Structural and biochemical analysis of xylan O-acetyltransferase 1 (XOAT1) show that XOAT1 catalyzes xylan acetylation through formation of an acyl-enzyme intermediate via a double displacement bi-bi mechanism.
Abstract
Xylans are a major component of plant cell walls. O-Acetyl moieties are the dominant backbone substituents of glucuronoxylan in dicots and play a major role in the polymer-polymer interactions that are crucial for wall architecture and normal plant development. Here, we describe the biochemical, structural, and mechanistic characterization of Arabidopsis (Arabidopsis thaliana) xylan O-acetyltransferase 1 (XOAT1), a member of the plant-specific Trichome Birefringence Like (TBL) family. Detailed characterization of XOAT1-catalyzed reactions by real-time NMR confirms that it exclusively catalyzes the 2-O-acetylation of xylan, followed by nonenzymatic acetyl migration to the O-3 position, resulting in products that are monoacetylated at both O-2 and O-3 positions. In addition, we report the crystal structure of the catalytic domain of XOAT1, which adopts a unique conformation that bears some similarities to the α/β/α topology of members of the GDSL-like lipase/acylhydrolase family. Finally, we use a combination of biochemical analyses, mutagenesis, and molecular simulations to show that XOAT1 catalyzes xylan acetylation through formation of an acyl-enzyme intermediate, Ac–Ser-216, by a double displacement bi-bi mechanism involving a Ser-His-Asp catalytic triad and unconventionally uses an Arg residue in the formation of an oxyanion hole.
INTRODUCTION
The plant cell wall provides a structural scaffold that surrounds each plant cell, defining the size and shape of the enclosed cells and resultant tissues. The plant cell wall is a composite material comprised of cellulose microfibrils, hemicellulosic polysaccharides, pectins, proteins, glycoproteins, and aromatic molecules (including lignins). Xylans are one of the most abundant plant polysaccharides on earth and are hence considered to be key targets for biomass modification to enhance the production of biofuels, bioproducts, and biomaterials (Smith et al. 2017). Most cell wall polysaccharides, with the exception of (1,3;1,4)-β-glucan and cellulose, are O-acetylated (Pauly and Ramírez 2018). All xylans produced by vascular plants are substituted with glycosyl or acyl groups on an identical backbone structure composed of 1,4-linked β-d-xylopyranosyl (Xyl) residues. The nature and pattern of these substituents vary depending on the plant tissue and species (Smith et al. 2017). For example, in dicots, O-acetylated glucuronoxylan (AcGX) is the most abundant hemicellulosic polysaccharide, whose backbone is substituted with 1,2-linked α-d-glucuronic acid (GlcA) and/or its 4-O-methyl derivative (MeGlcA). Approximately 60% of the backbone residues are O-acetylated, making (Me)GlcA the most abundant substituent of xylan. More detailed analysis of xylan acetylation in the Arabidopsis (Arabidopsis thaliana) Columbia (Col-0) accession showed that 44% of Xyl residues are monoacetylated at O-2 or O-3, 6% are bisubstituted at both O-2 and O-3, and ∼75% of the (Me)GlcA substituted backbone residues are also acetylated at the O-3 position (Chong et al. 2014). Furthermore, tandem mass spectrometry (MS/MS) analysis of enzymatically released neutral xylooligosaccharides revealed that acetyl residues are present on every alternate Xyl along the backbone, whereas a similar systematic, alternating pattern of (Me)GlcA and acetyl moieties is also observed on the released acidic xylooligosaccharides (Chong et al. 2014). This data suggests that acetylation may play a key role in the systematic addition of substituents along the polymer backbone.

Although the specific structural and biological roles of polysaccharide acetylation in the plant cell wall remain unclear, O-acetylation is thought to be a determinant of AcGX hydrophobicity, partially dictating interactions between xylan and other wall polymers, including cellulose and lignin (Busse-Wicher et al. 2014; Johnson et al. 2017). For example, the presence of O-acetyl substituents decreases adsorption of xylan to cellulosic surfaces, indicating that xylan-cellulose and xylan-lignin interactions may be modulated by the degree and patterning of substituents (Köhnke et al. 2011; Kang et al. 2019). The effect of acetylation patterning on xylan interactions is unknown, but a similar pattern can be found in xylans from the primary cell wall of grasses, which are heavily substituted with arabinosyl residues at O-2 or O-3 or at both O-2 and O-3 (Smith et al. 2017). Recent studies on poplar (Populus trichocarpa) genotypes with different cell wall compositions suggest that there is a close interaction between lignin and xylan and that the degree of xylan acetylation influences the interaction between these major cell wall polymers, thus impacting pretreatment efficiencies (Johnson et al. 2017). Furthermore, acetyl groups might sterically hinder some hydrolytic enzymes and thus decrease the accessibility of those enzymes to their polysaccharide targets (Biely 2012). Taken together, it is evident that xylan acetylation affects cell wall architecture and mechanical strength. Therefore, having a better sense of acetylation mechanisms in plants will form the basis for a deeper understanding into the interplay between polysaccharide primary structure and cell wall architecture, growth, and development. Advances in this area may overcome biomass recalcitrance to enzymatic saccharification, leading to the development of improved design schema for engineering efforts or targeted genomics approaches for the conversion of cell wall rich plant biomass into sustainable bioproducts.
Until recently, little was known about the process of plant cell wall polysaccharide O-acetylation. Despite the importance of this modification in both cell wall structure and organization, the identity of the acetyl donor substrates or the existence of donor intermediates and the exact roles of many of the proteins involved in polysaccharide acetylation remain unknown. At least four protein families participate in the acetylation pathway in plants, including the Trichome Birefringence-Like (TBL) protein family, reduced wall acetylation (RWA) proteins (Manabe et al. 2013), the Altered XYloglucan 9 (AXY9) protein (Schultink et al. 2015), and a newly described GDSL acetylesterase family (Pauly and Ramírez 2018).
Members of the TBL family function as polysaccharide O-acetyltransferases and catalyze O-acetylation of specific cell wall polymers (Yuan et al. 2016b, 2016c; Zhong et al. 2017, 2018; Stranne et al. 2018). ESKIMO1/TBL29 (At3g55990) is one of the most well-characterized members of this family. Plants carrying loss of function alleles in Arabidopsis (eskimo1, tbl29-1, tbl29-2) produce xylan with 50% to 60% less O-acetylation, have collapsed xylem vessels (Xiong et al. 2013; Lefebvre et al. 2011), and are tolerant to salt, drought, and freezing stresses. Our earlier studies on this enzyme provided direct biochemical evidence that TBL29/ESK1 is a xylan specific O-acetyltransferase that catalyzes the addition of O-acetyl groups to the 2-position of xylosyl backbone residues in vitro, establishing the precise molecular function of this enzyme (Urbanowicz et al. 2014) and led to the proposed name xylan O-acetyltransferase 1 (XOAT1). Recently, an eloquent report by Grantham and coworkers showed that the even pattern of xylan acetylation is absent in the esk1 mutant, indicating that XOAT1 is necessary for patterning of acetyl esters on xylan in Arabidopsis (Grantham et al. 2017). Since the initial biochemical analysis of XOAT1, several other members of the TBL family have also been shown to play a role in the regiospecific acetylation of xylan in Arabidopsis, including TBL3 (XOAT4), TBL28 (XOAT2), TBL30 (XOAT3), TBL31 (XOAT5), TBL32 (XOAT6), TBL33 (XOAT7), TBL34 (XOAT8), and TBL35 (XOAT9; Yuan et al. 2016a, 2016b, 2016c; Zhong et al. 2017).
In plants, acetyl-CoA cannot readily cross membranes and is independently produced in plastids, mitochondria, peroxisomes, and the cytosol, but not in the Golgi apparatus. Members of the RWA family function nonspecifically in polysaccharide O-acetylation at a biosynthetic step before TBLs and have been proposed to transport an activated form of acetate into the Golgi using cytosolic acetyl-CoA pools (Manabe et al. 2013). However, there is no evidence to date that known polysaccharide O-acetylating systems, including RWA, OatA, and AlgI, function as acetyl-CoA transporters (recently reviewed by Pauly and Ramírez, 2018; Brott et al., 2019). Currently, these systems are proposed to function by translocating acetyl groups derived from cytosolic acetyl-CoA or an unknown donor in both plants and microbes, but not acetyl-CoA itself, across membranes (Brott et al., 2019).
Understanding the precise function of enzymes involved in plant polysaccharide biosynthesis, including glycosyltransferases, acetyltransferases, and methyltransferase at the molecular level, is essential for gaining fundamental knowledge into how these biocatalysts work together to build architecturally complex structures such as the cell wall. Structural information of enzymes involved in polysaccharide biosynthesis, especially those involved in the addition of methyl and acetyl substituents present in plant cell wall polysaccharides, is missing in structural databases despite their significant roles in plant growth and development. Recently, mammalian cell expression has been successfully used to express plant glycosyltransferases involved in xyloglucan biosynthesis in sufficient quantities for structural and biochemical characterization, including Arabidopsis fucosyltransferase-1 (FUT1) and xyloglucan xylosyltransferase (XXT; Urbanowicz et al. 2017; Culbertson et al. 2018). Here, we applied this strategy for structural characterization of the xylan O-acetyltransferase XOAT1. We show that the structure of XOAT1 is characterized by a deep cleft on the surface of the protein, separating the molecule into two unequal lobes. A Ser-216–His-465–Asp-462 catalytic triad is located at the bottom of this cleft. Molecular simulations of XOAT1 in its substrate-bound states as well as experimental characterization of the acyl-enzyme intermediate provide evidence for a double-displacement mechanism.
RESULTS AND DISCUSSION
Biochemical Insights into XOAT1 Catalysis
Members of the plant-specific domain of unknown function (DUF) 231 family, also referred to as the trichome birefringence-like (TBL) family, are Golgi-localized polysaccharide O-acetyltransferases (Pauly and Ramírez 2018). XOAT1 comprises a single NH2-terminal transmembrane (T) domain, followed by an NH2-terminal variable region (NV), an N-terminal Cys-rich Pfam domain (PF14416) that is part of the plant-specific TBL region, and the DUF231 domain; XOAT1 also contains six predicted N-glycosylation sites (Figure 1A). We previously developed a mass spectrometry (MS)-based xylan O-acetyltransferase assay (Figure 1B), using acetyl‐CoA as the acetyl donor and 2‐aminobenzamide β‐1,4‐xylohexaose (Xyl6‐2AB) as the acceptor substrate to study enzymes involved in polysaccharide O-acetylation in vitro (Urbanowicz et al. 2014). The peptidoglycan O-acetyltransferase from Neisseria gonorrheae PatB and the secondary cell wall polysaccharide O-acetyltransferase from Bacillus cereus PatB1 were recently shown to display donor substrate promiscuity and use chromogenic acetyl-donor substrates in vitro (Sychantha et al., 2018; Brott et al., 2019). To determine whether other activated acetyl substrates may function as donor substrates for XOAT1, we performed in vitro xylan acetylation reactions to compare the ability of p-nitrophenyl acetate (pNP-Ac), acetylsalicylic acid (ASA), acetyl-CoA, and 4-methylumbelliferylacetate (4MU-Ac) to function as donor substrates for XOAT1. Matrix‐assisted laser desorption ionization time‐of‐flight mass spectrometry (MALDI‐TOF MS) analysis of the reaction products indicated that recombinant XOAT1 catalyzes the transfer of O-acetyl moieties from all four substrates to Xyl6‐2AB. When compared to acetyl-CoA, pNP-Ac, ASA, and 4MU-Ac all serve as better donor substrates in vitro (Figure 1C). This is consistent with a recent report by Ye and colleagues that was published during the preparation of this manuscript (Zhong et al. 2020).
