Abstract
Tobacco cigarette smoking is associated with increased sudden death risk, perhaps through adverse effects on ventricular repolarization. The effect of electronic (e-)cigarettes on ventricular repolarization is unknown. The objective of the study was to test the hypothesis that tobacco cigarettes and e-cigarettes have similar adverse effects on electrocardiogram (ECG) indexes of ventricular repolarization and these effects are attributable to nicotine. ECG recordings were obtained in 37 chronic tobacco cigarette smokers, 43 chronic e-cigarette users, and 65 nonusers. Primary outcomes, Tpeak to Tend (Tp-e), Tp-e/QT ratio, and Tp-e/QTc ratio, were measured in tobacco cigarette smokers pre-/post-straw control and smoking one tobacco cigarette and in e-cigarette users and nonusers pre-/post-straw control and using an e-cigarette with and without nicotine (different days). Mean values of the primary outcomes were not different among the three groups at baseline. In chronic tobacco cigarette smokers, all primary outcomes, including the Tp-e (12.9 ± 5.0% vs. 1.5 ± 5%, P = 0.017), Tp-e/QT (14.9 ± 5.0% vs. 0.7 ± 5.1%, P = 0.004), and Tp-e/QTc (11.9 ± 5.0% vs. 2.1 ± 5.1%, P = 0.036), were significantly increased pre-/post-smoking one tobacco cigarette compared with pre-/post-straw control. In chronic e-cigarette users, the Tp-e/QT (6.3 ± 1.9%, P = 0.046) was increased only pre/post using an e-cigarette with nicotine but not pre/post the other exposures. The changes relative to the changes after straw control were greater after smoking the tobacco cigarette compared with using the e-cigarette with nicotine for Tp-e (11.4 ± 4.4% vs. 1.1 ± 2.5%, P < 0.05) and Tp-e/QTc (9.8 ± 4.4% vs. −1.6 ± 2.6%, P = 0.05) but not Tp-e/QT(14.2 ± 4.5% vs. 4.2 ± 2.6%, P = 0.061) . Heart rate increased similarly after the tobacco cigarette and e-cigarette with nicotine. Baseline ECG indexes of ventricular repolarization were not different among chronic tobacco cigarette smokers, electronic cigarette users and nonusers. An adverse effect of acute tobacco cigarette smoking on ECG indexes of ventricular repolarization was confirmed. In chronic e-cigarette users, an adverse effect of using an e-cigarette with nicotine, but not without nicotine, on ECG indexes of ventricular repolarization was also observed.
NEW & NOTEWORTHY Abnormal ventricular repolarization, as indicated by prolonged Tpeak-end (Tp-e), is associated with increased sudden death risk. Baseline ECG indexes of repolarization, Tp-e, Tp-e/QT, and Tp-e/QTc, were not different among tobacco cigarette (TC) smokers, electronic cigarette (EC) users, and nonsmokers at baseline, but when TC smokers smoked one TC, all parameters were prolonged. Using an electronic cigarette with nicotine, but not without nicotine, increased the Tp-e/QT. Smoking induces changes in ECG indexes of ventricular repolarization associated with increased sudden death risk.
Listen to this article’s corresponding podcast at: https://ajpheart.podbean.com/e/smoking-effects-on-ventricular-repolarization/.
Keywords: electronic cigarettes, nicotine, smoking, sudden death, tobacco cigarettes, ventricular repolarization
INTRODUCTION
Tobacco cigarette smoking is the most important modifiable risk factor for cardiovascular disease and the leading preventable cause of sudden death in the United States. Surprisingly, the risk of sudden death from tobacco cigarette smoking is largely independent of smoking burden and reverts to that of a nonsmoker soon after smoking cessation (15, 30). These observations have led to the hypothesis that smoking-related sudden death is a direct toxic effect of constituent(s) in tobacco cigarette smoke, rather than a consequence of progressive coronary artery disease. Although controversial, electronic (e-)cigarettes are widely perceived as a safer alternative to lethal tobacco cigarettes, and switching from tobacco cigarettes to e-cigarettes has been advocated as a harm reduction strategy (18, 25). Toxic constituents in e-cigarette emissions, generated from heating a mixture of solvents, flavorings, and nicotine, are orders of magnitude lower than in tobacco cigarette smoke, that is, except for nicotine (16, 17, 24). Similar plasma nicotine levels are achievable in e-cigarette users and tobacco cigarette smokers (32, 37).
The mechanisms by which tobacco cigarettes trigger sudden death are unknown, but some studies in tobacco cigarette smokers have reported abnormal ventricular repolarization, which increases vulnerability to ventricular arrhythmias, especially in the setting of ischemia (2, 3, 5, 10, 12, 20, 21, 34). The increase in sympathetic tone and heart rate attributable to nicotine in cigarette smoke could unmask abnormal ventricular repolarization in predisposed individuals (41). Furthermore, smoking may precipitate coronary vasospasm and ischemia, which could also result in prolongation of ventricular repolarization (2). Even in the absence of sympathetic excitation, nicotine may directly prolong ventricular repolarization. In studies of isolated ventricular myocytes, nicotine has been shown to directly inhibit potassium channels (8, 38, 39). Potassium channel inhibition has been shown to prolong ventricular repolarization, thereby potentially increasing risk of ventricular arrhythmias and sudden death (8, 38, 39). All of these potential mechanisms attributable to tobacco cigarettes could also apply to e-cigarettes, since e-cigarettes contain nicotine and e-cigarette use increases sympathetic tone and heart rate (26).
Traditionally, the QT interval, easily identified and measured on the ECG, has been used to define ventricular repolarization. Even a minor prolongation of the QT interval, which remains within the normal range, has been associated with increased risk for ventricular arrhythmias and sudden death in specific clinical settings, such as following acute myocardial infarction (2, 11). However, the QT interval includes both ventricular depolarization and repolarization and thus may lack the sensitivity and precision to detect subtle but important alterations in ventricular repolarization (6, 11, 13, 28), prompting a search for alternate ECG indexes of ventricular repolarization.
The peak of the T wave to the end of the T wave (Tp-e interval) represents only ventricular repolarization (6, 7, 19), and prolongation of the Tp-e interval has been shown to be a better predictor of ventricular arrhythmias and sudden death than the QT interval or the QT interval corrected for heart rate (QTc, Bazett’s) in several clinical settings (4, 6, 9, 11, 19, 22, 23, 28, 33, 35, 43). In fact, the Tp-e interval is prolonged in many cohorts with increased risk of sudden death, including patients with congenital long QT syndrome, Brugada’s syndrome, obstructive sleep apnea, coronary artery disease, hypertrophic cardiomyopathy, inducible ventricular arrhythmias, survivors of sudden cardiac death, and, importantly, in otherwise healthy people who smoke tobacco cigarettes (4, 9, 19, 21–23, 28, 33–35, 40).
The purpose of this study was to test the hypothesis that ECG indexes of ventricular repolarization are prolonged in chronic tobacco cigarette smokers or e-cigarette users compared with age-matched nonusers. Furthermore, we hypothesized that acutely smoking a tobacco or electronic cigarette would acutely and similarly prolong ECG indexes of ventricular repolarization, likely attributable to increases in plasma nicotine, thus challenging the concept that switching from tobacco cigarettes to e-cigarettes would lead to harm reduction.
