Abstract
Ferroptosis is an iron-dependent cell death modality driven by oxidative phospholipid damage. In contrast to apoptosis, which enables organisms to eliminate targeted cells purposefully at specific times, ferroptosis appears to be a vulnerability of cells that otherwise use high levels of polyunsaturated lipids to their advantage. Cells in this high polyunsaturated lipid state generally have safeguards that mitigate ferroptotic risk. Since its recognition, ferroptosis has been implicated in degenerative diseases in tissues including kidney and brain, and is a targetable vulnerability in multiple cancers—each likely characterized by the high polyunsaturated lipid state with insufficient or overwhelmed ferroptotic safeguards. In this Perspective, we present progress towards defining the essential roles and key mediators of lipid peroxidation and ferroptosis in disease contexts. Moreover, we discuss gaps in our understanding of ferroptosis, and list key challenges that have thus far limited the full potential of targeting ferroptosis for improving human health.
Introduction
Ferroptosis is an iron-dependent, nonapoptotic cell death modality characterized by aberrant accumulation of lipid hydroperoxides and associated lipophilic reactive oxygen species (ROS) in cellular membranes (Dixon et al., 2012). Prior to the introduction of the term “ferroptosis”, which first described the cell death process in fibroblasts co-transformed by mutant HRAS and treated with Ras-selective lethal (RSL) compounds erastin and 1S,3R-RSL3 (RSL3) (Dixon et al., 2012), cell death of ferroptotic nature had been demonstrated by several findings in the context of cysteine deprivation, iron toxicity, and hepatic necrosis 5–6 decades ago (Bannai et al., 1977; Eagle, 1955a, 1955b; Golberg et al., 1962; Mitchell et al., 1973). Recently, the emergence of a series of ferroptosis-specific chemical modulators significantly accelerated the discovery of ferroptosis in contexts of degenerative diseases and cancer (Alim et al., 2019; Friedmann Angeli et al., 2014; Hangauer et al., 2017; Li et al., 2017; Viswanathan et al., 2017; Yang et al., 2014; Zou et al., 2019a). Modulating ferroptosis is an appealing therapeutic strategy for developing novel treatments, which could involve induction or inhibition of ferroptosis depending on the pathological context.
The path to ferroptosis
The introduction of polyunsaturated fatty acids (PUFA) into cellular membranes through evolution in aerobic life yielded advantages to certain cells, yet the facile oxidation of PUFAs to lipid hydroperoxides created an existential crisis. While PUFA lipids provide distinct membrane fluidity and are precursors of diverse signaling molecules, their 1,4-diene structural elements are susceptible to facile hydroperoxidation. In vitro, lipid hydroperoxidation is initiated by a reactive iron species-mediated hydrogen abstraction from the methylene group flanked by C-C double bonds that leads to a bis-allylic (pentadienyl) radical (Sevanian et al., 1990; Ursini et al., 1989; Winterbourn, 1995) (Figure 1). These pentadienyl radicals can react with dioxygen (O2) to form lipid peroxyl radicals, which can then react with another neighboring PUFA phospholipid, forming a lipid hydroperoxide and propagating the radical chain reaction, in a process called autoxidation. Additional reactive iron species will convert lipid hydroperoxides to various radical species, including oxy, peroxy, and carbon radicals that are extremely reactive towards proteins, lipids, and other metabolites. While these radical species are thought to be the terminal effectors of ferroptotic cell death, this remains to be definitively shown.
Figure 1. Chemical basis of lipid peroxidation and ferroptosis.
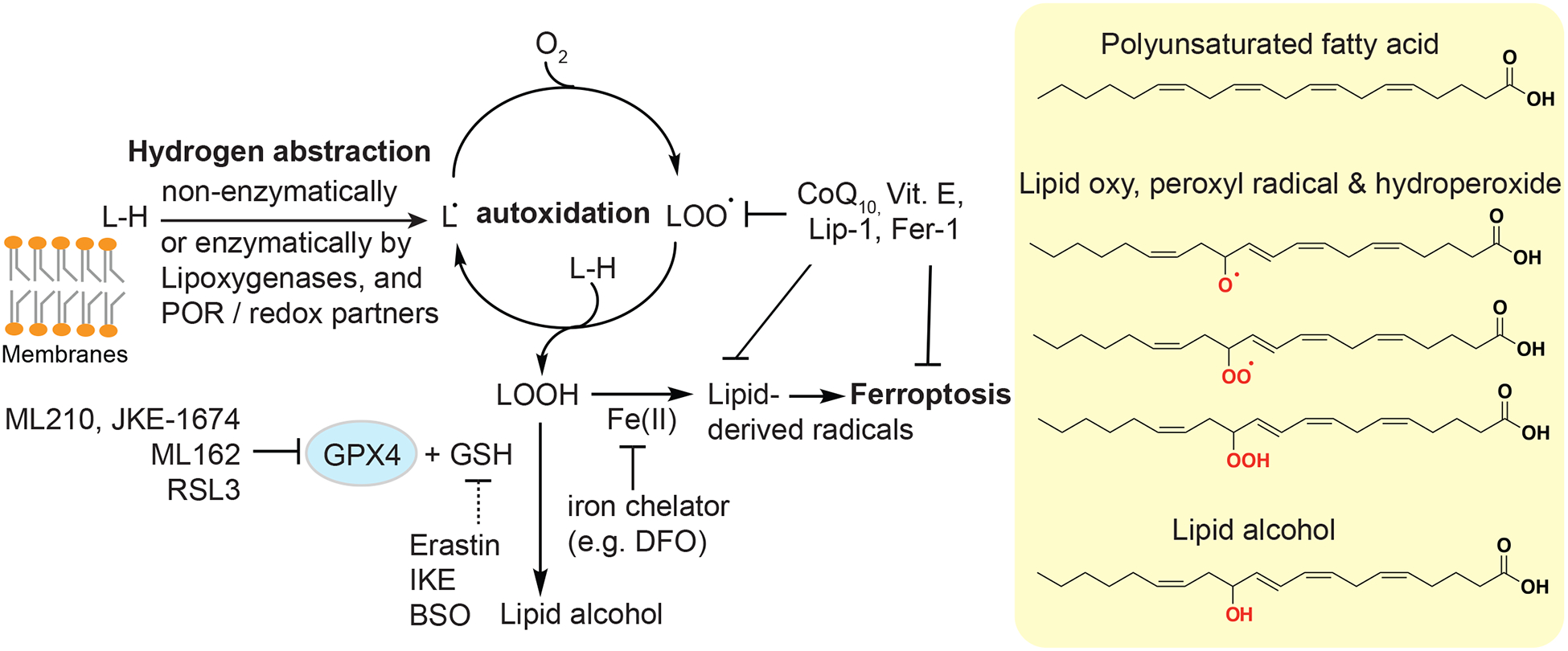
Abbreviations: GPX4, glutathione peroxidase 4; L-H, symbol for polyunsaturated lipids; Vit. E, vitamin E; Lip-1, liproxstatin-1; Fer-1, ferrostatin-1; IKE, imidazole ketone erastin; DFO, deferoxamine; BSO, L-Buthionine-(S,R)-Sulfoximine; GSH, reduced glutathione.
At the cellular level, lipid peroxidation typically initiates in the endoplasmic reticulum, and can rapidly spread to other subcellular structures (Kagan et al., 2017). Lipid hydroperoxides and byproducts alter the electric potential, fluidity, and permeability of membranes to cause osmotic balance, membrane breakage, and ultimately cell death. Given the central role of iron and lipid peroxidation in ferroptosis, this form of cell death is experimentally characterized by the restoration of cellular viability by iron chelation and lipophilic radical-trapping antioxidants (Conrad and Pratt, 2019; Dixon et al., 2012). Another potentially relevant feature of ferroptosis, though it awaits to be validated in many contexts, is the wave-like death pattern in adherent cell cultures in vitro or synchronized cell death in tissues ex vivo which may result from the propagative nature of autoxidation (Kim et al., 2016; Linkermann et al., 2014).