Figure 1.
Biochemical Properties of XOAT1.
(A) Scaled XOAT1 domain architecture described in this study. T: NH2-terminal transmembrane domain; NV: NH2-terminal variable region; TBL: TBL domain; DUF231: DUF231 domain. The transmembrane helix was predicted using TMHMM Server v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM/; Krogh et al., 2001). Prediction of the N-glycosylation sites (red diamonds) were performed using the NetNGlyc 1.0 Server (http://www.cbs.dtu.dk/services/NetNGlyc/; Blom et al., 2004). Scale bar = 100 amino acids.
(B) In vitro reaction scheme of xylan O-acetylation catalyzed by XOAT1.
(C) Donor substrate promiscuity of XOAT revealed by MALDI-TOF MS analysis of acetylated Xyl6-2AB generated by incubating XOAT1 with Xyl6-2AB acceptors and different acetyl-donors, acetyl-CoA, para-nitrophenyl acetate (pNP-Ac), acetylsalicylic acid (ASA), and 4-methylumbelliferylacetate (4MU-Ac) for 6 h. Each transfer of O-acetyl group increases the mass of Xyl6-2AB by 42 Da, as indicated by annotating [M+H]+ ions.
(D) Percentage of acyl-XOAT1 formed upon reaction with different acetyl donors. Error bars indicate mean ± sd from three technical replicates from one representative experiment. Statistical significance was determined with the two-tailed Student’s t test. *P < 0.05; **P < 0.01.
(E) Real-time 1H NMR analysis of XOAT1-catalyzed reaction by using acetyl-CoA and xylohexaose (Xyl6) as substrates. The thickened green line corresponds to the spectrum of the reaction at the 6-h time point, whereas the thickened purple line indicates the end of the 20-h reaction.
(F) Acetyl group migration analysis through real-time 1H NMR after removal of XOAT1. The thickened red line corresponds to the starting point for monitoring acetyl group migration after removal of XOAT1 after a 6-h reaction, whereas the thickened purple line represents the end point of monitoring the migration (14 h after transfer).
The preference for non–acetyl-CoA cosubstrates is consistent with what was observed for PatB (Moynihan and Clarke 2013) and calls into question the identity of the natural acetyl donors for this enzyme family in planta. However, the observation that XOAT1 can use surrogate compounds as donor substrates also facilitated the development of an in vitro xylan acetyltransferase assay utilizing the chromogenic acetyl donor pNP-Ac, adapted from methods described for bacterial polysaccharide acetyltransferases (Brott et al., 2019). We performed spectrophotometric assays to evaluate the O-acetyltransferase activity of XOAT1 by using pNP-Ac as a donor substrate together with increasing concentrations of xylopentaose (Xyl5) as an acceptor to validate our assays (Supplemental Figure 1). The release of pNP increased in parallel with the concentration of Xyl5 added to the reactions, indicating that the presence of Xyl5 facilitates the activity of XOAT1 as a xylan O-acetyltransferase and confirmed that this is an efficient method to determine the biochemical properties of xylan acetyltransferases. Next, we evaluated the ability of XOAT1 to use xylobiose and xylooligosaccharides with degrees of polymerization from two to six as acceptors, and showed that xylotriose is the smallest acceptor that XOAT1 can O-acetylate (Supplemental Figure 2), consistent with prior biochemical analyses of XOAT1 using 14C-labeled acetyl-CoA (Zhong et al. 2017).
XOAT1 O-Acetylates Xylan Through the Formation of a Covalent Acetyl-Enzyme Intermediate
To determine whether an acyl-enzyme intermediate forms during the reaction, we incubated different donors with XOAT1 in the absence of acceptor. We then digested the enzyme with trypsin and analyzed the fragments by liquid chromatography followed by tandem mass spectrometry (LC-MS/MS). The obtained peptides containing Ser-216 (210-MMFVGDSLNR-219) appeared as a mixture of the acetylated and unmodified peptides with a difference in molecular weight of 42 Da (shifted by m/z 21 for the doubly charged ion where z = 2; Supplemental Figure 3) corresponding to an attachment of an acetyl group. We mapped peptide sequences by MS/MS analysis, confirming that Ser-216 was the acetylation site, as shown in the higher energy collisional dissociation fragmentation spectra (Supplemental Figure 4). Further analysis of the peak areas of the extracted ion chromatographs indicated that ∼59% of the acetylated peptide population formed when acetyl-CoA was used as a donor substrate for XOAT1, whereas 73%, 74%, and 83% of peptide populations were acetylated when pNP-Ac, ASA, and 4MU-Ac were used as donors, respectively (Figure 1D). It is worth noting that the preferential donor substrates for XOAT1, revealed by quantification of the formation of the acyl-enzyme intermediates, is consistent with MALDI-TOF MS analysis of the products of the in vitro assays (Figure 1C), revealing that acetyl-CoA is the least preferred donor for XOAT1 catalysis. Furthermore, the formation of an acetyl-enzyme intermediate at Ser-216 (S216) suggested a double displacement mechanism for XOAT1-catalyzed acetyl transfer.
XOAT1 is an Obligate 2-O-Acetyltransferase
The spontaneous migration of acetyl groups can occur on xylooligosaccharides between the O-2 and O-3 positions of xylosyl backbone residues (Kabel et al. 2003) and can even rapidly migrate to position 4 on the nonreducing end (Biely et al. 2004). This makes it difficult to examine the regiospecificity of O-acetyltransferases by solely analyzing the products after the reaction is completed (Zhong et al. 2017). Therefore, the interpretation of data regarding polysaccharide acetylation can be difficult, and it is unclear whether equilibrium conditions observed are present in the native plant polysaccharide itself or are established during isolation procedures. Indeed, Kabel et al. have specifically shown that freeze-drying and/or redissolving the isolated material promotes acetyl migration on xylooligosaccharides, confirming that the position and distribution of O-acetyl moieties cannot be extrapolated to the xylan structures present in the plant (Kabel et al. 2003). Hence, the phenomenon of acetyl migration complicates determining the enzymatic regiospecificity of XOAT1. To unambiguously confirm the regiospecificity of these enzymes, we developed an experimental approach that can rapidly monitor spontaneous changes in the product during catalysis and after product formation. In these assays, we measured product formation starting 10 min after mixing all reaction components in real time at 30-min intervals and over 20 h (Figures 1E and 1F), confirming that the enzyme is an obligate 2-O-acetyltransferase. Studies by Ye et al. relied on measuring the final product after long reaction times (16 h), which would be highly affected by spontaneous migration (Zhong et al. 2017), as we observed here based on the relative increase in 3-O peak amplitude in longer incubations (Figure 1E).
The migration phenomenon of acyl groups and the regiospecificity of acetylesterases have been widely observed and studied (Kabel et al. 2003; Biely et al. 2004; Lassfolk et al. 2019; Michalak et al. 2020). We monitored XOAT1-catalyzed O-acetylation of xylohexaose (Xyl6) by real-time Proton nuclear magnetic resonance (1H NMR or RT-NMR) spectroscopy using an optimized protocol to effectively control the pH of the reaction (Figure 1E): the resonance of the methyl protons of the acetyl groups attached to the O-2 position (δ 2.176) appeared at the beginning and dominated throughout the incubation period, confirming that XOAT1 catalyzes O-acetylation at the O-2 position of xylosyl backbone residues. This is a similar trend that we reported in our previous study (Urbanowicz et al. 2014). The resonance corresponding to 3-O-acetyl groups (δ 2.160) only came after the appearance of the 2-O-acetyl resonance, suggesting acetyl group migration possibly occurring on xylopyranose units. To confirm that the acetyl group migration occurring in our reactions is driven by a nonenzymatic mechanism, we allowed enzymatic reactions to proceed for 6 h (bold red line), at which time we 1) removed XOAT1 using a spin filter and 2) continuously monitored the intensities of resonances corresponding to 2-O and 3-O-acetyl groups by RT-NMR spectroscopy for an additional 14 h (Figure 1F). In contrast to the spectra obtained during the XOAT1-catalyzed reaction, we observed a decrease of the resonance of 2-O-acetyl groups in parallel with an increase of the resonance of the 3-O-acetyl moieties, indicating that spontaneous migration of acetyl groups from the O-2 to O-3 position occurred after the XOAT1-catalyzed addition of acetyl groups to the O-2 position. These results unambiguously confirm the regiospecificity of XOAT1 in vitro by minimizing the confounding effects of spontaneous acetyl group migration.
Crystal Structure Reveals Atomistic Architecture of XOAT1
We generated three truncated forms of XOAT1 as fusion proteins containing an NH2‐terminal signal sequence followed by an 8xHis tag, an AviTag, ‘superfolder’ GFP, and the Tobacco Etch Virus (TEV) protease recognition site followed by truncated coding regions of Arabidopsis XOAT1 (Supplemental Figures 5A and 5B). We designed one construct with a truncation that removed the NH2‐terminal cytoplasmic tail and predicted transmembrane domain (amino acids 44–487, XOAT1-fl). A second construct encodes the catalytic domain and lacks the N-terminal variable region (amino acids 133-487, XOAT1-cat), and the third comprises solely the N-terminal variable region (amino acids 44-133, XOAT1-nv). Expression and secretion of GFP‐XOAT1-fl (∼121 mg L−1) and GFP-XOAT1-cat (∼92 mg L−1) in HEK293S (GnTI‐) cells yielded high levels of secreted recombinant fusion protein based on GFP fluorescence (Supplemental Table 1; Moremen et al. 2018). The activities of XOAT1-fl and XOAT1-cat were virtually the same. Reactions performed with XOAT1-nv did not result in the production of acetylated products, as expected (Supplemental Figure 5C). We therefore used XOAT1-nv as a control protein for comparative analysis using chromogenic substrates in later experiments. Taken together, these data indicate that the core catalytic domain comprises the TBL and DUF231 domains, and confirms that the N-terminal variable region does not play a direct role in catalysis.
