Abstract
Background
Frail older adults living in residential aged care facilities (RACFs) usually experience comorbidities and are frequently prescribed multiple medications. This increases the potential risk of inappropriate prescribing and its negative consequences. Thus, optimising prescribed medications in RACFs is a challenge for healthcare providers.
Objective
Our aim was to systematically review interventions that increase the appropriateness of medications used in RACFs and the outcomes of these interventions.
Methods
Systematic review and meta-analysis of randomised control trials (RCTs) and cluster randomised control trials (cRCTs) were performed by searching specified databases (MEDLINE, PubMed, Google scholar, PsycINFO) for publications from inception to May 2019 based on defined inclusion criteria. Data were extracted, study quality was assessed and statistically analysed using RevMan v5.3. Medication appropriateness, hospital admissions, mortality, falls, quality of life (QoL), Behavioural and Psychological Symptoms of Dementia (BPSD), adverse drug events (ADEs) and cognitive function could be meta-analysed.
Results
A total of 25 RCTs and cRCTs comprising 19,576 participants met the inclusion criteria. The studies tested various interventions including medication review (n = 13), staff education (n = 9), multi-disciplinary case conferencing (n = 4) and computerised clinical decision support systems (n = 2). There was an effect of interventions on medication appropriateness (RR 0.71; 95% confidence interval (CI): 0.60,0.84) (10 studies), and on medication appropriateness scales (standardised mean difference = − 0.67; 95% CI: − 0.97, − 0.36) (2 studies). There were no apparent effects on hospital admission (RR 1.00; 95% CI: 0.93, 1.06), mortality (RR 0.98; 95% CI: 0.86, 1.11), falls (RR 1.06; 95% CI: 0.89,1.26), ADEs (RR 1.04; 95% CI: 0.96,1.13), QoL (standardised mean difference = 0.16; 95% CI:-0.13, 0.45), cognitive function (weighted mean difference = 0.69; 95% CI: − 1.25, 2.64) and BPSD (RR 0.68; 95% CI: 0.44,1.06) (2 studies).
Conclusion
Modest improvements in medication appropriateness were observed in the studies included in this systematic review. However, the effect on clinical measures was limited to drive strong conclusions.
Keywords: Medication optimisation, Intervention, Elderly, Systematic review, Aged care facility, Clinical outcomes, Meta-analysis
Background
Inappropriate medication prescription encompasses misprescribing, overprescribing, and underprescribing. Misprescribing involves the use of medication that significantly increases the risk of adverse drug events (ADEs) and involves incorrect dose, frequency, administration and duration. Use of medications that are likely to cause drug-drug interactions or drug-disease interactions is also an aspect of misprescribing. Overprescribing involves the use of medications without clear indications. Underprescribing is the omission of clinically-indicated medication that may have potential benefit for treatment of the disease [1].
Residents of aged care facilities (RACFs) are often frail and have multiple comorbidities. On average RACF residents take more medications than younger age groups, and more than community-dwelling elderly with similar disease complications [2]. They are frequently prescribed multiple medications that can increase the risk of ADEs, morbidity and mortality [3, 4]. Moreover, the majority of these residents have dementia and the use of psychotropic drugs is typically high. Age-related changes in pharmacodynamics and pharmacokinetics, multiple co-morbidities, and the presence of polypharmacy are the main factors often associated with ageing that makes optimisation of drug therapy a complex task. Furthermore,
Previous studies indicate that about 40% of prescriptions for RACF residents may be suboptimal or inappropriate [5]. Consequently, there is a heightened risk of adverse drug reactions (ADRs), hospitalisations, and medical expense [3, 6]. Therefore, there is an urgent need to improve prescribing and to optimise drug therapy for older people living in care homes [5, 7].
Medication optimisation is a person-centred approach designed to ensure medication safety and improved clinical outcomes via effective use of medicine [8, 9]. A range of interventions for optimisation of prescribed medications in RACFs have been developed to potentially optimise prescribing. These include medication review, education programs, the use of clinical decision support technology, and multidisciplinary case-conferencing. These interventions have been evaluated to determine the effect of optimising prescribing in nursing homes and in older people with dementia, but the results were not pooled statistically [10–12], and the nursing home specific data require updating. The 2011 review concluded that in nursing homes, educational interventions including academic detailing seems to show most promise [12]. The other 2011 review found that education and pharmacist drug review may reduce inappropriate drug use under certain circumstances [11]. The other 2018 review of 18 experimental studies specific to older people living with dementia in any setting concluded that the improvement of medication appropriateness is supported by emerging evidence, and the impact of these interventions on dementia patients’ outcomes required more research [10].
We therefore aimed to systematically review the available interventions conducted by a health professional that aimed to increase the appropriateness of medications used in residential aged care facilities and to evaluate their effects on medication appropriateness and residents’ clinical outcomes.
Methods
This systematic review was conducted and reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13]. A PRISMA checklist can be found in Additional file 1. The review was registered with the international prospective register of systematic reviews PROSPERO CRD42020148669.
Data sources and search strategy
An electronic search of the literature was conducted from inception to May 2019 using the following databases — MEDLINE, PubMed, Google scholar, PsycINFO. A combination of the following keywords and MeSH terms were used: “Optimize OR improve OR maximize OR optimization AND medication OR drugs OR medicines AND side effects OR safety OR administration OR review AND nursing homes OR residential OR aged care”. The reference lists of the relevant articles and reviews were hand-searched to further identify any additional studies. The complete search strategy is presented in Additional file 2.
Study selection
The title and abstract of all retrieved articles were initially reviewed to find those potentially relevant to the study area. The abstract of the selected papers was assessed against five inclusion criteria: (i) randomised controlled trials (RCTs) and cluster randomised controlled trials (cRCTs); (ii) residents’ age 60 and older; settings are residential aged care facility or nursing homes or residential continuing care hospitals; (iii) interventions to increase the appropriateness of medications used in nursing homes (iv) reported in English; (v) published between 1980 and 2019.
Data extraction and quality assessment
Details of the included articles were independently extracted by two authors (H.A, and L.F). Data included details of the authors, publication year, country, study design, age, setting, sample size, intervention, follow-up, outcomes, and summary of results. Table 1 depicts the study characteristics of the included studies.
Table 1.
Study characteristics of the included studies
| Study ID | Author | Year | Country | Design | Setting | Sample size | Age | Intervention | Follow-up | Outcomes | Summary of results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Avorn et al. [14] | 1992 | USA | Cluster RCT | Nursing homes |
NHs = 12 NHR = 823 |
65 and older |
- physicians who is there prescribing of psychoactive drugs was above threshold at the baseline evaluation were invited by pharmacists for separate sessions (3 interactive visits). - All physicians of NHR received 6 literature summaries (insomnia, behavioural problems) in 3 mailings. - 4 training sessions were delivered to nurses/ nursing assistants on geriatric pharma psychology, alternatives to psychoactive drugs |
5 months |
-psychoactive drug use scores -proportion of residents using antipsychotics. |
-psychoactive drug use mean in intervention 27% compared to 80% in control group (P = 0.02). -antipsychotics ceased in intervention 32% versus 14% in control. - no of days/patient/ month greatly reduced in intervention than control. -no of non-recommended hypnotics ceased and substituted with alternative drugs/discontinued completely were 45% in intervention versus 21% control. |
| 2 | Rovner et al. [15] | 1996 | USA | RCT | Nursing homes |
1 NH (250 bed community NH) NHR = 89 |
65 and older |
-As ap art of a dementia care program: implementation of new prescribing guidelines based on protocol for psychotropic drug management -Educational rounds weekly for 1 h to discuss patient’s behavioural disorders, and medical status |
6 months |
- antipsychotic drug - behaviour disorders -restraint use, - and cognitive/ functional status. |
-Statistically significant 71% reduction in agitation with intervention versus 49% with control |
