Abstract
The current measures to control foot-and-mouth disease (FMD) include vaccination, movement control and slaughter of infected or susceptible animals. One of the difficulties in controlling FMD by vaccination arises due to the substantial diversity found among the seven serotypes of FMD virus (FMDV) and the strains within these serotypes. Therefore, vaccination using a single vaccine strain may not fully cross-protect against all strains within that serotype, and therefore selection of appropriate vaccines requires serological comparison of the field virus and potential vaccine viruses using relationship coefficients (r 1 values). Limitations of this approach are that antigenic relationships among field viruses are not addressed, as comparisons are only with potential vaccine virus. Furthermore, inherent variation among vaccine sera may impair reproducibility of one-way relationship scores. Here, we used antigenic cartography to quantify and visualize the antigenic relationships among FMD serotype A viruses, aiming to improve the understanding of FMDV antigenic evolution and the scope and reliability of vaccine matching. Our results suggest that predicting antigenic difference using genetic sequence alone or by geographical location is not currently reliable. We found co-circulating lineages in one region that were genetically similar but antigenically distinct. Nevertheless, by comparing antigenic distances measured from the antigenic maps with the full capsid (P1) sequence, we identified a specific amino acid substitution associated with an antigenic mismatch among field viruses and a commonly used prototype vaccine strain, A22/IRQ/24/64.
Introduction
Foot-and-mouth disease (FMD) is a highly transmissible vesicular disease of cloven-hoofed animals with a high morbidity rate in adult livestock. In addition to causing production losses, FMD can have a severe economic impact in endemic countries through, among other things, restriction of trade with FMD-free countries. Incursion from endemic countries into disease-free areas also carries high animal welfare and economic costs (Thompson et al., 2002).
Current measures to control the disease in FMD-free countries are driven largely by international trade regulations and include vaccination, movement controls and slaughter of infected and susceptible animals. In endemic countries, and where movement restrictions and slaughter are not always feasible, vaccination often plays a central role in FMD control. One of the difficulties in controlling FMD through vaccination arises due to the extent of broad intra- and inter-serotype antigenic diversity that exists for the seven serotypes of FMD virus (FMDV) (Kitching et al., 2005). Currently, inactivated vaccines do not protect against all strains within a particular serotype (Doel, 2003). This is particularly problematic for FMDV serotype A, which is one of the most antigenically diverse serotypes (Knowles et al., 2009). Therefore, accurate antigenic matching between field viruses and potential vaccine viruses is essential to ensure effective vaccination.
FMDV belongs to the genus Aphthovirus in the family Picornaviridae. There are seven serotypes within FMDV (serotypes O, A, SAT1, SAT2, SAT3, C and Asia 1) and each of these is split into topotypes and lineages. Virus strains are placed into different topotypes based on geographical location and on having approximately 85 % identity in the VP1 protein (for SAT viruses this is 20 %). Topotypes are further classified into lineages based on sequencing, vaccine matching and geographical regions. The P1 region of the genome encodes four capsid proteins: VP1, VP2, VP3 and VP4. The first three (VP1, VP2 and VP3) make up the virus outer surface and tolerate a significant proportion of amino acid changes without obvious functional impairment (Haydon et al., 2001). VP1 is the most accessible protein for the host immune system and contains the binding site (RGD motif), which attaches to the integrin receptor on epithelial cells and is located on a highly mobile G–H loop (Logan et al., 1993; Jackson et al., 2003, 2004). The G–H loop is the most variable site on the capsid and, along with other surface-exposed regions of VP1, VP2 and VP3, constitutes the five antigenic sites of FMDV serotype A strains that have been mapped using mAbs (Thomas et al., 1988; Baxt et al., 1989; Bolwell et al., 1989; Crowther et al., 1993).
Although sequencing the viral capsid genes is straightforward and rapid, predicting antigenic variation using sequence data alone has been challenging (Maree et al., 2011), with some limited success using regression models to predict neutralization values from sequence and structural data for serotype SAT1 (Reeve et al., 2010). Recent work focusing on using structure-based B-cell epitope prediction programs to determine antigenic residues has also shown some success, especially when multiple programs are used (Borley et al., 2013). The challenge is in part because certain amino acid substitutions may have a disproportionate antigenic effect. Therefore, serological tests are routinely used to estimate which vaccine strains may protect against field viruses using relationship coefficients (r 1 values) (Booth et al., 1978; Hamblin et al., 1986). These tests provide an estimation of the antigenic similarity between field and vaccine viruses, but they do not show relationships among FMDV strains and are dependent on the sera used (Mattion et al., 2009).
Antigenic cartography enables reliable quantitative and visual interpretation of pathogen binding assay data with the benefit of incorporating multiple serum–virus relationships when measuring antigenic distances among viruses. This method was first applied to human influenza A (H3N2) viruses (Smith et al., 2004a) and has since been applied to other viruses including swine influenza virus (de Jong et al., 2007), human influenza A (H1N1) virus (Lorusso et al., 2011), equine influenza virus (Lewis et al., 2011), lyssaviruses (Horton et al., 2010) and enterovirus A71 (Huang et al., 2009). Here, using antigenic cartography, we quantified the antigenic relationships among a panel of FMD serotype A viruses, representing the global diversity within the serotype. Antigenic relationships among field viruses were compared with differences in their full capsid P1 sequences. This identified a single amino acid substitution associated with an immunologically important antigenic change.
Results
Phylogenetic analysis
The maximum-likelihood (ML) phylogenetic relationships inferred from the capsid sequences highlighted the three topotypes within serotype A (Fig. 1) (Knowles & Samuel, 2003). Viruses grouped mostly according to geographical regions and by the year in which they were isolated. European/South American isolates appeared to share a common ancestor with the Asian topotype. A strain from Libya, A/LIB/14/2009, was an exception, as, although isolated from Africa, it clustered here with the Asian topotype as part of the A/ASIA/Iran-05 lineage.
