Abstract
The complement-regulatory protein CD46 is the primary receptor for human adenovirus type 35 (HAdV-35) and can regulate human immune-cell activation. CD4+ T-cells are critical for initiating and maintaining adaptive immunity elicited by infection or vaccination. It was reported previously that HAdV-35 can bind these cells and suppress their activation. The data reported here demonstrate that recombinant trimeric HAdV-35 knob proteins alone can induce CD46 receptor downregulation and inhibit interleukin-2 production and proliferation of human CD4+ T-cells in vitro similarly to mAbs specific to the CD46 region bound by HAdV-35 knobs. A mutant knob protein with increased affinity for CD46 compared with the wild-type knob caused equivalent effects. In contrast, a CD46-binding-deficient mutant knob protein did not inhibit T-cell activation. Thus, the capacity of HAdV-35 to attenuate human CD4+ T-cell activation depends predominantly on knob interactions with CD46 and can occur independently of infection.
The primary cellular attachment receptor for the species B human adenovirus (HAdV) type 35 is CD46. This complement-regulatory protein is expressed on nearly all nucleated human cells and protects cells from autologous complement-mediated lysis. CD46 is also a modulator of monocyte and T-cell activation, although many of its functions remain enigmatic (Cardone et al., 2011; Karp et al., 1996; Kemper & Atkinson, 2007; Marie et al., 2002). HAdV-35 fiber–knob trimers, positioned at the carboxyl terminus of each of the 12 fiber shafts extending from the capsid vertices, bind CD46 at sites mapped within membrane-distal short consensus repeats (SCRs) 1 and 2 (Fleischli et al., 2005; Gaggar et al., 2003, 2005). As CD46 binding occurs between two knob monomers, HAdV-35 probably engages multiple CD46 molecules (Nemerow et al., 2009). In addition to binding complement components and viral proteins, CD46 contains prototypic phosphorylation sites encoded within its two cytoplasmic tail isoforms and can transduce extracellular signals to intracellular downstream signalling cascades and impact cell function (Cardone et al., 2010; Joubert et al., 2009; Wang et al., 2000).
Elicitation of adaptive immunological memory after virus or vaccine exposure depends largely on optimal priming of CD4+ T-cell help. Naive CD4+ T-cells co-stimulated by dendritic cells (DCs) through T-cell receptor (TCR)-associated CD3 side chains and CD28 undergo proliferation and provide help signals, such as interleukin (IL)-2, that support the development of effector and memory CD8+ T-cells. Understanding how HAdV-35 may affect these events is of particular interest because replication-incompetent HAdV-35 vectors are being used to elicit cellular immune responses in vaccine trials (Liu, 2010). It was reported previously that HAdV-35 can inhibit CD4+ T-cell proliferation and IL-2 production by binding CD46 (Adams et al., 2011).
This study aimed to expand on these findings using well-characterized trimeric HAdV-35 knob proteins displaying different affinities for CD46. Using recombinant knobs in place of whole HAdV-35 particles facilitated a more specific analysis of the interaction of CD46 on T-cells, as infection, secondary virus-binding properties and potential steric hindrance of large virions on CD3/CD28 engagement could be excluded. The knobs were expressed in Escherichia coli and purified by nickel–nitrilotriacetic acid agarose chromatography. Knob trimerization and purity were then confirmed by SDS-PAGE and binding to CD46 was assessed by surface plasmon resonance (Wang et al., 2007, 2008, 2010). The wild-type knob bound CD46 with a K D of 14.64 nM. A mutated knob that bound CD46 with 23-fold higher affinity (K D = 0.63 nM) was constructed with Asp207Gly and Thr245Ala substitutions that principally caused a slower dissociation rate (referred to as high-affinity knob). A suitable control knob protein with ablated CD46-binding capacity was generated by substituting the critical CD46-interacting Arg279 residue with Cys (referred to as control knob). This mutation does not affect knob trimerization and, unlike substituting with His, blocks direct knob interaction with the critical Glu63 residue of the SCR1 domain of CD46 (Gustafsson et al., 2006; Wang et al., 2007).
