Abstract
The sympathetic nervous system modulates cardiac function by controlling key parameters such as chronotropy and inotropy. Sympathetic control of ventricular function occurs through extrinsic innervation arising from the stellate ganglia and thoracic sympathetic chain. In the healthy heart, sympathetic release of norepinephrine (NE) results in positive modulation of chronotropy, inotropy, and dromotropy, significantly increasing cardiac output. However, in the setting of myocardial infarction or injury, sympathetic activation persists, contributing to heart failure and increasing the risk of arrhythmias, including sudden cardiac death. Methodologies for detection of norepinephrine in cardiac tissue are limited. Present techniques rely on microdialysis for analysis by high-performance liquid chromatography coupled to electrochemical detection (HPLC-ED), radioimmunoassay, or other immunoassays, such as enzyme-linked immunosorbent assay (ELISA). Although significant information about the release and action of norepinephrine has been obtained with these methodologies, they are limited in temporal resolution, require large sample volumes, and provide results with a significant delay after sample collection (hours to weeks). In this study, we report a novel approach for measurement of interstitial cardiac norepinephrine, using minimally invasive, electrode-based, fast-scanning cyclic voltammetry (FSCV) applied in a beating porcine heart. The first multispatial and high temporal resolution, multichannel measurements of NE release in vivo are provided. Our data demonstrate rapid changes in interstitial NE profiles with regional differences in response to coronary ischemia, sympathetic nerve stimulation, and alterations in preload/afterload.
NEW & NOTEWORTHY Pharmacological, electrical, or surgical regulation of sympathetic neuronal control can be used to modulate cardiac function and treat arrhythmias. However, present methods for monitoring sympathetic release of norepinephrine in the heart are limited in spatial and temporal resolution. Here, we provide for the first time a methodology and demonstration of practice and rapid measures of individualized regional autonomic neurotransmitter levels in a beating heart. We show dynamic, spatially resolved release profiles under normal and pathological conditions.
Keywords: cardiac, cyclic voltammetry, electrochemistry, epinephrine, norepinephrine, sympathetic nervous system
INTRODUCTION
Cardiac sympathetic activation occurs during stress, including exercise, to improve cardiac output. However, in the setting of cardiac injury, a decrease in cardiac output reflexively results in chronic sympathetic activation, which can lead to progression of heart failure and development of ventricular arrhythmias (11). Norepinephrine (NE) is the primary neurotransmitter released from post-ganglionic sympathetic efferents (17). Heart failure is known to result in elevated myocardial NE levels, which portend a worse prognosis and are associated with cardiac mortality, ventricular arrhythmias, and sudden cardiac death (8). Therefore, NE can serve as an important biomarker of the status of cardiac disease. However, present methods to detect NE have significant limitations, and as a result, measurements of NE levels have not been routinely used clinically to assess the dynamic status of cardiac disease and to adjust therapies.
Present methodologies for the measure of NE in cardiac tissue rely largely on the collection of interstitial fluids by microdialysis (20). This method places a known length of semipermeable membrane tubing in the myocardial wall through which fluid is perfused. Localized NE passes through the wall of the dialysis tubing and is collected in the perfusate. This approach results in collection of a fraction of the localized NE (∼18%) and is limited in temporal resolution to the rate of perfusion and collection of the necessary volume for detection, with a typical period of 3 min or so (13). Sample analysis for NE content may be through varied methods, including high-performance liquid chromatography, coupled to electrochemical detection (HPLC-ED), enzyme-linked immunosorbent assay (ELISA), radioenzymatic assay, or mass spectrometry (4, 13, 29). All these approaches require specialized sample preparation and handling and are susceptible to sample degradation during processing before detection. Moreover, typically, many samples collected from parallel experiments are required for adequate sample for analysis. Thus, resulting values represent a signal average of responses across varied sources, a process that may fail to consider microenvironments within the heart and variability of responses from one source to another. While providing a vast knowledge of the nature of autonomic control of the heart under varied conditions, traditional approaches to NE measurement are limited in key aspects of temporal resolution, sample preparation, resolution of variation of response, and time for signal processing.
Therefore, as an alternative, cardiac imaging modalities such as positron emission tomography (PET) (10) and metaiodobenzylguanidine (MIBG) (9) have been developed to assess sympathetic innervation. However, these modalities provide a one-time static measurement, are costly, and suffer from poor resolution. Thus, while having the potential to provide important diagnostic and prognostic information on the status of autonomic control of the heart, such imaging approaches provide little data on dynamic responsiveness of the heart in reflex response to stress.
In this study, a novel adaptation of a dynamic approach for electrochemical detection of NE levels in vivo is presented. The approach is based on fast scanning cyclic voltammetry (FSCV), a method utilized to measure catecholamine release from isolated cells (21, 25) or from tissues (16, 30, 33). Briefly, an electrode is placed near the source of the transmitter and its potential driven though the oxidation/reduction potentials by a voltage clamp circuit. Thus, as the electrode potential is driven positive to the oxidation potential for NE, the NE is oxidized to a quinone product. The oxidation reaction generates electrons that are then measured as a compensating current in the voltage clamp and report the detection of molecules of NE. Driving the electrode potential back to a negative polarization reduces the quinone product to regenerate the catecholamine (6). Traditionally for these applications, electrodes for NE measurement were made of small diameter carbon fibers encased in a pulled borosilicate glass capillary or polypropylene tube to stabilize and insulate the brittle carbon fiber electrode, and electrode placement was with the aid of a micromanipulator. Although this configuration is very effective at measuring voltammetric currents in isolated cell or tissue applications, it suffers from several limitations that make measurements in a large, moving preparation impossible (e.g., probe length and flexibility, head stage design, proximity requirement, and reference electrode placement). The primary objective of this study was to evolve an FSCV technology that circumvents limitations associated traditional electrodes utilized for FSCV as well as traditional FSCV amplifier design to record local interstitial NE at high temporal resolution from multiple regions of the beating heart. With this new approach in hand, we overcome the spatiotemporal limitations of traditional microdialysis-based approaches outlined above. Moreover, we are able to measure dynamic NE on a single-event basis in a single subject without the need for data pooling and averaging of responses across several experiments. The power of this approach is to provide specific readouts of individual responses to cardiac stressors, for which there is considerable variability between subjects.
MATERIALS AND METHODS
Instrumentation.
We designed a multichannel amplifier that incorporated a low-resistance feedback resistor in the voltage clamp circuit to charge the greater capacitance of the long, flexible electrode, while still supporting a sufficient dV/dt scan rate. Our custom amplifier design was based on the NPI VA-10M, multichannel amplifier (NPI Electronic, Tamm, Germany). We employed a three-electrode design to accommodate placement of sensing electrodes in the myocardium and reference/ground electrodes in the chest wall. The amplifier was fitted with a 5× command potential input to allow scans of ≤1.2 V to allow measure of epinephrine and for specific isolation of NE over other catecholamines (32). The command potential was issued through software via the digital-to-analog converter channels and the signal acquired through the analog-to-digital converter channels of a HEKA LIH 8 + 8 analog-to-digital/digital-to-analog device (HEKA Elektonic, Holliston, MA). Other unique features of the amplifier included a switchable feedback resistor for each of the four acquisition channels, allowing for the choice of 1 MΩ or 10 MΩ feedback circuit to accommodate electrode variability. A single head stage with a common ground/reference circuit for all four acquisition channels was also developed to place the device near the chest in a single physical unit. All data reported here were collected with the 1 MΩ feedback resistor setting.