Fig. 1.

Unrooted ML tree of 40 full P1 capsid nucleotide sequences using GTR+G+I in mega 5.10. Bootstrap replicates (1000) were performed, and only bootstrap values above 70 % are shown. The three different topotypes have been coloured: Asia (blue), Africa (purple) and Europe/South America (EURO-SA; green). *** indicates viruses that contained a proline at position 149. Bar, nucleotide substitutions per site.
Antigenic analysis
Antigenic relationships among the panel of 46 viruses are illustrated in a three-dimensional antigenic map (Fig. 2a). Colouring the viruses by year, topotype and geographical origin revealed no distinct clustering of viruses with time, distance or genetic relationship (Fig. 2). This was particularly true for the sublineage A/ASIA/Iran-05BAR-08, for which there was large intra-lineage antigenic variation; for example, there were 2.81 antigenic units (AU) between A/IRN/23/2009 and A/LIB/14/2009 (1 AU equals a twofold dilution). The antigenic map shown by year also highlights the antigenic diversity of strains seen even within the same year. Viruses A/IRN/1/2007 and A/IRN/36/2007 were both isolated in Iran during the same year and, with only 3.93 % nucleotide differences in the VP1 region, are considered to be the same strain. However, they were 2.21 AU apart.
Fig. 2.
Three-dimensional antigenic maps of the 46 FMDV serotype A viruses representing the global diversity of serotype A FMDV. Viruses (spheres) and antisera (open cubes) were positioned such that the distance from each serum to each virus is determined by the neutralization titre. Multidimensional scaling was used to position both sera and viruses relative to each other, so orientation of the map within the axes is free. Bar, 1 AU (equivalent to a twofold dilution in antibody titre). All views are shown at a different orientation to highlight the patterns seen (viewed using Pymol; DeLano Scientific LLC). (a) Viruses coloured according to different topotypes: Africa (red), Asia, (blue) and Europe/South America (green). (b) Viruses coloured to highlight the A/ASIA/Iran-05 lineage (green). (c) Viruses coloured by year: up to and including 2003 (blue), 2004 (turquoise), 2005 (light blue), 2006 (green), 2007 (dark green), 2008 (orange) and 2009 (red).
Comparison of antigenic and genetic data
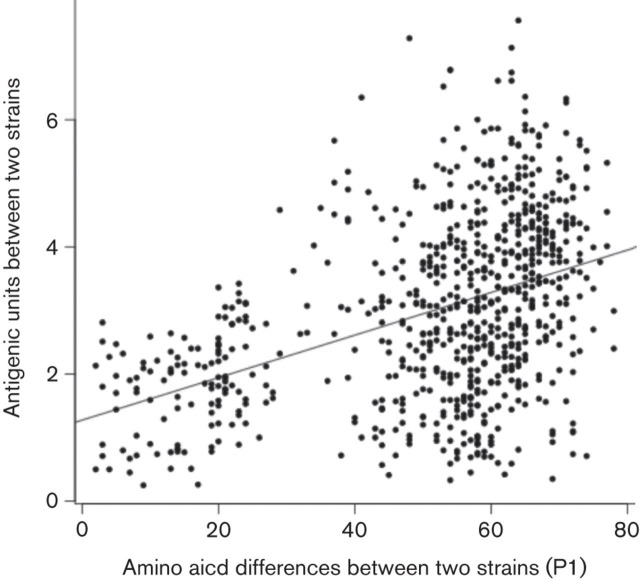
Using antigenic cartography, the antigenic distances among all viruses were calculated (Table S1, available in JGV Online), and these distances were used to compare antigenic and genetic data. Quantitative comparison of antigenic distance with the number of amino acid substitutions in P1 showed a significant but low correlation (r = 0.42, confidence interval (CI) 0.36–0.48, P<2.2×10−16) (Fig. 3). A linear regression model applied to the antigenic and genetic data predicted a mean value of 42 (5.7 %) amino acid changes having to occur for every change in AU.
Fig. 3.
Pair-wise antigenic and genetic distance (P1) between viruses. The antigenic distances were derived from the three-dimensional antigenic map of FMDV serotype A. Pearson’s correlation coefficient was 0.42 (95 % CI is 0.36–0.48; P<2.2×10−16). The Spearmans’s rank correlation (ρ) was equal to 43 %.
Antigenic effect depends on the position and nature of amino acid substitutions. Therefore, the sequence and antigenic data were investigated further to detect evidence for any amino acid substitutions with large antigenic effects. Amino acid sequences for all 46 viruses on the antigenic map were aligned and systematically analysed for specific substitutions common to all sequences in each antigenic group on the map. Specific attention was paid to previously identified antigenic sites and other surface-exposed residues. Using this approach, a single amino acid substitution, a proline at the third amino acid after the RGD domain of VP1, aa 149 (P149), defined a single cluster of antigenically related viruses (Fig. 4). The viruses in this cluster were from different geographical regions, spanning more than 30 years (Fig. 1). The closest vaccine virus to this cluster is prototype vaccine virus A22/IRQ/24/64, which also contains a proline at this position. Measurement of antigenic distance from this vaccine virus demonstrated that, for these 46 viruses, P149 was a strong predictor of antigenic match (and therefore presumed protection) to A22/IRQ/24/64, and there were only three viruses that did not match this pattern: A/SAU/15/2005, A/IRQ/24/2009 and A/SUD/3/77.
Fig. 4.
Three-dimensional antigenic map of FMDV serotype A isolates (see Fig. 2). Viruses are coloured by amino acid at position 149: proline (red), A/IRQ/24/64 (orange), others (blue), not sequenced due to confidentiality agreement (white). The cubes indicate the different sera.