| 3 | Meador et al. [16] | 1997 | USA | RCT | Nursing homes |
NHs = 12 NHR = 1311 |
65 and older |
-geropsychiatrist delivered educational visit to physicians (45-60 min) -NH staff received 5–6 1-h programmes over 1 week delivered by nurse educator. -after 1 month, follow up sessions - when requested, evening meetings for families. |
6 months |
-Proportion of APs drug use in days/ 100 /days of stay. -severity and presence of behavioural symptoms. |
-APs use per 100 days at baseline in intervention gp decreased from 25.3 days to 19.7 per 100. -Aps reduction is 23% in intervention gp to control gp. −33% NHR in intervention gp had their antipsychotics ceased. |
| 4 | Schmidt et al. 1&2 [17, 18] | 1998 | Sweden | Cluster RCT | Nursing homes |
NH = 33 NHR = 1854 |
65 and older | -Monthly multidisciplinary meetings led by pharmacist for 12 months | 12 months |
-Proportion of residents with psychotropics -non recommended hypnotics, antidepressants. |
−19% of residents in the intervention gp ceased APs (p = 0.007). −37% of residents ceased non- recommended hypnotics in intervention gp (p < 0.001). |
| Schmidt et al. [19] | 2000 | Sweden | Cluster RCT | Nursing homes |
NH = 36 NHR = 1549 |
65 and older | Nursing homes participated in 1995 were followed up. | three-year follow-up | Medication appropriateness |
-proportion of residents prescribed non-recommended hypnotics were lower (14.0%) compared to previous study 1995 (19.0%). - in1998 5% of residents were prescribed non recommended hypnotics compared to control gp (10.1%). |
|
| Claesson et al. [20] | 1998 | Sweden | Cluster-RCT | Nursing homes |
NH = 33 NHR = 1854 |
65 and older |
- regular multidisciplinary meetings (physician, pharmacist, NH nurses/assistant) reviewed resident’s drug use on a monthly basis over 12 months. -education for selected pharmacists (5 occasions = 65.5 h), topics were drug use in elderly, geriatrics. |
14 months | Medication-related problems |
-NH residents were prescribed on average 7.7o (range: 6–11) medications. - laxatives (70%) -psychotropic drugs (77%, range: 50–95%). |
|
| 5 | Furniss et al. [21] | 2000 | England. | Cluster RCT | Nursing homes |
NH = 14 NHR = 330 residents: (172 ctrl, 158 Int) |
65 and older |
-Medication review led by pharmacist. - pharmacist review the medications at NH, GPs surgery, or over phone. -Pharmacist collected details of current medication, medical history and current problem identified by nursing home staff. - 3 weeks post-medicine review, NH were revisited to identify any problems and to ascertain on whether changes had been implemented. |
8 months |
-no of prescribed medications -Types of medications, reason for using neuroleptic medications. -hospital admission (in-patient days) -MMSE -GDS -BASDEC -CRBRS -Falls and death |
- 239 of recommendations accepted by GP (91.6%). -change of medications =144 -In total MMSE were declined. - Mean CRBRS scores increased in Int compared to ctrl - deaths in ctrl were higher than Int NHs. |
| 6 | Stein et al. [22] | 2001 | USA | Cluster RCT | Nursing homes |
NH = 20 NHR = 147 |
65 and older |
-Staff training sessions (30 min) -Study physician visited/telephone to all primary care physicians -physicians received messages about NSAIDs risks and benefits , algorithm for stopping NSAIDs, or aternatives such as paracetamol or topical agents and non-pharmacological management for pain. |
3 months |
NSAIDs and paracetamol Use in the past week |
-Mean number of days of NSAIDs use deceased in Int gp from 7.0–1.9 days compared to ctrl gp (7.0–6.2 days), P = 0.0001 - paracetamol use in Int gp increased (3.1 days) compared to ctrl (0.31 days), P = 0.0001. |
| 7 | Roberts et al. [23] | 2001 | Australia | Cluster RCT | Nursing homes |
NH = 52 NHR = 3230 |
65 and older |
-nurse education (6–9 problem-based education sessions) including geriatric medications and common problems in long care such as depression & pain. -supported by bulletins, wall charts and clinical pharmacist visits. - clinical pharmacist average contact 26 h/NH -clinical pharmacist reviewed drug regimen for 500 residents selected by home staff. |
12 months |
-Mortality rate -hospital admission -Drug use -ADEs -Medication-related problems |
-mean no of psycholeptics administered /resident in Int gp decreased (− 0.14,95% CI − 0.28-0.0, p = 0.044) - in the intervention group mean number of benzodiazepines Administered/ resident reduced (− 0.06, 95% CI − 0.06 to 0.04, p = 0.29). |
| 8 | Crotty et al. (a) [24] | 2004 | Australia | Cluster RCT | Aged care facility |
NH = 10 NHR = 154 |
65 and older |
−2 multidisciplinary case conferences were conducted 6–12 weeks. -pharmacists, geriatrician, residential care staff, GP, and a representative of the Alzheimer’s Association of South Australia. -medication review prepared by the resident’s GP before case conference. |
7 months | -MAI score |
-Mean MAI score in Int gp 4.1 (2.1–6.1) versus 0.4 (0.4–1.2) in ctrl gp. - benzodiazepines: mean MAI score in int.gp 0.73 (0.16–1.30) versus − 0.38 (−1.02 to 0.27) in ctrl gp. |
| 9 | Crotty et al. (b) [25] | 2004 | Australia | RCT |
long-term care facility/hospital discharge |
NH = 85 NHR = 110 Discharged from 3 hospitals |
65 and older |
-pharmacist transition coordinator transfers the medication-related information to the family physician and community pharmacist. -case conference at facility within month of transfer include pharmacist, nurse, family physician, community pharmacist, |
8 weeks |
-MAI score -Hospital admission -Medication related problems -ADEs, -falls |
-No change in MAI score in Int gp 2.5, 95% CI1.4–3.7) -In ctrl gp MAI score had worsened 6.5, 95%CI 3.9–9.1) |
| 10 | Crotty et al. (c) [26] | 2004 | Australia | RCT | Residential care facilities |
NH = 20 NHR = 715 |
65 and older |
-Educational intervention: two (30 min) outreach visits of pharmacists to doctors. - presenting detailed audit information on psychotropic use, stroke risk reduction, and fall rates. −4 (2 h training sessions) for link nurse in each facility. |
7 months |
-MAI score -Hospital admissions - MRP |
-No significant difference in psychotropic drug use before &after intervention (0.89,95%CI 0.69–1.15). -PRN of antipsychotics drug use increased in Int gp compared to ctrl gp (4.95,95%CI 1.69–14.50). - No significant difference in BZD drug use before & after intervention (0.89,95%CI 0.69–1.15). - No significant difference in falls (1.17, 95%CI 0.86–1.58). |
| 11 | Fossey et al. [27] | 2006 | UK | Cluster RCT | Nursing homes |
NH = 12 NHR = 349 |
65 and older |
-Training and support to care staff on non-pharmacological interventions, alternatives to neuroleptic use. -Medication review by Led by old age psychiatrist, senior nurse every 3 months -contact between psychiatrist and prescribers to provide and wrote prescribing recommendations |
12 months |
-Proportion of residents receiving neuroleptics. -CMAI - QoL |
- reduction in neuroleptic use/resident (19.1, 95% CI 0.5–37.7%, P = 0.045) --Neuroleptic use decrease 24% in exp. (47 to 23%) but increased in ctrl 7.6% (49.7 to 42.1%). -No significant changes in CMAI |
| 12 | Zermansky et al. [28] | 2006 | UK | RCT |
Nursing homes and residential homes |
NH = 65 NHR = 661 |
65 and older |
- Pharmacist medication review by using the resident’s medical record. - consultation with the resident’s and carer. -pharmacist forward written recommendations to GP. |
6 months |
-no. of changes in medication/patient -Hospital admissions -Medication-related problems -Medicine costs -Number of medicines per participant - Mortality - Falls - SMMSE -Barthel index -GP consultations |
- Increase in mean number of drug changes/patient ctrl: 2.4 versus 3.1 in Int (P < 0.01) -no of falls reduced significantly - pharmacist recommendations accepted (75.6%), and 76.6% of these recommendations were implemented. |
| 13 | Gurwitz et al. [29] | 2008 |
USA and Canada |
Cluster RCT | Two large long-term care facilities. |
Facility = 2 Residents = 1118 |
65 and older |
-Computer program (order entry with clinical decision support system). - more than 600 potentially serious drug-drug interactions alerts were reviewed. -no of ADEs were identified (preventable events including errors and drug-drug interactions were determined). -alerts included in the CDSSs were assessed to determine if any of them could have prevented the prescribing of these drugs. |
1 year in one facility and 6 months in the other |
-Number of preventable ADEs - ADEs severity - ADEs preventability |
-None ADEs = (1.06,95% CI 0.92–1.23) Preventable ADEs = (1.02,95% CI 0.81–1.30) |
| 14 | Field et al. [30] | 2009 | Canada | Cluster RCT |
long-term care facility |
-One long-term care Facility - 22 long-stay units Residents= 833 |
65 and older |
The 22 long-stay units were randomly assigned - for Intervention units’ prescriber: Alerts related to medication prescribing for residents with renal insufficiency were displayed. -Control units: Alerts hidden and tracked - The types alerts were: maximum recommended daily dose/frequency of administration, medication to be avoided, and missing information. |
12 months | -Proportion of final drug orders alert that were appropriate |
-Appropriate final drug orders proportion were high in Int (1.2, 95% CI 1.0–1.4) for frequency. -for drugs that should be avoided (2.6, 95% CI 1.4–5.0). for missing information (1.8, 95% CI 1.1 to 3.4). -Appropriate final drug orders Significant in Int (1.2 95% CI 1.0–1.4). |