Platinum (Pt) wire electrodes, 30 cm in length and 127 µm in diameter, perfluoroalkoxy (PFA) coated for an insulator (A-M Systems, Sequim, WA), served as sensing elements for in vivo FSCV. On one end, the PFA coating was stripped to reveal ∼5 mm of bare wire that was then crimped into a 1-mm gold-plated connector pin. The wire pin joint was stabilized by flowing a small amount of solder into the joint (Fig. 1A). Admittance analysis was performed on multiple Pt electrodes, and we found that the phase offset for these electrodes, at the scan rate utilized to collect data in this report, varied electrode to electrode, but was between 33 and 51 degrees. Additionally, in vivo data were corrected for tissue impedance and electrode phase offset. These parameters were accounted for in analysis of voltammograms and oxidation currents.
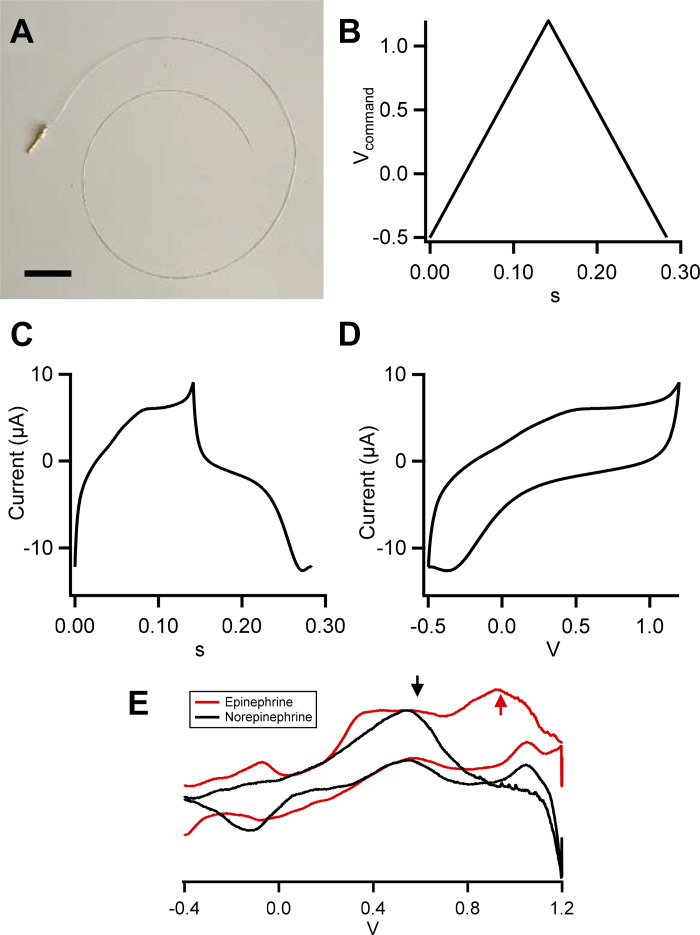
Fig. 1.
Platinum electrodes for fast-scanning cyclic voltammetry (FSCV). A: platinum wire electrode as used in this study is shown. Electrodes are 30 cm of platinum wire, 150 µm in diameter, and perfluoroalkoxy (PFA) coated. One end is stripped of ∼2 to 3 mm of PFA and soldered onto a 1-mm-diameter male gold-plated connector pin (scale bar, 2 cm). B: electrodes are driven with a sawtooth command voltage waveform, from −0.5 to 1.2 V, at a rate of 12 V/s. C: sample current recording obtained from an electrode bathed in bicarbonate-buffered saline is shown. D: resulting full voltammogram from the record shown in C. E: sample voltammograms obtained in vitro with norepinephrine (black; 250 µM in Tris-buffered saline) or epinephrine (red; 250 µM in Tris-buffered saline). Arrows indicate peak oxidation potentials for both norepinephrine (NE) and epinephrine (Epi; black) and the secondary potential unique to Epi (red), demonstrating the difference in voltammogram oxidation currents that serve to separate norepinephrine from epinephrine.
Acquisition and analysis software.
Software for driving command potential and data acquisition was custom written in IGOR Pro (version 7.08; WaveMetrics, Lake Oswego, OR). The LIH 8 + 8 issued command voltage and acquired data from the custom NPI VA-10M amplifier. Filter and gain were telegraphed from the amplifier. These values and recording parameters were written into the headers of the data waves for record keeping. Data were filtered at 1 kHz through a two-pole analog Bessel filter and digitized at 10 kHz. The command potential for FSCV was a sawtooth waveform between −0.5 and 1.2 V, issued at 12 V/s, for an effective cycle rate of ∼3.5 Hz. Collected data were baseline subtracted by an average voltammogram composed of 10 cycles before the experimental perturbation. Data for each channel were converted into a kymograph with command voltage plotted against time, with each column representing a single scan. Current amplitude was indicated by color. A horizontal line profile, representing current amplitude at a given command potential, was extracted at the oxidation potential for norepinephrine and corrected for the phase offset due introduced by the electrode impedance. An additional initial current artifact, due to equilibration of the electrode redox status under the sawtooth command potential, was subtracted for presentation. Data were saved in a three-dimensional pooled data wave for further statistical analysis and archive.
In vitro measurements.
For in vitro measurements, electrodes were held by a coarse manual manipulator and their tips placed in a laminar flow superfusion chamber (with 2.5–3 mL in total fluid volume). Electrodes were superfused at a constant rate of ∼2 mL/min with bicarbonate-buffered saline (BBS) of the following composition (in mM): 140 NaCl, 26 NaHCO3, 3.5 glucose, 3 CaCl2, 2 KCl, and 2 MgCl2. Calcium chloride was added from stock solution (3 M) before recording to avoid precipitation as CaCO3. The saline was constantly bubbled with 5% CO2 and 95% O2 to maintain the pH level around 7.4. Variable concentrations of NE were sequentially perfused into the chamber with concentrations ranging between 0 and 1 μM. Oxidation currents were determined for each level of NE. Stability of recording over 6 h was assessed by repeating measuring a constant given level of NE (100, 250, and 500 nM) in Tris [tris(hydroxymethyl)aminomethane]-buffered saline (TBS; 132 mM NaCl, 40 mM Tris, 11.2 mM glucose, 4.2 mM KCl, 2 mM CaCl2, and 0.7 mM MgCl2) at pH 7.4.
Norepinephrine specificity over epinephrine (Epi) is determined by the shape of the recorded voltammogram. An in vitro example is provided in Fig. 1E. Both NE and Epi exhibit a primary oxidation potential at ∼0.4 V. This primary oxidation potential will vary slightly as a function of electrode impedance, which introduces a slight phase offset, but the primary peak is readily identifiable (Fig. 1E, black arrow). Epinephrine is a secondary amine and exhibits a second oxidation current at greater positive potential (Fig. 1E, red arrow) that is not present in the NE voltammogram. This analytic approach is well capable of separating NE from Epi signals.