In eukaryotes, the risk of PUFA-lipid peroxidation is safeguarded by a molecular defense system involving the selenoenzyme glutathione peroxidase 4 (GPX4) (Ursini et al., 1982). Unlike other GPXs, GPX4 is the only known cellular enzyme that uses phospholipid hydroperoxides as substrates and reduces them to lipid alcohols with glutathione (GSH) as a cofactor (Friedmann Angeli et al., 2014; Ingold et al., 2018; Ursini et al., 1982; Yang et al., 2014). In single-celled parasites such as Trypanosoma brucei (Bogacz and Krauth-Siegel, 2018), the role of GPX4 is executed by a functional homolog tryparedoxin peroxidase (Tpx). The activity of GPX4 and orthologs maintains lipid hydroperoxides below threshold levels that trigger self-propagation and cell death (Figure 1). In addition, cellular lipophilic antioxidants such as vitamin E and ubiquinol, the reduced form of CoQ10, inhibit lipid peroxidation and ferroptosis with their radical-trapping activities (Bersuker et al., 2019; Doll et al., 2019; Matsushita et al., 2015; Zilka et al., 2017). Hence, ferroptosis has been referred to as a cell “sabotage” mechanism that protect cell integrity under normal conditions, but when compromised, leads to cell death as a result of sustained cellular physiology and metabolism (Green, 2019). This is in contrast to cell “suicide” programs such as apoptosis, which involve cellular pathways that have evolved to promote the elimination of certain cells in the whole organism at specific times (Green, 2019).
The chemical bas is of ferroptosis supports wide opportunities for inhibiting or instigating this pathway via chemical intervention (Conrad and Pratt, 2019). For instance, erastin, an inhibitor of SLC7A11 (also known as system xc-), the cystine/glutamate antiporter (Dixon et al., 2012, 2014; Dolma et al., 2003), and GPX4 inhibitors including ML210, ML162, and RSL3, can induce ferroptosis in certain high-PUFA cell types (Eaton et al.,2020; Viswanathan et al., 2017; Yang et al., 2014; Zou et al., 2019a). On the other hand, ferroptosis is inhibited by lipophilic radical-trapping antioxidants such as liproxstatin-1 (Dixon et al., 2012; Friedmann Angeli et al., 2014; Zilka et al., 2017), or by iron chelators, such as deferoxamine (DFO) (Dixon et al., 2012; Zilka et al., 2017) (Figure 1). Noteworthily, ferrostatin-1, one of the first established ferroptosis inhibitors, acts both as a radical-trapping agent and an unusual iron chelator (Dixon et al. 2012; Zilka et al., 2017; Miotto et al. 2020). Specific chemical modulators of ferroptosis also empowered preclinical and clinical studies that aim to target this pathway for therapeutic benefits.
Ferroptosis in physiology
There is still a gap in our understanding of the physiological role of ferroptosis. On one hand, GPX4−/− mice are embryonically lethal (Brütsch et al., 2015; Imai et al., 2003), suggesting that protecting against ferroptosis is critical for mammalian development. On the other hand, ferroptotic cell death per se is not known to be directly involved in developmental processes, and systemic ferroptosis inhibition with liproxstatin-1 administration in adult mice for an extended time period did not result in apparent adverse effects (Devisscher et al., 2018; Friedmann Angeli et al., 2014).Nonetheless, ferroptosis plays important roles in defending against pathogens in both animals and plants. For instance, lipid peroxidation and ferroptosis are used to guard against Plasmodium infection in mice (Kain et al., 2020), which is suppressed by erastin treatment. In plants, ferroptosis is activated in the blast fungus Magnaporthe oryzae during rice development (Dangol et al., 2019; Shen et al., 2019). Ferroptosis-like cell death also takes place in Arabidopsis thaliana in response to environmental stress such as heat-shock (Distéfano et al., 2017).
In addition to fending off parasites, ferroptosis is also important for defending against tumorigenesis. Two key tumor suppressor proteins, p53 (TP53) (Jiang et al., 2015) and BAP1 (Zhang et al., 2018a), independently suppress SLC7A11 expression. SLC7A11 downregulation significantly increases the susceptibility to ferroptosis in nascent neoplastic cells, thus keeping tumorigenesis in check. Importantly, GPX4+/− mice survive longer than GPX4+/+ mice, apparently due to delayed lymphoma, a common malignancy in aging mice. This finding supports a role for GPX4 insufficiency-induced ferroptosis in suppressing spontaneous oncogenesis (Ran et al., 2007). A second form of tumor suppression occurs in the context of CD8+ cytotoxic T lymphocyte (CTL)-mediated tumor killing in mouse melanoma models (Wang et al., 2019), where CTLs reactivated by immune checkpoint blockers trigger ferroptotic cell death in the tumor cells and evoke tumor regression.
Ferroptosis in pathology
Ferroptosis is implicated in multiple human degenerative diseases characterized by dysregulated cell death. Several vital organs, including the kidney, brain, liver, heart, and lung, appear to be highly susceptible to ferroptosis under pathological conditions. This is likely due to the increased PUFA-lipid contents in cells from the brain, kidney, and heart (Harayama and Riezman, 2018), frequent iron overload in the liver (Wang et al., 2017), or the well-oxygenated microenvironment in the lung. In addition to tissue intrinsic factors, the form of tissue damage appears to be common denominators for increased risk of ferroptosis. Recently characterized, ferroptosis-relevant pathologies include:
Ischemia/reperfusion injury (IRI), traumatic tissue injury, and hemorrhagic stroke. IRI-triggered ferroptosis is reported in the kidney (Friedmann Angeli et al., 2014; Huang et al., 2019; Su et al., 2019), liver (Carlson et al., 2016; Yamada et al., 2020), intestine (Li et al., 2019), brain (Guan et al., 2019), and heart (Fang et al., 2019). The efficacy of anti-ferroptosis agents in protecting acute kidney injury is demonstrated by several FDA-approved drugs, including rifampicin, promethazine, omeprazole, indole-3-carbinol, carvedilol, propranolol, estradiol, and thyroid hormones that exhibit lipid peroxyl radical-scavenging activities (Mishima et al., 2019), in addition to liproxstatin-1 (Friedmann Angeli et al., 2014). Moreover, ferroptosis also contributes to traumatic brain injury (TBI), stroke (Alim et al., 2019; Wenzel et al., 2017), and seizure-induced hippocampal damage (Mao et al., 2019). Notably, IRI, TBI, and stroke share features of abrupt blood-supply blockade, which may activate hypoxia response initially, reprogram cellular lipid metabolism, and induce a burst in oxidative stress levels following re-oxygenation in the tissue. Uncontrolled iron release to the circulation during hemolysis may also contribute to ferroptosis in normal and non-nucleated cells such as platelets (NaveenKumar et al., 2018). Together, the involvement of ferroptosis in IRI and traumatic injury-induced tissue damage is likely a general mechanism, and testing the protective role of ferroptosis inhibitory agents in these settings merits further investigation.
Tissue degeneration, particularly neurodegeneration. Ferroptosis is implicated in neurodegenerative diseases including Alzheimer’s disease (Hambright et al., 2017; Zhang et al., 2018b), Parkinson’s disease (Do Van et al., 2016), Pelizaeus-Merzbacher Disease (Nobuta et al., 2019), Huntington’s Disease (Skouta et al., 2014), and amyotrophic lateral sclerosis (ALS) (Devos et al., 2019). The high susceptibility to ferroptosis in neurons and glial cells is correlated with their high polyunsaturated lipid levels (Harayama and Riezman, 2018; Nobuta et al., 2019), although the triggering of cell death may be a cumulative event due to chronically reduced antioxidant capacity in these cells (Maher, 2018).
