Abstract
Noncoding RNAs (ncRNAs) are broadly described as RNA molecules that are not translated into protein. The investigation of dysregulated ncRNAs in human diseases such as cancer, neurological, and cardiovascular diseases has been under way for well over a decade. Micro-RNAs and long noncoding RNAs (lncRNAs) are the best characterized ncRNAs. These ncRNAs can have profound effects on the regulation of gene expression during cardiac development and disease. Importantly, ncRNAs are significant regulators of gene expression in several congenital heart diseases and can positively or negatively impact cardiovascular development. In this review, we focus on literature involving micro-RNAs and lncRNAs in the context of pediatric cardiovascular diseases, preclinical models of heart failure, and cardiac development.
Keywords: cardiac development, heart failure, long noncoding RNA, micro-RNA, pediatric
INTRODUCTION
In the last 20 years, several studies have demonstrated the importance of noncoding RNA (ncRNA) in the regulation of gene expression in health and disease. Micro-RNAs (miRNAs, miRs) were one of the first characterized ncRNAs with broad biological significance in several species. Since then, other ncRNAs have been discovered, including long noncoding RNAs (lncRNAs) and circular RNAs. miRNAs and lncRNAs are the best characterized of the ncRNAs. Several studies have shown that expression of these ncRNAs is dysregulated in several diseases, including various types of cancers and neurological and cardiovascular diseases. In addition, ncRNAs have profound roles in organogenesis. This mini-review will focus on the role of miRNAs and lncRNAs in cardiac development and pediatric heart failure, including congenital heart disease (CHD), and on recent studies demonstrating the complex regulatory role of ncRNAs in normal and abnormal cardiac development. A summary of these processes and targets is described in Table 1.
Table 1.
Summary of ncRNAs dysregulated in cardiac development and pediatric heart failure
| ncRNA | Study Model | Expression | Potential Targets/Interactors | Cardiac Effect | Study Author and Year | Ref. No. |
|---|---|---|---|---|---|---|
| miR-19a | Embryonic zebrafish | Downregulated | S1PR1 | Promote cardiac developmental defects | Guzzolino et al. 2018 | 9 |
| miR-9 | Cardiac tissue of children with CHD; zebrafish; cultured HEK-293T cells | Upregulated | TBX5 rs6489956 | Affects transcription activity of TBX5 rs6489956 | Wang et al. 2017 | 41 |
| miR-30a | Cardiac tissue of children with CHD; zebrafish; cultured HEK-293T cells | Upregulated | TBX5 rs6489956 | Affects translational activity of TBX5 rs6489956 | Wang et al. 2017 | 41 |
| miR-199b | Ovine RVH model | Downregulated | Dyrk1a | Decreases NFAT transcription activity | Kameny et al. 2018 | 10 |
| miR-29a | Ovine RVH model | Upregulated | Col1A and Col3A1 | Antifibrotic effect | Kameny et al. 2018 | 10 |
| miR-199a-5p | Children with cyanotic CHD and acyanotic CHD; cultured human myocardial cells | Downregulated | GRP78 and ATF6 | Myocardial protection during hypoxia | Zhou et al. 2017 | 48 |
| CAREL | Mice; mice cells; iPS cells; HEK-293 cells | Increased | miR-296, various other miRNAs predicted | Diminished cardiac repair | Cai et al. 2018 | 3 |
| Ppp1r1b | Mice; C2C12 cells; children with TOF | Inverse relationship | Tcap, other mRNAs | Congenital heart defects | Touma et al. 2016 | 37 |
| HA117 | Children with TOF | Increased | Unknown | Lower ; increased duration cardiopulmonary bypass; lower McGoon’s ratio; lower Nakata index; lower LVEDVI and LVEF | Wang et al. 2018 | 43 |
| EGOT | H9c2 cells | Increased | Cyclin D1, other mRNAs | Increased cardioprotection against hypoxia | Zhang et al. 2018 | 46 |
| ENSMUST00000117266 | Mice | Decreased | Multiple mRNAs | Decreased cardiomyocyte proliferative activity; cardiac development | Sun et al. 2017 | 34 |
ATF6, activating transcription factor 6; CAREL, cardiac regeneration-related lncRNA; CHD, congenital heart disease; Col1A, collagen type 1A1; Col3A1, collagen type 3A1; Dyrk1a, dual-specificity tyrosine phosphorylation-regulated kinase 1a; EGOT, eosinophil granule ontogeny transcript; GRP78, glucose-regulated protein; HEK-293T; human embryonic kidney-293T; iPS, induced pluripotent stem cells; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; miRNA, micro-RNA; NFAT, nuclear factor of activated cell; RVH, right ventricular hypertrophy; , peripheral capillary oxygen saturation; S1PR1, sphingosine-1-phosphate receptor; TBX5, T-box transcription factor; Tcap, Titin-Cap; TOF, Tetralogy of Fallot.
miRNAs
miRNAs are regulatory molecules consisting of ~22 noncoding nucleotides that regulate gene expression by targeting complementary sequences present mainly in the 3′-untranslated region (3′-UTR) of messenger RNAs (mRNAs), resulting in mRNA degradation or translational repression of targeted transcripts (1) (see Fig. 1 for overview). Transcription of miRNAs is mostly mediated by RNA polymerase II (RNA pol II) to form pri-miRNAs in the nucleus, which are subsequently cleaved by a protein complex called Drosha into pre-miRNA. Exportin 5 recognizes pre-miRNAs in the nucleus and transports them to the cytoplasm, where they are further processed by the endonuclease Dicer into their double-stranded structure. The mature miRNAs are loaded into RNA-induced silencing complex (RISC). RISC contains a specific protein, Argonaute 2 (AGO2), which orchestrates the interaction of the mature miRNA with its target mRNA (40, 44, 49). Several studies have shown that miRNAs are critical components of the cardiogenic regulatory network and play crucial roles in the proliferation, differentiation, and morphogenesis of the developing heart (reviewed in Ref. 44), as well as onset and progression of heart failure (reviewed in Ref. 39). The biological consequence of dysregulation of these miRNAs and their cardiac-specific targets is summarized here.