In vivo measurements.
All animal experiments were approved by the University of California Los Angeles Animal Research Committee and performed in accordance with guidelines set forth by the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (8th ed., 2011). Adult Yorkshire pigs, (total n = 4; 2 males and 2 females) were sedated with intramuscular telazol (4–6 mg/kg), intubated, and mechanically ventilated. General anesthesia was maintained with inhaled isoflurane (1.5–2.5%) and intravenous boluses of fentanyl (total: 10–30 µg/kg) during surgical preparation. Continuous intravenous saline was infused through the femoral vein throughout the experiments to maintain volume homeostasis. Arterial blood pressure was measured via a femoral arterial line. Heart rate was monitored by lead II ECG. Left ventricular systolic pressure was measured using a pressure monitoring pigtail catheter (5 Fr) inserted into the left ventricle (LV) via the left carotid artery and connected to a PCU-2000 pressure control system (Millar Instruments, Houston, TX). Arterial blood gas was tested hourly, and adjustments of ventilation and/or administration of sodium bicarbonate were made as necessary to maintain acid-base homeostasis.
A median sternotomy was performed to expose the heart as well as the stellate ganglia, inferior vena cava (IVC), and descending thoracic aorta. Snare occluders were placed around the great vessels (inferior vena cava and descending aorta) and at the first diagonal branch of the left anterior descending coronary artery (LAD). The stellate ganglia were isolated behind the parietal pleura, and bipolar electrodes were placed into each stellate ganglion and connected to a stimulator with an isolation unit (S88 and PSIU6; Grass Technologies, Warwick, RI). For each stellate ganglion, cardiac-related threshold was defined as the current that evoked a 10% increase in heart rate or systolic blood pressure at 4-Hz frequency and 4-ms pulse width. A bipolar cardiac pacing catheter was inserted into the right ventricle via the right jugular vein and connected to a Micropace system (EPS320; Micropace, Canterbury, NSW, Australia) for ventricular pacing. Following the completion of surgery, general anesthesia was changed to α-chloralose (50 mg/kg iv bolus with 10 mg·kg−1·h−1 continuous iv infusion).
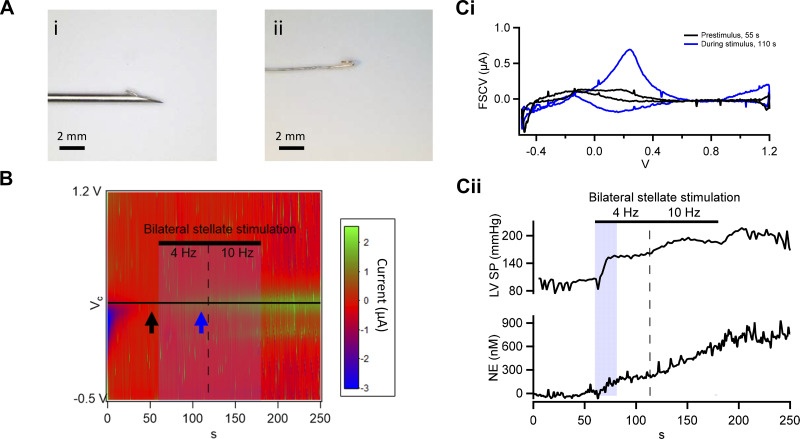
For insertion into the wall of the heart, FSCV Pt insulated wire electrodes were threaded through a 25-gauge hypodermic needle. The tip of the electrode was pushed to protrude ∼0.5 mm beyond the needle tip and was bent back along the shank of the needle to create a barb akin to a fish hook (Fig. 3A, i and ii). The needle was then inserted into the midmyocardium of the ventricle wall and the needle withdrawn, leaving the electrode inserted in the ventricle wall. For the purposes of regional analyses, anterior refers to ventral and posterior refers to dorsal aspect of the animal. Electrodes were placed at four sites covering the basal, apical, anterior, and lateral parts of the LV. This configuration produced minimal damage of the heart wall and resulted in stable electrode placement for the duration of the experimental protocol, often lasting 6 h. Ground and reference electrodes (two 18-gauge syringe needles) were inserted in the chest wall in intercostal muscle tissue. Following deployment of the multiple FSCV probes, they were cycled for 20 min before experimental procedures to establish a stable baseline. Hemodynamics and interstitial NE responses were then measured concurrently at baseline, in response to a given intervention, and then into the recovery phase. Baseline and peak NE concentrations for each data set provided in Figs. 3–5 are presented in Table 1.
Fig. 3.
Rapid in vivo measurement of norepinephrine (NE) by fast-scanning cyclic voltammetry (FSCV) during bilateral stellate stimulation. A: electrode prepared for in vivo use is shown. The platinum wire is threaded through a 25-gauge hypodermic needle and bent back to form a barb (A,i) before insertion into the muscle wall (scale bar, 2 mm). The same electrode is shown with the needle withdrawn (A,ii; scale bar, 2 mm). B: kymograph showing oxidative FSCV currents; each voltammogram (−0.5 to 1.2 V) represented by columns, sequentially as a function of time and color coded for current amplitude. C,i: representative voltammograms pulled before (black) and during (blue) bilateral stellate stimulation (BSS; 4 Hz, 2 ms, 3× threshold, first 1 min, increasing to 10 Hz for last minute) show the emergence of the NE oxidative current at 380 mV. C,ii: peak FSCV currents pulled from each voltammogram as a function of time. Current values were calibrated on a point-by-point basis against the standard calibration curve to provide interstitial norepinephrine changes through the duration of BSS. Measured left ventricular systolic pressure (LVSP; top curve) and calibrated change in NE (bottom curve) in response to BSS are coplotted for comparison.