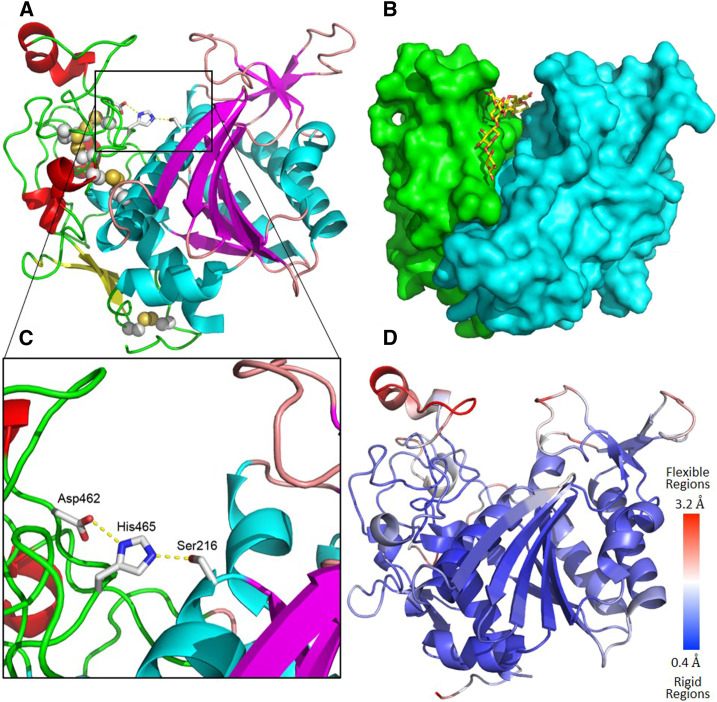
We determined the crystal structure of the XOAT1 catalytic domain (XOAT1-cat) by the single wavelength anomalous dispersion method using anomalous signals from sulfur atoms (S-SAD) at CuKα wavelength (1.54 Å). The XOAT1-cat domain monomer has approximate dimensions of 45Å × 48Å × 55Å and features an overall fold that is unique based on a search for related structures using PDBeFold (http://www.ebi.ac.uk/msd-srv/ssm/ssmcite.html). Overall, there are three β-sheets composed of seven (β4, β5, β6, β3, β9, β12, β13), four (β7, β8, β10, β11), and two (β1 and β2) β-strands; nine α-helices including a ‘broken’ one; and three α-helical turns (4-residue fragments stabilized by a single H-bond). The protein topology is presented in Supplemental Figure 6. A deep cleft divides the molecule into two unequal lobes. It is worth noting that the larger lobe formed by amino acids 197-366, 403-433, and 470-486 contains almost all secondary structure elements found in the protein: two parallel/antiparallel β-sheets, comprising seven and four β-strands, respectively, and eight large α-helices (all helices except H8). The smaller lobe includes amino acid residues 133-196, 367-402, and 434-469 and is mostly unstructured with only one short, broken α-helix (H8), three α-helical turns (αT1, αT2 and αT3), and a small two-strand antiparallel β-sheet (β1 and β2). All these unstructured loops are held together by four disulfide bridges (amino acids 140/191, 162/227, 171/467, 384/463). No disulfide bridges are present in the larger, more structured lobe (Figure 2). The deep cleft on the surface of the protein molecule is the most likely substrate binding site, with a putative classic catalytic triad (Ser-216–His-465–Asp-462) characteristic of Ser proteases (Dodson and Wlodawer 1998) at the bottom (Figures 2A to 2C). In agreement, mutations in these residues abolished XOAT1 activity (Zhong et al. 2017), and our structural data now confirms that these residues are in fact part of a classic catalytic triad. Two flexible loops form the walls of the cleft, comprised of amino acids 443-448 on one side and 273-281 on the other (Figure 2D).
Figure 2.
Overall Structure of XOAT1.
(A) Cartoon representation of the XOAT1-cat fold. Pink loops, cyan α-helices, and magenta β-strands are shown for the major lobe and green loops; red α-helices and yellow β-strands are shown for the minor lobe. Disulfides are shown as spheres. The sidechains of the catalytic triad Asp-462–His-465–Ser-216 are shown in stick representation.
(B) Surface representation reveals a putative substrate binding groove. The major lobe is shown in cyan, and the minor lobe is colored green. A xylodecaose bound in the groove in MD simulations is shown in stick representation.
(C) Zoomed in active site.
(D) Cartoon representation of the XOAT1-cat colored by flexibility of the protein regions based on MD simulations.
There are six N-glycans appended to the surface of XOAT1-cat. Four of the glycans are well-defined single N-acetylglucosamine (GlcNAc) sugars attached to Nδ atoms of Asn residues at positions 151, 241, 255, and 393 and 412, as expected after treatment with endo-β-N-acetylglucosaminidase F, which cleaves the β-1,4-link between the core N-acetylglucosamine residues of N-linked glycans. Asp-412 showed weak electron density for the fifth single GlcNAc modification; when added to the model, it refined to a strained conformation according to Privateer carbohydrate validation program (Agirre et al. 2015). After modeling and refining this density with and without GlcNAc, we decided that it was real but with an unlikely conformation caused by the weak and noisy electron density. The sixth glycan appended to Asn-425 is longer, with two GlcNAc units visible in the electron density maps that could be modeled, followed by at least one Man unit visible but not modeled due to weak and scattered electron density. Because most of the glycans are involved in the crystal contacts or are located in the immediate proximity to the crystal contacts, trimming of glycan structures to a single GlcNAc at four out of five respective sites was critical for successful crystallization of XOAT1-cat.
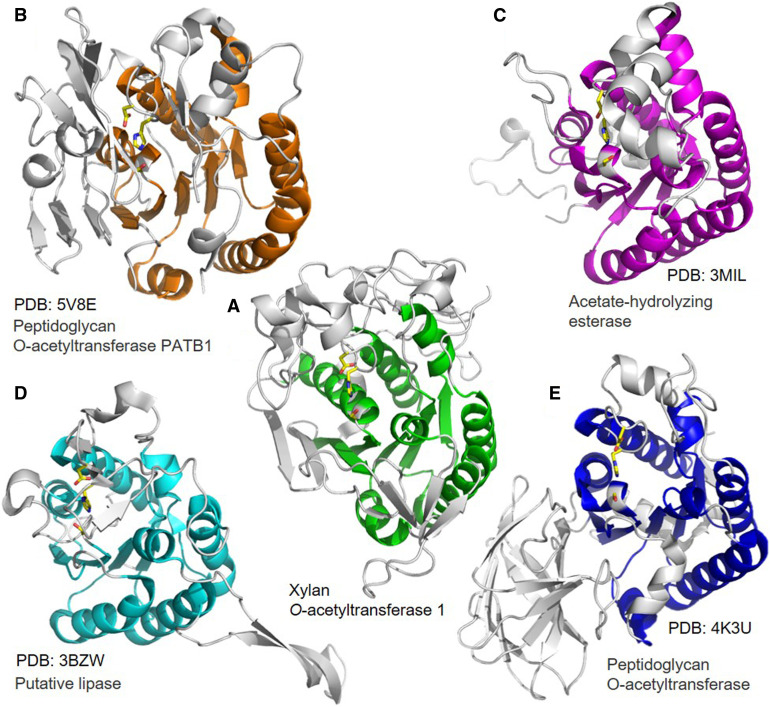
The catalytic triad (Ser-Asp-His) located in the active site cleft of XOAT1 was originally identified in Ser proteases and is found in several dissimilar enzymes and protein folds that cleave amide or ester bonds via nucleophilic attack (Dodson and Wlodawer 1998). A search for similar structures using the PDBeFOLD server indicated that the overall fold of XOAT1-cat is not present in any previously reported structures in the Protein Data Bank (PDB). However, some subregions of the enzyme, especially the central β-sheet region in the vicinity of the catalytic triad, have some similarities to known structures, despite sharing less than 10% sequence identity. Structural alignments performed with PDBeFold found two structures with a Z-score above six – a putative lipase from B. thetaiotamicron (PDB ID 3bzw, Z-score 8.2, root‐mean‐square deviation [RMSD] of 2.71 Å over 155 Cα atoms) and peptidoglycan O-acetylesterase from N. meningitidis (PDB ID 4k3u, Z-score 6.9, RMSD of 2.84 Å over 176 Cα atoms). Both proteins belong to the Pfam GDSL-like lipase/acylhydrolase family and share the α/β/α arrangement of the larger ‘structured’ lobe of XOAT1-cat, including five of the seven β-strands in the largest β-sheet flanked with seven α-helices on both sides (Figure 3). The central β-sheet region of XOAT1-cat and some of the surrounding α-helices also share some similarities upon structural alignment with two functionally similar enzymes: isoamyl acetate hydrolyzing esterase (PDB ID:3mil) from yeast (Saccharomyces cerevisiae; Ma et al. 2011) and peptidoglycan O-acetyltransferase from Bacillus cereus (PATB1; PDB ID: 5v8e; Sychantha et al. 2018). Isoamyl acetate hydrolyzing esterase performs the hydrolysis of acetyl esters, whereas PatB1 transfers an acetyl moiety from acetylated donor molecules, such as pNP-Ac onto the C3 hydroxyl of terminal β-GlcNAc residues of peptidoglycan. PatB1 is hence somewhat related by function to XOAT1. A conserved Ser-His-Asp catalytic triad also carries out catalytic activity in these four similar proteins. It is interesting to note that the order of appearance of the secondary structure domains, as well as the catalytic triad in the amino acid sequence, is Ser-Asp-His (with Ser closer to the N terminus and His closer to the C terminus) for the putative lipase, the peptidoglycan O-acetyltransferase from N. meningitidis, isoamyl acetate hydrolyzing esterase, and XOAT1. However, even though PatB1 is an acetyltransferase acting on a glycosyl moiety, the arrangement of the secondary structural elements, as well as the catalytic triad, is shifted to Asp-His-Ser (with Asp closer to the N terminus and Ser closer to the C terminus). Hence, this structural similarity of PatB1 with XOAT1 and the other similar proteins may be evidence of convergent evolution for use of a common catalytic triad, despite low primary sequence similarity among the enzymes (≤10%). Similar observations were previously made for Ser proteases containing a similar conserved catalytic triad (Buller and Townsend 2013).
Figure 3.
Active Site and Partial Conservation of Structural Domains in XOAT1.