| 15 | Patterson et al. [31] | 2010 | Ireland | Cluster RCT | Nursing homes |
NH = 11 NHR = 334 |
65 and older |
-intervention homes were visited monthly by trained pharmacists for 1 year. Resident’s information was collected from records, GP and community pharmacist. Interviews were conducted with the residents and next of kin to assess the need for medicines. - applied an algorithm to assess appropriateness of psychoactive medication and worked with GPs to improve the prescribing of these medications. |
Monthly for 12 months |
-Proportion of residents prescribed inappropriate psychoactive medications. -no of falls |
- At 12 months, residents taking inappropriate psychoactive medications in Int gp (19.5%) decreased compared to ctrl gp (50%) intervention homes (0.26, 95% CI 0.14–0.49) -No change the falls rate |
| 16 | Testad et al. [32] | 2010 | Norway | Cluster RCT | Nursing homes |
NH = 4 NHR = 211 |
65 and older |
-Education and training program (2 days seminar and monthly group guidance for six months). |
12 months |
-% of residents using antipsychotic drugs - Restraint use |
-No statically significant difference in antipsychotic use. - Significant reduction in Aggression in Int gp at 6 & 12 month follow-up. -Significant reduction in proportion of residents restrained at 6 months but not at 12 months. |
| 17 | Lapane et al. [33] | 2011 |
United States |
Cluster RCT | Nursing homes |
NH = 25 NHR = 3321 |
65 and older |
- GRAM is automatically generated to assist consultant pharmacists identify residents at risk for delirium/ falls -Detailed instruction of consultant pharmacists providing targeted medication review for all residents at high-risk. - Reports within 24 h of admission and used during monthly review. |
12 months |
− Mortality − Hospital admission potentially due to ADEs. |
-Mortality rate /1000 resident-months, HR: 0.90 (adjusted HR 0.89, 95% CI 0.73–1.08) -Hospital admission/1000 resident-months, HR: 1.13 (adjusted HR 1.11, 95% CI 0.94–1.31). |
| 18 | Pope et al. [34] | 2011 | UK, Ireland | RCT | Nurse-managed continuing-care |
NHR = 10 nurse-managed continuing-care Residents = 225 |
65 and older |
-medical assessment by a geriatrician, and using Beer’s criteria for multidisciplinary panel medication review. - recommendations forwarded to the GP. - after 6 months, reassessment occurred |
6 months |
-no of drugs prescribed -mortality -medication cost |
−92.7% of patients received medication recommendations and 80.1% accepted. - total number of medications/ patient/d reduced in Int gp (11.64–11.09 compared to ctrl 11.07–11.5). |
| 19 | Kersten et al. [35] | 2013 | Norway | RCT | Nursing homes |
NH = 22 NHR = 87 |
65 and older |
-A paper-based review with a view to reduce ADS scores were conducted by clinical pharmacist. -clinical pharmacist discuss discontinue or replace an anticholinergic drug with the physician before changes were implemented. |
8 weeks |
- Cognitive function - anti-cholinergic side-effects |
- cognitive function not improved - anti-cholinergic side-effects not improved |
| 20 | Milos et al. [36] | 2013 | Switzerland | RCT | Nursing homes or community | NHR = 279 | 75 years or older |
Pharmacists-led medication review that included assessment of relevant parts of (EMRs) and collection of patient’s blood sample data. - clinical pharmacist-initiated medication reviews based on the background information to identify DRPs. |
2 months |
- no of PIMs. - DRPs |
−6% decreased in PIM in Int gp -Total no of DRPs in the intervention group was 431 [mean 2.5 (1.5) / patient (range 0–9) - No significant difference between the no of DRPs in nursing home patients [mean 2.53 (1.33)] and community-dwelling patients [mean 2.55 (1.29)] Significant in changes in the actions taken by the physician were for lowered dosage. |
| 21 | Frankenthal et al. [37] | 2014 | Israel | RCT | chronic care geriatric facility |
NH = 1 NHR = 359 |
65 and older |
-medication review conducted by pharmacist -to identify PIMs and PPOs medications screened with STOPP/ START criteria then followed up with recommendations to the chief physician. - chief physician decided to accept or not. |
12 Months |
-medication appropriateness -mortality -hospital admission -QoL -MRP -medication cost |
-significant decreased in the average number of drugs prescribed in Int gp (P < .001). - significant decreased in the average number of falls in Int gp (P = .006). -decrease in the average drug costs in Int gp by US$29. - hospitalization, FIM scores, and QoL were same in both groups. |
| 22 | García-Gollarte, et al. [38] | 2014 | Spain | Cluster-RCT | Nursing homes |
NH = 36 NHR = 716 |
65 and older |
−30 doctors received educational intervention. - The educational intervention included general drug use in elderly, STOPP START workshop, and adverse drug reactions in older people. -participants also received educational material and references - on-demand support (via phone) for 6 months provided by the educator. |
6 Months |
- Medication appropriateness (STOPP-START) -Hospital admissions Medication appropriateness (STOPP-START) -Falls |
- The mean number of inappropriate drugs was higher in ctrl gp (1.29–1.56) compared to Int gp (0.81–1.13). -no of falls increased in the ctrl gp from 19.3–28% and not significantly change in the intervention group from 25.3–23.9%. |
| 23 | Pitkala et al. [39] | 2014 | Finland | Cluster-RCT | Assisted living facilities |
Facility = 20 Residents = 227 |
65 and older |
-two 4-h interactive training sessions for nursing staff aimed to enable nurses to recognize potentially harmful medications and corresponding adverse drug events. -the second 4-h sessions: case-study-based. - nurses in this intervention were asked to identify potential MDR and highlight these to the consulting doctor. |
12 months |
-Medication appropriateness -Hospital admissions -Mortality -QoL -MMSE |
-mean number of potentially harmful drugs lowered in int gp (−0.43, 95% CI-0.71 to −0.15) and not changed in ctrl gp (+ 0.11, 95% CI − 0.09 to + 0.31) (P = .004). -HR QoL decreased in Int gp (− 0.038, 95% CI − 0.054 to − 0.022) compared to ctrl gp (− 0.072,95% CI − 0.089 to − 0.055) (P = .005). -hospital admission decreased significantly in int gp (1.4 days/person/year, 95% CI 1.2 to −1.6) compared to ctrl gp (2.3 days/person/year; 95% CI 2.1to −2.7), RR = 0.60, 95% CI 0.49 to − 0.75, P < .001). |
| 24 | Connolly et al. [40] | 2015 | New Zealand | Cluster-RCT | RACFs |
NH = 36 NHR = 1998 |
65 and older |
- Gerontology nurse specialist delivered staff education and clinical coaching. - benchmarking of resident indicators including restraint use, falls, etc.). - multidisciplinary team meeting (1 h) monthly for the first 3 months. |
14 months |
- Hospital admissions (ambulatory sensitive hospitalisations, total acute admissions). -Mortality |
-no differences between Int and ctrl gp in rates of ambulatory sensitive hospitalisations admission (1.07; 95% CI 0.85–1.36; P = 0.59). -no difference in mortality (1.11; 95% CI 0.76–1.61; P = 0.62). |
| 25 | Potter et al. [41] | 2016 | Australia | RCT | RACFS |
Facility = 4 Residents = 95 |
65 and older |
-medication review followed by discontinuing non-beneficial medications conducted by a GP and a geriatrician/clinical Pharmacologist - During deprescribing, the GP reviewed participants weekly. |
12 months |
-no of falls -mortality -no of fallers -cognitive function -QoL |
-mortality 26% in int gp and 40% in ctrl gp (HR 0.60, 95%CI 0.30 to 1.22). -QoL Changes in Int gp (− 1.0 ± 4.3) compared to ctrl gp (− 1.0 ± 4.7). -Falls -Patients with one or more falls in int gp (0.56, 95% CI 0.42–0.69) compared to ctrl gp(0.65, 95% CI 0.50–0.77), (p = 0.40) |
Abbreviations: RCT Randomised Controlled Trials, NHR Nursing Home Residents, NHs Nursing Homes, CDSSs Computerised Clinical Decision Support Systems, CI Confidence Interval, GP General Practitioner, no number, min minutes, hr. hour, APs Antipsychotics, gp group, ctrl control, Int intervention, MMSE Mini-Mental State Exam, GDS Geriatric Depression Scale, BASDEC Brief Assessment Schedule Depression Cards, CRBRS Crichton-Royal Behaviour Rating Scale, P, p value, NSAIDs Non-Steroidal Anti-Inflammatory Drugs, ADEs Adverse Drug Events, MAI Medication Appropriateness Index, MRP Medication-related Problem, PRN pro re nata (when necessary), BZD Benzodiazepine, CMAI Cohen-Mansfield Agitation Inventory, QoL Quality of Life, exp experiment group, % percentage, GRAM Geriatric Risk Assessment Med Guide, HR Hazard Ratio, ADS Anticholinergic Drug Scale, EMRs electronic medical records, PIMs Potential Inappropriate medications, PPOs potential prescription omissions, STOPP/START Screening Tool of Older Person’s potentially inappropriate Prescriptions and Screening Tool to Alert doctors to Right Treatment, FIM Functional Independence Measure
Assessment of risk of Bias
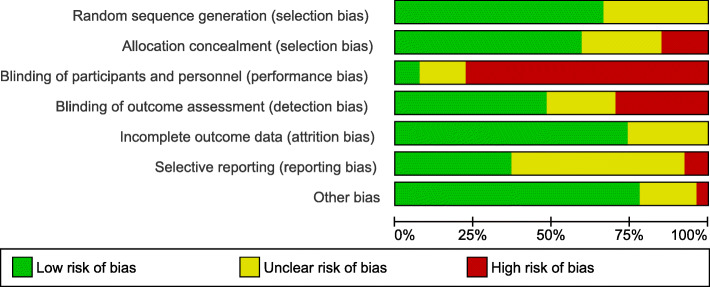
The quality of each article and risk of bias were assessed independently by the two reviewers (H.A, and L.F). For assessing risk of bias we used the Cochrane Collaboration’s tool [42]. The studies assessed based on standard criteria: adequate sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Risk of bias tables provided in RevMan v5.3 was used to assess reporting bias. See Risk of Bias in Included Studies Section (Figs. 2 and 3).
Fig. 2.
Risk of bias graph
Fig. 3.
