Abstract
Discrete calcium signals within the vascular endothelium decrease with age and contribute to impaired endothelial-dependent vasodilation. Calreticulin (Calr), a multifunctional calcium binding protein and endoplasmic reticulum (ER) chaperone, can mediate calcium signals and vascular function within the endothelial cells (ECs) of small resistance arteries. We found Calr protein expression significantly decreases with age in mesenteric arteries and examined the functional role of EC Calr in vasodilation and calcium mobilization in the context of aging. Third-order mesenteric arteries from mice with or without EC Calr knockdown were examined for calcium signals and constriction to phenylephrine (PE) or vasodilation to carbachol (CCh) after 75 wk of age. PE constriction in aged mice with or without EC Calr was unchanged. However, calcium signals and vasodilation to endothelial-dependent agonist carbachol were significantly impaired in aged EC Calr knockdown mice. Ex vivo incubation of arteries with the ER stress inhibitor tauroursodeoxycholic acid (TUDCA) significantly improved vasodilation in mice lacking EC Calr. Our data suggests diminished vascular Calr expression with age can contribute to the detrimental effects of aging on endothelial calcium regulation and vasodilation.
NEW & NOTEWORTHY Calreticulin (Calr) is responsible for key physiological processes in endoplasmic reticulum, especially in aging tissue. In particular, endothelial Calr is crucial to vascular function. In this study, we deleted Calr from the endothelium and aged the mice up to 75 wk to examine changes in vascular function. We found two key differences: 1) calcium events in endothelium were severely diminished after muscarinic stimulation, which 2) corresponded with a dramatic decrease in muscarinic vasodilation. Remarkably, we were able to rescue the effect of Calr deletion on endothelial-dependent vasodilatory function using tauroursodeoxycholic acid (TUDCA), an inhibitor of endoplasmic reticulum stress that is currently in clinical trials.
Keywords: aging, calreticulin, endothelial cell, ER stress, vasodilation
INTRODUCTION
Endothelial dysfunction of small resistance arteries occurs with advancing age and is independently associated with a 5-year risk of death, myocardial infarction, and stroke in humans (14). An aging vasculature is characterized by a long-term rise in blood pressure, diminished endothelial-dependent vasodilation (9), and decreased spontaneous calcium signals in the endothelium (5). Regulation of calcium signaling is an important component of homeostatic vascular function. In particular, the discrete localization and frequency of calcium events within the endothelial cell (EC) can have a profound outcome on overall vascular function due to spatial differences in calcium-activated receptors, channels, and organelles, including the endoplasmic reticulum (ER) (21, 28).
Calreticulin (Calr) is a multifunctional protein, best known for calcium binding and its role as an endoplasmic reticulum (ER) protein folding chaperone (10, 16). These functions allow Calr to respond to cellular stress both inside and outside the ER. Our previous work has shown Calr is highly expressed in small resistance arteries, specifically in myoendothelial junctions (MEJs) (4), the portion of the arterial wall where ECs and smooth muscle cells (SMCs) make cytosolic contact via gap junctions (27). We reported that knockdown of EC Calr resulted in an impaired ability to respond to smooth muscle cell (SMC) stimulation both in terms of a subsequent EC calcium mobilization but also impaired negative feedback to limit vasoconstriction. Importantly, the capacity of the resistance arteries to respond to endothelial agonists [i.e., carbachol (CCh)] was not impaired (4). In these previous experiments, adult male mice between 10 and 20 wk of age were used to study Calr localization and function in the absence of pathology; thus the role of EC Calr in vascular aging has not been explored.
Evidence suggests that decreased expression of ER chaperones, including Calr, is associated with aging (6, 26) and oxidative stress (3). To exacerbate the purported decrease of Calr with aging, we used an inducible, EC-specific Calr knockout mouse (EC Calr Δ/Δ) to investigate calcium signaling and vasoreactivity in small resistance arteries after mice were 75 wk of age (~15 mo old). We hypothesized that the detrimental effects of aging would be accelerated in EC Calr Δ/Δ mice and result in impaired calcium mobilization and vascular function regardless of agonist stimulation.
MATERIALS AND METHODS
Animals.
All experiments were approved by the University of Virginia Animal Care and Use Committee. EC Calr fl/fl and EC Calr Δ/Δ male mice were generated as described previously (4). Male mice were maintained on normal chow and water ad libitum with a 12-h light-dark cycle. At ~6 wk of age, littermates were injected with vehicle (EC Calr fl/fl) or tamoxifen (EC Calr Δ/Δ) for 10 consecutive days (0.1 mL, 10 mg/mL solution in peanut oil) and aged until they were 75–85 wk of age. Wild-type C57BL/6 male mice were purchased from Taconic. For these experiments, young mice are considered to be 10–20 wk of age and aged mice are considered between 75 and 85 wk of age.
At time of experiments, mice were euthanized with carbon dioxide. Third-order mesenteric arteries (lumen diameter: ~100–200 µm) were excised and placed into cold Krebs-Hepes buffer at pH 7.40–7.42 with 2 mM Ca2+ (118.4 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 4 mM NaHCO3, 1.2 mM KH2PO4, 10 mM HEPES, and 6 mM glucose).
Calcium imaging.
Arteries were dissected free of connective tissue, cut longitudinally, and pinned endothelial side up (en face) on Sylgard blocks, incubated with Fluo-4AM and imaged/analyzed as previously described (4, 25) with a focus on the number of calcium events occurring within 5 μm of internal elastic lamina (IEL) holes in one field of view over 2 min. Phenylephrine (PE) and CCh were used at 10 μM (Sigma). For amplitude analysis, individual values represent averaged peak fluorescence amplitude of all the calcium events in an artery (F/F0; baseline = 1). Spatial spread was determined using full-width at half-maximal (FWHM) amplitude as described in Ref. 24. Individual points represent averaged spatial spread of all the events in one artery.