Structural alignment of XOAT1 ([A]; Center) with peptidoglycan O-acetyltransferase from Bacillus cereus (PATB1; PDB ID: 5V8E; [B], top left), yeast Isoamyl acetate hydrolyzing esterase (PDB ID:3MIL; [C], top right), a putative lipase from Bacteroides thetaiotamicron (PDB ID 3BZW; [D], bottom left), and peptidoglycan O-acetylesterase from Neisseria meningitidis (PDB ID 4K3U; [E], bottom right). The secondary structure domains that demonstrate good alignment with XOAT1 are depicted in solid colors, whereas the nonaligned parts are grayed out. All structures share the Ser-His-Asp catalytic triad, which is shown in licorice representation with the carbons, oxygen, and nitrogen atoms colored in yellow, red, and blue, respectively.
Mutagenic Analysis Implicate Key Amino Acids in XOAT Function
The C-terminal portion of XOAT1, comprising the latter half of the plant-specific TBL domain and the entire DUF231 domain, is categorized in the Pfam database as a GDSL/SGNH-like acyl-esterase (PF13839; Supplemental Figure 7). The SGNH-hydrolase family is characterized by the presence of four invariant residues (Ser-Gly-Asn-His) in conserved blocks (I, II, III, and V). In these enzymes, the Ser in block I and the Asp and His residues in block V form the catalytic triad, whereas the backbone amide of the Gly and side-chain amides of Asn residues in blocks II and III serve as hydrogen bond donors to stabilize the tetrahedral oxyanion intermediate (Akoh et al. 2004). Sequence analysis of plant TBL proteins revealed no conserved motifs characteristic of SGNH blocks II or III (Supplemental Figure 7) in Arabidopsis (Arabidopsis thaliana). The TBL and DUF231 domains contain the conserved GDSL and DxxH motifs of blocks I and V, respectively, that together form the putative Ser-216–His-465–Asp-462 catalytic triad positioned at the bottom of the active site cleft (Figure 2; Supplemental Figure 7). We observed that the position filled by Leu in the GDSL motif can be occupied by any nonring forming basic amino acid residue in plant proteins. Directly downstream of the block I, there is a conserved RNQxxS motif that we termed plant-specific TBL-block II. In plant TBL proteins, the block V DxxH motif is part of a larger, highly conserved DCxHWCLPGxxD consensus region. We also identified a highly conserved, plant-specific RxDxH motif that we termed plant-specific TBL-block III (Supplemental Figure 7).
To evaluate the involvement of these conserved amino acids in the TBL family that may play an important role in catalysis, we performed site-directed mutagenesis of these residues in XOAT1 by substituting them with Ala. As a control, we also mutated the catalytic residues (S216A, H465A, and D462A), and verified that they abolish O-acetyltransferase activity as revealed by MALDI-TOF MS analysis of the reaction products (Figure 4A). This confirmed that S216A, H465A, and D462A are loss-of-function mutations that lack both transferase and esterase activity (Figure 4B) and confirm the utility of our in vitro assay. It is important to note that the esterase activity discussed here is indicated by release of p-nitrophenyl in the absence of acceptor, which occurs upon formation of the acyl-enzyme intermediate. Therefore, the combined assays allow us to measure both portions of the reaction: formation of the acyl-intermediate and transfer of the O-acetyl moiety from Ac–Ser-216 to the xylosyl acceptor. When we incubated XOAT1 with four electrophilic mechanism-based inhibitors of Ser proteases, N-p-tosyl-L-phenylalanyl chloromethyl ketone, 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, phenylmethylsulfonyl fluoride (PMSF) failed to abrogate the catalytic activity of XOAT1 as an O-acetyltransferase, as acetylated products had been detected by MALDI-TOF MS after the reaction, although methanesulfonyl fluoride (MSF) did. Importantly, these data suggest that either XOAT1 uses a different active site conformation compared to other Ser proteases or that the majority of these compounds are unable to access the active site pocket (Supplemental Figure 8). A similar phenomenon was observed previously: in this case, PMSF was not able to completely inactivate Neisseria gonorrheae PatB (Moynihan and Clarke 2014), whereas MSF abrogated both esterase and transferase activities of PatB in Bacillus cereus (Sychantha et al. 2018).
Figure 4.
Enzymatic Activity of XOAT1 Mutant Variants Compared with Wild-Type XOAT1.
(A) MALDI-TOF MS of the reaction products produced by XOAT1 and its mutant variants. Each transfer of an acetyl group (Ac) increases the mass of the Xyl6-2AB acceptor by 42 Da as annotated [M+H]+ ions.
(B) Comparative analysis of the esterase and transferase activities of XOAT1 and its variants, determined by measuring the release of pNP from pNP-Ac (5 mM) formed in the absence (gray) or in the presence (black) of the acceptor substrate Xyl4 (5 mM). Error bars indicate mean ± sd from four technical replicates. Statistical significance of each variant compared with the corresponding wild-typed constructs (XOAT1-cat and XOAT1aa57-486) was determined with the two-tailed Student’s t test. **P < 0.01.
(C) Close-up view of the mutated residues at the XOAT1 active site.
An important factor for the transition state of the mechanism mediated by the Ser-His-Asp triad is the oxyanion hole that stabilizes the excess negative charge on the oxygen of the acetyl group. The backbone amide of the Gly and side-chain amide of an Asn residue in SGNH blocks II and III stabilize the putative tetrahedral oxyanion through hydrogen bonding in SGNH hydrolases (Akoh et al. 2004). However, residues that would be characteristic of blocks II and III that are important for formation of the oxyanion hole in the SGNH-hydrolase family are completely absent in the plant TBL family. A recent study suggested that Arg-359 of the O-acetyltransferase PatB1 from Bacillus cereus is a key component of the oxyanion hole in that enzyme (Sychantha et al. 2018). The conserved RNQxxS motif of TBL-block II contains an Arg residue (Arg-219) that is correctly positioned in the active site and may function to stabilize the negative charges on the acetyl oxyanion during the formation of the tetrahedral reaction intermediates (Figure 3C).
We designed an R219A variant of XOAT1 to investigate its role in catalysis. The R219A variant cannot catalyze the transfer of acetyl groups to Xyl6-2AB (Figure 3A), indicating that Arg-219 plays an important role in XOAT1 catalysis. The hydrophilic side chain of Arg-219 is positioned at the surface of the active site cleft, suggesting that this residue may play a role in forming the correct conformation of the catalytic site, and might be important for the oxyanion hole formation. Furthermore, Arg-219 was observed to be an important active site residue involved in binding the donor molecule in the molecular dynamics (MD) simulations. However, it is worth noting that even though the R219A variant abolished transferase activity, as evidenced by MALDI-TOF MS of the reaction products (Figure 3A), it retained esterase activity, indicating that formation of the acyl-enzyme intermediate can still proceed to some extent (Figure 3B). Furthermore, this esterase activity increases when xylo-oligo acceptors are present in the reaction, as revealed by the spectrophotometric assay (Figure 3B), despite its inability to transfer an O-acetyl moiety to the acceptor (Figure 3A). The increased esterase activity of R219A may indicate that the binding of the acceptor substrate stabilizes folding of XOAT1 or contributes to formation of the oxyanion hole during the transfer of the acetyl group to the acceptor. Alternatively, in the absence of the guanidinium group of Arg-219, the oligosaccharide may bind in an unproductive geometry that directs the movement of a water molecule to a position from which it can attack the Ser-bound acetyl group, facilitating its hydrolysis. Thus, the binding of xylo-oligo substrates increases the esterase activity of R219A, and this mutation completely disrupts transferase activity, meaning that Arg-219 is critical for transferase activity and likely plays a role in limiting ester hydrolysis.
In most SGNH hydrolases, the side-chain amide of a conserved Asn residue in SGNH block III is also important to maintain the stability of the oxyanion hole by serving as a hydrogen bond donor for stabilization of the transition state. Although we hypothesize that the guanidinium moiety of an Arg residue in XOAT1, like PatB1, is a key contributor to formation of the oxyanion hole, we also observe the presence of an Asn residue (Asn-220) that is located close to the Ser-His-Asp catalytic triad (Figure 3C)–with its side chain amide pointing away from the active site. Thus, we evaluated the plausible role of Asn-220; however, the N220A variant retained both acetyltransferase and esterase activity, despite spatial proximity. At the end of the reaction, we detected acetylated oligosaccharide products by MALDI-TOF MS (Figure 3A) and detected (deacetylated) pNP spectrophotometrically (Figure 3B), both in the presence and absence of acceptor substrate. This result indicates that the side chain amide of Asn-220 is not involved in stabilizing the intermediate tetrahedral structure of the substrate during xylan O-acetylation. In addition, the N220A mutant has significantly more acetylhydrolase activity than either of the catalytically active controls, as indicated by the amount of pNP released in the absence of acceptor substrate (Figure 3B). These data suggest that Asn-220 might play a role in protecting the acyl-enzyme intermediate from hydrolysis, which would decrease the efficiency of the enzyme at low acceptor substrate concentrations.
Asp-215 resides directly next to the catalytic Ser-216, which is the site of nucleophilic attack during both stages of the mechanism (Figure 3C). Mutation of Asp-215 to Ala (D215A) abolished both the esterase and transferase activities of XOAT1 (Figure 3A). In addition, the level of released pNP in the hydrolysis assay was similar to that of the catalytically inactive control XOAT1-nv (Figure 3B). Because Asp-215 lies very close to the catalytic site, it may contribute to stabilizing the active site conformation. Mutation of Asp-215 resulted in a ∼75% decrease in production of soluble secreted protein (Supplemental Table 1), indicating that this mutation may also lead to unstable protein folding, as observed previously with FUT1 (Urbanowicz et al. 2017). The RxDxH motif that we termed TBL-block III contains a highly conserved His-437 residue that may play a role in substrate interactions. Mutation of His-437 to Ala (H437A) resulted in the production of nonacetylated products based on detection using MALDI-TOF MS; however, only 42% and 72% of hydrolysis activities remained in the H437A variant when reactions were respectively performed with and without the acceptor substrate (Figure 3B).
Computational Modeling Illuminates Active Site Characteristics and Reaction Mechanism
Experimental efforts to obtain substrate-bound structures of XOAT1 with either a donor molecule (pNP-Ac or acetyl-CoA) or an acceptor (xylo-bi/tri/tetra/penta/hexa-oses) were unsuccessful due to complications during the crystallization process (Supplemental Information Section I). Despite numerous attempts to crystallize the longer XOAT1-fl construct, only the XOAT1-cat domain formed crystals, with no space left in the crystal lattice for the N-terminal variable region, even if it had been disordered. We hypothesize that this tight packing interfered with our attempts to obtain a XOAT1-xylooligosaccharide co-complex. Given this lack of structural information on the substrate-bound states of XOAT1, understanding the molecular details of the mechanism and mode of action required alternative strategies. Molecular simulations have previously been used to provide information about substrate binding poses and the catalytic mechanism in cases where experimental characterization of enzyme-substrate complexes had failed (Bharadwaj et al. 2013). As outlined in the following paragraphs, molecular simulations reveal the putative active site in XOAT1 and the ability of its substrate binding groove to stabilize acceptor and donor substrates.
