Chloroplast tRNA modification influences protein translation, leading to pleiotropic developmental defects in rice, as revealed by analysis of a natural allele of tRNA-modifying GTPase gene PDD.
Abstract
The modification of tRNA is important for accurate, efficient protein translation. A number of tRNA-modifying enzymes were found to influence various developmental processes in distinct organisms. However, few genetic or molecular studies have focused on genes encoding tRNA-modifying enzymes in green plant organelles. Here, we discovered that PDDOL, a natural variation allele of PLEIOTROPIC DEVELOPMENTAL DEFECTS (PDD), leads to pleiotropic developmental defects in a near-isogenic line (NIL) generated by introgressing the wild rice Oryza longistaminata into the rice (Oryza sativa) cv 187R. Map-based cloning revealed that PDD encodes an evolutionarily conserved tRNA-modifying GTPase belonging to the tRNA modification E family. The function of PDD was further confirmed by genetic complementation experiments and mutant analysis. PDD mRNA is primarily expressed in leaves, and PDD is localized to chloroplasts. Biochemical analyses indicated that PDD187R forms homodimers and has strong GTPase activity, whereas PDDOL fails to form homodimers and has weak GTPase activity. Liquid chromatography–coupled tandem quadrupole mass spectrometry revealed that PDD is associated with the 5-methylaminomethyl-2-thiouridine modification of chloroplast tRNA. Furthermore, compared to 187R, NIL-PDDOL has severely reduced levels of proteins involved in photosynthesis and ribosome biogenesis but increased levels of plastid-encoded RNA polymerase subunits. Finally, we demonstrate that the defect due to PDDOL alters chloroplast gene expression, thereby affecting communication between the chloroplast and the nucleus.
INTRODUCTION
Rice (Oryza sativa) is one of the most important crops worldwide, serving as a major staple food crop for humans and as a model system to decipher the molecular mechanisms regulating plant development and growth. In addition to the two cultivated rice species, there are 21 wild species in the Oryza genus (Vaughan et al., 2003), providing ample natural genetic resources for studying gene function and improving agronomic traits in rice. To date, studies using various wild rice species as donors to create near-isogenic lines (NILs) via interspecific introgression have identified a number of genes involved in establishing rice architecture, inflorescence formation, stress responses, and awn development (Jin et al., 2008; Zhu et al., 2013; Hirabayashi et al., 2015; Hua et al., 2015).

Accurate and efficient protein translation is essential for ensuring that proteins perform their normal functions in cells. tRNA is a vital component of the translation machinery, as it delivers the corresponding amino acid to the elongating peptide chain based on codon–anticodon recognition (Giegé et al., 2012). To correctly decipher the genetic code, tRNA is extensively modified at various sites (Björk and Hagervall, 2005). To date, >100 modifications in tRNAs have been identified, most of which contribute to translational accuracy and efficiency (Björk et al., 1999; Gustilo et al., 2008; Cantara et al., 2011). For example, in yeast (Saccharomyces cerevisiae) and nematodes (Caenorhabditis elegans), the modification of uridine at position 34 (U34) in cytoplasmic tRNAs influences the translation rate and protein aggregation (Nedialkova and Leidel, 2015). In mice and humans, the modification of adenosine at position 37 in mitochondrial tRNAs governs protein translation and is associated with a mitochondrial-related disease (Wei et al., 2015). In rice, modifications of cytoplasmic pre-tRNAHis regulate the translation efficiency of specific mRNAs (Chen et al., 2019).
Modifications of tRNAs are catalyzed by tRNA-modifying enzymes, which are evolutionarily conserved in eukaryotic and prokaryotic organisms (Ferré-D’Amaré, 2003). In a given genome, ∼1 to 10% of genes are annotated as encoding tRNA-modifying enzymes (El Yacoubi et al., 2012). Among these genes, members of the tRNA modification E (TrmE) family are thought to directly modify the fifth carbon atom of the wobble uridine (U34) in certain tRNAs, such as tRNALysUUU, tRNAGluUUC, tRNAGlnUUG, tRNALeuUAA, and tRNAArgUCU (Moukadiri et al., 2014). These tRNAs can decode two-family box triplets ending in A or G (Armengod et al., 2012). In Escherichia coli, TrmE modifies U34 in tRNA to 5-carboxymethylaminomethyl-(2-thio)uridine, which can be decarboxylated to 5-methylaminomethyl-(2-thio)uridine by TrmC in certain tRNAs (Cabedo et al., 1999). In yeast, the TrmE protein MSS1 is localized to mitochondria and shares a similar function with TrmE in E. coli (Decoster et al., 1993). In human, although TrmE (GTPBP3) is also targeted to mitochondria, it modifies U34 in tRNA to 5-taurinomethyl-(2-thio)uridine (Villarroya et al., 2008; Asano et al., 2018). Although several studies have suggested that these modifications can influence the accuracy of codon–anticodon recognition and/or reading frame maintenance during translation (Yokoyama et al., 1985; Krüger et al., 1998; Brégeon et al., 2001), strong genetic and molecular evidence for this hypothesis is lacking.
Phylogenetic analysis revealed that TrmE proteins in green plants form a single monophyletic group with homologs from cyanobacteria, which are ancestors of chloroplasts (Suwastika et al., 2014), suggesting that plant TrmE proteins might modify chloroplast tRNAs to ensure translation in chloroplasts. The chloroplast, the site for photosynthesis, plays an important role in plant growth (Waters and Langdale, 2009). This semiautonomous organelle has its own genome and protein synthesis machinery. In general, the chloroplast genome encodes 30 tRNAs and ∼80 unique proteins (Chumley et al., 2006). Three decades ago, two-dimensional homochromatography and thin layer chromatography revealed a few modified nucleotides in several chloroplast tRNAs in soybean (Glycine max), barley (Hordum vulgare), and the alga Codium fragile (Pillay et al., 1984; Schön et al., 1986; Francis et al., 1989). However, to date, few studies have focused on the functions of chloroplast tRNA-modifying enzymes, besides TADA, which is involved in the deamination of adenine to inosine, affecting efficient chloroplast translation in Arabidopsis (Arabidopsis thaliana; Delannoy et al., 2009; Karcher and Bock, 2009).
Here, taking advantage of natural genetic variation in the African wild rice species Oryza longistaminata, we uncovered the crucial roles of the PLEIOTROPIC DEVELOPMENTAL DEFECTS (PDD) gene in rice growth and development. We demonstrate that PDD is a conserved TrmE family protein localized to rice chloroplasts and that it participates in the modification of chloroplast tRNAs, thereby affecting translation and plant development.
RESULTS
NIL-PDDOL Shows Pleiotropic Developmental Defects
To take advantage of natural genetic variation to identify functional genes in rice, we constructed a set of NILs from backcross progenies derived from a cross between rice cv 187R (O. sativa subsp indica) as the recurrent parent and African wild rice species O. longistaminata as the donor parent (Supplemental Figure 1A). In this population, we identified a NIL with severe pleiotropic developmental defects (detailed below). Genetic and phenotypic analyses of F2 progenies derived from F1 plants produced by crosses between this NIL and 187R revealed that the defects were caused by a single recessive nuclear locus (F2 segregation: 134 normal plants; 39 defective plants; 3:1 ratio: P = 0.46). Based on the phenotypic defects, we named the allele in the 187R background PDD187R. The allele in O. longistaminata was named PDDOL, and the NIL was named NIL-PDDOL.
Phenotypic analysis showed that, before the five-leaf stage, newly emerging leaves in NIL-PDDOL were albino and gradually turned green but with an albino margin during seedling development (Figures 1A and 1B). However, after the five-leaf stage, newly formed leaves had a normal green color (Figure 1B; Supplemental Figure 1B). The photosynthetic pigment contents were much lower in NIL-PDDOL than in 187R at the three-leaf stage (Figure 1C) but recovered to normal levels to those in 187R at later stages of development (Supplemental Figure 1C). Given the close relationship between photosynthetic pigments and chloroplast development, we compared the ultrastructures of chloroplasts in 187R and NIL-PDDOL by transmission electron microscopy. 187R leaf cells harbored normal chloroplasts with well-organized lamellar structures and normally stacked grana and thylakoid membranes (Figures 1D and 1E). By contrast, cells within the white sectors of NIL-PDDOL were severely vacuolated and lacked well-organized lamellar structures (Figures 1F and 1G). However, cells in the green sectors of NIL-PDDOL at later stages of development had well-developed chloroplasts (Supplemental Figure 1D). We also compared the photosynthetic capacities of 187R and NIL-PDDOL. The Fv/Fm value, representing the maximum quantum yield of PSII photochemistry, was significantly lower in NIL-PDDOL than in 187R at the three-leaf stage (Supplemental Figure 1E).
Figure 1.
Phenotypic Analysis of NIL-PDDOL.
(A) and (B) Phenotypes of 187R (left) and NIL-PDDOL seedlings (right) at the three-leaf stage (A) and five-leaf stage (B). The white boxes above the seedlings show magnified views of the areas of 187R and NIL-PDDOL leaves in the red rectangles. Bar in the top panels of (A) and (B) = 2 mm; bar in the bottom panel of (A) = 2 cm; bar in the bottom panel of (B) = 4 cm.
(C) Pigment contents in 187R and NIL-PDDOL leaves at the three-leaf stage. Data represent mean ± sd from three independent biological replicates (from different seedling leaves). ***, P < 0.001 (Student’s t test). Chl a, chlorophyll a; Chl b, chlorophyll b.
(D) to (G) Transmission electron microscopy images of cells from 187R (see [D] and [E]) and white leaves of NIL-PDDOL (see [F] and [G]) at the three-leaf stage. The red arrow indicates normally stacked grana. Bar in (D) and (F) = 1 μm; bar in (E) and (G) = 200 nm.
(H) Phenotypes of 187R (left) and NIL-PDDOL (right) at the mature stage. Bar = 10 cm.
(I) to (J) Plant height (I) and tiller number (J) in 187R and NIL-PDDOL. Before heading, plant height to the leaf tip was measured. After heading, plant height to the panicle tip was measured. The data are presented as mean ± sd. ***, P < 0.001 (n = 20 plants; Student’s t test).
(K) Phenotypes of the stem structures of 187R (left) and NIL-PDDOL (right). The red arrows indicate the node position. Bar = 10 cm.
(L) Comparison of 187R (left) and NIL-PDDOL (right) panicles and internode length. The data are presented as mean ± sd. ***, P < 0.001 (n = 20 plants; Student’s t test).
(M) Cross sections of the uppermost internodes of 187R and NIL-PDDOL. Bar = 50 μm.
Moreover, NIL-PDDOL plants showed sharply reduced plant height and tiller number throughout their life cycles (Figures 1H to 1J). NIL-PDDOL panicles were much shorter and had fewer branches than 187R panicles (Figure 1K; Supplemental Figure 1F; Supplemental Figures 2A and 2B). All internodes were significantly shorter in NIL-PDDOL than in 187R (Figures 1K and 1L). Microscopic observation of internode cross sections revealed that the short stature of NIL-PDDOL is caused by smaller cell size (Figure 1M). Additionally, although the grain size of NIL-PDDOL was similar to that of 187R (Supplemental Figure 1G), NIL-PDDOL plants displayed reduced grain number per panicle, seed setting rate, and 1000-grain weight (Supplemental Figures 2C to 2E), thereby leading to greatly reduced yield per plant (Supplemental Figure 2F).