Fig. 1.
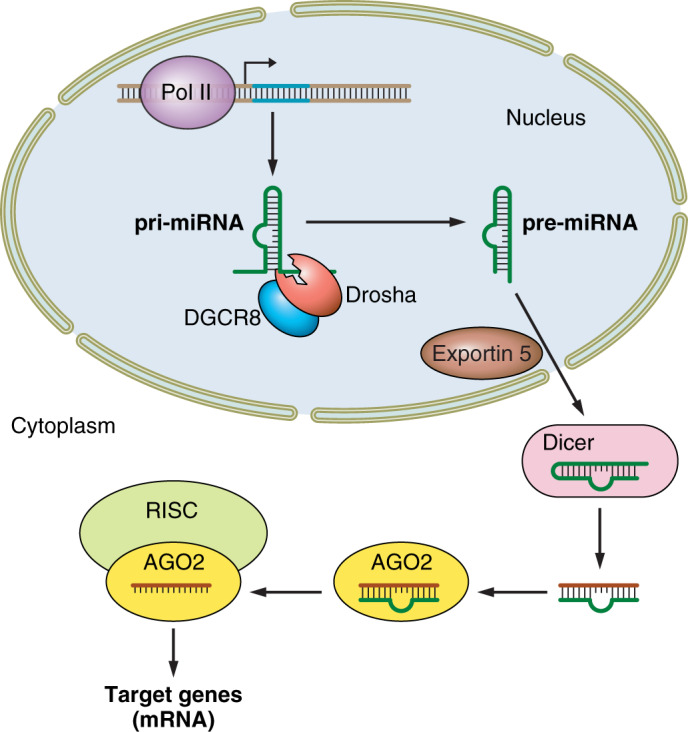
Micro-RNA (miRNA) transcription, processing, and function. Pri-miRNAs are transcribed from DNA by RNA polymerase II and processed to pre-miRNA by double-stranded RNA-specific endoribonuclease (Drosha) and DGCR8 microprocessor complex subunit (DGCR8). Exportin 5 exports the pre-miRNA to the cytoplasm, where the hairpin is removed by double-stranded RNA-specific endoribonuclease (Dicer). Following association with Argonaute 2 (AGO2), the miRNA duplex is cleaved to a single strand and becomes part of the RNA-induced silencing complex (RISC) complex, which can degrade mRNA targets and/or prevent translation.
miRNAs in Congenital Heart Disease
Congenital heart disease (CHD) is one of the most common birth defects in newborns (38). It refers to a range of possible heart anomalies, including septal defects, valve defects, and outflow tract anomalies. CHD can be due to genetic, epigenetic, and environmental factors (22). Several miRNAs are involved in cardiac development, and altered expression of specific miRNAs is associated with CHD and other cardiac development abnormalities (reviewed in Ref. 36). This review will focus on recent studies showing the relationship between miRNAs and pathological changes in response to CHD caused by T-box transcription factor 5 (TBX5) loss of function [Holt Oram syndrome (HOS)], cyanotic heart disease, and in response to maternal diabetes.
TBX5 Loss of Function Negatively Affects miRNA Expression, Which Contributes to the Development of CHD
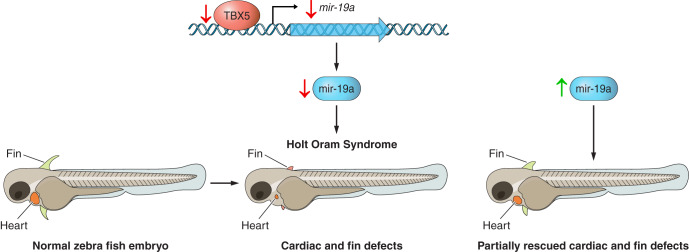
miRNA-19a levels are decreased in Holt Oram syndrome (HOS), a CHD characterized by cardiac and upper limb deformities due to a loss-of-function mutation in the TBX5 gene, a transcription factor involved in cardiac and limb development (16). Congestive heart diseases, such as atrial septal defect (ASD) and ventricular septal defect (VSD), are observed in 75% of HOS patients. A recent study in a HOS embryonic zebrafish model with cardiac and pectoral fin defects due to knockdown of TBX5 gene expression showed downregulation of miR-19a and that miR-19a expression was directly regulated by TBX5. The cardiac and fin defect observed in this model were partially rescued by replacing miR-19a (5) (see Fig. 2). A subsequent study by the same authors showed that cardiac defects due to downregulation of miR-19a resulted in upregulation of the sphingosine-1-phosphate receptor (S1PR1), and that downregulation of S1PR1 can partially rescue the detrimental effects of TBX5 loss of function (9). These data suggest that miR-19a might be used as a novel diagnostic and therapeutic tool for CHD-related birth deformities.
Fig. 2.
T-box transcription factor 5 (TBX5) and miR-19 in Holt Oram syndrome. Downregulation of the TBX5 gene negatively affects expression of miR-19a and causes cardiac and fin defects in a zebrafish model of Holt Oram syndrome. Replacement of miR-19a partially rescued the cardiac and fin defects observed due to downregulation of the TBX5 gene.
Similarly, another study in children from a Han Chinese population with CHD showed that miR-9 and miR-30a can differentially regulate expression of TBX5 based on variants identified in the 3′-UTR of the gene. In this study, the presence of variant rs6489956 (c.*1101C>T) on the 3′-UTR of TBX5 was significantly associated with increased CHD susceptibility in 1,177 CHD patients compared with 990 subjects in the control group. Allele analysis of three genotypes (TT, CT, and CC) showed reduced TBX5 mRNA expression and protein levels in cardiac tissue, as well as in cultured cells associated with the T allele. miR-9 and miR-30a were identified as regulators of TBX5 expression through the rs6489956 variant, both in vivo and in vitro at the transcriptional and translational level, respectively. Moreover, although TBX5 rs6489956 was associated with different types of CHD, the highest statistically significant difference was observed in patients with septal defect (ASD and VSD) (41). These data suggest that polymorphisms in the 3′-UTR of genes involved in cardiac development, such as TBX5, can affect their expression, resulting in CHD.