Two approaches were used to assess the specificity of the trimeric HAdV-35 knobs. All experiments were approved by the Karolinska Institutet Ethical Review Board. In the first approach, human monocyte-derived DCs that are highly susceptible to HAdV-35 were exposed to 10 infectious HAdV-5.35–GFP particles per cell, with or without soluble HAdV-35-derived knobs (0.1–1.0 µg ml−1) that had been added to the cells 15 min previously. The knobs were compared with two mouse anti-human CD46 mAbs (5 µg ml−1, both isotype IgG1), clones 13.42 (BMA) and M177 (Hycult), which bind SCR1 and 2, respectively, and inhibit HAdV-35 infection (Loré et al., 2007). After a 24 h incubation at 37 °C in which virus, knobs and mAbs remained in the cultures and were not washed away, GFP expression was measured by flow cytometry. The wild-type HAdV-35 knob, but not the mutant knob deficient in CD46 binding, reduced the frequency of GFP+ DCs equivalently to CD46 mAbs (Fig. 1a). The high-affinity knob mutant showed a tendency to be even more efficient than the wild-type knob at blocking infection. These data indicate that the knobs bound CD46 and blocked HAdV-5.35 infection according to their expected specificities (Wang et al., 2008).
Fig. 1.
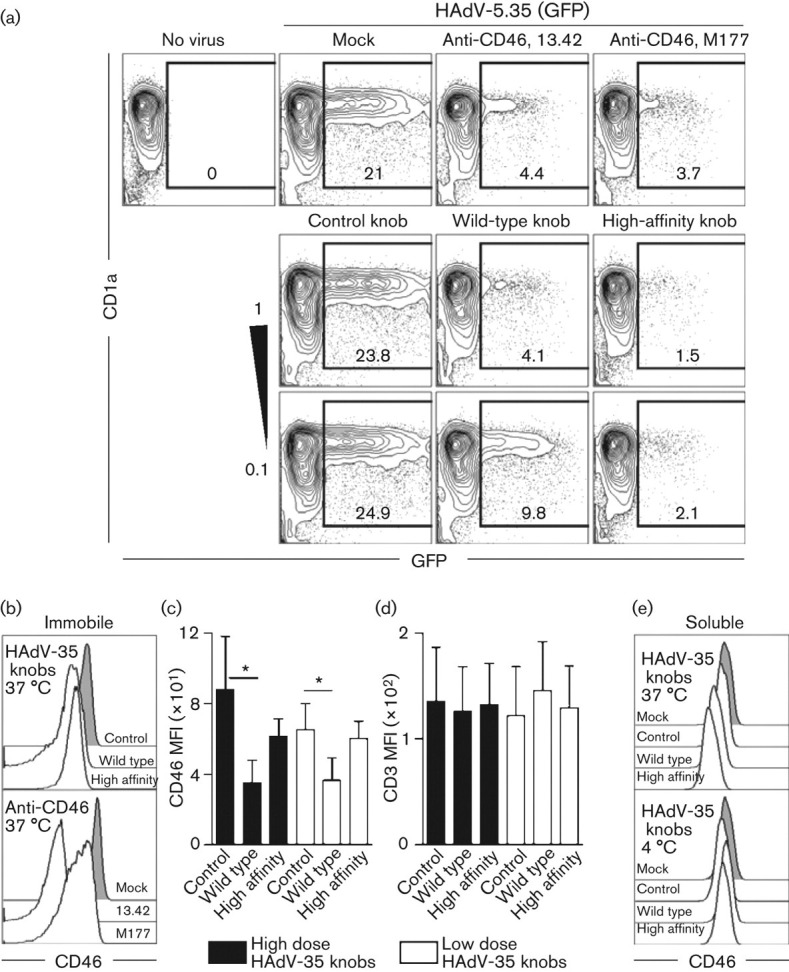
Knobs interfere with HAdV-35 infection and downregulate CD46. (a) DCs were exposed to HAdV-35–GFP with or without soluble CD46 mAbs (top row) or HAdV-35-derived knobs (middle row, 1 µg ml−1; bottom row, 0.1 µg ml−1) that were either wild-type, mutated to increase affinity for CD46 by 23-fold (referred to as high-affinity knob) or mutated to ablate CD46 binding (referred to as control knob). Flow-cytometry plots are representative of five independent experiments. Numbers indicate frequencies (%) of CD1a+/CD14+ DCs expressing GFP. (b) Histograms depict representative CD46 expression on CD4+ T-cells after exposure to mock treatment, immobile knobs (10 µg ml−1) or anti-CD46 mAb (5 µg ml−1). (c, d) Graphs summarize (c) CD46 and (d) CD3 mean fluorescence intensity (MFI) after high (filled bars, 10 µg ml−1) and low (empty bars, 5 µg ml−1) doses of knobs from three independent experiments (mean±sd). Paired t-test; *P<0.05. (e) Histograms depict representative CD46 expression on CD4+ T-cells after exposure to soluble knobs (5 µg ml−1) at 4 °C for 15 min or at 37 °C for 24 h.
