Abstract
Background
Drought is a major limiting factor seriously influencing worldwide soybean production and its impact on yield, morphological and physiological traits depend on the timing it occurs and the intensity of water shortage. Only limited research has however been conducted on identifying the drought-tolerant genotypes at different growth stages (vegetative growth phase, reproductive growth phase and the whole growth phase) as well as evaluate the effectiveness and reliability of multiple phenotypic and yield-related characteristics in soybean.
Results
Two pot experiments and a 2-year field experiment were conducted to evaluate soybean drought tolerance at different growth stages. The membership function value of drought tolerance (MFVD) was used to identify drought-resistant cultivars during vegetative growth phase and reproductive growth stage; the relative drought index (RDI) of yield was used to assess drought-resistant cultivars during the whole growing period. In this study, regression models built based on MFVD indicated that the variation of drought tolerant coefficient (DC) of R/S, TRL, LAI and RSR could explain 73.70% of the total variation at vegetative growth phase. However, higher heritability only found in LAI and RSR, indicating the two traits could serve as reliable criteria for drought evaluation. Similarly, the DC of SPP, YPP, PH, PB, MSNN and STB could explain 94.30% of the total variation in MFVD according to stepwise multiple linear regression analyses at reproductive growth phase. Thus, these six traits were identified as indicators for screening drought resistance genotypes in soybean. In addition, correlation analysis revealed that the MFVD was significantly positively correlated with the DCRB, DCR/S, DCRSA, DCRSR and DCRBR at vegetative growth phase and DCYPP, DCSPP, DCRB, and DCPB at reproductive growth phase. This indicated that these traits were closely related to the drought resistance of plants.
Conclusions
LD24, JD36 and TF31 of vegetative growth phase, and TD37 and LD26 of reproductive growth phase were identified with drought tolerant and highly drought tolerant, respectively. Moreover, 30 accessions with drought tolerance were screened in the field trial and could be applied for the drought resistance of other genotypes by cross-breeding.
Keywords: Soybean, Screening drought tolerance genotypes, Membership function value, Drought-tolerant coefficient
Background
Soybean (Glycine max L. Merr.), as an indispensable source of protein, oil and micronutrients in human diets and animal fodders, has become a crucial and economical agricultural crop in the world based on its excellent nutritional value and health benefits [1]. However, drought stress, which is the most important abiotic restriction, can have devastating effects on the stability and productivity of soybean in many semi- and arid areas of the world [2, 3]. It has been extensively reported that drought stress can directly induce a wide range of injury symptoms in plants, such as the inhibition of plant photosynthesis [4, 5], increased oxidative [6], and changes in metabolism [7]. Furthermore, drought stress led to the decrease in leaf area, pod yield, plant height, 1000-seed weight, harvest index, seed yield, etc. [8]. It was estimated that about 40% soybean yield decrease was caused by drought stress [9]. Depending on hybrid characteristics, soybeans use about 450–700 mm of rain fall during the growing season [10]. Improving the drought resistance of varieties is thus a key measure for reducing yield losses and stabilizing crop production under drought condition. The effects of drought stress on plant depend not only on the characteristics (duration, intensity) of the stress but also on the timing of occurrence relative to the development cycle of the plant. The most critical period for water stress in soybean was the flowering stage and the period following flowering [11]. The yield formation was sensitive when mild water deficit happened during the seedling phase, and became more sensitive to serious water deficit occurring at the flowering-podding phase in soybean [8]. Therefore, it is necessary to evaluate the response of soybean with different genetic germplasms to drought stress at diverse growth stages.
Drought tolerance is defined as the relative yield of a genotype compared to other genotypes subjected to the same drought stress [12]. Although yield was the primary characteristic for measurement of drought resistance on water deficit condition in many crops [13–15], the secondary characteristic might be specifically appropriate to improve selection response to drought stress condition. Relative water content, chlorophyll content, and ascorbic acid could be used as secondary indicators for selecting drought-tolerant genotypes [16, 17]. In this study, a plant is said to have drought tolerance if it can maintain better phenotypic traits and achieve higher yields under drought conditions. Drought-tolerant coefficient (DC), supply a measure of drought effects based on the reduction of each trait under water stress conditions in comparison to well-watered conditions, and therefore used for identification of drought-tolerant genotypes [18–20]. The membership function value of drought tolerance (MFVD) calculated from DC provided a comprehensive evaluation method for drought resistance of materials based on multi-indicator determination [21, 22]. According to this method for drought resistance assessment, several studies have reported that some physiological traits such as photosynthetic rate, leaf chlorophyll content, superoxide dismutase (SOD) activity and some yield-related traits such as spikelet number, grain number per spike, grain yield per plant were affected by soil water stress, and have been considered as evaluation parameters of drought resistance in other crops [23–26]. The direct impact on soybean that occurs due to drought stress are the decrease in yield and its component such as number of pods, number of seeds and seed weight [27]. Soybean plants subjected to water stress during flowering and vegetative growth stages had significantly lower total dry matter and yields [28]. Therefore, it is essential to carry out drought tolerance evaluation of soybean phenotypic and yield-related traits.
Plant root architecture has been also reported to be associated with water stress tolerance in various crops [29, 30]. The ability of a plant to modify its root distribution to exploit deeper stored soil water may be an important mechanism to avoid drought [31]. Deep rooting, root length density and root distribution have been identified as drought adaptive traits [32, 33] which can be used as selection criteria for drought resistance in other crops. However, the assessment on some important morphological and physiological traits, such as root length, root area and leaf area index (LAI) of soybean lines under different water regimes to examine thoroughly how soybean genotypes respond to drought in terms of these traits has not been clearly demonstrated.
The present investigation was carried out to identify the suitable soybean genotypes for drought tolerance at different growth stages combining with the value of membership function (MFVD) and drought-tolerant coefficient (DC). Moreover, this study also aimed to evaluate the use of multiple phenotypic and yield-related traits as secondary indices for drought resistance assessment.
Results
Response of traits measured and calculated to water stress
Assessing drought tolerance at vegetative growth stage (Expt. 1)
The variation was confirmed by the average value, standard deviation (SD) and the drought-tolerant coefficient (DC) of each trait when water was controlled at vegetative growth phase (VGP) were presented in the Table 1. In response to water-stressed (WS) regime, the mean value of 7 traits decreased but the others increased compared with that in the well-watered (WW) regime. Leaf area index (LAI) showed the lowest DC (0.59) which declined by 41.16%, indicating that LAI was the most sensitive trait to water stress in this group of genotypes. In addition, decreased in WS conditions the mean values of plant height (PH), shoot biomass (SB), root biomass (RB), total root length (TRL), root surface area (RSA) and root volume (RV) by 20.13, 38.53, 5.82, 13.41, 14.58 and 9.94%, respectively, but increased those of root/shoot ratio (R/S), root average diameter (RD), root length ratio (RLR), root surface area ratio (RSR), root volume ratio (RVR), root average diameter ratio (RDR), root biomass ratio (RBR) by 50.00, 14.55, 25.89, 22.49, 25.14, 83.33, and 38.09%, respectively, as compared with the WW regime (Table 1). This indicated that the adverse effects of drought stress on shoots were greater than roots. Furthermore, there was significant difference between the DC of RDR and DC of other traits according to Duncan’s multiple comparisons (P < 0.05).
Table 1.
Means value, standard deviation (SD) of traits under well-watered (WW) and water-stressed (WS) regimes and the drought-tolerant coefficient (DC) of each trait at vegetative growth stage
| Traits | Mean ± SD (WW) | Mean ± SD (WS) | Mean ± SD (DC) |
|---|---|---|---|
| PH (cm) | 45.05 ± 1.78 | 35.98 ± 1.93 | 0.80 ± 0.01 ef |
| LAI | 3.11 ± 0.44 | 1.83 ± 0.13 | 0.59 ± 0.05 f |
| SB (g plant−1) | 7.06 ± 1.03 | 4.34 ± 0.25 | 0.62 ± 0.06 f |
| RB (g plant−1) | 1.89 ± 0.18 | 1.78 ± 0.04 | 0.95 ± 0.07 de |
| R/S | 0.28 ± 0.02 | 0.42 ± 0.01 | 1.53 ± 0.04 b |
| TRL (m) | 51.15 ± 1.81 | 44.29 ± 2.37 | 0.87 ± 0.02 e |
| RSA (cm2) | 969.69 ± 62.85 | 828.29 ± 61.42 | 0.86 ± 0.10 e |
| RV (cm3) | 14.69 ± 0.83 | 13.23 ± 1.10 | 0.91 ± 0.12 e |
| RD (mm) | 0.55 ± 0.02 | 0.63 ± 0.04 | 1.15 ± 0.11 cd |
| RLR (m/g) | 5.91 ± 0.55 | 7.44 ± 0.15 | 1.38 ± 0.19 c |
| RSR (cm2/g) | 113.21 ± 8.43 | 138.67 ± 8.38 | 1.23 ± 0.05 c |
| RVR (cm3/g) | 1.75 ± 0.35 | 2.19 ± 0.18 | 1.29 ± 0.36 bc |
| RDR (mm/g) | 0.06 ± 0.01 | 0.11 ± 0.01 | 1.87 ± 0.07 a |
| RBR | 0.21 ± 0.01 | 0.29 ± 0.01 | 1.37 ± 0.03 bc |
Means followed by the same letter within the third column are not significantly different (p ≤ 0.05) as determined by Duncan’s multiple-range test (n = 3)
Assessing drought tolerance at the reproductive growth stage (Expt. 2)
Phenotypic variation was confirmed by the average phenotypic value, SD and the DC of each trait when water was controlled at reproductive growth phase (RGP) (Table 2). The mean value of 8 traits decreased, while only one trait increased under water-stressed regime. Root biomass (RB) showed the largest drought coefficient (1.01) which increased by 2.22%, demonstrating that this trait was the less impacted by drought conditions in this group of cultivars. The mean values of hundred seeds weight (HSW) and main stem node number (MSNN) were reduced in WS conditions by 0.16 and 4.83%, respectively. And there was no significant difference between the drought-tolerant coefficient (DC) of the two and the DC of RB according to Duncan’s multiple-range test, indicating that the three agronomic traits were less affected by soil moisture. Moreover, for these varieties, WS treatment decreased the mean values of plant height (PH), pods per plant (PPP), seeds per plant (SPP), yield per plant (YPP), stem biomass (STB), pod biomass (PB) by 8.49, 19.09, 25.05%, 24.24.80, 26.23, and 20.98%, respectively, as compared with WW treatment.