METHODS
As detailed below, ECG recordings were obtained during the period of 2015–2018 in a large cohort of subjects participating in our clinical investigations (NCT03072628, NCT02740595, and NCT02724241) of tobacco product use; indexes of ventricular repolarization were analyzed in these ECG recordings for the present study.
Human Subjects
In these studies, ECG recordings were obtained from healthy male and female subjects, ages 21–45 yr, who were 1) chronic (≥12 mo) tobacco cigarette smokers, 2) chronic (≥12 mo) e-cigarette users, or 3) nonusers. All participants were required to meet the following criteria: 1) sinus rhythm; 2) not competitive or trained athletes; 3) not pregnant; 4) not taking prescription medications regularly (oral contraceptives were allowed); 5) alcohol intake less or equal to two drinks per day, and no illicit drug use, including marijuana, determined through screening questionnaire, and confirmed at each visit with a urine toxicology test; 6) no self-reported chronic illness, including asthma, hypertension, heart disease, diabetes, or hyperlipidemia; 7) nonobese [body mass index (BMI) <30 kg/m2]; and 8) no regular exposure to secondhand smoke in nontobacco cigarette smokers. End-tidal CO was measured in e-cigarette users to detect those who were surreptitiously using tobacco cigarettes. On the day of the written informed consent, before the day of the first experimental session, all subjects were familiarized and acclimated to the experimental set-up. The experimental protocol was approved by the Institutional Review Board at the University of California, Los Angeles, and written informed consent was obtained from each participant.
Parallel Group Study
In a Parallel Group comparison, three groups were compared: 1) chronic tobacco cigarette smokers, 2) chronic e-cigarette users, and 3) nonusers. The resting ECG intervals reflecting ventricular repolarization (see outcomes in Statistical Analysis) were compared among the three groups.
Crossover Study
Acute tobacco cigarette smoking.
Chronic tobacco cigarette smokers participated in up to two acute smoking sessions in random order separated by 4 wk: 1) smoking one tobacco cigarette (own brand), and 2) puffing on an empty straw (control).
Acute e-cigarette use.
Chronic EC users and nonusers participated in up to four 30-min acute exposure sessions in random order separated by 4 wk: 1) empty e-cigarette (control), 2) e-cigarette with nicotine, 3) e-cigarette without nicotine, and 4) nicotine inhaler (a “clean” source of nicotine, without flavorings or solvents; estimated 4 mg nicotine delivered per cartridge (29).
Smoking Topography
Tobacco cigarette.
Subjects puffed on an empty straw or smoked one tobacco cigarette in 7 min, a typical time interval to smoke one tobacco cigarette.
Electronic cigarette and nicotine inhaler.
Standardized puff topography consisting of 4-s puffs with a ~30-s interpuff interval for 30 min (60 puffs) was used, since, we have reported that this topography was tolerable and sufficient to increase plasma nicotine levels, even in inexperienced nonuser controls (26). According to the package insert and company literature (29), utilizing this same topography the nicotine inhaler was expected to achieve very similar plasma nicotine levels seen with our second-generation e-cigarette device (26).
E-Cigarette Device
In our earliest studies (2015), subjects used Greensmoke cigalike e-cigarette device (the highest rated e‐cigarette brand in the United States sold online at the time of the study design) with tobacco-flavored liquid and the solvents vegetable glycerin/propylene glycol (VG/PG) with 1) 1.2% nicotine, 2) 0% nicotine (same solvents and flavoring), and 3) empty (no liquid; sham control). In 2016 we switched to a more efficient nicotine delivery system, the second‐generation pen‐like device (1.0 Ω, eGo‐One by Joyetech, Irvine, CA), strawberry-flavored VG/PG liquid with 1) 1.2% nicotine, 2) 0% nicotine (same solvents and flavoring), or 3) empty (no liquid; straw control).
Nicotine and Cotinine Plasma Levels
Before and after tobacco cigarette, e-cigarette, nicotine inhaler, or control exposures, blood was drawn according to laboratory specifications and sent to the University of California, Los Angeles Clinical Laboratories for nicotine (half-life 1–2 h) and cotinine (half-life 16–20 h) levels.
Technique for ECG Recording
Five ECG electrodes were placed on the chest in standard five-lead telemetry configuration, right and left upper and lower chest, and one just to the right of the lower right sternal border. Recording electrodes were standard silver-silver chloride conductors (RedDot, 3M Health Care, St. Paul, MN). Five-minute ECG recordings from two leads (typically II and V1), using a high-resolution digital recording system (1,000-Hz sampling frequency, LabPro7, AdInstruments), were obtained, free from significant artifact or noise.
Analysis of ECG Recordings
The ECG Analysis Module software measures the QT interval from the earliest onset of the QRS to the end of the T wave, and the Tp-e is measured from the peak of the T wave to the end of the T wave. The peak of the T wave is defined as the highest point. If the T wave is negative, the nadir, rather than the peak, is used (6, 14). The end of the T wave is defined as the intersection of the tangent to the down slope of the T wave and the isoelectric line when not followed by a U wave, or if distinct from the U wave. U waves are not included in the Tp-e interval. Leads in which the T wave amplitude was <1.5 mm were excluded from analysis (28). The Tp-e was measured in each lead, and the lead with the maximum Tp-e at baseline was used for statistical analysis (28, 31). The same lead was used following intervention. Tp-e and QT intervals were measured, and Tp-e/QT ratio, Tp-e/QTc ratio, and QTc interval (Bazett’s) were calculated.
Outliers.
For a given subject and time, we examined normal quantile plots for primary and secondary outcomes to determine if there were outliers. Outliers were detected by the normal quantile plots only for outcomes involving Tp-e. Therefore, to avoid bias, outcomes involving Tp-e were summarized for a given individual exposure and period with a trimmed average, since the data followed a normal distribution. Trimmed values were computed to remove the effect of outliers due to protocol violations (patient moving or coughing) or ectopic beats, during the 5-min continuous ECG recordings. The trimmed average is the mean value after the lowest 2.5% and highest 2.5% of the values are removed. If an outlier occurs, it will be in the lower or upper 2.5% of the distribution.
Reliability.
There is no consensus regarding the “best” or preferred lead for Tp-e analysis, and no systematic comparison among the leads has been reported (4, 22, 34). Methodology has varied in the approach to the measurement of the Tp-e and QT intervals. Early reports describe two observers using magnifying glasses and calipers to make manual measurements (4, 9, 34). Alternatively, digitized data, either from a 12-lead ECG or a 24-h 3-channel ECG recording, has been analyzed with the aid of commercially available software (23, 28). In our study, ECG recordings were analyzed using commercially available software, ECG Analysis Module (LabPro7, AdInstruments) by two experienced investigators (M. Ip. and E. Diamantakos) who were blinded to study group and exposure type. On average, 300 beats were analyzed per lead. The within subject coefficient of variation (CV) for each investigator and the interclass correlation coefficient (ICC) between investigators for each outcome over time and overall was computed; within subject CV was <0.1% and the corresponding ICC was >99.9%.