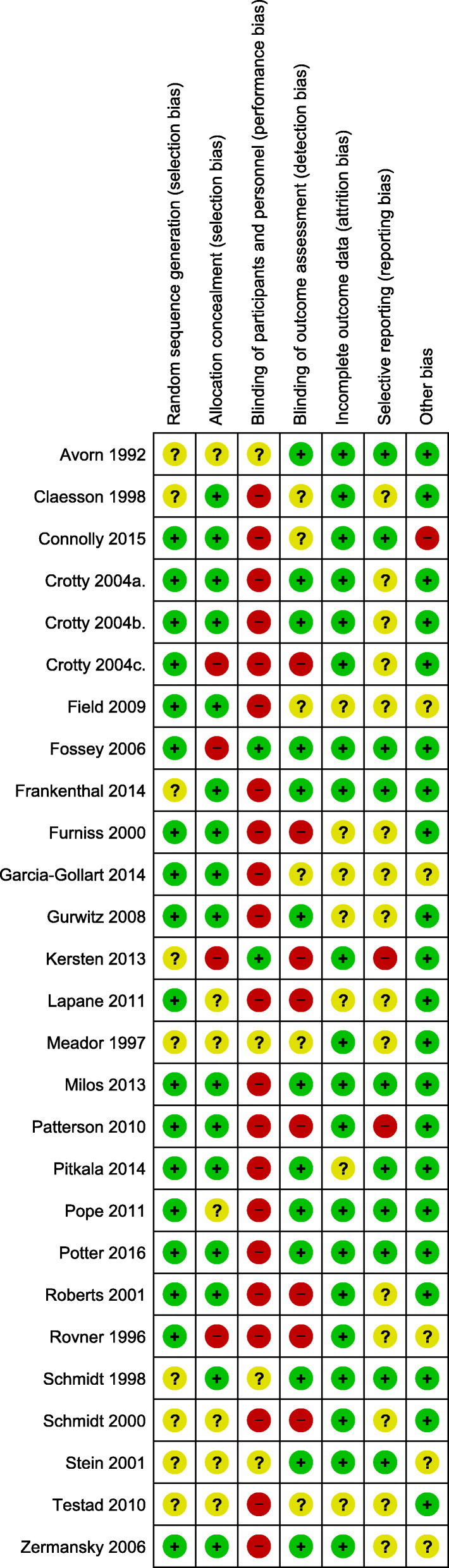
Risk of bias summary
Statistical analysis
Outcome measures such as medication appropriateness, hospitalisation, mortality and other outcomes were assessed for heterogeneity and were pooled for meta-analysis using fixed effects methods if little heterogeneity was found or using random effects methods if heterogeneity was present (P value < .05). The software Review Manager (RevMan) version 5.3 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) was used. The risk ratios (RR) for dichotomous outcomes with 95% confidence intervals (CIs) were calculated. Heterogeneity was measured by I2. Continuous outcomes were expressed as mean difference (MD) and standardized mean difference (SMD) between groups with a 95% confidence interval (95% CI). Funnel plots were used to assess possible publication bias (Additional file 3: Figs. S1 to S9). Effect estimates were considered statistically significant of the p value was less than 0.05 (2 tailed).
Results
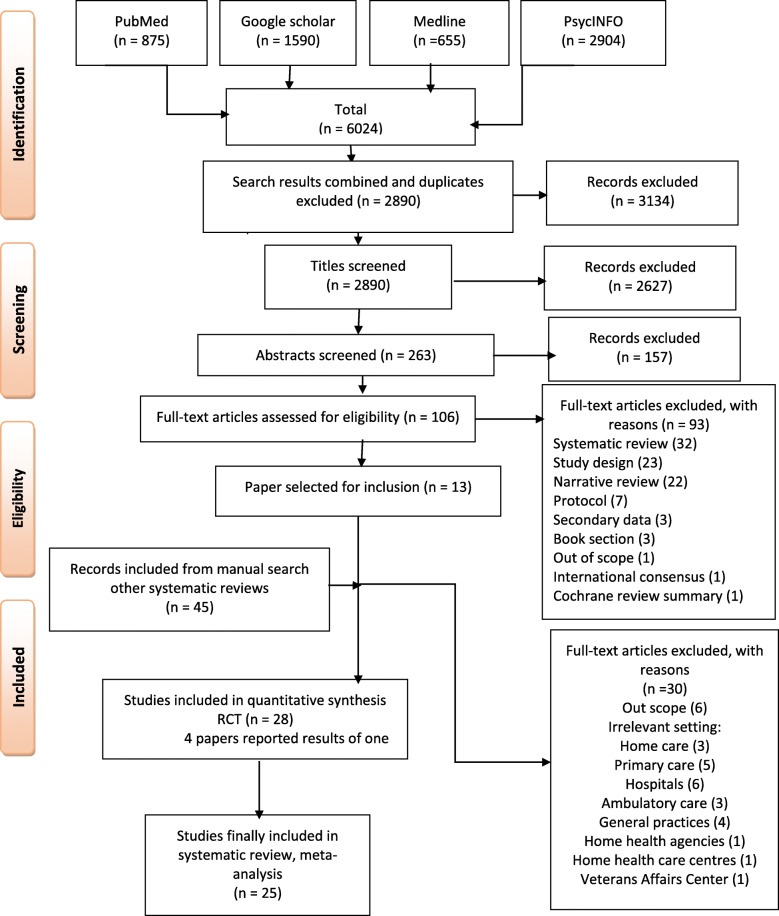
The literature search provided a total of 6024 potentially relevant publications. Following independent screening for eligibility, 106 articles were assessed for eligibility of which 25 RCTs and cRCTs were included in this systematic review. The flowchart of the literature search is represented in Fig. 1.
Fig. 1.
PRISMA flow diagram
Study design
The design consisted of 15 studies [14, 17, 19–24, 27, 29–33, 38–40] comprising five cRCTs and 10 RCTs [15, 16, 25, 26, 28, 34–37, 41].
Country and settings
Trials in residential aged care settings or residential continuing care hospitals (long-term care) were conducted in the USA (n = 5) [14–16, 22, 33], Australia (n = 5) [23–26, 41], UK (n = 3) [21, 27, 28], Norway (n = 2) [32, 35], and one each in Canada [30], Israel [37], Sweden (4 papers reported results of one) [17–20], Finland [39], Spain [38], Switzerland [36], Ireland [31], New Zealand [40] and one combined between USA and Canada [29] and one combined between UK and Ireland [34].
Participants
Studies included involved older people living in residential aged care facilities aged 60 years and older with a mean age range of 81.2 to 87.2 years.
Interventions
Various interventions applied by pharmacist, physician or a multidisciplinary team (physicians, pharmacists, and nurses) in the included studies were evaluated. Methods to review residents’ medications were presented in 12 studies [21, 23, 25, 28, 31, 33–38, 41]; nine studies [14–16, 22, 26, 27, 32, 39, 40] investigated the impact of staff education, four studies [17, 20, 24, 25, 40] evaluated the implementation of multi-disciplinary case conferencing, and two studies [29, 30] evaluated computerised clinical decision support systems.
Outcomes
Most outcome measures in the reviewed studies were reported as (a) medication appropriateness (n = 16) [14–17, 19, 23–27, 30, 31, 35–39], hospital admission (n = 11) [21, 23, 25, 28, 33, 34, 37–41], mortality (n = 9) [21–23, 28, 33, 34, 37, 39, 40], medication-related problems (n = 7) [18, 20, 21, 23, 25, 28, 36, 37], falls (n = 7) [26–28, 31, 33, 38, 41], quality of life (n = 5) [27, 34, 37, 39, 41], Behavioural and Psychological Symptoms of Dementia BPSD (n = 4) [15, 16, 27, 32], ADEs (n = 2) [25, 29], and cognitive function (n = 2) [15, 35].
Medication appropriateness
Medication appropriateness was assessed in 11,470 residents encompassing 16 RCTs [14–17, 19, 23–27, 30, 31, 35–39] by different tools including Medication Appropriateness Index (MAI) [24–26], STOPP-START criteria [37, 38], indicators of appropriate neuroleptic prescribing in nursing homes [27], Beers criteria, Anticholinergic Drug Score (ADS), number of psychotropic medications and non-steroidal anti-inflammatory drugs (NSAIDs( [39].
Other outcomes
Hospital admission
Eleven studies [21, 23, 25, 28, 33, 34, 37–41] specified hospital admission as an outcome measure. Furniss et al. [21] reported in-patient days as hospital admission. Roberts et al. [23] investigated the proportion of hospitalised residents. Crotty et al. [25] reported hospital usage based on unplanned visits to emergency department and hospital readmission. Zermansky et al. [28] reported hospitalisation rate during a 6-month period per resident. Lapane et al. [33] investigated any hospitalisation and potential ADE-related hospitalisation in a randomised cluster trial. Pope et al. [34] reported the number of admissions to acute hospital. Frankenthal et al. [37] reported hospital admissions. Garcia-Gollarte et al. [38] reported the total number of days spent in hospital. Pitkala et al. [39] reported hospital days/resident/year. Connolly et al. [40] reported all acute admissions and ambulatory sensitive hospitalisations. Potter et al. [41] reported hospital admission as the proportion of residents experiencing an unplanned hospital admission.