Dysregulation of miRNAs in Congenital Heart Disease Promotes Myocardial Protection
A recent study showed that patients with cyanotic congenital heart defect have reduced cardiac expression of miR-199a-5p compared with acyanotic congenital heart defect patients. Downregulation of miR-199a-5p leads to an increase in expression of glucose-regulated protein, 78 kDa (GRP78), and activating transcription factor 6 (ATF6), the two major genes expressed in response to endoplasmic reticulum stress. These genes modulate the unfolded protein response (UPR) during hypoxia. This suggests that downregulation of miR-199a-5p is involved in myocardial protection during hypoxia by favoring the expression of UPR-related genes and promotes a novel adaptative mechanism in response to chronic hypoxia in patients with cyanotic CHD. Furthermore, reduced expression of miR-199a-5p was also observed in human myocardial cells cultured in hypoxic conditions compared with cells cultured in normoxic conditions. The authors proposed that miR-199a-5p may be used as a potential clinical diagnostic and therapeutic target for the protection of cardiomyocytes following hypoxia (48).
A study in an ovine model of CHD evaluated expression of miRNAs in adaptive right ventricular hypertrophy (RVH) in response to pulmonary arterial hypertension. miR-199b and miR-29a were differentially expressed in adaptive RVH compared with maladaptive RVH. There was decreased expression of miR-199b, which resulted in increased expression of its direct target gene (Dyrk1a). This resulted in eventual reduction in nuclear factor of activated cell (NFAT)/calcineurin signaling in the right ventricular (RV) tissue of adaptive RVH (10). In contrast, in maladaptive RVH, an increase in the expression of this miR with activation of the NFAT/calcineurin pathway is observed (28). Different than what is seen with miR-199b, expression of miR-29a was increased in adaptive RV, but not in maladaptive. Increased miR-29 lead to reduction of its targets (collagen type A1 and type 3A1) with decreased fibrosis in adaptive RVH, suggesting that the regulation of miRNA and gene expression required for adaptive RVH is distinct from that for maladaptive RVH. These results suggest that miRNAs may serve as a prognostic and therapeutic tool in preventing pathological remodeling of the RV (10).
miRNAs in Cardiac Development Defects Due to Maternal Diabetes
Previous studies have shown that pre-gestational maternal diabetes mellitus (PGDM) (type 1 and type 2) can induce an increase in the risk of birth defects, including CHD (23). In addition to morphological deformities, maternal diabetes can significantly reduce systolic function in the developing heart (32). Hyperglycemia generates high levels of cellular oxidative stress, leading to excessive apoptosis in the developing heart (45) and altering the expression of genes essential for proper cardiac development (24). However, the role of PGDM factors, other than hyperglycemia, in the development of CHD remains largely unknown (32). In a recent study, a diabetic mouse model was used to investigate dysregulation of miRNAs in embryonic heart tissue following oxidative stress in maternal PGDM. miRNA array revealed altered expression profile of 149 miRNAs in the embryonic heart tissue from diabetic mothers compared with control animals. Increased expression of miR-142-3p, miR-144-3p, and miR-448-3p, and decreased expression of miR-322-5p, miR-27a-3p, and miR-181b-5p in developing hearts exposed to maternal diabetes was confirmed by RT-PCR. Furthermore, 2,111 genes were predicted targets of the dysregulated miRNAs, of which 284 are transcription regulators. Transcription factors like Cited2, Zeb2, Mef2c, Smad4, and Ets1 were found to be targeted by PDGM-altered miRNAs. Prediction analysis showed miRNAs altered in the developing heart due to maternal diabetes are involved in cardiac development pathways (STAT3 and IGF-I). Furthermore, the authors crossed diabetic female animals with transgenic male mice overexpressing the antioxidant enzyme superoxide dismutase 1 (SOD1) to generate SOD1-overexpressing embryo in diabetic and nondiabetic conditions. Expression of miRNAs and transcription factors dysregulated in cardiac tissue of embryos exposed to maternal diabetes was normalized in the SOD1-overexpressing embryos, suggesting that the observed altered expression of miRNAs and embryonic cardiac development deformities following maternal diabetes could be due to oxidative stress (6).
Similarly, Shi et al. (32) generated a diabetic mouse model to study the effect of maternal exosomal miRNA in the development of embryonic heart tissue exposed to maternal PGDM. Decreased fertility rate, increased still birth and fetal absorption, as well as morphological deformities in postnatal animals were observed in the diabetic group compared with the normal control group. In addition, histological results of the embryonic heart tissue revealed myocardial hypertrophy, ventricular hypoplasia, and VSD. The authors analyzed the expression of maternal exosomes, and, although there were no changes in exosomal protein levels, 218 exosomal miRNAs (126 upregulated and 92 downregulated) were significantly different in the exosomes from diabetic mice. Out of these miRNAs, six upregulated miRNAs (miR-122-5p, -192-5p, -99a-5p, -328-3p, -423-3p, and -133a-5p), and seven downregulated miRNAs (miR-423-5p, -23a-3p, -320-3p, -146a-5p, -221-3p, -30c-5p, and -350-3p) showed highly statistically significant differences between the two groups. Maternal exosomes were observed in fetal tissues, including heart and placenta, confirming that exosomes can cross the maternal fetal barrier. Furthermore, the authors injected normal pregnant mice with exosomes extracted from diabetic pregnant mice via tail vein at different embryonic stages, and the cardiac development deformities observed in the diabetic pregnant mice were recapitulated. The authors suggested that miR-133, miR-30, miR-99, and miR-23 could be the main contributors to the abnormal cardiac developments observed in the fetus of diabetic mice since they are known to be involved in cardiac development (32).