Table 2.
Means value, standard deviation (SD) of traits under well-watered (WW) and water-stressed (WS) regimes and the drought-tolerant coefficient (DC) of each trait at reproductive growth phase
| Traits | Mean ± SD (WW) | Mean ± SD (WS) | Mean ± SD (DC) |
|---|---|---|---|
| PH (cm) | 96.35 ± 11.70 | 88.81 ± 9.40 | 0.92 ± 0.02 abc |
| MSNN | 15.54 ± 1.31 | 15.11 ± 0.92 | 0.97 ± 0.02 ab |
| PPP | 32.94 ± 13.51 | 27.66 ± 6.00 | 0.88 ± 0.18 abc |
| SPP | 67.65 ± 28.18 | 54.10 ± 18.41 | 0.82 ± 0.10 bc |
| HSW (g) | 19.35 ± 2.67 | 19.32 ± 2.18 | 0.99 ± 0.03 a |
| YPP (g plant−1) | 12.61 ± 4.71 | 10.15 ± 3.24 | 0.82 ± 0.04 c |
| RB (g plant− 1) | 3.61 ± 0.58 | 3.69 ± 1.03 | 1.01 ± 0.12 a |
| STB (g plant−1) | 9.72 ± 2.71 | 7.70 ± 1.97 | 0.79 ± 0.04 c |
| PB (g plant−1) | 4.67 ± 1.84 | 3.86 ± 1.29 | 0.84 ± 0.06 bc |
Means followed by the same letter within the third column are not significantly different (p ≤ 0.05) as determined by Duncan’s multiple-range test (n = 3)
Relationship of the same trait between WS and WW treatments
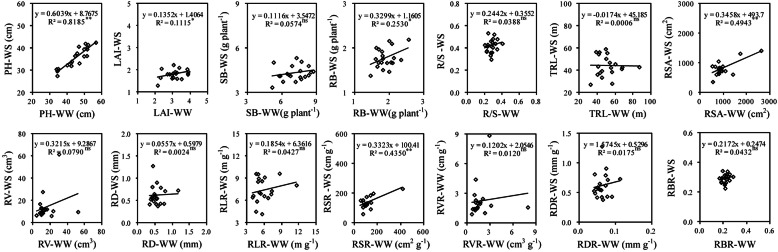
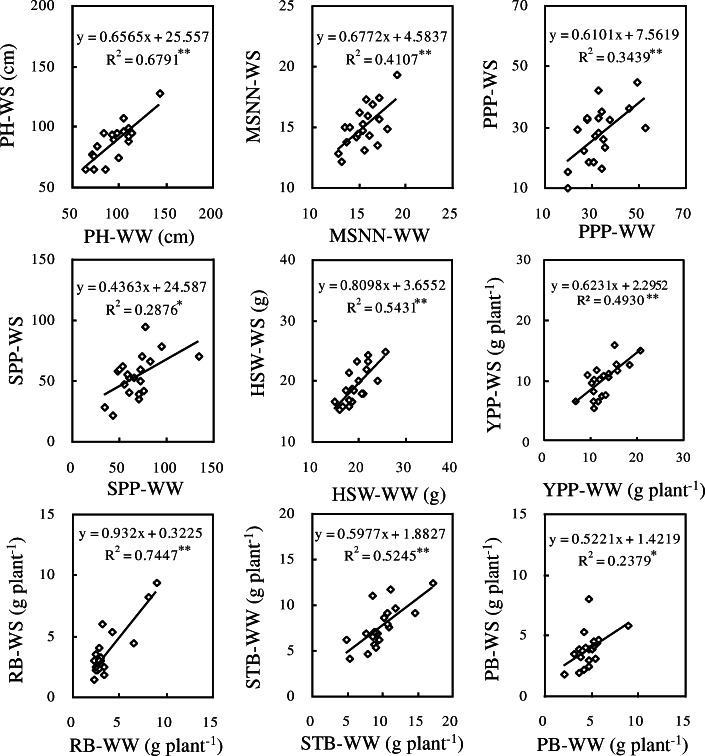
Significant and positive correlations were observed between WS and WW regimes for the same traits including PH (r2 = 0.819, P < 0.01), LAI (r2 = 0.192, P < 0.05), RB (r2 = 0.253, P < 0.05), RSA (r2 = 0.494, P < 0.01) and RSR (r2 = 0.435, P < 0.01) when water was controlled at vegetative growth phase (Fig. 1), and thus the above five parameters could serve as reliable characters in drought screening. At reproductive growth stage, correlations between WW and WS regimes for the same indicators in all the investigated traits (PH, r2 = 0.679, P < 0.01; MSNN, r2 = 0.411, P < 0.01; PPP, r2 = 0.344, P < 0.01; SPP, r2 = 0.288, P < 0.05; HSW, r2 = 0.543, P < 0.01; YPP, r2 = 0.493, P < 0.01; RB, r2 = 0.745, P < 0.01; STB, r2 = 0.525, P < 0.01; PB, r2 = 0.238, P < 0.05) were all significant (Fig. 2). At the whole growth stage, it was also found that the same indicators of all investigated agronomic traits (PH, r2 = 0.681, P < 0.01; FPH, r2 = 0.536, P < 0.01; MSNN, r2 = 0.693, P < 0.01; BR, r2 = 0.514, P < 0.01) of Shenyang were positively correlated with those of Chaoyang (Fig. 3).
Fig. 1.
Relationship of the same trait between WS and WW treatments at vegetative growth phase. ns r is no significance; * r is significance at level of 0.05; ** r is significance at levels of 0.01. The same as below
Fig. 2.
Relationship of the same trait between WS and WW treatments at reproductive growth phase
Fig. 3.
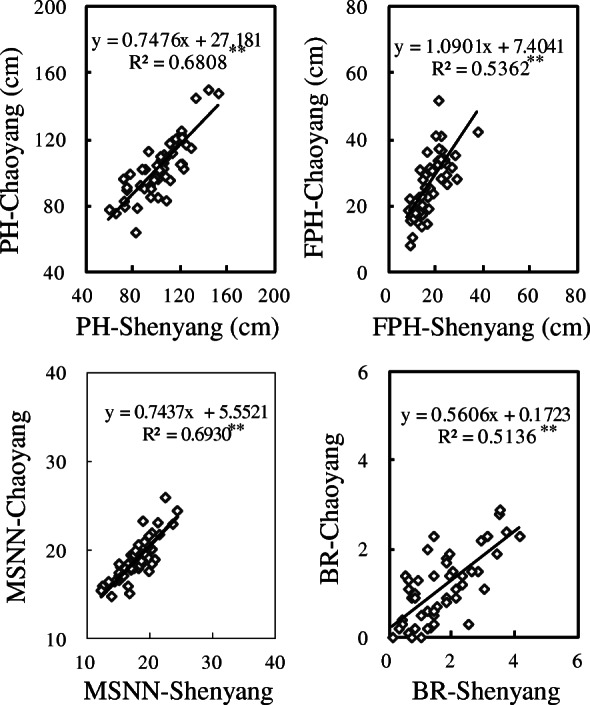
Relationship of the same trait between well-watered condition (Shenyang) and water stress condition (Chaoyang)
Genetic variation and broad sense heritability of the investigated traits
Assessing drought tolerance at vegetative growth stage (Expt. 1)
The ANOVA revealed that there were significant effects (P < 0.05) for 9 traits including plant height (PH), leaf area index (LAI), shoot biomass (SB), root/shoot ratio (R/S), root surface area (RSA), root length ratio (RLR), root surface area ratio (RSR), root average diameter ratio (RDR), root biomass ratio (RBR) between two water regimes and 11 tested traits including PH, LAI, SB, total root length (TRL), root surface area (RSA), root volume (RV), root average diameter (RD), root length ratio (RLR), root surface area ratio (RSR), root volume ratio (RVR), root average diameter ratio (RDR) among 20 cultivars (Table 3). Only 5 traits (TRL, RV, RD, RVR, RDR) varied greatly (P < 0.05) between variety and water regime interactions. The genetic variation coefficient (CVg) ranged from 16.49 to 38.95% on WW regime and from 15.32 to 41.62% on WS regime for the 14 traits, respectively (Table 3). The lowest broad sense heritability (H2) was estimated for R/S (0.34), suggesting that the soil moisture made a larger contribution to the variation of the trait. The higher H2 estimates, in these traits were obtained for PH, LAI, RB, RSA and RSR with a significant linear relationship between WS and WW regimes (Fig. 1 and Table 3).
Table 3.