To test this prediction on a larger dataset, 166 complete or partial VP1 sequences from a global panel of serotype A virus strains spanning 24 years (1987–2010) were analysed along with their corresponding r 1 value data against A22/IRQ/24/64 [data from the World Reference Laboratory/European Union Reference Laboratory (WRL/EURL) for FMD]. A proline at position 149 remained a strong predictor of vaccine match to A22/IRQ/24/64 (as determined by an r 1 value ≥0.3), with only 15 % of vaccine matches not being predicted based on the amino acid at position 149 of VP1 (Fig. 5). Of the 53 matched samples (r 1 value ≥0.3), only three did not have a proline at position 149 of VP1.
Fig. 5.
Boxplot of 166 r 1 values obtained from the WRL/EURL for FMD against A22/IRQ/24/64 vaccine virus. The amino acid at position 149 of VP1 and the resulting r 1 value are shown. An r 1 value ≥0.3 is suggestive of a vaccine match against A22/IRQ/24/64 (horizontal line).
Of the 15 % of serotype A viruses for which the vaccine matching could not be predicted based on the amino acid at position 149 of VP1, nearly half (10/22) were from one sublineage within the genetic lineage A/ASIA/Iran-05, i.e. BAR-08. A phenylalanine at aa 66 of VP1 and a glutamic acid at aa 64 of VP2 differentiated the three A/ASIA/Iran-05BAR-08 isolates from all other viruses. If the A/ASIA/Iran-05BAR-08 sublineage was not included in the analysis, the P149 substitution correctly predicted vaccine virus matching to A22/IRQ/24/64 for 92 % of the 166 viruses analysed.
Discussion
Here, we have for the first time both quantified and visualized the antigenic variability among FMDV serotype A strains, allowing the detection of an important amino acid substitution possibly responsible for the antigenic match to a widely used vaccine strain, A22/IRQ/24/64. An advantage of using antigenic cartography over r 1 values is the use of multiple neutralization values, rather than against one homologous serum as used for r 1 values to infer antigenic relationships. This allows antigenic relationships among all field viruses and multiple vaccine viruses to be assessed simultaneously. Antigenic maps made using a specifically generated body of virus neutralization test (VNT) data showed that there were discrepancies between antigenic, genetic and geographical relationships of viruses.
Antigenic cartography assumes that multiple epitopes or multiple antibodies against the same epitope can be represented as a single point. If the viruses were most parsimoniously represented by multiple points, corresponding to the different antigenic epitopes, then we would expect to see a large error associated with the position of the viruses and poor prediction of titres from the map.
Comparing the antigenic data with P1 capsid sequence data suggested that P149 is strongly associated with a match to vaccine A22/IRQ/24/64. Even in the one lineage (A/ASIA/Iran-05BAR-08) with low r 1 values against A22/IRQ/24/64 despite the amino acid at position 149, P149 was still associated with a discernible antigenic effect. This is probably due to multiple epitopes contributing to the overall antigenicity of the virus. In addition, this residue is in close vicinity to the VP1 aa 148, which has been reported to be critical in the A24 strain by mAb escape mutant studies (Mahapatra et al., 2011). It is possible that substitutions in this area, especially from a proline, a known helix breaker, may be responsible for the change in overall conformation of the protein leading to antigenic variation in these viruses. This lineage highlights the importance of continuous monitoring of the antigenic variation in circulating viruses and the need to understand the interactions and contributions among antigenic sites on the FMDV capsid.
What effect this proline has on the structure of FMDV serotype A strains is not known, as this region of VP1 is too flexible to be structurally modelled reliably (Fry et al., 2005). However, this region has been suggested previously to be part of antigenic site one for serotype A (Thomas et al., 1988; Baxt et al., 1989). For FMDV serotype O, where this region has been modelled using a reduced structure of the G–H loop and crystallography, there is an α-helix in this region (Logan et al., 1993). This work on serotype O would suggest that a proline at aa 149 of VP1 might interfere with a similar α-helix on serotype A viruses, resulting in this antigenically distinct group.
The antigenic map allows comparison between existing and new vaccine viruses. Using the antigenic map, we identified a cluster of viruses closely related to vaccine virus A/IRQ/24/64. A22/IRQ/24/64 has been one of the most widely used vaccine viruses in the Middle East, but recently a new lineage of FMDV (A/ASIA/Iran-05) has emerged (Knowles et al., 2009), and viruses isolated from this lineage (A/IRN/2005 and A/TUR/2006) have been incorporated into new vaccines. The A/IRN/2005 vaccine virus is 2.26 AU away from the vaccine virus A22/IRQ/24/64, and the two A/TUR/2006 vaccine viruses are 2.81 AU and 3.05 AU away from A22/IRQ/24/64. There are limited data and no sequences available for the A/IRN/2005 vaccine virus, due to confidentiality agreements. The A/TUR/2006 vaccine viruses group phylogenetically with the other A/ASIA/Iran-05 viruses.
Although a new vaccine virus A/ASIA/Iran-05 has been developed, vaccine virus A22/IRQ/24/64 appears still to protect against some new virus strains. Therefore, it would appear that there is co-circulation of multiple antigenic strains occurring in the Middle East. This implies that new vaccine strains that are developed in response to antigenic shift away from current vaccine viruses cannot fully replace the use of older vaccines. Also, currently there is incomplete information available from most endemic countries as to what vaccines are used, where and how often vaccination takes place and what species are targeted. Importantly, if there is co-circulation of multiple antigenic strains, a co-ordinated vaccination regime to eliminate disease locally will require scientific and strategic planning and implementation.
Predicting vaccine match using the number of amino acid substitutions alone is not accurate (Fig. 3). It appears that FMDV serotype A is able to sustain a large number of amino acid changes that do not affect the antigenicity of the virus. Also, the opposite appears to be true, where a single amino acid can cause a proportionally significant structural change resulting in the virus escaping the host’s humoral immunity.