Experimental Protocol
After abstaining from caffeine, exercise, and tobacco product use for at least 12 h, participants were placed in a supine position in the Human Physiology Laboratory located in the University of California, Los Angeles Clinical Translational Research Center. No cell phones or digital stimuli were permitted during the study, and no talking was permitted during the ECG recording. After blood draw and instrumentation, and 10 min of quiet rest, up to 10 min of continuous ECG recordings were obtained. Participants then underwent the assigned exposure, and then ECG recordings were repeated, and blood samples were obtained.
Statistical Analysis (Using Statistical Software SAS 9.4 and R 3.5.2)
Outcomes for ECG analysis.
primary outcomes.
Primary outcomes were the trimmed average of the Tp-e interval, Tp-e/QT ratio, or Tp-e/QTc ratio over the 5-min recording period for each subject. These variables are indicative of spatial dispersion of refractoriness and were chosen since they have been most consistently reported to be predictive of arrhythmia risk in several cohorts (9, 28, 43).
secondary outcomes.
Secondary outcomes were QT and QTc intervals, common indexes of ventricular repolarization, but perhaps less sensitive than the primary outcomes above (6, 11, 19, 23, 28, 33, 43). Additionally, the trimmed standard deviation of the Tp-e, QT, and QTc intervals over time, indicative of temporal dispersion of refractoriness, were compared among the groups. The Tp-e, Tp-e/QT ratio and Tp-e/QTc ratio outcomes were first summarized across the recording period for each subject by their trimmed average since they have stable normal distributions within subject and period after a small number of outliers are removed. The parallel group mean comparisons among three groups were carried out using ANOVA models or analysis of covariance models with gender as the covariate. For the crossover study, mean postexposure minus mean preexposure (baseline) change in each outcome was compared using a repeated measure (mixed) model. When the change in the outcomes was compared between groups (chronic tobacco cigarette smokers vs. e-cigarette users), the change in each outcome was relative to the change with straw control. The mean change outcomes in the crossover design were compared using a robust repeated measure (mixed) model with fixed treatment, visit (period), and order (treatment × visit interaction) effects as well as random subject effects to adjust for possible effects of visit and allow for the nonindependence among observations on the same subject (R function rlmer in library robustlmm). A robust version of the mixed model was used to reduce the effect of possible outliers and corresponds to using trimmed means. Additionally, the association of trimmed average Tp-e and trimmed average Tp-e change from baseline versus postexposure plasma nicotine and cotinine levels were assessed by computing Spearman correlation.
Sample size/power calculations.
Based on Tp-e means and SDs from our data and conservatively assuming a low correlation across sessions, a sample size of n = 20 had 80% power for confirming mean changes of 15% from baseline, and a sample size of n = 30 had 80% power for confirming mean changes of 13% from baseline. Other ECG indexes of ventricular repolarization required even fewer subjects. The sample size is computed using the usual two-sided α = 0.05.
RESULTS
Human Subjects
A total of 145 participants, including 37 chronic tobacco cigarette smokers, 43 chronic e-cigarette users, and 65 nonusers were included in this analysis. All chronic e-cigarette users reported using e-cigarettes with nicotine. Baseline characteristics are displayed in Table 1. Plasma cotinine levels were not significantly different in the tobacco cigarette group compared with chronic e-cigarette group, consistent with a similar daily smoking burden.
Table 1.
Baseline characteristics
| TC Smokers | EC Users | Nonusers | P Value (Overall) | |
|---|---|---|---|---|
| n | 37 | 43 | 65 | |
| Age, yr | 26.7 ± 0.9 | 28.0 ± 0.9 | 0.27 | |
| Sex (men/women) | 26/11 | 27/16 | 29/36 | 0.03 |
| BMI, kg/m2 | 24.3 ± 0.4 | 24.6 ± 0.6 | 23.2 ± 0.4 | 0.09 |
| Race | 0.71 | |||
| Asian, n | 9 | 11 | 13 | |
| Black, n | 3 | 2 | 6 | |
| Hispanic, n | 1 | 5 | 9 | |
| Pacific Islander, n | 0 | 0 | 1 | |
| White, n | 23 | 23 | 32 | |
| Not stated, n | 1 | 3 | 4 | |
| Plasma cotinine, ng/ml | 129.1 ± 38.4 | 109.2 ± 54.8* | 0 |
Values are means ± SE. P values for age, body mass index (BMI), and (log) plasma cotinine were computed using one-way ANOVA. P values for sex and race were computed using Fisher’s exact test. EC, electronic cigarette; TC, tobacco cigarette.
P = 0.35, TC smokers vs. EC users.
Parallel Group Study
Primary or secondary outcomes representative of ECG indexes of ventricular repolarization were not different among the three groups at baseline (Table 2), even when controlled for sex.
Table 2.
Parallel study: primary and secondary outcomes
| TC Smokers | EC Users | Nonusers | P Value | |
|---|---|---|---|---|
| n | 37 | 43 | 65 | |
| Tp-e, ms | 52.5 ± 2.1 | 57.5 ± 1.8 | 54.4 ± 1.4 | 0.16 |
| Tp-e/QT | 150.3 ± 7.2 | 166.9 ± 6.2 | 161.3 ± 5.0 | 0.22 |
| Tp-e/QTc | 148.3 ± 6.5 | 159.2 ± 5.6 | 156.8 ± 4.5 | 0.42 |
| QT, ms | 351.5 ± 5.1 | 347.0 ± 4.4 | 341.7 ± 3.6 | 0.27 |
| QTc, ms | 350.0 ± 3.9 | 360.0 ± 3.4 | 350.0 ± 2.7 | 0.10 |
| SDn Tp-e, ms | 6.4 ± 1.0 | 5.6 ± 0.9 | 7.0 ± 0.7 | 0.51 |
| SDn Tp-e/QT | 17.1 ± 2.9 | 15.4 ± 2.5 | 19.3 ± 2.0 | 0.21 |
| SDn Tp-e/QTc | 18.0 ± 2.8 | 15.6 ± 2.5 | 19.5 ± 2.0 | 0.47 |
| HR, beats/min | 61.6 ± 3.0 | 66.5 ± 3.8 | 63.7 ± 2.2 | 0.48 |
Values are means ± SE and are adjusted for sex using a robust two-way ANOVA model. EC, electronic cigarette; HR, heart rate; SDn, trimmed standard deviation; TC, tobacco cigarette; Tp-e, interval from T peak to T end.
Crossover Study
Chronic tobacco cigarette smokers.
increase in plasma nicotine levels following acute exposure.
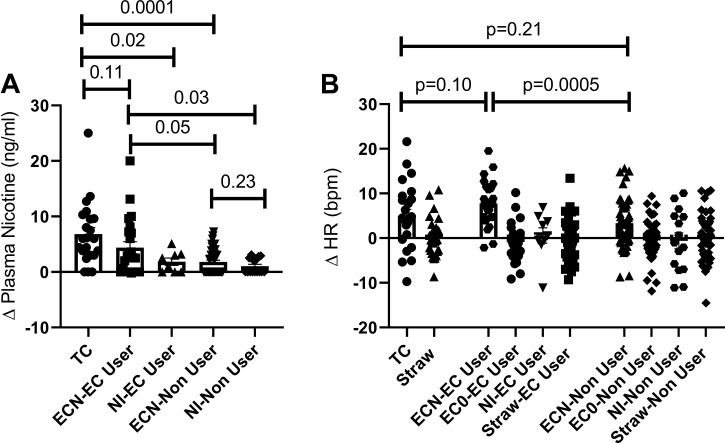
The increase in plasma nicotine levels from baseline was not different in chronic tobacco cigarette smokers after smoking one tobacco cigarette and in chronic e-cigarette users after using the e-cigarette with nicotine but was significantly greater in these groups compared with the other exposures (Fig. 1A).