lncRNAs
lncRNAs are a class of ncRNAs that are defined by two major distinguishing features: 1) lack of translation into protein, and 2) length > 200 base pairs. Currently, lncRNAs are viewed as a regulatory molecule with emerging importance in human diseases and development (12, 14, 31). In addition to being a relatively recent discovery, some unique traits of lncRNAs have made them notoriously difficult to study, such as being relatively low in abundance and possessing long stretches of nucleotide sequence that are not well conserved across species (18, 19, 26). As such, the exact mechanisms by which lncRNAs exert their regulatory action, and the full spectrum of human diseases in which they play a role, are still being elucidated.
lncRNAs Modulate Gene Expression Through Diverse Mechanisms
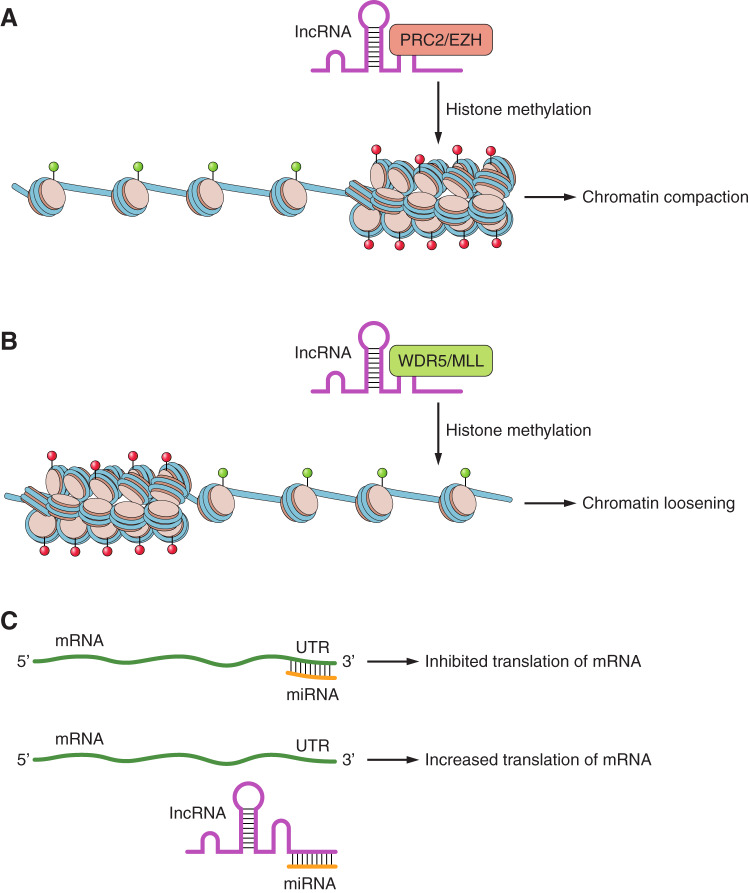
Several mechanisms are involved in lncRNA-mediated changes in gene expression, and an in-depth examination of their function is outside the scope of this review. However, below we will highlight several high-profile studies that elucidated how lncRNAs exert control of gene expression in general and in cardiac biology. Broadly, lncRNAs are known to affect gene expression by acting as cis- or trans-acting regulators via interactions with other chromatin-modifying proteins (17) (see Fig. 3 for overview). lncRNAs can, through diverse epigenetic mechanisms, suppress gene expression, as in the case of the Xist lncRNA, or promote gene expression, as in the case of HOTTIP-regulation of homeobox (HOX).
Fig. 3.
Long noncoding RNAs (lncRNAs) influence chromatin state and gene expression. lncRNAs recruitment and association with a histone (blue) tail (yellow) modifying enzyme that deposits repressive histone 3 trimethylation of lysine 27 marks (H3K27me3; red), resulting in chromatin compaction (A), or deposits activating histone 3 trimethylation of lysine 4 marks (H3K4me3; green; B). C: presence of a lncRNA acting as a competing endogenous RNA (ceRNA) binding factor, which can sponge microRNAs (miRNAs) and increase translation of miRNA targets. MLL, mixed-lineage leukemia; PRC2, polycomb repressor 2 complex.
Xist silencing of the X chromosome (XC) is a well-recognized mechanism of cis-regulated lncRNA mediated-silencing (7). During X-chromosome inactivation (XCI), Xist uses its structural conformation, rather than sequence complementarity, to spread across the XC. As XCI progresses, Xist’s RNA domains complex with the polycomb repressor 2 complex (PRC2) and act in concert to enrich chromatin with the repressive chromatin modification histone 3 lysine 27 trimethylation (H3K27me3), resulting in silenced chromatin (7).
HOX genes exist in clusters across several chromosomes and are well known governors of embryonic development and organogenesis (13, 25). The lncRNA HOTTIP acts in a mechanistically similar manner, but instead activates gene expression by recruiting the methyltransferase complex WDR5-mixed-lineage leukemia (MLL), which enriches the chromatin landscape proximal to the HOXA locus with histone 3 lysine 4 trimethylation (H3K4me3), resulting in transcriptional poise (42). Interestingly, the HOXC locus is also associated with the HOTAIR lncRNA. HOTAIR was the first lncRNA discovered to act in a trans-regulatory manner, albeit its regulation of gene expression is mechanistically similar to the example of Xist-mediated gene silencing. HOTAIR associates with several histone-modulating proteins and complexes, including PRC2, resulting in increased enrichment of H3K27me3 and the subsequent silencing of the HOXD cluster of genes, which reside on a different chromosome (29).