The future use of FMDV antigenic cartography may include choosing a field strain as a potential new vaccine virus. However, although the position of a vaccine virus relative to field viruses will give an indirect guide to vaccine coverage, the position of a homologous serum against a vaccine is more appropriate to determining whether the vaccine virus is a good candidate, as we are interested in the antigenic response of cattle (see Table S1 for differences between virus-to-virus and virus-to-serum distances). This adds complexity, as the homologous serum is not always at the same location on the map as the vaccine virus. Finding a suitable vaccine may therefore require choosing more than one virus until the desired position of serum is achieved, as the position of the sera on the map may be distant from the group of field viruses for which the vaccine virus was intended. Individual animals will have a slightly different immunological response to a vaccine virus, but experience with influenza antigenic maps suggests that the sera are located in approximately the same area.
Antigenic cartography allows the antigenic evolution to be visualized and compared with the genetic evolution. This study has been shown to be useful not only in describing the antigenic variation but also in discriminating the antigenic and genetic relationships. This is particularly true for vaccine matching with A22/IRQ/24/64, which can be predicted from a single substitution at position 149 of VP1.
Methods
Virus propagation.
Forty-six FMDV serotype A isolates from the WRL/EURL for FMD (Pirbright, UK) were used in this study (Table 1). Once received from the WRL/EURL, the majority of viruses were propagated once or twice in a pig kidney cell line (IB-RS-2) with the maximum number of propagations being four passages. However, some viruses had been propagated prior to being obtained for use in this study. This was done in order to obtain a virus titre above 103 TCID50. Tissue-culture supernatant was harvested when more than 85 % cytopathic effect was noted, and the viruses were stored in glycerol (50 %, v/v) at −20 °C for the working virus stock or −80 °C for long-term use.
Table 1.
Serotype A FMDVs used in this study
| Topotype | Lineage | Virus name | GenBank accession no. | Sequence reference |
| Asia | A15 | A15/Bangkok/TAI/60 | KF112918 | This work |
| A22 | A22/IRQ/24/64 | AY593763 | Carrillo et al. (2005) | |
| Iran-05 | A/IRN/41/2003 | KF112906 | This work | |
| Iran-05 | A/IRN/2005 | not available | - | |
| Iran-05 | A/IRN/1/2005 | EF494486 | Klein et al. (2007) | |
| Iran-05 | A/IRN/31/2005 | KF112907 | This work | |
| Iran-05 | A/IRN/36/2007 | Pending | Upadhyaya et al. (2013) | |
| Iran-05 | A/IRN/7/2004 | Pending | Upadhyaya et al. (2013) | |
| Iran-05 | A/PAK/23/2009 | KF112915 | Upadhyaya et al. (2013) | |
| Iran-05 | A/SAU/15/2005 | Pending | Upadhyaya et al. (2013) | |
| Iran-05 | A/TUR/20/2006 | KF112923 | This work | |
| Iran-05 | A/TUR/24/2007 | Pending | Upadhyaya et al. (2013) | |
| Iran-05 | A/TUR/4/2006 | KF112922 | This work | |
| Iran-05AFG-07 | A/BAR/2/2009 | KF112900 | This work | |
| Iran-05AFG-07 | A/PAK/2/2009 | KF112915 | This work | |
| Iran-05ARD-07 | A/TUR/7/2008 | KF112924 | This work | |
| Iran-05BAR-08 | A/IRN/23/2009 | KF112908 | This work | |
| Iran-05BAR-08 | A/IRQ/24/2009 | KF112909 | This work | |
| Iran-05BAR-08 | A/LIB/14/2009 | KF112913 | This work | |
| Iran-96 | A/IRN/1/96 | KF152935 | This work | |
| Iran-99 | A/IRN/22/99 | Pending | Upadhyaya et al. (2013) | |
| Unnamed | A/IRN/10/2003 | KF112905 | This work | |
| Unnamed | A/IRN/32/2001 | Not available | – | |
| Sea-97 | A/LAO/7/2006 | Pending | Upadhyaya et al. (2013) | |
| Sea-97 | A/May/97 | Not available | – | |
| Sea-97 | A/TAI/1/2006 | KF112920 | This work | |
| Sea-97 | A/VIT/4/2004 | KF112927 | This work | |
| Thai-87 | A/TAI/118/87 | KF112919 | This work | |
| Africa | G-I | A/KEN/42/66 | KF112910 | This work |
| G-I | A/KEN/1/2003 | KF112911 | This work | |
| G-I | A/KEN/22/2009 | KF112912 | This work | |
| G-II | A/EGY/1/72 | KF112901 | This work | |
| G-II | A/ETH/9/2008 | KF112903 | This work | |
| G-III | A21/Lumbwa/KEN/3/64 | AY593761 | Carrillo et al. (2005) | |
| G-IV | A/SUD/3/77 | KF112916 | This work | |
| G-IV | A/ERI/3/98 | Not available | – | |
| G-IV | A/SUD/1/2006 | KF112917 | This work | |
| G-IV | A/TOG/9/2005 | KF112921 | This work | |
| G-V | A/NGR/2/73 | KF112914 | This work | |
| G-VII | A/EGY/1/2006 | KF112902 | This work | |
| G-VII | A/UGA/13/66 | KF112925 | This work | |
| G-VIII | A23/Kitale/KEN/64 | AY593766 | Carrillo et al. (2005) | |
| Europe/South America | A11 | A11/Germany/c.29 (AGB) | KF112904 | This work |
| A12 | A12/Kent/UK/199/32 | KF112926 | This work | |
| A24 | A24/Cruzeiro/BRA/55 | AY593768 | Carrillo et al. (2005) | |
| A-81 | A/Alem/ARG/81 | KF112899 | This work |
Sequencing and phylogenetic analysis.