Map-Based Cloning and Identification of PDD
To identify the gene causing the pleiotropic defects of NIL-PDDOL, we performed map-based cloning. Using 200 F2 plants derived from a cross between NIL-PDDOL and 187R, the PDD locus was initially mapped to the long arm of chromosome 8 (Figure 2A). To finely map PDD, we crossed NIL-PDDOL with 93-11 (O. sativa subsp indica). By analyzing ∼9000 BC1F2 plants, the PDD gene was further narrowed down to a 35-kb genomic region containing five candidate genes (CGs) in the Nipponbare reference genome (Figure 2A). Among these genes, CG1 and CG3 are tandemly duplicated genes. CG2, CG4, and CG5 are annotated as encoding an expressed protein, tRNA modification GTPase TrmE, and peptidase, respectively (Supplemental Table 1). Based on genome annotations and available mRNA expression data, we reasoned that CG4 or CG5 might be the responsible gene.
Figure 2.
Map-Based Cloning and Characterization of the PDD Gene.
(A) Molecular cloning of the PDD gene. The PDD locus was narrowed down to a 35-kb genomic DNA region on the chromosome 8 (Chr.8), which contained five CGs (CG1 to CG5). The number of recombinants identified is shown below each marker.
(B) CG4 construct used for complementation. HPT, hygromycin phosphotransferase gene; LB, left border; NOS, nopaline synthase terminator; RB, right border.
(C) and (D) Phenotypes of complementation lines (CP-1 and CP-2) at the seedling stage (C) and the mature stage (D). Bar in (C) = 2 cm; bar in (D) = 20 cm.
We performed genetic complementation experiments to identify which gene is indeed PDD. We generated two independent binary constructs containing CG4 or CG5 genomic fragments with their own promoters from 187R (Figure 2B; Supplemental Figure 3D) and separately transformed these constructs into NIL-PDDOL. In total, we obtained 12 independent T0 lines for the CG4 construct and 10 independent T0 lines for the CG5 construct. The phenotypic defects of NIL-PDDOL were restored in transgenic plants harboring the CG4 (LOC_Os08g31460) complementation construct (Figures 2C and 2D), including the pigment contents, tiller number, and plant height (Supplemental Figures 3A to 3C). By contrast, the construct harboring the CG5 genomic fragment failed to rescue the developmental defects of NIL-PDDOL (Supplemental Figure 3E). Therefore, the CG4 (LOC_Os08g31460) locus corresponds to PDD.
To further confirm the function of PDD, we generated pdd mutants via clustered regularly interspaced short palindromic repeats (CRISPR)/ Cas9–mediated genome editing (Xie et al., 2015). We designed two distinct guide RNAs targeting the first and second exons of PDD (Figure 3A). After transforming the construct harboring the guide RNAs into 187R, we obtained 15 independent T0 lines. After genotyping, we identified five distinct genome-edited alleles at both sites in PDD in three T0 lines (pdd-1/+, pdd-2/pdd-3, and pdd-4/pdd-5; Figure 3A), which were predicted to produce truncated proteins (Supplemental Figure 4). Unfortunately, although the pdd-2/pdd-3 and pdd-4/pdd-5 plants lived for a short time, both died before the five-leaf stage, suggesting that the combined mutant alleles are too deleterious to allow survival to adulthood. However, in a population containing 96 individual plants from the next generation of pdd-1/+, we obtained 4 pdd-1 homozygous plants that survived and completed their life cycles.
Figure 3.
Genotypes and Phenotypes of Genome-Edited pdd Mutants.
(A) Genotypes of the pdd mutants obtained by CRISPR/Cas9.
(B) and (C) Phenotypes of the pdd-1 mutant at the seeding stage (B) and the mature stage (C). Bar in (B) = 2 cm; bar in (C) = 20 cm.
(D) and (E) Plant height (D) and tiller number (E) of 187R, NIL-PDDOL, and pdd-1. The data are shown as box-plot graphs. Lines across the box show the median values. Bottom and top boxes indicate the 25th percentile to the 75th percentile. Whiskers represent the maximum and minimum values. ***, P < 0.001 (n = 20 plants; Student’s t test).
(F) Seedling growth phenotypes of 187R, NIL-PDDOL, and pdd-1. Bar = 5 cm.
(G) Quantitative analysis of the proportions of seedlings from 96 187R, NIL-PDDOL, and pdd-1 seeds. The data are shown as mean ± sd from three independent biological replicates. n.s., no significant difference; ***, P < 0.001 (Student’s t test).
Genotyping revealed that pdd-1 contained a 1-bp T insertion in the first exon and a 1-bp A insertion in the second exon of PDD, which were predicted to produce an mRNA with a premature termination codon (Figure 3A), potentially leading to the production of a truncated protein with only 35 amino acids from the N terminus (Supplemental Figure 4). To determine whether these two small DNA polymorphisms resulted in nonsense-mediated mRNA decay in pdd-1, we measured the mRNA levels of PDD in 187R and pdd-1 via RT-qPCR. The level of PDD mRNA was not affected in pdd-1 (Supplemental Figure 3F), implying that the mutations in pdd-1 did not alter PDD mRNA abundance. Phenotypic analysis showed that pdd-1 exhibited similar but more severe developmental defects compared to NIL-PDDOL (Figures 3B to 3E; Supplemental Figures 2A to 2F), further confirming that the causal gene in NIL-PDDOL is PDD.
In addition, genetic analysis of a population of seeds derived from a heterozygous pdd-1/+ plant revealed that the proportion of homozygous individuals (∼16%) was much smaller than expected (25%; P = 0.017), implying that a portion of homozygous embryos might have failed to develop into mature seeds. Furthermore, comparative phenotypic analysis of seed germination and plant growth in 187R, NIL-PDDOL, and pdd-1 revealed that most pdd-1 seeds germinated but failed to form seedlings, unlike 187R and NIL-PDDOL (Figures 3F and 3G), further demonstrating that PDD is essential for normal rice development. These results also indicate that the natural variant in the African wild rice species is a relatively weak allele.
PDD Encodes a Chloroplast-Localized TrmE Family Protein
In the rice genome, PDD is annotated as a tRNA-modifying GTPase belonging to the TrmE family. Phylogenetic analysis showed that most organisms from bacteria to humans contain only one TrmE gene (Supplemental Figure 5), implying that the copy number of TrmE tends to be under strict natural selection during evolution.
Bioinformation analysis of protein structure revealed that PDD possesses a predicted chloroplast-targeting signal; one conserved N-terminal domain of ∼120 amino acid residues; a central G domain of ∼170 residues; and C-terminal region containing a highly conserved CxGK motif, where x represents any residue (Figure 4A; Supplemental Figure 5). Multiple protein sequence alignment revealed two novel conserved regions (named R1 and R2) in the N-terminal domain of this protein (Figure 4A). The G domain contains four typical motifs (G1 to G4), which is characteristic of GTPases (Figure 4A). In addition, PDD is highly conserved among plant species, sharing >80% sequence identity with homologs in Brachypodium distachyon, maize (Zea mays), and sorghum (Sorghum bicolor) and 60.9% identity with its homolog in Arabidopsis (Supplemental Figure 6).
Figure 4.

PDD Encodes a Chloroplast-Localized TrmE Subfamily Protein.
(A) Schematic diagram of the structure of PDD and multiple sequence alignment of conserved motifs among PDD homologs. CTS, chloroplast-targeting signal, M. musculus, Mus musculus (house mouse); S. pombe, Schizosaccharomyces pombe (fission yeast).
(B) Expression analysis of PDD in various tissues by RT-qPCR. Data were normalized to ACTIN1 expression. The bars represent mean ± sd of triplicate experiments. L-S, leaves at the seedling stage; L-T, leaves at the tillering stage; P, panicle; R, root; S, stem; SH-S, sheath at the seedling stage; SH-T, sheath at the tillering stage.
(C) GUS staining of seeds at 1 d after germination (a), seedlings at the four-leaf stage (b), root (c), mature leaves (d), panicle (e), spikelet (f), and pistil (g). Bar in (a) and (f) = 1 mm; bar in (b), (d), and (e) = 1 cm; bar in (c) and (g) = 200 μm.
(D) Subcellular localization of PDD187R-eGFP in rice protoplasts. (Top) Negative control using empty vector. (Bottom) Localization of PDD187R-eGFP fusion protein. Bar = 10 μm.
To explore the molecular function of PDD, we investigated its mRNA expression pattern in various tissues of 187R plants by RT-qPCR. The PDD transcript was preferentially expressed in green tissues during development, including leaves at the seedling stage and leaves and sheaths at the tillering stage, but with much lower expression in other tissues such as roots, stems, and panicles (Figure 4B). In addition, we generated a binary construct with the 2167-bp promoter region of PDD187R driving the β-glucuronidase (GUS) reporter gene and used it to transform the japonica rice var Nipponbare. We detected GUS signals in various tissues at different stages of development, such as leaves, the embryos of seeds, roots, panicles, and ovaries (Figure 4C). To examine the subcellular localization of PDD, we constructed the Pro35S:PDD187R-eGFP fusion vector and introduced it into rice protoplasts. The GFP signals colocalized with the autofluorescence signals from chlorophyll (Figure 4D), indicating that PDD is a chloroplast-localized protein.
Natural Variations in PDDOL Affect Homodimer Formation and GTPase Activity
To identify the genetic variations between PDD187R and PDDOL, we cloned and compared their genomic sequences, including their promoter regions. Sequence alignment revealed 468 polymorphisms, including single-nucleotide polymorphisms (SNPs) and insertions/deletions, in this region between the two varieties (Table 1; Supplemental Figure 7). The protein-coding region in PDDOL contains one 6-bp deletion and 28 SNPs compared to PDD187R (Table 1; Supplemental Figure 7B). These DNA polymorphisms are predicted to cause two amino acid deletions at the beginning of the N terminus and five amino acid alterations along the entire protein region (Figure 5A; Supplemental Figure 7C). To determine whether the PDDOL version is defective in vivo, we generated a binary construct containing the PDDOL coding sequence driven by the 2167-bp native promoter from PDD187R (Figure 5B). After transforming the vector into NIL-PDDOL, we identified 16 independent transgenic plants. All 16 plants displayed highly similar phenotypes to NIL-PDDOL plants (Figures 5C and 5D), indicating that PDDOL is a dysfunctional protein.
Table 1. Sequence Polymorphisms between PDD187R and PDDOL.
| Sequence | PDD187R (bp) | PDDOL (bp) | No. of Variations | No. of SNPs | No. of Insertions | No. of Deletions |
|---|---|---|---|---|---|---|
| Promoter | 2085 | 2809 | 286 | 249 | 25 | 12 |
| Genomic | 4007 | 3771 | 182 | 159 | 15 | 8 |
| Coding | 1659 | 1653 | 29 | 28 | 0 | 1 |
Figure 5.

Sequence and Functional Variation Analysis of PDD187R and PDDOL.
(A) Amino acid variations between PDD187R and PDDOL. CTS, chloroplast-targeting signal.
(B) Diagram of the vector used for transformation. RB, right border; LB, left border; HPT, hygromycin phosphotransferase gene; NOS, nopaline synthase terminator.
(C) and (D) Phenotypes of transgenic lines (nos. 1 and 2) at the seedling stage (C) and the mature stage (D). Bar in (C) = 2 cm; bar in (D) = 10 cm.
(E) Examination of homodimer formation of PDD187R and PDDOL by Y2H assay. AD, activation domain; BD, DNA binding domain.
(F) Examination of homodimer formation of PDD187R and PDDOL by BiFC assay. Yellow fluorescent protein (YFP) signals were observed and imaged by confocal microscopy. The left-most panels show bright-field images. The second panels show chlorophyll autofluorescence signals. The third panels show YFP signals. The last panels show merged chlorophyll autofluorescence images with YFP signals and bright-field images. Bar = 20 μm.
