Abstract
The relationship between Epstein-Barr virus (EBV) and the germinal centre (GC) of the asymptomatic host remains an enigma. The occasional appearance of EBV-positive germinal centres in some patients, particularly those with a history of immunosuppression, suggests that EBV numbers in the GC are subject to immune control. The relationship, if any, between lymphoid hyperplasia with EBV-positive germinal centres and subsequent or concurrent lymphomagenesis remains to be clarified. As far as the development of EBV-associated Hodgkin's lymphoma is concerned, the suppression of virus replication, mediated by LMP1 on the one hand, and the loss of B-cell receptor signalling on the other, appears to be an important pathogenic mechanism. A further important emerging concept is that alterations in the microenvironment of the EBV-infected B-cell may be important for lymphomagenesis.
Introduction
The Epstein–Barr virus (EBV) infects most of the world’s adult population. In most cases primary infection and virus persistence are asymptomatic, the virus having evolved a sophisticated strategy to exist long term in the B-cell pool. However, EBV can contribute to the development of several human B-cell lymphomas, which include Hodgkin's lymphoma (HL), Burkitt's lymphoma, and a subset of diffuse large B-cell lymphomas, and can potently transform resting B-cells in vitro (Young & Murray, 2003; Young & Rickinson, 2004; Oyama et al., 2003, 2007). Two questions central to our understanding of the origins of EBV-associated B-cell lymphomas are (1) how the host and virus interact to allow benign persistent latent infection, and (2) how perturbation of this normal homeostasis leads to neoplastic transformation. This review will summarize current knowledge of how the EBV life cycle is regulated in the B-cells of the asymptomatic host. It will also discuss how the disruption of normal B-cell homeostasis can contribute to the development of B-cell lymphomas, focussing on several novel pathogenic mechanisms in EBV-associated HL, which include the suppression of the virus lytic cycle and the activation of collagen receptor signalling.
In vitro transformation of B-cells by EBV
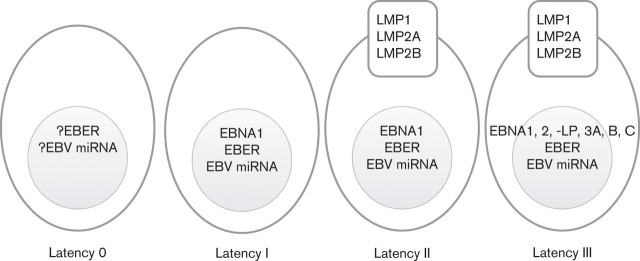
Much of what we know of the role of EBV in B-cell transformation comes from the study of lymphoblastoid cell lines (LCLs), cell lines generated by the in vitro infection of resting B-cells. These in vitro transformed B-cells express a limited subset of viral gene products, the so-called latent genes. They include six nuclear antigens (EBNAs 1, 2, 3A, 3B, 3C and -LP), three latent membrane proteins (LMPs 1, 2A and 2B), two major groups of viral microRNA (miRNA) (Amoroso et al., 2011) and the non-coding Epstein–Barr-encoded RNA (EBER); the latter of which is a target for in situ-based assays to detect EBV in tissue sections (Wu et al., 1990). This pattern of virus latency is often referred to as latency III or the growth programme (Fig. 1). Experiments with recombinant EBV lacking individual latent genes have shown that EBNA2 and LMP1 are required for the in vitro transformation of B-cells, and have highlighted a critical role for EBNA-LP, EBNA3A and EBNA3C in this process (Rickinson & Kieff, 2001). EBNA3A and EBNA3C have recently emerged as potential oncogenes, which in combination repress transcription of pro-apoptotic and senescence-inducing genes (Anderton et al., 2008; Skalska et al., 2010; Maruo et al., 2011). In contrast, EBNA3B is dispensable for B-cell transformation and has recently been reported to have a potential tumour suppressor function in vivo (White et al., 2012).
Fig. 1.
Different forms of EBV latency. EBV, Epstein-Barr virus; EBNA, EBV nuclear antigen; LMP, latent membrane protein; EBER, EBV-encoded RNAs; miRNA, microRNA.