Mortality
Nine studies [21–23, 28, 33, 34, 37, 39, 40] included mortality as an outcome measure. Furniss et al. [21] reported mortality as a number of deaths over 8-months,by Zermansky et al. [28] over 6-months. The number of deaths was reported by Stein et al. [22] over a 3-month evaluation period, by Pope et al. [34] over a 6-month period, and by Frankenthal et al. [37] over 1 year. Roberts et al. [23] reported residents’ cumulative survival and death proportion for 1 year. Lapane et al. [33] calculated the average percentage of mortality per 1000 person-months. Pitkala et al. [39] used a Cox proportional hazard model to calculate hazard ratios. Connolly et al. [40] reported death risk ratio over 14 months.
Falls
Seven studies [26–28, 31, 33, 38, 41] included falls as an outcome measure. Crotty et al. [26] calculated the percentage of residents who fell in 3 months prior. Fossey et al. [27] reported the proportion of residents who had at least one fall over a 12-month period. Zermansky et al. [28] reported number of falls over 6 months. Patterson et al. [31] calculated falls rate per 100 resident-months. Lapane et al. [33] reported the number of people falling over 12 months. Garcia-Gollarte et al. [38] reported the number of falls and fallers post-intervention. Potter et al. [41] reported the proportion of patients with one or more falls.
Medication - related problems
Seven studies [18, 20, 21, 23, 25, 28, 36, 37] included medication - related problems as an outcome measure. Claesson and Schmidt et al. [18, 20] reported the type and frequency of drug-related problems discussed by clinical teams and their recommendations. Furniss et al. [21] reported the total number of recommendations made by the pharmacist, and the accepted recommendations by the general physician (GP) and the actual changes in medications. Roberts et al. [23] calculated the number of drug changes. Crotty et al. [25] categorised medication-related problems to different categories such as high dose, administration time and no indication. Zermansky et al. [28] measured the recommendations made by the pharmacist against the number of accepted/rejected recommendations of the doctor. Milos et al. [36] measured the percentage of medications changed. Frankenthal et al. [37] measured the number of recommendations accepted by the GP according to the STOPP-START criteria. There was no extractable data for this outcome and therefore meta-analysis was not performed.
Quality of Life (QoL)
Five studies [27, 34, 37, 39, 41] reported patient Quality of Life (QoL). Fossey et al. [27] reported rating for wellbeing in residents. Pope et al. [34] measured QoL by asking patients with Abbreviated Mental Test Score (AMTS ≥8) or staff who were familiar with the patient about whether the intervention had been of benefit. Frankenthal et al. [37] used the Medical Outcomes Study 12-item Short-form Health survey (SF-12). Pitkala et al. [39] used the 15-dimensional instrument of health-related QoL (15D). Potter et al. [41] used self-reported QoL assessed with Quality of Life in Alzheimer’s Dementia (QOLAD).
Behavioural and Psychological Symptoms of Dementia (BPSD)
Four studies [15, 16, 27, 32] assessed Behavioural and Psychological Symptoms of Dementia (BPSD). Rovner et al. [15] reported behaviour disorder. Fossey et al. [27] reported aggression events in past 12 months and Testad et al. [32] used the Cohen-Mansfield agitation inventory tool (CMAI) to measure agitated behaviour of residents. Meador et al. [16] used Nursing Home Behaviour Problem Scale (NHBPS).
ADEs
Two studies [25, 29] reported ADEs. One study defined ADE as an injury resulting from the use of a drug [29]. Crotty et al. [25] investigated number of ADEs during the 8-week follow-up period.
Cognitive function
Two studies [15, 35] included cognitive function as an outcome measure. Rovner et al. [15] used the Norwegian version of the global cognitive test Mini-Mental Sate Examination (MMSE) to assess cognition. Kersten et al. [35] used the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD)‘s 10-word list test for delayed recall and recognition and MMSE.
Risk of bias in included studies
Assessment of the risk of bias is summarised in (Figs. 2 and 3). Except for nine studies with unclear risk [14, 16, 17, 19, 20, 22, 32, 35, 37], the remaining 16 studies had low risk of selection bias. Performance bias was high in 21 (77.8%) studies [15, 19–21, 23–26, 28–34, 36–41]; detection bias was high in eight (29.6%) studies [15, 19, 21, 23, 26, 31, 33, 35], and allocation concealment was found in four (14.8%) studies [15, 26, 27, 35]. In most of the studies, blinding of participants and staff was not possible due to the nature of the intervention.
Effectiveness of the interventions
Medication appropriateness
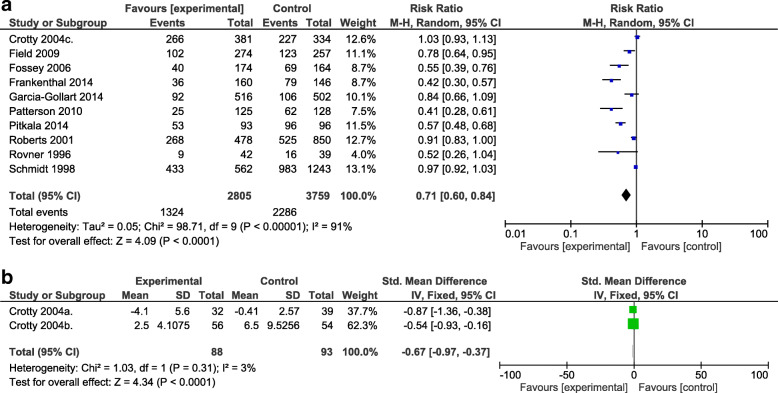
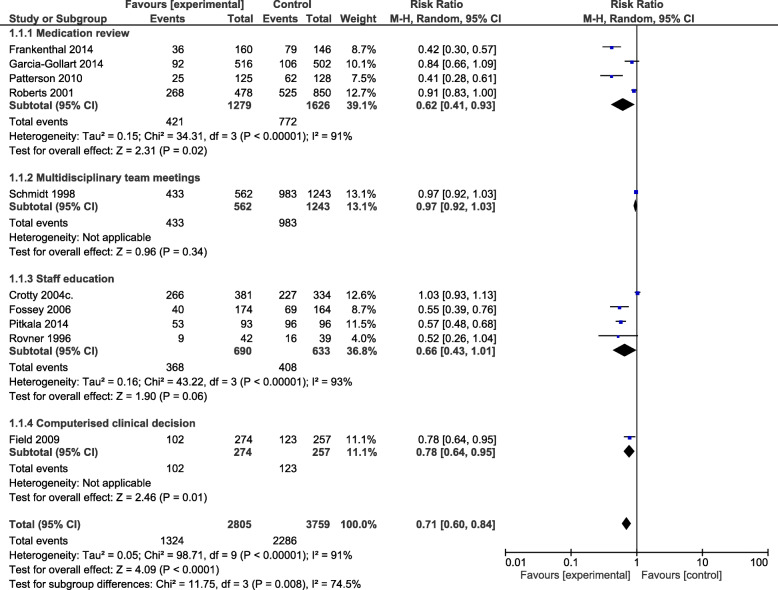
Meta-analysis of medication appropriateness (Fig. 4) including 6754 residents [15, 17, 23–27, 30, 31, 37–39] showed a significant improvement on medication appropriateness (RR 0.71; 95% confidence interval (CI): 0.60, 0.84, despite high heterogeneity (P < 0.00001; I2 = 91%). This outcome was assessed for the intervention subtypes of staff education (RR 0.66, 95% CI:0.43, 1.01), implementation of multi-disciplinary case conferencing (RR 0.97,95% CI:0.92, 1.03) computerised clinical decision support systems (RR 0.78, 95% CI:0.64, 0.95) and medication review (RR 0.62 95% CI:0.41, 0.93) (See Fig. 5).
Fig. 4.
a: A meta-analysis of the effect of interventions on medication appropriateness. b: Standardised mean difference in the change of MAI score comparing experimental (intervention) group and control group
Fig. 5.
Subgroup analysis of the effect of interventions medication review, multi-disciplinary team meetings, staff education and computerised clinical decision
The standardised mean difference of medication appropriateness scales for the remaining two Crotty et al. studies [24, 25] was calculated separately (standardised mean difference = − 0.67; 95% CI: − 0.97, − 0.36) with a heterogeneity of I2 = 3%.
Other outcomes
Hospital admission
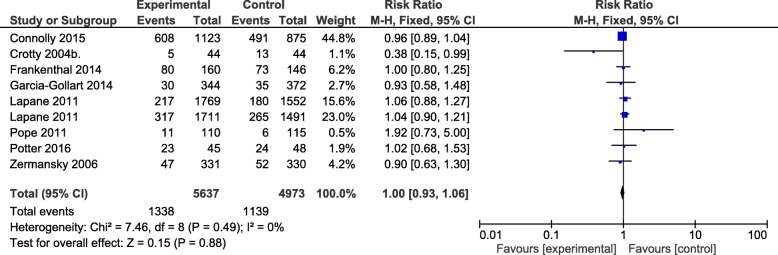
Meta-analysis of hospital admission (Fig. 6) as an outcome measure investigated in 11,272 residents resulted in the analysis of eight studies [25, 28, 33, 34, 37, 38, 40, 41] (10,610 residents), which showed that interventions have no effect on hospital admission RR = 1.00, 95% CI: 0.93,1.06) with a heterogeneity of I2 = 0%.
Fig. 6.