(G) Relative GTPase activity of PDD187R and PDDOL in vitro. The data are shown as mean ± sd from three independent biological replicates. ***, P < 0.001 (Student’s t test).
To investigate whether the variations in PDDOL affect its mRNA abundance, we measured PDD expression in 187R and NIL-PDDOL via RT-qPCR. The mRNA levels were higher in NIL-PDDOL than in 187R (Supplemental Figure 3F), suggesting that PDDOL might have a stronger promoter or that its mRNA stability might be altered. Furthermore, given that there were two amino acid deletions and one amino acid substitution in the predicted chloroplast-targeting signal, we investigated whether the subcellular localization of this protein is affected in NIL-PDDOL. When we introduced the Pro35S:PDDOL-eGFP construct into rice protoplasts, GFP signals still localized to the chloroplast (Supplemental Figure 8A), as observed for PDD187R (Figure 4D), indicating that the chloroplast localization of PDDOL was not affected by these changes.
The N-terminal domain primarily mediates the dimerization of TrmE family members, and the G domain is responsible for GTP binding and hydrolysis (Scrima et al., 2005). To determine which domain of PDDOL leads to the dysfunction of this protein, we examined the dimerization and GTPase activity of PDD187R and PDDOL. A yeast two-hybrid (Y2H) assay revealed that PDD187R successfully formed homodimers, but PDDOL did not (Figure 5E). A bimolecular fluorescence complementation (BiFC) analysis in wild tobacco (Nicotiana benthamiana) epidermal cells also showed that PDD187R formed dimers in chloroplasts, further confirming the notion that PDD is a chloroplast-localized protein. By contrast, BiFC showed that PDDOL lost the ability to form dimers (Figure 5F). Finally, to determine whether the GTPase activity of PDDOL is also affected, we expressed and purified both PDD187R and PDDOL in E. coli and examined their GTPase activities in vitro using a GTPase colorimetric assay. PDD187R had strong GTPase activity (Figure 5G; Supplemental Figure 8B), but PDDOL had significantly lower GTPase activity than PDD187R (Figure 5G; Supplemental Figure 8B), implying that the GTPase activity of PDDOL is also impaired. In summary, our results demonstrate that the variations in PDDOL lead to both the inability of this protein to form homodimers and its reduced GTPase activity.
PDD Is Involved in the mnm5s2U Modification of Chloroplast tRNAs in Rice
Given the similar sequences and protein properties of PDD and its homologs in the TrmE family (Figures 4A and 5E to 5G), we reasoned that PDD might modify tRNAs in rice chloroplasts. To explore this hypothesis, we performed liquid chromatography–coupled tandem quadrupole mass spectrometry (LC-MS/MS) to measure the tRNA modifications in both 187R and NIL-PDDOL chloroplasts. First, we purified total tRNAs from chloroplasts isolated from fresh leaves of 187R and NIL-PDDOL seedlings at the three-leaf stage. Subsequently, these tRNAs were completely digested to individual nucleosides for LC-MS/MS sample injection (Figure 6A). We examined 23 distinct types of tRNA modifications using multiple reaction monitoring mode (Supplemental Table 2), including six types that were found to be associated with TrmE proteins in various species (Supplemental Figure 9). Our analysis identified 18 modified nucleosides with well-separated, distinct ion peaks (Figures 6B to 6D). A comparison of the modification levels between 187R and NIL-PDDOL revealed no obvious differences in the relative quantities of 17 types of modifications (Figures 6E to 6G). However, 5-methylaminomethyl-2-thiouridine (mnm5s2U) modification levels were significantly lower in NIL-PDDOL than in 187R (Figure 6G). We then examined the modification status of chloroplast tRNAs in transgenic complementation lines of NIL-PDDOL. The levels of modified mnm5s2U nucleosides in these lines were fully recovered to that in 187R (Figure 6H). These results indicate that PDD is associated with the mnm5s2U modification of chloroplast tRNA in rice.
Figure 6.

Detection and Data Analysis of Chloroplast tRNA Modification Status.
(A) Scheme used for the chloroplast tRNA modification detection experiments.
(B) to (D) LC-MS/MS chromatogram of the 18 modified nucleosides with well-separated ion peaks. m1A, 1-methyladenosine; m2A, 2-methyladenosine; m6A, N6-methyladenosine; D, dihydrouridine; m5U, 5-methyluridine; m1G, 1-methylguanosine; m2G, N2-methylguanosine; m7G, 7-methylguanosine; m5C, 5-methylcytidine; m22G, N2,N2-dimethylguanosine; Ψ, pseudouridine; I, inosine; Am, 2'-O-methyladenosine; ncm5U, 5-carbamoylmethyluridine; t6A, N6-threonylcarbamoyladenosine; Cm, 2'-O-methylcytidine; ac4C, N4-acetylcytidine.
(E) to (G) Relative abundance of the modified nucleosides shown in abbreviated form in 187R and NIL-PDDOL. Data represent mean ± sd from three independent biological replicates. *, P < 0.05 (Student’s t test). Cm, 2'-O-methylcytidine; m2A, 2-methyladenosine; Am, 2'-O-methyladenosine; m1G, 1-methylguanosine; m2G, N2-methylguanosine; ac4C, N4-acetylcytidine; Ψ, pseudouridine; I, inosine; m5C, 5-methylcytidine; m1A, 1-methyladenosine; m22G, N2,N2-dimethylguanosine; m7G, 7-methylguanosine; m5U, 5-methyluridine; D, dihydrouridine; ncm5U, 5-carbamoylmethyluridine; m6A, N6-methyladenosine; t6A, N6-threonylcarbamoyladenosine.
(H) Relative abundance of mnm5s2U in 187R, NIL-PDDOL, and the complementation lines (CP) chloroplast tRNA from seedlings at the three-leaf stage. Data represent mean ± sd from three independent biological replicates. n.s., no significant difference; *, P < 0.05 (Student’s t test).
(I) Relative abundance of mnm5s2U in 187R and NIL-PDDOL chloroplast tRNA from seedlings at the six-leaf stage. Data represent mean ± sd from three independent biological replicates. *, P < 0.05 (Student’s t test).
In addition, to investigate whether the tRNA modification is recovered in green leaves of NIL-PDDOL at later stages of development, we measured the levels of mnm5s2U in 187R and NIL-PDDOL chloroplasts isolated from seedlings at the six-leaf stage. The level of mnm5s2U in chloroplast tRNA was still lower in NIL-PDDOL than in 187R (Figure 6I), indicating that the level of the mnm5s2U modification is not recovered at later stages of development.
Levels of Chloroplast Proteins Associated with Photosynthesis and Ribosome Biogenesis Are Greatly Reduced in NIL-PDDOL
Given that tRNA modifications can affect the fidelity and efficiency of translation, we measured the levels of four key proteins involved in photosynthesis, including PSI subunit PsaB, PSII subunits D1 and D2, and the large subunit of ribulose-1,5-bis-phosphate carboxylase/oxygenase (Rubisco; RbcL), in 187R and NIL-PDDOL leaves at the three-leaf stage by immunoblot analysis. In contrast to 187R, D1 and D2 were nearly absent in NIL-PDDOL, accompanied by a striking decrease in RbcL and PsaB levels (Figure 7A). These results indicate that proteins involved in both the light reaction and carbon fixation reaction of photosynthesis are greatly affected in NIL-PDDOL. Furthermore, we measured the levels of proteins associated with the transcriptional and translational systems in chloroplasts, including subunits of plastid-encoded RNA polymerase (PEP; RpoA, RpoB, and RpoC2) and subunits of the chloroplast ribosome (RPS2, RPS3, RPS12, RPL2, and RPL16), in both 187R and NIL-PDDOL. Unexpectedly, the levels of RpoA, RpoB, and RpoC2 were higher in NIL-PDDOL than in 187R (Figure 7A), suggesting that the transcriptional system might be enhanced in NIL-PDDOL chloroplast. However, the protein levels of the ribosome subunits were markedly reduced in NIL-PDDOL, especially RPS2, RPS3, and RPL2 (Figure 7A), indicating that the accumulation of ribosome subunits is greatly impaired in NIL-PDDOL chloroplasts.
Figure 7.
Accumulation of Chloroplast Proteins Is Sharply Reduced in NIL-PDDOL.
(A) Immunoblot analysis of chloroplast proteins in 187R and NIL-PDDOL at the three-leaf stage. HSP90 was used as an internal control.
(B) Expression analysis of chloroplast genes by RT-qPCR. Data were normalized to ACTIN1 expression. The bars represent mean ± sd of triplicate experiments. ***, P < 0.001 (Student’s t test).
(C) rRNA analysis of 187 and NIL-PDDOL using an Agilent 2100 Bioanalyzer. FU, fluorescence units.
To determine whether the altered protein levels in NIL-PDDOL were caused by corresponding changes in gene expression, we compared the mRNA levels of genes encoding the above-mentioned proteins in 187R and NIL-PDDOL by RT-qPCR. The mRNA levels of photosynthesis-related genes transcribed by PEP, including psaB, D1, D2, and rbcL, were significantly reduced in NIL-PDDOL (Figure 7B). However, compared to 187R, the mRNA levels of genes associated with the transcriptional and translational machinery, such as rpoA, rpoB, rpoC2, rps2, rps3, rpl2, and rpl16, which are transcribed by nuclear-encoded RNA polymerase (NEP), were greatly increased in NIL-PDDOL (Figure 7B).
The observation that the accumulation of chloroplast ribosome subunit proteins was reduced but their mRNA expression levels were enhanced in NIL-PDDOL is similar to findings for several mutants defective in chloroplast ribosome biogenesis (Bang et al., 2012; Wang et al., 2016; Zhang et al., 2017). Therefore, we analyzed the composition and content of rRNAs in 187R and NIL-PDDOL using an Agilent 2100 Bioanalyzer. The chloroplast ribosome has one 50S large subunit and one 30S small subunit, containing 23S, 16S, 5S, and 4.5S rRNAs and ribosomal proteins (Bieri et al., 2017). Both 16S and 23S rRNA levels were dramatically lower in NIL-PDDOL than in 187R (Figure 7C), suggesting that chloroplast ribosome biogenesis is greatly affected in NIL-PDDOL. To investigate whether plastid-encoded protein levels recover at later stages of development, we analyzed the levels of the above-mentioned proteins in the green leaves of both 187R and NIL-PDDOL plants at the six-leaf stage. The levels of these proteins in NIL-PDDOL almost fully recovered to those in 187R at this later stage of development (Supplemental Figure 10).
The Balance of Chloroplast mRNA Transcription Is Severely Disturbed in NIL-PDDOL
To globally investigate the effects of PDD on mRNA accumulation in chloroplasts, we compared the expression profiles of chloroplast genes in 10-d-old (three-leaf stage) 187R and NIL-PDDOL seedlings by high-throughput whole-transcriptome sequencing (RNA-seq) with three biological replicates (see Methods). Forty-eight chloroplast genes exhibited a more than twofold difference in expression levels (27 with reduced transcript levels; 21 with increased transcript levels) in NIL-PDDOL compared to 187R (Figure 8; Supplemental Data Set 1). The differential expression patterns detected by RNA-seq were consistent with data for selected genes obtained by RT-qPCR (Figures 7B and 8), validating the reliability of the RNA-seq data. Chloroplast genes can be divided into three classes based on their transcription: class I genes are predominantly transcribed by PEP, class II genes are transcribed by both NEP and PEP, and class III genes are exclusively transcribed by NEP (Hajdukiewicz et al., 1997). Interestingly, the mRNA levels of class I genes were reduced in NIL-PDDOL, such as genes encoding PSI-related proteins, PSII-related proteins, and rbcL (Figure 8), indicating that PEP activity is defective in NIL-PDDOL. However, the expression levels of class III genes, which encode the subunits of PEP and chloroplast ribosomal proteins, were largely increased in NIL-PDDOL (Figure 8), indicating that the transcriptional activity mediated by NEP is enhanced in these plants.
