.6 ± 13.2 | 51.2 ± 14.9 |
| Non-HDL Cholesterol (mg/dL) | 105.2 ± 17.5 | 139.9 ± 14.6 | 182.6 ± 29.0 | 138.9 ± 38.1 |
| LDL Cholesterol (mg/dL) | 92.4 ± 17.0 | 122.9 ± 15.2 | 158.0 ± 27.8 | 121.2 ± 33.8 |
| Triglycerides (mg/dL) | 63.8 ± 31.0 | 85.1 ± 47.6 | 121.0 ± 60.0 | 87.2 ± 51.9 |
means and standard deviations for continuous measurements; percent for categorical data.
Participants from Offspring exam 1 (follow-up to exam 8) and participants from Offspring exam 2 (follow-up to exam 9, only subjects who did not attend exam 1).
Participants with available non-HDL cholesterol and lipid treatment data.
Average follow-up for CVD survival time from second exam.
Non-HDL values for participants on treatment inflated by non-parametric adjustment.
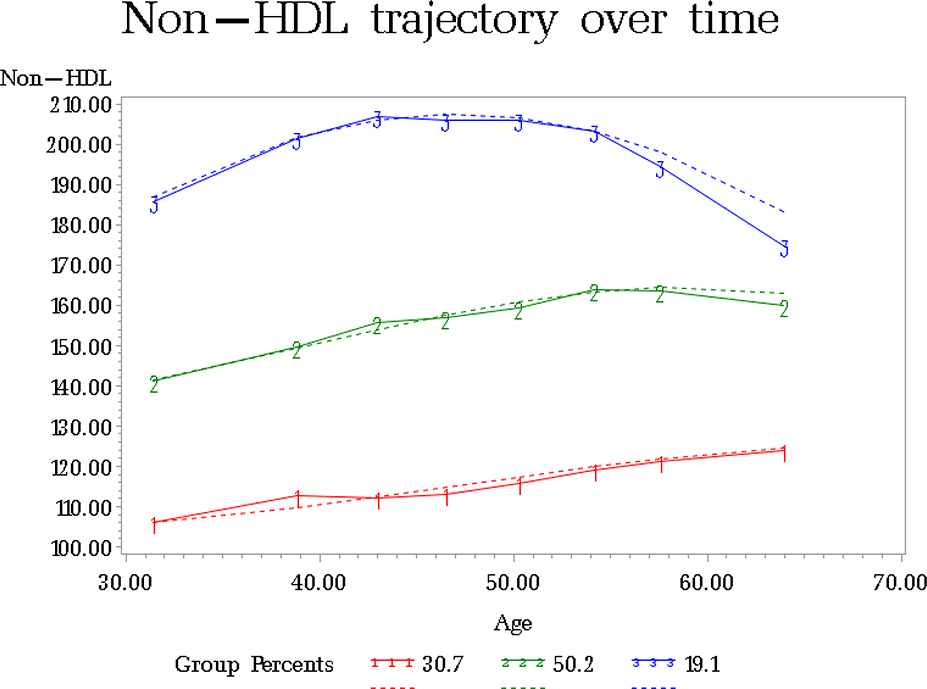
Our group-based model identified three mutually-exclusive cholesterol trajectories. It assigned individuals based on their non-HDL-C levels at baseline and 7 subsequent examinations (average follow-up of 32.6 years). Estimated (dotted line) and crude (solid line) changes of non-HDL-C levels over time, adjusted for lipid treatment on follow-up, are presented in Figure 1.
Figure 1:
Average non-HDL cholesterol levels over time for subgroups of participants clustered according to group-based model estimation.
Group-based model estimation was produced using Traj Procedure in SAS software. Dotted lines indicate estimated values and solid lines crude changes over time. Non-HDL values for participants on treatment inflated by non-parametric adjustment.