Fig. 1.
Changes in nicotine and heart rate. A: the increase in plasma nicotine levels was not different in chronic tobacco cigarette smokers after smoking 1 tobacco cigarette (n = 25) and in chronic e-cigarette users after using the e-cigarette with nicotine (n = 26) but was significantly greater in these groups compared with the other exposures. B: the increase in heart rate was similar in chronic tobacco cigarette smokers after smoking 1 tobacco cigarette and in chronic e-cigarette users after using the e-cigarette with nicotine. Means compared using repeated measure (mixed) ANOVA model controlling for nonindependence via random subject effects. EC, electronic cigarette; ECN, electronic cigarette with nicotine; EC0, electronic cigarette without nicotine; HR, heart rate; NI, nicotine inhaler; TC, tobacco cigarette.
increase in heart rate following acute exposure.
The increase in heart rate was similar in chronic tobacco cigarette smokers after smoking one tobacco cigarette and in chronic e-cigarette users after using the e-cigarette with nicotine (Fig. 1B). The change in heart rate was correlated with the increase in plasma nicotine levels (tobacco cigarette smokers, rs = 0.464 P = 0.002, e-cigarette users; rs = 0.375 P = 0.04; and nonusers rs = 0.263 P = 0.03).
changes in ecg indexes of ventricular repolarization in chronic tobacco cigarette smokers after acute tobacco cigarette smoking.
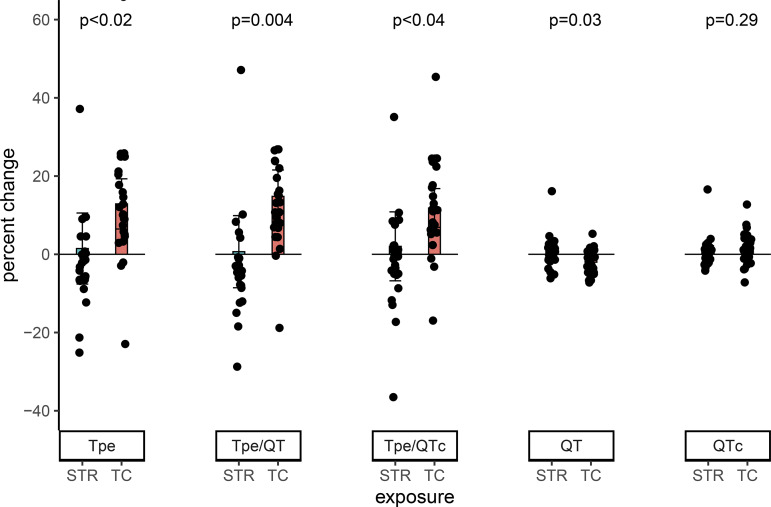
In chronic tobacco cigarette smokers, all primary outcomes indicative of ventricular repolarization, including Tpe, a measure of spatial dispersion of refractoriness (27, 42), and Tpe/QT and Tpe/QTc, significantly prolonged after smoking one tobacco cigarette compared with puffing on an empty straw. This significant prolongation was observed when each parameter was compared as a percent change from baseline (Fig. 2) or as an absolute change (data not shown).
Fig. 2.
Changes in ECG indexes of ventricular repolarization in chronic tobacco cigarette smokers after acute tobacco cigarette smoking. In chronic tobacco cigarette smokers, all primary outcomes indicative of ventricular repolarization significantly increased after smoking 1 tobacco cigarette compared with puffing on an empty straw. Observations with percent change >50% omitted for display purposes only. Means compared using a robust repeated measure (mixed) model adjusting for visit and controlling for nonindependence via random subject effects. STR, straw control; TC, tobacco cigarette; Tp-e; Tpeak to Tend interval.
Of the secondary outcomes, the QT interval significantly decreased after smoking one tobacco cigarette compared with puffing on an empty straw, but this decrease was eliminated when corrected for heart rate (QTc) (Fig. 2). The variability of the Tpe, Tpe/QT, and Tpe/QTc (temporal dispersion of refractoriness) all tended to increase after smoking one tobacco cigarette compared with puffing on an empty straw (Table 3). The increase in Tpe/QT, the decrease in QT interval, and the variability of the Tpe and Tpe/QT were all significantly correlated with the increase in plasma nicotine (Tpe/QT rs = 0.34 P = 0.03; QT rs = −0.320 P = 0.04; variability of the Tpe rs = 0.302 P = 0.05; and variability of Tpe/QT rs = 0.344 P = 0.02).
Table 3.
Crossover study: secondary outcomes
| SDn Tp-e, ms | SDn Tp-e/QT | SDn Tp-e/QTc | |
|---|---|---|---|
| TC Smokers | |||
| TC | 1.16 ± 0.72 | 3.21 ± 2.02 | 2.43 ± 2.24 |
| Straw | −0.49 ± 0.74 | −1.29 ± 2.08 | −1.90 ± 2.31 |
| P value | 0.062 | 0.066 | 0.096 |
| EC Users | |||
| ECN | 1.67 ± 0.46 | 5.06 ± 1.23 | 3.35 ± 1.08 |
| EC0 | 0.68 ± 0.45 | 2.58 ± 1.21 | 1.79 ± 1.06 |
| NI | 0.12 ± 0.89 | 0.50 ± 2.36 | 0.61 ± 2.07 |
| Straw control | 0.53 ± 0.44 | 1.23 ± 1.18 | 1.01 ± 1.04 |
| P value | 0.83 | 0.70 | 0.88 |
| Nonusers | |||
| ECN | 0.98 ± 0.35 | 2.78 ± 0.96 | 2.22 ± 0.86 |
| EC0 | 0.44 ± 0.35 | 0.98 ± 0.97 | 0.76 ± 0.87 |
| NI | 0.19 ± 0.63 | 0.97 ± 1.74 | 1.58 ± 1.56 |
| Straw control | 0.55 ± 0.44 | 1.52 ± 1.22 | 1.15 ± 1.10 |
| P value | 0.78 | 0.71 | 0.76 |
Values are means ± SE and are adjusted for sex using a robust two-way analysis of variance model. EC, electronic cigarette; ECN, EC with nicotine; EC0, EC without nicotine; NI, nicotine inhaler; SDn, trimmed standard deviation; TC, tobacco cigarette; Tp-e, interval from T peak to T end.
Chronic e-cigarette users.
changes in ecg indexes of ventricular repolarization in chronic e-cigarette users after acute e-cigarette or nicotine inhaler use.