Damage induced by specific chemicals or pathogens. For instance, nephrotic folic acid over-dosing induces ferroptosis in renal nephrons (Linkermann et al., 2014; Martin-Sanchez et al., 2017), whereas doxorubicin induces heart damage that can be alleviated by the iron chelator dexrazoxane (Fang et al., 2019; Yu et al., 2019). In the lung, ferroptosis of epithelial cells can be induced by smoke in patients with chronic obstructive pulmonary disease (COPD) (Yoshida et al., 2019), and by infection with Mycobacterium tuberculosis (Amaral et al., 2019) or lipoxygenase-expressing Pseudomonas aeruginosa (Dar et al., 2018). Cigarette smoke extract can induce ferroptosis in vascular smooth muscle cells as well (Sampilvanjil et al., 2020). The list of ferroptosis-eliciting chemicals and pathogens will likely expand.
Overall, ferroptosis is not only induced by pharmacological or genetic intervention, but also occurs in tissues under a wide range of pathophysiological conditions, highlighting that inhibiting ferroptosis could be beneficial for treating these diseases. In addition to the known ferroptosis-relevant tissue malfunctions discussed above, lipid peroxidation and ferroptosis-like events occur more broadly in other human diseases, such as atherosclerosis (Berliner and Heinecke, 1996), diabetes mellitus (Davì et al., 2005), Alexander’s disease (Castellani et al., 1998), inflammatory bowel disease (Achitei et al., 2013), prion disease (Brazier et al., 2006; Milhavet et al., 2000), and chronic alcohol exposure (Shaw, 1989; Shaw et al., 1981). Hence, a full understanding on the mechanisms of lipid peroxidation and ferroptos is has tremendous translational value.
Ferroptosis in cancer
Cancer cell lines from several lineages exhibit intrinsic sensitivity to ferroptosis, including clear-cell renal cell and ovarian carcinoma (Miess et al., 2018; Zou et al., 2019a), triple-negative breast cancer (Doll et al., 2017), adrenocortical carcinomas (Belavgeni et al., 2019), diffuse large B-cell lymphoma (Yang et al., 2014), and cancer cells from the liver (Louandre et al., 2013; Sun et al., 2016), pancreas (Badgley et al., 2019), and colorectum (Singhal et al., 2019). Moreover, susceptibility to ferroptosis can be acquired during cell state transitions in cancer, such as the development of therapy resistance (Hangauer et al., 2017; Tsoi et al., 2018; Viswanathan et al., 2017). Though the mechanisms underlying this sensitivity shift remain to be fully established, inducing ferroptosis represents a potential avenue for overcoming cancer-drug resistance.
Additionally, several unique cancer cell states exhibit key features that resemble ferroptosis-sensitive states. For instance, metastasis-initiating cells disseminated to distant organs generally exhibit high levels of oxidative stress (Piskounova et al., 2015; Tasdogan et al., 2020), and demand increased PUFAs to maintain stemness, motility, and intracellular signaling (Zou et al., 2019b). One pathway of meeting the demand in PUFA lipids in brain metastasis cells is to uptake free PUFAs secreted by astrocytes (Zou et al., 2019b). Whether these cancer cells are sensitive to ferroptosis requires further characterization.
On the other hand, other existing anti-cancer therapies may act partly by triggering ferroptosis in the target cells, including sorafenib in liver cancer (Louandre et al., 2013), artesunate in pancreatic cancer (Eling et al., 2015), and lapatinib in breast cancer cells (Ma et al., 2016). Ionizing radiation sensitizes cells to ferroptosis in cancer cells (Ye et al. 2020; Lang et al. 2019; Lei et al. 2020) as well as during bone marrow transplantation (Zhang et al., 2020). Ferroptosis is also an effector pathway for cancer immunotherapy (Wang et al., 2019). Together, inducing ferroptosis in cancer represents a promising strategy to lower tumor burden and overcome therapy resistance.
Mechanisms of ferroptosis evasion
Though inducing ferroptosis holds great potential in curbing the progression of human cancers, many cancer cells are intrinsically resistant to ferroptosis induction. While three rate-limiting factors are required to drive a ferroptosis-susceptible cell state, including PUFA-based phospholipids (Doll et al., 2017; Kagan et al., 2017; Yang et al., 2016), reactive iron (Gao et al., 2015), and cellular oxidants, reprogramming each of these metabolic pathways marks the common routes to confer resistance to ferroptosis in cells (Figure 2):
-
Reducing polyunsaturated lipid levels. For instance, breast cancer cells exhibiting lower expression levels of the PUFA-selective esterification enzyme, acyl-CoA synthetase, long-chain family member 4 (ACSL4), are less susceptible to ferroptosis induction (Doll et al., 2017). The expression of ACSL4 can also be suppressed by α6β4 integrin-mediated Src and STAT3 pathway activation (Brown et al., 2017).
Despite the clear contribution of PUFA-lipids to ferroptosis, how cells selectively regulate PUFA-lipid levels is poorly understood. In renal cell carcinoma cells, hypoxia-inducible factor 2-ɑ (HIF-2ɑ) positively and selectively regulates PUFA-lipids by activating hypoxia-induced, lipid droplet-associated protein (HILPDA) (Zou et al., 2019a) (Figure 2), although the biochemical basis for HILPDA’s PUFA-selectivity remains unclear. The pro-ferroptosis role of HIF-2ɑ is recapitulated in colorectal cancers (Singhal et al., 2019). Considering the complex physiological roles of polyunsaturated lipids, cells likely shape their PUFA-lipidome by their intrinsic cell states and by responding to environmental stimuli.
Restricting iron availability. For instance, mammary epithelial and breast carcinoma cells can resist ferroptosis by restricting iron accessibility via prominin 2 (PROM2) up-regulation (Brown et al., 2019) (Figure 2), whereas lung adenocarcinoma cells express high levels of iron-sulfur cluster biosynthesis enzyme cysteine desulfurase (NFS1) to store iron into iron-responsive proteins, therefore limiting iron required for lipid peroxidation (Alvarez et al., 2017).
Augmenting cellular antioxidant capacity and/or downregulating ROS production. Cancer cells can replenish cellular ubiquinol by upregulating apoptosis inducing factor, mitochondrion-associated, 2 (AIFM2, recently renamed as ferroptosis suppressor protein 1, or FSP1) (Bersuker et al., 2019; Doll et al., 2019), which leads to a ferroptosis-resistant state. Recently, AIFM2 is also shown to contribute to ferroptosis resistance via recruiting the endosomal sorting complexes required for transport (ESCRT)-III machinery for membrane repair (Dai et al. 2020). Alternatively, ALK+ lymphoma cells resist lipid peroxidation by shunting the cholesterol biosynthesis pathway towards production of more squalene, a cellular intrinsic antioxidant (Garcia-Bermudez et al., 2019). Additionally, the activation of the nuclear factor, erythroid 2-like 2 (NRF2, NFE2L2) antioxidant program is perhaps a general mechanism of surviving under oxidative stress (Dodson et al., 2019; Sun et al., 2016). NRF2 activates a wide range of antioxidant enzymes and regulators, including SLC7A11 (Dodson et al., 2019; Fan et al., 2017). Though less understood, reducing the level of receptor-interacting serine/threonine-protein kinase 1 (RIPK1) results in glutathione up-regulation and resistance to necroptosis, a programmed form of necrosis, and ferroptosis in fibroblasts from patients of cleavage-resistant RIPK1-induced autoinflammatory syndrome (Tao et al., 2020). Ferroptosis can also be suppressed via intercellular mechanisms involving the cadherin-mediated neurofibromatosis type 2 (NF2) and Hippo pathway activation (Wu et al., 2019).
NO•-mediated scavenging of lipid radicals. The nitroxygenation of eicosatetraenoyl-phosphatidylethanolamine intermediates and oxidatively truncated lipid species during ferroptosis was recently demonstrated in the dynamic regulation of ferroptosis susceptibility in macrophages and microglia, where NO• donors or inducible nitric oxide synthase (iNOS) expression induces resistance of M2 macrophages to ferroptosis (Kapralov et al. 2020). The involvement of the iNOS/NO• pathway in ferroptosis regulation in other contexts is worth future investigation.