Figure 8.
Differential Expression of Chloroplast-Encoded Genes in 187R and NIL-PDDOL.
The expression levels of the genes shown in magenta were detected by RT-qPCR, as shown in Figure 7B. FC, fold change.
Expression of ∼4000 Nuclear Genes Is Altered in NIL-PDDOL
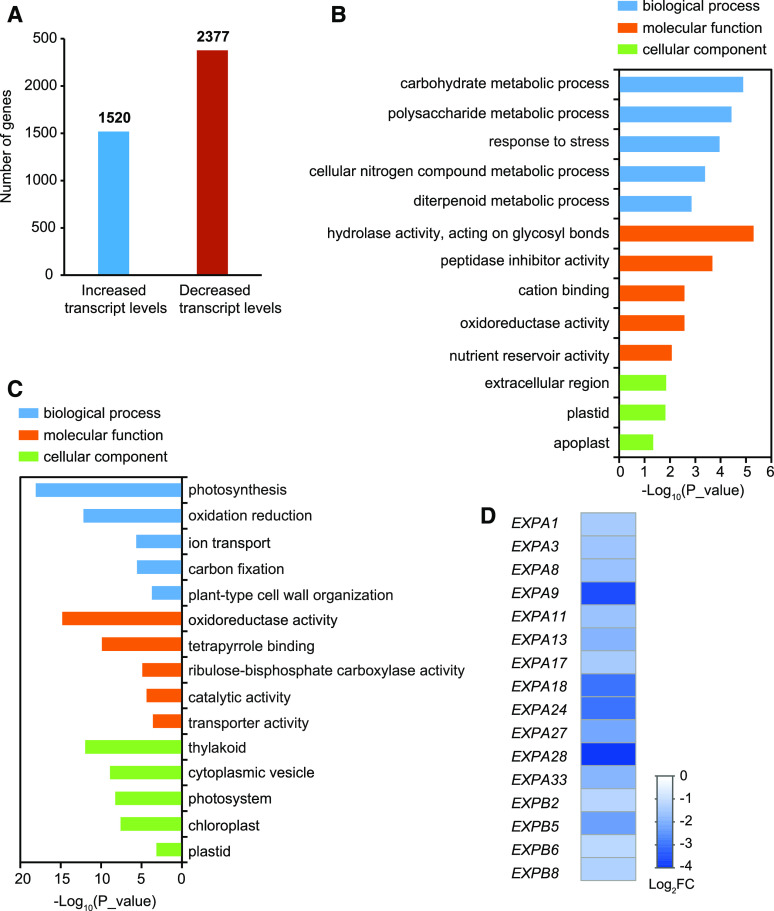
Chloroplast development is regulated by the coordinated expression of both chloroplast and nuclear genes (Pogson and Albrecht, 2011). Changes in developmental and/or gene expression status in the chloroplast can evoke massive changes in nuclear gene expression (Chi et al., 2015). To investigate the global changes in nuclear gene expression in NIL-PDDOL, we further analyzed our RNA-seq data. The expression of 1520 nuclear genes was increased and the expression of 2377 nuclear genes was reduced in NIL-PDDOL compared to 187R (P < 0.05; log2 (fold change) > 1 or log2 (fold change) < –1; Figure 9A; Supplemental Data Set 2).
Figure 9.
RNA-Seq Analysis of Nucleus-Encoded Genes in 10-d-Old 187R and NIL-PDDOL Seedlings.
(A) Number of differentially expressed genes in NIL-PDDOL compared to 187R (log2FC > 1 or log2FC < –1).
(B) Gene ontology analysis of genes with increased expression levels in NIL-PDDOL compared to 187R.
(C) Gene ontology analysis of genes with reduced expression levels in NIL-PDDOL compared to 187R.
(D) Heatmap of expansin genes with reduced expression levels in NIL-PDDOL compared to 187R.
We further categorized the genes with differential expression levels by gene ontology (GO) analysis using AgriGO (Supplemental Data Set 3; Tian et al., 2017), including genes in the biological process, molecular function, and cellular component categories. Among the upregulated genes in NIL-PDDOL versus 187R, biological processes involved in carbohydrate/polysaccharide metabolism, response to stress, cellular nitrogen compound metabolism, and diterpenoid metabolism were significantly enriched (Figure 9B). In the molecular function category, GO terms hydrolase activity, peptidase inhibitor activity, and cation binding were the most highly enriched (Figure 9B). In the cellular component category, extracellular region, plastid, and apoplast were the most highly enriched (Figure 9B).
Among the downregulated genes in NIL-PDDOL versus 187R, the most highly enriched GO terms in the biological process category were photosynthesis, oxidation–reduction, ion transport, carbon fixation, and plant-type cell wall organization (Figure 9C). For the molecular function category, the most highly enriched terms included oxidoreductase activity, tetrapyrrole (the biochemical precursors of chlorophylls) binding, Rubisco activity, catalytic activity, and transporter activity (Figure 9C). In the cellular component category, the top five GO terms were thylakoid, cytoplasmic vesicle, photosystem, chloroplast, and plastid (Figure 9C). These results suggest that the expression of photosynthesis-associated nuclear genes (PhANGs) is repressed in NIL-PDDOL. Furthermore, this analysis indicated that the PhANGs with reduced expression in NIL-PDDOL primarily participate in PSI and PSII, light harvesting, carbon fixation, and electron carrier processes (Supplemental Data Set 4). In addition, the GO term plant-type cell wall organization included 16 genes encoding expansin family proteins (Supplemental Table 3), which are closely associated with plant cell growth (Cosgrove, 2000). The expression of all 16 genes was greatly reduced in NIL-PDDOL versus 187R, as revealed in a heatmap of the RNA-seq data (Figure 9D) and confirmed by RT-qPCR (Supplemental Figure 11); these results are consistent with the reduced cell size in NIL-PDDOL (Figure 1M).
DISCUSSION
The Chloroplast tRNA-Modifying Enzyme PDD Is Essential for Normal Development in Flowering Plants
tRNAs, which function as critical components of protein translational systems in cells, are usually extensively modified at multiple sites, ensuring their accurate performance. Many genes have evolved encoding tRNA-modifying enzymes, occupying ∼1 to 10% of a given genome (El Yacoubi et al., 2012). The loss of function of certain cytoplasmic tRNA-modifying enzymes can result in developmental defects, such as embryonic lethality in Drosophila melanogaster and mouse (Chen et al., 2009b; Walker et al., 2011), neuronal dysfunction in C. elegans and human (Freude et al., 2004; Chen et al., 2009a), and defective growth (Hu et al., 2010; Mehlgarten et al., 2010; Philipp et al., 2014; Jin et al., 2019) and stress responses (Zhou et al., 2013; Wang et al., 2017) in plants. In addition, mutations in genes encoding tRNA-modifying enzymes localized to organelles can greatly affect various developmental processes. For example, the knockout of the mouse gene Cdk5rap1, encoding a mitochondrial tRNA-modifying enzyme, led to respiratory defects and myopathy (Wei et al., 2015). However, few studies have focused on the biological functions of tRNA-modifying enzymes in chloroplasts, another important organelle in green plants (Delannoy et al., 2009; Karcher and Bock, 2009).
Here, we demonstrated the biological function of the nucleus-encoded tRNA-modifying enzyme PDD in rice, which is localized to the chloroplast. We uncovered the natural allele PDDOL, which we integrated into rice cv 187R from an African wild rice by genetic introgression (Supplemental Figure 1A), leading to pleiotropic developmental defects (Figure 1). Also, pdd-1 homozygotes and two heteroallelic combinations (pdd-2/pdd-3 and pdd-4/pdd-5), which were obtained by CRISPR/Cas9-based genome editing, showed poor survival due to severe genetic disruptions (Figure 3). These results suggest that PDD is essential for normal rice development and that PDDOL is a weak allele. Considering that the wild rice parent O. longistaminata produces green leaves during early development, we hypothesize that other mechanisms in O. longistaminata might exist that compensate for the dysfunction of PDDOL. Alternatively, it is also possible that nucleo-cytoplasmic incompatibility between the introduced PDDOL and the native chloroplast genome contributes to the phenotypes of NIL-PDDOL.
The CRISPR/Cas9-generated pdd-1 allele, a severely impaired allele generated in this study, is predicted to produce a 35–amino acid truncated protein (Supplemental Figure 4). The pdd-2, pdd-4, and pdd-5 alleles are predicted to generate distinct peptides lacking half of the N-terminal domain and the entire G domain (Supplemental Figure 4), suggesting that both the N-terminal and G domains are essential for PDD function. By contrast, both the PDDOL and pdd-3 alleles encode nearly full-length PDD proteins similar to that in 187R, with a few amino acid substitutions or deletions (Figure 5; Supplemental Figure 4). In detail, PDDOL contains two amino acid deletions and five amino acid alterations, leading to two residue changes (Arg145Gly and Ala191Asp) in the N-terminal domain and one amino acid substitution (Glu376Gln) in the G domain (Figure 5A). The pdd-3 allele is predicted to generate a protein lacking three amino acid residues at positions 13, 14, and 159 (Supplemental Figure 4). Protein sequence analysis revealed that the Leu at position 159, along with the Ala at position 191, are highly conserved in green plants and that the Glu at position 376 is relatively conserved in most plants (Supplemental Figure 6). Therefore, we speculate that the inability of PDDOL to form homodimers may be caused by an Ala191Asp change and that the Glu at position 376 is important for normal GTPase activity.
In human and yeast, TrmE family proteins are targeted to mitochondria (Decoster et al., 1993; Villarroya et al., 2008). Here, we showed that PDD localizes to chloroplasts in rice. These findings suggest that TrmE family proteins predominantly function in semiautonomous organelles in eukaryotes. Chloroplasts and mitochondria are endosymbiotic organelles that were derived from cyanobacterium and α-proteobacterium, respectively (Bock and Timmis, 2008). Phylogenetic analysis revealed that TrmE proteins in animals and fungi are grouped with α-proteobacteria, whereas TrmEs in green plants form a separate monophyletic class with the cyanobacterial clade (Suwastika et al., 2014), supporting the functional diversity of TrmEs in different organelles. However, the genomes of the green plants analyzed in this study only contained TrmEs from cyanobacteria (Supplemental Figure 5), suggesting that they have lost the mitochondrial copies of TrmE during evolution. Indeed, when we cotransfected rice protoplasts with the mitochondrial marker F1-ATPase-γ:RFP (Niwa et al., 1999) and Pro35S:PDD187R-eGFP, the PDD187R-eGFP fusion protein was capable of localizing to the mitochondria (Supplemental Figure 12). Therefore, perhaps cyanobacteria-derived TrmE has taken over the function of TrmE from the mitochondrial ancestor in green plants. The molecular and biological functions of TrmE in rice mitochondria should be comprehensively studied in the future.
tRNA Modification in Chloroplasts Might Be Required for Accurate and Efficient Translation of Various Proteins Associated with the PEP Transcriptional System and Ribosome Biogenesis
TrmE proteins modify the wobble uridines in tRNAs that decode NNA/NNG codons of the mixed codon family (Moukadiri et al., 2014). The rice chloroplast genome contains five tRNAs with these features, including cp-tRNAArgUCU, cp-tRNAGluUUC, cp-tRNAGlnUUG, cp-tRNALeuUAA, and cp-tRNALysUUU (Hiratsuka et al., 1989). Several decades ago, biochemical analysis indicated that the wobble uridines of cp-tRNAGluUUC in barley are modified to mnm5s2U (Schön et al., 1986). Here, using LC-MS/MS and functional genetic analysis, we discovered that the TrmE protein PDD is involved in the mnm5s2U modification in rice chloroplast tRNAs (Figure 6). In addition, based on studies in other species, the mnm5s2U modification is only thought to occur in wobble uridines (Armengod et al., 2014). These findings suggest that PDD might participate in wobble uridine modifications in rice chloroplast tRNAs.