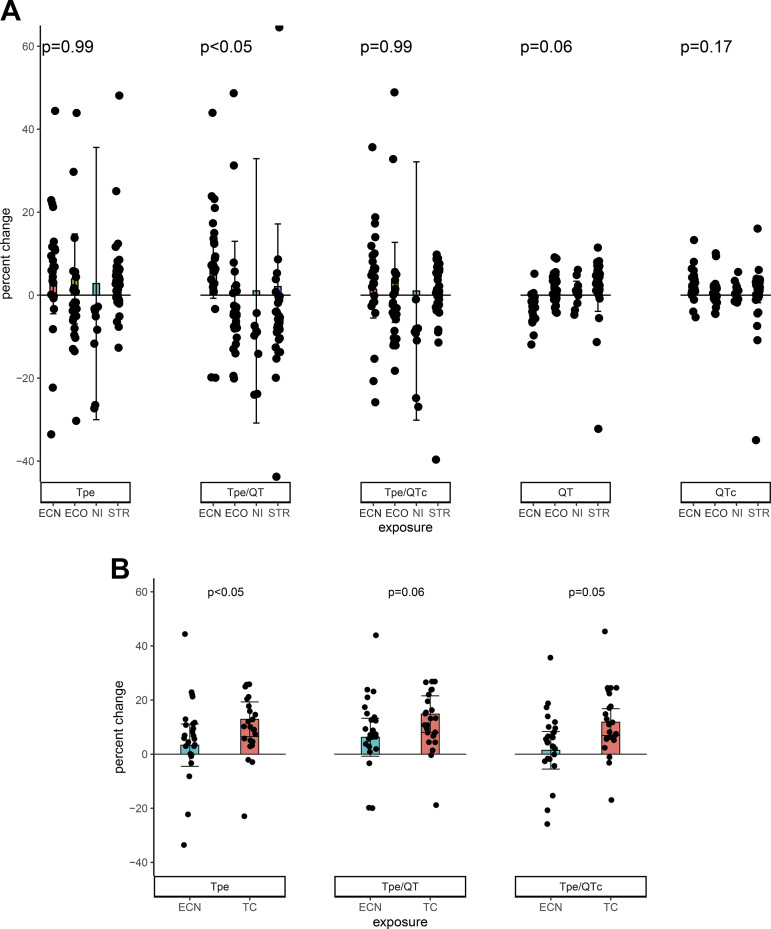
In chronic e-cigarette users, the primary outcome of Tpe/QT was increased after using an e-cigarette with nicotine but not after any of the other exposures. This significant prolongation was observed only when Tpe/QT was compared as a percent change from baseline (Fig. 3A), not as an absolute change (data not shown). The other primary outcomes, Tp-e and Tp-e/QTc ratio, or secondary outcomes (Fig. 3 and Table 3) were not changed by any of the acute exposures. None of the primary or secondary outcomes were correlated with the increase in plasma nicotine (data not shown).
Fig. 3.
Changes in ECG indexes of ventricular repolarization in chronic e-cigarette users. A: after acute e-cigarette or nicotine inhaler use. In chronic e-cigarette users, the primary outcome of Tp-e/QT was increased after using an e-cigarette with nicotine but not after any of the other exposures (e-cigarette without nicotine, n = 27; nicotine inhaler n = 9; straw, n = 40). The other primary outcomes, Tp-e and Tp-e/QTc ratio, were not changed by any of the acute exposures. Observations with percent change greater than 50% and less than −40% omitted for display purposes only. Means compared using a robust repeated measure (mixed) model adjusting for visit and controlling for nonindependence via random subject effects. B: comparison of the effect of smoking a tobacco cigarette versus using an e-cigarette with nicotine on ECG indexes of ventricular repolarization. The increases in Tpe and Tpe/QTc, but not Tpe/QT, were significantly greater after smoking the tobacco cigarette compared with using the e-cigarette with nicotine. No observations omitted. Means compared using a robust repeated measure (mixed) model adjusting for visit and controlling for nonindependence using random subject effects. EC, electronic cigarette; ECN, electronic cigarette with nicotine; EC0, electronic cigarette without nicotine; NI, nicotine inhaler; STR, straw control; TC, tobacco cigarette; Tp-e, Tpeak to Tend interval.
Chronic tobacco cigarette smokers versus chronic e-cigarette users.
comparison of the effect of smoking a tobacco cigarette versus using an e-cigarette with nicotine on ecg indexes of ventricular repolarization.
The prolongation in Tp-e and Tp-e/QTc, but not Tp-e/QTc, was significantly greater after smoking the tobacco cigarette compared with using the e-cigarette with nicotine.
Nonusers.
changes in ecg indexes of ventricular repolarization in nonusers after acute e-cigarette or nicotine inhaler use.
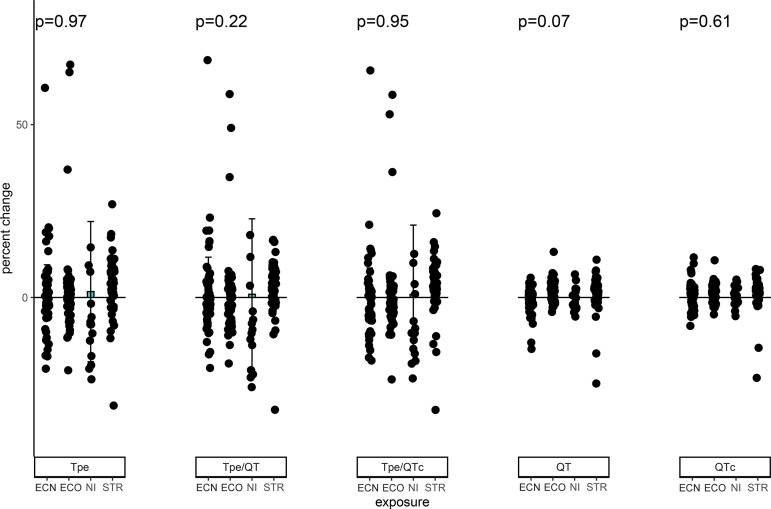
In nonusers, there was no significant change in any of the primary or secondary outcomes, compared as a percent change (Fig. 4 and Table 2) or as an absolute change (data not shown), after any of the acute exposures.
Fig. 4.
Changes in ventricular repolarization in nonusers after acute e-cigarette or nicotine inhaler use. In nonusers, there was no significant change in any of the primary outcomes, Tp-e, Tp-e/QT, and Tp-e/QTc or secondary outcomes, QT and QTc (Bazett’s), as measure by percent change after any of the acute exposures (e-cigarette without nicotine, n = 53; EC without nicotine, n = 49; nicotine inhaler n = 15; straw, n = 49). Observations with percent change >80% and <-40% omitted for display purposes only. Means compared using a robust repeated measure (mixed) model adjusting for visit and controlling for nonindependence via random subject effects. EC, electronic cigarette; ECN, electronic cigarette with nicotine; EC0, electronic cigarette without nicotine; NI, nicotine inhaler; STR, straw control; Tp-e, Tpeak to Tend interval.
DISCUSSION
In this study, we first compared several indexes of ventricular repolarization in abstinent chronic tobacco cigarette smokers, abstinent chronic e-cigarette users and nonusers and found no difference in any ECG index of ventricular repolarization among the groups. These findings are consistent with prior reports in chronic tobacco cigarette smokers who had refrained from smoking before ECG-recordings, in whom ECG indexes of ventricular depolarization, including the QT interval, QTc (Bazett’s) interval, and dispersion of the QT interval were not different from nonsmokers at baseline (3, 5). In this report, we extended these prior studies by including indexes of spatial dispersion of ventricular repolarization, the Tp-e, Tp-e/QT ratio and Tp-e/QTc ratio, which also were not different from nonsmokers at baseline. Finally, we extended these comparisons to include a large cohort of chronic e-cigarette users, and again, the primary and secondary ECG indexes of ventricular repolarization were not different from nonusers or tobacco cigarette smokers at baseline.