Latent EBV infection in the B-cells of normal carriers
Although the virus efficiently transforms B-cells in vitro, >90 % of the human population carry EBV asymptomatically. Primary infection with EBV is usually also asymptomatic, but in some cases can result in infectious mononucleosis (IM) (Balfour et al., 2013). Once infected, the individual will remain so for life.
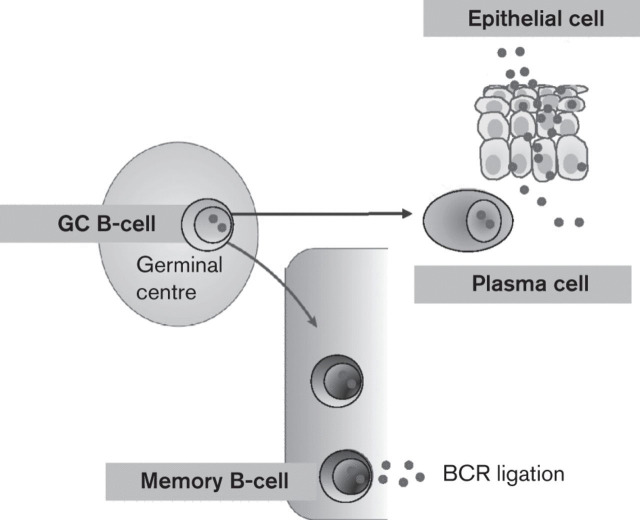
The discovery that EBV is present in memory B-cells, where virus gene expression is probably completely silenced (Hochberg et al., 2004), suggested a model in which the virus persists by hijacking the normal mechanism of memory B-cell homeostasis (Babcock et al., 1998). However, the mechanism by which EBV gains access to the memory B-cell pool remains controversial. It has been proposed that the virus uses the growth programme to drive newly infected resting B-cells into the cell cycle so that they can differentiate into the resting memory state via a germinal centre (GC) reaction (Thorley-Lawson, 2001; Thorley-Lawson et al., 2008; Thorley-Lawson & Gross, 2004) (Fig. 2). The GC is a region of secondary lymphoid tissue in which antigen-activated B-cells undergo proliferation, class switch recombination and somatic hypermutation, leading to antigen selection and affinity maturation (MacLennan, 1994; Klein & Dalla-Favera, 2008). In the GC, failure to successfully compete for antigen and T-cell help leads to apoptosis (Klein & Dalla-Favera, 2008).
Fig. 2.
The fate of EBV-infected GC B-cells. The normal fate of the non-malignant EBV-infected GC B-cell is unclear but like normal GC B-cells they are thought to be able to differentiate into either plasma cells or memory cells. This could be important for the normal viral life cycle because differentiation to memory cells is associated with latency and long-term persistence, whereas differentiation to plasma cells has been shown to induce the lytic cycle and is probably important for the replication of EBV in the oropharynx.
EBV infection in GC B-cells
EBV-infected GC B-cells have been shown to express a more restricted pattern of virus gene expression than is observed in LCLs; in this form of latency, often referred to as latency II or the default programme, LMP1 and LMP2 are expressed, but the EBNAs, with the exception of EBNA1, are not (Babcock et al., 2000; Thorley-Lawson & Gross, 2004; Roughan & Thorley-Lawson, 2009) (Fig. 1). LMP1 and LMP2 have been shown to possess, respectively, the CD40 and B-cell receptor (BCR) signalling functions necessary for GC survival and together are thought to contribute to the survival of EBV-infected B-cells in the GC and presumably their subsequent differentiation into memory B-cells (Casola et al., 2004; Caldwell et al., 1998; Gires et al., 1997; He et al., 2003; Panagopoulos et al., 2004; Swanson-Mungerson et al., 2005; Vockerodt et al., 2008). The default programme is also expressed in several EBV-associated cancers, including HL.