Finally, lncRNAs can affect gene expression by acting as a competing endogenous RNA molecules (ceRNA), effectively becoming miRNA “sponges” (35). Through this mechanism, lncRNAs bind to miRNAs and sequester them, limiting their functional availability of miRNAs and preventing their function. There are several examples of lncRNAs acting as sponge in the heart and affecting several processes, including fibrosis and hypertrophy.
lncRNAs Are Essential Members of Regulatory Gene Networks in Cardiac Development
Several seminal studies in mice exemplify the importance of lncRNAs as potent modulators of gene expression and illustrate their roles within larger regulatory networks. The Braveheart lncRNA was shown to be an important upstream activator of mesoderm posterior 1 (Mesp1). Mesp1 is a critically important gene, and a central member of a larger functionally similar regulatory gene network that is required to promote the differentiation of embryonic pre-cardiac mesoderm into cardiovascular cells (12). The lncRNA Fendrr has been shown to control development of the heart and its walls via the anchoring of epigenetic landscape-modifying factors, such as polycomb repressor complex 2 (PRC2) and trithorax group (TrxG)/MLL to promoters of lateral plate mesoderm genes. Collectively with Fendrr, the sustained positioning of PRC2 and TrxG/MLL, whose functions are mediated via the recruitment of gene-silencing H3K27me3 and gene-activating H3K4me3, exert transcriptional control of mesoderm differentiation and constitute a major driver of cardiac development (8).
Hand2 is a transcription factor that has a critical role in the development of cardiac ventricles. For transcription by RNA Pol II, Hand2’s promoter requires sustained acetylation, supported by the binding of both the lncRNA Upperhand (Uph) and the GATA4 transcription factor (2). In addition, Hand2’s expression also requires that Uph be cotranscribed in cis, solidifying the role of Uph in cardiac development. Taken together, several lncRNAs have been identified as important regulatory elements in cardiac development, and their identification represents a significant milestone in the field.
lncRNAs Are Differentially Expressed During Development and Hypothesized to Influence Cardiac Regeneration and Repair
Neonatal mice are capable of regenerating cardiac tissue in the first 6 days of life (P1–P6), including after injury (27); however, the capacity is lost by the 7th day (P7). A study by Chen et al. (4) examining the regenerative capacity of the heart in neonatal mice determined that 685 lncRNAs and 1,833 mRNAs were significantly different when comparing P1 versus P6 mice. To investigate what may be cis-regulated by significantly differently expressed lncRNAs, an analysis was conducted on the transcripts that originate proximally to lncRNA origins. Among the resulting pathways, metabolic pathway-related genes were significantly altered when comparing regeneration-capable P1 versus regeneration-incapable P7 hearts. Additionally, phosphatidylinositol 3-kinase (PI3K)-Akt, which are involved in cardio-proliferation, and pathways known to be important to cardiac regeneration, such as Hippo and Wnt signaling, were also enriched, as were cAMP, calcium signaling, cGMP-PKG, and adrenergic signaling. Although not mechanistic, these coexpression data suggest that lncRNA regulation of mRNAs may play a significant role in the development of the mammalian heart and govern cardiac regeneration.
Cai et al. (3) investigated whether experimental manipulation of lncRNAs can regulate the capacity of cardiac regeneration in a mouse model of myocardial infarction (MI). Similar to the aforementioned studies, investigators compared the significantly different lncRNAs in developing mice at P1 versus P7 and identified 128 significantly differentially expressed lncRNAs with more than a twofold change. A small subset of eight lncRNAs were identified as being highly enriched (>5-fold), with one lncRNA found to be expressed in the cytoplasm of cardiomyocytes, referred to as cardiac regeneration-related lncRNA (CAREL). Both CAREL transgenic and adenovirus overexpressor mice were used to examine the effect of increased cardiac CAREL. In young mice, overexpression of CAREL through either methodology resulted in lower capacity to repair cardiac damage after apical resection compared with wild-type cohorts, with transgenic mice also displaying decreased cardiac function as measured by echo. When CAREL levels were decreased by adenoviral shRNA (Ad-shCAREL) before MI induction, both young and adult mice displayed improved ejection fraction, improved fractional shortening, increased cardiomyocyte proliferation, and enhanced cardiac regeneration. Additionally, it was bioinformatically predicted and experimentally validated that CAREL has a binding site for miR-296 and was shown to act as a ceRNA. The effects of miR-296 depletion via antagomiR abolished the proliferation effect of Ad-shCAREL. Furthermore, investigators also found that expression of the human conserved sequence of CAREL (chCAREL) in 1) human induced pluripotent stem cells (hiPS), 2) AC16 human cardiac cell line, and 3) induced pluripotent stem cell-derived cardiac progenitor cells recapitulated the attenuation of cardiomyocyte proliferation seen in mice. Furthermore, investigators knocked down chCAREL in hiPS-derived cardiomyocytes and observed increased cellular proliferation. These data suggest that CAREL and miR-296 comprise a novel regulatory axis that modulates cardiac proliferation and repair.
Sun et al. (34) conducted a profiling study of lncRNAs expressed in the heart of mice at P1, P7, and P28. These authors identified 765 and 4,855 significantly different lncRNAs between P1 versus P7, and P7 versus P28, respectively. Based on validation experiments and coexpression analyses, the authors further investigated the highly expressed ENSMUST00000117266 lncRNA, which was hypothesized to be a regulator of cardiac development. siRNA knockdown of ENSMUST00000117266 in neonatal mouse cardiomyocytes resulted in a significant increase in the number of cells in the G0/G1 phase and a decrease in the number of cells in the G2/M phase of the cell cycle, suggesting that this lncRNA plays a role in regulating cardiomyocyte development and proliferation.