For phylogenetic analyses, full capsid sequences were obtained for 40 of the 46 viruses used for antigenic interpretation. Eleven viruses had been sequenced previously (Carrillo et al., 2005; Klein et al., 2007), 29 viruses were sequenced as part of this study from the same stock viruses used for VNT and four viruses could not be sequenced due to confidentiality agreements with pharmaceutical companies (Table 1).
Total RNA from the virus working stocks was extracted using an RNeasy kit (Qiagen). Reverse transcription was performed using a SuperScript III reverse transcriptase kit (Invitrogen). PCR was then carried out using a KOD Hot-start Polymerase kit (Novagen) according to the manufacturer’s instructions with primer pairs L463F (5′-ACCTCCRACGGGTGGTACGC-3′) and NK72R (5′-GAAGGGCCCAGGGTTGGACTC-3′) or L463F and EUR2B52R (5′-GACATGTCCTCCTGCATCTGGTTGAT-3′), resulting in an amplified product of approximately 2500 bp. These PCR amplicons were purified using an Illustra GFX PCR DNA and Gel Band Purification kit (GE Healthcare) according to the manufacturer’s instructions. Internal sequencing primers were then used to sequence the entire P1 region on both strands (primer sequences available on request). Cycle sequencing was carried out using an ABI 3730 sequencer (Applied Biosystems). Sequences were checked manually, and consensus sequences were determined from at least one forward and one reverse sequence for all regions. Consensus nucleotide sequences of the viruses were aligned using the clustal w multiple sequence alignment program (Thompson et al., 1994). The predicted amino acid sequences were translated using BioEdit version 7.0.1 (Hall, 1999).
The optimum evolutionary molecular model for these data was determined to be the general time reversible model with gamma shape parameter and a proportion of invariant sites (GTR+G+I; jMODELTEST) (Guindon & Gascuel, 2003; Posada, 2008). mega version 5.10 (Tamura et al., 2011) was used to construct the ML tree with the topology optimized using subtree pruning-regrafting (SPR level 5). Bootstrap resampling (1000 cycles) was also carried out to assess the robustness of individual nodes.
Polyclonal sera.
Six bovine sera, collected 21 days post-vaccination, were supplied by the WRL/EURL for FMD for antigenic characterization. The vaccines (A/ERI/98, A/IRN/96, A/IRN/99, A/IRN/2005 and A/MAY/97) were inactivated and were oil adjuvant based (Merial). The prototype vaccine A22/IRQ/24/64 was aqueous based and was prepared by WRL/EURL FMD and the Onderstepoort Veterinary Institute (South Africa). No significant difference could be detected between the log of the r 1 value generated using antisera raised against the aqueous-based and oil adjuvant-based A22/IRQ/24/64 vaccine (paired t-test, P = 0.30, n = 18). Each serum was inactivated at 56 °C for 1 h prior to use in the VNT. All sera were stored at −20 °C.
VNT.
For antigenic analyses, the ability of each serum to neutralize each virus was assessed using a VNT. The test was undertaken either using a two-dimensional (Rweyemamu et al., 1976) or a one-dimensional VNT technique as recommended by the Office International des Epizooties (OIE, 2008). The two-dimensional VNT was modified for diagnostic vaccine matching by the WRL/EURL using five virus doses instead of the nine described in earlier publications. Briefly, the vaccine antiserum was diluted twofold vertically in a 96-well plate. Five different virus doses were then added horizontally across the plate, resulting in the sera being titrated against all five virus doses. The five virus doses were calculated from the TCID50 virus titre determined on a separate plate. For each of these virus doses, the 50 % end point serum neutralization titre was then calculated. The serum titre at a virus dose of 100 TCID50 was then predicted using linear regression. The one-dimensional VNT technique suggests using one pre-aliquotted virus dose of 100 TCID50 to neutralize anti-FMDV sera (OIE, 2008). Each test was repeated at least twice and all duplicates had to be within a twofold dilution of each other. Due to this modified method being faster, saving resources and giving similar results to the two-dimensional VNT, it was used for the majority of VNTs carried out in this study (WRL/EURL for FMD, unpublished data).
Calculation of serological relationship coefficient.
The relationship coefficients (r 1 value) between the field virus and A22/IRQ/24/64 were calculated for comparison with antigenic distances measured using antigenic cartography. These r 1 values were defined as the division of the heterologous (field virus) neutralization value by the homologous (vaccine virus) neutralization value. An r 1 value ≥0.3 was considered indicative of a vaccine virus antigenically matched with the field virus (i.e. the vaccine virus can be expected to protect an animal from disease caused by infection with the field virus tested) (Rweyemamu et al., 1976).
Antigenic cartography.
The antigenic variation among FMDV serotype A isolates was both quantified and visualized using a method described previously (Smith et al., 2004b). Briefly, neutralization titres between a virus and a serum were converted to a target distance by taking the difference between the log2 reciprocal neutralization titre against a virus and the maximum log2 reciprocal titre achieved by that serum against any other virus. Thus, a serum having a high neutralization titre against a virus has a lower target distance to that virus than a serum with a lower neutralization titre. Calculating target distances in this way allowed quantification and visualization of the titre differences among viruses in twofold dilutions (denoted as 1 AU), no matter what the magnitude of the actual titre. All viruses and sera were then positioned on the map by minimizing the difference between the target distances and map distances using multidimensional scaling (Smith et al., 2004b). The position of each virus and antiserum is therefore determined by the relationship and position of all other viruses and sera. To minimize local optima, multiple random restarts (100 repeats) of the conjugant gradient optimization method were used (Flannery et al., 1988).