The frequency of EBV-infected GC B-cells in normal persistently infected individuals is very low; only around one-half of germinal centres from such individuals have been shown to be EBV-positive, on average harbouring only 3.5 EBV-infected cells per GC (Roughan et al., 2010). In keeping with this, it has been shown that viral loads in GC B-cells isolated from persistently infected individuals are much lower than those found in memory B-cells from the same donors (Chaganti et al., 2009). Previous studies that have used EBER in situ hybridization to document EBV-positive cells in the lymph nodes (Niedobitek et al., 1992) and the tonsils (Hudnall et al., 2005) of persistently infected individuals have shown that EBER-positive cells are mainly restricted to the extrafollicular areas, and are only rarely seen in GC. Similarly, most EBV-infected B-cells within the lymphoid tissues of patients with IM are located outside lymphoid follicles (Niedobitek et al., 1992, 1997; Kurth et al., 2000, 2003; Anagnostopoulos et al., 1995; Chaganti et al., 2009).
Lymphoid hyperplasia with EBV-positive germinal centres
Although EBV-positive GC B-cells are rare in the lymphoid tissues of healthy virus carriers and IM patients, a number of studies have reported instances of reactive lymphadenopathy in which lymphoid tissues display an expansion of EBV-positive cells within one or more GC (Dojcinov et al., 2011; Niedobitek et al., 1992; Martín et al., 2011, 2012; Araujo et al., 1999). This condition might represent an exaggerated form of the natural persistent state in which there is failure to control EBV infection in GC B-cells; in support of this possibility, eight cases of lymphoid hyperplasia with EBV-positive germinal centres were reported by Dojcinov et al. (2011), as part of the spectrum of adult late onset EBV-associated B-cell lymphoproliferations which are presumed to have in common an age-related decline in EBV-specific immunity.
Lymphoid hyperplasia with EBV-positive germinal centres has also been reported in association with HL. For example, it has been found concurrently with a diagnosis of EBV-positive HL (Martín et al., 2011, 2012), and at relapse following an initial diagnosis of EBV-positive HL (Martín et al., 2011, 2012). Although this might support the possibility that lymphoid hyperplasia with EBV-positive germinal centres represents a stage in the evolution of HL, one cannot rule out the possibility that these are simply benign EBV-driven lymphoproliferations which share some of the histological characteristics of classical HL, including the presence of ‘Hodgkin/Reed–Sternberg (HRS)-like cells’ (Dojcinov et al., 2011).
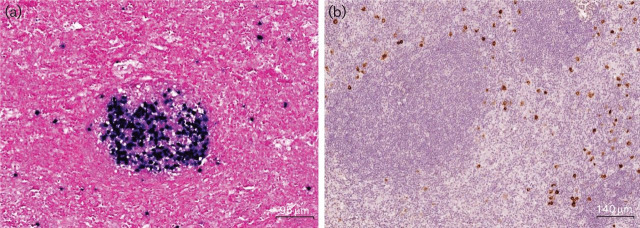
Our own analysis of five cases of lymphoid hyperplasia with EBV-positive germinal centres has revealed that the expansion of EBV-positive B-cells in germinal centres can be extensive and highly variable in adjacent follicles (Fig. 3, Table 1). Two of our five cases arose in patients with a history of immunosuppression (common variable immunodeficiency and human immunodeficiency virus (HIV) infection; cases 2 and 5, respectively; Table 1), and two further cases were found in association with EBV-positive HL (cases 1 and 4). Furthermore, while there are conflicting reports about latent gene expression in the EBV-infected GC B-cells of such cases, we did not observe LMP1 or LMP2A expression in EBER-positive germinal centres; this might suggest that the expansion of EBV-positive B-cells, at least in our cases, was antigen- rather than LMP1/2-driven.
Fig. 3.
Rare cases of reactive lymphadenopathy harbouring EBV-positive germinal centres. (a) EBER-positive cells in a GC with some scattered interfollicular EBER-positive cells in an enlarged cervical lymph node (case 1; see Table 1). The number of EBER-positive cells per GC was highly variable, including completely negative germinal centres. (b) Interfollicular EBER-positive cells express LMP1, while EBER-positive cells in GCs are LMP1-negative. A similar pattern was observed for LMP2A.