lncRNAs Are Involved in and Modulate the Severity of Congenital Heart Diseases
As the name implies, individuals with the CHD Tetralogy of Fallot (TOF) display a tetrad of abnormal cardiac morphology that includes 1) a VSD, 2) pulmonary valve stenosis, 3) overriding aorta, and 4) RVH (33). Nearly all patients also display cellular dysplasia of the RV outflow tract (RVOT), which includes the pulmonary artery. Two prognostic predictors of TOF outcome are McGoon’s ratio and the Nakata index, which aim to describe the adequacy of the pulmonary artery (adequacy positively correlating with increasing values). Wang et al. (43) examined the association between the expression of the lncRNA HA117 in tissue samples from the RVOT of TOF patients and the clinical manifestation and prognosis of TOF using the 1) Nakata index, 2) McGoon’s ratio, 3) left ventricular (LV) ejection fraction (LVEF), and 4) LV end-diastolic volume index (LVEDVI). Their findings indicate that patients with high expression of HA117 displayed a lower McGoon’s ratio, Nakata index, LVEDVI, and LVEF relative to patients with low HA117 expression. Furthermore, higher values of the prognostic indexes were negatively correlated with HA117 expression. Examination of clinical perioperative data also revealed higher expression of HA117 associated with long duration of cardiopulmonary bypass and intensive care unit stay. An examination of postoperative data 6 mo after surgery showed patients with lower expression of HA117 displayed increased peripheral capillary oxygen saturation () compared with higher expression of HA117, which was associated with lower saturation levels, suggesting HA117 expression may influence recovery and/or function.
Touma et al. (37) used deep sequencing to investigate the expression of lncRNAs in the neonatal mouse heart and was one of the seminal studies to determine that lncRNA expression is associated with distinct developmental time points. Furthermore, their studies showed that mRNA and lncRNA expression was largely similar between the LV and RV. Examination of the data with weighted gene coexpression network analysis (WGCNA) revealed that the most dramatic changes in gene expression occur during the first 3 days of development (P0–P3), with only moderate changes occurring between P3 and P7. WGCNA also identified lncRNAs that behave similarly to hub genes based on their co expression with mRNAs, which suggests a few lncRNAs influence the expression of many mRNAs. The analysis revealed lncRNA/mRNA pairs that are plausible regulators of cardiac development; some of which are also conserved in human hearts with CHD. Most notably, investigators were able to identify an inverse relationship between the expression of the Ppp1r1b lncRNA and the Titin-Cap (Tcap) mRNA and demonstrate that the ratio of the Ppp1r1b:Tcap can be used to differentiate between TOF and VSD. This suggests that dynamic interaction between mRNA and lncRNA strongly influences cardiac development and severity in CHD.
Knockdown of the EGOT lncRNA Attenuates the Therapeutic Effect of rhBNP in H9c2 Cells
B-type natriuretic peptide (BNP) is a peptide produced in and released by the LVs and RVs in response to unusual dilation or stretching and is also used as a clinical marker of cardiac pathology (15). Recombinant human BNP (rhBNP) is being investigated in a pediatric clinical trial aiming to determine if rhBNP improves postcorrective TOF surgery due to its ability to increase cardiac output (47). However, as is the case with many therapeutics, the underlying molecular mechanism of action is not known.
A study by Zhang et al. (46) demonstrated in H9c2 cells that the increased apoptosis autophagy and decreased cell viability induced by hypoxia could be significantly reduced by treatment with rhBNP. Furthermore, a reversal of the protective effects of rhBNP could be observed by siRNA-mediated knockdown of eosinophil granule ontogeny transcript (EGOT). The study demonstrated that treatment of rhBNP may exert its protective effect by downstream activation of the PI3K/AKT/mammalian target of rapamycin (mTOR) pathway that is reversible by siRNA-mediated downregulation of EGOT. It was also shown that levels of cyclin D1, which have been shown to be protective against heart failure post-MI (4) in other studies, were also reduced in response to downregulation of EGOT in H9c2 cells, suggesting that the therapeutic effect of rhBNP is linked to the lncRNA EGOT. This finding showcases the potential utility for lncRNAs as attenuators of disease. EGOT and other lncRNAs that can ameliorate the detrimental effects of hypoxia would be especially beneficial in cases where patients suffer from hypoxemia, including, but not limited to, TOF.
Conclusions
Emergent data have identified miRNAs and lncRNAs as potent signaling and regulatory molecules with diverse actions in both pediatric cardiovascular diseases and cardiac development. Much of what we know in these areas is a by-product of adult research in that the majority of investigations seeking to understand their role are focused on diseases relevant to adult populations (MI, lack of regenerative capacity), but involve the use of young models (neonatal rat ventricular myocytes, neonatal mice). It is possible to extrapolate from these adult studies and utilize these findings as a foundational framework to design pediatric studies. However, it is known that there are distinct differences in adult and pediatric cardiovascular disease etiologies (21, 30), and that adult therapies applied to pediatric patients have not substantially improved pediatric outcomes (11). Given their repeated implication in pediatric cardiovascular diseases, their potential usage as prognostic markers (20), and preclinical/clinical trials focused on heart disease etiologies prevalent in adults (such as MI or ischemia-reperfusion injury), an emphasis on pediatric-related investigations specific to CHD and/or pediatric heart failure is needed to improve understanding of age-dependent molecular mechanisms of cardiovascular diseases and develop age-specific treatment options.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-139968, HL-126928, and K24-HL-150630.
DISCLOSURES
C. C. Sucharov is the scientific founder of, and has equity in, miRagen, Inc., and is the founder and scientific advisor at CoramiR. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
L.T. and F.H. prepared figures; L.T., F.H., and C.C.S. drafted manuscript; L.T., F.H., and C.C.S. edited and revised manuscript; L.T., F.H., and C.C.S. approved final version of manuscript.