Analysis of variance (ANOVA), genetic variation coefficient (CVg) and broad sense heritability (H2) of each trait on two water conditions of 20 varieties at vegetative growth phase
| Variation Source |
df | Means of squares | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PH (cm) | LAI | SB | RB | R/S | TRL | RSA | RV | RD | RLR | RSR | RVR | RDR | RBR | ||
| Replication (R) | 2 | 138.04 | 3.11 | 16.09 | 0.47 | 0.01 | 638.84 | 80,066.06 | 298.45 | 0.08 | 0.16 | 7099.86 | 6.40 | 0.00 | 0.00 |
| Water (W) | 1 | 2470.67*** | 49.59* | 223.29* | 0.34 | 0.63*** | 1410.16 | 589,699.18* | 18.64 | 0.17 | 77.34* | 16,762.72* | 6.77 | 0.05* | 0.19*** |
| Variety (V) | 19 | 186.08*** | 0.58** | 2.48** | 0.25 | 0.01 | 358.16** | 657,814.55*** | 500.60*** | 0.12*** | 10.28** | 16,765.40*** | 9.18*** | 0.00*** | 0.00 |
| W × V | 19 | 16.67 | 0.36 | 1.71 | 0.11 | 0.01 | 374.67** | 187,211.60 | 282.48** | 0.11*** | 6.78 | 5149.34 | 7.38*** | 0.00*** | 0.00 |
| Error | 38 | 12.09 | 0.26 | 1.13 | 0.15 | 0.01 | 157.98 | 112,709.22 | 108.32 | 0.03 | 4.35 | 3403.59 | 2.51 | 0.00 | 0.00 |
| CVg(%) (WW) | 16.49 | 25.51 | 23.06 | 23.30 | 27.66 | 27.69 | 30.36 | 38.95 | 36.08 | 34.84 | 38.24 | 38.77 | 34.08 | 21.04 | |
| CVg(%) (WS) | 15.32 | 21.31 | 22.00 | 20.92 | 23.32 | 27.75 | 30.74 | 38.08 | 37.02 | 31.89 | 32.29 | 41.62 | 39.82 | 16.36 | |
| H2 | 0.91 | 0.62 | 0.44 | 0.63 | 0.34 | 0.35 | 0.74 | 0.58 | 0.45 | 0.47 | 0.72 | 0.47 | 0.43 | 0.36 | |
*,**,*** Mean of squares significant at p < 0.05, 0.01, and 0.001, respectively
Assessing drought tolerance at the reproductive growth stage (Expt. 2)
Analysis of variance, genetic variation coefficient (CVg) and broad sense heritability (H2) of each trait under WS and WW regimes at reproductive growth phase were given in Table 4. Extremely significant differences (P < 0.01) in plant height (PH), main stem node number (MSNN), pods per plant (PPP), seeds per plant (SPP), hundred seeds weight (HSW), yield per plant (YPP), root biomass (RB), stem biomass (STB), and pod biomass (PB) occurred between the tested varieties. The great differences were also observed in PH, MSNN, SPP, HSW, STB and PB between the interactions. The CVg ranged from 13.29 to 47.99% under WW condition and from 12.87 to 50.30% under WS condition for 9 traits, respectively. The maximum and minimum broad sense heritability (H2) was estimated for PH (0.90) and PB (0.71), respectively. The H2 values for the 9 investigated traits were all more than 0.71, for instance, MSNN (0.81), SPP (0.73), etc. This indicated that the variations of the traits were mostly due to genetic differences and these traits were highly heritable.
Table 4.
ANOVA, CVg and H2 of each trait on two water conditions of 20 varieties at reproductive growth phase
| Variation Source |
df | Means of squares | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PH (cm) | MSNN | PPP | SPP | HSW | YPP | RB | STB | PB | ||
| Replication (R) | 2 | 4449.43 | 49.58 | 4184.43 | 21,640.29 | 234.28 | 632.88 | 26.02 | 217.40 | 98.10 |
| Water (W) | 1 | 1706.30* | 5.63 | 837.41* | 5504.69* | 0.02 | 181.11* | 0.19 | 123.54* | 19.62* |
| Variety (V) | 19 | 1669.39*** | 14.17*** | 368.50*** | 1717.99*** | 41.92*** | 46.92*** | 22.44*** | 32.44*** | 8.51*** |
| W × V | 19 | 182.18*** | 3.11*** | 96.20 | 534.98* | 6.44** | 8.37 | 1.68 | 5.48* | 2.94** |
| Error | 38 | 43.72 | 0.58 | 90.16 | 293.81 | 2.94 | 9.82 | 1.38 | 2.68 | 1.30 |
| CVg(%) (WW) | 22.89 | 13.29 | 47.88 | 51.73 | 19.67 | 46.00 | 16.06 | 38.62 | 47.99 | |
| CVg(%) (WS) | 20.10 | 12.87 | 47.71 | 47.04 | 18.63 | 42.93 | 27.93 | 39.34 | 50.30 | |
| H2 | 0.90 | 0.81 | 0.74 | 0.73 | 0.86 | 0.82 | 0.88 | 0.84 | 0.71 | |
*,**,*** Mean of squares significant at p < 0.05, 0.01, and 0.001, respectively
Assessing drought tolerance at the whole growth stage (Expt. 3)
Variety (V), location (L) and L × V interactions had significant effects (P ≤ 0.05) for yield, plant height (PH), first pod height (FPH), branches (BR) in 2 years (Table 5). The main effects associated with variety and L × V interaction term were also significant (P ≤ 0.05) with respect to main stem node number (MSNN) in both years. However, location did not significantly affect MSNN in 2014, suggesting that MSNN was less affected by the different rainfall conditions. The CVg for the 5 investigated traits ranged from 13.77 to 72.83% in Shenyang and from 15.72 to 88.00% in Chaoyang during 2014–2015, respectively (Table 5). In addition, CVg values of 3 traits, i.e., yield, PH, MSNN ranged between 10 and 30%, and for first pod height (FPH) and branches (BR), CVg values were more than 30% in 2014–2015. Higher H2 was observed for the five traits tested in the 2 years, indicating that the phenotypic variations of these traits in this group of genotypes were mostly due to genetic differences and they were highly heritable traits.
Table 5.
ANOVA, CVg and H2 of each trait under well-watered condition (Shenyang) and water stress condition (Chaoyang) of 50 varieties in both years
| Variation Source | df | Means of squares | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | ||||||||||
| Yield (kg ha−1) | PH (cm) | FPH (cm) | MSNN | BR | Yield (kg ha−1) | PH (cm) | FPH (cm) | MSNN | BR | ||
| Replication (R) | 2 | 94,924.81 | 181.05 | 47.54 | 5.29 | 0.36 | 1646.295 | 161.05 | 141.67 | 0.06 | 0.07 |
| Location (L) | 1 | 8,300,616.00* | 4901.29* | 4434.42* | 103.57 | 6.57* | 9372584* | 1889.55* | 7868.95* | 44.96* | 47.88* |
| Variety (V) | 49 | 880,734.50*** | 1979.29*** | 414.44*** | 42.99*** | 4.53*** | 46.92*** | 2170.80*** | 293.17*** | 32.11*** | 6.07*** |
| L × V | 49 | 269,710.70*** | 235.37*** | 116.45*** | 5.51*** | 0.97*** | 1,287,688.00*** | 451.74*** | 93.81*** | 6.07*** | 1.64*** |
| Error | 96 | 94,624.68 | 10.14 | 7.05 | 1.58 | 0.27 | 318,273.50 | 7.52 | 7.95 | 0.66 | 0.22 |
| CVg(%) (Shenyang) | 13.77 | 20.54 | 43.09 | 18.07 | 72.52 | 19.95 | 16.06 | 41.48 | 15.73 | 72.83 | |
| CVg(%) (Chaoyang) | 21.32 | 15.72 | 43.11 | 14.28 | 88.00 | 22.65 | 27.93 | 36.19 | 12.69 | 83.64 | |
| H2 | 0.74 | 0.89 | 0.78 | 0.88 | 0.81 | 0.83 | 0.93 | 0.75 | 0.84 | 0.78 | |
*,**,*** Mean of squares significant at p < 0.05, 0.01, and 0.001, respectively
Identification and classification of drought tolerance among diverse genotypes
Assessing drought tolerance at vegetative growth stage and reproductive growth stage (Expts. 1 and 2)
The MFVD can be used as a comprehensive index to evaluate the drought tolerance of soybean genotypes according to the 14 characteristics during the vegetative growth period and the 9 traits for the reproductive growth period (Table 6). Among the 20 soybean accessions, 3 accessions (LD24, JD36 and TF31) showed drought tolerance (Level 2) when water was controlled at vegetative growth phase, 2 accessions (TD37 and LD26) showed highly drought tolerance (Level 1) water controlled at reproductive growth stage. Thus, these accessions could be used as resources for drought tolerance improvement in soybean breeding. Besides, 3 accessions (LD10, FD17 and LD21) and 1 accession (LD17) was screened for susceptible (Level 4) to water stress during vegetative growth phase and reproductive growth phase, respectively.
Table 6.
Subordinate function values and class to drought resistance on genotypes tested when water was controlled at vegetative growth phase and reproductive growth phase
| Cultivar Name | LD10 | LD15 | LD17 | LD18 | LD21 | LD23 | LD24 | LD26 | JD36 | JD37 | KY11 | KY12 | TF29 | TF31 | TD37 | TD40 | TD49 | DD12 | SN10 | FD17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MFVD of VGP | 0.33 | 0.36 | 0.47 | 0.40 | 0.33 | 0.37 | 0.52 | 0.38 | 0.53 | 0.43 | 0.39 | 0.41 | 0.40 | 0.49 | 0.37 | 0.45 | 0.40 | 0.35 | 0.41 | 0.33 |
| Level | 4 | 3 | 3 | 3 | 4 | 3 | 2 | 3 | 2 | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 4 |
| MFVD of RGP | 0.48 | 0.51 | 0.43 | 0.55 | 0.52 | 0.51 | 0.45 | 0.61 | 0.53 | 0.49 | 0.54 | 0.47 | 0.51 | 0.44 | 0.61 | 0.45 | 0.49 | 0.53 | 0.52 | 0.53 |
| Level | 3 | 3 | 4 | 3 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 3 |
Assessing drought tolerance at the whole growth stage (Expt. 3)
Fifty soybean genotypes were classified into four groups based on relative drought index (RDI) of yield and yield in semi-arid region in the field experiments during the 2 years (Fig. 4 and Table 7). Group I was comprised of the drought tolerant and high yield genotypes with higher RDI values than average (0.93) of 50 genotypes and higher yield than the average (2785.95 kg ha− 1). This group had 23 cultivars including LD24, JD36 and TF31 that have been screened with drought tolerance at vegetative growth phase. The group II comprised of non-drought tolerant and high yield genotypes which produced more than average yield of 50 genotypes at semi-arid zone, but the response to drought stress was lower than the average. There were only 2 genotypes within group II. The group III involved drought tolerance and low yield cultivars produced higher than average soybean yield, but the resistance to drought stress was above the average. There were 7 cultivars in group III including TD37 and LD26 screened with highly drought tolerance when water was controlled at reproductive growth phase. The group IV consisted of non-drought tolerance and low yield accessions including 18 genotypes that showed lower yield and RDI values than the average.