Table 1.
Details of five patients with reactive lymphadenopathy containing EBV-positive GCs
+, Positive; −, negative; HPF, high power field.
| Case no. | Sex | Age | Tissue | Diagnosis | Serology | EBER | LMP1/LMP2A IHC |
| 1 | M | 27 | Lymph node | EBV+ classical HL. In retrospect considered as EBV-driven lymphoproliferation. Patient relapsed 4 years later with similar histology and gene expression findings | EBV IgG+ | Reactive lymphoid hyperplasia. EBER+ cells in up to 4 GCs per section (range 2–40+ cells per GC) and EBER+ interfollicular lymphocytes (range 24–63+ cells per High power field (HPF)) | LMP1 and LMP2A expression detected in interfollicular lymphocytes but not in EBER+ GC |
| 2 | M | 49 | Tonsil | Common variable immunodeficiency. EBV+ classical HL 20 years earlier. In retrospect differential diagnosis EBV+ HL or atypical reaction to EBV | EBV IgG+, IgM− | Reactive lymphoid hyperplasia. EBER+ cells in up to 2 GCs per section (range 22–133+ cells per GC). EBER+ cells within and surrounding crypt epithelium. EBER+ interfollicular lymphocytes (range 69–196+ cells per HPF) | LMP1, but not LMP2A, expression detected in interfollicular lymphocytes. EBER+ GC LMP1− and LMP2A- |
| 3 | M | 20 | Lymph node | PET+ enlarged lymph nodes left jaw angle and some smaller PET+ nodes in neck and both axillae. No IM symptoms. Diagnosed as reactive lymphadenopathy | Not known | Reactive lymphoid hyperplasia. EBER+ cells in up to 8 GCs per section (range 7–100+ cells per GC). EBER+ interfollicular lymphocytes (range 0–15+ cells per HPF) | LMP1 and LMP2A expression detected in interfollicular lymphocytes but not in EBER+ GC |
| 4 | F | 23 | Lymph node | EBV+ classical HL 5 years earlier (with some EBER+ GCs). In retrospect doubt about original HL diagnosis; differential diagnosis EBV reactive lymphadenopathy. Further cervical lymph node biopsy 1 year later shows same histology and EBER+ GC | EBV IgG+, IgM− | Reactive lymphoid hyperplasia. EBER+ cells in up to 2 GCs per section (range 16–52+ cells per GC). EBER+ interfollicular lymphocytes (range 0–58+ cells per HPF) | LMP1 and LMP2A undetectable in EBER+ GC and in interfollicular cells. Subsequent cervical lymph node biopsy showed no LMP2A and LMP1 expression in EBER+ GC. In this case interfollicular lymphocytes expressed both LMP1 and LMP2A |
| 5 | M | 32 | Tonsil | HIV+. Enlarged adenoid. Concurrent hairy leukoplakia | EBV IgG+, IgM− | Reactive lymphoid hyperplasia. EBER+ cells in up to 7 GCs per section (range 20–180+ cells per GC). EBER+ interfollicular lymphocytes (range 2–5+ cells per HPF) | LMP1 and LMP2A undetectable in GC and interfollicular cells |
Replication of EBV in B-cells
In LCLs, the virus can switch from latency to induce its replicative cycle. At any one time, LCLs contain a small proportion of cells that spontaneously enter the lytic cycle. The lytic cycle can be induced in these cells by various treatments including the cross-linking of the BCR (Kieff & Rickinson, 2001).Terminal differentiation into plasma cells can also initiate the viral replicative cycle (Anagnostopoulos et al., 1995; Crawford & Ando et al., 1986; Niedobitek et al., 1997, 2000; Laichalk & Thorley-Lawson, 2005) (Fig. 2). Thus, the EBV lytic cycle can be triggered in B-cells by two distinct routes, either by activating BCR signalling or by terminal differentiation into plasma cells. The former probably represents an acute reactivation in response to stress that allows the virus to escape before the cell dies, whereas the latter is likely to be the natural route to EBV replication in tonsils (Laichalk & Thorley-Lawson, 2005).