Pressure myography.
Arteries were dissected free of connective tissue, and both ends were cannulated on glass micropipettes and pressurized to 80 mmHg. Concentration responses to PE and CCh were performed as previously described (4). Tauroursodeoxycholic acid (TUDCA; 30 μM in DMSO, Sigma) or vehicle was incubated abluminally and superfused for 30 min on small, isolated arteries. Endothelial and smooth muscle viability was confirmed via constriction to 30 mM KCl and dilation to 1 μM NS309, an activator of intermediate and small conductance calcium-activated potassium channels on the endothelium. Tone was calculated as (calcium-free diameter – baseline before PE)/calcium-free diameter. A Danish MyoTechnology 114P pressure myograph system and MyoVIEW software was used to collect data.
Immunoblot.
All second- and third-order mesenteric arteries from one mouse were dissected out and pooled together for each n value and dounce homogenized in RIPA protein lysis buffer with protease inhibitor cocktail (Sigma). Samples were run on a 10% SDS-PAGE denaturing gel and transferred to nitrocellulose and probed with anti-Calr Ab (Thermo Fisher no. PA3-900) and for total protein (Li-Cor), and developed on a Li-Cor imager. To normalize the Calr values, the fluorescent signal of the total protein stain was quantified for the entire lane. Calr expression was calculated as the ratio of Calr intensity to total protein. We have previously validated this antibody via immunofluorescence on EC Calr Δ/Δ mouse arteries and Western blotting of endothelial cells after Calr siRNA (4).
Histology.
Third-order mesenteric arteries from EC Calr fl/fl and EC Calr Δ/Δ mice were excised and fixed in 4% PFA overnight, processed, and embedded in paraffin. Four-micrometer-thick sections were stained with Masson’s trichrome or hematoxylin and eosin.
Analysis of internal elastic lamina holes.
Third order mesenteric arteries were cut open longitudinally and stained with Alexa 647 hydrazide and DAPI. Arteries were viewed en face with an Olympus FV1000, and the number of holes per field of view was counted manually. Four different fields of view were averaged per mouse, and four mice were used per genotype and age.
Quantitative PCR.
Thoracic aortas were excised, cleaned of perivascular fat, and snap frozen. Tissue was homogenized, RNA was extracted, and quantitative (q)PCR was completed and analyzed as previously described (4). Mouse primers (Sigma) were used for Calr were as follows: forward: AAAATCCTGAATACAAGGGC and reverse: CATAGATATTTGCATCGGGG; and for loading control β2-microglobin (B2M) as follows: forward: GTATGCTATCCAGAAAACCC and reverse: CTGAAGGACATAATCTGACATC.
Statistical analysis.
Graph Pad Prism 8 was used for statistical analysis. Data are presented as means ± SE. P < 0.05 was considered statistically significant (denoted with an asterisk). Unpaired Student’s t test was used to compare groups of two. For other comparisons, two-way ANOVA with repeated measures with Tukey’s post hoc test was used.
RESULTS
Vascular calreticulin expression decreases with age.
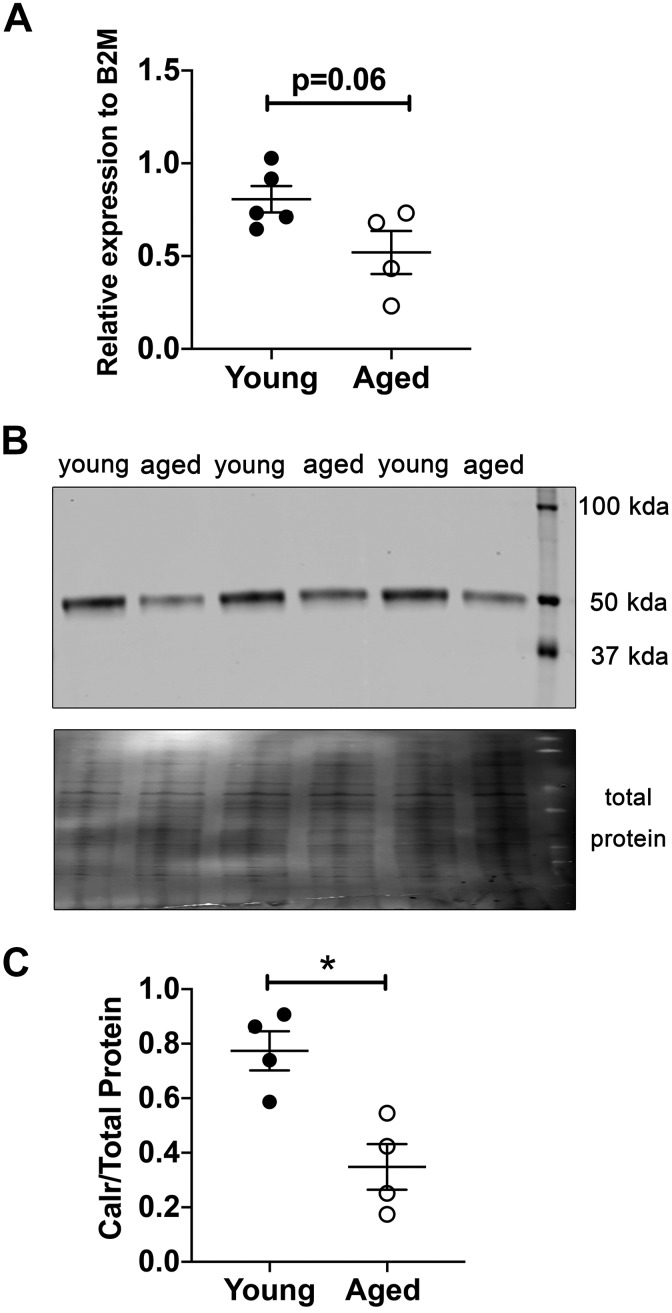
Initially we sought to determine if advanced age induced changes in Calr protein expression in large arteries from C57BL/6 mice. There was a trend in decreased Calr mRNA expression in aortas from aged mice (75–85 vs. 10–20 wk old; Fig. 1A). In small mesenteric resistance arteries that have fewer layers of smooth muscle compared with aortas, Calr protein expression was significantly decreased (Fig. 1B). Due to the important role of resistance artery ECs in regulating blood pressure and flow, we hypothesized deletion of Calr from ECs may exacerbate the effects of aging. To that end, we found that EC-specific deletion of Calr significantly decreased mouse survival up to ~71 wk of age (Fig. 2A). For this reason, we examined resistance artery function in mice lacking calreticulin from the endothelium.