The top trajectory included 19.1% of all individuals. Their starting mean non-HDL-C was 186.6 ± 34.1 mg/dl and remained elevated throughout the follow-up, reaching 194.5 ± 31.5 mg/dL at the second to last (examination 7) and 175.0 ± 27.0 mg/dl at the last visit (examination 8, Table 2). Notably, 53.0% of these individuals were on statin therapy at examination 7 and 80.8% at examination 8. The middle trajectory was the largest, including 50.2% individuals, with mean baseline adjusted non-HDL-C of 141.4 ± 25.2 mg/dl. Non-HDL-C levels increased linearly in this subgroup, reaching 160.1±20.7 mg/dl at the end of follow-up (Table 2). The last subgroup included 30.7% of individuals with the lowest cholesterol levels. At baseline their mean non-HDL-C was 105.4 ± 21.2 mg/dl and it remained low throughout follow-up, reaching 123.4 ± 25.5 mg/dl at the last examination. Statin usage among these individuals at the last two examinations was 1.21% and 10.4%, respectively.
Table 2:
Non-HDL cholesterol (mg/dL) levels and percent of participants on lipid treatment at exams 1 to 8 across model-based categories.
| Category | Sample Size | Follow-up | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Exam 1 | Exam 2 | Exam 3 | Exam 4 | Exam 5 | Exam 6 | Exam 7 | Exam 8 | ||
| Low | N=766 | 105.4±21.2 | 112.2±20.7 | 111.9±21.1 | 112.8±21.1 | 115.4±21.2 | 118.6±22.4 | 120.9±24.7 | 123.4±25.5 |
| 0.0% | 0.0% | 0.0% | 0.1% | 0.2% | 0.3% | 1.2% | 10.4% | ||
| Mid | N=1278 | 141.4±25.2 | 149.9±23.8 | 155.9±24.1 | 157.0±23.1 | 159.5±21.9 | 164.0±24.2 | 163.5±24.4 | 160.1±20.7 |
| 0.1% | 0.1% | 0.0% | 0.5% | 1.6% | 7.0% | 17.5% | 49.4% | ||
| High | N=472 | 186.6±34.1 | 201.8±32.7 | 207.5±29.2 | 207.1±33.2 | 206.6±44.6 | 203.8±32.0 | 194.5±31.5 | 175.0±27.0 |
| 0.0% | 0.8% | 1.7% | 8.5% | 18.9% | 32.1% | 53.0% | 80.8% | ||
| Age at exam (years) | 31.4±4.1 | 38.8±4.5 | 43.0±4.8 | 46.5±4.7 | 50.3±4.6 | 54.2±4.8 | 57.7±4.2 | 64.0±4.3 | |
means and standard deviations for non-HDL cholesterol and age data. Non-HDL values for participants on treatment inflated by non-parametric adjustment.
The clear separation of trajectories in early adulthood and the relative stability of observed lipid levels prompted us to determine if these findings can be translated into simple, clinically actionable insights. Accordingly, we investigated to what extent lipid measurements before age 40 can reliably predict future non-HDL-C levels. After excluding those with LDL-C≥190 mg/dL (already recommended for lipid-lowering treatment by most guidelines), we classified individuals as “high” non-HDL-C if the mean of their first two measurements (the first one at mean age 31.4 and the second at mean age 38.8) was at or above 160 mg/dl, a level roughly corresponding to the 10th percentile of non-HDL C in the highest group identified by the trajectory group modeling (that is about 90% of all non-HDL C measurements in the highest trajectory exceeded 160 mg/dl). Those whose mean non-HDL C was below 130 mg/dl were classified as “low”. The 130 mg/dl level corresponded to the 88th percentile of non-HDL C in the lowest group identified by trajectory modeling (that is about 88% of all non-HDL C measurements in the lowest trajectory were below 130 mg/dl). We then calculated the mean non-HDL-C levels over the remaining life-course (the third through eighth examination) and investigated their distribution among individuals classified as “high” and “low”.