The immediate-early EBV gene, BZLF1, provides the trigger for the induction of the lytic cycle and alone is sufficient to activate downstream lytic genes and viral replication (Countryman et al., 1985; Takada et al., 1986). The latent membrane proteins can regulate entry to the lytic cycle. Thus, while the virus lytic cycle can be activated by LMP2A, it is inhibited by LMP1 (Miller et al., 1994; Schaadt et al., 2005; Engels et al., 2012; Adler et al., 2002; Prince et al., 2003).
EBV-associated HL
The association between EBV and a subset of classical HL was first established when it was shown that EBV DNA is present in the malignant HRS cells of this disease (Weiss et al., 1989). EBV rates in HL tumours from North America and Europe have subsequently been shown to vary between 20 and 50 %, whereas much higher rates are observed in underdeveloped countries. EBV-positive rates are also generally higher: in males compared with females; in Asians and Hispanics compared with whites or blacks (Glaser et al., 1997); and in the UK in South Asian children compared with non-South Asian children (Flavell et al., 2001). In developed countries, the proportion of EBV-positive cases is higher in older people and in children, especially in those under 10 years of age, whereas the lowest rates of EBV-positive disease are found in young adults (Glaser et al., 1997; Jarrett et al., 1991; Armstrong et al., 1998).
Although HRS cells are of B-cell origin, they lack a functional BCR, as well as the downstream components required for the transmission of the BCR signal. In some cases the loss of BCR expression can be explained by the presence of destructive IgV gene mutations (Bräuninger et al., 2003; Küppers, 2005). Nearly all cases of HL harbouring these so-called ‘crippling’ mutations are EBV-positive (Bräuninger et al., 2006). This observation suggests that HRS precursors with such mutations can survive and eventually undergo malignant transformation only if infected by EBV. In support of this, EBV has been shown to be able to rescue BCR-negative GC B-cells from apoptosis (Bechtel et al., 2005; Mancao et al., 2005; Chaganti et al., 2005).
EBV-infected HRS cells are known to express LMP1 and LMP2. LMP1 could be especially important in the pathogenesis of HL since it constitutively activates several of the pathways, including NF-κB, JAK/STAT and phosphatidylinositol 3-kinase/AKT, which are known to be aberrantly activated in HRS cells (Bargou et al., 1997; Kube et al., 2001; Dutton et al., 2005).The potential importance of LMP1 in the pathogenesis of HL is underscored by our observation that LMP1 expression in primary human GC B-cells, the presumed progenitors of HRS cells, can contribute up to one-quarter of the transcriptional changes observed when HL cell lines are compared with isolated GC B-cells (Vockerodt et al., 2008).
Suppression of the EBV lytic cycle as a pathogenic mechanism in EBV-associated HL
We have hypothesized that the suppression of virus replication, which would otherwise lead to cell death, is an important pathogenic event in the development of EBV-associated lymphomas, such as HL. As described above, the EBV lytic cycle can be initiated in B-cells following either cross-linking of the BCR or their differentiation into plasma cells. We have found evidence which suggests that both mechanisms are disabled in EBV-infected HRS cells.