Figure 2. Mechanisms mediating ferroptosis sensitivity and resistance in cells.
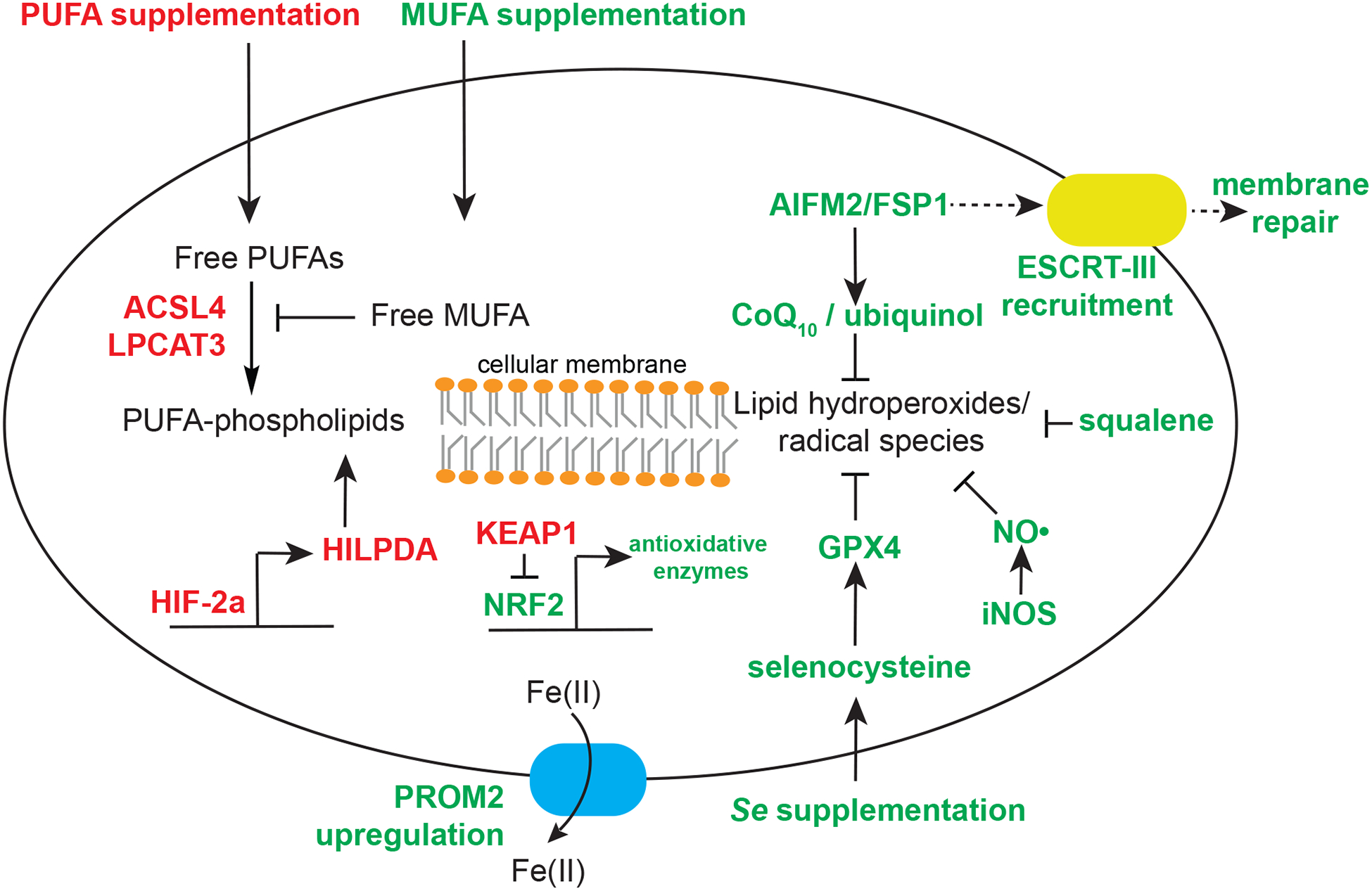
Red: genes, metabolites, and methods that increase cellular sensitivity to ferroptosis. Green, genes, metabolites, and methods that decrease cellular sensitivity to ferroptosis. Abbreviations: PUFA, polyunsaturated fatty acids; MUFA, monounsaturated fatty acids; PROM2, Prominin2; AIFM2/FSP1, apoptosis inducing factor, mitochondrion-associated, 2 (recently renamed as ferroptosis suppressor protein 1); HILPDA, hypoxia-induced, lipid droplet associated protein; HIF-2ɑ, hypoxia-inducible factor 2ɑ; ACSL4, acyl-CoA synthetase, long-chain family member 4; LPCAT3, lysophosphatidylcholine acyltransferase 3; ESCRT, endosomal sorting complexes required for transport machinery; iNOS, inducible nitric oxide synthase.
Conclusions and Future Perspective
The last eight years mark an era of rapid progress in defining the biochemical basis of ferroptosis, developing novel chemical tools, and identifying ferroptosis-relevant disease contexts. To gain a full understanding on the mechanisms of this cell death modality, we pose the following questions that we hope will stimulate the next phase of ferroptosis research:
-
What is the basis of lipid peroxidation? Lipid peroxidation can occur non-enzymatically via autoxidation or enzymatically. While excessive non-enzymatic lipid peroxidation can lead to membrane damage and nonspecific cell death, whether this process is the dominating pathway to ferroptotic cell death in certain contexts remains unclear, and future research is required to examine the relative contribution of these two peroxidation mechanisms in ferroptosis.
On the other hand, the cellular enzymes involved in directly catalyzing lipid peroxidation remain mysterious. Prior research largely focused on characterizing the activity and specificity of lipoxygenases (encoded by arachidonate lipoxygenases, or ALOXs), which are non-heme iron-containing dioxygenases that catalyze dioxygenation of PUFAs in a stereo-and regio-specific manner (Kuhn et al. 2015). However, the direct involvement of lipoxygenases in ferroptosis execution is recently questioned by the intrinsic radical trapping activities of small-molecule lipoxygenase inhibitors, and by the limited ALOX mRNA expression in a wide collection of ferroptosis-susceptible cancer cells (Shah et al. 2018; Zou et al. 2020). Meanwhile, alternative enzymes including cytochrome P450 oxidoreductase (POR) were identified as crucial and general ferroptosis factors in multiple cancer lineages (Zou et al. 2020) (Figure 1). While the POR redox partners that directly interact with PUFA-phospholipids remain unclear, the context-specific contributions of lipoxygenases, POR, and other lipid peroxidation mediators merit further investigation. Another relevant question is the relative contribution of regioselective versus nonspecific lipid peroxidation in ferroptosis.
What are the identities and properties of the death-executing molecules resulting from PUFA-lipid peroxidation, and what types of membrane damage are necessary/sufficient to cause cell death? One plausible mechanism is that death is executed by the formation of adducts of electrophilic oxidatively truncated lipids with one or more nucleophilic protein targets.
Are all polyunsaturated lipids equal in initiating and propagating lipid peroxidation? Prior studies suggest that arachidonic acid (C20:4)- or adrenic acid (C22:4)-containing phosphatidylethanolamines (PE), but not phosphatidylcholines (PC) with the same side chains, are responsible for ferroptosis (Kagan et al., 2017). However, considering that PEs are largely located on the inner leaflet (Yamaji-Hasegawa and Tsujimoto, 2006), whereas PCs are most frequently on the outer leaflet of cellular membranes, it remains unknown as to whether these distinctions result from differences in intrinsic chemical reactivity or physical accessibility. Overall, within each lipid class, the chemical and biological properties that may shape their ability to undergo oxidation presumably include, but are not limited to chemical reactivity, cellular abundances, subcellular distribution, membrane asymmetry, packing conformation, and neighboring lipid environment.