A meta-analysis of the effect of interventions on hospital admission
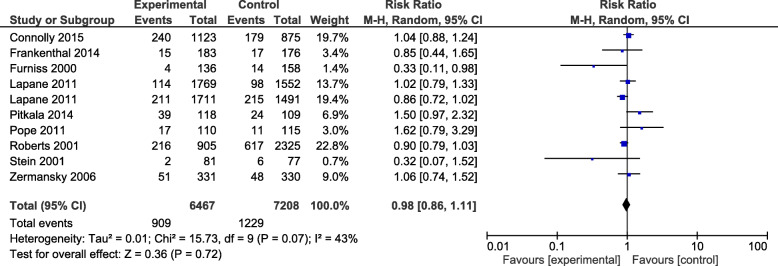
Mortality
Meta-analysis of mortality (Fig. 7) as an outcome measure investigated in 13,675 residents [21–23, 28, 33, 34, 37, 39, 40] showed no significant difference between the intervention group and control group (RR 0.98, 95% CI: 0.86,1.11, P = 0.07) with a heterogeneity of I2 = 43%.
Fig. 7.
A meta-analysis of the effect of interventions on mortality
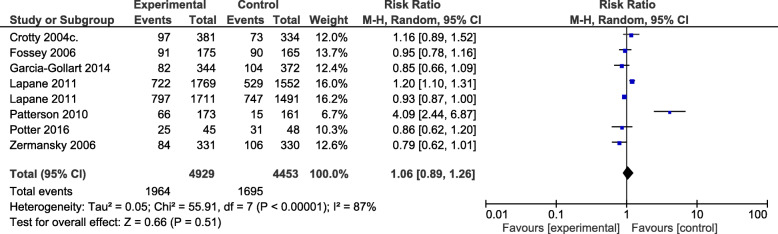
Falls
Meta-analysis of falls (Fig. 8) as an outcome measure investigated in 9382 residents [26–28, 31, 33, 38, 41] showed that interventions had no effect on falls (RR = 1.06; 95%CI: 0.89,1.26) with a heterogeneity of I2 = 87%.
Fig. 8.
A meta-analysis of the effect of interventions on falls
Medication- related problems
Claesson and Schmidt et al. [18, 20] found 819 medication -related problems in 395 participants resulting in an action taken in 90% (737) with withdrawal of a drug in 368 (45%) and change of medications in 162 (20%). Furniss et al. [21] reported that 239 (92%) of 261 recommendations were accepted by the GP resulting in change in therapy in 144 patients. The most common reasons for recommendations (33%) were the medication indication was no longer present. Roberts et al. [23] found that medication reviews resulted in changes to medications in 54 (39%) of residents. Crotty et al. [25] reported that the most common medication-related problem identified in control and intervention groups was that the residents were allocated to a new family physician when transfer to long-term care facility (n = 35, 62.5% intervention; n = 41, 75.9% control). Zermansky et al. [28] found a significant difference in the mean number of drug changes per resident (mean 3.1, SD = 2.7 for intervention and mean 2.4, SD = 2.6 for control) (P < 0.0001). Milos et al. [36] found similar number of drug-related problems between community-dwelling patients (mean 2.55, SD = 1.29) and nursing home residents (mean 2.53, SD = 1.33) (p = 0.767). 56% of drug-related problems resulted in an action taken and change of medications (mean 1.44, SD = 1.33) with no difference between the community dwelling and the nursing home patients (p = 0.946). Frankenthal et al. [37] made 327 recommendations - 245 in 129 residents based on STOPP and 82 in 65 residents based on START. The physician accepted 82.4% of STOPP recommendations and 92.6% of START recommendations.
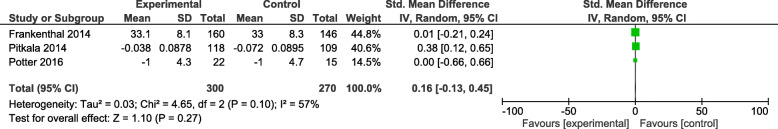
Quality of Life (QoL)
Meta-analysis of quality of life (Fig. 9) outcomes in 570 residents, of a total of 1141 residents that included QoL as an outcome measure, found that the interventions had no effect on residents’ QoL (standardised mean difference = 0.16 95% CI:-0.13, 0.45) with a heterogeneity of I2 = 57% between trials [37, 39, 41]. Pitkala et al. [39] reported that health-related QoL in the intervention group (− 0.038, 95% CI: − 0.054, − 0.022) declined more slowly than in control group during 12-month follow-up (− 0.072, 95% CI: − 0.089, − 0.055). Frankenthal et al. [37] found no significant difference between groups in the physical average score (intervention mean 33.1 ± 8.1, control mean 33 ± 8.3, p = 0.09) and mental components (intervention mean 37.7 ± 1.7, control mean 39.6 ± 11.3 p = 0.70) of the SF-12 questionnaire.
Fig. 9.
A meta-analysis of the effect of interventions on quality of life (QoL)
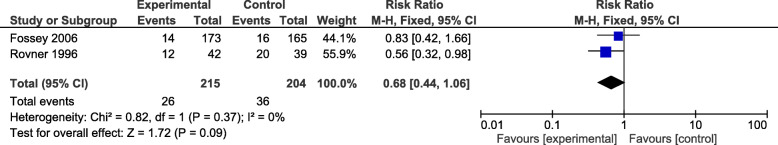
BPSD
Of 1941 residents examined, pooled analysis of BPSD of two studies [15, 27] comprising 419 residents showed no significant change after the intervention (RR 0.68, 95% CI: 0.44,1.06; I2 =0%) (Fig. 10).
Fig. 10.
A meta-analysis of the effect of interventions on Behavioural and Psychological Symptoms of Dementia (BPSD)
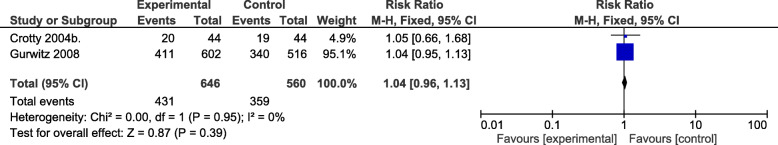
ADEs
Of the 1206 residents [25, 29] examined for ADEs after the intervention. Neither of the two RCTs reported any statistically significant differences in ADEs between the intervention and control groups. The RR for all ADEs was 1.04 (95% CI: 0.96,1.13; I2 =0%) (Fig. 11).
Fig. 11.
A meta-analysis of the effect of interventions on adverse drug events (ADEs)
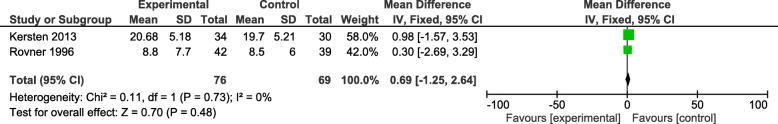
Cognitive function
Meta-analysis of 145 residents [15, 35] indicated that the interventions had no effect on cognitive function (weighted mean difference = 0.69, 95%CI: − 1.25, 2.64) (Fig. 12). No heterogeneity was detected between trials (I2 =0).
Fig. 12.
A meta-analysis of the effect of interventions on cognitive function
Discussion
This systematic review examined how a wide variety of interventions optimise medications prescribed in nursing homes, when applied either individually or through multi-faceted approaches. Our meta-analysis of available data showed that the interventions implemented in the included studies can improve medication appropriateness in older residents, although heterogeneity was high among included studies. Whilst these results were promising, the impact on the residents’ clinical outcomes was undetectable. There was limited evidence for effectiveness of interventions in reducing hospitalisation, all-cause mortality, falls, ADEs, cognitive function or BPSD.
There are several published systematic reviews on clinical outcomes of different interventions conducted in aged care homes. In a review by Forsetlund and colleagues [11], the authors found that both educational outreach/educational interventions and medication review by pharmacists under certain situations could reduce inappropriate drug use in nursing homes. However, they reported that the evidence was of poor quality and too low to assess the effect of the interventions on health outcomes. A review by Loganathan et al. [12] grouped the interventions into four groups (staff education including academic detailing, multi-disciplinary team meetings, medication review, and computerised clinical decision support system). No one interventional strategy was found to be effective. However, the most promising intervention seems to be education including academic detailing. That review reported that multifaceted interventions are likely to be required to improve prescribing in care homes [12]. A narrative review by Shafiee et al. [10], which included 18 studies, seven of them RCTs, found that the interventions may improve medication appropriateness in people with dementia in any settings, but the evidence for the effect of the interventions on health outcomes remained uncertain.
Our findings on clinical outcomes are in line with that of previous reviews [43, 44], which found no evidence for the interventions impacting resident’s clinical outcomes such as ADEs, mortality, QoL and hospital admission. Since the elderly often exhibit non-specific clinical symptoms such as depression, constipation, falls and confusion, it is difficult to detect ADEs as opposed to the general condition of the residents. Another possible reason for the lack of significant effect of interventions on falls and ADEs may be the potential for underreporting of incidents that were obtained from nursing records. The lack of effect of interventions on QoL in the treatment group compared to the control may be attributed to the wide variation in the length of the follow up period (3–12 months) [43].
Interventions that focus on individual team members may had limited effectiveness in busy clinical environment. For example, the educational intervention delivered by a pharmacist [26] failed to have any significant effect on major outcomes. The investigators demonstrated that the lack of effect was attributed to staff attrition, short study duration, and not all the physicians in the recruited homes participated in the study.