Modifications of tRNA anticodons, especially at the wobble position, play critical roles in maintaining the fidelity and efficiency of mRNA translation (Ranjan and Rodnina, 2016). The tRNA wobble uridine modification mediated by TrmE family proteins is thought to mainly prevent base pairing with C/U and/or prevent ribosomal frameshifting during translation, thereby maintaining the accuracy of protein translation (Yokoyama et al., 1985; Brégeon et al., 2001). Alternatively, tRNA modifications associated with TrmE family members can greatly influence the efficiency of anticodons in reading cognate codons (Krüger et al., 1998; Johansson et al., 2008; Westhof et al., 2014). For example, the hypomodified mt-tRNALeuUAA lacking 5-taurinomethyluridine failed to decode the UUG codon efficiently (Kirino et al., 2004). In addition, superwobbling can occur in chloroplasts, in which the set of tRNAs is reduced (Alkatib et al., 2012). Several tRNA modifications can prevent superwobbling (Suzuki et al., 2011). However, it is not known whether the mnm5s2U modification at U34 prohibits superwobbling in green plants.
Here, we found that the levels of PEP subunit proteins were higher NIL-PDDOL than in 187R (Figure 7A). However, RNA-seq analysis revealed that the expression levels of the genes that are transcribed by the PEP system were greatly reduced in NIL-PDDOL (Figure 8), suggesting that PEP activity might be impaired in NIL-PDDOL, primarily due to the defect in PDD. We speculate that some PEP subunits might have reduced activities due to translational errors caused by superwobbling and/or frameshifting in NIL-PDDOL during translation. Therefore, it is possible that the mnm5s2U modification is required to prevent superwobbling. In addition, the mRNA levels of chloroplast ribosome subunit genes were much higher in NIL-PDDOL than in 187R (Figure 7B). However, the levels of the corresponding proteins were markedly reduced in NIL-PDDOL (Figure 7A). These findings suggest that the translation efficiency of proteins involved in ribosome biogenesis is severely affected in NIL-PDDOL. In summary, we propose that PDD-mediated chloroplast tRNA modifications affect multiple aspects of chloroplast translation.
Chloroplast Transcription and Translation Status in NIL-PDDOL Trigger Retrograde Signaling to Repress PhANG Gene Expression
Chloroplast development and gene expression are under nuclear control via anterograde signaling. A signaling system derived from chloroplasts named retrograde signaling also functions in plants; this system transmits information about the developmental and functional state of the chloroplast to the nucleus to regulate nuclear gene expression (Chan et al., 2016). The retrograde signaling system is a complex network containing multiple signals and pathways, including a class of signals triggered by chloroplast gene expression (Hernández-Verdeja and Strand, 2018). PhANG expression is suppressed in mutants with defects in the chloroplast transcriptional machinery, such as sig2 and sig6 (Woodson et al., 2013). Treatment with the chloroplast translation inhibitor lincomycin also represses PhANG expression (Susek et al., 1993). These findings indicate that the status of chloroplast transcription and translation can trigger retrograde signaling to repress PhANG expression.
In this study, we demonstrated that the functional defect of PDDOL leads to a decrease in mnm5s2U tRNA modification, potentially impairing the translation process in chloroplasts (Figures 6 and 7). RNA-seq and RT-qPCR revealed that the expression of chloroplast-encoded genes is greatly affected in NIL-PDDOL, with most PEP-transcribed genes showing reduced expression levels and NEP-transcribed genes showing increased expression levels (Figures 7B and 8). These results indicate that PEP activity is impaired and NEP activity is enhanced in NIL-PDDOL. Moreover, the expression levels of PhANG are significantly reduced in NIL-PDDOL (Supplemental Data Set 4). Based on these observations, we reasoned that in NIL-PDDOL, information about impaired transcription and translation in the chloroplast is transmitted to the nucleus by retrograde signaling, causing the nucleus to increase NEP activity in the chloroplast, thereby enhancing the expression of genes related to the transcriptional and translational machinery in the chloroplast. On the other hand, the reduced expression levels of PhANG and expansin genes in NIL-PDDOL might facilitate plant survival by allowing the cells to conserve energy by reducing plant growth.
Taken together, we propose a working model for the molecular mechanism underlying PDD function in 187R and impaired PDDOL function in NIL-PDDOL (Figure 10). According to our model, in 187R, PDD187R modifies the uridines in chloroplast tRNAs into mnm5s2U, ensuring accurate and/or efficient translation in the chloroplast to promote normal plant development. In NIL-PDDOL, the defective function of PDDOL results in decreased chloroplast tRNA modification, consequently affecting the fidelity and/or efficiency of translation in the chloroplast. Thus, aberrant chloroplast translation leads to impaired ribosome biogenesis and reduced levels of photosynthetic proteins, resulting in the albino phenotype of NIL-PDDOL. In addition, in response to retrograde signaling, the transcriptional levels of PhANG and expansin genes in the nucleus decrease in NIL-PDDOL, leading to smaller cell size and dwarf plant stature.
Figure 10.
A Proposed Working Model of the Molecular Function of PDD.
In 187R, PDD187R correctly modifies the chloroplast tRNA’s U34 to mnm5s2U, ensuring accurate and/or efficient translation in the chloroplast to promote normal plant development. In NIL-PDDOL, the functional defect of PDDOL results in reduced levels of mnm5s2U modification in chloroplast tRNAs, leading to translational errors in the chloroplast. Aberrant chloroplast translation results in impaired ribosome biogenesis, reduced levels of photosynthetic proteins, and impaired PEP activity. The transcriptional levels of PhANG and expansin genes in the nucleus are reduced by retrograde signaling.
METHODS
Plant Materials and Growth Conditions
The F1 population generated by a cross between 187R (Oryza sativa subsp indica) and African wild rice (Oryza longistaminata) was obtained from Rongbai Li. The plants were grown in a paddy field in Shanghai (31°27′N, summer season, temperate climate), Taicang (31°45′N, summer season, temperate climate), and Sanya (18°16′N, winter season, subtropical climate), China under local cultivation conditions. For experiments at the seedling stage, the plants were grown in a growth chamber at 30/26°C under a 10-h-light/14-h-dark cycle using one-half-strength Murashige and Skoog medium as a nutrient source. An 18-W white light-emitting diode full-spectrum grow light was used (color temperature value, 6500 K; light intensity, 320 μmol photons m−2 s−1).
Photosynthetic Pigment Content and Fv/Fm Measurements
Chlorophyll and carotenoid contents were measured with a spectrophotometer. Briefly, fresh leaves (∼0.05 g) were collected and immersed in 8 mL of chlorophyll extraction buffer (95% [v/v] ethanol: acetone:water = 5:4:1) for 24 h in the dark with gentle shaking (Peng et al., 2012). The absorbance of the supernatants at 663, 645, and 470 nm was measured with a microplate spectrophotometer (Synergy II; BioTek). Each sample was measured with three biological replicates from different seedling leaves, and each biological replicate sample was measured three times using the same supernatant. For the Fv/Fm measurements, seedlings at the three-leaf stage were dark adapted for at least 1 h. The Fv/Fm values were then determined using a portable photosynthesis system (LI-6400XT; LI-COR Biosciences) according to the manufacturer’s instructions.
Transmission Electron Microscopy
The third leaves from 187R and NIL-PDDOL plants were cut into small pieces and fixed in 2.5% (v/v) glutaraldehyde in 0.1 M phosphate buffer at 4°C for 16 h. After three rinses with phosphate buffer, the samples were incubated in 1% (w/v) osmic acid at room temperature for 5 h and washed with phosphate buffer. The samples were dehydrated through an ethanol series (30, 50, 70, 80, 90, 95, 100, and 100% [v/v]). The ethanol was subsequently replaced by a series of Spurr’s resin dilutions (25, 50, 75, and 100% [v/v]). Finally, the samples were embedded in Spurr’s resin at 60°C for 48 h. Ultrathin sections were cut with a diamond knife in a Leica UC7 ultramicrotome and collected on copper grids. The sections were stained with uranyl acetate and observed under a Hitachi HT7700 transmission electron microscope.
Paraffin Sectioning and Observation of Cell Size
The uppermost internodes of 187R and NIL-PDDOL were fixed in formaldehyde–acetic acid–ethanol solution for 24 to 48 h, dehydrated in a graded ethanol series, cleared with HistoChoice (1328-H103; Amresco), and embedded in Paraplast Plus (P3683; Sigma-Aldrich). Sections (10 µm in thickness) were obtained with a Leica RM2245 microtome, transferred onto poly-l-Lys–coated glass slides, deparaffinized with a HistoChoice series, and dehydrated through an ethanol series. The cell size was photographed by bright-field microscopy (Scope A1; Zeiss).
Seed Germination and Plant Growth Assay
Ninety-six seeds per sample were soaked in water at 30°C for 2 d, placed on wet filter paper in a Petri dish, and incubated at 30°C for 2 d. The seeds were transferred to a growth chamber and incubated under standard conditions. After 10 d in the growth chamber, we counted the number of seedlings and calculated the proportions of seedlings from 96 seeds. Each sample was measured with three independent biological replicates.
Map-Based Cloning of PDD
Primary mapping was performed using 200 F2 plants derived by genetic crosses between NIL-PDDOL and 187R. To fine map the PDD locus, 93-11 was crossed with NIL-PDDOL. Recombinants were screened for the RM1309 and RM8264 markers using 4104 plants from the BC1F2 population, and the PDD locus was ultimately mapped to the interval between 420SNP and 480SNP using 4608 additional BC1F2 individuals.
Vector Construction and Plant Transformation
We created a complementation vector by amplifying a 6135-bp genomic DNA fragment of PDD including the 2096-bp promoter region from 187R using primers cPDD-F and cPDD-R. The DNA fragment was inserted into the pCAMBIA 1304 vector using BamHI/KpnI sites. We constructed a vector by amplifying the 2096-bp promoter sequence of PDD187R and the 1653-bp coding region sequence of PDDOL. The DNA fragments were inserted into the pCAMBIA 1304 vector using HindIII/EcoRI sites. The recombinant plasmid was introduced into NIL-PDDOL via Agrobacterium-mediated transformation (Hiei and Komari, 2008).
We used the CRISPR/Cas9 multiplex editing system to generate genome-edited mutants of PDD (Xie et al., 2015). The specific spacer sequences were selected using the CRISPR-PLANT database (http://www.genome.arizona.edu/crispr/; Xie et al., 2014). The construct was generated as described by Xie et al. (2014). The synthesized fragments with tandemly arrayed tRNA-guide RNA architecture were inserted into pRGEB32. We confirmed the construct by sequencing and transformed it into 187R via Agrobacterium-mediated transformation (Hiei and Komari, 2008). Several rice transformations were conducted by the Hefei Jiangu Biotechnology.