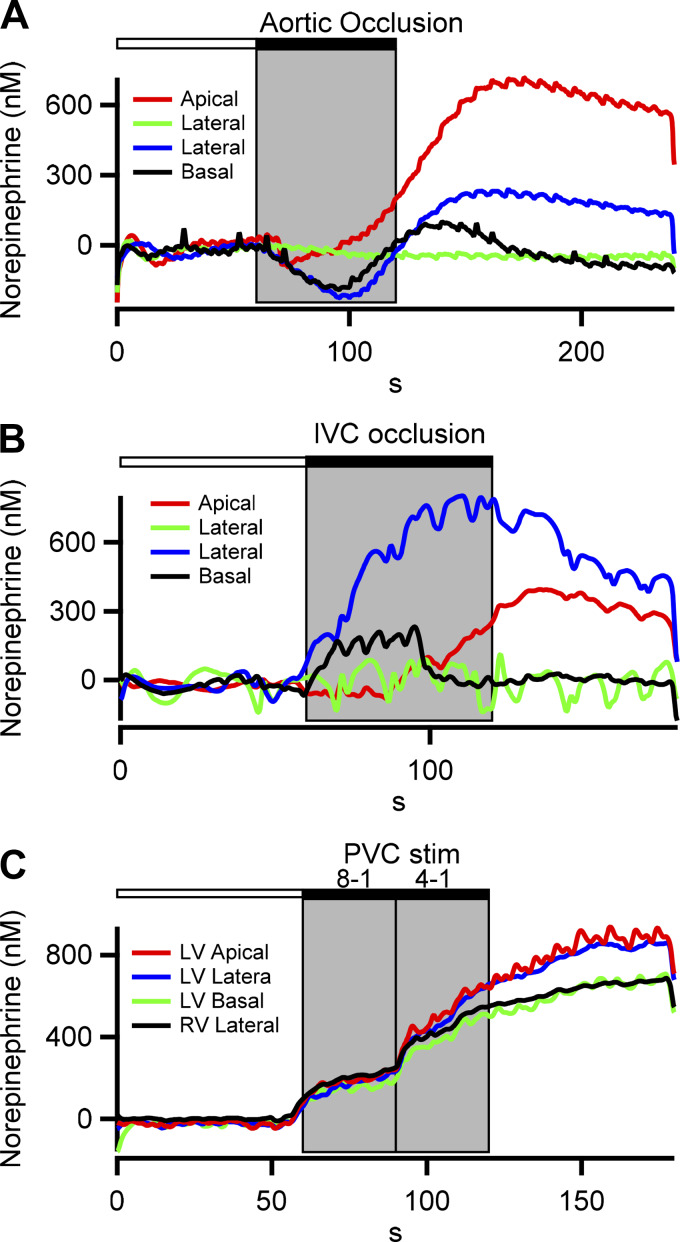
Fig. 5.
Spatial norepinephrine dynamics under hemodynamic and pacing stress. Time-resolved norepinephrine measurements from different ventricle locations under 3 different perturbations are plotted. Complementary hemodynamic measures for each recording are provided in Table 2. A: response to transient occlusion of the descending aorta is shown. The increase in afterload (increased left ventricular peak systolic pressure; see Table 2) results in a baroreflex-mediated decrease in interstitial norepinephrine from baseline. Rate of pressure change in the left ventricle (LV) remains relatively constant. Following release of the occlusion, a rebound effect in norepinephrine is observed. B: decrease in preload (intracardiac pressure reduced), associated with transient occlusion of the inferior vena cava (IVC), results in a reflex-mediated increase in interstitial norepinephrine. Corresponding hemodynamics provided in Table 2 show decreased left ventricular systolic pressure (LVSP) and dP/dt during IVC occlusion. C: response to 2 levels of premature ventricular contraction (PVC) induction (8:1, 4:1) is shown. PVC induction is transduced by the cardiac afferents, resulting in a reflex increase in interstitial norepinephrine levels and coincident increased LVSP and LV dP/dt (Table 2). RV, right ventricle.
Table 1.
Estimated baseline and peak norepinephrine levels for all raw data traces
| Figure No. | Baseline Norepinephrine, nM | Peak Norepinephrine, nM |
|---|---|---|
| Fig. 3 | 6.0 | 646.9 |
| Fig. 4 (green, black, red, blue) | 5.8, 4.3, 6.2, 6.0 | 76.3, 25.4, 725.0, 393.7 |
| Fig. 5A,i (red, blue, green, black) | 9.9, 6.8, 7.2, 5.5 | 716.8, 239.5, 99.9, 18.6 |
| Fig. 5B,i (red, blue, green, black) | −5.7, −2.5, −1.2, −2.2 | 394.7, 802.3, 112.0, 232.1 |
| Fig. 5C,i (red, blue, green, black) | −3.6, −2.4, −4.9, 5.9 | 938.2, 866.9, 708.4, 663.7 |
Baseline values were measured before onset of stimulation and peak values as the maximum estimated norepinephrine concentration any time following the stimulation. Note that very small currents fall on a steep component of the standard curve, and negative baseline values are due to noise in the signal.
Cardiac stressors.
The transient cardiac stressors tested included bilateral stellate ganglia stimulation for 4 min (4 Hz, 4-ms pulse width, 2× threshold, first 2 min and increasing to 10 Hz for last 2 min), inferior vena cava occlusion (decrease preload) for 60 s, descending aorta occlusion (increase afterload) for 60 s, occlusion of the left anterior descending coronary artery for one minute, and intermittent ventricular stimulation to induce variably coupled premature ventricular contractions (PVC) at every eight heart beats for 30 s and then every 4 heart beats for 30 s. A minimum of 15 min was allowed between stressors for recovery of cardiac function to baseline.
Electrocardiogram (ECG), hemodynamic data, and stimulus markers (reflecting intervention onsets and offsets) were input to a data acquisition system (Power1401; Cambridge Electronic Design-CED, Cambridge, UK). Data were analyzed offline using the software Spike2 (Cambridge Electronic Design). Data streams from the voltammetry and CED data acquisition systems were manually time-synchronized at the time of data collection and merged during subsequent offline analysis. At the completion of the experiments, animals were euthanized under anesthesia by inducing ventricular fibrillation via application of direct current to the heart.
RESULTS
Electrode design and characterization.
Acquisition and analysis software were developed in-house to drive a custom-designed four-channel voltage clamp amplifier. PFA-insulated platinum wires, 150 µM in diameter and 30 cm in length, were used as flexible FSCV electrodes (Fig. 1A). A sawtooth command waveform (Fig. 1B) drove the recorded voltammograms (Fig. 1, C and D). Recordings were performed in bicarbonate-buffered saline (BBS) to mimic the interstitial conditions of the myocardium. A sample voltammogram of an electrode in BBS displays a hysteresis at a scan rate of 12 V/s from −0.5 V to 1.2 V (Fig. 1D). This command potential range is wide enough to measure norepinephrine (NE) as well as other potential catecholamines (e.g., epinephrine; Fig. 1E), and the scan rate provides a sample rate of ∼3.53 Hz (Fig. 1E).
In vitro assessments of electrode sensitivity and stability.