In the second approach, the knobs were tested for their capacity to directly downregulate surface CD46 expression on CD4+ T-cells, similar to what has been reported for HAdV-35 and CD46 mAbs, as well as for other viruses that bind CD46 on different cells (Adams et al., 2011; Bartz et al., 1996; Sakurai et al., 2007; Santoro et al., 1999; Schnorr et al., 1995; Yant et al., 1997). On lymphoid cells, CD46 internalizes rapidly following direct ligand binding (Crimeen-Irwin et al., 2003). Here, surface CD46 expression was measured by flow cytometry on purified CD4+ T-cells that had been exposed to anti-CD46 mAb (5 µg ml−1) or knobs at low (5 µg ml−1) or high (10 µg ml−1) doses for 24 h at 37 °C. Plastic plate-immobilized wild-type knobs and high-affinity mutant knobs reduced CD46 expression compared with cells treated with the CD46-binding-deficient control knob, although only the wild-type knob reached statistical significance (Fig. 1b, c). The degree of CD46 downregulation caused by wild-type knobs was similar to that found with CD46 mAbs (Fig. 1b) and HAdV-35 (Adams et al., 2011). The control knob with deficient CD46 binding induced modest downregulation compared with untreated cells, which may indicate that a low degree of CD46 binding remains. Importantly, expression of CD3 on T-cells was unaffected by all treatments (Fig. 1d). In soluble form, the low dose of both wild-type knobs and high-affinity knobs caused CD46 downregulation (Fig. 1e). The absence of CD46 downregulation after binding of knobs at 4 °C (Fig. 1e) indicates that downregulation represents receptor internalization and that the knobs do not interfere with binding of the staining anti-CD46 mAb (eBioscience, clone 8E2). In summary, these two experimental approaches confirmed that the wild-type and high-affinity knobs are able to interfere with HAdV-5.35 infection and mediate internalization of surface CD46.