Fig. 4.
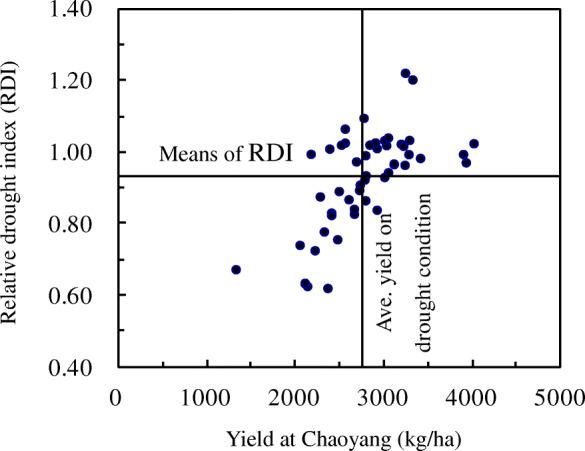
Identification and classification to drought resistance on genotypes tested of the whole growth phase
Table 7.
Identification and classification to drought resistance on genotypes tested of the whole growth stage
| Drought tolerance and high yield | Non-drought tolerance and high yield | Drought tolerance and low yield | Non-drought tolerance and low yield |
|---|---|---|---|
| DD14, JD36, JD37, LD18, LD24, LD25, LD29, LD30, SN12, SN16, SN17, TF31, TD39, TD43, TD45, TD46, TD48, TD49, TD50, TD55, TD56, TD57, YW9. |
LX2, TD47. |
DD11, KY11, LD17, LD23, LD26, LD31, TD37. | DD12, DD13, DD15, FD17, KY12, L08–28, LD15, LD21, LD22, LD28, SN8, SN10, SN11, TD38, TD40, TD42, XY11, YW6. |
Correlation between MFVD and DC of each trait
Assessing drought tolerance at vegetative growth stage (Expt. 1)
Correlation coefficients between the membership function value of drought tolerance (MFVD) and the drought-tolerant coefficient (DC) of each trait at vegetative growth stage were analyzed (Table 8). The DCRB, DCR/S, DCRSA, DCRLR, DCRSR and DCRBR showed highly positive correlations with the MFVD (Table 8; P < 0.05). Moreover, correlation coefficients between the DC of each trait and those of others were also analyzed and shown in Table 8. The highest correlation of DC was found between RBR and R/S (r = 1.00; P < 0.01), meaning that RBR was closely related to R/S. In addition, DCPH, DCLAI and DCSB were also positively correlated with each other. In contrast, a negative correlation occurred between DCR/S and DCLAI, DCSB.
Table 8.
Correlation coefficients between the membership function value of drought tolerance (MFVD) and the drought-tolerant coefficient (DC) of each trait at vegetative growth stage
| MFVD | DCPH | DCLAI | DCSB | DCRB | DCR/S | DCTRL | DCRSA | DCRV | DCRD | DCRLR | DCRSR | DCRVR | DCRDR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DCPH | − 0.14 | |||||||||||||
| DCLAI | −0.22 | 0.75** | ||||||||||||
| DCSB | −0.10 | 0.83** | 0.89** | |||||||||||
| DCRB | 0.47* | 0.31 | 0.24 | 0.18 | ||||||||||
| DCR/S | 0.66** | − 0.40 | − 0.49* | − 0.63** | 0.62** | |||||||||
| DCTRL | 0.40 | 0.35 | 0.11 | 0.15 | 0.41 | 0.21 | ||||||||
| DCRSA | 0.52* | 0.14 | − 0.03 | − 0.10 | 0.38 | 0.43 | 0.56** | |||||||
| DCRV | −0.35 | − 0.10 | − 0.03 | 0.05 | − 0.06 | − 0.42 | − 0.08 | − 0.14 | ||||||
| DCRD | 0.17 | 0.28 | 0.23 | 0.23 | 0.48* | 0.21 | 0.07 | 0.17 | 0.10 | |||||
| DCRLR | 0.47* | 0.00 | −0.28 | −0.28 | 0.19 | 0.40 | 0.87** | 0.65** | −0.07 | −0.08 | ||||
| DCRSR | 0.53* | −0.14 | − 0.32 | −0.41 | 0.22 | 0.56** | 0.48* | 0.93** | −0.10 | 0.13 | 0.73** | |||
| DCRVR | −0.26 | − 0.29 | −0.24 | − 0.20 | −0.13 | − 0.28 | −0.15 | − 0.16 | 0.94** | − 0.11 | −0.01 | − 0.42 | ||
| DCRDR | 0.25 | −0.01 | −0.05 | − 0.09 | 0.35 | 0.39 | −0.04 | 0.27 | −0.13 | 0.92** | 0.00 | 0.34 | −0.16 | |
| DCRBR | 0.65** | −0.45* | −0.52* | − 0.67** | 0.59** | 1.00** | 0.19 | 0.43* | −0.13 | 0.21 | 0.41 | 0.58** | −0.27 | 0.40 |
* r is significance at level of 0.05; ** r is significance at level of 0.01; *** r is significance at level of 0.001
Assessing drought tolerance at the reproductive growth stage (Expt. 2)
Correlation coefficients between the MFVD and DC of each trait at reproductive growth stage were listed in Table 9. The great positive correlation occurred between MFVD and DCSPP (r = 0.47; P < 0.05), DCYPP (r = 0.42; P < 0.05), DCPB (r = 0.41; P < 0.05) and DCRB (r = 0.46; P < 0.05). Besides, correlation coefficients between the DC of each trait were available in Table 9 when water was controlled at reproductive growth stage. Significant positive correlations of DC of tested traits were observed between DCYPP and DCPH, DCYPP and DCMSNN, DCYPP and DCPPP, DCYPP and DCSPP, DCYPP and DCSTB, and DCYPP and DCPB, revealing that these traits were closely related to yield per plant in soybean. The significant correlations also occurred between DCPH and DCMSNN (r = 0.63; P < 0.01), DCPPP and DCSPP (r = 0.96; P < 0.01), DCPPP and DCPB (r = 0.91; P < 0.01) (Table 9).
Table 9.
Correlation coefficients between the MFVD and the DC of each trait at reproductive growth stage
| MFVD | DCPH | DCMSNN | DCPPP | DCSPP | DCHSW | DCYPP | DCRB | DCSTB | |
|---|---|---|---|---|---|---|---|---|---|
| DCPH | 0.22 | ||||||||
| DCMSNN | 0.05 | 0.63** | |||||||
| DCPPP | 0.03 | 0.49* | 0.57** | ||||||
| DCSPP | 0.47* | 0.55** | 0.61** | 0.96** | |||||
| DCHSW | 0.15 | −0.38 | −0.28 | −0.64** | −0.62** | ||||
| DCYPP | 0.42* | 0.56** | 0.61** | 0.84** | 0.93** | −0.36 | |||
| DCRB | 0.46* | −0.09 | 0.00 | −0.15 | −0.19 | − 0.25 | −0.32 | ||
| DCSTB | 0.21 | 0.66** | 0.33 | 0.47* | 0.56** | −0.49* | 0.50* | 0.05 | |
| DCPB | 0.41* | 0.44* | 0.51* | 0.91** | 0.87** | −0.53* | 0.84** | −0.15 | 0.43* |
* r is significance at level of 0.05; ** r is significance at level of 0.01; *** r is significance at level of 0.001
Drought tolerance explained by multiple DC of traits
Assessing drought tolerance at vegetative growth stage (Expt. 1)
Multiple linear stepwise regression to explain the MFVD prediction with the accepted 4 limiting DC of traits at vegetative growth phase were estimated and listed in Table 10. The results showed that the MFVD variation was explained 36.60, 21.00, 10.20, and 5.90% by DC of R/S, TRL, LAI and RSR, respectively. As a result, 73.70% of the variation in MFVD was caused by these DC of 4 traits. Regression coefficient, standard error, T-value and probability of the 4 accepted variables at vegetative growth phase were calculated and displayed in Table 11. The prediction equation for MFVD with accepted 4 limiting DC of traits was as:
where YMFVDV was the membership functions value of drought tolerance for one cultivar at VGP, A1, A2, A3 and A4 were the DC value for R/S, TRL, LAI and RSR, respectively.
Table 10.
Multiple linear stepwise regression to explain the MFVD with DC of each trait at vegetative growth phase and reproductive growth phase
| Variables | Step | Variable entered | Partial R2 | Model R2 | Sig. |
|---|---|---|---|---|---|
|
MFVD (VGP) |
1 | DCR/S(A1) | 0.366 | 0.366 | *** |
| 2 | DC TRL (A2) | 0.210 | 0.776 | * | |
| 3 | DC LAI (A3) | 0.102 | 0.838 | ** | |
| 4 | DC RBR (A4) | 0.059 | 0.868 | * | |
|
MFVD (RGP) |
1 | DCSPP (B1) | 0.446 | 0.902 | *** |
| 2 | DCYPP (B2) | 0.201 | 0.999 | ** | |
| 3 | DCPH(B3) | 0.116 | 0.902 | *** | |
| 4 | DCPB (B4) | 0.066 | 0.953 | * | |
| 5 | DCMSNN (B5) | 0.067 | 0.987 | *** | |
| 6 | DCSTB (B6) | 0.047 | 0.969 | * |
MFVD (VGP) The membership function value of drought tolerance of vegetative growth phase, MFVD (RGP) The membership function value of drought tolerance of reproductive growth phase *,**,*** Significant at p < 0.05, 0.01, and 0.001, respectively
Table 11.