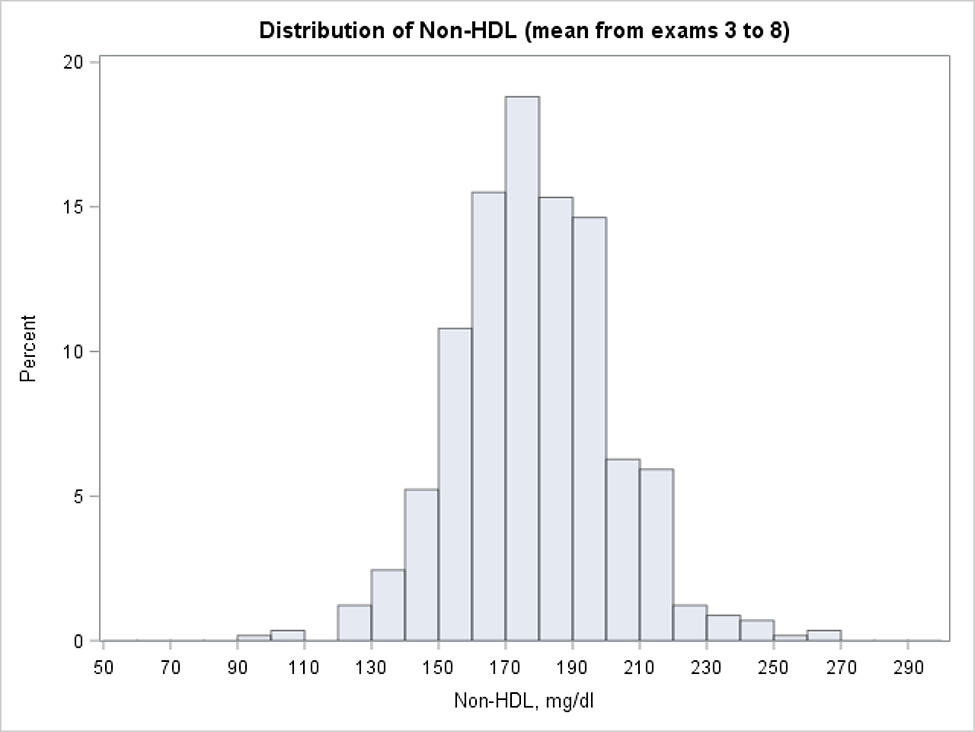
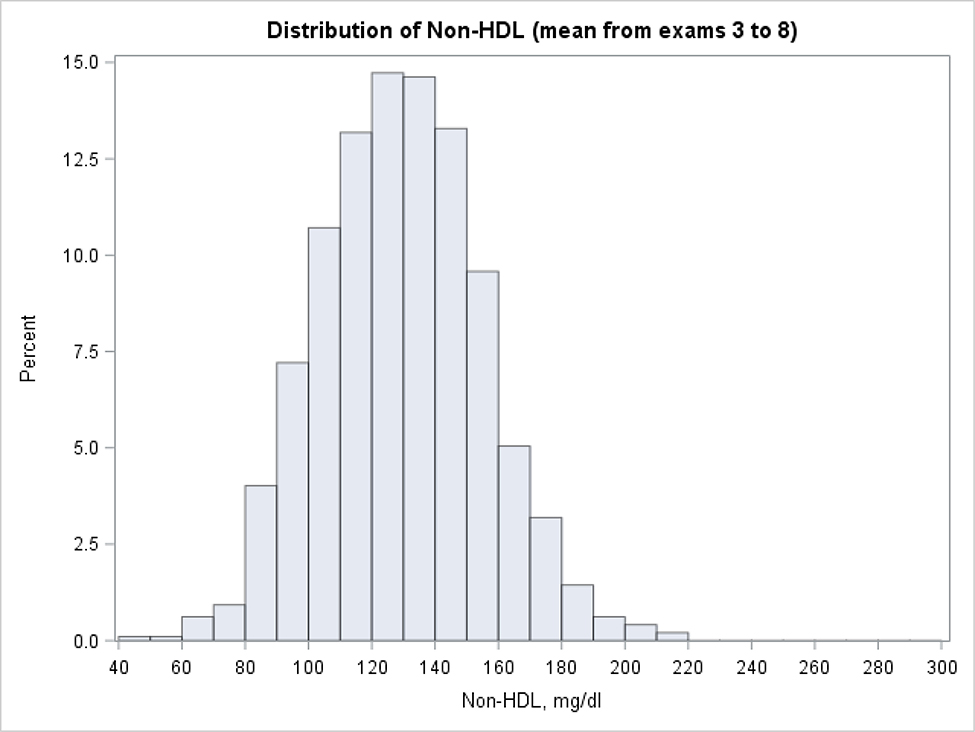
Using this approach 24.9% individuals were classified as “high young adulthood non-HDL-C” and 33.1% were classified as “low young adulthood non-HDL-C”. The histograms with the distribution of mean non-HDL-C levels for the “high” and “low” groups are presented in Figure 2 and 3 (respectively) and the percentiles of the distribution are given in Table 3. More than 80% of individuals with “high” non-HDL-C average at the first two examinations had long-term mean of non-HDL-C exceeding 160 mg/dl. In the cohort with “low” mean non-HDL-C at the first two examinations, the great majority (88%) had a mean non-HDL-C which did not cross 160 mg/dL. When the analyses were repeated with the mean non-HDL-C level based on the first 3 examinations, the mean non-HDL-C at examinations 4–8 also exceeded 160 mg/dL in 80% individuals among those with “high” mean at the first three measurements. The mean from examinations 4–8 remained below 160 mg/dl among 90% of those with “low” mean at the first three exams. Agreement between participants’ group assignment to non-HDL-C categories, when first two or three measurements were taken into account, was very high (reclassification between categories < 7.5%).