Very few of the interventions were based on strong theoretical foundations. An exception was the educational study by Pitkälä et al. [39], who suggested that the use of constructive learning theory to recognise potentially harmful medications was more likely to change practice in healthcare than using lectures alone. Deficits in the education of health careworkers were thought to be important. Forsetlund et al. [11] suggested that health care providers receive inadequate training in geriatric medications in their education. Therefore, any intervention for minimising medications usually requires some form of education.
Although our primary focus was on clinical outcomes, some interventions demonstrated a decrease in medication-related costs. Frunsis et al. [21] reported a reduction in the cost of medicine per resident over 4 months period by 27.47 GB Pounds in the intervention group. Roberts et al. [23] reported savings in drug cost (64 AUD/ resident/year in intervention group) in the clinical pharmacy program. Frankenthal et al. [37] found a significant reduction in the average monthly costs of medications in the intervention group ILS 279 ± 171.9 compared to baseline ILS 382.7 ± 279 (P < 0.001) at 12 months follow-up period. Pope et al. [34] reported a net reduction in medication cost in intervention group over a 6-month period. While, Crotty et al. [24] reported similar Pharmaceutical Benefit Scheme (PBS) monthly drug costs of regular medications between groups (mean AUD 359 in intervention versus AUD 303 in control (P = 0.837). These interventions require resources and therefore, evaluating these interventions economically and their cost-effectiveness should be considered in future research.
Strengths and limitations
This systematic review was based on a comprehensive search of the literature that was limited to 25 studies with robust design (RCT, cRCT) and compared to previous reviews on related topics [12, 44], our sample size may be regarded as sufficiently powered.
Another strength of this review was a focus only on residents in care homes. The nursing home population is at heightened risk of receiving multiple drugs because of their comorbidities. Therefore, evaluating specific available interventions optimising medications in this setting is required. We were able to complete meta-analyses to pooled the overall effects.
This study is not without limitations. We included only English language publications, which may lead to potential omission of other interventions. Although MEDLINE, PubMed, Google scholar, and PsycINFO databases were searched for relevant articles, some studies indexed in other databases may have been missed. We identified several additional articles manually which may indicate poor indexing of older studies and a lack of consistent terminology.
Due to the nature of the interventions, performance and detection biases may have resulted from the difficulty in maintaining blinding. Meta-analysis of some studies was difficult due to the variations in the measurement of specific outcomes. Certain outcomes, such as cognitive function, were examined in a limited number of articles, reducing the power of the analysis. Further, certain studies were small or had short study periods, which may potentially limit the effect of an intervention on the outcomes. We attempted to evaluate medication-related problems such as drug interactions, number of pharmacist recommendations etc. but these outcomes were not consistently reported in the studies and this our ability to draw any robust conclusions was limited.
Heterogeneity was notable among some studies included in the meta-analysis. The factors that caused this heterogeneity were difficult to discern. Due to differences in training, the characteristics of the nursing home residents, healthcare culture, the number of physicians’ visits and their usual practices the ability to generalise findings from one country to another is difficult.
Implications for research and practice
In view of the considerable investment in strategies aimed at improving medication appropriateness in RACFs worldwide, our findings question the value of such interventions based on the apparent lack of outcomes that may be meaningful to RACF residents.
Large, high quality RCT studies are required to identify effective interventions to optimise medications used in RACFs. Regarding physicians or staff acceptance of the intervention, only limited information was provided in the studies. Further qualitative study utilising semi-structured interviews may provide useful information to obtain the opinion of healthcare professionals with regard to process outcomes, such as whether the intervention was perceived to be successful or not, and to identify the potential means to overcome the barriers to changing professional behaviour by this method. More intensive interventions on medical and care staff with more stringent monitoring may be required.
Conclusion
This systematic review found that multifaceted interventions including medication review, staff education/training, multi-disciplinary case-conferencing and clinical decision support technology could improve the appropriateness of medications at RACFs. However, evidence for the effect of these interventions on residents’ clinical outcomes was scarce and no conclusion could be drawn. More robust clinical studies are required to ascertain the health outcomes benefits.
Supplementary information
Additional file 3 Funnel plots (pdf). Figure S1. Medication appropriateness funnel plot (10studies). Figure S2. Medication appropriateness funnel plot (2 studies). Figure S3. Hospital admission funnel plot. Figure S4. Mortality funnel plot. Figure S5. Falls funnel plot. Figure S6. Quality of life (QoL) funnel plot. Figure S7. Behavioural and Psychological Symptoms of Dementia (BPSD) funnel plot. Figure S8. Adverse drug events (ADEs) funnel plot. Figure S9. Cognitive function funnel plot.
Acknowledgments
Not applicable.
Abbreviations
- RACFs
Residential aged care facilities
- RCTs
Randomised control trials
- cRCTs
Cluster randomised control trials
- QoL
Quality of life
- BPSD
Behavioural and Psychological Symptoms of Dementia
- ADEs
Adverse drug events
- CI
Confidence interval
- RR
Risk ratio
- MD
Mean difference
- SMD
Standardized mean difference
- ADRs
Adverse drug reactions
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- MAI
Medication appropriateness index
- ADS
Anticholinergic drug score
- NSAIDs
Non-steroidal anti-inflammatory drugs
- AMTS
Abbreviated mental test score
- 15 D
15-dimensional instrument of health-related QoL
- QOLAD
Quality of Life in Alzheimer’s Dementia
- CMAI
Cohen-Mansfield agitation inventory tool
- NHBPS
Nursing Home Behaviour Problem Scale
- MMSE
Mini-Mental Sate Examination
- CERAD
Consortium to Establish a Registry for Alzheimer’s Disease
- PIP
Potentially inappropriate prescriptions
- PBS
Pharmaceutical Benefit Scheme
Authors’ contributions
HA and LF had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: HA, LF, CE and AS. Analysis and interpretation of data: LF, HA. Initial drafting of the manuscript: HA, LF, CE, and AS. Statistical analysis: HA, LF and CE and AS. Study supervision: LF, CE, and AS. All authors have read and approved the final version of this manuscript.
Funding
Hend Almutairi is supported by University of Western Australia International Fee Scholarship (Uifs) And University Postgraduate Award (Upa).
Leon Flicker is supported by a Medical Research Future Fund Next Generation Clinical Researchers Practitioner Fellowship (1155669).
The actual review received no specific funding. The funding sources had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hend Almutairi, Email: phhend@hotmail.com.
Andrew Stafford, Email: andrew.stafford@curtin.edu.au.
Christopher Etherton-Beer, Email: christopher.etherton-beer@uwa.edu.au.
Leon Flicker, Email: leon.flicker@uwa.edu.au.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12877-020-01634-4.