REFERENCES
- 1.Ambros V. microRNAs: tiny regulators with great potential. Cell 107: 823–826, 2001. doi: 10.1016/S0092-8674(01)00616-X. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KM, Anderson DM, McAnally JR, Shelton JM, Bassel-Duby R, Olson EN. Transcription of the non-coding RNA upperhand controls Hand2 expression and heart development. Nature 539: 433–436, 2016. doi: 10.1038/nature20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai B, Ma W, Ding F, Zhang L, Huang Q, Wang X, Hua B, Xu J, Li J, Bi C, Guo S, Yang F, Han Z, Li Y, Yan G, Yu Y, Bao Z, Yu M, Li F, Tian Y, Pan Z, Yang B. The long noncoding RNA CAREL controls cardiac regeneration. J Am Coll Cardiol 72: 534–550, 2018. doi: 10.1016/j.jacc.2018.04.085. [DOI] [PubMed] [Google Scholar]
- 4.Chen YM, Li H, Fan Y, Zhang QJ, Li X, Wu L-J, Chen ZJ, Zhu C, Qian LM. Identification of differentially expressed lncRNAs involved in transient regeneration of the neonatal C57BL/6J mouse heart by next-generation high-throughput RNA sequencing. Oncotarget 8: 28052–28062, 2017. doi: 10.18632/oncotarget.15887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiavacci E, D’Aurizio R, Guzzolino E, Russo F, Baumgart M, Groth M, Mariani L, D’Onofrio M, Arisi I, Pellegrini M, Cellerino A, Cremisi F, Pitto L. MicroRNA 19a replacement partially rescues fin and cardiac defects in zebrafish model of Holt Oram syndrome. Sci Rep 5: 18240, 2015. doi: 10.1038/srep18240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong D, Zhang Y, Reece EA, Wang L, Harman CR, Yang P. microRNA expression profiling and functional annotation analysis of their targets modulated by oxidative stress during embryonic heart development in diabetic mice. Reprod Toxicol 65: 365–374, 2016. doi: 10.1016/j.reprotox.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engreitz JM, Pandya-Jones A, McDonel P, Shishkin A, Sirokman K, Surka C, Kadri S, Xing J, Goren A, Lander ES, Plath K, Guttman M. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science 341: 1237973, 2013. doi: 10.1126/science.1237973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grote P, Wittler L, Hendrix D, Koch F, Währisch S, Beisaw A, Macura K, Bläss G, Kellis M, Werber M, Herrmann BG. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell 24: 206–214, 2013. doi: 10.1016/j.devcel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzzolino E, Chiavacci E, Ahuja N, Mariani L, Evangelista M, Ippolito C, Rizzo M, Garrity D, Cremisi F, Pitto L. Post-transcriptional modulation of sphingosine-1-phosphate receptor 1 by miR-19a affects cardiovascular development in zebrafish. Front Cell Dev Biol 6: 58, 2018. doi: 10.3389/fcell.2018.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kameny RJ, He Y, Zhu T, Gong W, Raff GW, Chapin CJ, Datar SA, Boehme JT, Hata A, Fineman JR. Analysis of the microRNA signature driving adaptive right ventricular hypertrophy in an ovine model of congenital heart disease. Am J Physiol Heart Circ Physiol 315: H847–H854, 2018. doi: 10.1152/ajpheart.00057.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantor PF, Abraham JR, Dipchand AI, Benson LN, Redington AN. The impact of changing medical therapy on transplantation-free survival in pediatric dilated cardiomyopathy. J Am Coll Cardiol 55: 1377–1384, 2010. doi: 10.1016/j.jacc.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 12.Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, Ding H, Butty VL, Torrey L, Haas S, Abo R, Tabebordbar M, Lee RT, Burge CB, Boyer LA. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell 152: 570–583, 2013. doi: 10.1016/j.cell.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lappin TRJ, Grier DG, Thompson A, Halliday HL. HOX genes: seductive science, mysterious mechanisms. Ulster Med J 75: 23–31, 2006. [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, Dai Q, Chen W, He C. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 10: 93–95, 2014. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA; Breathing Not Properly Multinational Study Investigators . Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 347: 161–167, 2002. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 16.McDermott DA, Fong JC, Basson CT. Holt-Oram Syndrome (Online). GeneReviews; https://www.ncbi.nlm.nih.gov/pubmed/20301290 [23 Jan 2020]. [Google Scholar]
- 17.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet 10: 155–159, 2009. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 18.Mercer TR, Dinger ME, Sunkin SM, Mehler MF, Mattick JS. Specific expression of long noncoding RNAs in the mouse brain. Proc Natl Acad Sci USA 105: 716–721, 2008. doi: 10.1073/pnas.0706729105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol 20: 300–307, 2013. doi: 10.1038/nsmb.2480. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto SD, Karimpour-Fard A, Peterson V, Auerbach SR, Stenmark KR, Stauffer BL, Sucharov CC. Circulating microRNA as a biomarker for recovery in pediatric dilated cardiomyopathy. J Heart Lung Transplant 34: 724–733, 2015. doi: 10.1016/j.healun.2015.01.979. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto SD, Stauffer BL, Nakano S, Sobus R, Nunley K, Nelson P, Sucharov CC. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. Eur Heart J 35: 33–41, 2014. doi: 10.1093/eurheartj/ehs229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muntean I, Togănel R, Benedek T. Genetics of Congenital Heart Disease: Past and Present. Biochem Genet 55: 105–123, 2017. doi: 10.1007/s10528-016-9780-7. [DOI] [PubMed] [Google Scholar]