Fig. 1.
Aged murine third-order mesenteric arteries express less calreticulin (Calr). A: Calr mRNA expression trends lower in aged aortic arteries. (Young n = 5; Aged n = 4; P = 0.06 via unpaired t test). B: representative blot showing bands for Calr protein at 50 kDa. C: Protein expression of calreticulin was quantified in young male and aged male normalized to total protein (n = 4/group; *P = 0.008 via unpaired t test). B2M, β2-microglobin.
Fig. 2.
A: aged endothelial cell (EC) calreticulin (Calr) Δ/Δ have significantly worse survival over time (*P < 0.05 via Gehan-Breslow-Wilcoxon survival test). In the ~500 days of aging, mice lacking EC Calr died in greater numbers (n = 20/group).
In the context of aging, EC Calr deletion does not affect phenylephrine-mediated vascular function.
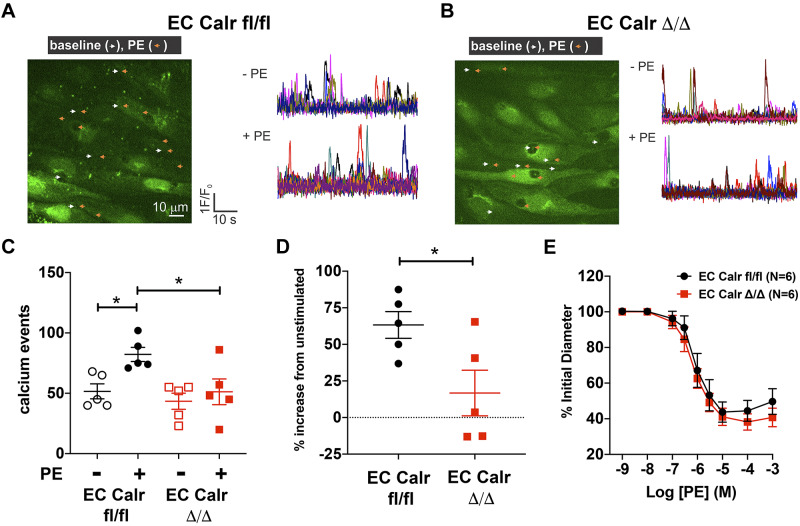
To determine the impact of EC Calr deletion on physiological function, we quantified the number of discrete, spontaneous, and/or agonist-induced calcium events localized to the MEJ in an ex vivo, en face arterial preparation in the presence and absence of agonist stimulation. The calcium events within the IEL limit SMC-mediated vasoconstriction via negative feedback (8, 11, 21). The number of baseline calcium events was not different between genotypes (Fig. 3, A and B). PE increased the number of calcium events within IEL holes of aged WT arteries (Fig. 3, A and B). However, aged EC Calr Δ/Δ mice did not significantly increase IEL hole-localized calcium signals in response to PE (Fig. 3, C and D). There is no genotype difference in ex vivo PE vasoconstriction (Fig. 3E, P = 0.14 via two-way ANOVA).
Fig. 3.
Aged endothelial cell (EC) calreticulin (Calr) Δ/Δ arteries do not increase calcium events to phenylephrine and vasoconstriction is unchanged. A and B: representative images and calcium event traces of 3rd-order mesenteric arteries from aged EC Calr fl/fl and Δ/Δ male micein an en face preparation. ECs were loaded with Fluo-4AM (green) to visualize calcium release into the cytosol. Calcium events were imaged and quantified before (white arrows) and during 10 μM phenylephrine (PE) exposure (orange arrows). C: in aged EC Calr fl/fl mice, PE stimulation caused a significant increase in events versus baseline (P = 0.0018, two-way ANOVA with Tukey’s multiple comparisons) and PE-stimulated EC Calr Δ/Δ arteries (P = 0.0017). D: the percent increase in PE-stimulated calcium events was significantly lower in EC Calr Δ/Δ mice (P = 0.03, unpaired t test); n = 5 arteries from 5 mice/group. E: 3rd-order mesenteric arteries were excised and maintained at 80 mmHg in a pressure myograph. After endothelial and smooth muscle viability was checked, increasing concentrations of PE were added to the bath and lumen diameter was recorded. PE constriction in aged mice with or without EC Calr was unchanged (n = 6 arteries from 6 mice/group = 0.14, via two-way ANOVA). *P < 0.05.