We first showed that XOAT1 demonstrates increased activity with longer xylan oligosaccharides (with degree of polymerization >3) and catalyzes the addition of multiple acetyl moieties onto a single acceptor, necessitating an active site that can accommodate larger acceptors (Supplemental Figure 2). MD simulations of XOAT1 in the absence of substrates reveal significant flexibility in loops surrounding the putative substrate binding groove. The presence of a Ser-His-Asp triad at the base of this loop further strengthens the hypothesis that the cleft is an extension of the active site and enables binding oligosaccharide substrates. By analogy to Ser proteases and esterases (Akoh et al. 2004), the Ser residue acts as a nucleophile assisted by the His residue, which acts as a base whose excess positive charge is stabilized by a hydrogen bond with the Asp residue (Figure 5). Supplemental Figure 9 depicts the two key hydrogen bonds that are necessary for the proper functioning of this catalytic triad. In the absence of substrates, we observed that the acid-base distance (Asp-462 O to His-465 H) is within 2 Å throughout the MD simulation, whereas the base-nucleophile distance is frequently (22% of the simulation time) within 3 Å (Figure 6). It must be noted that Asp-462 and His-465 are a part of the same minor lobe of XOAT1, whereas the nucleophile is situated on an α-helix that is part of the major lobe (Figure 2C). This may explain the observation that the acid-base distances are shorter than the base-nucleophile distances.
Figure 5.
Reaction Mechanism of XOAT1.
Proposed reaction mechanism for acetylation of xylan oligosaccharides by XOAT1. Rearrangement of the pi electrons within the His residue are not shown.
Figure 6.
Molecular Dynamics Simulation Results Corroborate Reaction.
(A) A snapshot from unbiased simulations of XOAT1 in its donor bound state reveals catalytically competent configurations for the 1st stage of the reaction mechanism. The donor molecule is shown in licorice representation with carbons colored magenta along with the important residues stabilizing it. Distances for the proton transfer and nucleophilic attack are shown with dashed lines.
(B) Distances between protons on O-2 (magenta) and O-3 (blue) of the Xyl ring closest to the catalytic triad indicate that the O-2 proton is consistently closer to the His-465 nitrogen.
(C) A snapshot from an unbiased simulation of XOAT1 in its acceptor (xylodecaose)-bound state. Three Xyl units closest to the active site are shown in licorice representation with the carbons colored yellow along with the important residues stabilizing it. The distance for proton transfer to generate the nucleophile on the Xyl unit is shown with dashed lines. Note that snapshots depicted in (A) and (C) are prereaction stationary states observed in MD simulations.
Separate MD simulations of XOAT1 in the donor- and acceptor-bound states revealed the ability of the active site and the putative binding groove to accommodate the surrogate donor molecule (pNP-Ac) and the acceptor xylodecaose substrate over simulation times in excess of 50 ns. These observations of substrate-bound configurations from unbiased MD simulations, combined with data from mutagenesis experiments, elucidate the catalytic mode of action of XOAT1. Structural alignments of subregions of the XOAT1 crystal structure with other proteins revealed conservation of important structural domains, as well as the putative catalytic triad among these enzymes (Figure 3). Furthermore, MD simulations of XOAT1 in the absence of substrates, and in the donor- and acceptor-bound states, established the stability of the catalytic triad, as well as the ability of the active site to bind the acceptor and donor molecules. The mutagenesis experiments further substantiated the indispensable role of the Ser-216–His-465–Asp-462 catalytic triad and helped identify residues that may contribute to formation of an oxyanion hole. Based on these observations, we propose that XOAT1 uses a double displacement bi-bi reaction to catalyze xylan 2-O-acetylation consisting of two stages, as illustrated in Figure 5. The first stage of catalysis involves acetylation of Ser-216 upon binding of the acetyl donor, in this case the surrogate donor pNP-Ac is depicted. Coordination of His-465 with the unprotonated Asp 462 allows His-465 to act as a general base, facilitating the nucleophilic attack of the acetyl group of the donor by Ser-216. Arg-219 itself contributes to formation of an oxyanion hole that stabilizes the putative tetrahedral transition state. The transfer (via His-465) of the Ser-216-proton (red font) onto the phenolic oxygen of the donor facilitates release of the deacylated donor (p-nitrophenol as shown) and acetylated Ser-216 (acyl-enzyme intermediate), which is supported by the LC-MS/MS data (Figure 1D; Supplemental Figures 3 and 4), demonstrating that XOAT is able to use all acetyl donors investigated in formation of the acyl-enzyme intermediate.
SUMMARY
Unraveling the identity, selectivity, and catalytic mechanisms of plant biocatalysts involved in cell wall formation is vital to understanding the roles these enzymes play in generating structures critical to the composite nature of the plant cell wall, which is fundamental to exploiting wall structural plasticity in agronomically productive crops. Xylan forms an integral part of the wall and defines its architecture and structural properties. Enzymes that catalyze the addition of substituents, such as acetyl groups to the xylan backbone (e.g., XOAT1), are of significant interest as they play key roles in shaping the polysaccharide’s physical properties. The importance of this single enzyme in plant growth and development is substantiated by the fact that mutation of XOAT1 in Arabidopsis results in plants with a 40% reduction in xylan O-acetylation that exhibit collapsed xylem vessels and that manifest several pleiotropic phenotypes related to stress responses (Pauly and Ramírez 2018).
We expressed the soluble catalytic domain of XOAT1 (XOAT-cat, XOAT133-487) for structural analysis by transient transfection of suspension culture HEK293S cells using a fusion protein strategy similar to our prior studies on mammalian and plant glycosyltransferases (Urbanowicz et al. 2017; Moremen et al. 2018). The catalytic domain of XOAT1 adopts a unique structure, bearing some similarities the α/β/α topology of members of the GDSL-like lipase/acylhydrolase family, and comprises three β-sheets composed of seven, four, and two β-strands, nine α-helices, and three α-helical turns. A deep cleft divides the molecule into two unequal lobes that open to accommodate the mono O-acetylation of linear β-1,4-xylan and contains a Ser-216–His-465–Asp-462 catalytic triad at its base. Real time NMR experiments confirm XOAT1 specifically transfers O-acetyl moieties solely to the O-2 position of the xylan backbone. Further, we applied this technique to study post-synthesis acetyl migration in real time and showed that the O-acetyl at the O-2 position migrates spontaneously to the O-3 position in the absence of enzyme. We propose a double displacement bi-bi mechanism involving the Ser-216–His-465–Asp-462 triad, which is substantiated by both molecular simulation results and biochemical experiments that confirmed the formation of the acyl-enzyme intermediate, Ac–Ser-216. In addition, we propose that XOAT1 likely unconventionally uses an Arg (Arg-219) residue to conduct the role of an oxyanion hole in the mechanism.
These mechanistic revelations about an enzyme involved in plant polysaccharide acetylation and the biosynthesis of the predominant hemicellulosic polysaccharide xylan represent a major leap forward in understanding the biochemical mechanisms used by plants to synthesize their cell walls. This work lays the basis for further studies exploring the nature of enzymes involved in polysaccharide acetylation, including the interplay between glycosyl and nonglycosyl substituent patterning along glycopolymer backbones and how the regiospecificity of O-acetyltransferases impacts plant cell wall properties.
METHODS
Expression and Purification of Fusion Proteins
Fusion protein expression and purification was accomplished as described previously by Urbanowicz et al. (2014). Briefly, we used total RNA extracted from Arabidopsis (Arabidopsis thaliana; Col-0) inflorescence stems to generate cDNAs using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific). We then PCR-amplified the sequences of the truncated XOAT1 coding-region from the cDNAs using the following primer pairs: 5′- AACTTGTACTTTCAAGGCTCAAAACCTCACGACGTC-3′ and 5′- ACAAGAAAGCTGGGTCCTAACGGGAAATGATACGTGT-3′. We cloned the resulting PCR product into a mammalian expression vector, pGEn2-DEST, as previously described by Moremen et al. (2018). We then purified the expression plasmids and transformed them into HEK cells (FreeStyle™ 293-F cell line, Life Technologies; HEK293S GnTI- cells, catalog number CRL-3022, ATCC) as previously described by Moremen et al. (2018).
Recombinant proteins were secreted into the culture medium by HEK293 cells, and purified using HisTrap HP columns and an ÄKTA protein purification system (GE Healthcare Life Sciences). For crystallization, the purified His8-sfGFP-XOAT1-cat preparation was treated with recombinant His-tagged GFP-TEV protease and His-tagged EndoF1, and purified as previously described by Urbanowicz et al. (2017).We further purified tag-free XOAT1 samples using Source 15Q anion exchange chromatography with 20 mM Tris, pH 7.5, and 0 to 0.35 M NaCl gradient. Finally, we used a Superdex 75 pg (26/60) size exclusion chromatography column in 20 mM Tris (pH 7.5), 100 mM NaCl to complete the purification. We performed SDS-PAGE followed by Coomassie Brilliant Blue R-250 (Bio-Rad) staining to confirm the purity of the proteins. For the activity assays, we dialyzed proteins in HEPES sodium salt-HCl (75 mM, pH 7.0) with Chelex-100 resin (5 g/L, Bio-Rad) to remove any divalent metal contaminants. After dialysis, we concentrated the proteins using Amicon Ultra centrifugal filter devices (30-kD molecular weight cutoff, EMD Millipore), and quantified protein concentrations by Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific). The resulting fusion proteins comprise NH2-terminal signal peptides, 8×His-tags, AviTag recognition sites, Green fluorescent proteins, TEV protease recognition sites, and the catalytic domains of the corresponding enzymes.
We generated mutated variants of XOAT1 by site-directed mutagenesis using the Q5 Site-Directed Mutagenesis Kit (New England Biolabs) according to the manufacturer's instructions using pGEN2-DEST-XOAT1 as a template. Oligonucleotide primers used to generate the base changes and expression levels of the wild-type and mutant variants are listed in Supplemental Table 1. We confirmed the introduction of mutations by sequencing (Eurofins).