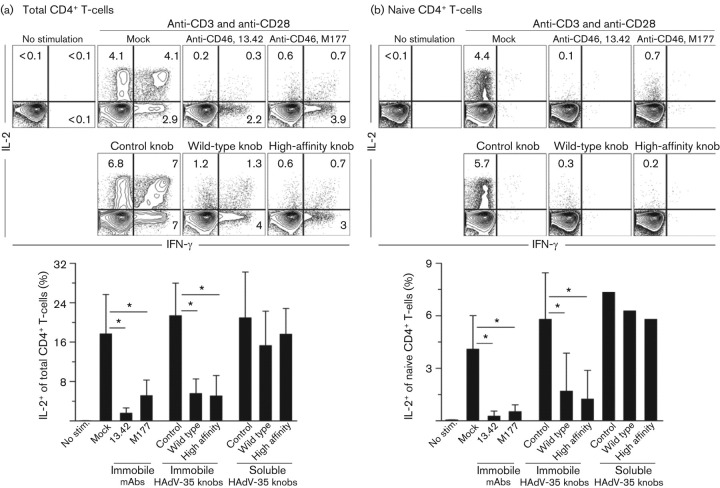
As the knobs showed the expected binding specificity for CD46, they provided a useful reagent to test whether knob interactions with CD46 alone were sufficient for HAdV-35 to suppress human naive CD4+ T-cell proliferation, as was reported previously (Adams et al., 2011). To test the capacity of the knobs to affect T-cell proliferation, purified naive CD4+ T-cells (>95 % CD3+CD4+CCR7+CD45RA+) were activated with immobilized anti-CD3 and anti-CD28 mAbs (1 µg ml−1; BD) with or without immobile or soluble CD46 mAb (5 µg ml−1) or knobs (1 and 5 µg ml−1). Proliferation was then measured in triplicate on day 5 by 16 h [3H]thymidine incorporation (measured as c.p.m.). CD46 mAbs were used as controls to confirm earlier findings (Adams et al., 2011). Similar to the SCR1-binding CD46 mAb clone 13.42, but unlike the SCR2-binding clone M177, the presence of immobile wild-type and high-affinity knobs caused a substantial dose-dependent reduction in naive CD4+ T-cell proliferation (Fig. 2a). The knob mutated to ablate CD46 binding had no effect. Increasing knob affinity for CD46 made no discernible difference, as the mutant knob with high affinity for CD46 inhibited proliferation equivalently to the wild-type knob. This finding suggests that the affinity of the wild-type knob is sufficient to reduce CD3/28-dependent activation. Similar to CD46 mAb and HAdV-35 particles, treatment of cells with knobs did not induce cell necrosis or apoptosis based on annexin V and 7-AAD co-staining (data not shown), indicating that reduced cell survival was not the cause of deficient proliferation. In contrast to the apparent capacity of soluble HAdV-35 to suppress T-cell activation (Adams et al., 2011), this was not evident for soluble knobs (Fig. 2b). A possible explanation is that multivalent knob interactions with CD46, provided by the structural arrangement of 12 knob trimers on each virus capsid, is required. Structure data support the notion that the composition of the capsid proteins may enable HAdV-35 whole particles to cross-link CD46 more efficiently than soluble knobs (Nemerow et al., 2009; Pache et al., 2008; Wang et al., 2007). In addition, the observation that plate immobilization of the recombinant knobs was necessary may not be unexpected, as immobilization of CD3 and CD28 mAbs was necessary to induce robust T-cell signalling (data not shown).
Fig. 2.

Immobile HAdV-35 knobs attenuate T-cell proliferation. Graphs depict CD3/CD28-induced proliferation of naive CD4+ T-cells with or without (a) immobile or (b) soluble anti-CD46 mAb or knobs. High (5 µg ml−1) and low (1 µg ml−1) doses of knobs, represented by black wedges, were used in each experiment. Results are representative of (a) three or (b) two independent experiments performed in triplicate (mean±sd).