Figure 2:
Distribution of long term non-HDL cholesterol levels among subjects with high lipid values at first two exams (mean from exam 1 and 2 ≥ 160 mg/dl, participants with LDL-C≥=190 at first two exams excluded).
Long term non-HDL levels calculated as a mean value from exams 3 to 8. Non-HDL values for participants on treatment inflated by non-parametric adjustment.
Figure 3:
Distribution of long term non-HDL cholesterol levels (mean from exams 3 to 8) among subjects with low lipid values at first two exams (mean from exam 1 and 2 < 130 mg/dl, participants with LDL-C≥190 at first two exams excluded).
Long term non-HDL levels calculated as a mean value from exams 3 to 8. Non-HDL values for participants on treatment inflated by non-parametric adjustment.
Table 3:
Distribution of long term means of non-HDL cholesterol among subjects with low (< 130mg/dl) and high (≥ 160mg/dl) levels at early exams (participants with LDL-C>=190 at first two exams excluded)
| Subgroup | Percentiles | ||||||
|---|---|---|---|---|---|---|---|
| 5th | 10th | 25th | 50th | 75th | 90th | 95th | |
| <130 mg/dl | 87.8 | 97.0 | 111.2 | 129.1 | 146.8 | 161.3 | 173.4 |
| ≥160 mg/dl | 145.8 | 150.5 | 162.6 | 177.0 | 192.8 | 208.9 | 216.7 |
Percentiles of non-HDL cholesterol levels in mg/dl units. Categories computed based on the mean from the first two exams. Long-term non-HDL levels calculated as the mean from exams 3 to 8. Non-HDL values for participants on treatment inflated by non-parametric adjustment.
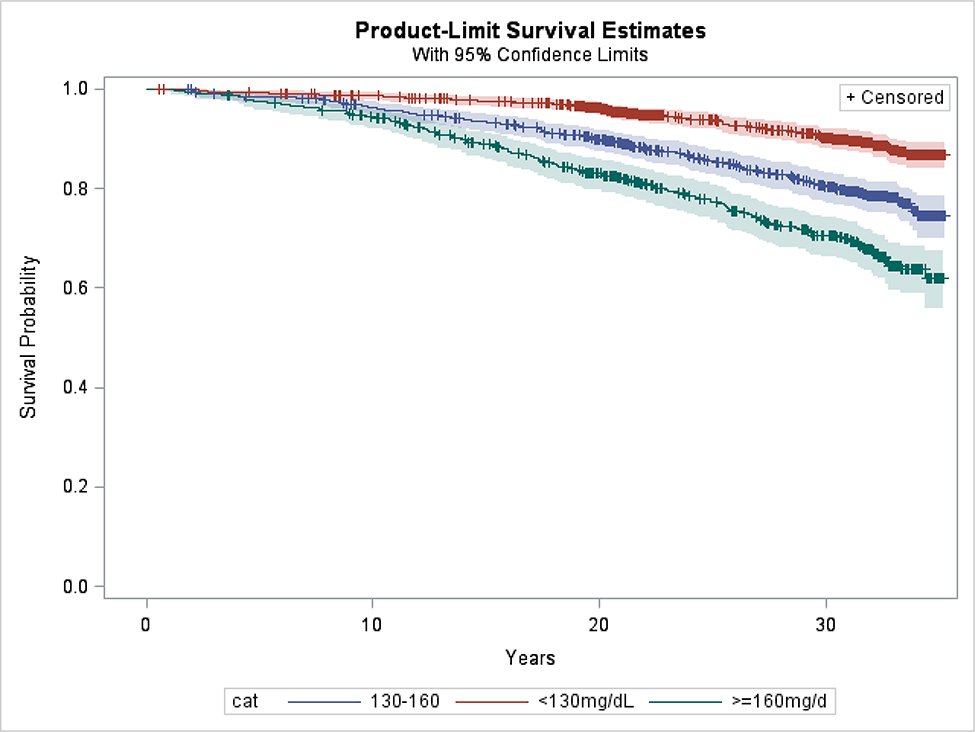
We also investigated the difference in new onset CVD events in those classified as “high” versus “low” non-HDL-C based on the mean of the first two examinations. The corresponding Kaplan-Meier survival curves are presented in Figure 4. The 25-year cardiovascular event rates were 22.6% for the “high” non-HDL C group, 14.6% for the “intermediate” and 6.4% for the “low” group (log-rank p-value < 0.001). Hazard ratios for incidence of CVD extracted from Cox model adjusted for standard cardiovascular risk factors (age, sex, SBP, hypertension treatment and smoking status) were also statistically significant: HR=1.30, 95% CI: 1.04, 1.63, p=0.022 and HR=2.29, 95% CI: 1.75, 3.00, p<0.001, for high vs. mid and high vs. low groups, respectively.
Figure 4:
Kaplan-Meier curves for categories of non-HDL cholesterol (participants with LDL-C≥=190 at first two exams excluded).
categories calculated as mean non-HDL value from exam 1 and 2. Non-HDL values for participants on treatment inflated by non-parametric adjustment.