Mathematically, the antigenic maps were not limited to the second and third dimension, and Euclidean (‘straight line’) distances can be taken between points in any dimension. Therefore, blind prediction and self-consistency experiments were undertaken to determine the optimal dimension for visualizing these data. Antigenic maps were made with a random 10 % of the titres omitted. These omitted values were then predicted using maps in dimensions two to five. The mean prediction error was similar for each dimension [0.88±0.16 AU (mean±sem) for the second dimension, 0.85±0.15 AU for the third dimension, 0.95±0.16 AU for the fourth dimension and 0.92±0.17 AU for the fifth dimension], suggesting no discernible mean advantage in precision using higher dimensions. The mean difference between titres predicted by the antigenic map and the titre from the VNT was therefore less than a twofold dilution. The maximum difference seen between the predicted titre and the titre from the VNT was 2.61 AU for the second dimension and 1.17 AU for the third dimension, and therefore the third dimension was chosen.
Testing vaccine matching prediction using sequence.
A larger dataset of r 1 values and VP1 sequences for 166 viruses tested against vaccine virus A22/IRQ/24/64 was used to test predictions for vaccine matching. Neutralization data were generated by the WRL/EURL for FMD using a two-dimensional VNT with pooled sera from five cattle bled 21 days post-vaccination with an oil adjuvant-based A22/IRQ/24/64 vaccine. Complete or partial VP1 sequences for all 166 were determined previously (Armstrong et al., 1994; Knowles et al., 2007; Schumann et al., 2008; Valarcher et al., 2008; Ayelet et al., 2009; Knowles et al., 2009; Abdul-Hamid et al., 2011; Habiela et al., 2010; Waheed et al., 2011; Knowles et al., 2012).
Supplementary Data
Acknowledgements
Thanks are due to Bob Statham and the WRL/EURL FMD team for their advice and expertise on the VNT and r 1 values. A. B. L. was supported by a fellowship from the Cambridge Infectious Diseases Consortium of the Veterinary Training and Research Initiative (Defra grant VT0105). J. L. N. W. is supported by the Alborada Trust and the Research and Policy for Infectious Disease Dynamics program of the Science and Technology Directorate, Department of Homeland Security and Fogarty International Center. D. L. H. is partially supported by the Cambridge Infectious Diseases Consortium Veterinary Training and Research Initiative (Defra grant VT0105) and EU FP7 Research Infrastructure Grant ‘European Virus Archive (EVA)’ (grant 228292). N. J. K. was partially supported by a Defra UK grant (SE2939). D. J. S. and C. A. R. acknowledge the support of EU FP7 grants EMPERIE (223498). C. A. R. acknowledges the support of the NIH Director’s Pioneer Award DP1-OD000490-0 and D. J. S., C. A. R. and J. L. N. W. are also supported by ANTIGONE (278976). C. A. R. was supported by a University Research Fellowship from the Royal Society.
Footnotes
The GenBank accession numbers for the FMDV capsid sequences determined in this study are: KF112918 (A15/Bangkok/TAI/60), KF1120906 (A/IRN/41/2003), KF112907 (A/IRN/31/2005), KF112923 (A/TUR/20/2006), KF112922 (A/TUR/4/2006), KF112900 (A/BAR/2/2009), KF112915 (A/PAK/2/2009), KF112924 (A/TUR/7/2008), KF112908 (A/IRN/23/2009), KF112909 (A/IRQ/24/2009), KF112913 (A/LIB/14/2009), KF152935 (A/IRN/1/96), KF112905 (A/IRN/10/2003), KF112920 (A/TAI/1/2006), KF112927 (A/VIT/4/2004), KF112919 (A/TAI/118/87), KF112910 (A/KEN/42/66), KF112911 (A/KEN/1/2003), KF112912 A/KEN/22/2009), KF112901 (A/EGY/1/72), KF112903 A/ETH/9/2008), KF112916 (A/SUD/3/77), A/SUD/1/2006 (KF112917), A/TOG/9/2005 (KF112921), A/NGR/2/73 (KF112914), KF112902 (A/EGY/1/2006), KF112925 (A/UGA/13/66), KF112904 (A11/Germany/c.29), KF112926 (A12/Kent/UK/199/32) and KF112899 (A/Alem/ARG/81).
Two supplementary tables are available with the online version of this paper.
References
- Abdul-Hamid N. F., Hussein N. M., Wadsworth J., Radford A. D., Knowles N. J., King D. P. Phylogeography of foot-and-mouth disease virus types O and A in Malaysia and surrounding countries. Infect Genet Evol. 2011;11:320–328. doi: 10.1016/j.meegid.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Armstrong R. M., Samuel A. R., Carpenter W. C., Kant R., Knowles N. J. A comparative study of serological and biochemical methods for strain differentiation of foot-and-mouth disease type A viruses. Vet Microbiol. 1994;39:285–298. doi: 10.1016/0378-1135(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Ayelet G., Mahapatra M., Gelaye E., Egziabher B. G., Rufeal T., Sahle M., Ferris N. P., Wadsworth J., Hutchings G. H., Knowles N. J. Genetic characterization of foot-and-mouth disease viruses, Ethiopia, 1981–2007. Emerg Infect Dis. 2009;15:1409–1417. doi: 10.3201/eid1509.090091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxt B., Vakharia V., Moore D. M., Franke A. J., Morgan D. O. Analysis of neutralizing antigenic sites on the surface of type A12 foot-and-mouth disease virus. J Virol. 1989;63:2143–2151. doi: 10.1128/jvi.63.5.2143-2151.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell C., Clarke B. E., Parry N. R., Ouldridge E. J., Brown F., Rowlands D. J. Epitope mapping of foot-and-mouth disease virus with neutralizing monoclonal antibodies. J Gen Virol. 1989;70:59–68. doi: 10.1099/0022-1317-70-1-59. [DOI] [PubMed] [Google Scholar]