We next probed the effects of acute exposures on ECG indexes of ventricular repolarization within each group. In the group of chronic tobacco cigarette smokers, smoking one tobacco cigarette significantly increased plasma nicotine levels and heart rate compared with puffing on a straw control. An increase in heart rate is normally associated with a shortening of ventricular repolarization. Paradoxically, immediately after chronic tobacco cigarette smokers smoked one tobacco cigarette compared with puffing on an empty straw (control), all primary outcomes indicative of abnormal ventricular repolarization, specifically Tp-e, Tp-e/QT ratio, and Tp-e/QTc ratio, prolonged significantly. Importantly, the percentage increase in each outcome indicative of increased spatial dispersion of repolarization was similar to the percent increase detected in other cohorts with increased risk of sudden death (4, 9, 22, 23, 28, 33, 35, 40). These findings are consistent with prior reports in chronic tobacco cigarette smokers who had not refrained from smoking before ECG recordings, in whom ECG indexes of spatial dispersion of refractoriness, including the Tp-e, Tp-e/QT ratio, and Tp-e/QTc ratio, were also prolonged compared with nontobacco cigarette smokers (21, 34).
These findings that tobacco cigarette smokers do not exhibit abnormal ventricular repolarization at baseline, but that smoking even a single tobacco cigarette does induce significant and pervasive abnormalities in ECG indexes of ventricular repolarization associated with increased arrhythmia risk, may explain prior observations that 1) the risk of cardiac death following smoking cessation reverts toward that of a nonsmoker soon after quitting tobacco cigarette smoking, and 2) smoking only one to three tobacco cigarettes/day markedly increases cardiovascular mortality, approaching that of individuals who smoke one to three packs per day (15, 30). It remains unknown if the adverse effect of tobacco cigarette smoking on ECG indexes of ventricular repolarization is attributable to nicotine or 1 or more of the 7,000 other constituents present in tobacco cigarette smoke. It would be interesting to repeat acute exposure studies utilizing tobacco cigarettes without nicotine to help clarify the role of nicotine versus non-nicotine constituents in tobacco cigarette smoke.
When chronic e-cigarette users used the e-cigarette with nicotine, plasma nicotine levels increased similarly compared with the increase in plasma nicotine when chronic tobacco cigarette smokers smoked one tobacco cigarette. Immediately after using the e-cigarette with nicotine, only one of the three primary outcomes, the Tp-e/QT ratio, was significantly increased compared with straw control, and the mean increase (6.3 ± 1.9%) in the Tp-e/QT ratio was less than half that seen in chronic tobacco cigarette smokers after smoking a tobacco cigarette (14.9 ± 5.0%). While the increase in this ECG index of ventricular repolarization falls within the range of percentage increase in the Tp-e/QT ratio in other cohorts with increased sudden death risk, it is at the lower end (4, 9, 22, 23, 28). The clinical significance of an abnormality in any of the three parameters indicative of increased spatial dispersion of repolarization is unknown.
The increases in two of the three primary outcomes were significantly greater after smoking a tobacco cigarette compared with using the e-cigarette with nicotine (Fig. 3B). This greater effect cannot be attributed to a greater acute exposure to tobacco cigarette compared with e-cigarette emissions since the increase in plasma nicotine levels was similar in each cohort after their respective acute exposures. Additionally, the increase in heart rate was similar, in fact tended to be greater, in chronic e-cigarette users after using the e-cigarette with nicotine compared with the chronic tobacco cigarette smokers after smoking one tobacco cigarette. This observation suggests that one or more of the 7,000 non-nicotine constituents in tobacco cigarette smoke likely contributes to the more marked acute prolongation of ventricular repolarization associated with acute tobacco cigarette smoking.
In the group of nonusers, immediately after using the e-cigarette with nicotine compared with straw control, heart rate increased significantly. In fact, the increase in heart rate was not different from the increase in heart rate after chronic tobacco cigarette smokers smoked one tobacco cigarette, although the increase in plasma nicotine levels were significantly lower. The robust increase in heart rate in nonusers after using the e-cigarette with nicotine despite only a small increase in plasma nicotine levels could be attributable to an alerting response provoked by using a potentially noxious device. In the nonuser group, after using the e-cigarette with nicotine compared with straw control, none of the primary outcomes indicative of spatial dispersion in ventricular repolarization were significantly increased. It remains unknown if nonusers, like chronic e-cigarette users, would develop abnormalities in ventricular repolarization after using an e-cigarette with nicotine if a greater exposure (as indicated by plasma nicotine levels) were achieved.
Limitations
In this study, only indirect ECG indexes of ventricular repolarization were measured, which are likely less sensitive than directly measuring spatial ventricular repolarization or action potential duration. Nonetheless, even with these noninvasive, indirect measurements, we were able to detect significant adverse effects of smoking. Two ECG leads, rather than all 12 possible leads, were collected and analyzed for ECG indexes of ventricular repolarization. Recording more leads is unlikely to have altered our findings, since the methodology for collecting ECG recordings was the same in each cohort. The detection of abnormal ECG indexes of ventricular repolarization may be difficult, even in patients diagnosed with inherited channelopathies diagnostic of congenital long QT syndrome, since its phenotypic expression is influenced by heart rate and autonomic tone. Abrupt changes in heart rate triggered by provocative testing with brisk standing or exercise testing can unmask diminished repolarization reserve (1, 36, 41), and thus aid in diagnosis. Provocative testing was not done in these studies but could be performed in future studies to unmask abnormal ECG indexes of ventricular repolarization in smokers with concealed abnormalities of at rest (41). Using the nicotine inhaler only slightly increased in plasma nicotine levels, and thus was not an adequate test of this “pure” form of nicotine, without solvents or flavors. This may be explained by our participants’ unfamiliarity with the nicotine inhaler, as well as by the fact that only 5% of nicotine from the nicotine inhaler reaches the lungs; the rest is more slowly absorbed through the oral mucosa (29). Finally, the latest generation, pod-like e-cigarette (Juul), which purportedly delivers nicotine at a steeper rate than earlier generation e-cigarette devices, was not tested in these studies. Studies of the effects of these latest, pod-like e-cigarettes on ECG indexes of ventricular repolarization are warranted.
Conclusions.
In summary, in these studies of 145 healthy participants, resting heart rate and ECG indexes of ventricular repolarization were not different in tobacco cigarette smokers or e-cigarette users compared with nonusers at baseline. Smoking a tobacco cigarette or an e-cigarette acutely increased heart rate. Furthermore, an adverse effect of acute tobacco cigarette smoking on ECG indexes of ventricular repolarization was confirmed. In chronic e-cigarette users, an adverse effect of using an e-cigarette with nicotine, but not without nicotine, on one of the ECG indexes of ventricular repolarization was also present. If one does not currently smoke, one should not use e-cigarettes, since they may have adverse cardiovascular effects that are associated with increased sudden death risk.