Analyses evaluating the benefit of early statin therapy revealed that treatment of all subjects in the “high” non-HDL-C subgroup would reduce the CVD event rate of 22.6% by 58.0% to 9.5%, which is between the “intermediate” and “low” non-HDL-C groups. The corresponding number needed to treat in the “high” non-HDL-C group to prevent a CVD event would be approximately 8. Sensitivity analyses performed on 1258 participants with non-missing records for non-HDL-C levels at all exams did not materially differ from analyses conducted on the entire cohort. The group-based model identified the same trajectories of non-HDL-C over time, with 22.1%, 52.4% and 25.5% belonging to the three categories (Appendix Figure 1). More than 78% of individuals with mean non-HDL-C at the first two examinations ≥ 160 mg/dl had mean non-HDL C at exams 3–8 exceeding 160 mg/dl. Among those with mean non-HDL C below 130 mg/dl at the first two examinations, 92% had their mean non-HDL C for exams 3–8 below 160 mg/dl. The 25-year cardiovascular event rates were 22.9% for the “high” non-HDL C group and 5.9% for the “low” group (log-rank p-value < 0.001). The results remained robust in the analyses which excluded individuals who received lipid-lowering medications on follow-up.
Discussion
In this analysis we found that non-HDL C levels in young adulthood (25–40) was highly predictive of high lifetime non-HDL C level. Indeed, we have shown that non-HDL C trajectories remain fairly flat between ages 30 and 60. Moreover, two measurements of non-HDL C between age 25 and 40 are sufficient to confidently categorize individuals as high or low non-HDL C for the next 25–30 years. If the first two values are high, there is an 80% likelihood the average non-HDL-C would be ≥160 mg/dl during the next 30 years. If the first two values are low, there is an almost 90% likelihood, the average non-HDL C would remain below 160 mg/dl during the next 30 years. The high non-HDL-C group made up approximately a quarter of our total sample while the low non-HDL-C group made up a third of the total. These divisions were associated with dramatic differences in outcomes: the “high” non-HDL-C subgroup experienced more than 1 in 5 chance of a cardiovascular event over the next 25 years whereas the low non-HDL-C subgroup experienced less than a 1 in 17 chance of a cardiovascular event over the same timeframe. These event rates are particularly striking given the young age of our cohort at the beginning of the follow-up, an age at which the 10-year risk of cardiovascular events determined by conventional algorithms is generally low.