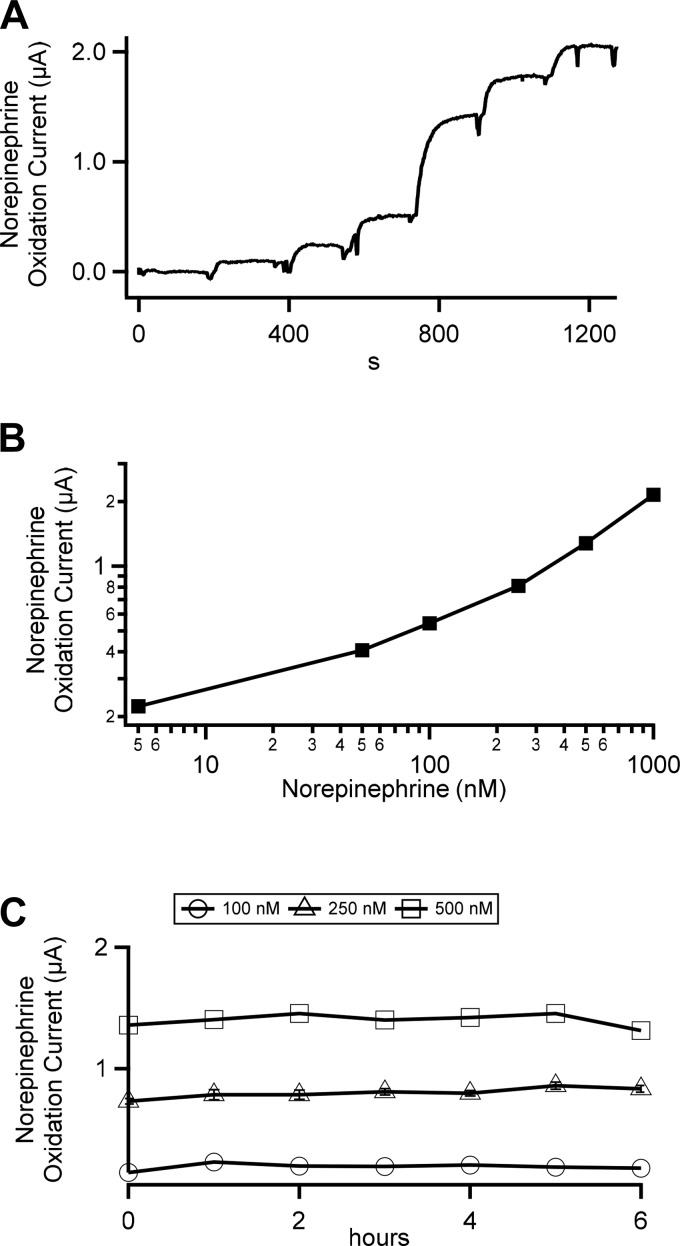
We superfused electrodes with BBS supplemented with increasing concentrations of NE (0 to 1 μM) in a laminar flow chamber. Peak currents at the NE oxidation potential were measured and plotted (Fig. 2A). Peak NE oxidation currents for each concentration are plotted against NE concentration and provide a standard calibration curve (Fig. 2B). To account for nonlinearity of the standard curve, acquired data are matched point for point to their intersection with the standard curve. The result reports a change in NE concentration from baseline. Next, we tested the stability of our recording configuration by recording peak currents at the NE oxidation potential by repeating addition of the given concentrations of NE over 6 h and found the electrodes to be stable over this period (Fig. 2C), where there was no significant degradation in measure signal for any of the three NE levels tested (100, 250, and 500 nM).
Fig. 2.
In vitro calibration of platinum wire fast-scanning cyclic voltammetry (FSCV) electrodes. A: electrode was placed in a flow chamber and superfused with bicarbonate-buffered saline at ∼2 mL/min. The superfusate was sequentially supplemented with norepinephrine of the following concentrations (5, 50, 100, 250, 500, and 1,000 nM). The electrode was driven with the command voltage waveform shown in Fig. 1. Resulting oxidation currents for norepinephrine were measured from the voltammograms and are plotted against time. B: norepinephrine oxidation currents were measured under each norepinephrine concentration to provide a standard curve for calibration of FSCV currents recorded in vivo. C: oxidation currents were measured at 1-h intervals over a 6-h time frame in bicarbonate-buffered saline supplemented with norepinephrine (100, 250, and 500 nM).
In vivo assessments of electrode sensitivity and stability.
A platinum electrode was inserted into the left ventricle (LV) midmyocardium with the aid of a hypodermic needle (Fig. 3A,i) and the needle withdrawn, leaving the sensing element of the electrode embedded (Fig. 3A,ii shows the electrode tip). Interstitial NE levels were evaluated at baseline and in response to bilateral stellate ganglion stimulation. Data are presented as a kymograph (Fig. 3B), with y-axis columns representing the upstroke of the sawtooth command potential and time represented on the x-axis. Current magnitude is color-coded. The black horizontal line represents the peak oxidation potential for NE. There is emergence of a signal during stellate ganglia stimulation, which persists somewhat after stimulation, indicating increased NE at the electrode tip. Example voltammograms (current versus command potential) are provided in Fig. 3C. The black voltammogram was measured at baseline (time point indicated by the black arrow in Fig. 3B) and the blue during stellate ganglia stimulation (time point indicated by the blue arrow in Fig. 3B). Currents were pulled from the kymograph, as a function of time, at the peak NE oxidation potential (black horizontal line in Fig. 3B) and calibrated against the standard curve to provide time-resolved, evoked changes in NE concentration (Fig. 3C,ii). Average preperturbation baseline NE level was 6.0 nM (measured over a 20-s window before stimulation) and rose significantly as a function of stellate stimulation (Table 1). This approximate 646 nM increase in NE is greater than values obtained through other techniques (i.e., radioimmunoassay; see Refs. 20 and 27) but does not suffer from sample dilution or potential degradation after collection and, therefore, is considered reflective of physiological interstitial concentrations. In simultaneous hemodynamic measurements, complementary time-resolved increases in LV peak systolic pressure (LVSP) mirror the increased NE. For further complementary comparison, heart rate (HR) and LV-developed pressure (dP/dt) were also measured. Their values at baseline and during stimulation, respectively, were, HR 99.5 and 187 beats/min and LV developed pressure 1,310 and 1,789 mmHg/s, each indicating a complementary response.
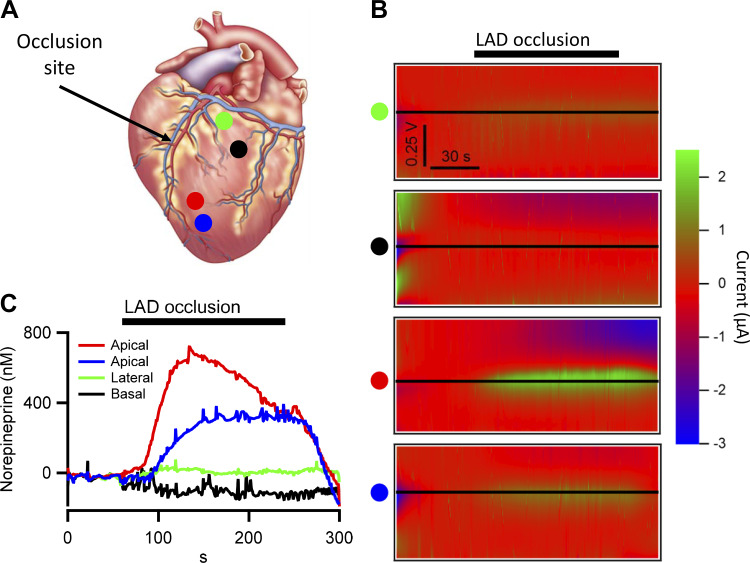
A major goal of our study was to leverage the localized measurement of interstitial NE at the tip of each electrode across multiple regions of the myocardium. This approach thus provides high time resolution measure of localized NE levels that are fundamental to the potential for arrhythmias and progression into heart failure. We conducted experiments utilizing four independent acquisition channels to provide a gross spatial map of NE levels across the left ventricle in response to acute occlusion of the left anterior descending (LAD) coronary artery (180 s duration). LAD occlusion results in loss of circulation beyond the occlusion site and induces regional myocardial ischemia (e.g., in LV apex in this example). Subsequent activation of local nociceptors produces a reflex sympathoexcitation (10, 23, 24), which results in reflex release of NE from sympathetic efferent neurons projecting to the heart. In this example, electrodes were placed caudal to the site of vessel occlusion (indicated by black arrow, Fig. 4A) within basal regions of the LV, whose circulation remains intact (indicated by green and black dots; Fig. 4A), and another set of electrodes were placed apical to the site of occlusion where circulation is blocked (indicated by red and blue dots). FSCV was performed spanning a time frame 60 s before, during occlusion, and into the reperfusion phase. Figure 4B provides the kymographs for each channel (indicated by the colored dot to the left of each kymograph). As in Fig. 3, black horizontal lines indicate the peak potential for NE oxidation. Line profiles for current magnitude were pulled as a function of time from the kymographs, calibrated against the standard curve, and plotted (Fig. 4C). These data demonstrate that myocardium apical to the occlusion site (red and blue dots) exhibited a strong elevation in interstitial NE, whereas those regions of the left ventricle receiving normal circulation (green and black dots) did not demonstrate an increase in NE levels beyond baseline. Thus, our approach is capable of providing spatially resolved, high temporal resolution readouts of local NE release under cardiac ischemia and stress.