Sequencing and Phylogenetic Analysis
Gene annotation was performed using the Michigan State University rice annotation database (http://rice.plantbiology.msu.edu/). Domain analysis was performed using InterPro (http://www.ebi.ac.uk/interpro/search/sequence-search). The chloroplast-targeting signal was predicted using ChloroP 1.1 (http://www.cbs.dtu.dk/services/ChloroP/). Protein sequences homologous to PDD were identified using BLASTP against the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov/). Multiple sequence alignments were generated using MEGA version 6.0, and the phylogenetic tree (Supplemental Figure 5) was constructed with MEGA version 6.0 using the neighbor-joining method and default values. Bootstrap support values were calculated from 1000 replicates and are listed on the branch nodes. The sequence alignments and tree files are provided as Supplemental Files 1 and 2, respectively.
RNA Extraction and RT-qPCR
Total RNA was extracted from the samples with TRIzol Reagent (15596018; Invitrogen) according to the manufacturer’s instructions. First-strand cDNA was synthesized from 2 μg of total RNA using a FastQuant RT kit (KR106; Tiangen). The cDNA used to detect the expression levels of plastid-encoded genes was synthesized using random hexamer primers. RT-qPCR was conducted using SuperReal PreMix Plus (FP205; Tiangen) on a StepOnePlus real-time PCR system (Applied Biosystems). The PCR conditions were 95°C for 15 min followed by 40 cycles of 95°C for 10 s and 60°C for 30 s. The relative expression levels of the target RNAs were calculated by the 2–ΔΔCt method using ACTIN1 (LOC_Os03g50885) as an internal control to normalize different samples. Each sample used three biological replicates by extracting RNA from different batches of seedlings.
Subcellular Localization
The coding sequences of PDD187R and PDDOL were amplified and cloned into the N terminus of eGFP under the control of the cauliflower mosaic virus 35S promoter using EcoRI/SalI sites (Tang et al., 2014). The recombinant vector was transformed into rice protoplasts as previously described by Zhang et al. (2011). GFP fluorescence in the transformed protoplasts was visualized under a confocal laser-scanning microscope (TCS SP8; Leica). F1-ATPase-γ:RFP (Niwa et al., 1999) was used as a mitochondria localization marker.
Histochemical Staining of GUS Expression
The ProPDD187R:GUS vector was constructed by cloning the 2167-bp promoter region of PDD187R into the pCAMBIA1300 vector using HindIII/BamHI sites to drive GUS expression. The construct was transformed into japonica rice var Nipponbare plants via Agrobacterium-mediated transformation. Various tissues of ProPDD187R:GUS T2 transgenic plants were incubated in 90% (v/v) acetone on ice for 10 min, washed several times with GUS staining buffer [10 mM EDTA, pH 8.0, 29 mM Na2HPO4, 21 mM NaH2PO4, 1 mM K4Fe(CN)6, 1 mM K3Fe(CN)6, 0.1% (v/v) Triton X-100, 0.05% (w/v) 5-bromo-4-chloro-3-indolyl-β-d-glucuronide, and 20% (v/v) methanol], submerged in the staining buffer, and placed in a vacuum chamber for 20-min periods of vacuum infiltration. The samples were incubated overnight in staining buffer at 37°C. Ethanol was used to remove the chlorophyll after GUS staining.
Y2H Assay
Y2H assays were performed using the Matchmaker Gold Yeast Two-Hybrid system (630489; Clontech). The coding sequences of PDD187R and PDDOL were cloned into pGBKT7 using EcoRI/SalI sites and cloned into pGADT7 using EcoRI/BamHI sites. The constructs were transformed into yeast strain Y2H gold (pGBKT7 constructs) or Y187 (pGADT7 constructs) using the lithium acetate/polyethylene glycol method. Transformants were mated on 2× yeast peptone dextrose adenine liquid medium for 24 h, followed by selection on synthetic dropout medium (SD)/-Trp-Leu plates for 36 h. Transformants were selected on SD/-His-Ade-Trp-Leu plates to test for positive interactions. pGADT7+pGBKT7 was used as the negative control.
BiFC Assay
The full-length coding sequences of PDD from 187R and NIL-PDDOL were cloned into pXY103/pXY104 using BamHI/SalI sites (Su et al., 2017). The constructs were transformed into Agrobacterium GV3101 cells. Transformants were harvested once the OD600 reached 2.0 and resuspended in MES/MgCl2/acetosyringone solution to a final OD600 of 1.0. Various combinations of cell suspensions were mixed at a 1:1 ratio and used to infiltrate young Nicotiana benthamiana leaves. The leaves were excised and visualized under a confocal microscope (TCS SP8; Leica) following 48 h of incubation.
Protein Purification and GTPase Activity Assay
The coding sequences of PDD187R and PDDOL were cloned into a modified pET28a vector with a His6-small ubiquitin-related modifier (SUMO) tag at the N terminus using EcoRI/SalI sites (Yang et al., 2018). The plasmids were transformed into Escherichia coli strain C41 (DE3) for expression. A 20-mL aliquot of overnight bacterial cultures was inoculated into 1 L of Luria-Bertani medium and cultured at 37°C. When the OD600 reached 0.6 to 0.8, protein expression was induced by adding 0.2 mM isopropyl β-d-thiogalactopyranoside, followed by incubation at 18°C for 18 h. The cells were harvested by centrifugation at 4500 rpm for 20 min and resuspended in lysis buffer (20 mM Tris-HCl, pH 8.0, 500 mM NaCl, and 25 mM imidazole, pH 8.0). Following disruption in a JN-02C cell crusher, the cell debris was removed by centrifugation (17,000 rpm) at 4°C for 1 h, and the supernatant was loaded onto a nickel-chelating column. The protein with His6-SUMO tag was eluted from the column using elution buffer (20 mM Tris-HCl, pH 8.0, 500 mM NaCl, and 500 mM imidazole, pH 8.0). After overnight treatment with SUMO protease Ulp1 at 4°C, the cleaved His6-SUMO tag was removed using a second nickel-chelating column. Finally, PDD187R and PDDOL were detected by SDS-PAGE and used in the GTPase activity assay.
The GTPase assay was performed using a commercial GTPase assay kit (602-0120; Innova Biosciences) following the manufacturer’s instructions, with minor modifications. Briefly, 200 μL of reaction buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM KCl, 2.5 mM MgCl2, 0.5 mM GTP, and 100 μg of protein was incubated at 30°C for 10 min. After stopping the reaction by adding Gold mix and stabilizer, the plate was incubated at room temperature for 30 min in the dark and the absorbance was read at a wavelength of 650 nm. GFP protein was used as the negative control. We calculated relative enzyme activity as recommended.
Chloroplast Isolation, tRNA Extraction, and Purification
Chloroplasts were isolated from ∼10-d-old 187R and NIL-PDDOL seedlings using a modified Suc density gradient centrifugation method (Takamatsu et al., 2018). The entire process was carried at 4°C. Briefly, 20 g of fresh leaves was cut into ∼1-cm pieces and homogenized in 400 mL of isolation buffer (50 mM Tris-HCl, pH 8.0, 0.35 M Suc, 7 mM EDTA, 5 mM DTT, and 0.1% [w/v] BSA). The homogenate was filtered twice through two layers of Miracloth (475855; Millipore). The filtrate was centrifuged at 1000g for 10 min. After resuspending the pellet in 6 mL of isolation buffer, the suspension was slowly layered onto a cushion of 20/45% (w/v) Suc in 50 mM Tris-HCl, pH 8.0, 0.3 M sorbitol, and 7 mM EDTA. Following centrifugation at 2000g for 30 min, the green layer at the 20% Suc and 45% Suc interface was carefully collected, diluted by adding three volumes of isolation buffer, and centrifuged at 3000g for 10 min. RNAs smaller than 200 nucleotides were extracted from the chloroplast pellet using RNAiso for small RNA (9753A; Takara). The band containing tRNAs was sliced from a 15% (v/v) Tris–boric acid–EDTA-urea gel, and the tRNAs were recovered using a ZR small-RNA PAGE Recovery Kit (R1070; Zymo Research).
tRNA Digestion and LC-MS/MS Analysis
The nucleosides were analyzed by LC-MS/MS as described by Wang et al. (2017), with minor modifications. Briefly, ∼5 μg tRNA was digested with 2 U of P1 nuclease (N8630; Sigma-Aldrich) and 1.5 U of calf intestine alkaline phosphatase (CAP-111; Toyobo) in 20 mM Hepes-KOH, pH 7.0, at 37°C for 3 h. The samples were diluted in Milli-Q water (Synergy; Millipore) to a concentration of 15 ng/μL, and 10-μL samples were injected into the LC-MS/MS machine. All digested tRNA samples were analyzed using three biological replicates from different batches of seedlings.
The LC-20A HPLC system with an Inertsil ODS-3 column (2.1 × 150 mm, 5-μm particle size; Shimadzu) was used for nucleoside separation. The binary solvent system composed by 2 mM ammonium acetate (solution A) and methanol (solution B) was used as the mobile phase. The gradient was as follows: 0 to 10 min, 0 to 50% of B; 10 to 13 min, 50 to 100% of B; 13 to 23 min, 100% of B; 23 to 23.1 min, 100 to 5% of B; 23.1 to 30 min, 5 to 0% of B. Solution A (100%) was applied for 10 min to re-equilibrate the column before the next sample was injected. An API 4000 Q-Trap mass spectrometer (Applied Biosystems) was used for detection. Electrospray ionization mass spectrometry was conducted in positive ion mode. Multiple reaction monitoring mode was used to determine parent-to-product ion transitions. The relative abundance of each modified nucleoside was calculated by the ratio to the sum of A, U, C, and G.
Protein Extraction, SDS-PAGE, and Immunoblot Analysis
Total proteins were isolated from the leaves of ∼10-d-old 187R and NIL-PDDOL seedlings. The tissues were ground in liquid nitrogen and thawed in lysis buffer (7 M urea, 2 M thiourea, and 30 mM Tris). Cell debris was removed by centrifugation at 12,000g for 15 min at 4°C. Sample amounts were standardized by fresh weight. Total proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes, immunoblotted with various antibodies, and detected using Clarity Western ECL Substrate (1705060; Bio-Rad). Heat shock protein HSP90 was used as an internal reference (Li et al., 2011). Anti-PsaB (catalog no. AS10 695; dilution 1:1000), D1 (catalog no. AS05 084; dilution 1:1600), D2 (catalog no. AS06 146; dilution 1:5000), RbcL (catalog no. AS03 037; dilution 1:5000), and RPL2 (catalog no. AS15 2876; dilution 1:3000) antibodies were purchased from Agrisera (https://www.agrisera.com/). Anti-RpoA (catalog no. AbP80103-A-SE; dilution 1:1000), RpoB (catalog no. AbP80101-A-SE; dilution 1:1000), RpoC2 (catalog no. AbP80094-A-SE; dilution 1:500), RPS3 (catalog no. AbP80377-A-SE; dilution 1:500), and HSP90 (catalog no. AbM51099-31-PU; dilution 1:10,000) antibodies were obtained from BGI (http://www.proteomics.org.cn/). Anti-RPS2 (catalog no. PHY0427S; dilution 1:500), RPS12 (catalog no. PHY0434S; dilution 1:500), and RPL16 (catalog no. PHY0431S; dilution 1:500) antibodies were obtained from PhytoAB (http://www.phytoab.com/).