LMP1 suppresses plasma cell differentiation in EBV-infected B-cells
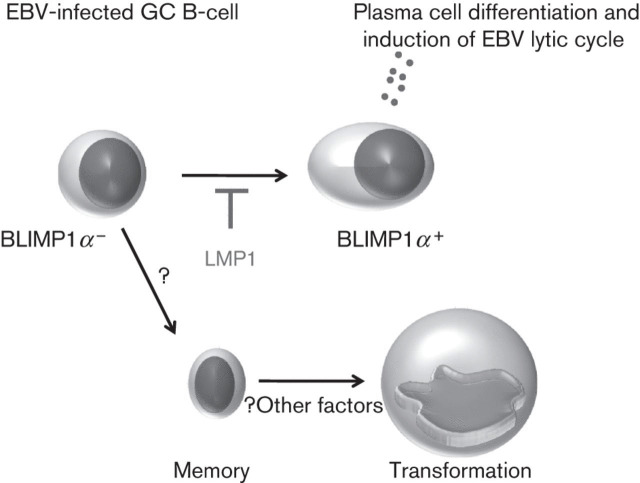
As described above, plasma cell differentiation leads to EBV replication in B-cells. Plasma cell differentiation is known to be controlled by the coordinated expression of B-cell-associated transcription factors. One of these transcription factors is BLIMP1α. BLIMP1α is encoded by the PRDM1 gene and is essential for plasma cell differentiation. We have shown that LMP1 can downregulate BLIMP1α expression in primary human GC B-cells, an effect that was accompanied by the partial disruption of the BLIMP1α transcriptional programme, including the aberrant induction of myc, the repression of which is required for terminal B-cell differentiation (Vrzalikova et al., 2011; Lin et al., 2000). Furthermore, this downregulation appeared to be important for the suppression of the virus lytic cycle since the re-expression of BLIMP1α induced the lytic cycle in EBV-transformed B-cells. These observations are consistent with previous reports showing that LMP1 can block entry into the viral lytic cycle in B-cells (Adler et al., 2002; Prince et al., 2003). We have speculated that the disruption of normal BLIMP1α functions could be an essential step in the pathogenesis of EBV-associated germinal centre-derived lymphomas, preventing not only the terminal differentiation of germinal centre-derived progenitors, but also virus replication (Fig. 4).
Fig. 4.
Model in which LMP1 blocks plasma cell differentiation. BLIMP1α is required for plasma cell differentiation. Plasma cell differentiation of EBV-infected B-cell leads to induction of the virus lytic cycle. LMP1 can suppress BLIMP1α expression, thereby preventing terminal differentiation to plasma cells. LMP1 can also presumably drive cells into the memory B-cell pool.
Absence of the BCR signalling machinery prevents BCR-mediated lytic cycle entry in HRS cells
The alternative route to virus replication in B-cells is induced following activation of the BCR. LMP2A is a BCR mimic and can induce the lytic cycle in B-cells. However, EBV-infected HRS cells do not have a functional BCR receptor and lack expression of other critical downstream components of BCR signalling. We have shown that LMP2A and BCR signalling in primaryhuman GC B-cells results in overlapping transcriptional changes, many of which are also observed when primary HRS cells are compared with normal GC B-cells. These data support the contention that LMP2A can provide a surrogateBCR-like signal in HRS cells. However, we also found that because HRS cells lack downstream BCR signalling components, LMP2A cannot induce the EBV lytic cycle in these cells (Vockerodt et al., 2013). These observations have allowed us to develop a model in which LMP2A-expressing HRS cell progenitors lacking BCR signalling functions are protected from entry to the virus lytic cycle which in turn could lead to their positive selection during the evolution of EBV-associated HL.
Collagen receptor signalling in the pathogenesis of EBV-associated HL
As described above, LMP1 is reported to be expressed in the EBV-infected GC B-cells of normal healthy EBV carriers, where it is thought to provide a CD40-like survival signal. However, LMP1 is also reported to be an oncogene that can constitutively activate several cell signalling pathways known to be aberrantly expressed in HRS cells. In an attempt to resolve this apparent paradox, we revisited our previous analysis of the transcriptional changes induced by LMP1 in normal GC B-cells (Vockerodt et al., 2008). During the course of this analysis, we noted that LMP1 upregulated expression of the discoidin domain receptor 1 (DDR1), a receptor tyrosine kinase (RTK) which can be activated by collagen (Cader et al., 2013). This was of interest because HL tissues are frequently extensively infiltrated by collagen. We were then able to show that short-term exposure to collagen was sufficient to activate DDR1 and promote the survival of lymphoma cells in vitro (Cader et al., 2013). These observations are important because they suggest that the oncogenic activities of LMP1 may, at least in part, be dependent on cues from the microenvironment.