Fig. 4.
In vivo norepinephrine measured during transient myocardial ischemia. Fast-scanning cyclic voltammetry (FSCV) was used to measure interstitial norepinephrine levels at various locations in the left ventricle in response to transient left anterior descending (LAD) artery occlusion. A: location of platinum (Pt) wire electrodes are shown and color coded by location. Green and black electrode sites are within the basal aspects of the left ventricle with normal perfusion and blue and red sites within the ischemic zone. B: kymographs are provided for each probe location showing oxidative FSCV currents at baseline during the coronary occlusion and continuing into the reperfusion phase. Black horizontal line indicates the norepinephrine current for each probe. C: dynamic changes in interstitial NE levels in response to transient occlusion of the LAD coronary artery are plotted for each probe location. Note the regional disparity with interstitial NE increasing within the ischemic zone (red and blue) and remaining at baseline in areas of the left ventricle, where perfusion was unaffected (green and black).
Finally, NE measurements under varied autonomic and cardiac interventions were correlated to hemodynamic responses measured simultaneously in the same test preparation. Four electrodes were placed across the left ventricle, one basal, one apical, and two lateral. NE release was evaluated during transient occlusions of the descending aorta (AO; an increase in afterload; Fig. 5A) or inferior vena cava (IVC; a decrease in preload; Fig. 5B) and induction of premature ventricular contractions via programmed pacing (PVC; Fig. 5C). As expected, the aortic occlusion resulted in reflex-mediated decreased interstitial NE levels, followed by a rebound after release of the occlusion. Additionally, both inferior vena cava occlusion and ectopic stimulation increased interstitial NE as expected (Table 1). Hemodynamic parameters (LVSP, HR, and dP/dt) mirrored the evoked changes in NE concentration (Table 2). Thus, NE measured by FSCV, in the beating heart, correlates with well-characterized physiological responses to autonomic stressors.
Table 2.
Complementary hemodynamic parameters for norepinephrine data traces in Fig. 5
| Stressor (Corresponding Part of Fig. 5) | LVSP (Baseline, Stimulated), mmHg | LV Developed Pressure (Baseline, Stimulated), mmMg/s |
|---|---|---|
| Aortic occlusion (A) | 115, 156 | 1,733, 1,655 |
| IVC occlusion (B) | 106, 72 | 1,850, 1,044 |
| PVC stimulation (C) | 133, 152 | 1,584, 1,787 |
Left ventricular (LV) peak systolic pressure (LVSP; mmHg) and developed LV pressure (dP/dt, mmHg/s) were measured before and during (baseline, stimulated) indicated stressors simultaneously with the fast-scanning cyclic voltammetry recordings presented in the indicated corresponding part of Fig. 5. IVC, inferior vena cava; PVC, premature ventricular contraction.
DISCUSSION
Electrochemical approaches for catecholamine detection have been well established in the fields of neuroscience and analytic chemistry. Steady-state (i.e., fixed potential) amperometric detection of catecholamine release from isolated neuroendocrine chromaffin cells represented a breakthrough in the study of the molecular basis of neurotransmitter exocytosis (7, 19). Indeed, this implementation of electrochemical detection exhibits sub-millisecond resolution, and this has been a key tool in the study of fusion pore regulation in the secretion process that is able to measure the rate of release of catecholamine through single fusion pores (12, 31). However, steady-state amperometry suffers from the limitation that it cannot determine which type of oxidizable substance is being released (6), and thus it is not appropriate for tissue-level studies where multiple oxidizable molecules may be present.
Fast-scanning cyclic voltammetry (FSCV) relies on scanning the probe potential through the range of oxidation potentials of many substances. Identification of which substance is oxidizing is accomplished through measuring the specific oxidation potential (i.e., separating norepinephrine from dopamine) or by measuring the full spectrum of oxidation reactions (i.e., norepinephrine from epinephrine). In this dynamic electrochemical approach, the electrode potential is driven by a voltage clamp circuit with a dynamic command potential spanning the oxidation-reduction potentials for NE. Thus, as the electrode potential is driven in a positive dV/dt past the oxidation potential for NE, the NE is oxidized to a quinone product and releases two electrons. These electrons are then measured as a compensating current in the voltage clamp and report the detection of a single molecule of NE (25). Driving the electrode potential back to a negative polarization reduces the quinone product to regenerate the catecholamine. In our application, we devised a form of FSCV appropriate to measure norepinephrine at discrete locations in the myocardium with minimal tissue damage, fast sample frequency, and rapid data analysis and in multiple parallel channels. We developed and characterized long (30 cm), flexible, platinum PFA-insulated electrodes, allowing us to reach the heart in an open-chest porcine model. Additionally, we revised the circuitry design of a commercially available, multichannel voltammetry amplifier to meet the accommodate capacitance of the platinum electrodes and to provide a stable and accurate reference potential for the voltage clamp circuitry.
Platinum electrodes are very commonly used in nerve recordings. They are flexible, available in a variety of diameters, provide a low level of reactivity, and do not readily corrode. Thus, they exhibit several characteristics required to be used on the dynamic context of open-chest heart recordings. One of the proprieties of platinum is that they are not a purely capacitative material, meaning that when a voltage is applied, they do transfer charge into the surrounding tissue. This characteristic defines a limitation on the electronics used to clamp the electrodes to the desired command potential. One must be able to push significant current to charge the capacitance of the electrode to clamp it to the command potential. As described in materials and methods, we collaborated with NPI Electronic to build a custom device for this purpose. This amplifier incorporates four individual and separately controlled voltage clamp channels with switchable gain, filter, and command potential inputs. The single head stage connects to and drives four independent electrodes but utilizes a single reference/ground circuit for all four channels. We designed the head stage to be switchable between a 1 and 10 MΩ feedback resistor, which is low enough to push significant current required and high enough to provide a reliable voltage clamp of the electrode while providing a large range.