Crystallization
We initially obtained XOAT1-cat crystals with sitting drop vapor diffusion using a 96-well plate with PEG ion HT screen from Hampton Research. We added 50 μL of well solution to the reservoir made drops with 0.2 μL of well solution and 0.2 μL of protein solution using a Phoenix crystallization robot (Art Robbins Instruments). We grew the crystals at 20°C using an optimization screen containing 0.1 M MES, pH 6.0 to 7.0, and 15 to 22% (w/v) PEG 3350, 0.02 M Calcium chloride dihydrate, 0.02 M Cadmium chloride hydrate, and 0.02 M Cobalt (II) chloride hexahydrate. The protein solutions contained 5.62 mg/mL of protein, 20 mM Tris (pH 7.5), 100 mM NaCl, and 20 mM 4-nitrophenyl acetate. We soaked crystals in well solution with additional 10% (w/v) glycerol and 5% (w/v) ethylene glycol for additional cryo-protection.
Data Collection and Processing
We flash-froze the XOAT1-cat crystals in a nitrogen gas stream at 100 K before home source data collection using an in-house Bruker X8 MicroStar X-Ray generator with Helios mirrors and Bruker Platinum 135 CCD detector. We indexed and processed the data with the Bruker Suite of programs version 2014.9 (Bruker AXS).
Structure Solution and Refinement
We converted intensities into structure factors and flagged 5% of the reflections for Rfree calculations using programs F2MTZ, Truncate, CAD, and Unique from the CCP4 package of programs (Winn et al. 2011). We used Crank2 (Skubák and Pannu, 2013) to solve the structure using sulfur Single-wavelength anomalous dispersion (Hendrickson and Teeter, 1981). We performed refinement and manual correction using REFMAC5 (Murshudov et al. 2011) version 5.8.135 and Coot (Emsley et al. 2010) version 0.8.2. We used the MOLPROBITY method (Chen et al. 2010) to analyze the Ramachandran plot. We calculated root mean square deviations (rmsd) of bond lengths and angles from ideal values of Engh and Huber stereo chemical parameters (Engh and Huber 1991). We validated carbohydrate structures using Privateer (Agirre et al. 2015). We calculated Wilson B-factor using CTRUNCATE version 1.15.10 (Winn et al. 2011). The data collection and refinement statistics are shown in Supplemental Table 2.
Donor and Acceptor Substrate Specificities of XOAT1
To determine the activity of acetyltransferase on different donor substrates, we performed the standard assays (15 µL) using acetylsalicylic acid (1 mM), acetyl-CoA (1 mM), or pNP-Ac (1 mM) as donors, and Xyl6-2AB (0.25 mM) as acceptor substrates in HEPES sodium salt-HCl (75 mM, pH 6.8) with purified proteins (4 µM). We incubated the reactions at room temperature for indicated periods of time, and then analyzed the products by Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-TOF MS) using a Microflex LT spectrometer (Bruker) as previously described by Urbanowicz et al. (2014). Briefly, we mixed aliquots (5 µL) of the reactions with Dowex-50 cation exchanger resin (1 μL suspension in water), and incubated the mixture at room temperature for 30 min. We pelleted the resin by centrifugation at 2000 g at room temperature for 1 min. Next, 1 μL of the supernatant was directly mixed with 1 μL of 20 mg/mL 2,5-dihydroxybenzoic acid in 50% methanol (DHB) matrix solution (20 mg/mL 2,5-dihydroxybenzoic acid in 50% [v/v] methanol) directly on a stainless steel MALDI target plate, then concentrated to dryness using a hair dryer. We generated each positive ion spectrum by summation of 200 laser shots.
We purchased xylooligosaccharides (Xyl2, Xyl3, Xyl4, Xyl5, and Xyl6) from Megazyme. We determined the acceptor substrate specificities by assays as above but with increased amount of acetylsalicylic acid (5 mM) as the donor and nonlabeled xylo-oligosaccharides (3 mM), Xyl2, Xyl3, Xyl4, Xyl5, and Xyl6 as the acceptor substrates. We analyzed acetylated products by MALDI-TOF MS. We also examined the acetyltransferase activity of XOAT1 on xylo-oligosaccharide by carrying out hydrolysis assays using pNP-Ac (5 mM) as the donor, and xylopentaose (Xyl5) with different concentrations (0, 1, 5, and 25 mM) as the acceptor substrate in HEPES sodium salt-HCl (75 mM, pH 6.8) with purified proteins (5 µM). We incubated the reactions for 1 h and determined the hydrolysis of pNP-Ac in the reaction by measuring the absorbance of the released para-nitrophenol (pNP) at 350 nm, using Epoch Microplate Spectrometer (BioTek) every minute during the reaction period.
Determination of Catalytic Activity of XOAT1 Constructs and Variants
To evaluate the O-acetyltransferase activities of different XOAT1 constructs and its mutated variants, we performed activity assays by incubating the purified enzymes (4 µM) with acetylsalicylic acid (1 mM) and Xyl6-2AB (0.25 mM) in HEPES sodium salt-HCl (75 mM, pH 6.8) for 2 h. We analyzed the acetylated products by MALDI-TOF MS as described previously. To further investigate whether XOAT1 variants possess xylan O-acetyltransferase and/or O-acetylesterase activities, we incubated the enzymes (5 µM) with or without nonlabeled Xyl4 (5 mM) as the acceptor and pNP-Ac (5 mM) as the donor substrate for 60 min. We quantified the amount of released pNP in the reaction by measuring the absorbance at 350 nm using Epoch Microplate Spectrometer (BioTek) as described above. We used the noncatalytic truncated protein containing only the N-terminal variable region and lacking the catalytic domain, XOAT1-nv (Supplemental Figuress 5B and 5C) as control. We obtained the transferase and esterase activity of all XOAT1 variants by subtracting the amount of pNP released in the control sample containing XOAT1-nv from each standard reaction containing different XOAT1 variants with or without the acceptor.
Peptide Analysis of acyl-XOAT1 by LC-MS/MS
We performed in vitro assays by incubating XOAT1 (4 µM) with four different acetyl donors (1 mM): acetyl-CoA, acetyl salicylic acid, para-nitrophenyl acetate, and 4-methylumbelliferyl acetate in HEPES sodium salt buffer (pH 6.8; 75 mM) for 4 min at room temperature. We then stopped the reactions quickly by flash freezing in a dry ice/ethanol cooling bath (–78°C). We prepared all donor substrate stocks in DMSO, with a final percentage of DMSO in all reactions of 5% (v/v). Control reactions had no donor substrate added.
We reduced, alkylated, and then digested all samples with trypsin (sequencing grade; Promega) in Tris-HCl (50 mM, pH 8.2) buffer overnight at 37°C. After protease digestion, we desalted and filtered all samples before LC-MS analysis. We performed NanoLC-MS/MS on an Orbitrap Fusion Tribrid Mass Spectrometer (Thermo Fisher Scientific) coupled to an Ultimate3000 RSLCnano low flow liquid chromatography system (Thermo Fisher Scientific), and equipped with a nanospray ion source. We injected prepared peptide samples onto a loading column (Thermo Fisher Scientific, C18, volume 2 µL) before being transferred to a separation column (Acclaim PepMap 100 C18 – 0.075 × 150 mm with 3 µm particle size). We performed the separation under a linear gradient from 5% (v/v) to 100% (v/v) reagent B (80% [v/v] Acetonitrile, 0.1% [v/v] formic acid) at a flow rate of 0.3 µL/min. We ran the analysis in automatic mode collecting an MS scan (full FTMS at 150-2000 m/z; resolution 120,000) followed by data-dependent MS/MS scans (CID) at a cycle time of 3 s We processed the data manually using QualBrowser (Thermo Fisher Scientific).
RT-NMR Analysis of Acetyl Group Migration
To verify the positional specificity of XOAT1-cat, we monitored the reaction of O-acetylation of Xyl6 using real-time 1H NMR spectroscopy. We mixed 200 µg Xyl6 with 4 mM Ac-CoA and 9 µM purified enzyme in 100 mM potassium phosphate buffer (pH 6.8) in D2O (99.9%; Cambridge Isotope Laboratories), and rapidly transferred the reaction (110 uL total volume) to the NMR spectrometer. Previously, we used potassium bicarbonate as a buffer in our real-time NMR experiments (Urbanowicz et al., 2014); however, biocarbonate buffers are unstable and become alkaline upon exposure to atmospheric CO2, increasing the rate of acetyl migration in solution. Due to this observation, we have now optimized our real-time NMR conditions and used phosphate buffer, which is a more stable buffer. Data acquisition started 10 min after initiating the reaction, and recorded data at 298 K with an Agilent-NMR spectrometer operating at 600 MHz equipped with a 5-mm NMR cold probe. The 1D 1H spectra consist of 16 transients and were acquired with water presaturation every 30 min over a 20-h period. The spectral array generated represents the reaction progress. We quantified the amount of acetylated xylosyl residues generated during the reaction by integrating the resonance peaks corresponding to the methyl protons of the acetyl groups attached to O-2 and O-3 of the xylosyl residues, and the calculation was based on the initial concentration of Ac-CoA added into the reaction. We processed the data processing using MestReNova software (Mestrelab Research S.L., Universidad de Santiago de Compostela, Spain).
We monitored nonenzymatic acetyl group migration in real-time by 1H NMR. To generate an adequate amount of acetylated Xyl6 that meets the sensitivity of the NMR spectrometer, we performed scaled-up reactions in parallel to those prepared for real-time NMR analysis of catalysis. Briefly, we generated acetylated Xyl6 by incubating XOAT1 (9 µM) with nonlabeled Xyl6 (200 µg) and Ac-CoA (4 mM) in 100 mM potassium phosphate buffer in D2O (99.9%; Cambridge Isotope Laboratories) for 6 h at room temperature. We then removed the enzyme via diafiltration by passing the mixture through a 30-kD centrifugal filter device (Amicon, Merck Millipore) by centrifugation in a swing out rotor at 3700 g at 4°C. We confirmed the absence of XOAT1 in the filtrate, and its complete retention in the filter device, by both SDS-PAGE using a precast Mini-PROTEIN TGX Stain-Free Gel (Bio-Rad Laboratories, Inc.) and by measuring the direct absorbance at 280 nm using a NanoDrop Spectrophotometer (Thermo Fisher Scientific). We transferred the filtrate immediately to the NMR spectrometer, and monitored the signals that correspond to the CH3 of the O-acetyl substituents at different positions by arrayed 1H NMR according to the following process. We recorded data at 298 K with an Agilent-NMR spectrometer equipped with a 5-mm NMR cold probe operating at 600 MHz. Each 1D 1H spectra consist of 16 transients, acquired with water presaturation every 30 min for 14 h overnight. The spectral array generated represents the reaction progress. Samples were never subjected to lyophilization.