Where is lipid peroxidation initiated in the cell, and what is the structural basis supporting initiation? Several organelles including endoplasmic reticulum (Kagan et al., 2017), mitochondria (Gao et al., 2019), and lysosome (Gao et al., 2018; Torii et al., 2016) have been implicated in ferroptosis. Although lipid peroxidation likely spreads among organelles rapidly, the precise contributions of each of these organelles and their sequence of events remain unclear.
What are the physiological roles of ferroptosis? Though ferroptosis appears to be dispensable for mammalian early development, the roles of ferroptosis in tissue homeostasis, neuronal network formation and maintenance, learning and memory, immunity, and normal aging in adult life await to be fully examined.
It remains largely unknown whether acquired resistance to ferroptosis would occur in cancer patients who are exposed to ferroptosis-inducing agents. Considering the high relapse rate after treatment with most targeted therapies and the metabolic plasticity of cancer cells, ferroptosis resistance will likely emerge as well. What are the molecular paths to acquired ferroptosis resistance? What will the the rapeutic options be for these patients? If the involvement of ferroptosis in cytotoxic T cell-mediated tumor killing (Wang et al., 2019) is generalizable to many other cancer types besides melanoma, adopting a ferroptosis-resistant state in cancer cells may attenuate the efficacy of immunotherapy. Hence, understanding the mechanisms of acquired ferroptosis resistance in vivo has important therapeutic value.
In addition to these mechanistic questions, several therapeutic challenges remain before the power of ferroptosis-targeted strategies can be harnessed fully to benefit human health:
-
Small molecule ferroptosis inducers that are more potent and suitable for in vivo use are urgently needed. Imidazole ketone erastin (IKE) prepared as nanoparticles and cyst(e)inase, an engineered enzyme, have been reported to have promise, with appreciable activity in mouse tumor models (Zhang et al., 2019; Badgley et al., 2020). Among the GPX4 inhibitors, ML210 and its derivatives including JKE-1674 emerged as the most selective covalent inhibitors of GPX4 (Eaton et al.,2020) (Figure 1), although the current compounds exhibit poor or imperfect pharmacological properties when administered to animal models in vivo.
If a bioavailable ferroptosis inducer becomes available, what will the therapeutic window and toxicity profile be in humans? Based on mouse and limited human genetic results, it is possible that toxicities will emerge in tissues like the kidney, liver, and central nervous system. Thorough pharmacological analyses are required to evaluate the timing and extent of drug-induced toxicities, and to assess whether these toxicities can be mitigated by optimizing drug dosing and scheduling. Furthermore, better molecular markers that are predictive of ferroptosis sensitivity in vivo are needed to assist in stratifying patients for their likelihood of responding to ferroptosis-modulating agents.
Efficacious, bioavailable ferroptosis inhibitors targeting novel elements of ferroptosis biology are needed. Several categories of ferroptosis inhibitors that target different segments of the lipid peroxidation cascade have been developed: lipophilic RTAs including liproxstatin (Friedmann Angeli et al., 2014), ferrostatin (Dixon et al., 2012; Skouta et al., 2014) and analogs (Devisscher et al., 2018; Hofmans et al., 2016), monounsaturated fatty acids that displace PUFAs from phospholipids (Magtanong et al., 2019), inhibitors of PUFA-lipid biosynthetic enzymes such as ACSL4 (Doll et al., 2017), and selenium supplementation which augments GPX4 protein expression (Alim et al., 2019). Despite the report that no toxicities have been seen with chronic ferroptosis inhibition in mice to date (Devisscher et al., 2018; Friedmann Angeli et al., 2014), it seems likely that toxicities will emerge in humans undergoing ferroptosis preventive therapies. The physiological effects of long-term lipid peroxidation blockade in humans remain to be characterized, and strategies designed to be tissue-specific may be needed. Furthermore, while current ACSL4 inhibitors such as thiazolidinediones are also PPAR- γ agonists and likely will exhibit off-target effects (Doll et al., 2017), the use of ferrostatin and liproxstatin in vivo has been limited by their pharmacokinetic properties (Devisscher et al., 2018; Hofmans et al., 2016). Future research is required to develop ferroptosis inhibitors that are compatible with clinical use.
More effective and accessible biomarkers for reporting ferroptosis occurrence in patients and biological samples are needed. At the moment, functional assays utilizing ferroptosis inhibitors are required to establish whether certain cell death events involve the ferroptosis cascade, precluding the utility of this strategy in humans or tissue samples. Previously proposed biomarkers include prostaglandin E synthase 2 (PTGS2) and ChaC glutathione-specific gamma-glutamylcyclotransferase 1 (CHAC1) mRNA induction (Dixon et al., 2014; Yang et al., 2014). However, due to the lack of a clear understanding on the molecular relationship and a robust correlation between these genes and ferroptosis, the utility of these markers in the clinic is limited. Immunostaining analysis of 4-hydroxynonenal (4-HNE), a lipid peroxidation byproduct, offers a useful approximation of lipid peroxidation (Paradis et al., 1997; Riahi et al., 2010); nonetheless, the poor stability of 4-HNE in tissues and low sensitivity of the assay preclude this method from broader usage. Meanwhile, advances in redox phospholipidomics may enable more direct detection of lipid peroxidation in body fluids and tissue biopsy (Kagan et al., 2017, 2019).
With the resolution of these issues, we will have a clearer understanding on how cells cope with lipid peroxidation and survive under oxidative stress in pathophysiological conditions, and we will be closer to having novel ferroptosis-targeted therapies. Moreover, considering the various environmental stressors cells face in a complex organism, additional metabolic cascades that impact the state and fate of cells, like the peroxidation of polyunsaturated lipids described here, are likely to be discovered.
ACKNOWLEDGEMENTS
We thank Whitney S. Henry, Haoxin Li, Tanaz Sharifnia, John K. Eaton and Emily. T. Graham for insightful discussions and suggestions. This work was supported by the NCI’s Cancer Target Discovery and Development (CTD2) Network (grant number U01CA217848, awarded to S.L.S.). Y.Z. was supported by the National Cancer Institute of the National Institutes of Health under Award Number K99CA248610. We apologize to those whose work could not be discussed here due to space restrictions.
Footnotes
DECLARATION OF INTERESTS
S.L.S. serves on the Board of Directors of the Genomics Institute of the Novartis Research Foundation (“GNF”); is a shareholder and serves on the Board of Directors of Jnana Therapeutics; is a shareholder of Forma Therapeutics; is a shareholder and advises Kojin Therapeutics, Kisbee Therapeutics, Decibel Therapeutics and Eikonizo Therapeutics; serves on the Scientific Advisory Boards of Eisai Co., Ltd., Ono Pharma Foundation, Exo Therapeutics, and F-Prime Capital Partners; and is a Novartis Faculty Scholar. Kojin Therapeutics in particular explores the medical potential of cell plasticity related to ferroptosis. Y.Z. declares no competing interests related to this work.