Perspectives and Clinical Relevance
Neural control of the heart reflects a hierarchy of interdependent reflex loops involving intrathoracic and central nervous system neural networks (3). The efferent outputs for the cardiac nervous system are the parasympathetic and sympathetic neurons (18, 22). At rest, there is a parasympathetic predominance that shifts to a sympathetic dominance during high levels of stress (2, 22). Cardiac disease disrupts not only heart muscle but also the cardiac nervous system (1, 26, 28). Both the progression of heart failure and the potential for sudden cardiac death are associated with excessive sympathocardiac excitation (9, 11) with resultant elevated interstitial cardiac NE (4). Heterogeneous and high levels of sympathetic output to the heart are major risk factors for morbidity and mortality (9, 11, 14). Although direct nerve recordings of sympathetic firing provide an index of neuronal activity (15), measurement of catecholamine levels directly within the heart would provide the most relevant measure of neurotransmitter-receptor interactions, especially when evaluating autonomic tone or assessing regional NE release. This has a high degree of relevance, especially in structural heart disease, where heterogeneities in the cardiac electrical substrate are amplified by disparate levels of NE, leading to high risk for ventricular arrhythmias, including tachycardia/fibrillation (11), and where an increased cardiac sympathetic tone as indicated by increased NE levels portends a poor prognosis (8). Current approaches for functional readouts of cardiac NE using microdialysis cannot be easily performed in humans and are severely limited in their spatial/temporal readout capability and most often provide data only after significant time delay. Cardiac sympathetic imaging suffers from poor regional resolution in addition to significant time delay and is costly.
Using our approach and multiple interfaces dispersed throughout the left ventricle, we demonstrate that it is possible to obtain high-resolution dynamic readouts of catecholamine interstitial levels at baseline and in response to stress with FSCV from a single subject. Proof of concept for this approach is shown in response to direct electrical stimulation of the sympathetic postganglionic projections to the heart, transient myocardial ischemia, and changes in preload and afterload and in response to induced premature ventricular contractions, all interventions that can alter sympathetic output to the heart. As expected, some of interventions evoked similar changes in NE throughout the ventricles (e.g., stellate ganglia stimulation and PVCs). Other stressors, especially regional myocardial ischemia, caused disparate release of NE. A major strength of this approach that lends itself to eventual clinical application is that it is able to resolve responses from single subjects with high spatiotemoral resolution. This point is critical to allow for characterization of individual cardiopathologies with nonuniform tissue damage, as expected in myocardial infarction. General information from a population response does not provide such individualized information. Importantly, we show that these regional NE readouts as provided by FSCV are stable over time and have a dynamic range that covers the NE concentrations up to and including pathological levels (5). When paired with high-density recording of regional cardiac electrical/mechanical function, this technology holds great promise in unraveling the mechanisms underlying arrhythmia formation and pump failure.
Finally, in an effort to test broader application and extend the FSCV-based approach in vivo as described here, we have further tested this approach in other model organisms, tissues, and extracellular fluids where norepinephrine modulates physiological activities. These studies are in progress but include vascular compartments of pig and mouse, adipose tissues in mice, and rat urine. Promising results have been obtained and indicate that the FSCV measure of norepinephrine will be applicable across a broad set of conditions.
Limitations.
Electrode-to-electrode variable impedance and capacitative phase shift of the signal occur, requiring potential offset and amplitude compensation during analysis. In its present iteration, the chest must be open for insertion of the electrode interface, and only one active recording site is available from each electrode. These experiments were performed under general anesthesia, which may diminish NE release and reflex responses to cardiac interventions. Further studies should consider a microarray interface to provide transmural readouts of NE levels and a vascular insertion route so that FSCV can be utilized in the closed-chest preparation.
GRANTS
This work was supported by National Institute of Biomedical Imaging and Bioengineering Multiple Principal Investigator Grant UO1-EB-025138 (to J.L.A., C.S., K.S., and M.V.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.V., K.S., J.L.A., and C.S. conceived and designed research; S.-A.C., N.K., and C.S. performed experiments; S.-A.C., M.V., N.K., J.L.A., and C.S. analyzed data; M.V., K.S., J.L.A., and C.S. interpreted results of experiments; S.-A.C. and C.S. prepared figures; C.S. drafted manuscript; S.-A.C., M.V., K.S., J.L.A., and C.S. edited and revised manuscript; S.-A.C., M.V., K.S., J.L.A., and C.S. approved final version of manuscript.
REFERENCES
- 1.Ajijola OA, Hoover DB, Simerly TM, Brown TC, Yanagawa J, Biniwale RM, Lee JM, Sadeghi A, Khanlou N, Ardell JL, Shivkumar K. Inflammation, oxidative stress, and glial cell activation characterize stellate ganglia from humans with electrical storm. JCI Insight 2: 94715, 2017. doi: 10.1172/jci.insight.94715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ardell JL, Andresen MC, Armour JA, Billman GE, Chen PS, Foreman RD, Herring N, O’Leary DS, Sabbah HN, Schultz HD, Sunagawa K, Zucker IH. Translational neurocardiology: preclinical models and cardioneural integrative aspects. J Physiol 594: 3877–3909, 2016. doi: 10.1113/JP271869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ardell JL, Armour JA. Neurocardiology: Structure-Based Function. Compr Physiol 6: 1635–1653, 2016. doi: 10.1002/cphy.c150046. [DOI] [PubMed] [Google Scholar]
- 4.Ardell JL, Foreman RD, Armour JA, Shivkumar K. Cardiac sympathectomy and spinal cord stimulation attenuate reflex-mediated norepinephrine release during ischemia preventing ventricular fibrillation. JCI Insight 4: 131648, 2019. doi: 10.1172/jci.insight.131648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arora RC, Cardinal R, Smith FM, Ardell JL, Dell’Italia LJ, Armour JA. Intrinsic cardiac nervous system in tachycardia induced heart failure. Am J Physiol Regul Integr Comp Physiol 285: R1212–R1223, 2003. doi: 10.1152/ajpregu.00131.2003. [DOI] [PubMed] [Google Scholar]
- 6.Chow RH, von Rüden L. Electrochemical detection of secretion from single cells. In: Single-Channel Recording (2nd ed.), edited by Sakmann B and Neher E. New York: Plenum Press, 1995, chapt. 11, p. 245–275. [Google Scholar]