Regression coefficient, standard error, T-value and probability of the accepted DC of each trait that can be used to predict the MFVD based on the stepwise regression analysis at vegetative growth phase and reproductive growth phase
| Variables | Variable entered | Regression coefficients |
Standard error |
T | Sig. |
|---|---|---|---|---|---|
|
MFVD (VGP) |
Constant | 0.280 | 0.054 | 5.150 | *** |
| DCR/S (A1) | −0.176 | 0.059 | −2.960 | * | |
| DC TRL(A2) | 0.110 | 0.020 | 5.400 | *** | |
| DCLAI (A3) | 0.082 | 0.019 | 4.310 | *** | |
| DCRBR (A4) | −0.024 | 0.014 | −1.770 | * | |
|
MFVD (RGP) |
Constant | −0.421 | 0.014 | −30.890 | *** |
| DCSPP (B1) | 0.165 | 0.008 | 21.390 | *** | |
| DCYPP (B2) | 0.108 | 0.006 | 18.650 | *** | |
| DCPH (B3) | 0.048 | 0.005 | 9.800 | *** | |
| DCPB (B4) | 0.060 | 0.004 | 13.670 | *** | |
| DCMSNN (B5) | 0.174 | 0.009 | 18.560 | *** | |
| DCSTB (B6) | 0.130 | 0.004 | 30.500 | *** |
*,**,*** Significant at p < 0.05, 0.01, and 0.001, respectively
Assessing drought tolerance at reproductive growth stage (Expt. 2)
Multiple linear stepwise regression to explain the MFVD prediction with the accepted 6 DC of traits at reproductive growth phase were estimated and listed in Table 10. The results showed that the MFVD variation was explained 44.60, 20.10, 11.60, 6.60, 6.70, and 4.70% by DC of SPP, YPP, PH, PB, MSNN and STB, respectively. In total, 94.30% of the variation in MFVD was ascribed to these 6 DC of traits. Regression coefficients for the 6 accepted variables were calculated and shown in Table 11. The prediction equation for MFVD with 6 limiting DC of traits was as:
where YMFVDR was the membership functions value of drought tolerance for one cultivar at RGP, B1, B2, B3, B4, B5 and B6 were the DC value for SPP, YPP, PH, PB, MSNN and STB, respectively.
Discussion
Soybean is one of the major and wide spread crops in the world and is rather sensitive to water stress. Many drought-tolerant varieties have been developed for other crops [17, 20, 34, 35]. However, study on a set of accurate, stable, simple and systematic method and index system for identification and selection of drought tolerance in soybean is still limited. It is an important step to improve the identification of soybean drought-tolerant germplasm by screening a number of key and stable traits with high heritability for the identification of drought tolerance in soybean.
Drought stress caused reductions in plant height, pods per plant, 100-kernel weight, yield per plant, LAI, biological yield, root length, root volume, etc. [4, 36–39]. Soybean yield is mainly a function of number of plants, dry matter production, seed numbers and seed size. Water stress have been reported to reduce seed weight, total biomass, pods per plant, seeds per plant, seeds per pod, 100-grain weight, and ultimately caused a decline in soybean yield [40–42]. In this study, some drought traits measured among 20 soybean accessions under WS regimes also showed remarkable decline in PH, LAI, SB, RB, TRL, RSA, RD and RV water controlled at vegetative growth phase. In addition, decline in PH, PPP, SPP, YPP, STB and PB was observed when water was controlled at reproductive growth phase. In contrast, striking increase occurred in R/S, RD, RLR, RSR, RVR, RDR and RBR under water stress condition, as compared with the well water treatment. This showed that drought stress enhanced the proportion of root distribution throughout the plant [43, 44].
The present study from Expt.2 and Expt.3 also showed that all the investigated agronomic traits in the WW treatments were positively correlated with those of the WS treatment. The similar results were obtained by Liu et al. [45]. However, in Expt.1 of this study, only the five traits, i.e., PH, LAI, SB, RSA and RSR had high and positive correlations (P < 0.05) between WW and WS conditions. This could be attributed to the previous experimental conditions where water stress was weaker than the present study. In addition, the differences in the tested crops and the measured indicators were also the main reason.
The significant differences in variety and water stress were observed on plant height, LAI, shoot biomass, root biomass, R/S, total root length, etc. [46–49]. The ANOVA analysis in Expt.1 also indicated that there were significant differences for some traits including PH, LAI, SB, RSA, RLR, RSR, etc. between among 20 cultivars and two water regimes. The genetic variation for the trait under selection and a higher heritability of the trait are necessary for breeding and the trait utility within the selection process [23]. In Expt.1, higher heritability found in PH, RSA, RSR, LAI and RB indicated that the five traits were highly heritable, and strong positive correlation was observed among the five parameters under normal and drought conditions. There were significant differences in plant height, main stem node number, yield, and internode length in soybean between cultivars [1]. The Expt. 2 demonstrated that the significant differences were found for indicators such as PH, MSNN, PPP, SPP, HSW, YPP, RB, STB, and PB between variety treatments, and all these indicators presented higher H2 values. Therefore, the 9 secondary characteristics could be considered as criteria for drought resistance assessment.
Previous research has conclusively indicated positive correlations between MFVD and the drought-tolerant coefficient of some traits including plant height, grain number per spike, biological yield per plant, grain yield per plant, thousand kernel weight in wheat [23], which is also supported by our results; the conclusion stated clearly that MFVD was significantly positively correlated with the DCRB, DCR/S, DCRSA, DCRSR and DCRBR during vegetative growth period and DCYPP, DCRB, and DCPB during reproductive growth period. It is therefore concluded that the less the values of these traits decreased under drought stress, the more drought-tolerant the genotypes were. In addition, the DC of some traits were positively correlated with each other such as DCPH and DCLAI, DCPH and DCSB, DCTRL and DCRSA, DCPH and DCMSNN, DCMSNN and DCPPP, DCYPP and DCSPP. As compared with different drought criteria, we revealed that root-related indicators had relatively higher correlation with drought tolerance of plant than other ones tested, thus we regarded the root system as more valuable standard for evaluating the drought resistance of soybean. Similar studies have also pointed out the important role of roots in enhancing plant resistance to drought stress [50–52].
The drought resistance is a complex characteristic. A single characteristic cannot reflect the complex traits of the drought resistance mechanism, so more traits should be considered to evaluate the drought resistance in soybean. Stepwise multiple linear regression analyses revealed that the drought-tolerant coefficient of R/S, TRL, LAI and RSR could explain 73.70% of the total variation in MFVD at VGP, but higher heritability only found in LAI and RSR indicated that the 2 traits could serve as reliable indicators in drought evaluation. This again showed that the root system plays an important role in improving the drought resistance. Root traits contribute to drought avoidance of plants, which is relevant to avoid agricultural drought and sustain crop performance [53]. When soil moisture is insufficient, root traits are also essential for maintaining crop yields [54]. Many scientists have also claimed that modifying the root system structure will enhance crop yields and achieve a new green revolution [55, 56]. In addition, stepwise multiple linear regression analyses illustrated that the DC of SPP, YPP, PH, PB, MSNN and STB could explain 94.30% of the variation in MFVD at RGP. As a result, those six traits could be used as a combination to screen soybean genotypes for drought resistance.
Conclusions
Even though drought-tolerant indices have many advantages, it is more and more widely used in combination with the value of membership function to screen drought resistant varieties in many crops. The present investigation was carried out to identify drought tolerance genotypes integrated the two methods which is needed for the development of soybean varieties in the arid and semi-arid areas. As a result, 3 accessions (LD24, JD36 and TF31) with drought tolerant during vegetative growth phase and 2 cultivars (TD37 and LD26) with highly drought resistant at reproductive growth stage were screened according to MFVD under WS and WW conditions, respectively. Meanwhile, based on DC and yield in the semi-arid zone, 50 genotypes were classified as drought tolerance and high yield (DD14, JD36, JD37, LD18, LD24, LD25, LD29, LD30, SN12, SN16, SN17, TF31, TD39, TD43, TD45, TD46, TD48, TD49, TD50, TD55, TD56, TD57, YW9), non-drought tolerance and high yield (LX2, TD47), drought tolerance and low yield (DD11, KY11, LD17, LD23, LD26, LD31, TD37), and non-drought tolerance and low yield (DD12, DD13, DD15, FD17, KY12, L08–28, LD15, LD21, LD22, LD28, SN8, SN10, SN11, TD38, TD40, TD42, XY11, YW6). In comparison WS, WW treatment increased the mean values of 7 characteristics but decreased that of the others at vegetative growth stage, and increased that of 8 traits but only decreased that of 1 trait at reproductive growth stage for 20 varieties. Correlation analysis showed that the MFVD was significantly and positively correlated with DCRB, DCR/S, DCRSA, DCRLR, DCRSR and DCRBR of vegetative growth phase and DCYPP and DCRB of reproductive growth stage. The results from ANOVA analysis showed that there were significant differences (P < 0.05) for 9 traits (PH, LAI, SB, R/S, RSA, RLR, RSR, RDR, RBR) between two water regimes, for 11 tested indicators (PH, LAI, SB, TRL, RSA, RV, RD, RLR, RSR, RVR, RDR) among 20 cultivars, for 5 properties (TRL, RV, RD, RVR, RDR) between the interactions when water was controlled at vegetative growth stage. While there was significant difference (P < 0.05) for only one trait (PH) between two water regimes, for all 9 investigated features (PH, MSNN, PPP, SPP, HSW, YPP, RB, STB, PB) among 20 cultivars, and for 5 indices (PH, MSNN, SPP, HSW, STB, STB) between the interactions when water was controlled at reproductive growth phase. The H2 for the investigated traits was estimated by genetic variance and phenotypic variance in two water treatments. However, the H2 of only one trait (PH) was higher than 0.9 and that of two traits (RSA, RSR) were more than 0.7 during vegetative growth stage, while that of the 9 investigated traits were all more than 0.7 during reproductive growth stage. This indicated that the variations of these traits were mostly due to genetic differences and they were highly heritable traits. There was a significant and positive linear relationship between WS and WW condition for the five traits (PH, LAI, SB, RSA and RSR) at vegetative growth stage and for all the agronomic traits at reproductive growth stage and the whole growth stage.