- Booth J. C., Rweyemamu M. M., Pay T. W. Dose–response relationships in a microneutralization test for foot-and-mouth disease viruses. J Hyg (Lond) 1978;80:31–42. doi: 10.1017/S0022172400053377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borley D. W., Mahapatra M., Paton D. J., Esnouf R. M., Stuart D. I., Fry E. E. Evaluation and use of in-silico structure-based epitope prediction with foot-and-mouth disease virus. PLoS ONE. 2013;8:e61122. doi: 10.1371/journal.pone.0061122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo C., Tulman E. R., Delhon G., Lu Z., Carreno A., Vagnozzi A., Kutish G. F., Rock D. L. Comparative genomics of foot-and-mouth disease virus. J Virol. 2005;79:6487–6504. doi: 10.1128/JVI.79.10.6487-6504.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther J. R., Farias S., Carpenter W. C., Samuel A. R. Identification of a fifth neutralizable site on type O foot-and-mouth disease virus following characterization of single and quintuple monoclonal antibody escape mutants. J Gen Virol. 1993;74:1547–1553. doi: 10.1099/0022-1317-74-8-1547. [DOI] [PubMed] [Google Scholar]
- de Jong J. C., Smith D. J., Lapedes A. S., Donatelli I., Campitelli L., Barigazzi G., Van Reeth K., Jones T. C., Rimmelzwaan G. F., other authors Antigenic and genetic evolution of swine influenza A (H3N2) viruses in Europe. J Virol. 2007;81:4315–4322. doi: 10.1128/JVI.02458-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doel T. R. FMD vaccines. Virus Res. 2003;91:81–99. doi: 10.1016/S0168-1702(02)00261-7. [DOI] [PubMed] [Google Scholar]
- Flannery B., Teukolsky S., Vetterling W. Numerical Recipes in C. Cambridge: Cambridge University Press; 1988. [Google Scholar]
- Fry E. E., Newman J. W., Curry S., Najjam S., Jackson T., Blakemore W., Lea S. M., Miller L., Burman A., other authors Structure of Foot-and-mouth disease virus serotype A1061 alone and complexed with oligosaccharide receptor: receptor conservation in the face of antigenic variation. J Gen Virol. 2005;86:1909–1920. doi: 10.1099/vir.0.80730-0. [DOI] [PubMed] [Google Scholar]
- Guindon S., Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Habiela M., Ferris N. P., Hutchings G. H., Wadsworth J., Reid S. M., Madi M., Ebert K., Sumption K. J., Knowles N. J., other authors Molecular characterization of foot-and-mouth disease viruses collected from Sudan. Transbound Emerg Dis. 2010;57:305–314. doi: 10.1111/j.1865-1682.2010.01151.x. [DOI] [PubMed] [Google Scholar]
- Hall T. BioEdit: a user friendly biolgoical sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Hamblin C., Barnett I. T., Crowther J. R. A new enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against foot-and-mouth disease virus. II. Application. J Immunol Methods. 1986;93:123–129. doi: 10.1016/0022-1759(86)90442-4. [DOI] [PubMed] [Google Scholar]
- Haydon D. T., Samuel A. R., Knowles N. J. The generation and persistence of genetic variation in foot-and-mouth disease virus. Prev Vet Med. 2001;51:111–124. doi: 10.1016/S0167-5877(01)00210-0. [DOI] [PubMed] [Google Scholar]
- Horton D. L., McElhinney L. M., Marston D. A., Wood J. L., Russell C. A., Lewis N., Kuzmin I. V., Fouchier R. A., Osterhaus A. D., other authors Quantifying antigenic relationships among the lyssaviruses. J Virol. 2010;84:11841–11848. doi: 10.1128/JVI.01153-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. W., Hsu Y. W., Smith D. J., Kiang D., Tsai H. P., Lin K. H., Wang S. M., Liu C. C., Su I. J., Wang J. R. Reemergence of enterovirus 71 in 2008 in Taiwan: dynamics of genetic and antigenic evolution from 1998 to 2008. J Clin Microbiol. 2009;47:3653–3662. doi: 10.1128/JCM.00630-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T., King A. M., Stuart D. I., Fry E. Structure and receptor binding. Virus Res. 2003;91:33–46. doi: 10.1016/S0168-1702(02)00258-7. [DOI] [PubMed] [Google Scholar]
- Jackson T., Clark S., Berryman S., Burman A., Cambier S., Mu D., Nishimura S., King A. M. Integrin αvβ8 functions as a receptor for foot-and-mouth disease virus: role of the β-chain cytodomain in integrin-mediated infection. J Virol. 2004;78:4533–4540. doi: 10.1128/JVI.78.9.4533-4540.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitching R. P., Hutber A. M., Thrusfield M. V. A review of foot-and-mouth disease with special consideration for the clinical and epidemiological factors relevant to predictive modelling of the disease. Vet J. 2005;169:197–209. doi: 10.1016/j.tvjl.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Klein J., Hussain M., Ahmad M., Normann P., Afzal M., Alexandersen S. Genetic characterisation of the recent foot-and-mouth disease virus subtype A/IRN/2005. Virol J. 2007;4:122. doi: 10.1186/1743-422X-4-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles N. J., Samuel A. R. Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 2003;91:65–80. doi: 10.1016/S0168-1702(02)00260-5. [DOI] [PubMed] [Google Scholar]
- Knowles N. J., Wadsworth J., Reid S. M., Swabey K. G., El-Kholy A. A., El-Rahman A. O. A., Soliman H. M., Ebert K., Ferris N. P., other authors Foot-and-mouth disease virus serotype A in Egypt. Emerg Infect Dis. 2007;13:1593–1596. doi: 10.3201/eid1310.070252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles N. J., Nazem Shirazi M. H., Wadsworth J., Swabey K. G., Stirling J. M., Statham R. J., Li Y., Hutchings G. H., Ferris N. P., other authors Recent spread of a new strain (A-Iran-05) of foot-and-mouth disease virus type A in the Middle East. Transbound Emerg Dis. 2009;56:157–169. doi: 10.1111/j.1865-1682.2009.01074.x. [DOI] [PubMed] [Google Scholar]