Our present results extend those of Navar et al. [5], which demonstrated that a higher number of years of exposure to elevated levels of non-HDL C before age 55 was a strong predictor of future CVD risk between age 55 and age 70 and those of Abdullah et al. (15), which demonstrated that an elevated LDL-C and non-HDL-C in a low short term-risk group was associated with a substantial long term event CVD event rate. Here we show that the non-HDL-C risk category can be reliably determined using two measurements before age 40, stressing the importance of preventive lipid measurements in young adulthood. We also established that the “high young adulthood non-HDL” designation is associated with more than 20% risk of CVD in the next 25 years.
Our results are important as current guidelines emphasize lipid lowering intervention for primary prevention based predominately on shorter-term (10 year) CVD risk. Unfortunately, because age so dominates the calculation of risk, the short-term risk model will not effectively prevent the substantial number of cardiovascular events that occur before age 60, the age after which calculated risk increases so dramatically. (3) Moreover, the risk calculation applies only to individuals above age 40, because calculated 10-year risk before that age is very small. Consequently, the current 10-year risk-based approach to prevention reduces the chance to impede the development of the atherosclerotic lesions that begin and mature between 30 to 60 years of age but cause clinical events only thereafter. The challenge, therefore, is to develop a cost-effective model to identify younger individuals at lower 10-year risk who have a reasonable probability of substantially benefitting from earlier onset of lipid lowering therapy.
The Benefit model of cardiovascular prevention incorporates both baseline risk and the relative risk reduction associated with lowering cholesterol in identifying those who should consider preventive lipid lowering therapy. (16) The calculation of benefit is derived from both the baseline risk and the baseline level of LDL-C. The Benefit model, therefore, acknowledges the importance of risk but adds substantial additional emphasis to the causes of risk. The current study lends support to consideration of the assessment of benefit over the longer-term (14), especially for younger adults. Indeed, our analysis demonstrates that treatment of the subgroup with “high” non-HDL-C in young adulthood would produce a substantial drop in CVD events with an NNT of approximately 8. However, prevention is not limited to pharmacological interventions. Intensive life style intervention should be part of preventive therapy in all and the first approach in young individuals with adverse risk profiles.
We note that while there were marked differences in CVD risk, non-HDL-C levels, on average, rose in all three subgroups. The decrease in non-HDL-C in the “high” subgroup at the last examination is likely the result of statin therapy and might indicate that our adjustment for treatment was conservative. Still, there were individuals in the “intermediate” and “low” subgroups, whose levels of non-HDL-C rose significantly over the period of observation and, if substantial and sustained, this should not be ignored. Important limitations in our knowledge remain. Most critically, there are no long-term randomized clinical trial results of statin therapy to rely on and, realistically, there never will be. Nevertheless, the risks of statin therapy do appear to be low. The most common, muscle ache and soreness, almost always resolve when the medication is discontinued. There is a small risk of diabetes mellitus but this apparently applies principally to those who were already on the pathway to develop this disorder. (17) There is, therefore, the possibility that statin therapy during this prediabetic period may actually diminish long term risk. Importantly, notwithstanding that almost all the statin RCTs were 5 years or less in duration, no guideline recommends that statin therapy for groups that have been shown to benefit be limited to this period. Moreover, statins have now been used in substantial numbers of patients for decades without clear new signals of risk. Nevertheless, formal estimates of long-term risks of statin therapy have not been completed.