Methods
The pot experiments
A total of 20 soybean varieties from Liaoning Academy of Agricultural Science and Tieling Academy of Agricultural Science were selected in the present study to determine their drought tolerance properties at the vegetative growth stage (Expt. 1) and the reproductive growth stage (Expt. 2) under water stress conditions. The origin and description of 20 soybean varieties are shown in Table 12. The controlled experiments were conducted in a greenhouse of Liaoning Academy of Agricultural Science in Shenyang, Liaoning, China (41°49′ N, 123°32′ W). For both experiment seeds were sowed in plastic pots (28 cm average diameter and 28 cm average tall) contained 15 kg air dry brown soil from 0 to 20 cm plough layer. Soil samples were collected before planting to analyze the characteristics shown in Table 13. Six soybean seeds were sown in each pot and the population was thinned to two plants per pot when the first trifoliate leaf emerged. The pots were arranged in the electric movable greenhouse which was opened in the sunny day to maintain living conditions in a natural environment and closed in the rainy days to avoid the rain soaking plastic basin.
Table 12.
The origin and description of 20 soybean studies varieties
| Cultivar Name | Origin | Description |
|---|---|---|
| LD10 | Shenyang, China | Commercial cultivar (LD3 × L82–5185) |
| LD15 | Shenyang, China | Commercial cultivar (L85062 × ZCY18) |
| LD17 | Shenyang, China | Commercial cultivar (LD3 × L92-2738 M) |
| LD18 | Shenyang, China | Commercial cultivar (L89094 × L93040) |
| LD21 | Shenyang, China | Commercial cultivar (L8878× L93009) |
| LD23 | Shenyang, China | Commercial cultivar (LD10 × L91086) |
| LD24 | Shenyang, China | Commercial cultivar (LD3 × YPZ) |
| LD26 | Shenyang, China | Commercial cultivar (L8880 × IOA22) |
| JD36 | Jinzhou, China | Commercial cultivar (JD2 × TF18) |
| JD37 | Jinzhou, China | Commercial cultivar (MC25 × L9825) |
| KY11 | Kaiyuan, China | Commercial cultivar (KJ7528× GZM) |
| KY12 | Kaiyuan, China | Commercial cultivar (K8525 × KJ8157) |
| TF29 | Tieling, China | Commercial cultivar (8114 × 84059) |
| TF31 | Tieling, China | Commercial cultivar (LD3 × Resnick) |
| TD37 | Tieling, China | Commercial cultivar (89034–10 × TF29) |
| TD40 | Tieling, China | Commercial cultivar (89078 × 92035) |
| TD49 | Tieling, China | Commercial cultivar (93058–19 × TF29) |
| DD12 | Dandong, China | Commercial cultivar (D806 × LD10) |
| SN10 | Shenyang, China | Commercial cultivar (SN92–16 × SN91–44) |
| FD17 | Fushun, China | Commercial cultivar (F82–47× DJ1) |
Table 13.
Some initial properties of the soils in each experiment
| Experiment number | Location | pH | OM (%) |
Total N(%) | Total P(%) |
Total K(%) |
Avai.N (mg kg−1) |
Avai.P (mg kg−1) |
Avai.K (mg kg− 1) |
|---|---|---|---|---|---|---|---|---|---|
| Expts. 1, 2 | Shenyang | 6.90 | 1.18 | 0.12 | 0.08 | 2.42 | 100 | 16.8 | 98 |
|
Expt. 3 (2014) |
Shenyang | 6.60 | 2.68 | 0.12 | 0.18 | 2.64 | 100 | 25.4 | 135 |
| Chaoyang | 6.90 | 2.12 | 0.11 | 0.16 | 2.78 | 94.0 | 22.8 | 164 | |
|
Expt. 3 (2015) |
Shenyang | 6.60 | 2.58 | 0.13 | 0.17 | 2.58 | 110 | 24.8 | 148 |
| Chaoyang | 6.90 | 2.24 | 0.11 | 0.17 | 2.75 | 102 | 23.4 | 175 |
Two series of pot experiments were arranged in two factors randomized complete block design with three replicates. The water treatments included well-watered (WW) and water-stressed (WS) regimes. The soil water content of WW regime was maintained at field capacity, and that of WS regime was 50% of the field capacity. Twenty domestic soybean genotypes used in the experiments. Two pots for each genotype with two water regimes were grown next to each other in pairs. For each pair the treatments were randomly assigned to each pot. The soil water content and field capacity were determined before the experiment, and then calculate the weight of pot and soil for each water treatment (plant weight is ignored). The pots were weighed every 2 d to maintain soil moisture at the target weight by rewatering.
Expt. 1
Water-stressed (WS) was applied 30 days when the plants had three fully expanded leaves and ended at flowering phase. Thereafter, plants were harvested and a total of 14 traits were investigated and calculated. Definition of traits and their description of measurement are listed in Table 14.
Table 14.
Trait name and description of their measurement and calculation
| Experiment No. | Trait names and description of their measurement and calculation |
|---|---|
| Expt. 1 | Plant height (PH, cm), the average height of the two individuals per pot from cotyledonary node to the main stem top. Leaf area index (LAI), total leaf area per unit ground area, and leaf area was measured with a leaf area meter (LI-3100C, LI-COR, Lincoln, NE, USA). Shoot biomass (SB, g plant−1) and root biomass (RB, g plant−1), the average of above ground dry weight and that of root dry weight of the two plants per pot after drying to constant weight at 80 °C in a drying oven, respectively. Root/shoot ratio (R/S), calculated as root dry weight/shoot dry weight; Total root length (TRL, m), root surface area (RSA, cm2), root average diameter (RD, mm) and root volume (RV, cm3), calculated on the average of two individuals per pot and measured using WinRHIZO (EPSON 1680, WinRHIZO Pro2003b, Regent Instruments Inc., Quebec, Canada). Root length ratio (RLR, m/g), total root length/total biomass. Root surface area ratio (RSR, cm2/g), root surface area/total biomass; Root volume ratio (RVR, cm3/g), root volume/total biomass; root average diameter ratio (RDR, mm/g), root diameter/total biomass; root biomass ratio (RBR), root biomass/total biomass. |
| Expt. 2 | Plant height (PH, cm), the average height of the two individuals per pot from cotyledonary node to the main stem top. Main stem node number (MSNN), Pods per plant (PPP) and Seeds per plant (SPP), measured on the average of 2 plants in the pot at maturity; Hundred seeds weight (HSW, g), calculated the weight of 100 seeds; Yield per plant (YPP, g plant−1), the average yield of the two individuals per pot. Root biomass (RB, g plant− 1), Stem biomass (STB, g plant− 1) and Pod biomass (PB, g plant− 1), measured on the average of 2 plants which were separated into bean, root, stem and pod determined after drying, respectively. |
| Expt. 3 | Plant height (PH, cm), First pod height (FPH, cm), Main stem node number (MSNN) and Branches (BR), were estimated in 5 plants per plot. |
Expt. 2
Water-stressed (WS) was applied 50 days at the beginning of flowering R1 until the first physiological maturity pod appearance R7. After all plants maturity, plants were harvested and a total of 9 traits were investigated which are listed in Table 14.
Expt. 3 (field studies)
Fifty soybean genotypes from Liaoning Academy of Agricultural Science and Tieling Academy of Agricultural Science including 18 varieties in Expts. 1 and 2 were evaluated for drought tolerance in Chaoyang (41°30′ N, 120°29′ W, 170 m a.s.l.) located in the semi-arid zone and Shenyang (41°82′N, 123°55′W, 52.9 m a.s.l.) located at humid and sub-humid region, representing different rainfall characteristics in 2014–2015. Both Chaoyang and Shenyang are temperate continental monsoon climate type with severe and dry winter, and high temperature and concentrated rainfall in summer. The annual average sunshine, the mean temperature, and frost free days in a normal year in Chaoyang are around 2900 h, 6 °C and 135 d, respectively, and those in Shenyang are around 2400 h, 8 °C and 150 d, respectively.
The field experiment was arranged in complete block design replicated three times with two locations (Shenyang representing well-watered condition is located in a humid and semi-humid area; Chaoyang representing water stress condition is located in a semi-arid area). Both Chaoyang and Shenyang, 50 genotypes were sown in field plots, and each plot consisted of four 6 m rows, in East-West orientation, with 0.60 m inter-row spacing. Plots were over-seeded with hand planters and seedlings thinned to a final stand of 166,700 plants ha− 1. The plots were fertilized with 45–70-60 kg ha− 1 in the form of N-P2O5-K2O before planting. The soil samples of two locations were typically brown soil that was taken before planting to analyze the characteristics given in Table 13.
After maturity, an area of 6.0 m2 was harvested by hand from the two central rows from each plot. The whole harvested area was used to determine yield. Five plants from the second row were harvested and a total of 4 traits were investigated which were listed in Table 14.