HAdV-35 and CD46 mAb were also shown previously to inhibit IL-2 production by T-cells (Adams et al., 2011). To test whether knobs alone could mediate similar effects, purified total and naive CD4+ T-cells were activated with immobile anti-CD3/CD28 mAbs with or without immobile CD46 mAbs or immobile or soluble knobs. Five hours later, production of IL-2 and gamma interferon (IFN-γ) (mAbs from BD) was measured intracellularly by flow cytometry as described previously (Adams et al., 2011). As expected from previous findings (Adams et al., 2011), both CD46 mAb clones reduced IL-2 production significantly (upper quadrants) in total (Fig. 3a) and naive (Fig. 3b) CD4+ T-cell populations. This suggests that the binding region and signalling strengths of both mAbs are sufficient to block IL-2 production (Fig. 3), whereas only the 13.42 clone could interfere with proliferation under these conditions (Fig. 2). Similar to CD46 mAb, immobile wild-type and high-affinity knobs inhibited IL-2 production significantly in total CD4+ T cells by 74 and 76 %, respectively, compared with the CD46-binding-deficient mutant knob (Fig. 3a). The knobs showed a similar effect on the naive subset (Fig. 3b). The functional changes were therefore consistent in both populations of T-cells and indicate a substantial reduction in the frequency of IL-2-producing cells caused by the CD46-binding knobs. A noticeable difference was not observed between wild-type and high-affinity knobs. The knob deficient in CD46 binding and none of the knobs in soluble form affected IL-2 production (Fig. 3). The results were confirmed when ELISA was used to assay secreted IL-2 after 24 h (data not shown). As expected, the effector cytokine IFN-γ was produced predominantly within the total CD4+ T-cell population and not by the naive subset (Fig. 3a, b). Engagement of CD46 led to ablation of IFN-γ-producing cells within the IFN-γ/IL-2 double-positive population (upper right quadrants), but had less noticeable effect on the single-producing IFN-γ population (lower right quadrants). These results are consistent with our previous report that used HAdV-35 and CD46 mAb, as well as an additional report showing that CD46 can modulate IFN-γ in CD8+ T-cells (Oliaro et al., 2006). Taken together, our data demonstrate that HAdV-35 knobs can attenuate proliferation and IL-2 production and that a single amino acid (Arg279Cys) mutation in the knob ablates their capacity to modulate primary T-cell activation.
Fig. 3.
Immobile HAdV-35 knobs attenuate IL-2 production. (a) Total CD4+ T-cells or (b) the naive subset were CD3/CD28-activated with or without immobile or soluble anti-CD46 mAb (1 µg ml−1) or HAdV-35-derived knobs (5 µg ml−1). Flow-cytometry plots are from one representative experiment of three with immobile CD46 mAbs or knobs. Numbers indicate frequencies of CD3+/CD4+ live cells expressing intracellular IL-2 and IFN-γ. Graphs summarize three independent experiments (mean±sd) except for the columns in (b) where there are no error bars; here, only one experiment was performed (mean). Paired t-test; *P<0.05.
Increasing knob valency for CD46 by plate-immobilization seems to be needed to negatively alter CD4+ T cell activation in vitro, but alternative mechanisms may be plausible. First, there may be a spatial requirement for HAdV-35 knob engagement of CD46 to occur proximally (as in the immobile setting) rather than distally (as in soluble form) to the CD3/CD28 complex in order to attenuate CD4+ T cell activation. Second, there may be a temporal requirement for CD46 ligation to occur simultaneously with CD3/CD28 signalling, since mAb engagement of CD46 15 min prior to CD3/28 stimulation does not lead to reduced proliferation (data not shown). However, further dissection of the molecular pathways originating from CD46 expressed on CD4+ T cells will be necessary to reveal potential roles for cis versus trans receptor engagement as well as for temporal aspects of CD46 engagement relative to CD3/CD28 engagement (Hawkins & Oliaro, 2010).
HAdV-35 was demonstrated to regulate critical naive CD4+ T-cell functions via interactions with its receptor CD46 (Adams et al., 2011). The data reported here demonstrate that knob interactions with CD46 alone can be solely responsible for attenuating CD4+ T-cell proliferation and IL-2 production, rather than infection or other capsid-binding properties. The well-characterized recombinant HAdV-35 knob proteins used here represent more suitable reagents than anti-CD46 mAbs and whole HAdV-35 particles for studying the functional consequences of HAdV-35 binding to CD46 on human immune cells. It will be interesting to determine whether these immune-modulatory effects are conserved among CD46-using viruses such as AdV-11, which has high-avidity knob binding to CD46 and imparts substantial conformational change on the receptor (Persson et al., 2007). The capacity of HAdV-35 knobs to affect immune-cell function directly may impact host immune responses towards the virus in vivo, although this remains to be tested. In a recent study, immunization of non-human primates with species B HAdV-34 and HAdV-35 vectors induced lower cellular immune responses to encoded antigens than non-CD46-using species C HAdV-5 and HAdV-6 vectors (Colloca et al., 2012). Our data presented here may also be relevant for the use of HAdV-35 knobs in immune therapy (Wang et al., 2010).