GRANTS
This work was supported by Tobacco-Related Disease Research Program Grants TRDRP 23XT-0006H (to H.R.M.), 25IR-0024H (to H.R.M.), TRDRP 28IR-0065 (to H.R.M.), and TRDRP T29IP0319 (to H.R.M.) and by National Center for Advancing Translational Science, University of California, Los Angeles, Clinical and Translational Science Institute Grant UL1-TR-001881.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.R.M. conceived and designed research; H.R.M. interpreted results of experiments; H.R.M., and J.G. prepared figures; M.I., E.D., K.H., J.G., and H.R.M. analyzed data; K.H., Y.C., R.S.M., K.H.N., and E.T. performed experiments; H.R.M. drafted manuscript; J.G. edited and revised manuscript; M.I., E.D., K.H., Y.C., R.S.M., K.H.N., E.T., J.G., and H.R.M. approved final version of manuscript.
REFERENCES
- 1.Adler A, van der Werf C, Postema PG, Rosso R, Bhuiyan ZA, Kalman JM, Vohra JK, Guevara-Valdivia ME, Marquez MF, Halkin A, Benhorin J, Antzelevitch C, Wilde AA, Viskin S. The phenomenon of “QT stunning”: the abnormal QT prolongation provoked by standing persists even as the heart rate returns to normal in patients with long QT syndrome. Heart Rhythm 9: 901–908, 2012. doi: 10.1016/j.hrthm.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahnve S. QT interval prolongation in acute myocardial infarction. Eur Heart J 6, Suppl D: 85–95, 1985. doi: 10.1093/eurheartj/6.suppl_d.85. [DOI] [PubMed] [Google Scholar]
- 3.Akbarzadeh MA, Yazdani S, Ghaidari ME, Asadpour-Piranfar M, Bahrololoumi-Bafruee N, Golabchi A, Azhari A. Acute effects of smoking on QT dispersion in healthy males. ARYA Atheroscler 10: 89–93, 2014. [PMC free article] [PubMed] [Google Scholar]
- 4.Alizade E, Yesin M, Yazicioglu MV, Karaayvaz EB, Atici A, Arslan S, Avci A, Acar G, Tabakci M, Izci S, Pala S. Evaluation of Tp-e interval, Tp-e/QT ratio, and Tp-e/QTc ratio in patients with asymptomatic arrhythmogenic right ventricular cardiomyopathy. Ann Noninvasive Electrocardiol 22: 1, 2017. doi: 10.1111/anec.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrássy G, Szabo A, Dunai A, Simon E, Nagy T, Trummer Z, Tahy A, Varro A. Acute effects of cigarette smoking on the QT interval in healthy smokers. Am J Cardiol 92: 489–492, 2003. doi: 10.1016/S0002-9149(03)00678-7. [DOI] [PubMed] [Google Scholar]
- 6.Antzelevitch C. Heterogeneity and cardiac arrhythmias: an overview. Heart Rhythm 4: 964–972, 2007. doi: 10.1016/j.hrthm.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antzelevitch C. T peak-Tend interval as an index of transmural dispersion of repolarization. Eur J Clin Invest 31: 555–557, 2001. doi: 10.1046/j.1365-2362.2001.00849.x. [DOI] [PubMed] [Google Scholar]
- 8.Bébarová M, Horáková Z, Kula R. Addictive drugs, arrhythmias, and cardiac inward rectifiers. Europace 19: 346–355, 2017. doi: 10.1093/europace/euw071. [DOI] [PubMed] [Google Scholar]
- 9.Castro Hevia J, Antzelevitch C, Tornés Bárzaga F, Dorantes Sánchez M, Dorticós Balea F, Zayas Molina R, Quiñones Pérez MA, Fayad Rodríguez Y. Tpeak-Tend and Tpeak-Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol 47: 1828–1834, 2006. doi: 10.1016/j.jacc.2005.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Alessandro A, Boeckelmann I, Hammwhöner M, Goette A. Nicotine, cigarette smoking and cardiac arrhythmia: an overview. Eur J Prev Cardiol 19: 297–305, 2012. doi: 10.1177/1741826711411738. [DOI] [PubMed] [Google Scholar]
- 11.Deyell MW, Krahn AD, Goldberger JJ. Sudden cardiac death risk stratification. Circ Res 116: 1907–1918, 2015. doi: 10.1161/CIRCRESAHA.116.304493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dilaveris P, Pantazis A, Gialafos E, Triposkiadis F, Gialafos J. The effects of cigarette smoking on the heterogeneity of ventricular repolarization. Am Heart J 142: 833–837, 2001. doi: 10.1067/mhj.2001.118737. [DOI] [PubMed] [Google Scholar]
- 13.Dobson CP, Kim A, Haigney M. QT Variability Index. Prog Cardiovasc Dis 56: 186–194, 2013. doi: 10.1016/j.pcad.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Emori T, Antzelevitch C. Cellular basis for complex T waves and arrhythmic activity following combined I(Kr) and I(Ks) block. J Cardiovasc Electrophysiol 12: 1369–1378, 2001. doi: 10.1046/j.1540-8167.2001.01369.x. [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg I, Jonas M, Tenenbaum A, Boyko V, Matetzky S, Shotan A, Behar S, Reicher-Reiss H; Bezafibrate Infarction Prevention Study Group . Current smoking, smoking cessation, and the risk of sudden cardiac death in patients with coronary artery disease. Arch Intern Med 163: 2301–2305, 2003. doi: 10.1001/archinte.163.19.2301. [DOI] [PubMed] [Google Scholar]
- 16.Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P 3rd, Benowitz NL. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: a longitudinal within-subjects observational study. Nicotine Tob Res 19: 160–167, 2016. doi: 10.1093/ntr/ntw160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P 3rd, Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 23: 133–139, 2014. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green SH, Bayer R, Fairchild AL. Evidence, policy, and e-cigarettes–will England reframe the debate? N Engl J Med 374: 1301–1303, 2016. doi: 10.1056/NEJMp1601154. [DOI] [PubMed] [Google Scholar]
- 19.Gupta P, Patel C, Patel H, Narayanaswamy S, Malhotra B, Green JT, Yan GX. T(p-e)/QT ratio as an index of arrhythmogenesis. J Electrocardiol 41: 567–574, 2008. doi: 10.1016/j.jelectrocard.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Ileri M, Yetkin E, Tandoğan I, Hisar I, Atak R, Senen K, Cehreli S, Demirkan D. Effect of habitual smoking on QT interval duration and dispersion. Am J Cardiol 88: 322–325, 2001. doi: 10.1016/S0002-9149(01)01653-8. [DOI] [PubMed] [Google Scholar]
- 21.İlgenli TF, Tokatlı A, Akpınar O, Kılıçaslan F. The effects of cigarette smoking on the Tp-e interval, Tp-e/QT ratio and Tp-e/QTc ratio. Adv Clin Exp Med 24: 973–978, 2015. doi: 10.17219/acem/28114. [DOI] [PubMed] [Google Scholar]
- 22.Kilicaslan F, Tokatli A, Ozdag F, Uzun M, Uz O, Isilak Z, Yiginer O, Yalcin M, Guney MS, Cebeci BS. Tp-e interval, Tp-e/QT ratio, and Tp-e/QTc ratio are prolonged in patients with moderate and severe obstructive sleep apnea. Pacing Clin Electrophysiol 35: 966–972, 2012. doi: 10.1111/j.1540-8159.2012.03439.x. [DOI] [PubMed] [Google Scholar]