Estimation of drought-tolerant coefficient (DC) and membership function value of drought tolerance (MFVD)
The drought tolerance coefficient (DC) is calculated according to the following equation: data ratio derived from the WS and WW regimes of the same genotype for each trait [23, 57, 58].
where DCijr is the drought-tolerant coefficient of the j-th trait for the i-th cultivar in the r-th replication; Tijwsr and Tijwwr are the value of the j-th trait for the i-th cultivar evaluated under WS and WW treatments in the r-th replication, respectively; DCij is the average value of drought-tolerant coefficient of j-th trait for the i-th cultivar.
Soybean drought tolerance was also evaluated by the membership function value. This methodology gives a comprehensive assessment by using the membership functions based on the theory of fuzzy mathematics. The membership function of a fuzzy set is a generalization of the indicator function in classical sets; it represents the degree of truth as an extension of valuation [59]. For any set T, a membership function on T is any function from T to the real unit interval [0,1]. According to the DC, the modified MFVD was calculated following the equations:
where Fij is the membership function value of the j-th trait for i-th cultivar for drought tolerance; DCjmax and DCjmin were the maximum value and minimum value of the drought resistant coefficient for the j-th trait, respectively; Fi is the average value of the membership function of measured traits for the i-th cultivar for drought tolerance.
Drought tolerance is divided into five levels according to the average value () and standard deviation (SD) of MFVD in two series of pot experiments. Class and level to drought resistance are listed in Table 15.
Table 15.
Class and level to drought resistance of soybean genotypes according to the and SD of MFVD
| Level | Fi | Class |
|---|---|---|
| 1 | Fi ≥ + 1.64SD | Highly drought tolerant |
| 2 | + 1.64SD > Fi ≥ + SD | Drought tolerant |
| 3 | + SD > Fi ≥ - SD, | Moderate drought tolerant |
| 4 | - SD > Fi ≥ - 1.64SD | Susceptible |
| 5 | Fi < - 1.64SD | Highly susceptible |
Evaluation and classification of drought tolerance of soybean genotypes at the whole growth stage based on yield relative drought index (RDI) and yield in semi-arid areas in the field experiment. RDI of yield was calculated as [60]:
where Yiws and Yiww were the yield of tested i-th genotype and Ymws and Ymws were the mean yield of all genotypes under water-stressed (semi-arid region) and well-watered (humid and sub-humid region) conditions, respectively.
Data analysis
Multiple linear regression was performed with SAS 8.0 statistical software to construct the select indices of MFVD using multiple DC of some traits. Analysis of variance (ANOVA), correlation and heritability (H2) analyses were carried out for all the data sets using SPSS 16.0 statistical software, and significance differences was determined at the significances of 0.05, 0.01, 0.001 probability level using the Duncan’s tests.
Variation was partitioned into relevant sources of variation to test for differences among genotypes. The linear model was used as follows:
where Yrki: observation corresponding to kth level of water factor, the ith level of genotypic factor and the rth replication; μ: general mean; Rr: the rth replication effect; Wk: the kth level of water treatment effect; Gi: the ith genotypic treatment effect; WGki: interaction between the ith level of genotypic treatment and the kth level of water treatment; and εrki: the residual error.
The genetic variation coefficient (CVg) of each trait was calculated following the equation:
where SD is standard deviation, is the average value of the trait under the same water-controlled conditions.
The broad sense heritability (H2) of each trait was calculated following the equation:
Where Vg is genotypic variance, Vwg is the interaction variance between genotype and water treatment, Ve is error variance.
Acknowledgements
We thank Chuang Wang, Jiahong Wang and Yulu Yuan for their help with field and laboratory work.
Abbreviations
- WS
Water-stressed
- WW
Well-watered
- MFVD
Membership function value of drought tolerance
- DC
Drought-tolerant coefficient
- VGP
Vegetative growth phase
- RGP
Reproductive growth phase
- H2
Broad sense heritability
- OM
Organic matter
- Avai.
Available
- N
Nitrogen
- P
Phosphorus
- K
Potassium
- DCPH
Drought-tolerant coefficient of plant height
- DCLAI
Drought-tolerant coefficient of leaf area index
- DCSB
Drought-tolerant coefficient of shoot biomass
- DCRB
Drought-tolerant coefficient of root biomass
- DCR/S
Drought-tolerant coefficient of root/shoot ratio
- DCTRL
Drought-tolerant coefficient of total root length
- DCRSA
Drought-tolerant coefficient of root surface area
- DCRV
Drought-tolerant coefficient of root volume
- DCRD
Drought-tolerant coefficient of root average diameter
- DCRLR
Drought-tolerant coefficient of root length ratio
- DCRSR
Drought-tolerant coefficient of root surface area ratio
- DCRVR
Drought-tolerant coefficient of root volume ratio
- DCRDR
Drought-tolerant coefficient of root average diameter ratio
- DCRBR
Drought-tolerant coefficient of root biomass ratio
- DCMSNN
Drought-tolerant coefficient of main stem node number
- DCSPP
Drought-tolerant coefficient of seeds per plant
- DCPPP
Drought-tolerant coefficient of pods per plant
- DCHSW
Drought-tolerant coefficient of hundred seeds weight
- DCSTB
Drought-tolerant coefficient of stem biomass
- DCPB
Drought-tolerant coefficient of pod biomass
- DCYPP
Drought-tolerant coefficient of yield per plant
Authors’ contributions
CY and WW designed the experiments. SS, CW, XS and SL carried out the experiments. FW analyzed experimental results. CY drafted the manuscript. HL revised it. All authors contributed to and approved the final manuscript.
Funding
The authors would like to thank the financial support from the National Key R&D Plan Project (No. 2016YFD0100201–01), National Agriculture Ministry of China (No. CARS-004-CES11), National Science and Technology Ministry of China (No.2016ZX08004–005), Natural Science Foundation of Liaoning Province (2020-MS-047), and Liaoning Provincial Central Leading Local Development Project (2019JH6/10400001).
Availability of data and materials
All datasets generated or analyzed during this study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chunjuan Yan, Email: yanchunjuan1983@163.com.
Wenbin Wang, Email: wangwenbin2018@163.com.
References
- 1.Liu K. Soybeans: chemistry, technology, and utilization. Gaithersburg: Aspen Publishers; 1997. pp. 1–22. [Google Scholar]
- 2.Shaheen T, Rahman M, Riaz MS, Zafar Y, Rahman M. 8-soybean production and drought stress. Abiotic Biotic Stress Soybean Production. 2016;1:177–196. [Google Scholar]
- 3.Sinclair T, Marrou H, Soltani A, Vadez V, Chandolu KC. Soybean production potential in Africa. Global Food Sec. 2014;3(1):31–40. [Google Scholar]
- 4.Ohashi Y, Nakayama N, Saneoka H, Fujita K. Effects of drought stress on photosynthetic gas exchange, chlorophyll fluorescence and stem diameter of soybean plants. Biol Plant. 2006;50(1):138–141. [Google Scholar]
- 5.Tang Y, Sun X, Wen T, Liu M, Yang M, Chen X. Implications of terminal oxidase function in regulation of salicylic acid on soybean seedling photosynthetic performance under water stress. Plant Physiol Biochem. 2017;112:19–28. doi: 10.1016/j.plaphy.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Porcel R, Ruiz-Lozano JM. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. J Exp Bot. 2004;55:1743–1750. doi: 10.1093/jxb/erh188. [DOI] [PubMed] [Google Scholar]
- 7.Du Y, Zhao Q, Chen L, Yao X, Zhang W, Zhang B, Xie F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol Biochem. 2020;146:1–12. doi: 10.1016/j.plaphy.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Cui Y, Jiang S, Jin J, Ning S, Feng P. Quantitative assessment of soybean drought loss sensitivity at different growth stages based on S-shaped damage curve. Agric Water Manag. 2019;213:821–832. [Google Scholar]
- 9.Specht JE, Hume DJ, Kumudini SV. Soybean yield potential-a genetic and physiological perspective. Crop Sci. 1999;39:1560–1570. [Google Scholar]
- 10.Dogan E, Kirnak H, Copur O. Deficit irrigations during soybean reproductive stages and CROPGRO-soybean simulations under semi-arid climatic conditions. Field Crop Res. 2007;103(2):154–159. [Google Scholar]
- 11.Meckel L, Egli DB, Phillips RE, Radcliffe D, Leggett JE. Effect of moisture stress on seed growth in soybeans. Agron J. 1984;75:1027–1031. [Google Scholar]
- 12.Hall AE. Is dehydration tolerance relevant to genotypic differences in leaf senescence and crop adaptation to dry environments? In: Close TJ, Bray EA, editors. Plant responses to cellular dehydration during environmental stress. California: Academic; 1993. pp. 1–10. [Google Scholar]
- 13.Kumar A, Bernier J, Verulkar S, Lafitte HR, Atlin GN. Breeding for drought tolerance: direct selection for yield, response to selection and use of drought-tolerant donors in upland and lowland-adapted populations. Field Crop Res. 2008;107(3):221–231. [Google Scholar]
- 14.Adebayo MA, Menkir A. Assessment of hybrids of drought tolerant maize (Zea mays L.) inbred lines for grain yield and other traits under stress managed conditions. Nigerian J Genet. 2014;28(2):19–23. [Google Scholar]