Acknowledgements
This study was supported by Vetenskapsrådet, the Swedish Governmental Agency for Innovation Systems (Vinnova), the Swedish Society of Medicine and the Wenner-Gren Foundation.
References
- Adams W. C., Gujer C., McInerney G., Gall J. G., Petrovas C., Karlsson Hedestam G. B., Koup R. A., Loré K. Adenovirus type-35 vectors block human CD4+ T-cell activation via CD46 ligation. Proc Natl Acad Sci U S A. 2011;108:7499–7504. doi: 10.1073/pnas.1017146108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz R., Brinckmann U., Dunster L. M., Rima B., Ter Meulen V., Schneider-Schaulies J. Mapping amino acids of the measles virus hemagglutinin responsible for receptor (CD46) downregulation. Virology. 1996;224:334–337. doi: 10.1006/viro.1996.0538. [DOI] [PubMed] [Google Scholar]
- Cardone J., Le Friec G., Vantourout P., Roberts A., Fuchs A., Jackson I., Suddason T., Lord G., Atkinson J. P., other authors Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol. 2010;11:862–871. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone J., Le Friec G., Kemper C. CD46 in innate and adaptive immunity: an update. Clin Exp Immunol. 2011;164:301–311. doi: 10.1111/j.1365-2249.2011.04400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca S., Barnes E., Folgori A., Ammendola V., Capone S., Cirillo A., Siani L., Naddeo M., Grazioli F., other authors Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med. 2012;4:115ra2. doi: 10.1126/scitranslmed.3002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimeen-Irwin B., Ellis S., Christiansen D., Ludford-Menting M. J., Milland J., Lanteri M., Loveland B. E., Gerlier D., Russell S. M. Ligand binding determines whether CD46 is internalized by clathrin-coated pits or macropinocytosis. J Biol Chem. 2003;278:46927–46937. doi: 10.1074/jbc.M308261200. [DOI] [PubMed] [Google Scholar]
- Fleischli C., Verhaagh S., Havenga M., Sirena D., Schaffner W., Cattaneo R., Greber U. F., Hemmi S. The distal short consensus repeats 1 and 2 of the membrane cofactor protein CD46 and their distance from the cell membrane determine productive entry of species B adenovirus serotype 35. J Virol. 2005;79:10013–10022. doi: 10.1128/JVI.79.15.10013-10022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggar A., Shayakhmetov D. M., Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- Gaggar A., Shayakhmetov D. M., Liszewski M. K., Atkinson J. P., Lieber A. Localization of regions in CD46 that interact with adenovirus. J Virol. 2005;79:7503–7513. doi: 10.1128/JVI.79.12.7503-7513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson D. J., Segerman A., Lindman K., Mei Y. F., Wadell G. The Arg279Gln [corrected] substitution in the adenovirus type 11p (Ad11p) fiber knob abolishes EDTA-resistant binding to A549 and CHO-CD46 cells, converting the phenotype to that of Ad7p. J Virol. 2006;80:1897–1905. doi: 10.1128/JVI.80.4.1897-1905.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins E. D., Oliaro J. CD46 signaling in T cells: linking pathogens with polarity. FEBS Lett. 2010;584:4838–4844. doi: 10.1016/j.febslet.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Joubert P. E., Meiffren G., Grégoire I. P., Pontini G., Richetta C., Flacher M., Azocar O., Vidalain P. O., Vidal M., Lotteau V. Autophagy induction by the pathogen receptor CD46. Cell Host Microbe. 2009;6:354–366. doi: 10.1016/j.chom.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Karp C. L., Wysocka M., Wahl L. M., Ahearn J. M., Cuomo P. J., Sherry B., Trinchieri G., Griffin D. E. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- Kemper C., Atkinson J. P. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- Liu M. A. Immunologic basis of vaccine vectors. Immunity. 2010;33:504–515. doi: 10.1016/j.immuni.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Loré K., Adams W. C., Havenga M. J., Precopio M. L., Holterman L., Goudsmit J., Koup R. A. Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. J Immunol. 2007;179:1721–1729. doi: 10.4049/jimmunol.179.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie J. C., Astier A. L., Rivailler P., Rabourdin-Combe C., Wild T. F., Horvat B. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nat Immunol. 2002;3:659–666. doi: 10.1038/ni810. [DOI] [PubMed] [Google Scholar]