In vitro Assays of XOAT1 with Protease Inhibitors
We performed in vitro assays with protease inhibitors following the standard assays mentioned previously but with addition of 80 μM N-p-tosyl-L-phenylalanyl chloromethyl ketone, 0.8 mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, 4 mM PMSF, or 5 mM MSF in the reaction. We used acetylsalicylic acid at a final concentration of 1 mM as the donor substrate in the assays. After overnight incubation, we analyzed the acetylated products by MALDI-TOF MS.
Computational Modeling
Docking and molecular dynamics simulations have been successful in gaining insight into plausible substrate binding poses and identify the important active site residues that aid substrate binding (Bharadwaj et al. 2013; Urbanowicz et al. 2017). We used autoDock4 and AutoDockTools4 software package for the docking the acceptor (xylodecaose) and donor (pNP-Ac) molecules to the 1.85 Å XOAT1 structure (Morris et al. 2009). We identified plausible binding poses as starting points for molecular dynamics simulations. We incorporated the four disulfide bonds identified in the crystal structure, between residues 465-386, 469-173, 229-164, and 142-193; we estimated the protonation states of titratable amino acid residues using the H++ web server (Anandakrishnan et al. 2012). We generated fully solvated systems for the protein in its apo and bound states. We used the CHARMM36 forcefield for the protein (Huang and MacKerell 2013), xylodecaose substrate (Guvench et al. 2009), the CGENFF force field for pNP-Ac (Vanommeslaeghe et al. 2010), and the TIP3P forcefield for modeling water (Jorgensen et al. 1983). We built three systems for XOAT in different states: 1) apo state (no substrate bound), 2) donor-bound state (XOAT1 with six pNP-Ac molecules bound), and 3) acceptor-bound state (XOAT1 with acetylated Ser 216 and a xylodecaose substrate bound). We used the CGENFF protocol to obtain parameters for the acetylated Ser residue in the acceptor bound state (Vanommeslaeghe et al. 2010). We obtained the system set-ups for the bound states based on preliminary docking studies; hence the simulation procedure for the protein-substrate complexes involved multiple minimizations and short equilibration runs with restraints on the substrate. The short equilibration runs involved sequential runs starting with a fixed substrate, followed by restraints on the substrate, which were gradually released for unrestrained production runs. The production runs for data analysis involved 60-ns unbiased runs with all H-atoms restrained using the SHAKE algorithm, a 2-fs time step, periodic boundary conditions, a nonbonded cutoff of 11 Å, the particle mesh Ewald for long range electrostatics, and frames saved every 100 ps (Ryckaert et al. 1977; Darden et al. 1993). All MD simulations used the domdec MD engine that is part of the CHARMM MD package (Brooks et al. 2009; Hynninen and Crowley 2014). We analyzed the trajectories for root mean square fluctuations, interaction energies, catalytically important distances, and volumetric occupancies. We performed root-mean-square fluctuations and distance calculations with pytraj (python version of CPPTRAJ; Roe and Cheatham 2013) and calculated volumetric occupancies using the volmap tool in VMD (Humphrey et al. 1996).
Statistical Analysis
All data are present as mean ±sd except for Supplemental Figure 1 (the quantitative data of the duplicates and their average are displayed). We determined statistical significance with the two-tailed Student’s t test through Microsoft Excel Version 15.32 (2017). *P < 0.05; **P < 0.01. The number of replicates is provided in the figure legends for each experiment and in Supplemental File 4.
Accession Numbers
The atomic coordinates of XOAT1-cat have been deposited into the Protein DataBank (accession code 6CCI; http://www.rcsb.org/pdb). Sequence data for the genes that were described in this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following Arabidopsis AGI locus identifiers: At5g06700 (TBR); At3g12060 (TBL1); At1g60790 (TBL2); At5g01360 (TBL3/XOAT4); At5g49340 (TBL4); At5g20590 (TBL5); At3g62390 (TBL6); At1g48880 (TBL7); At3g11570 (TBL8); At5g06230 (TBL9); At3g06080 (TBL10); At5g19160 (TBL11); At5g64470 (TBL12); At2g14530 (TBL13); At5g64020 (TBL14); At2g37720 (TBL15); At5g20680 (TBL16); At5g51640 (TBL17/YLS7); At4g25360 (TBL18); At5g15900 (TBL19); At3g02440 (TBL20); At5g15890 (TBL21); At3g28150 (TBL22/AXY4L); At4g11090 (TBL23/MOAT1); (TBL24/MOAT2); At1g01430 (TBL25/MOAT3); At4g01080 (TBL26/MOAT4); At1g70230 (TBL27/AXY4); At2g40150 (TBL28/XOAT2); At3g55990 (TBL29/XOAT1/ESK1); At2g40160 (TBL30/XOAT3); At1g73140 (TBL31/XOAT5); At3g11030 (TBL32/XOAT6); At2g40320 (TBL33/XOAT7); At2g38320 (TBL34/XOAT8); At5g01620 (TBL35/XOAT9); At3g54260 (TBL36); At2g34070 (TBL37); At1g29050 (TBL38); At2g42570 (TBL39); At2g31110 (TBL40); At3g14850 (TBL41); At1g78710 (TBL42); At2g30900 (TBL43); At5g58600 (TBL44/PMR5); At2g30010 (TBL45).
SUPPLEMENTAL DATA
Supplemental Figure 1. Activity curves of XOAT1 as an O-acetyltransferase.
Supplemental Figure 2. MALDI-TOF MS of the acetylated xylo-oligosacharides generated by XOAT1.
Supplemental Figure 3. Quantitation of the XOAT1 acyl-enzyme intermediate.
Supplemental Figure 4. MS/MS higher energy collisional dissociation fragmentation spectra of m/z corresponding to the acetylated peptide MMFVGDSLNR (m/z 606.2840 z=2), where S indicates the site of modification.
Supplemental Figure 5. Domain architectures of the designed expression constructs of XOAT1 and their corresponding O-acetyltransferase activities.
Supplemental Figure 6. Protein topology diagram for XOAT1-cat.
Supplemental Figure 7. Sequence alignment of the Arabidopsis TBL protein family.
Supplemental Figure 8. MALDI-TOF MS of the reaction products produced by XOAT1 with Ser protease inhibitors.
Supplemental Figure 9. Stability of the catalytic triad.
Supplemental Table 1. Primer sequences for gene cloning and site-directed mutagenesis for generation of the expression constructs.
Supplemental Table 2. X-ray data collection and refinement statistics. Statistics for the highest resolution bin are shown in parenthesis.
Supplemental Table 3. t Test results.
Supplemental File 1. (fasta format). Arabidopsis TBL protein sequences.
Supplemental File 2. (fasta format). Alignment of Arabidopsis TBLs.
Supplemental File 3. Newick tree of the alignment of Arabidopsis TBLs.
Supplemental File 4. XOAT1 PDB validation report.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
Funding was provided in part by the BioEnergy Science Center (BESC) and the Center for Bioenergy Innovation (CBI), and from the U.S. Department of Energy (DOE) Bioenergy Research Centers supported by the Office of Biological and Environmental Research in the DOE Office of Science. This work was supported by the DOE | Laboratory Directed Research and Development (LDRD; program at NREL), the U.S. Department of Energy (DOE) (authored in part by Alliance for Sustainable Energy, the Manager and Operator of the National Renewable Energy Laboratory; under contracts DE-AC36-08GO28308 and DE-AC05-000R22725), and the HHS | National Institutes of Health (NIH; grants P41GM103390, P01GM107012, and 1S10OD018530). The views expressed in the article do not necessarily represent the views of the DOE or the U.S. Government. The U.S. Government and the publisher, by accepting the article for publication, acknowledges that the U.S. Government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for U.S. Government purposes.
AUTHOR CONTRIBUTIONS
V.V.L., H.-T.W., V.S.B., M.A., M.J.P., J.-Y.Y., S.A.A.-H., Y.J.B., and B.R.U. designed and performed experiments, and analyzed data; V.S.B. and Y.J.B. performed computational simulations; M.A., M.E.H., K.W.M., and W.S.Y. designed experiments, analyzed and interpreted data, and edited the article; V.V.L., H.-T.W., V.S.B., M.A., Y.J.B., and B.R.U. wrote the article; Y.J.B. and B.R.U. conceived and led the project.