RNA-Seq and GO Enrichment Analysis
Total RNA was isolated from ∼10-d-old 187R and NIL-PDDOL seedlings, each with three biological replicates from different batches of seedlings. Each sample was quantified, and its quality was checked in an Agilent 2100 Bioanalyzer. The rRNA was removed from total RNA using a Ribo-Zero rRNA Removal Kit (Plant Leaf). The rRNA-depleted RNA was fragmented and reverse transcribed. First-strand cDNA was synthesized from the RNA using random primers. Second-strand cDNA was synthesized using Second Strand Synthesis Enzyme Mix (including dACG-TP/dUTP). The double-stranded cDNA was purified using AxyPrep Mag PCR Clean-up (Axygen) and treated with End Prep Enzyme Mix to repair both ends and to add a dA-tail in one reaction, followed by T-A ligation to add adaptors to both ends. Size selection of adaptor-ligated DNA was performed using AxyPrep Mag PCR Clean-up (Axygen), and ∼360-bp fragments (with an approximate insert size of 300 bp) were recovered. The library was constructed and sequenced using the Illumina Hisequation 2000 system. The raw sequencing data were collected and filtered. We obtained 95 million reads each from 187R and NIL-PDDOL for nucleus-encoded genes, and 33 million reads each from 187R and NIL-PDDOL were obtained for chloroplast-encoded genes. The significance of differentially expressed genes was determined using P-values < 0.05 and |log2 (fold change)| > 1. GO analysis was performed using the AgriGO website (Tian et al., 2017). Rice chloroplast genes were identified by referring to the chloroplast genome database (http://rocaplab.ocean.washington.edu/old_website/tools/cpbase/run).
Primer Sequences
The primers used in this study are listed in Supplemental Data Set 5.
Accession Numbers
Sequence data used to construct the phylogenetic tree were download from NCBI under the accession numbers listed in Supplemental Figure 5. Additional sequences of the PDD homologs used in Supplemental Figure 6 were download from NCBI and EnsemblPlants (http://plants.ensembl.org/index.html) under the following accession numbers: XP_015648320 (O. sativa); XP_003574461 (B. distachyon); XP_002444316 (S. bicolor); XP_008677280 (Z. mays); TraesCS5B02G032900 (Triticum aestivum); XP_002301037 (Populus trichocarpa); XP_003522812 (G. max); NP_177924 (Arabidopsis); XP_020521993 (Amborella trichopoda). The raw sequencing data have been submitted to the NCBI BioProject database (https://www.ncbi.nlm.nih.gov/bioproject/) under accession number PRJNA602187.
Supplemental Data
Supplemental Figure 1. Construction of NIL-PDDOL and additional phenotypic analysis of NIL-PDDOL.
Supplemental Figure 2. Quantitative analysis of the agronomic traits of 187R, NIL-PDDOL, and pdd-1.
Supplemental Figure 3. Phenotypic analysis of transgenic complementation lines harboring CG4 or CG5 and mRNA expression levels of PDD in 187R, NIL-PDDOL, and pdd-1.
Supplemental Figure 4. Protein sequence alignment of PDD in 187R and various genome-edited mutants.
Supplemental Figure 5. Phylogenetic analysis and protein structures of TrmE family members.
Supplemental Figure 6. Protein sequence alignment of homologs of PDD in various plant species.
Supplemental Figure 7. Alignment of the promoter sequences, coding sequences and protein sequences of PDD187R and PDDOL.
Supplemental Figure 8. Subcellular localization of PDDOL and GTPase colorimetric assay.
Supplemental Figure 9. Chemical structures of TrmE-related tRNA modifications in E. coli and human mitochondria.
Supplemental Figure 10. Immunoblot analysis of chloroplast proteins in 187R and NIL-PDDOL green leaves at the six-leaf stage (Supports Figure 7).
Supplemental Figure 11. Expression analysis of expansin genes via RT-qPCR.
Supplemental Figure 12. Subcellular localization of PDD187R-eGFP in mitochondria.
Supplemental Table 1. List of candidate genes in the mapped region and their putative functions.
Supplemental Table 2. LC-MS/MS parameters for analysis of modified nucleosides.
Supplemental Table 3. Expansin genes with reduced expression levels in NIL-PDDOL compared to 187R.
Supplemental Data Set 1. Chloroplast genes with reduced expression levels and increased expression levels in NIL-PDDOL compared to 187R.
Supplemental Data Set 2. Nuclear genes with reduced expression levels and increased expression levels in NIL-PDDOL compared to 187R.
Supplemental Data Set 3. GO analysis of nuclear genes with reduced or increased expression levels in NIL-PDDOL compared to 187R.
Supplemental Data Set 4. Nuclear genes involved in PSI and PSII, light harvesting, carbon fixation and electron carriers.
Supplemental Data Set 5. Primers used in this study.
Supplemental File 1. Sequence alignments of TrmE proteins.
Supplemental File 2. Phylogenetic tree file for TrmE proteins.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We appreciate Rongbai Li (Guangxi University) for providing the F1 plants generated from a cross between 187R (O. sativa subsp indica) and African wild rice (O. longistaminata). We thank Jianhua Gan and Xi Chen (School of Life Science, Fudan University) for help with protein purification. We are grateful to Jirong Huang (College of Life and Environmental Sciences, Shanghai Normal University) for providing the antibodies for PsaB, D1, D2, and RbcL. We also thank the anonymous reviewers for their constructive comments, which have significantly improved our article. This work was supported by a grant from the National Natural Science Foundation of China (31770351 to P.L. and 31671655 to X. L.), the 111 Project (grant D16014), and start-up funding from Henan Provincial University to P.L.
AUTHOR CONTRIBUTIONS
H.L. and D.R. performed map-based cloning, most experiments and data analyses. L.J. and X. Li. generated the constructs for functional studies. Y.Y., L.M., W.C., and A.M. helped with the phenotypic analysis and subcellular localization assays. N.J. performed bioinformatics analysis for transcriptome sequencing. X. Luo developed the rice NILs and constructed the mapping population. P.C. assisted to detect and analyze tRNA modifications via mass spectrometry. X. Luo, H.M., and J.Y. provided valuable advice and supervised the study. P.L. conceived the study, designed the experiments, interpreted data, supervised the project, and wrote the article.
Footnotes
Articles can be viewed without a subscription.
References
- Alkatib S., Scharff L.B., Rogalski M., Fleischmann T.T., Matthes A., Seeger S., Schöttler M.A., Ruf S., Bock R.(2012). The contributions of wobbling and superwobbling to the reading of the genetic code. PLoS Genet. 8: e1003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armengod M.E., Meseguer S., Villarroya M., Prado S., Moukadiri I., Ruiz-Partida R., Garzón M.J., Navarro-González C., Martínez-Zamora A.(2014). Modification of the wobble uridine in bacterial and mitochondrial tRNAs reading NNA/NNG triplets of 2-codon boxes. RNA Biol. 11: 1495–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armengod M.E., Moukadiri I., Prado S., Ruiz-Partida R., Benítez-Páez A., Villarroya M., Lomas R., Garzón M.J., Martínez-Zamora A., Meseguer S., Navarro-González C.(2012). Enzymology of tRNA modification in the bacterial MnmEG pathway. Biochimie 94: 1510–1520. [DOI] [PubMed] [Google Scholar]
- Asano K., et al. (2018). Metabolic and chemical regulation of tRNA modification associated with taurine deficiency and human disease. Nucleic Acids Res. 46: 1565–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang W.Y., Chen J., Jeong I.S., Kim S.W., Kim C.W., Jung H.S., Lee K.H., Kweon H.S., Yoko I., Shiina T., Bahk J.D.(2012). Functional characterization of ObgC in ribosome biogenesis during chloroplast development. Plant J. 71: 122–134. [DOI] [PubMed] [Google Scholar]
- Bieri P., Leibundgut M., Saurer M., Boehringer D., Ban N.(2017). The complete structure of the chloroplast 70S ribosome in complex with translation factor pY. EMBO J. 36: 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk G.R., Durand J.M., Hagervall T.G., Leipuvienė R., Lundgren H.K., Nilsson K., Chen P., Qian Q., Urbonavičius J.(1999). Transfer RNA modification: Influence on translational frameshifting and metabolism. FEBS Lett. 452: 47–51. [DOI] [PubMed] [Google Scholar]
- Björk G.R., Hagervall T.G. (2005). Transfer RNA modification. Ecosal Plus 1. [DOI] [PubMed] [Google Scholar]
- Bock R., Timmis J.N.(2008). Reconstructing evolution: Gene transfer from plastids to the nucleus. BioEssays 30: 556–566. [DOI] [PubMed] [Google Scholar]
- Brégeon D., Colot V., Radman M., Taddei F.(2001). Translational misreading: A tRNA modification counteracts a +2 ribosomal frameshift. Genes Dev. 15: 2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabedo H., Macián F., Villarroya M., Escudero J.C., Martínez-Vicente M., Knecht E., Armengod M.E.(1999). The Escherichia coli trmE (mnmE) gene, involved in tRNA modification, codes for an evolutionarily conserved GTPase with unusual biochemical properties. EMBO J. 18: 7063–7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantara W.A., Crain P.F., Rozenski J., McCloskey J.A., Harris K.A., Zhang X., Vendeix F.A., Fabris D., Agris P.F.(2011). The RNA modification database, RNAMDB: 2011 update. Nucleic Acids Res. 39: D195–D201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.X., Phua S.Y., Crisp P., McQuinn R., Pogson B.J.(2016). Learning the languages of the chloroplast: Retrograde signaling and beyond. Annu. Rev. Plant Biol. 67: 25–53. [DOI] [PubMed] [Google Scholar]
- Chen C., Tuck S., Byström A.S.(2009a). Defects in tRNA modification associated with neurological and developmental dysfunctions in Caenorhabditis elegans elongator mutants. PLoS Genet. 5: e1000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., et al. (2019). Translational regulation of plant response to high temperature by a dual function tRNAHis guanylyltransferase in rice. Mol. Plant 12: 1123–1142. [DOI] [PubMed] [Google Scholar]
- Chen Y.T., Hims M.M., Shetty R.S., Mull J., Liu L., Leyne M., Slaugenhaupt S.A.(2009b). Loss of mouse Ikbkap, a subunit of elongator, leads to transcriptional deficits and embryonic lethality that can be rescued by human IKBKAP. Mol. Cell. Biol. 29: 736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi W., Feng P., Ma J., Zhang L.(2015). Metabolites and chloroplast retrograde signaling. Curr. Opin. Plant Biol. 25: 32–38. [DOI] [PubMed] [Google Scholar]
- Chumley T.W., Palmer J.D., Mower J.P., Fourcade H.M., Calie P.J., Boore J.L., Jansen R.K.(2006). The complete chloroplast genome sequence of Pelargonium × hortorum: Organization and evolution of the largest and most highly rearranged chloroplast genome of land plants. Mol. Biol. Evol. 23: 2175–2190. [DOI] [PubMed] [Google Scholar]
- Cosgrove D.J.(2000). Loosening of plant cell walls by expansins. Nature 407: 321–326. [DOI] [PubMed] [Google Scholar]
- Decoster E., Vassal A., Faye G.(1993). MSS1, a nuclear-encoded mitochondrial GTPase involved in the expression of COX1 subunit of cytochrome c oxidase. J. Mol. Biol. 232: 79–88. [DOI] [PubMed] [Google Scholar]
- Delannoy E., Le Ret M., Faivre-Nitschke E., Estavillo G.M., Bergdoll M., Taylor N.L., Pogson B.J., Small I., Imbault P., Gualberto J.M.(2009). Arabidopsis tRNA adenosine deaminase arginine edits the wobble nucleotide of chloroplast tRNAArg(ACG) and is essential for efficient chloroplast translation. Plant Cell 21: 2058–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi B., Bailly M., de Crécy-Lagard V.(2012). Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu. Rev. Genet. 46: 69–95. [DOI] [PubMed] [Google Scholar]
- Ferré-D’Amaré A.R.(2003). RNA-modifying enzymes. Curr. Opin. Struct. Biol. 13: 49–55. [DOI] [PubMed] [Google Scholar]