Aged EC Calr Δ/Δ arteries have impaired calcium mobilization.
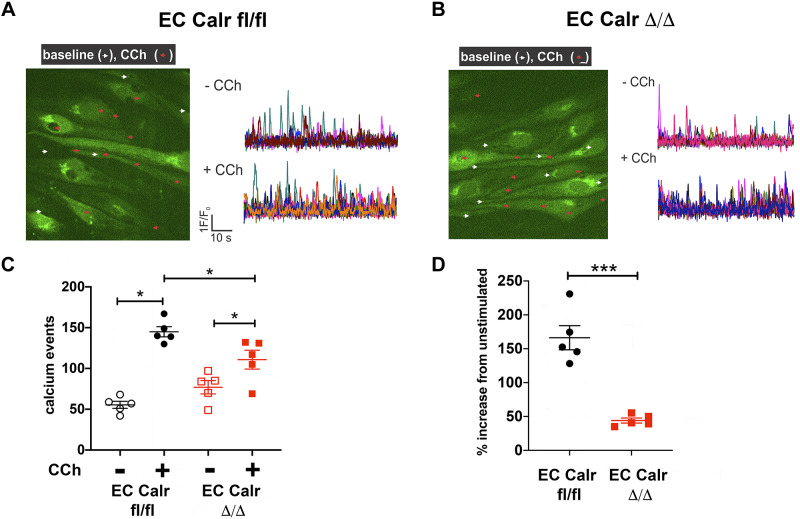
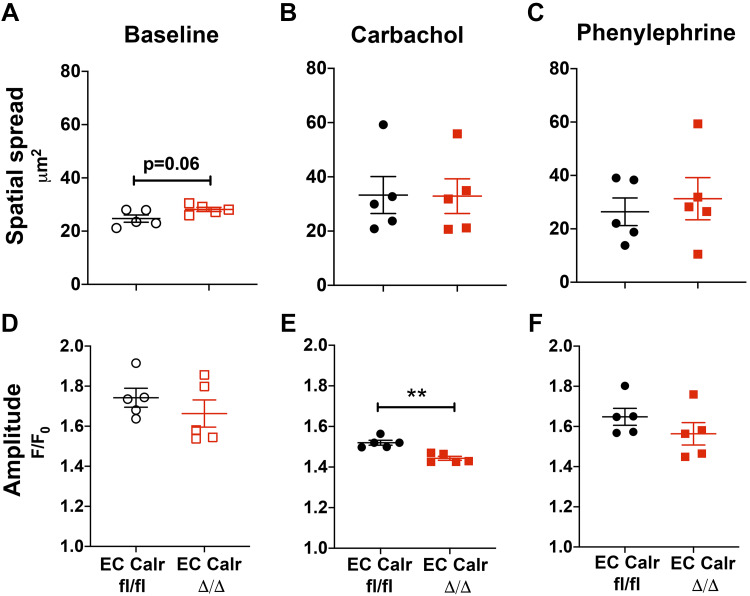
In young mice, EC Calr Δ/Δ did not affect calcium signals or dilation to carbachol (4), a muscarinic agonist and endothelial-dependent vasodilator similar to acetylcholine. Interestingly, the number of calcium events in response to CCh in aged mice triples (~50 to ~150 events/min), but in EC Calr Δ/Δ mice there is a significantly blunted calcium mobilization response (Fig. 4). To better understand the calcium signal properties, we quantified the average spatial spread of the calcium signals at baseline and in response to agonists. Spatial spread tended to be higher at baseline with EC Calr Δ/Δ but was unchanged with agonist addition (Fig. 5, A–C). The average fluorescence amplitude of the calcium signals in each artery was not different at baseline or with PE but was significantly decreased in EC Calr Δ/Δ upon CCh stimulation (Fig. 5, D–F).
Fig. 4.
Aged endothelial cell (EC) calreticulin (Calr) Δ/Δ arteries have fewer carbachol-stimulated calcium events. Representative images and calcium event traces of 3rd-order mesenteric arteries from aged EC Calr fl/fl (A) and Δ/Δ (B) male mice in an en face preparation. A: endothelial cells were loaded with Fluo-4AM (green) to visualize calcium release into the cytosol. Calcium events were imaged and quantified before (white arrows) and during 10μM carbachol (CCh) (red arrows). C: in aged arteries, CCh stimulation caused a significant increase in calcium events versus baseline (EC Calr fl/fl P < 0.0001, EC Calr Δ/Δ P = 0.006, 2 way ANOVA with Tukey’s multiple comparisons test). However, the magnitude of increase in CCh stimulated EC Calr Δ/Δ events was significantly lower than EC Calr fl/fl (P = 0.0058). D: the percent increase in CCh-stimulated calcium events was significantly lower in EC Calr Δ/Δ mice P = 0.001, unpaired t test; n = 5 arteries from 5 mice/group. *P < 0.05; ***P < 0.001.
Fig. 5.
Properties of endothelial calcium signals in aged endothelial cell (EC) calreticulin (Calr) Δ/Δ. Calcium events at baseline and in response to carbachol (CCh) or phenylephrine (PE) were recorded in internal elastic lamina (IEL) holes of en face mesenteric arteries. A–C: the average distance of all the calcium signals’ spread tends to be higher in EC Calr Δ/Δ at baseline, while relatively unchanged with addition of carbachol or phenylephrine. D: the average fluorescence amplitude of the calcium events occurring is not different at baseline. Amplitude is significantly lower in EC Calr Δ/Δ upon CCh stimulation (E) but not phenylephrine stimulation (F). **P = 0.0011, unpaired t test; n = 5 arteries from 5 mice per group.