An important limitation of our study is that based on age at entry, Framingham does not include data on younger subjects. Data from studies such as CARDIA demonstrate that lipid levels and, in particular apoB levels, at age 25 identify those with an increased risk of coronary calcification at age 50. (18) Indeed, the atherosclerotic process almost certainly is underway well before this, as numerous previous studies have documented. (19) Accordingly, lipid screening should begin earlier than in our study. (1) Additional limitations include lack of geographic and ethnic/racial diversity in the Framingham sample. Moreover, this is a retrospective analysis, with follow-up necessarily spanning a “historical” period (it is not possible to have 30-year follow up into the future). On the other hand, the Framingham data base provides a virtually unique quality of data collection over an extended time period when statins were not in common use, allowing for a “natural history” investigation with limited confounding due to medication use. Statin use did appear to affect the results of our study in that the average non-HDL-C was lower in the High group in examination 8 compared with examination 7. No adjustments could be made for compliance with therapy or duration of treatment. Nor could adjustment be made for the cardio-protective effect of statins, which may account for the decrease in the difference in relative event rates between the high and low non-HDL-C groups between examinations 7 and 8. Indeed, the net effect is that the final difference in the relative rates of events between the “high” and “low” groups represent a conservative estimate of the long-term effect of elevated levels of non-HDL-C. Finally, there is now considerable evidence, including from the Framingham Heart Study, that apoB is superior to non-HDL C and LDL C to estimate the atherogenic risk associated with the apoB lipoproteins but apoB was not consistently measured during the Framingham examinations. (20–23)
In summary, the present study demonstrates that lipid levels in young adulthood are highly predictive of future levels and that individuals can be reliably assigned to high and low non-HDL-C groups based on two measurements collected between age 25 and 40. Those with high non-HDL in young adulthood have significant (22.6%) risk for subsequent CVD in the next 25 years relative to 6.4% among those with low non-HDL in young life. This underscores the importance of lipid measuring non-HLD C level before age 40 and as well as helping to facilitate informed patient-provider discussion about the potential benefits of preventive lipid lowering efforts during the early midlife period.
Supplementary Material
Perspectives.
Competency in Medical Knowledge
Lipid levels in young adulthood are highly predictive of future levels and that individuals can be reliably assigned to high and low non-HDL-C groups based on two measurements collected between age 25 and 40.
Translational Outlook
Early lipid monitoring before age 40 would identify a majority of those with high likelihood for lifetime elevated lipid levels and high lifetime risk for CVD. This information could help facilitate informed patient-provider discussion about the potential benefits of preventive lipid lowering efforts during the early midlife period.
Acknowledgments
Funding: Doggone Foundation
Disclosure: GT has participated in advisory boards/speaker bureau for Sanofi, Amgen, Boehringer Ingelheim and Ionis. GT is funded as clinical research scholar from FRQS-Quebec. JTW is funded by NIH LRP K23 HL133601 and receives modest support from NGM Biopharmaceuticals. AN is funded by the National Heart, Lung, and Blood Institute grant K01HL133416. AN institution receives support from Sanofi, Regeneron, Amgen, Janssen, and Amarin. AN receives fees for consulting from Sanofi, Regeneron, NovoNordisk, Amarin, AstraZeneca, and Amgen. EP receives modest personal consultant support from Amgen, Sanofi, AstraZeneca, Merck, Janssen and Amarin. KMP receives fees from HunterRockhold and Ironwood. MJP received grants from Sanofi/Regeneron and Amgen, and fees from Merck and Boehringer Ingelheim.