- Francis M.A., Suh E.R., Dudock B.S.(1989). The nucleotide sequence and characterization of four chloroplast tRNAs from the alga codium fragile. J. Biol. Chem. 264: 17243–17249. [PubMed] [Google Scholar]
- Freude K., et al. (2004). Mutations in the FTSJ1 gene coding for a novel S-adenosylmethionine-binding protein cause nonsyndromic X-linked mental retardation. Am. J. Hum. Genet. 75: 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giegé R., Jühling F., Pütz J., Stadler P., Sauter C., Florentz C.(2012). Structure of transfer RNAs: similarity and variability. Wiley Interdiscip. Rev. RNA 3: 37–61. [DOI] [PubMed] [Google Scholar]
- Gustilo E.M., Vendeix F.A., Agris P.F.(2008). tRNA’s modifications bring order to gene expression. Curr. Opin. Microbiol. 11: 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdukiewicz P.T., Allison L.A., Maliga P.(1997). The two RNA polymerases encoded by the nuclear and the plastid compartments transcribe distinct groups of genes in tobacco plastids. EMBO J. 16: 4041–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Verdeja T., Strand Å.(2018). Retrograde signals navigate the path to chloroplast development. Plant Physiol. 176: 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiei Y., Komari T.(2008). Agrobacterium-mediated transformation of rice using immature embryos or calli induced from mature seed. Nat. Protoc. 3: 824–834. [DOI] [PubMed] [Google Scholar]
- Hirabayashi H., et al. (2015). qEMF3, a novel QTL for the early-morning flowering trait from wild rice, Oryza officinalis, to mitigate heat stress damage at flowering in rice, O. sativa. J. Exp. Bot. 66: 1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka J., et al. (1989). The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol. Gen. Genet. 217: 185–194. [DOI] [PubMed] [Google Scholar]
- Hu Z., Qin Z., Wang M., Xu C., Feng G., Liu J., Meng Z., Hu Y.(2010). The Arabidopsis SMO2, a homologue of yeast TRM112, modulates progression of cell division during organ growth. Plant J. 61: 600–610. [DOI] [PubMed] [Google Scholar]
- Hua L., et al. (2015). LABA1, a domestication gene associated with long, barbed awns in wild rice. Plant Cell 27: 1875–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Huang W., Gao J.P., Yang J., Shi M., Zhu M.Z., Luo D., Lin H.X.(2008). Genetic control of rice plant architecture under domestication. Nat. Genet. 40: 1365–1369. [DOI] [PubMed] [Google Scholar]
- Jin X., Lv Z., Gao J., Zhang R., Zheng T., Yin P., Li D., Peng L., Cao X., Qin Y., Persson S., Zheng B., et al. (2019). AtTrm5a catalyses 1-methylguanosine and 1-methylinosine formation on tRNAs and is important for vegetative and reproductive growth in Arabidopsis thaliana. Nucleic Acids Res. 47: 883–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.J., Esberg A., Huang B., Björk G.R., Byström A.S.(2008). Eukaryotic wobble uridine modifications promote a functionally redundant decoding system. Mol. Cell. Biol. 28: 3301–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher D., Bock R.(2009). Identification of the chloroplast adenosine-to-inosine tRNA editing enzyme. RNA 15: 1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino Y., Yasukawa T., Ohta S., Akira S., Ishihara K., Watanabe K., Suzuki T.(2004). Codon-specific translational defect caused by a wobble modification deficiency in mutant tRNA from a human mitochondrial disease. Proc. Natl. Acad. Sci. USA 101: 15070–15075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger M.K., Pedersen S., Hagervall T.G., Sørensen M.A.(1998). The modification of the wobble base of tRNAGlu modulates the translation rate of glutamic acid codons in vivo. J. Mol. Biol. 284: 621–631. [DOI] [PubMed] [Google Scholar]
- Li X., Bai H., Wang X., Li L., Cao Y., Wei J., Liu Y., Liu L., Gong X., Wu L., Liu S., Liu G.(2011). Identification and validation of rice reference proteins for western blotting. J. Exp. Bot. 62: 4763–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlgarten C., Jablonowski D., Wrackmeyer U., Tschitschmann S., Sondermann D., Jäger G., Gong Z., Byström A.S., Schaffrath R., Breunig K.D.(2010). Elongator function in tRNA wobble uridine modification is conserved between yeast and plants. Mol. Microbiol. 76: 1082–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moukadiri I., Garzón M.J., Björk G.R., Armengod M.E.(2014). The output of the tRNA modification pathways controlled by the Escherichia coli MnmEG and MnmC enzymes depends on the growth conditions and the tRNA species. Nucleic Acids Res. 42: 2602–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedialkova D.D., Leidel S.A.(2015). Optimization of codon translation rates via tRNA modifications maintains proteome integrity. Cell 161: 1606–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa Y., Hirano T., Yoshimoto K., Shimizu M., Kobayashi H.(1999). Non-invasive quantitative detection and applications of non-toxic, S65T-type green fluorescent protein in living plants. Plant J. 18: 455–463. [DOI] [PubMed] [Google Scholar]
- Peng Y., Zhang Y., Lv J., Zhang J., Li P., Shi X., Wang Y., Zhang H., He Z., Teng S.(2012). Characterization and fine mapping of a novel rice albino mutant low temperature albino 1. J. Genet. Genomics 39: 385–396. [DOI] [PubMed] [Google Scholar]
- Philipp M., John F., Ringli C.(2014). The cytosolic thiouridylase CTU2 of Arabidopsis thaliana is essential for posttranscriptional thiolation of tRNAs and influences root development. BMC Plant Biol. 14: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay D.T., Guillemaut P., Weil J.H.(1984). Nucleotide sequences of three soybean chloroplast tRNAsLeu and re-examination of bean chloroplast tRNA2Leu sequence. Nucleic Acids Res. 12: 2997–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson B.J., Albrecht V.(2011). Genetic dissection of chloroplast biogenesis and development: An overview. Plant Physiol. 155: 1545–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan N., Rodnina M.V.(2016). tRNA wobble modifications and protein homeostasis. Translation (Austin) 4: e1143076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön A., Krupp G., Gough S., Berry-Lowe S., Kannangara C.G., Söll D.(1986). The RNA required in the first step of chlorophyll biosynthesis is a chloroplast glutamate tRNA. Nature 322: 281–284. [DOI] [PubMed] [Google Scholar]
- Scrima A., Vetter I.R., Armengod M.E., Wittinghofer A.(2005). The structure of the TrmE GTP-binding protein and its implications for tRNA modification. EMBO J. 24: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Cheng Z., Huang J., Lin J., Copenhaver G.P., Ma H., Wang Y.(2017). Arabidopsis RAD51, RAD51C and XRCC3 proteins form a complex and facilitate RAD51 localization on chromosomes for meiotic recombination. PLoS Genet. 13: e1006827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susek R.E., Ausubel F.M., Chory J.(1993). Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell 74: 787–799. [DOI] [PubMed] [Google Scholar]
- Suwastika I.N., Denawa M., Yomogihara S., Im C.H., Bang W.Y., Ohniwa R.L., Bahk J.D., Takeyasu K., Shiina T.(2014). Evidence for lateral gene transfer (LGT) in the evolution of eubacteria-derived small GTPases in plant organelles. Front. Plant Sci 5: 678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Nagao A., Suzuki T.(2011). Human mitochondrial tRNAs: Biogenesis, function, structural aspects, and diseases. Annu. Rev. Genet. 45: 299–329. [DOI] [PubMed] [Google Scholar]
- Takamatsu T., Baslam M., Inomata T., Oikawa K., Itoh K., Ohnishi T., Kinoshita T., Mitsui T.(2018). Optimized method of extracting rice chloroplast DNA for high-quality plastome resequencing and de novo assembly. Front. Plant Sci 9: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Luo D., Zhou D., Zhang Q., Tian D., Zheng X., Chen L., Liu Y.G.(2014). The rice restorer Rf4 for wild-abortive cytoplasmic male sterility encodes a mitochondrial-localized PPR protein that functions in reduction of WA352 transcripts. Mol. Plant 7: 1497–1500. [DOI] [PubMed] [Google Scholar]
- Tian T., Liu Y., Yan H., You Q., Yi X., Du Z., Xu W., Su Z.(2017). agriGO v2.0: A GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 45 (W1): W122–W129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan D.A., Morishima H., Kadowaki K.(2003). Diversity in the Oryza genus. Curr. Opin. Plant Biol. 6: 139–146. [DOI] [PubMed] [Google Scholar]
- Villarroya M., et al. (2008). Characterization of human GTPBP3, a GTP-binding protein involved in mitochondrial tRNA modification. Mol. Cell. Biol. 28: 7514–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J., Kwon S.Y., Badenhorst P., East P., McNeill H., Svejstrup J.Q.(2011). Role of elongator subunit Elp3 in Drosophila melanogaster larval development and immunity. Genetics 187: 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li D., Gao J., Li X., Zhang R., Jin X., Hu Z., Zheng B., Persson S., Chen P.(2017). The 2′-O-methyladenosine nucleoside modification gene OsTRM13 positively regulates salt stress tolerance in rice. J. Exp. Bot. 68: 1479–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., et al. (2016). WHITE PANICLE1, a Val-tRNA synthetase regulating chloroplast ribosome biogenesis in rice, is essential for early chloroplast development. Plant Physiol. 170: 2110–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M.T., Langdale J.A.(2009). The making of a chloroplast. EMBO J. 28: 2861–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F.Y., et al. (2015). Cdk5rap1-mediated 2-methylthio modification of mitochondrial tRNAs governs protein translation and contributes to myopathy in mice and humans. Cell Metab. 21: 428–442. [DOI] [PubMed] [Google Scholar]
- Westhof E., Yusupov M., Yusupova G.(2014). Recognition of Watson-Crick base pairs: constraints and limits due to geometric selection and tautomerism. F1000Prime Rep. 6: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson J.D., Perez-Ruiz J.M., Schmitz R.J., Ecker J.R., Chory J.(2013). Sigma factor-mediated plastid retrograde signals control nuclear gene expression. Plant J. 73: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K., Minkenberg B., Yang Y.(2015). Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc. Natl. Acad. Sci. USA 112: 3570–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K., Zhang J., Yang Y.(2014). Genome-wide prediction of highly specific guide RNA spacers for CRISPR-Cas9-mediated genome editing in model plants and major crops. Mol. Plant 7: 923–926. [DOI] [PubMed] [Google Scholar]
- Yang C., Wu R., Liu H., Chen Y., Gao Y., Chen X., Li Y., Ma J., Li J., Gan J.(2018). Structural insights into DNA degradation by human mitochondrial nuclease MGME1. Nucleic Acids Res. 46: 11075–11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S., Watanabe T., Murao K., Ishikura H., Yamaizumi Z., Nishimura S., Miyazawa T.(1985). Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc. Natl. Acad. Sci. USA 82: 4905–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Su J., Duan S., Ao Y., Dai J., Liu J., Wang P., Li Y., Liu B., Feng D., Wang J., Wang H.(2011). A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Cui X., Wang Y., Wu J., Gu X., Lu T.(2017). The RNA editing factor WSP1 is essential for chloroplast development in rice. Mol. Plant 10: 86–98. [DOI] [PubMed] [Google Scholar]
- Zhou W., Karcher D., Bock R.(2013). Importance of adenosine-to-inosine editing adjacent to the anticodon in an Arabidopsis alanine tRNA under environmental stress. Nucleic Acids Res. 41: 3362–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Tan L., Fu Y., Liu F., Cai H., Xie D., Wu F., Wu J., Matsumoto T., Sun C.(2013). Genetic control of inflorescence architecture during rice domestication. Nat. Commun. 4: 2200. [DOI] [PMC free article] [PubMed] [Google Scholar]