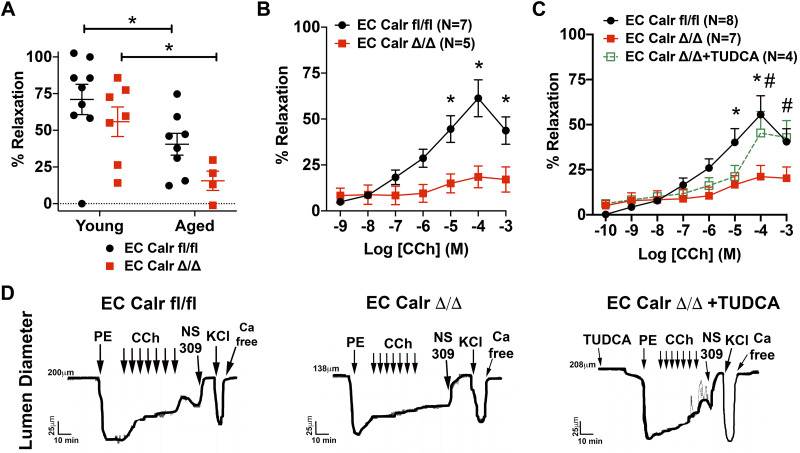
Aging impairs vasodilation and EC Calr Δ/Δ arteries have an exacerbated impairment that is restored upon acute ER stress inhibition with TUDCA.
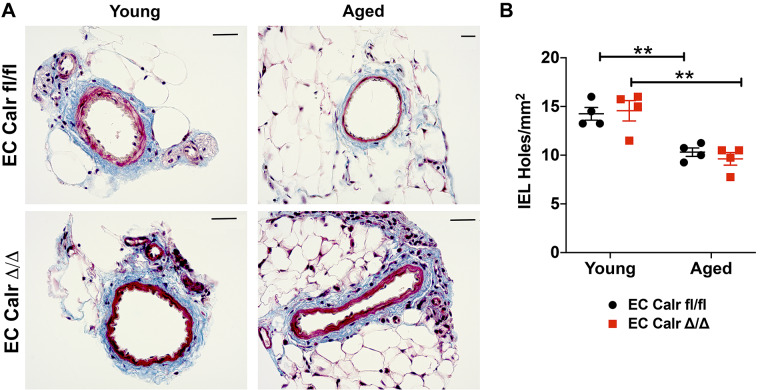
CCh-mediated calcium events lead to vasodilation. In aged mice, maximum dilation to 10 µM CCh was significantly impaired with aging in both groups (Fig. 6A). Aged EC Calr Δ/Δ mice had a significantly impaired dilation to CCh over a range of concentrations in comparison to their similarly aged counterparts (Fig. 6B). Because of the well-known role Calr plays in ER stress (17, 18), we hypothesized the diminished dilation to CCh in aged EC Calr Δ/Δ mice was due to increased ER stress. Acute ex vivo incubation of EC Calr Δ/Δ arteries with the ER stress inhibitor TUDCA rescued endothelial-mediated vasodilation to CCh to the level of aged EC Calr intact mice (Fig. 6C) over a range of concentrations. The effect of EC Calr deletion with aging is specific to CCh as it does not affect dilation or constriction to other commonly used dilatory or constrictive agonists (Fig. 7, A–D) or result in gross morphological differences between other aged arteries (Fig. 8, A and B). Thus calreticulin is an essential component to endothelial CCh calcium events and vasodilatory function in aging mice, possibly by mediating ER stress.
Fig. 6.
Aged endothelial cell (EC) calreticulin (Calr) Δ/Δ arteries have impaired endothelial-dependent vasodilation. A: maximimum vasodilation to 10 μM carbachol (CCh) is significantly blunted with aging (Young EC Calr fl/fl: n = 9 arteries from 9 mice; Young EC Calr Δ/Δ: n = 7 arteries from 7 mice; Aged EC Calr fl/fl: n = 8 arteries from 8 mice; Aged EC Calr Δ/Δ: n = 4 arteries from 4 mice). B: aging negatively impacts vasodilation in EC Calr Δ/Δ arteries (Aged EC Calr fl/fl: n = 7 arteries from 7 mice; Aged EC Calr Δ/Δ: n = 5 arteries from 5 mice; *P < 0.05, two-way ANOVA with Sidak’s multiple comparisons). C: incubation of aged EC Calr Δ/Δ arteries with 30 μM tauroursodeoxycholic acid (TUDCA) (n = 4) significantly improves dilation to CCh (*P < 0.05, Aged EC Calr fl/fl vs. Aged EC Calr del/del; #P < 0.05 Aged EC Calr Δ/Δ vs. Aged EC Calr Δ/Δ + TUDCA). Aged EC Calr fl/fl: n = 8 arteries from 8 mice; Aged EC Calr Δ/Δ: n = 7 arteries from 7 mice; Aged EC Calr Δ/Δ + TUDCA: n = 4 arteries from 4 mice. D: representative arterial lumen diameter traces that show vasoconstriction or vasodilation response to the agonists used.
Fig. 7.

Functional endothelial cells (ECs) and smooth muscle cells in ex vivo arteries from aged mice. Third-order mesenteric arteries were dissected free of fat and pressurized to 80 mmHg in an ex vivo pressure myograph; n = 6–8 arteries from 6 to 8 mice/group. A: dilation to 1 μM NS309 was intact and not different by genotype. B: both groups had equivalent constriction to 30mM KCl. C: before carbachol (CCh) dose responses, arteries were equivalently preconstricted with phenylephrine (PE). Calr, calreticulin D: before addition of agonists, initial myogenic tone at 80 mmHg was quantified.