Conclusions
EBV is associated with a range of different B-cell malignancies; however, the contribution of the virus to each of these tumours is probably different. For example, in the case of Burkitt’s lymphoma, EBV probably provides an anti-apoptotic signal which complements myc de-regulation. In contrast, in EBV-associated post-transplant lymphomas, in which T-cell control of EBV infection is severely impaired, the virus is thought to be the major driver of tumour cell growth and survival. In the case of HL, EBV is also thought to provide anti-apoptotic functions, this time facilitating the survival of BCR-negative HRS cells, or their precursors. Why EBV-infected HRS cells evolve to lose BCR functions is not known. Our studies suggest that this might be to avoid the induction of virus replication and the ensuing cell death which would otherwise be induced by cross-linking of the BCR. A further indication of the importance of suppressing virus replication in the pathogenesis of EBV-associated HL is provided by the observation that the viral oncogene, LMP1, can downregulate BLIMP1α, thereby supressing plasma cell differentiation and the alternative route to virus replication in B-cells.
Another unusual feature of HL not found to the same extent in other EBV associated B-cell lymphomas is the presence of an unusually florid microenvironment composed of numerous different non-malignant cell types and extracellular matrix components, such as collagen. This microenvironment contributes to disease pathogenesis not only by directly stimulating tumour cell growth and survival but potentially also by redirecting the functions of the EBV latent genes expressed by HRS cells. This is exemplified by the induction of the cell surface receptor DDR1 by LMP1; DDR1 is a growth- and survival-promoting tyrosine kinase that is activated in EBV-infected B-cells only in the presence of its ligand, collagen.
Acknowledgements
K. V., Z. C., M. V., E. N. and P. M. were supported by Leukaemia Lymphoma Research. K. V., P. M. and P. F. were also funded in part by the European Union (infrastructure support CZ.1.05/2.1.00/01.0030 and CZ.1.07/2.3.00/20.0019). G. M. was supported by the National Cancer Institute, Cairo University, Egypt and L.-F. Y. by University of Malaya UM.C/625/1/HIR/MOHE/DENT/23.
References
- Adler B., Schaadt E., Kempkes B., Zimber-Strobl U., Baier B., Bornkamm G. W. Control of Epstein-Barr virus reactivation by activated CD40 and viral latent membrane protein 1. Proc Natl Acad Sci U S A. 2002;99:437–442. doi: 10.1073/pnas.221439999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoroso R., Fitzsimmons L., Thomas W. A., Kelly G. L., Rowe M., Bell A. I. Quantitative studies of Epstein-Barr virus-encoded microRNAs provide novel insights into their regulation. J Virol. 2011;85:996–1010. doi: 10.1128/JVI.01528-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostopoulos I., Hummel M., Kreschel C., Stein H. Morphology, immunophenotype, and distribution of latently and/or productively Epstein-Barr virus-infected cells in acute infectious mononucleosis: implications for the interindividual infection route of Epstein-Barr virus. Blood. 1995;85:744–750. [PubMed] [Google Scholar]
- Anderton E., Yee J., Smith P., Crook T., White R. E., Allday M. J. Two Epstein-Barr virus (EBV) oncoproteins cooperate to repress expression of the proapoptotic tumour-suppressor Bim: clues to the pathogenesis of Burkitt’s lymphoma. Oncogene. 2008;27:421–433. doi: 10.1038/sj.onc.1210668. [DOI] [PubMed] [Google Scholar]