REFERENCES
- Achitei D, Ciobica A, Balan G, Gologan E, Stanciu C, and Stefanescu G (2013). Different profile of peripheral antioxidant enzymes and lipid peroxidation in active and non-active inflammatory bowel disease patients. Dig. Dis. Sci 58, 1244–1249. [DOI] [PubMed] [Google Scholar]
- Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E, Seravalli J, Ai Y, Sansing LH, Ste Marie EJ, et al. (2019). Selenium Drives a Transcriptional Adaptive Program to Block Ferroptosis and Treat Stroke. Cell 177, 1262–1279.e25. [DOI] [PubMed] [Google Scholar]
- Alvarez SW, Sviderskiy VO, Terzi EM, Papagiannakopoulos T, Moreira AL, Adams S, Sabatini DM, Birsoy K, and Possemato R (2017). NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature 551, 639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral EP, Costa DL, Namasivayam S, Riteau N, Kamenyeva O, Mittereder L, Mayer-Barber KD, Andrade BB, and Sher A (2019). A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis. J. Exp. Med 216, 556–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgley MA, Kremer D, Carlo Maurer H, DelGiorno KE, Lee H-J, Purohit V, Sagalovskiy I, Ma A, Kapillian J, Firl CEM, et al. (2019). “Induction of pancreatic tumor-selective ferroptosis through modulation of cystine import.” [Google Scholar]
- Bannai S, Tsukeda H, and Okumura H (1977). Effect of antioxidants on cultured human diploid fibroblasts exposed to cystine-free medium. Biochem. Biophys. Res. Commun 74, 1582–1588. [DOI] [PubMed] [Google Scholar]
- Belavgeni A, Bornstein SR, von Mässenhausen A, Tonnus W, Stumpf J, Meyer C, Othmar E, Latk M, Kanczkowski W, Kroiss M, et al. (2019). Exquisite sensitivity of adrenocortical carcinomas to induction of ferroptosis. Proc. Natl. Acad. Sci. U. S. A 116, 22269–22274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliner JA, and Heinecke JW (1996). The role of oxidized lipoproteins in atherogenesis. Free Radic. Biol. Med 20, 707–727. [DOI] [PubMed] [Google Scholar]
- Bersuker K, Hendricks J, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al. (2019). The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogacz M, and Krauth-Siegel RL (2018). Tryparedoxin peroxidase-deficiency commits trypanosomes to ferroptosis-type cell death. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazier MW, Lewis V, Ciccotosto GD, Klug GM, Lawson VA, Cappai R, Ironside JW, Masters CL, Hill AF, White AR, et al. (2006). Correlative studies support lipid peroxidation is linked to PrP(res) propagation as an early primary pathogenic event in prion disease. Brain Res. Bull 68, 346–354. [DOI] [PubMed] [Google Scholar]
- Brown CW, Amante JJ, Goel HL, and Mercurio AM (2017). The α6β4 integrin promotes resistance to ferroptosis. J. Cell Biol 216, 4287–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CW, Amante JJ, Chhoy P, Elaimy AL, Liu H, Zhu LJ, Baer CE, Dixon SJ, and Mercurio AM (2019). Prominin2 Drives Ferroptosis Resistance by Stimulating Iron Export. Dev. Cell 51, 575–586.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brütsch SH, Wang CC, Li L, Stender H, Neziroglu N, Richter C, Kuhn H, and Borchert A (2015). Expression of Inactive Glutathione Peroxidase 4 Leads to Embryonic Lethality, and Inactivation of the Alox15 Gene Does Not Rescue Such Knock-In Mice. Antioxidants & Redox Signaling 22, 281–293. [DOI] [PubMed] [Google Scholar]
- Carlson BA, Tobe R, Yefremova E, Tsuji PA, Hoffmann VJ, Schweizer U, Gladyshev VN, Hatfield DL, and Conrad M (2016). Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Biol 9, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani RJ, Perry G, Harris PL, Cohen ML, Sayre LM, Salomon RG, and Smith MA (1998). Advanced lipid peroxidation end-products in Alexander’s disease. Brain Res 787, 15–18. [DOI] [PubMed] [Google Scholar]
- Conrad M, and Pratt DA (2019). The chemical basis of ferroptosis. Nat. Chem. Biol 15, 1137–1147. [DOI] [PubMed] [Google Scholar]
- Dai E, Zhang W, Cong D, Kang R, Wang J, and Tang D (2020). AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochem. Biophys. Res. Commun 523, 966–971. [DOI] [PubMed] [Google Scholar]
- Dangol S, Chen Y, Hwang BK, and Jwa N-S (2019). Iron- and Reactive Oxygen Species-Dependent Ferroptotic Cell Death in Rice-Magnaporthe oryzae Interactions. The Plant Cell 31, 189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar HH, Tyurina YY, Mikulska-Ruminska K, Shrivastava I, Ting H-C, Tyurin VA, Krieger J, St Croix CM, Watkins S, Bayir E, et al. (2018). Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. J. Clin. Invest 128, 4639–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davì G, Falco A, and Patrono C (2005). Lipid peroxidation in diabetes mellitus. Antioxid. Redox Signal 7, 256–268. [DOI] [PubMed] [Google Scholar]
- Devisscher L, Van Coillie S, Hofmans S, Van Rompaey D, Goossens K, Meul E, Maes L, De Winter H, Van Der Veken P, Vandenabeele P, et al. (2018). Discovery of Novel, Drug-Like Ferroptosis Inhibitors with in Vivo Efficacy. J. Med. Chem 61, 10126–10140. [DOI] [PubMed] [Google Scholar]
- Devos D, Moreau C, Kyheng M, Garçon G, Rolland AS, Blasco H, Gelé P, Timothée Lenglet T, Veyrat-Durebex C, Corcia P, et al. (2019). A ferroptosis-based panel of prognostic biomarkers for Amyotrophic Lateral Sclerosis. Sci. Rep 9, 2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distéfano AM, Martin MV, Córdoba JP, Bellido AM, D’Ippólito S, Colman SL, Soto D, Roldán JA, Bartoli CG, Zabaleta EJ, et al. (2017). Heat stress induces ferroptosis-like cell death in plants. J. Cell Biol 216, 463–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS, et al. (2014). Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 3, e02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson M, Castro-Portuguez R, and Zhang DD (2019). NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol 23, 101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, et al. (2017). ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol 13, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Grocin AG, Xavier da Silva TN, Panzilius E, Scheel C, et al. (2019). FSP1 is a glutathione-independent ferroptosis suppressor. Nature. [DOI] [PubMed] [Google Scholar]
- Dolma S, Lessnick SL, Hahn WC, and Stockwell BR (2003). Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3, 285–296. [DOI] [PubMed] [Google Scholar]
- Do Van B, Gouel F, Jonneaux A, Timmerman K, Gelé P, Pétrault M, Bastide M, Laloux C, Moreau C, Bordet R, et al. (2016). Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol. Dis 94, 169–178. [DOI] [PubMed] [Google Scholar]
- Eagle H (1955a). Nutrition needs of mammalian cells in tissue culture. Science 122, 501–514. [DOI] [PubMed] [Google Scholar]
- Eagle H (1955b). The specific amino acid requirements of a human carcinoma cell (Stain HeLa) in tissue culture. J. Exp. Med 102, 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton JK, Furst L, Ruberto RA, Moosmayer D, Hilpmann A, Ryan MJ, Zimmermann K, Cai LL, Niehues M, Badock V, et al. (2020). Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles. Nat. Chem. Biol 10.1038/s41589-020-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eling N, Reuter L, Hazin J, Hamacher-Brady A, and Brady NR (2015). Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience 2, 517–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, Wirth A-K, Chen D, Wruck CJ, Rauh M, Buchfelder M, and Savaskan N (2017). Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis 6, e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X, et al. (2019). Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. U. S. A 116, 2672–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, et al. (2014). Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol 16, 1180–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Bai Y, Jia Y, Zhao Y, Kang R, Tang D, and Dai E (2018). Ferroptosis is a lysosomal cell death process. Biochem. Biophys. Res. Commun 503, 1550–1556. [DOI] [PubMed] [Google Scholar]