Fig. 8.
Endothelial cell (EC) calreticulin (Calr) Δ/Δ resistance arteries are not morphologically different young or aged. A: representative trichrome collagen staining of third order mesenteric artery cross sections. Scale bar = 25 μm in each image. B: the number of internal elastic lamina (IEL) holes were quantified in 4 distinct regions of an en face 3rd-order mesenteric artery. Aging significantly decreased the number of IEL holes with no genotype effect (n = 4 arteries from 4 mice/group; **P < 0.01 via two-way ANOVA with post hoc test).
DISCUSSION
Here we have demonstrated that 1) vascular Calr expression decreases with age in mouse aorta and mesenteric arteries, 2) EC Calr deletion in aged arteries impairs PE and CCh-mediated calcium signals specifically at holes in the IEL where MEJ form, 3) aging decreases vasodilation to a maximum concentration of CCh, 4) EC Calr deletion in aged arteries further impairs vasodilation to a range of CCh concentrations, and 5) incubating aged EC Calr Δ/Δ arteries with TUDCA ex vivo improves CCh vasodilation. Therefore, we propose that deletion of EC Calr could be a model of accelerated vascular aging via endothelial dysfunction, specifically at the MEJ.
Expression of major ER localized chaperone proteins decreases with age (6), although it is not clear if this is a cause or consequence of aging. It remains to be determined which cell type is responsible for the decreased expression of arterial Calr with age, although SMCs are more numerous than ECs in the vascular wall (20, 22). We see a similar decrease of Calr in both large and small arteries, despite the lack of functional MEJ in IEL holes of aorta in comparison with mesenteric arteries (23). It is also possible that the cellular or subcellular localization of EC Calr could also be affected by aging and contributes to the observed dysfunction.
Our previous data suggest EC Calr is differentially localized in small mesenteric arteries (i.e., within the ER in the EC monolayer and outside the ER at the MEJ) and its deletion from the entire EC modulates calcium dynamics in the MEJ (4). These data also demonstrated selective calcium impairment as young arteries could mobilize calcium to CCh but not PE stimulation. The diminished EC calcium response to PE would be expected to result in increased constriction to PE. The PE vasoconstrictive response is not significantly different in EC Calr Δ/Δ mice compared with control in the aged mice. When compared with our previous work (4), the magnitude of PE constriction in control mice is increased ~10–15% with age. This observation is in line with a greater contractile response in aged arteries exhibiting EC dysfunction. In contrast to young arteries, we show here that EC Calr deletion impairs calcium mobilization in response to both agonists (PE and CCh) and this impairment is evident before advanced age. Our data suggest that this could be due to increased ER stress when EC Calr is not present.
Calr has a range of physiological functions including the folding of nascent proteins and glycoproteins, maintaining calcium homeostasis and calcium buffering (17). The chaperoning function of Calr is facilitated through its interaction with ERp57 (ER protein of 57 kDa) and calnexin, an ER integral membrane protein with high homology. Calr has three key functional domains: the N domain, P domain, and C domain that contribute to its multifunctional capabilities. In particular, the N and P domains are essential for the molecular chaperone activity of Calr (13). The P domain not only interacts with ERp57 but also contains a high-affinity, low-capacity calcium binding site (2, 7, 15). In contrast, the C domain contains low-affinity, high-capacity calcium binding sites which confers the calcium buffering function to Calr. Indeed, Calr can bind nearly 50% of the ER calcium content (19), thus making loss-of-function mutations detrimental to maintenance of ER calcium levels and agonist-induced calcium efflux (17). For the current studies, EC deletion of Calr resulted in an EC-specific decrease in protein expression and thus function of all three domains. Due to the role of Calr in numerous cellular activities, it is difficult to pinpoint the current findings to loss of one particular domain/function; however, the blunted calcium responses to CCh and PE in aged Calr-deficient mice would suggest that the loss of the C domain and its calcium buffering capacity might play an important role in the observed EC dysfunction. In young mice, an indirect measurement of EC ER calcium did not show differences between wild-type and EC Calr Δ/Δ ER calcium load (4). Analysis of arterial calcium events in aged mice showed smaller calcium event amplitudes in EC Calr Δ/Δ specifically in response to CCh. This attenuated calcium response could be an explanation for the observed impairment in vasodilation due to differences in IP3R function and calcium efflux from internal stores as TRPV4 channels were inhibited when performing calcium imaging experiments. ER calcium load was not directly measured in these studies, so the direct impact of EC Calr Δ/Δ and acute TUDCA incubation on ER calcium content remains to be determined.
Aging-induced EC dysfunction can be characterized by fewer active sites of calcium signals and reduced frequency and duration of these calcium events, combined with fewer arterial IEL holes (5). In our experimental preparation, the number of baseline calcium events was not different between genotypes. This suggests that EC Calr participates in calcium mobilization in response to agonists but does not affect spontaneous localized calcium release that occurs under basal conditions. The average amplitude of calcium events at baseline (Fig. 5D) is also independent of EC Calr expression. These findings are in line with our previous study of young mice; the presence of EC Calr does not appear to influence basal calcium dynamics and becomes more relevant upon specific agonist stimulation. Spontaneous calcium signaling in IEL holes may still be maintained at 15 mo of age and is not diminished until 24 mo as seen in another study (5), so it is possible that further aging of the mice could delineate a role for EC Calr in an unstimulated state. We chose to study mice at 75–85 wk of age (15 mo) as vascular Calr expression is decreased and EC Calr deletion already had a significant impact on calcium signaling and vascular function at this timepoint.