- Nemerow G. R., Pache L., Reddy V., Stewart P. L. Insights into adenovirus host cell interactions from structural studies. Virology. 2009;384:380–388. doi: 10.1016/j.virol.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliaro J., Pasam A., Waterhouse N. J., Browne K. A., Ludford-Menting M. J., Trapani J. A., Russell S. M. Ligation of the cell surface receptor, CD46, alters T cell polarity and response to antigen presentation. Proc Natl Acad Sci U S A. 2006;103:18685–18690. doi: 10.1073/pnas.0602458103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pache L., Venkataraman S., Nemerow G. R., Reddy V. S. Conservation of fiber structure and CD46 usage by subgroup B2 adenoviruses. Virology. 2008;375:573–579. doi: 10.1016/j.virol.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Persson B. D., Reiter D. M., Marttila M., Mei Y. F., Casasnovas J. M., Arnberg N., Stehle T. Adenovirus type 11 binding alters the conformation of its receptor CD46. Nat Struct Mol Biol. 2007;14:164–166. doi: 10.1038/nsmb1190. [DOI] [PubMed] [Google Scholar]
- Sakurai F., Akitomo K., Kawabata K., Hayakawa T., Mizuguchi H. Downregulation of human CD46 by adenovirus serotype 35 vectors. Gene Ther. 2007;14:912–919. doi: 10.1038/sj.gt.3302946. [DOI] [PubMed] [Google Scholar]
- Santoro F., Kennedy P. E., Locatelli G., Malnati M. S., Berger E. A., Lusso P. CD46 is a cellular receptor for human herpesvirus 6. Cell. 1999;99:817–827. doi: 10.1016/S0092-8674(00)81678-5. [DOI] [PubMed] [Google Scholar]
- Schnorr J. J., Dunster L. M., Nanan R., Schneider-Schaulies J., Schneider-Schaulies S., ter Meulen V. Measles virus-induced down-regulation of CD46 is associated with enhanced sensitivity to complement-mediated lysis of infected cells. Eur J Immunol. 1995;25:976–984. doi: 10.1002/eji.1830250418. [DOI] [PubMed] [Google Scholar]
- Wang G., Liszewski M. K., Chan A. C., Atkinson J. P. Membrane cofactor protein (MCP; CD46): isoform-specific tyrosine phosphorylation. J Immunol. 2000;164:1839–1846. doi: 10.4049/jimmunol.164.4.1839. [DOI] [PubMed] [Google Scholar]
- Wang H., Liaw Y. C., Stone D., Kalyuzhniy O., Amiraslanov I., Tuve S., Verlinde C. L., Shayakhmetov D., Stehle T., other authors Identification of CD46 binding sites within the adenovirus serotype 35 fiber knob. J Virol. 2007;81:12785–12792. doi: 10.1128/JVI.01732-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Liu Y., Li Z., Tuve S., Stone D., Kalyushniy O., Shayakhmetov D., Verlinde C. L., Stehle T., other authors In vitro and in vivo properties of adenovirus vectors with increased affinity to CD46. J Virol. 2008;82:10567–10579. doi: 10.1128/JVI.01308-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Liu Y., Li Z. Y., Fan X., Hemminki A., Lieber A. A recombinant adenovirus type 35 fiber knob protein sensitizes lymphoma cells to rituximab therapy. Blood. 2010;115:592–600. doi: 10.1182/blood-2009-05-222463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yant S., Hirano A., Wong T. C. Identification of a cytoplasmic Tyr-X-X-Leu motif essential for down regulation of the human cell receptor CD46 in persistent measles virus infection. J Virol. 1997;71:766–770. doi: 10.1128/jvi.71.1.766-770.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]