- 23.Lubinski A, Lewicka-Nowak E, Kempa M, Baczynska AM, Romanowska I, Swiatecka G. New insight into repolarization abnormalities in patients with congenital long QT syndrome: the increased transmural dispersion of repolarization. Pacing Clin Electrophysiol 21: 172–175, 1998. doi: 10.1111/j.1540-8159.1998.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 24.Margham J, McAdam K, Forster M, Liu C, Wright C, Mariner D, Proctor C. Chemical composition of aerosol from an E-cigarette: a quantitative comparison with cigarette smoke. Chem Res Toxicol 29: 1662–1678, 2016. doi: 10.1021/acs.chemrestox.6b00188. [DOI] [PubMed] [Google Scholar]
- 25.Middlekauff HR. COUNTERPOINT: does the risk of electronic cigarettes exceed potential benefits? No. Chest 148: 582–584, 2015. doi: 10.1378/chest.15-0540. [DOI] [PubMed] [Google Scholar]
- 26.Moheimani RS, Bhetraratana M, Peters KM, Yang BK, Yin F, Gornbein J, Araujo JA, Middlekauff HR. Sympathomimetic effects of acute e-cigarette use: role of nicotine and non-nicotine constituents. J Am Heart Assoc 6: 6, 2017. doi: 10.1161/JAHA.117.006579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Opthof T, Coronel R, Wilms-Schopman FJ, Plotnikov AN, Shlapakova IN, Danilo P Jr, Rosen MR, Janse MJ. Dispersion of repolarization in canine ventricle and the electrocardiographic T wave: Tp-e interval does not reflect transmural dispersion. Heart Rhythm 4: 341–348, 2007. doi: 10.1016/j.hrthm.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 28.Panikkath R, Reinier K, Uy-Evanado A, Teodorescu C, Hattenhauer J, Mariani R, Gunson K, Jui J, Chugh SS. Prolonged Tpeak-to-tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol 4: 441–447, 2011. doi: 10.1161/CIRCEP.110.960658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfizer Nicotrol Inhaler. Kalamazoo, MI: Pharmacia & Upjohn Co., 2008. [Google Scholar]
- 30.Pope CA 3rd, Burnett RT, Krewski D, Jerrett M, Shi Y, Calle EE, Thun MJ. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation 120: 941–948, 2009. doi: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- 31.Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, van Herpen G, Wagner GS, Wellens H; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society . AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 119: e241–e250, 2009. doi: 10.1161/CIRCULATIONAHA.108.191096. [DOI] [PubMed] [Google Scholar]
- 32.Shahab L, Goniewicz ML, Blount BC, Brown J, McNeill A, Alwis KU, Feng J, Wang L, West R. Nicotine, carcinogen, and toxin exposure in long-term e-cigarette and nicotine replacement therapy users: a cross-sectional study. Ann Intern Med 166: 390–400, 2017. doi: 10.7326/M16-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimizu M, Ino H, Okeie K, Yamaguchi M, Nagata M, Hayashi K, Itoh H, Iwaki T, Oe K, Konno T, Mabuchi H. T-peak to T-end interval may be a better predictor of high-risk patients with hypertrophic cardiomyopathy associated with a cardiac troponin I mutation than QT dispersion. Clin Cardiol 25: 335–339, 2002. doi: 10.1002/clc.4950250706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taşolar H, Ballı M, Bayramoğlu A, Otlu YO, Cetin M, Altun B, Cakıcı M. Effect of smoking on Tp-e interval, Tp-e/QT and Tp-e/QTc ratios as indices of ventricular arrhythmogenesis. Heart Lung Circ 23: 827–832, 2014. doi: 10.1016/j.hlc.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Tse G, Gong M, Wong WT, Georgopoulos S, Letsas KP, Vassiliou VS, Chan YS, Yan BP, Wong SH, Wu WK, Ciobanu A, Li G, Shenthar J, Saguner AM, Ali-Hasan-Al-Saegh S, Bhardwaj A, Sawant AC, Whittaker P, Xia Y, Yan GX, Liu T. The Tpeak-Tend interval as an electrocardiographic risk marker of arrhythmic and mortality outcomes: a systematic review and meta-analysis. Heart Rhythm 14: 1131–1137, 2017. doi: 10.1016/j.hrthm.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 36.Viskin S, Postema PG, Bhuiyan ZA, Rosso R, Kalman JM, Vohra JK, Guevara-Valdivia ME, Marquez MF, Kogan E, Belhassen B, Glikson M, Strasberg B, Antzelevitch C, Wilde AA. The response of the QT interval to the brief tachycardia provoked by standing: a bedside test for diagnosing long QT syndrome. J Am Coll Cardiol 55: 1955–1961, 2010. doi: 10.1016/j.jacc.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagener TL, Floyd EL, Stepanov I, Driskill LM, Frank SG, Meier E, Leavens EL, Tackett AP, Molina N, Queimado L. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob Control 26, e1: e23–e28, 2017. doi: 10.1136/tobaccocontrol-2016-053041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Shi H, Wang Z. Nicotine depresses the functions of multiple cardiac potassium channels. Life Sci 65: PL143–PL149, 1999. doi: 10.1016/S0024-3205(99)00370-7. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Yang B, Zhang L, Xu D, Wang Z. Direct block of inward rectifier potassium channels by nicotine. Toxicol Appl Pharmacol 164: 97–101, 2000. doi: 10.1006/taap.2000.8896. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe N, Kobayashi Y, Tanno K, Miyoshi F, Asano T, Kawamura M, Mikami Y, Adachi T, Ryu S, Miyata A, Katagiri T. Transmural dispersion of repolarization and ventricular tachyarrhythmias. J Electrocardiol 37: 191–200, 2004. doi: 10.1016/j.jelectrocard.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Wong JA, Gula LJ, Klein GJ, Yee R, Skanes AC, Krahn AD. Utility of treadmill testing in identification and genotype prediction in long-QT syndrome. Circ Arrhythm Electrophysiol 3: 120–125, 2010. doi: 10.1161/CIRCEP.109.907865. [DOI] [PubMed] [Google Scholar]
- 42.Yagishita D, Chui RW, Yamakawa K, Rajendran PS, Ajijola OA, Nakamura K, So EL, Mahajan A, Shivkumar K, Vaseghi M. Sympathetic nerve stimulation, not circulating norepinephrine, modulates T-peak to T-end interval by increasing global dispersion of repolarization. Circ Arrhythm Electrophysiol 8: 174–185, 2015. doi: 10.1161/CIRCEP.114.002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamaguchi M, Shimizu M, Ino H, Terai H, Uchiyama K, Oe K, Mabuchi T, Konno T, Kaneda T, Mabuchi H. T wave peak-to-end interval and QT dispersion in acquired long QT syndrome: a new index for arrhythmogenicity. Clin Sci (Lond) 105: 671–676, 2003. doi: 10.1042/CS20030010. [DOI] [PubMed] [Google Scholar]