- 7.Chow RH, von Rüden L, Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature 356: 60–63, 1992. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- 8.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311: 819–823, 1984. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 9.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res 114: 1815–1826, 2014. doi: 10.1161/CIRCRESAHA.114.302589. [DOI] [PubMed] [Google Scholar]
- 10.Foreman RD, Garrett KM, Blair RW. Mechanisms of cardiac pain. Compr Physiol 5: 929–960, 2015. doi: 10.1002/cphy.c140032. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda K, Kanazawa H, Aizawa Y, Ardell JL, Shivkumar K. Cardiac innervation and sudden cardiac death. Circ Res 116: 2005–2019, 2015. doi: 10.1161/CIRCRESAHA.116.304679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fulop T, Doreian B, Smith C. Dynamin I plays dual roles in the activity-dependent shift in exocytic mode in mouse adrenal chromaffin cells. Arch Biochem Biophys 477: 146–154, 2008. doi: 10.1016/j.abb.2008.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hankes GH, Ardell JL, Tallaj J, Wei CC, Aban I, Holland M, Rynders P, Dillon R, Cardinal R, Hoover DB, Armour JA, Husain A, Dell’Italia LJ. Beta1-adrenoceptor blockade mitigates excessive norepinephrine release into cardiac interstitium in mitral regurgitation in dog. Am J Physiol Heart Circ Physiol 291: H147–H151, 2006. doi: 10.1152/ajpheart.00951.2005. [DOI] [PubMed] [Google Scholar]
- 14.Hanna P, Shivkumar K, Ardell JL. Calming the Nervous Heart: Autonomic Therapies in Heart Failure. Card Fail Rev 4: 92–98, 2018. doi: 10.15420/cfr.2018.20.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart EC, Head GA, Carter JR, Wallin BG, May CN, Hamza SM, Hall JE, Charkoudian N, Osborn JW. Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. Am J Physiol Heart Circ Physiol 312: H1031–H1051, 2017. doi: 10.1152/ajpheart.00703.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaffe EH, Marty A, Schulte A, Chow RH. Extrasynaptic vesicular transmitter release from the somata of substantia nigra neurons in rat midbrain slices. J Neurosci 18: 3548–3553, 1998. doi: 10.1523/JNEUROSCI.18-10-03548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jänig W. Functional anatomy of the peripheral sympathetic and parasympathetic system. In: The Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis, edited by Cambridge WJ. New York: Cambridge University Press, 2006, p. 13–34. [Google Scholar]
- 18.Janig W. The Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis. New York: Cambridge University Press, 2006. [Google Scholar]
- 19.Jankowski JA, Schroeder TJ, Holz RW, Wightman RM. Quantal secretion of catecholamines measured from individual bovine adrenal medullary cells permeabilized with digitonin. J Biol Chem 267: 18329–18335, 1992. doi: 10.21236/ADA251716. [DOI] [PubMed] [Google Scholar]
- 20.Killingsworth CR, Wei CC, Dell’Italia LJ, Ardell JL, Kingsley MA, Smith WM, Ideker RE, Walcott GP. Short-acting beta-adrenergic antagonist esmolol given at reperfusion improves survival after prolonged ventricular fibrillation. Circulation 109: 2469–2474, 2004. doi: 10.1161/01.CIR.0000128040.43933.D3. [DOI] [PubMed] [Google Scholar]
- 21.Leszczyszyn DJ, Jankowski JA, Viveros OH, Diliberto EJ Jr, Near JA, Wightman RM. Secretion of catecholamines from individual adrenal medullary chromaffin cells. J Neurochem 56: 1855–1863, 1991. doi: 10.1111/j.1471-4159.1991.tb03441.x. [DOI] [PubMed] [Google Scholar]
- 22.Levy MN, Martin PJ. Neural control of the heart. In: Handbook of Physiology, The Cardiovascular System, The Heart, edited by Berne RM. Bethesda, MD: American Physiological Society, 1979, vol. 1, sect. 2, p. 581–620. [Google Scholar]
- 23.Longhurst JC, Tjen-A-Looi SC, Fu LW. Cardiac sympathetic afferent activation provoked by myocardial ischemia and reperfusion. Mechanisms and reflexes. Ann NY Acad Sci 940: 74–95, 2001. doi: 10.1111/j.1749-6632.2001.tb03668.x. [DOI] [PubMed] [Google Scholar]
- 24.Malliani A, Lombardi F, Pagani M, Recordati G, Schwartz PJ. Spinal cardiovascular reflexes. Brain Res 87: 239–246, 1975. doi: 10.1016/0006-8993(75)90421-7. [DOI] [PubMed] [Google Scholar]
- 25.Pihel K, Schroeder TJ, Wightman RM. Rapid and selective cyclic voltammetric measurements of epinephrine and norepinephrine as a method to measure secretion from single bovine adrenal medullary cells. Anal Chem 66: 4532–4537, 1994. doi: 10.1021/ac00096a021. [DOI] [Google Scholar]
- 26.Rajendran PS, Nakamura K, Ajijola OA, Vaseghi M, Armour JA, Ardell JL, Shivkumar K. Myocardial infarction induces structural and functional remodelling of the intrinsic cardiac nervous system. J Physiol 594: 321–341, 2016. doi: 10.1113/JP271165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tallaj J, Wei CC, Hankes GH, Holland M, Rynders P, Dillon AR, Ardell JL, Armour JA, Lucchesi PA, Dell’Italia LJ. Beta1-adrenergic receptor blockade attenuates angiotensin II-mediated catecholamine release into the cardiac interstitium in mitral regurgitation. Circulation 108: 225–230, 2003. doi: 10.1161/01.CIR.0000079226.48637.5A. [DOI] [PubMed] [Google Scholar]
- 28.Vaseghi M, Salavatian S, Rajendran PS, Yagishita D, Woodward WR, Hamon D, Yamakawa K, Irie T, Habecker BA, Shivkumar K. Parasympathetic dysfunction and antiarrhythmic effect of vagal nerve stimulation following myocardial infarction. JCI Insight 2: 86715, 2017. doi: 10.1172/jci.insight.86715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaseghi M, Yamakawa K, Sinha A, So EL, Zhou W, Ajijola OA, Lux RL, Laks M, Shivkumar K, Mahajan A. Modulation of regional dispersion of repolarization and T-peak to T-end interval by the right and left stellate ganglia. Am J Physiol Heart Circ Physiol 305: H1020–H1030, 2013. doi: 10.1152/ajpheart.00056.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh PL, Petrovic J, Wightman RM. Distinguishing splanchnic nerve and chromaffin cell stimulation in mouse adrenal slices with fast-scan cyclic voltammetry. Am J Physiol Cell Physiol 300: C49–C57, 2011. doi: 10.1152/ajpcell.00332.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang CT, Bai J, Chang PY, Chapman ER, Jackson MB. Synaptotagmin-Ca2+ triggers two sequential steps in regulated exocytosis in rat PC12 cells: fusion pore opening and fusion pore dilation. J Physiol 570: 295–307, 2006. doi: 10.1113/jphysiol.2005.097378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf K, Zarkua G, Chan SA, Sridhar A, Smith C. Spatial and activity-dependent catecholamine release in rat adrenal medulla under native neuronal stimulation. Physiol Rep 4: e12898, 2016. doi: 10.14814/phy2.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolfe JT, Wang H, Perez-Reyes E, Barrett PQ. Stimulation of recombinant Cav3.2, T-type, Ca2+ channel currents by CaMKIIγC. J Physiol 538: 343–355, 2002. doi: 10.1113/jphysiol.2001.012839. [DOI] [PMC free article] [PubMed] [Google Scholar]