- Knowles N. J., He J., Shang Y., Wadsworth J., Valdazo-González B., Onosato H., Fukai K., Morioka K., Yoshida K., other authors Southeast Asian foot-and-mouth disease viruses in Eastern Asia. Emerg Infect Dis. 2012;18:499–501. doi: 10.3201/eid1803.110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis N., Daly J., Russell C., Horton D., Skepner E., Bryant N., Burke D., Rash A., Wood J., other authors The antigenic and genetic evolutionof equine influenza A (H3N8) virus from 1968 to 2007. J Virol Methods. 2011;85:12742–12749. doi: 10.1128/JVI.05319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan D., Abu-Ghazaleh R., Blakemore W., Curry S., Jackson T., King A., Lea S., Lewis R., Newman J., other authors Structure of a major immunogenic site on foot-and-mouth disease virus. Nature. 1993;362:566–568. doi: 10.1038/362566a0. [DOI] [PubMed] [Google Scholar]
- Lorusso A., Vincent A. L., Harland M. L., Alt D., Bayles D. O., Swenson S. L., Gramer M. R., Russell C. A., Smith D. J., other authors Genetic and antigenic characterization of H1 influenza viruses from United States swine from 2008. J Gen Virol. 2011;92:919–930. doi: 10.1099/vir.0.027557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra M., Seki C., Upadhyaya S., Barnett P. V., La Torre J., Paton D. J. Characterisation and epitope mapping of neutralising monoclonal antibodies to A24 Cruzeiro strain of FMDV. Vet Microbiol. 2011;149:242–247. doi: 10.1016/j.vetmic.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Maree F. F., Blignaut B., Esterhuysen J. J., de Beer T. A., Theron J., O’Neill H. G., Rieder E. Predicting antigenic sites on the foot-and-mouth disease virus capsid of the South African Territories types using virus neutralization data. J Gen Virol. 2011;92:2297–2309. doi: 10.1099/vir.0.032839-0. [DOI] [PubMed] [Google Scholar]
- Mattion N., Goris N., Willems T., Robiolo B., Maradei E., Beascoechea C. P., Perez A., Smitsaart E., Fondevila N., other authors Some guidelines for determining foot-and-mouth disease vaccine strain matching by serology. Vaccine. 2009;27:741–747. doi: 10.1016/j.vaccine.2008.11.026. [DOI] [PubMed] [Google Scholar]
- OIE . Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 6th edn. Paris: Office International Des Epizooties; 2008. Foot and mouth disease; pp. 190–217. [Google Scholar]
- Posada D. jModelTest: phylogenetic model averaging. Mol Biol Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Reeve R., Blignaut B., Esterhuysen J. J., Opperman P., Matthews L., Fry E. E., de Beer T. A., Theron J., Rieder E., other authors Sequence-based prediction for vaccine strain selection and identification of antigenic variability in foot-and-mouth disease virus. PLOS Comput Biol. 2010;6:e1001027. doi: 10.1371/journal.pcbi.1001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rweyemamu M. M., Pay T. W., Parker M. J. Serological differentiation of foot-and-mouth disease virus strains in relation to selection of suitable vaccine viruses. Dev Biol Stand. 1976;35:205–4. [PubMed] [Google Scholar]
- Schumann K. R., Knowles N. J., Davies P. R., Midgley R. J., Valarcher J. F., Raoufi A. Q., McKenna T. S., Hurtle W., Burans J. P., other authors Genetic characterization and molecular epidemiology of foot-and-mouth disease viruses isolated from Afghanistan in 2003–2005. Virus Genes. 2008;36:401–413. doi: 10.1007/s11262-008-0206-4. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Lapedes A. S., de Jong J. C., Bestebroer T. M., Rimmelzwaan G. F., Osterhaus A. D., Fouchier R. A. Mapping the antigenic and genetic evolution of influenza virus. Science. 2004a;305:371–376. doi: 10.1126/science.1097211. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Lapedes A. S., de Jong J. C., Bestebroer T. M., Rimmelzwaan G. F., Osterhaus A. D., Fouchier R. A. Mapping the antigenic and genetic evolution of influenza virus. Science. 2004b;305:371–376. doi: 10.1126/science.1097211. [DOI] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. mega5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. A., Woortmeijer R. J., Puijk W., Barteling S. J. Antigenic sites on foot-and-mouth disease virus type A10. J Virol. 1988;62:2782–2789. doi: 10.1128/jvi.62.8.2782-2789.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D., Muriel P., Russell D., Osborne P., Bromley A., Rowland M., Creigh-Tyte S., Brown C. Economic costs of the foot and mouth disease outbreak in the United Kingdom in 2001. Rev Sci Tech. 2002;21:675–687. doi: 10.20506/rst.21.3.1353. [DOI] [PubMed] [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J. clustal w: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya S., Ayelet G., Paul G., King D. P., Paton D. J., Mahapatra M. Genetic basis of antigenic variation in foot-and-mouth disease serotype A viruses from the Middle East. Vaccine. 2013;2013 doi: 10.1016/j.vaccine.2013.08.102. pii: S0264-410X(13)01214-0. doi: 10.1016/j.vaccine.2013.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valarcher J. F., Leforban Y., Rweyemamu M., Roeder P. L., Gerbier G., Mackay D. K., Sumption K. J., Paton D. J., Knowles N. J. Incursions of foot-and-mouth disease virus into Europe between 1985 and 2006. Transbound Emerg Dis. 2008;55:14–34. doi: 10.1111/j.1865-1682.2007.01010.x. [DOI] [PubMed] [Google Scholar]
- Waheed U., Parida S., Khan Q., Hussain M., Ebert K., Wadsworth J., Reid S., Hutchings G., Mahapatra M., other authors Molecular characterisation of foot-and-mouth diseae virus from Pakistan 2005–2008. Transbound Emerg Dis. 2011;58:166–172. doi: 10.1111/j.1865-1682.2010.01186.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.