The impairment in carbachol-mediated dilation was not due to preconstriction or tone artifacts as we confirmed that preconstriction to PE was equivalent between genotypes (Fig. 7C). Arteries included in the experiments all 1) had PE constriction at least 50% of the maximum diameter of the vessel, 2) had lumen diameters approximately resistance artery size (<220 µm), and 3) had vessels fully relaxed to NS309, which works though endothelial-dependent vasodilatory mechanisms. This indicates that the vasodilatory reserve was not permanently affected by preconstriction. The diminished myogenic tone in this manuscript should not affect the ability to extrapolate these data because the concentration effect curves plot the percent maximal diameter. This calculation accounts for the maximum artery size as measured in calcium free buffer and not just the percentage of the diameter immediately preconstriction, which could differ depending on the amount of myogenic tone
Aberrant EC calcium signaling can trigger cell senescence that leads to apoptosis (12). TUDCA’s mechanism of action involves prevention of ER stress-mediated apoptosis by modulating intracellular calcium levels (1, 29). Indeed, one of our key findings was that acute TUDCA administration restored vasodilation in mice lacking EC Calr, which could be due to improved EC calcium mobilization. There is currently an early phase 1, double-blind, clinical trial approved to study the benefits of TUDCA supplementation on EC function in aged humans (NCT04001647; started June 2019). The primary outcomes to be measured include endothelial-dependent blood flow response to an acetylcholine concentration response before and after 8 wk of TUDCA or placebo and markers of ER stress in EC biopsies. A limitation of our study is the lack of calcium event quantification in the presence of TUDCA, but this is a compelling next step, particularly if TUDCA improves EC-dependent dilation in 60- to 80-yr-old humans and/or decreases markers of ER stress. This finding in humans would support our findings that inhibition of ER stress improves dysfunction in aged arteries and underscore the effectiveness of inhibiting ER stress to limit vascular aging.
Taken together, the presence of EC Calr is important in maintaining homeostatic calcium dynamics during the aging process and the decrease in endothelial Calr with aging may contribute to impaired vasodilation.
GRANTS
This work supported by National Heart, Lung, and Blood Institute Grants HL-088554 (to B.E.I.), HL-007284 (to L.A.B.), and HL-142808 (to S.K.S.) and American Diabetes Association Grant ADA 1-19-JDF-111 (to P.B.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.A.B., P.B., and B.E.I. conceived and designed research; L.A.B., H.R.A.-P., K.H., J.M., S.R.J., E.M., M.E.G., and S.S. performed experiments; L.A.B., H.R.A.-P., K.H., J.M., S.R.J., M.E.G., P.B., and S.S. analyzed data; L.A.B. and B.E.I. interpreted results of experiments; L.A.B. prepared figures; L.A.B. and B.E.I. drafted manuscript; L.A.B., M.E.G., P.B., S.S., and B.E.I. edited and revised manuscript; L.A.B., P.B., S.S., and B.E.I. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Nenja Krüger and Ulrich Ruther at Heinrich Heine University of Düsseldorf for assistance with quantitative PCR, University of Virginia Research Histology Core, and Sheri VanHoose for artery processing, sectioning, and staining of slides
REFERENCES
- 1.Amaral JD, Viana RJ, Ramalho RM, Steer CJ, Rodrigues CM. Bile acids: regulation of apoptosis by ursodeoxycholic acid. J Lipid Res 50: 1721–1734, 2009. doi: 10.1194/jlr.R900011-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baksh S, Michalak M. Expression of calreticulin in Escherichia coli and identification of its Ca2+ binding domains. J Biol Chem 266: 21458–21465, 1991. [PubMed] [Google Scholar]
- 3.Bernard-Marissal N, Moumen A, Sunyach C, Pellegrino C, Dudley K, Henderson CE, Raoul C, Pettmann B. Reduced calreticulin levels link endoplasmic reticulum stress and Fas-triggered cell death in motoneurons vulnerable to ALS. J Neurosci 32: 4901–4912, 2012. doi: 10.1523/JNEUROSCI.5431-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biwer LA, Good ME, Hong K, Patel RK, Agrawal N, Looft-Wilson R, Sonkusare SK, Isakson BE. Non-endoplasmic reticulum-based calr (calreticulin) can coordinate heterocellular calcium signaling and vascular function. Arterioscler Thromb Vasc Biol 38: 120–130, 2018. doi: 10.1161/ATVBAHA.117.309886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boerman EM, Everhart JE, Segal SS. Advanced age decreases local calcium signaling in endothelium of mouse mesenteric arteries in vivo. Am J Physiol Heart Circ Physiol 310: H1091–H1096, 2016. doi: 10.1152/ajpheart.00038.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson RR, Dunning LM, Holtzman JL. The effect of aging on the chaperone concentrations in the hepatic, endoplasmic reticulum of male rats: the possible role of protein misfolding due to the loss of chaperones in the decline in physiological function seen with age. J Gerontol A Biol Sci Med Sci 61: 435–443, 2006. doi: 10.1093/gerona/61.5.435. [DOI] [PubMed] [Google Scholar]