- 23.Øyen N, Diaz LJ, Leirgul E, Boyd HA, Priest J, Mathiesen ER, Quertermous T, Wohlfahrt J, Melbye M. Prepregnancy diabetes and offspring risk of congenital heart disease: a nationwide cohort study. Circulation 133: 2243–2253, 2016. doi: 10.1161/CIRCULATIONAHA.115.017465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavlinkova G, Salbaum JM, Kappen C. Maternal diabetes alters transcriptional programs in the developing embryo. BMC Genomics 10: 274, 2009. doi: 10.1186/1471-2164-10-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pezzani L, Milani D, Manzoni F, Baccarin M, Silipigni R, Guerneri S, Esposito S. HOXA genes cluster: clinical implications of the smallest deletion. Ital J Pediatr 41: 31, 2015. doi: 10.1186/s13052-015-0137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ponjavic J, Ponting CP, Lunter G. Functionality or transcriptional noise? Evidence for selection within long noncoding RNAs. Genome Res 17: 556–565, 2007. doi: 10.1101/gr.6036807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science 331: 1078–1080, 2011. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy S, Zhao M, Hu DQ, Fajardo G, Hu S, Ghosh Z, Rajagopalan V, Wu JC, Bernstein D. Dynamic microRNA expression during the transition from right ventricular hypertrophy to failure. Physiol Genomics 44: 562–575, 2012. doi: 10.1152/physiolgenomics.00163.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129: 1311–1323, 2007. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rossano JW, Shaddy RE. Heart failure in children: etiology and treatment. J Pediatr 165: 228–233, 2014. doi: 10.1016/j.jpeds.2014.04.055. [DOI] [PubMed] [Google Scholar]
- 31.Sahakyan A, Yang Y, Plath K. The role of Xist in X-chromosome dosage compensation. Trends Cell Biol 28: 999–1013, 2018. doi: 10.1016/j.tcb.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi R, Zhao L, Cai W, Wei M, Zhou X, Yang G, Yuan L. Maternal exosomes in diabetes contribute to the cardiac development deficiency. Biochem Biophys Res Commun 483: 602–608, 2017. doi: 10.1016/j.bbrc.2016.12.097. [DOI] [PubMed] [Google Scholar]
- 33. Sprengers RW, Roest AA, Kroft LJ. Tetralogy of Fallot. : Congenital Heart Diseases in Adults. Medical Radiology, edited by Ley S, Ley-Zaporozhan J. Cham, Switzerland: Springer, 2017, p. 89–116, doi: 10.1007/174_2017_107. [DOI] [Google Scholar]
- 34.Sun X, Han Q, Luo H, Pan X, Ji Y, Yang Y, Chen H, Wang F, Lai W, Guan X, Zhang Q, Tang Y, Chu J, Yu J, Shou W, Deng Y, Li X. Profiling analysis of long non-coding RNAs in early postnatal mouse hearts. Sci Rep 7: 43485, 2017. doi: 10.1038/srep43485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature 505: 344–352, 2014. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian J, An X, Niu L. Role of microRNAs in cardiac development and disease. Exp Ther Med 13: 3–8, 2017. doi: 10.3892/etm.2016.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Touma M, Kang X, Zhao Y, Cass AA, Gao F, Biniwale R, Coppola G, Xiao X, Reemtsen B, Wang Y. Decoding the long noncoding RNA during cardiac maturation: a roadmap for functional discovery. Circ Cardiovasc Genet 9: 395–407, 2016. doi: 10.1161/CIRCGENETICS.115.001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Triedman JK, Newburger JW. Trends in congenital heart disease. Circulation 133: 2716–2733, 2016. doi: 10.1161/CIRCULATIONAHA.116.023544. [DOI] [PubMed] [Google Scholar]
- 39.Vegter EL, van der Meer P, de Windt LJ, Pinto YM, Voors AA. MicroRNAs in heart failure: from biomarker to target for therapy. Eur J Heart Fail 18: 457–468, 2016. doi: 10.1002/ejhf.495. [DOI] [PubMed] [Google Scholar]
- 40.Wahid F, Shehzad A, Khan T, Kim YY. MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta 1803: 1231–1243, 2010. doi: 10.1016/j.bbamcr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Wang F, Liu D, Zhang RR, Yu LW, Zhao J-Y, Yang XY, Jiang SS, Ma D, Qiao B, Zhang F, Jin L, Gui YH, Wang HY. A TBX5 3′UTR variant increases the risk of congenital heart disease in the Han Chinese population. Cell Discov 3: 1–13, 2017. doi: 10.1038/celldisc.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang KC, Yang YW, Liu B, Sanyal A, Corces-zimmerman R, Chen Y, Lajoie BR, Protacio A, Flynn RA, Gupta RA, Wysocka J, Lei M, Dekker J, Helms JA, Chang HY. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472: 120–124, 2013. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Q, Wang Z, Wu C, Pan Z, Xiang L, Liu H, Jin X, Tong K, Fan S, Jin X. Potential association of long noncoding RNA HA117 with tetralogy of Fallot. Genes Dis 5: 185–190, 2018. doi: 10.1016/j.gendis.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wojciechowska A, Braniewska A, Kozar-Kamińska K. MicroRNA in cardiovascular biology and disease. Adv Clin Exp Med 26: 865–874, 2017. doi: 10.17219/acem/62915. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y, Reece EA, Zhong J, Dong D, Shen W, Harman CR, Yang P. Type 2 diabetes mellitus induces congenital heart defects in murine embryos by increasing oxidative stress, endoplasmic reticulum stress, and apoptosis. Am J Obstet Gynecol 215: 366.e1–366.e10, 2016. doi: 10.1016/j.ajog.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang C, Pan S, Aisha A, Abudoukelimu M, Tang L, Ling Y. Recombinant human brain natriuretic peptide regulates PI3K/AKT/mTOR pathway through lncRNA EGOT to attenuate hypoxia-induced injury in H9c2 cardiomyocytes. Biochem Biophys Res Commun 503: 1186–1193, 2018. doi: 10.1016/j.bbrc.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 47.Zhang S, Wang Z. Effect of recombinant human brain natriuretic peptide (rhBNP) versus nitroglycerin in patients with heart failure: a systematic review and meta-analysis. Medicine (Baltimore) 95: e4757, 2016. doi: 10.1097/MD.0000000000004757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Y, Jia WK, Jian Z, Zhao L, Liu CC, Wang Y, Xiao Y. Downregulation of microRNA-199a-5p protects cardiomyocytes in cyanotic congenital heart disease by attenuating endoplasmic reticulum stress. Mol Med Rep 16: 2992–3000, 2017. doi: 10.3892/mmr.2017.6934. [DOI] [PubMed] [Google Scholar]
- 49.Zhu H, Fan GC. Extracellular/circulating microRNAs and their potential role in cardiovascular disease. Am J Cardiovasc Dis 1: 138–149, 2011. [PMC free article] [PubMed] [Google Scholar]