References
- Agirre J., Iglesias-Fernández J., Rovira C., Davies G.J., Wilson K.S., Cowtan K.D.(2015). Privateer: Software for the conformational validation of carbohydrate structures. Nat. Struct. Mol. Biol. 22: 833–834. [DOI] [PubMed] [Google Scholar]
- Akoh C.C., Lee G.-C., Liaw Y.-C., Huang T.-H., Shaw J.-F.(2004). GDSL family of serine esterases/lipases. Prog. Lipid Res. 43: 534–552. [DOI] [PubMed] [Google Scholar]
- Anandakrishnan R., Aguilar B., Onufriev A.V.(2012). H++ 3.0: Automating pK prediction and the preparation of biomolecular structures for atomistic molecular modeling and simulations. Nucleic Acids Res. 40: W537–W541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj V.S., Dean A.M., Maupin C.M.(2013). Insights into the glycyl radical enzyme active site of benzylsuccinate synthase: A computational study. J. Am. Chem. Soc. 135: 12279–12288. [DOI] [PubMed] [Google Scholar]
- Biely P.(2012). Microbial carbohydrate esterases deacetylating plant polysaccharides. Biotechnol. Adv. 30: 1575–1588. [DOI] [PubMed] [Google Scholar]
- Biely P., Mastihubová M., la Grange D.C., van Zyl W.H., Prior B.A.(2004). Enzyme-coupled assay of acetylxylan esterases on monoacetylated 4-nitrophenyl β-D-xylopyranosides. Anal. Biochem. 332: 109–115. [DOI] [PubMed] [Google Scholar]
- Blom N., Sicheritz-Pontén T., Gupta R., Gammeltoft S., Brunak S.(2004). Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4: 1633–1649. [DOI] [PubMed] [Google Scholar]
- Brooks B.R., et al. (2009). CHARMM: The biomolecular simulation program. J. Comput. Chem. 30: 1545–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brott A.S., Sychantha D., Clarke A.J.(2019). Assays for the enzymes catalyzing the O-acetylation of bacterial cell wall polysaccharides In Bacterial Polysaccharides. (New York: Humana Press; ), pp. 115–136. [DOI] [PubMed] [Google Scholar]
- Buller A.R., Townsend C.A.(2013). Intrinsic evolutionary constraints on protease structure, enzyme acylation, and the identity of the catalytic triad. Proc. Natl. Acad. Sci. USA 110: E653–E661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse-Wicher M., Gomes T.C., Tryfona T., Nikolovski N., Stott K., Grantham N.J., Bolam D.N., Skaf M.S., Dupree P.(2014). The pattern of xylan acetylation suggests xylan may interact with cellulose microfibrils as a twofold helical screw in the secondary plant cell wall of Arabidopsis thaliana. Plant J. 79: 492–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen V.B., Arendall W.B. III, Headd J.J., Keedy D.A., Immormino R.M., Kapral G.J., Murray L.W., Richardson J.S., Richardson D.C.(2010). MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66: 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong S.-L., Virkki L., Maaheimo H., Juvonen M., Derba-Maceluch M., Koutaniemi S., Roach M., Sundberg B., Tuomainen P., Mellerowicz E.J., Tenkanen M.(2014). O-acetylation of glucuronoxylan in Arabidopsis thaliana wild type and its change in xylan biosynthesis mutants. Glycobiology 24: 494–506. [DOI] [PubMed] [Google Scholar]
- Culbertson A.T., Ehrlich J.J., Choe J.-Y., Honzatko R.B., Zabotina O.A.(2018). Structure of xyloglucan xylosyltransferase 1 reveals simple steric rules that define biological patterns of xyloglucan polymers. Proc. Natl. Acad. Sci. USA 115: 6064–6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darden T., York D., Pedersen L.(1993). Particle mesh Ewald: An N⋅ log (N) method for Ewald sums in large systems. J. Chem. Phys. 98: 10089–10092. [Google Scholar]
- Dodson G., Wlodawer A.(1998). Catalytic triads and their relatives. Trends Biochem. Sci. 23: 347–352. [DOI] [PubMed] [Google Scholar]
- Emsley P., Lohkamp B., Scott W.G., Cowtan K.(2010). Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66: 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engh R.A., Huber R.(1991). Accurate bond and angle parameters for X-ray protein-structure refinement. Acta Crystallogr. A 47: 392–400. [Google Scholar]
- Grantham N.J., Wurman-Rodrich J., Terrett O.M., Lyczakowski J.J., Stott K., Iuga D., Simmons T.J., Durand-Tardif M., Brown S.P., Dupree R., Busse-Wicher M., Dupree P.(2017). An even pattern of xylan substitution is critical for interaction with cellulose in plant cell walls. Nat. Plants 3: 859–865. [DOI] [PubMed] [Google Scholar]
- Guvench O., Hatcher E.R., Venable R.M., Pastor R.W., Mackerell A.D.(2009). CHARMM additive all-atom force field for glycosidic linkages between hexopyranoses. J. Chem. Theory Comput. 5: 2353–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson W.A., Teeter M.M.(1981). Structure of the hydrophobic protein crambin determined directly from the anomalous scattering of sulphur. Nature 290: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., MacKerell A.D. Jr.(2013). CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 34: 2135–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey W., Dalke A., Schulten K. (1996). VMD: Visual molecular dynamics. J. Mol. Graph. 14: 33–8, 27–8. [DOI] [PubMed] [Google Scholar]
- Hynninen A.P., Crowley M.F.(2014). New faster CHARMM molecular dynamics engine. J. Comput. Chem. 35: 406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.M., Kim H., Ralph J., Mansfield S.D.(2017). Natural acetylation impacts carbohydrate recovery during deconstruction of Populus trichocarpa wood. Biotechnol. Biofuels 10: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen W.L., Chandrasekhar J., Madura J.D., Impey R.W., Klein M.L.(1983). Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79: 926–935. [Google Scholar]
- Kabel M.A., de Waard P., Schols H.A., Voragen A.G.(2003). Location of O-acetyl substituents in xylo-oligosaccharides obtained from hydrothermally treated Eucalyptus wood. Carbohydr. Res. 338: 69–77. [DOI] [PubMed] [Google Scholar]
- Kang X., Kirui A., Dickwella Widanage M.C., Mentink-Vigier F., Cosgrove D.J., Wang T.(2019). Lignin-polysaccharide interactions in plant secondary cell walls revealed by solid-state NMR. Nat. Commun. 10: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhnke T., Östlund A., Brelid H.(2011). Adsorption of arabinoxylan on cellulosic surfaces: Influence of degree of substitution and substitution pattern on adsorption characteristics. Biomacromolecules 12: 2633–2641. [DOI] [PubMed] [Google Scholar]
- Krogh A., Larsson B., von Heijne G., Sonnhammer E.L.(2001). Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305: 567–580. [DOI] [PubMed] [Google Scholar]
- Lassfolk R., Rahkila J., Johansson M.P., Ekholm F.S., Wärnå J., Leino R.(2019). Acetyl group migration across the saccharide units in oligomannoside model compound. J. Am. Chem. Soc. 141: 1646–1654. [DOI] [PubMed] [Google Scholar]
- Lefebvre V., Fortabat M.-N., Ducamp A., North H.M., Maia-Grondard A., Trouverie J., Boursiac Y., Mouille G., Durand-Tardif M.(2011). ESKIMO1 disruption in Arabidopsis alters vascular tissue and impairs water transport. PLoS One 6: e16645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Lu Q., Yuan Y., Ge H., Li K., Zhao W., Gao Y., Niu L., Teng M.(2011). Crystal structure of isoamyl acetate-hydrolyzing esterase from Saccharomyces cerevisiae reveals a novel active site architecture and the basis of substrate specificity. Proteins 79: 662–668. [DOI] [PubMed] [Google Scholar]
- Manabe Y., Verhertbruggen Y., Gille S., Harholt J., Chong S.-L., Pawar P.M., Mellerowicz E., Tenkanen M., Cheng K., Pauly M.(2013). RWA proteins play vital and distinct roles in cell wall O-acetylation in Arabidopsis. Plant Physiology 163: 1107–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak L., La Rosa S.L., Leivers S., Lindstad L.J., Røhr Å.K., Lillelund Aachmann F., Westereng B.(2020). A pair of esterases from a commensal gut bacterium remove acetylations from all positions on complex β-mannans. Proc. Natl. Acad. Sci. USA 117: 7122–7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moremen K.W., et al. (2018). Expression system for structural and functional studies of human glycosylation enzymes. Nat. Chem. Biol. 14: 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J.(2009). AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 30: 2785–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynihan P.J., Clarke A.J.(2013). Assay for peptidoglycan O-acetyltransferase: A potential new antibacterial target. Anal. Biochem. 439: 73–79. [DOI] [PubMed] [Google Scholar]
- Moynihan P.J., Clarke A.J.(2014). Mechanism of action of peptidoglycan O-acetyltransferase B involves a Ser-His-Asp catalytic triad. Biochemistry 53: 6243–6251. [DOI] [PubMed] [Google Scholar]
- Murshudov G.N., Skubák P., Lebedev A.A., Pannu N.S., Steiner R.A., Nicholls R.A., Winn M.D., Long F., Vagin A.A.(2011). REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 67: 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly M., Ramírez V.(2018). New insights into wall polysaccharide O-acetylation. Front Plant Sci 9: 1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe D.R., Cheatham T.E. III(2013). PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 9: 3084–3095. [DOI] [PubMed] [Google Scholar]
- Ryckaert J.-P., Ciccotti G., Berendsen H.J.C.(1977). Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 23: 327–341. [Google Scholar]
- Schultink A., Naylor D., Dama M., Pauly M.(2015). The role of the plant-specific ALTERED XYLOGLUCAN9 protein in Arabidopsis cell wall polysaccharide O-acetylation. Plant Physiol. 167: 1271–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubák P., Pannu N.S.(2013). Automatic protein structure solution from weak X-ray data. Nat. Commun. 4: 2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P.J., Wang H.-T., York W.S., Peña M.J., Urbanowicz B.R.(2017). Designer biomass for next-generation biorefineries: Leveraging recent insights into xylan structure and biosynthesis. Biotechnol. Biofuels 10: 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranne M., Ren Y., Fimognari L., Birdseye D., Yan J., Bardor M., Mollet J.C., Komatsu T., Kikuchi J., Scheller H.V., Sakuragi Y.(2018). TBL10 is required for O-acetylation of pectic rhamnogalacturonan-I in Arabidopsis thaliana. Plant J. 96: 772–785. [DOI] [PubMed] [Google Scholar]
- Sychantha D., Little D.J., Chapman R.N., Boons G.-J., Robinson H., Howell P.L., Clarke A.J.(2018). PatB1 is an O-acetyltransferase that decorates secondary cell wall polysaccharides. Nat. Chem. Biol. 14: 79–85. [DOI] [PubMed] [Google Scholar]
- Urbanowicz B.R., et al. (2017). Structural, mutagenic and in silico studies of xyloglucan fucosylation in Arabidopsis thaliana suggest a water-mediated mechanism. Plant J. 91: 931–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowicz B.R., Peña M.J., Moniz H.A., Moremen K.W., York W.S.(2014). Two Arabidopsis proteins synthesize acetylated xylan in vitro. Plant J. 80: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanommeslaeghe K., Hatcher E., Acharya C., Kundu S., Zhong S., Shim J., Darian E., Guvench O., Lopes P., Vorobyov I., Mackerell A.D. Jr.(2010). CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 31: 671–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn M.D., et al. (2011). Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong G., Cheng K., Pauly M.(2013). Xylan O-acetylation impacts xylem development and enzymatic recalcitrance as indicated by the Arabidopsis mutant tbl29. Mol. Plant 6: 1373–1375. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Teng Q., Zhong R., Haghighat M., Richardson E.A., Ye Z.-H.(2016a). Mutations of Arabidopsis TBL32 and TBL33 affect xylan acetylation and secondary wall deposition. PLoS One 11: e0146460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Teng Q., Zhong R., Ye Z.-H.(2016c). TBL3 and TBL31, two Arabidopsis DUF231 domain proteins, are required for 3-O-monoacetylation of xylan. Plant Cell Physiol. 57: 35–45. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Teng Q., Zhong R., Ye Z.H.(2016b). Roles of Arabidopsis TBL34 and TBL35 in xylan acetylation and plant growth. Plant Sci. 243: 120–130. [DOI] [PubMed] [Google Scholar]
- Zhong R., Cui D., Richardson E.A., Phillips D.R., Azadi P., Lu G., Ye Z.-H.(2020). Cytosolic Acetyl-CoA generated by ATP-citrate lyase is essential for acetylation of cell wall polysaccharides. Plant Cell Physiol. 61: 64–75. [DOI] [PubMed] [Google Scholar]
- Zhong R., Cui D., Ye Z.-H.(2017). Regiospecific acetylation of xylan is mediated by a group of DUF231-containing O-acetyltransferases. Plant Cell Physiol. 58: 2126–2138. [DOI] [PubMed] [Google Scholar]
- Zhong R., Cui D., Ye Z.-H.(2018). Xyloglucan O-acetyltransferases from Arabidopsis thaliana and Populus trichocarpa catalyze acetylation of fucosylated galactose residues on xyloglucan side chains. Planta 248: 1159–1171. [DOI] [PubMed] [Google Scholar]