- 7.Frickel EM, Riek R, Jelesarov I, Helenius A, Wuthrich K, Ellgaard L. TROSY-NMR reveals interaction between ERp57 and the tip of the calreticulin P-domain. Proc Natl Acad Sci USA 99: 1954–1959, 2002. doi: 10.1073/pnas.042699099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garland CJ, Bagher P, Powell C, Ye X, Lemmey HA, Borysova L, Dora KA. Voltage-dependent Ca2+ entry into smooth muscle during contraction promotes endothelium-mediated feedback vasodilation in arterioles. Sci Signal 10: eaal3806, 2017. doi: 10.1126/scisignal.aal3806. [DOI] [PubMed] [Google Scholar]
- 9.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension 27: 849–853, 1996. doi: 10.1161/01.HYP.27.4.849. [DOI] [PubMed] [Google Scholar]
- 10.Gold LI, Eggleton P, Sweetwyne MT, Van Duyn LB, Greives MR, Naylor SM, Michalak M, Murphy-Ullrich JE. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB J 24: 665–683, 2010. doi: 10.1096/fj.09-145482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong K, Cope EL, DeLalio LJ, Marziano C, Isakson BE, Sonkusare SK. TRPV4 (transient receptor potential vanilloid 4) channel-dependent negative feedback mechanism regulates Gq protein-coupled receptor-induced vasoconstriction. Arterioscler Thromb Vasc Biol 38: 542–554, 2018. doi: 10.1161/ATVBAHA.117.310038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia G, Aroor AR, Jia C, Sowers JR. Endothelial cell senescence in aging-related vascular dysfunction. Biochim Biophys Acta Mol Basis Dis 1865: 1802–1809, 2019. doi: 10.1016/j.bbadis.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Leach MR, Cohen-Doyle MF, Thomas DY, Williams DB. Localization of the lectin, ERp57 binding, and polypeptide binding sites of calnexin and calreticulin. J Biol Chem 277: 29686–29697, 2002. doi: 10.1074/jbc.M202405200. [DOI] [PubMed] [Google Scholar]
- 14.Lind L, Berglund L, Larsson A, Sundström J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation 123: 1545–1551, 2011. doi: 10.1161/CIRCULATIONAHA.110.984047. [DOI] [PubMed] [Google Scholar]
- 15.Martin V, Groenendyk J, Steiner SS, Guo L, Dabrowska M, Parker JM, Müller-Esterl W, Opas M, Michalak M. Identification by mutational analysis of amino acid residues essential in the chaperone function of calreticulin. J Biol Chem 281: 2338–2346, 2006. doi: 10.1074/jbc.M508302200. [DOI] [PubMed] [Google Scholar]
- 16.Michalak M, Corbett EF, Mesaeli N, Nakamura K, Opas M. Calreticulin: one protein, one gene, many functions. Biochem J 344: 281–292, 1999. doi: 10.1042/bj3440281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J 417: 651–666, 2009. doi: 10.1042/BJ20081847. [DOI] [PubMed] [Google Scholar]
- 18.Naidoo N. ER and aging-Protein folding and the ER stress response. Ageing Res Rev 8: 150–159, 2009. doi: 10.1016/j.arr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura K, Zuppini A, Arnaudeau S, Lynch J, Ahsan I, Krause R, Papp S, De Smedt H, Parys JB, Muller-Esterl W, Lew DP, Krause KH, Demaurex N, Opas M, Michalak M. Functional specialization of calreticulin domains. J Cell Biol 154: 961–972, 2001. doi: 10.1083/jcb.200102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norton CE, Sinkler SY, Jacobsen NL, Segal SS. Advanced age protects resistance arteries of mouse skeletal muscle from oxidative stress through attenuating apoptosis induced by hydrogen peroxide. J Physiol 597: 3801–3816, 2019. doi: 10.1113/JP278255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ottolini M, Hong K, Sonkusare SK. Calcium signals that determine vascular resistance. Wiley Interdiscip Rev Syst Biol Med 11: e1448, 2019. doi: 10.1002/wsbm.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandow SL, Looft-Wilson R, Doran B, Grayson TH, Segal SS, Hill CE. Expression of homocellular and heterocellular gap junctions in hamster arterioles and feed arteries. Cardiovasc Res 60: 643–653, 2003. doi: 10.1016/j.cardiores.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Sandow SL, Senadheera S, Bertrand PP, Murphy TV, Tare M. Myoendothelial contacts, gap junctions, and microdomains: anatomical links to function? Microcirculation 19: 403–415, 2012. doi: 10.1111/j.1549-8719.2011.00146.x. [DOI] [PubMed] [Google Scholar]
- 24.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonkusare SK, Dalsgaard T, Bonev AD, Hill-Eubanks DC, Kotlikoff MI, Scott JD, Santana LF, Nelson MT. AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Sci Signal 7: ra66, 2014. doi: 10.1126/scisignal.2005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stahon KE, Bastian C, Griffith S, Kidd GJ, Brunet S, Baltan S. Age-related changes in axonal and mitochondrial ultrastructure and function in white matter. J Neurosci 36: 9990–10001, 2016. doi: 10.1523/JNEUROSCI.1316-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Straub AC, Zeigler AC, Isakson BE. The myoendothelial junction: connections that deliver the message. Physiology (Bethesda) 29: 242–249, 2014. doi: 10.1152/physiol.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor MS, Francis M. Decoding dynamic Ca(2+) signaling in the vascular endothelium. Front Physiol 5: 447, 2014. doi: 10.3389/fphys.2014.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vang S, Longley K, Steer CJ, Low WC. The Unexpected Uses of Urso- and Tauroursodeoxycholic Acid in the Treatment of Non-liver Diseases. Glob Adv Health Med 3: 58–69, 2014. doi: 10.7453/gahmj.2014.017. [DOI] [PMC free article] [PubMed] [Google Scholar]