- Gao M, Monian P, Quadri N, Ramasamy R, and Jiang X (2015). Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 59, 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, and Jiang X (2019). Role of Mitochondria in Ferroptosis. Mol. Cell 73, 354–363.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bermudez J, Baudrier L, Bayraktar EC, Shen Y, La K, Guarecuco R, Yucel B, Fiore D, Tavora B, Freinkman E, et al. (2019). Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature 567, 118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golberg L, Martin LE, and Batchelor A (1962). Biochemical changes in the tissues of animals injected with iron. 3. Lipid peroxidation. Biochem. J 83, 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR (2019). The Coming Decade of Cell Death Research: Five Riddles. Cell 177, 1094–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Li X, Yang X, Yan J, Shi P, Ba L, Cao Y, and Wang P (2019). The neuroprotective effects of carvacrol on ischemia/reperfusion-induced hippocampal neuronal impairment by ferroptosis mitigation. Life Sci. 235, 116795. [DOI] [PubMed] [Google Scholar]
- Hambright WS, Fonseca RS, Chen L, Na R, and Ran Q (2017). Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol 12, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangauer MJ, Viswanathan VS, Ryan MJ, Bole D, Eaton JK, Matov A, Galeas J, Dhruv HD, Berens ME, Schreiber SL, et al. (2017). Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551, 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harayama T, and Riezman H (2018). Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol 19, 281–296. [DOI] [PubMed] [Google Scholar]
- Hofmans S, Vanden Berghe T, Devisscher L, Hassannia B, Lyssens S, Joossens J, Van Der Veken P, Vandenabeele P, and Augustyns K (2016). Novel Ferroptosis Inhibitors with Improved Potency and ADME Properties. J. Med. Chem 59, 2041–2053. [DOI] [PubMed] [Google Scholar]
- Huang L, Liao X, Sun H, Jiang X, Liu Q, and Zhang L (2019). Augmenter of liver regeneration protects the kidney from ischaemia-reperfusion injury in ferroptosis. J. Cell. Mol. Med 23, 4153–4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H, Hirao F, Sakamoto T, Sekine K, Mizukura Y, Saito M, Kitamoto T, Hayasaka M, Hanaoka K, and Nakagawa Y (2003). Early embryonic lethality caused by targeted disruption of the mouse PHGPx gene. Biochem. Biophys. Res. Commun 305, 278–286. [DOI] [PubMed] [Google Scholar]
- Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, Roveri A, Peng X, Porto Freitas F, Seibt T, et al. (2018). Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 172, 409–422.e21. [DOI] [PubMed] [Google Scholar]
- Jiang L, Kon N, Li T, Wang S-J, Su T, Hibshoosh H, Baer R, and Gu W (2015). Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520, 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Mao G, Qu F, Angeli JPF, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, et al. (2017). Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol 13, 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Tyurina YY, Sun WY, Vlasova II, Dar H, Tyurin VA, Amoscato AA, Mallampalli R, van der Wel PCA, He RR, et al. (2019). Redox phospholipidomics of enzymatically generated oxygenated phospholipids as specific signals of programmed cell death. Free Radic. Biol. Med 147, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain HS, Glennon EKK, Vijayan K, Arang N, Douglass AN, Fortin CL, Zuck M, Lewis AJ, Whiteside SL, Dudgeon DR, et al. (2020). Liver stage malaria infection is controlled by host regulators of lipid peroxidation. Cell Death Differ. 27, 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Zhang L, Ma K, Riegman M, Chen F, Ingold I, Conrad M, Turker MZ, Gao M, Jiang X, et al. (2016). Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat. Nanotechnol 11, 977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L, Liao P, Zhou J, Zhang Q, Dow A, et al. (2019). Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 9, 1673–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH, Ajani JA, Xiao Q, Liao Z, Wang H, et al. (2020). The role of ferroptosis in ionizing radiation induced cell death and tumor suppression. Cell Res. 30, 146–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Han X, Lan X, Gao Y, Wan J, Durham F, Cheng T, Yang J, Wang Z, Jiang C, et al. (2017). Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight 2, e90777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D, Liu D, Zhang F, Ning S, Yao J, et al. (2019). Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 26, 2284–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz P-S, et al. (2014). Synchronized renal tubular cell death involves ferroptosis. Proc. Natl. Acad. Sci. U. S. A 111, 16836–16841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louandre C, Ezzoukhry Z, Godin C, Barbare J-C, Mazière J-C, Chauffert B, and Galmiche A (2013). Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. International Journal of Cancer 133, 1732–1742. [DOI] [PubMed] [Google Scholar]
- Ma S, Henson ES, Chen Y, and Gibson SB (2016). Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 7, e2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magtanong L, Ko P-J, To M, Cao JY, Forcina GC, Tarangelo A, Ward CC, Cho K, Patti GJ, Nomura DK, et al. (2019). Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem Biol 26, 420–432.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P (2018). Potentiation of glutathione loss and nerve cell death by the transition metals iron and copper: Implications for age-related neurodegenerative diseases. Free Radic. Biol. Med 115, 92–104. [DOI] [PubMed] [Google Scholar]
- Mao X-Y, Jin M-Z, Li Q, Jia J-N, Sun Q-Y, Zhou H-H, Liu Z-Q, and Jin W-L (2019). Lysyl oxidase promotes neuronal ferroptosis exacerbating seizure-induced hippocampal damage. [Google Scholar]
- Martin-Sanchez D Ruiz-Andres O, Poveda J, Carrasco S, Cannata-Ortiz P, Sanchez-Niño MD, Ruiz Ortega M, Egido J, Linkermann A, Ortiz A, et al. (2017). Ferroptosis, but Not Necroptosis, Is Important in Nephrotoxic Folic Acid-Induced AKI. J. Am. Soc. Nephrol 28, 218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M, Freigang S, Schneider C, Conrad M, Bornkamm GW, and Kopf M (2015). T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med 212, 555–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miess H, Dankworth B, Gouw AM, Rosenfeldt M, Schmitz W, Jiang M, Saunders B, Howell M, Downward J, Felsher DW, et al. (2018). The glutathione redox system is essential to prevent ferroptosis caused by impaired lipid metabolism in clear cell renal cell carcinoma. Oncogene 37, 5435–5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhavet O, McMahon HE, Rachidi W, Nishida N, Katamine S, Mangé A, Arlotto M, Casanova D, Riondel J, Favier A, et al. (2000). Prion infection impairs the cellular response to oxidative stress. Proc. Natl. Acad. Sci. U. S. A 97, 13937–13942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto G, Rossetto M, Di Paolo ML, Orian L, Venerando R, Roveri A, Vučković AM, Bosello Travain V, Zaccarin M, Zennaro L, et al. (2020). Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox. Biol 28, 10.1016/j.redox.2019.101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima E, Sato E, Ito J, Yamada K-I, Suzuki C, Oikawa Y, Matsuhashi T, Kikuchi K, Toyohara T, Suzuki T, et al. (2019). Drugs Repurposed as Antiferroptosis Agents Suppress Organ Damage, Including AKI, by Functioning as Lipid Peroxyl Radical Scavengers. J. Am. Soc. Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, and Brodie BB (1973). Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J. Pharmacol. Exp. Ther 187, 211–217. [PubMed] [Google Scholar]
- NaveenKumar SK, SharathBabu BN, Hemshekhar M, Kemparaju K, Girish KS, and Mugesh G (2018). The Role of Reactive Oxygen Species and Ferroptosis in Heme-Mediated Activation of Human Platelets. ACS Chem. Biol 13, 1996–2002. [DOI] [PubMed] [Google Scholar]
- Nobuta H, Yang N, Ng YH, Marro SG, Sabeur K, Chavali M, Stockley JH, Killilea DW, Walter PB, Zhao C, et al. (2019). Oligodendrocyte Death in Pelizaeus-Merzbacher Disease Is Rescued by Iron Chelation. Cell Stem Cell 25, 531–541.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis V, Kollinger M, Fabre M, Holstege A, Poynard T, and Bedossa P (1997). In situ detection of lipid peroxidation by-products in chronic liver diseases. Hepatology 26, 135–142. [DOI] [PubMed] [Google Scholar]
- Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ, and Morrison SJ (2015). Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran Q, Liang H, Ikeno Y, Qi W, Prolla TA, Roberts LJ, Wolf N, VanRemmen H, and Richardson A (2007). Reduction in Glutathione Peroxidase 4 Increases Life Span Through Increased Sensitivity to Apoptosis. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 62, 932–942. [DOI] [PubMed] [Google Scholar]