References
- 1.O’Connor MN, Gallagher P, O’Mahony D. Inappropriate prescribing. Drugs Aging. 2012;29(6):437–452. doi: 10.2165/11632610-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Burns E, McQuillan N. Prescribing in care homes: the role of the geriatrician. TACD. 2011;2(6):353–358. doi: 10.1177/2040622311421438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamilton HJ, Gallagher PF, O'Mahony D. Inappropriate prescribing and adverse drug events in older people. BMC Geriatr. 2009;9(1):5–3. doi: 10.1186/1471-2318-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, et al. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet. 2007;370(9582):173–184. doi: 10.1016/S01406736(07)61091-5. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher P, Barry P, O'Mahony D. Inappropriate prescribing in the elderly. J Clin Pharm Ther. 2007;32(2):113–121. doi: 10.1111/j.1365-2710.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- 6.Jiwa M. Optimising medication in frail older people. Prescriber. 2018;29(3):18–24. doi: 10.1002/psb.1655. [DOI] [Google Scholar]
- 7.Anrys P, Strauven G, Boland B, Dalleur O, Declercq A, Degryse J-M, et al. Collaborative approach to optimise medication use for older people in nursing homes (COME-ON): study protocol of a cluster controlled trial. Implement Sci. 2015;11(1):35. doi: 10.1186/s13012-016-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadogan CA, Ryan C, Hughes CM. Appropriate polypharmacy and medicine safety: when many is not too many. Drug Saf. 2016;39(2):109–116. doi: 10.1007/s40264-015-0378-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NICE M, Prescribing CU . Medicines optimisation: the safe and effective use of medicines to enable the best possible outcomes. 2015. [PubMed] [Google Scholar]
- 10.Shafiee Hanjani L, Long D, Peel NM, Peeters G, Freeman CR, Hubbard RE. Interventions to optimise Prescribing in older people with dementia: a systematic review. Drugs Aging. 2019;36(3):247–267. doi: 10.1007/s40266-018-0620-9. [DOI] [PubMed] [Google Scholar]
- 11.Forsetlund L, Eike MC, Gjerberg E, Vist GE. Effect of interventions to reduce potentially inappropriate use of drugs in nursing homes: a systematic review of randomised controlled trials. BMC Geriatr. 2011;11:16. doi: 10.1186/1471-2318-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loganathan M, Singh S, Franklin BD, Bottle A, Majeed A. Interventions to optimise prescribing in care homes: systematic review. Age Ageing. 2011;40(2):150–162. doi: 10.1093/ageing/afq161. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 14.Avorn J, Soumerai SB, Everitt DE, Ross-Degnan D, Beers MH, Sherman D, et al. A randomized trial of a program to reduce the use of psychoactive drugs in nursing homes. N Engl J Med. 1992;327(3):168–173. doi: 10.1056/NEJM199207163270306. [DOI] [PubMed] [Google Scholar]
- 15.Rovner BW, Steele CD, Shmuely Y, Folstein MF. A randomized trial of dementia care in nursing homes. J Am Geriatr Soc. 1996;44(1):7–13. doi: 10.1111/j.1532-5415.1996.tb05631.x. [DOI] [PubMed] [Google Scholar]
- 16.Meador KG, Taylor JA, Thapa PB, Fought RL, Ray WA. Predictors of antipsychotic withdrawal or dose reduction in a randomized controlled trial of provider education. J Am Geriatr Soc. 1997;45(2):207–210. doi: 10.1111/j.1532-5415.1997.tb04509.x. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt IK, Claesson CB, Westerholm B, Nilsson LG. Physician and staff assessments of drug interventions and outcomes in Swedish nursing homes. Ann Pharmacother. 1998;32(1):27–32. doi: 10.1111/j.1532-5415.1998.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt I, B. Claesson C, Westerholm B, Nilsson LG, Svarstad BL. The impact of regular multidisciplinary team interventions on psychotropic prescribing in Swedish nursing homes. J Am Geriatr Soc 1998;46(1):77–82. DOI: 10.1177/106002809803200102. [DOI] [PubMed]
- 19.Schmidt IK, Fastbom J. Quality of drug use in Swedish nursing homes. Clin Drug Investig. 2000;20(6):433–446. doi: 10.2165/00044011-200020060-00006. [DOI] [PubMed] [Google Scholar]
- 20.Claesson CB, Schmidt IK. Drug use in Swedish nursing homes. Clin Drug Investig. 1998;16(6):441–452. doi: 10.2165/00044011-199816060-00004. [DOI] [PubMed] [Google Scholar]
- 21.Furniss L, Burns A, Craig SK, Scobie S, Cooke J, Faragher B. Effects of a pharmacist's medication review in nursing homes. Randomised controlled trial. Br J Psychiatry. 2000;176:563–567. doi: 10.1192/bjp.176.6.563. [DOI] [PubMed] [Google Scholar]
- 22.Stein CM, Griffin MR, Taylor JA, Pichert JW, Brandt KD, Ray WA. Educational program for nursing home physicians and staff to reduce use of non-steroidal anti-inflammatory drugs among nursing home residents: a randomized controlled trial. Med Care. 2001;39(5):436–445. doi: 10.1097/00005650-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Roberts MS, Stokes JA, King MA, Lynne TA, Purdie DM, Glasziou PP, et al. Outcomes of a randomized controlled trial of a clinical pharmacy intervention in 52 nursing homes. Br J Clin Pharmacol. 2001;51(3):257–265. doi: 10.1046/j.1365-2125.2001.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crotty M, Halbert J, Rowett D, Giles L, Birks R, Williams H, et al. An outreach geriatric medication advisory service in residential aged care: a randomised controlled trial of case conferencing. Age Ageing. 2004;33(6):612–617. doi: 10.1093/ageing/afh213. [DOI] [PubMed] [Google Scholar]
- 25.Crotty M, Rowett D, Spurling L, Giles LC, Phillips PA. Does the addition of a pharmacist transition coordinator improve evidence-based medication management and health outcomes in older adults moving from the hospital to a long-term care facility? Results of a randomized, controlled trial. Am J Geriatr Pharmacother. 2004;2(4):257–264. doi: 10.1016/j.amjopharm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Crotty M, Whitehead C, Rowett D, Halbert J, Weller D, Finucane P, et al. An outreach intervention to implement evidence based practice in residential care: a randomized controlled trial. BMC Health Serv Res. 2004;4(1):6–2. doi: 10.1186/1472-6963-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fossey J, Ballard C, Juszczak E, James I, Alder N, Jacoby R, et al. Effect of enhanced psychosocial care on antipsychotic use in nursing home residents with severe dementia: cluster randomised trial. Bmj. 2006;332(7544):756–761. doi: 10.1136/bmj.38782.575868.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zermansky AG, Alldred DP, Petty DR, Raynor DK, Freemantle N, Eastaugh J, et al. Clinical medication review by a pharmacist of elderly people living in care homes--randomised controlled trial. Age Ageing. 2006;35(6):586–591. doi: 10.1093/ageing/afl075. [DOI] [PubMed] [Google Scholar]
- 29.Gurwitz JH, Field TS, Rochon P, Judge J, Harrold LR, Bell CM, et al. Effect of computerized provider order entry with clinical decision support on adverse drug events in the long-term care setting. J Am Geriatr Soc. 2008;56(12):2225–2233. doi: 10.1111/j.1532-5415.2008.02004.x. [DOI] [PubMed] [Google Scholar]
- 30.Field TS, Rochon P, Lee M, Gavendo L, Baril JL, Gurwitz JH. Computerized clinical decision support during medication ordering for long-term care residents with renal insufficiency. J Am Med Inform Assoc. 2009;16(4):480–485. doi: 10.1197/jamia.M2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson SM, Hughes CM, Crealey G, Cardwell C, Lapane KL. An evaluation of an adapted U.S. model of pharmaceutical care to improve psychoactive prescribing for nursing home residents in northern Ireland (fleetwood northern Ireland study) J Am Geriatr Soc. 2010;58(1):44–53. doi: 10.1111/j.1532-5415.2009.02617.x. [DOI] [PubMed] [Google Scholar]
- 32.Testad I, Ballard C, Bronnick K, Aarsland D. The effect of staff training on agitation and use of restraint in nursing home residents with dementia: a single-blind, randomized controlled trial. J Clin Psychiatry. 2010;71(1):80–86. doi: 10.4088/JCP.09m05486oli. [DOI] [PubMed] [Google Scholar]
- 33.Lapane KL, Hughes CM, Daiello LA, Cameron KA, Feinberg J. Effect of a pharmacist-led multicomponent intervention focusing on the medication monitoring phase to prevent potential adverse drug events in nursing homes. J Am Geriatr Soc. 2011;59(7):1238–1245. doi: 10.1111/j.1532-5415.2011.03418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pope G, Wall N, Peters CM, O'Connor M, Saunders J, O'Sullivan C, et al. Specialist medication review does not benefit short-term outcomes and net costs in continuing-care patients. Age Ageing. 2011;40(3):307–312. doi: 10.1093/ageing/afq095. [DOI] [PubMed] [Google Scholar]
- 35.Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2013;68(3):271–278. doi: 10.1093/gerona/gls176. [DOI] [PubMed] [Google Scholar]
- 36.Milos V, Rekman E, Bondesson A, Eriksson T, Jakobsson U, Westerlund T, et al. Improving the quality of pharmacotherapy in elderly primary care patients through medication reviews: a randomised controlled study. Drugs Aging. 2013;30(4):235–246. doi: 10.1007/s40266-013-0057-0. [DOI] [PubMed] [Google Scholar]
- 37.Frankenthal D, Lerman Y, Kalendaryev E, Lerman Y. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. J Am Geriatr Soc. 2014;62(9):1658–1665. doi: 10.1111/jgs.12993. [DOI] [PubMed] [Google Scholar]
- 38.García-Gollarte F, Baleriola-Júlvez J, Ferrero-López I, Cuenllas-Díaz Á, Cruz-Jentoft AJ. An educational intervention on drug use in nursing homes improves health outcomes resource utilization and reduces inappropriate drug prescription. J Am Med Dir Assoc. 2014;15(12):885–891. doi: 10.1016/j.jamda.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Pitkala KH, Juola A-L, Kautiainen H, Soini H, Finne-Soveri UH, Bell JS, et al. Education to reduce potentially harmful medication use among residents of assisted living facilities: a randomized controlled trial. J Am Med Dir Assoc. 2014;15(12):892–898. doi: 10.1016/j.jamda.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Connolly MJ, Boyd M, Broad JB, Kerse N, Lumley T, Whitehead N, et al. The aged residential care healthcare utilization study (ARCHUS): a multidisciplinary, cluster randomized controlled trial designed to reduce acute avoidable hospitalizations from long-term care facilities. J Am Med Dir Assoc. 2015;16(1):49–55. doi: 10.1016/j.jamda.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Potter K, Flicker L, Page A, Etherton-Beer C. Deprescribing in frail older people: a randomised controlled trial. PLoS One. 2016;11(3):e0149984. doi: 10.1371/journal.pone.0149984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;34(3):5423–5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patterson SM, Cadogan CA, Kerse N, Cardwell CR, Bradley MC, Ryan C, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2014(10). DOI:org/10.1002/14651858.CD008165.pub4. [DOI] [PubMed]
- 44.Alldred DP, Kennedy MC, Hughes C, Chen TF, Miller P. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst Rev. 2016;2:CD009095. doi: 10.1002/14651858.CD009095.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 3 Funnel plots (pdf). Figure S1. Medication appropriateness funnel plot (10studies). Figure S2. Medication appropriateness funnel plot (2 studies). Figure S3. Hospital admission funnel plot. Figure S4. Mortality funnel plot. Figure S5. Falls funnel plot. Figure S6. Quality of life (QoL) funnel plot. Figure S7. Behavioural and Psychological Symptoms of Dementia (BPSD) funnel plot. Figure S8. Adverse drug events (ADEs) funnel plot. Figure S9. Cognitive function funnel plot.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional files.