Abbreviations
- ACC/AHA
American College of Cardiology/American Heart Association
- ApoB
Apolipoprotein B
- BMI
Body Mass Index
- CARDIA
The Coronary Artery Revascularization in Diabetes trial
- CVD
Cardiovascular Disease
- DBP
Diastolic Blood Pressure
- HDL-c
High-density Lipoprotein cholesterol
- HR
Hazard Ratio
- LDL-c
Low-density Lipoprotein cholesterol
- NNT
Number Needed to Treat
- Non-HDL-c
Non-high-density Lipoprotein cholesterol
- RCT
Randomized Clinical Trial
- SBP
Systolic Blood Pressure
Footnotes
Central Illustration: Figure 3 from the manuscript
References
- 1.Grundy SM, Stone NJ, Bailey AL, et al. Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018. [Google Scholar]
- 2.Pencina MJ, Navar-Boggan AM, D’Agostino RB, et al. Application of new cholesterol guidelines to a population-based sample. N. Engl. J. Med. 2014; 370:1422–1431. [DOI] [PubMed] [Google Scholar]
- 3.Sniderman AD, Thanassoulis G, Williams K, Pencina MJ. Risk of Premature Cardiovascular Disease vs the Number of Premature Cardiovascular Events. JAMA Cardiol 2016; 1:492–494. [DOI] [PubMed] [Google Scholar]
- 4.Sniderman AD, Toth PP, Thanassoulis G, Pencina MJ, Furberg CD. Taking a longer term view of cardiovascular risk: the causal exposure paradigm. BMJ 2014; 348:g3047–g3047. [DOI] [PubMed] [Google Scholar]
- 5.Navar-Boggan AM, Peterson ED, D’Agostino RB, Neely B, Sniderman AD, Pencina MJ. Hyperlipidemia in early adulthood increases long-term risk of coronary heart disease. Circulation 2015; 131:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cupples LA. Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurements. Framingham Heart Study 1987; 18:181–196. [Google Scholar]
- 7.Levy D, DeStefano AL, Larson MG, et al. Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the Framingham heart study. Hypertension 2000; 36:477–483. [DOI] [PubMed] [Google Scholar]
- 8.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med 2005; 24:2911–2935. [DOI] [PubMed] [Google Scholar]
- 9.Nagin DS. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychological Methods; 1999, 4(2), 139–157. [DOI] [PubMed] [Google Scholar]
- 10.Nagin DS. Group-Based Modeling of Development. Harvard University Press; 2009. [Google Scholar]
- 11.Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: a group-based method. Psychological Methods 2001; 6:18–34. [DOI] [PubMed] [Google Scholar]
- 12.Jones B, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001; 29:374–393. [Google Scholar]
- 13.Jones BL, Nagin DS. Advances in Group-Based Trajectory Modeling and an SAS Procedure for Estimating Them. Sociological Methods & Research 2016; 35:542–571. [Google Scholar]
- 14.Thanassoulis G, Sniderman AD, Pencina MJ. A Long-term Benefit Approach vs Standard Risk-Based Approaches for Statin Eligibility in Primary Prevention. JAMA Cardiol. 2018. November 1;3(11):1090–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdullah SM, Defina LF, Leonard D, et al. Long-term association of low-density lipoprotein cholesterol with cardiovascular mortality in individuals at low 10-year risk of atherosclerotic cardiovascular disease: results from the Cooper Center Longitudinal Study. Circulation 2018; 138:2315–2325. [DOI] [PubMed] [Google Scholar]
- 16.Thanassoulis G, Williams K, Altobelli KK, Pencina MJ, Cannon CP, Sniderman AD. Individualized Statin Benefit for Determining Statin Eligibility in the Primary Prevention of Cardiovascular Disease. Circulation 2016; 133:1574–1581. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Pradhan A, Macfadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 2012; 380:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkins JT, Li RC, Sniderman A, et al. Discordance between apolipoprotein B and LDL-cholesterol in young adults predicts coronary artery calcification: the CARDIA study. J Am Coll Cardiol. 2016;67(2):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson JG, Williams KJ, Gidding S, et al. Eradicating the burden of atherosclerotic cardiovascular disease by lowering apolipoprotein B lipoproteins Earlier in life. J. Am. Heart Assoc, 7 (2018), 10.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sniderman AD, Toth PP, Thanassoulis G, Furberg CD. An evidence-based analysis of the National Lipid Association recommendations concerning non-HDL-C and apoB. Journal of Clinical Lipidology 2016; 10:1248–1258. [DOI] [PubMed] [Google Scholar]
- 21.Cantey EP, Wilkins JT. Discordance between lipoprotein particle number and cholesterol content: an update. Curr Opin Endocrinol Diabetes Obes 2018; 25:130–136. [DOI] [PubMed] [Google Scholar]
- 22.Sniderman AD, Couture P, Martin SS, et al. Hypertriglyceridemia and cardiovascular risk: a cautionary note about metabolic confounding. J. Lipid Res. 2018; 59:1266–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pencina MJ, D’Agostino RB, Zdrojewski T, et al. Apolipoprotein B improves risk assessment of future coronary heart disease in the Framingham Heart Study beyond LDL-C and non-HDL-C. Eur J Prev Cardiol 2015;22:2047487315569411–1327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.