- 15.Seymen M, Yavuz D, Dursun A, Kurtar ES, Türkmen Ö. Identification of drought-tolerant pumpkin (Cucurbita pepo L.) genotypes associated with certain fruit characteristics, seed yield, and quality. Agric Water Manag. 2019;221:150–159. [Google Scholar]
- 16.Silva MA, Jifon JL, Sharma V, Silva JAG, Caputo MM, Damaj MB, Guimarães ER, Ferro MIT. Use of physiological parameters in screening drought tolerance in sugarcane genotypes. Sugar Technol. 2011;13:191–197. [Google Scholar]
- 17.Gunes A, Inal A, Adak MS, Bagci EG, Cicek N, Eraslan F. Effect of drought stress implemented at pre- or post-anthesis stage on some physiological parameters as screening criteria in chickpea cultivars. Russ J Plant Physiol. 2008;55:59–67. [Google Scholar]
- 18.Fischer KS, Edmeades GO, Johnson EC. Selection for improvement in maize yield under moisture deficits. Field Crop Res. 1989;22:227–243. [Google Scholar]
- 19.Regan BP, Cress WA, Staden J. Van root growth, water relations, abscisic acid and proline level of drought resistant and drought sensitive maize cultivars in response to water stress. S Afr Bot. 1993;59(1):98–104. [Google Scholar]
- 20.Classen MM, Shaw RH. Water deficit effects on corn: II. Grain components. Agron J. 1970;62:652–655. [Google Scholar]
- 21.Yan M, Huang W, Hu J, Lv Z, Lei S, Huang C. Evaluation of rice drought resistance by subordinate function. Hybrid Rice. 2009;24(5):76–79. [Google Scholar]
- 22.Peng Y, Shi G, Cui H. Evaluation of drought resistant of different processing tomato at seed germination stage under PEG-6000 stress. Seed. 2013;32(7):44–49. [Google Scholar]
- 23.Chen X, Min D, Yasir TA, Hu YG. Evaluation of 14 morphological, yield-related and physiological traits as indicators of drought tolerance in Chinese winter bread wheat revealed by analysis of the membership function value of drought tolerance (MFVD) Field Crop Res. 2012;137:195–201. [Google Scholar]
- 24.Meng QL, Guan ZB, Feng BL, Chai Y, Hu YG. Principal component analysis and fuzzy clustering on drought-tolerance related traits of foxtail millet (Setaria italica) Sci Agric Sin. 2009;42(8):2667–2675. [Google Scholar]
- 25.He XY, Wen RL, Wu CR, Zhou JG. Analysis of maize drought resistance at seeding stage by fuzzy subordination method. Southwest China J Agric Sci. 2008;21(1):52–56. [Google Scholar]
- 26.Song Q, Liu C, Bachir DG, Chen L, Hu Y. Drought resistance of new synthetic hexaploid wheat accessions evaluated by multiple traits and antioxidant enzyme activity. Field Crop Res. 2017;210:91–103. [Google Scholar]
- 27.Sadeghipour O, Abbasi S. Soybean response to drought and seed inoculation. World Appl Sci J. 2012;17(1):55–60. [Google Scholar]
- 28.Jha PK, Kumar SN, Inesa AVM. Responses of soybean to water stress and supplemental irrigation in upper indo-Gangetic plain: field experiment and modeling approach. Field Crop Res. 2018;219(15):76–86. [Google Scholar]
- 29.Shi G, Xia S, Ye J, Huang Y, Liu C, Zhang Z. PEG-simulated drought stress decreases cadmium accumulation in castor bean by altering root morphology. Environ Exp Bot. 2015;111:127–134. [Google Scholar]
- 30.Kashiwagi J, Krishnamurthy L, Purushothaman R, Upadhyaya HD, Gaur PM, Gowda CLL, Ito O, Varshney RK. Scope for improvement of yield under drought through the root traits in chickpea (Cicer arietinum L.) Field Crop Res. 2015;170:47–54. [Google Scholar]
- 31.Wright GC, Rao RCN. Peanut water relations. In: Smartt J, editor. The peanut crop. London: Chapman & Hall; 1994. pp. 281–325. [Google Scholar]
- 32.Matsui T, Singh BB. Root characteristics in cowpea related to drought tolerance at the seedling stage. Exp Agric. 2003;39:29–38. [Google Scholar]
- 33.Taiz L, Zeiger E. Stress physiology. In: Taiz L, Zeiger E, editors. Plant physiology. Sunderland: Academic; 2006. pp. 671–681. [Google Scholar]
- 34.Sadras VO, Lake L, Leonforteb A, McMurray LS, Paull JG. Screening field pea for adaptation to water and heat stress: associations between yield, crop growth rate and seed abortion. Field Crop Res. 2013;150:60–70. [Google Scholar]
- 35.Torres RO, McNally KL, Cruz CV, Serraj R, Henry A. Screening of rice genebank germplasm for yield and selection of new drought tolerance donors. Field Crop Res. 2013;147:12–22. [Google Scholar]
- 36.Mejia MN, Madramootoo CA, Broughton RS. Influence of water table management on corn and soybean yields. Agric Water Manag. 2000;46(1):73–89. [Google Scholar]
- 37.Rosadi RAB, Afandi, Senge M, Ito K, Adomako JT. Critical water content and water stress coefficient of soybean (Glycine max L. Merr.) under deficit irrigation. Paddy Water Environ. 2005;3(4):219–223. [Google Scholar]
- 38.Baghbani AA, Modarres-Sanavy SAM, Mashhadi-Akbar-Boojar M, AliMokhtassi-Bidgoli Towards improving the agronomic performance, chlorophyll fluorescence parameters and pigments in fenugreek using zeolite and vermicompost under deficit water stress. Ind Crop Prod. 2017;109:346–357. [Google Scholar]
- 39.Hosseini F, Mosaddeghi MR, Dexter AR. Effect of the fungus Piriformospora indica on physiological characteristics and root morphology of wheat under combined drought and mechanical stresses. Plant Physiol Biochem. 2017;118:107–120. doi: 10.1016/j.plaphy.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Harris DS, Schapaugh WT, Kanemasu ET. Genetic diversity in soybean for leaf canopy temperature and yield. Crop Sci. 1984;24:839–842. [Google Scholar]
- 41.Jumrani K, Bhatia VS. Impact of combined stress of high temperature and water deficit on growth and seed yield of soybean. Physiol Mol Biol Plants. 2018;24:37–50. doi: 10.1007/s12298-017-0480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sammons DJ, Peters DB, Hymowitz T. Screening soybeans for tolerance to moisture stress: a field procedure. Field Crop Res. 1980;3:321–335. [Google Scholar]
- 43.Onillon B, Durand JL, Gastal F, Tournebize R. Drought effects on growth and carbon partitioning in a tall fescue sward grown at different rates of nitrogen fertilization. Eur J Agron. 1995;4(1):91–99. [Google Scholar]
- 44.Purushothaman R, Krishnamurthy L, Upadhyaya HD, Vadez V, Varshney RK. Root traits confer grain yield advantages under terminal drought in chickpea (Cicer arietinum L.) Field Crop Res. 2017;201:146–161. doi: 10.1016/j.fcr.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu C, Yang Z, Hu Y. Drought resistance of wheat alien chromosome addition lines evaluated by membership function value based on multiple traits and drought resistance index of grain yield. Field Crop Res. 2015;179:103–112. [Google Scholar]
- 46.Zaifnejad M, Clark RB, Sullivan CY. Aluminum and water stress effects on growth and proline of sorghum. J Plant Physiol. 1997;150(3):338–344. [Google Scholar]
- 47.Purcell LC, Edwards JT, Brye KR. Soybean yield and biomass responses to cumulative transpiration: questioning widely held beliefs. Field Crop Res. 2007;101:10–18. [Google Scholar]
- 48.Li D, Liu H, Qiao Y, Wang Y, Cai Z, Dong B, Shi C, Liu Y, Li X, Liu M. Effects of elevated CO2 on the growth, seed yield, and water use efficiency of soybean (Glycine max L. Merr.) under drought stress. Agric Water Manag. 2013;129:105–112. [Google Scholar]
- 49.Adu MO, Asare PA, Yawson DO, Ackah FK, Amoah KK, Nyarko M, Andoh D. Quantifying variations in rhizosheath and root system phenotypes of landraces and improved varieties of juvenile maize. Rhizosphere. 2017;3:29–39. [Google Scholar]
- 50.Vadez V. Root hydraulics: the forgotten side of roots in drought adaptation. Field Crop Res. 2014;165:15–24. [Google Scholar]
- 51.Shan L, Yang C, Li Y, Duan Y, Geng D, Li Z, Zhang R, Duan G. Effects of drought stress on root physiological traits and root biomass allocation of Reaumuria soongorica. Acta Ecol Sin. 2015;35:155–159. [Google Scholar]
- 52.Chirino E, Ruiz-Yanetti S, Vilagrosa A, Mera X, Espinoza M, Lozano P. Morpho-functional traits and plant response to drought conditions in seedlings of six native species of ecuadorian ecosystems. Flora. 2017;233:58–67. [Google Scholar]
- 53.Serraj R, McNally KL, Slamet-Loedin I, Kohli A, Haefele SM, Atlin G, Kumar A. Drought resistance improvement in rice: an integrated genetic and resource management strategy. Plant Prod Sci. 2011;14:1–14. [Google Scholar]
- 54.Bengough AG, McKenzie BM, Hallett PD, Valentine TA. Root elongation, water stress and mechanical impedance: a review of limiting stresses and beneficial root tip traits. J Exp Bot. 2011;62:59–68. doi: 10.1093/jxb/erq350. [DOI] [PubMed] [Google Scholar]
- 55.Den HG, Van IG, Beeckman T, De SI. The roots of a new green revolution. Trends Plant Sci. 2010;15:600–607. doi: 10.1016/j.tplants.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 56.Lynch JP. Roots of the second green revolution. Aust J Bot. 2007;55:493–512. [Google Scholar]
- 57.Blum A. Breeding crop varieties for stress environments. Crit Rev Plant Sci. 1984;2:199–238. [Google Scholar]
- 58.Szira F, Balint AF, Borner A, Galiba G. Evaluation of drought-related traits and screening methods at different developmental stages in spring barley. J Agron Crop Sci. 2008;194(5):334–342. [Google Scholar]
- 59.Zadeh L. Fuzzy sets. Inf Control. 1965;8:338–353. [Google Scholar]
- 60.Fischer RA, Wood JT. Drought resistance in spring wheat cultivars III. Yield association with morphological traits. Aust J Agric Res. 1979;30:1001–1020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated or analyzed during this study are available from the corresponding author on reasonable request.