- Riahi Y, Cohen G, Shamni O, and Sasson S (2010). Signaling and cytotoxic functions of 4-hydroxyalkenals. Am. J. Physiol. Endocrinol. Metab 299, E879–E886. [DOI] [PubMed] [Google Scholar]
- Sampilvanjil A, Karasawa T, Yamada N, Komada T, Higashi T, Baatarjav C, Watanabe S, Kamata R, Ohno N, and Takahashi M (2020). Cigarette Smoke Extract Induces Ferroptosis in Vascular Smooth Muscle Cells. Am. J. Physiol. Heart Circ. Physiol [DOI] [PubMed] [Google Scholar]
- Sevanian A, Nordenbrand K, Kim E, Ernster L, and Hochstein P (1990). Microsomal lipid peroxidation: the role of NADPH—cytochrome P450 reductase and cytochrome P450. Free Radical Biology and Medicine 8, 145–152. [DOI] [PubMed] [Google Scholar]
- Shah R, Shchepinov MS, and Pratt DA (2018). Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent Sci 4, 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S (1989). Lipid peroxidation, iron mobilization and radical generation induced by alcohol. Free Radic. Biol. Med 7, 541–547. [DOI] [PubMed] [Google Scholar]
- Shaw S, Jayatilleke E, Ross WA, Gordon ER, and Leiber CS (1981). Ethanol-induced lipid peroxidation: potentiation by long-term alcohol feeding and attenuation by methionine. J. Lab. Clin. Med 98, 417–424. [PubMed] [Google Scholar]
- Shen Q, Liang M, Yang F, Deng YZ, and Naqvi NI (2019). Ferroptosis contributes to developmental cell death in rice blast. [DOI] [PubMed] [Google Scholar]
- Singhal R, Mitta SR, Olive KP, Lyssiotis CA, and Shah YM (2019). Hypoxia inducible factor-2α increases sensitivity of colon cancer cells towards oxidative cell death. [Google Scholar]
- Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, Rosenberg PA, Lo DC, Weinberg JM, Linkermann A, et al. (2014). Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J. Am. Chem. Soc 136, 4551–4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Jiang X, Yang C, Zhang J, Chen B, Li Y, Yao S, Xie Q, Gomez H, Murugan R, et al. (2019). Pannexin 1 mediates ferroptosis that contributes to renal ischemia/reperfusion injury. J. Biol. Chem 294, 19395–19404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, and Tang D (2016). Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 63, 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao P, Sun J, Wu Z, Wang S, Wang J, Li W, Pan H, Bai R, Zhang J, Wang Y, et al. (2020). A dominant autoinflammatory disease caused by non-cleavable variants of RIPK1. Nature 577, 109–114. [DOI] [PubMed] [Google Scholar]
- Tasdogan A, Faubert B, Ramesh V, Ubellacker JM, Shen B, Solmonson A, Murphy MM, Gu Z, Gu W, Martin M, et al. (2020). Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature 577, 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii S, Shintoku R, Kubota C, Yaegashi M, Torii R, Sasaki M, Suzuki T, Mori M, Yoshimoto Y, Takeuchi T, et al. (2016). An essential role for functional lysosomes in ferroptosis of cancer cells. Biochem. J 473, 769–777. [DOI] [PubMed] [Google Scholar]
- Tsoi J, Robert L, Paraiso K, Galvan C, Sheu KM, Lay J, Wong DJL, Atefi M, Shirazi R, Wang X, et al. (2018). Multi-stage Differentiation Defines Melanoma Subtypes with Differential Vulnerability to Drug-Induced Iron-Dependent Oxidative Stress. Cancer Cell 33, 890–904.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini F, Maiorino M, Valente M, Ferri L, and Gregolin C (1982). Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim. Biophys. Acta 710, 197–211. [DOI] [PubMed] [Google Scholar]
- Ursini F, Maiorino M, Hochstein P, and Ernster L (1989). Microsomal lipid peroxidation: mechanisms of initiation. The role of iron and iron chelators. Free Radic. Biol. Med 6, 31–36. [DOI] [PubMed] [Google Scholar]
- Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada K, Aguirre AJ, et al. (2017). Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547, 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, An P, Xie E, Wu Q, Fang X, Gao H, Zhang Z, Li Y, Wang X, Zhang J, et al. (2017). Characterization of ferroptosis in murine models of hemochromatosis. Hepatology 66, 449–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, et al. (2019). CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569, 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel SE, Tyurina YY, Zhao J, St Croix CM, Dar HH, Mao G, Tyurin VA, Anthonymuthu TS, Kapralov AA, Amoscato AA, et al. (2017). PEBP1 Wardens Ferroptosis by Enabling Lipoxygenase Generation of Lipid Death Signals. Cell 171, 628–641.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn CC (1995). Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol. Lett 82–83, 969–974. [DOI] [PubMed] [Google Scholar]
- Wu J, Minikes AM, Gao M, Bian H, Li Y, Stockwell BR, Chen Z-N, and Jiang X (2019). Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 572, 402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Karasawa T, Wakiya T, Sadatomo A, Ito H, Kamata R, Watanabe S, Komada T, Kimura H, Sanada Y, et al. (2020). Iron Overload as a Risk Factor for Hepatic Ischemia-Reperfusion Injury in Liver Transplantation: Potential role of ferroptosis. Am. J. Transplant [DOI] [PubMed] [Google Scholar]
- Yamaji-Hasegawa A, and Tsujimoto M (2006). Asymmetric distribution of phospholipids in biomembranes. Biol. Pharm. Bull 29, 1547–1553. [DOI] [PubMed] [Google Scholar]
- Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, et al. (2014). Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, and Stockwell BR (2016). Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. U. S. A 113, E4966–E4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye LF, Chaudhary KR, Zandkarimi F, Harken AD, Kinslow CJ, Upadhyayula PS, Dovas A, Higgins DM, Tan H, Zhang Y, et al. (2020). Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chem. Biol 15, 469–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Minagawa S, Araya J, Sakamoto T, Hara H, Tsubouchi K, Hosaka Y, Ichikawa A, Saito N, Kadota T, et al. (2019). Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat. Commun 10, 3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Ruan Y, Huang X, Dou L, Lan M, Cui J, Chen B, Gong H, Wang Q, Yan M, et al. (2019). Dexrazoxane ameliorates doxorubicin-induced cardiotoxicity by inhibiting both apoptosis and necroptosis in cardiomyocytes. Biochem. Biophys. Res. Commun [DOI] [PubMed] [Google Scholar]
- Zhang X, Xing X, Liu H, Feng J, Tian M, Chang S, Liu P, and Zhang H (2020). Ionizing radiation induces ferroptosis in granulocyte-macrophage hematopoietic progenitor cells of murine bone marrow. Int. J. Radiat. Biol 1–35. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shi J, Liu X, Feng L, Gong Z, Koppula P, Sirohi K, Li X, Wei Y, Lee H, et al. (2018a). BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol 20, 1181–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tan H, Daniels JD, Zandkarimi F, Liu H, Brown LM, Uchida K, O’Connor OA, and Stockwell BR (2019). Imidazole Ketone Erastin Induces Ferroptosis and Slows Tumor Growth in a Mouse Lymphoma Model. Cell Chem Biol 26, 623–633.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-H, Wang D-W, Xu S-F, Zhang S, Fan Y-G, Yang Y-Y, Guo S-Q, Wang S, Guo T, Wang Z-Y, et al. (2018b). α-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice. Redox Biol 14, 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilka O, Shah R, Li B, Friedmann Angeli JP, Griesser M, Conrad M, and Pratt DA (2017). On the Mechanism of Cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the Role of Lipid Peroxidation in Ferroptotic Cell Death. ACS Cent Sci 3, 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Palte MJ, Deik AA, Li H, Eaton JK, Wang W, Tseng Y-Y, Deasy R, Kost-Alimova M, Dančík V, et al. (2019a). A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat. Commun 10, 1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Watters A, Cheng N, Perry CE, Xu K, Alicea GM, Parris JLD, Baraban E, Ray P, Nayak A, et al. (2019b). Polyunsaturated Fatty Acids from Astrocytes Activate PPARγ Signaling in Cancer Cells to Promote Brain Metastasis. Cancer Discov. 9, 1720–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Li H, Graham ET, Deik AA, Eaton JK, Wang W, Sandoval-Gomez G, Clish CB, Doench JG, & Schreiber SL (2020) Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat Chem Biol 16(3), 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]