- Araujo I., Foss H. D., Hummel M., Anagnostopoulos I., Barbosa H. S., Bittencourt A., Stein H. Frequent expansion of Epstein-Barr virus (EBV) infected cells in germinal centres of tonsils from an area with a high incidence of EBV-associated lymphoma. J Pathol. 1999;187:326–330. doi: 10.1002/(SICI)1096-9896(199902)187:3<326::AID-PATH242>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Armstrong A. A., Alexander F. E., Cartwright R., Angus B., Krajewski A. S., Wright D. H., Brown I., Lee F., Kane E., Jarrett R. F. Epstein-Barr virus and Hodgkin’s disease: further evidence for the three disease hypothesis. Leukemia. 1998;12:1272–1276. doi: 10.1038/sj.leu.2401097. [DOI] [PubMed] [Google Scholar]
- Babcock G. J., Decker L. L., Volk M., Thorley-Lawson D. A. EBV persistence in memory B-cells in vivo. Immunity. 1998;9:395–404. doi: 10.1016/S1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- Babcock G. J., Hochberg D., Thorley-Lawson A. D. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B-cell. Immunity. 2000;13:497–506. doi: 10.1016/S1074-7613(00)00049-2. [DOI] [PubMed] [Google Scholar]
- Balfour H. H., Jr, Odumade O. A., Schmeling D. O., Mullan B. D., Ed J. A., Knight J. A., Vezina H. E., Thomas W., Hogquist K. A. Behavioral, virologic, and immunologic factors associated with acquisition and severity of primary Epstein-Barr virus infection in university students. J Infect Dis. 2013;207:80–88. doi: 10.1093/infdis/jis646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargou R. C., Emmerich F., Krappmann D., Bommert K., Mapara M. Y., Arnold W., Royer H. D., Grinstein E., Greiner A., other authors Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin’s disease tumor cells. J Clin Invest. 1997;100:2961–2969. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel D., Kurth J., Unkel C., Küppers R. Transformation of BCR-deficient germinal-center B-cells by EBV supports a major role of the virus in the pathogenesis of Hodgkin and posttransplantation lymphomas. Blood. 2005;106:4345–4350. doi: 10.1182/blood-2005-06-2342. [DOI] [PubMed] [Google Scholar]
- Bräuninger A., Spieker T., Mottok A., Baur A. S., Küppers R., Hansmann M. L. Epstein-Barr virus (EBV)-positive lymphoproliferations in post-transplant patients show immunoglobulin V gene mutation patterns suggesting interference of EBV with normal B-cell differentiation processes. Eur J Immunol. 2003;33:1593–1602. doi: 10.1002/eji.200323765. [DOI] [PubMed] [Google Scholar]
- Bräuninger A., Schmitz R., Bechtel D., Renné C., Hansmann M. L., Küppers R. Molecular biology of Hodgkin’s and Reed/Sternberg cells in Hodgkin’s lymphoma. Int J Cancer. 2006;118:1853–1861. doi: 10.1002/ijc.21716. [DOI] [PubMed] [Google Scholar]
- Cader F. Z., Vockerodt M., Bose S., Nagy E., Brundler M. A., Kearns P., Murray P. G. The EBV oncogene LMP1 protects lymphoma cells from cell death through the collagen-mediated activation of DDR1. Blood. 2013;122:4237–4245. doi: 10.1182/blood-2013-04-499004. [DOI] [PubMed] [Google Scholar]
- Caldwell R. G., Wilson J. B., Anderson S. J., Longnecker R. Epstein-Barr virus LMP2A drives B-cell development and survival in the absence of normal B-cell receptor signals. Immunity. 1998;9:405–411. doi: 10.1016/S1074-7613(00)80623-8. [DOI] [PubMed] [Google Scholar]
- Casola S., Otipoby K. L., Alimzhanov M., Humme S., Uyttersprot N., Kutok J. L., Carroll M. C., Rajewsky K. B-cell receptor signal strength determines B-cell fate. Nat Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- Chaganti S., Bell A. I., Pastor N. B., Milner A. E., Drayson M., Gordon J., Rickinson A. B. Epstein-Barr virus infection in vitro can rescue germinal center B-cells with inactivated immunoglobulin genes. Blood. 2005;106:4249–4252. doi: 10.1182/blood-2005-06-2327. [DOI] [PubMed] [Google Scholar]
- Chaganti S., Heath E. M., Bergler W., Kuo M., Buettner M., Niedobitek G., Rickinson A. B., Bell A. I. Epstein-Barr virus colonization of tonsillar and peripheral blood B-cell subsets in primary infection and persistence. Blood. 2009;113:6372–6381. doi: 10.1182/blood-2008-08-175828. [DOI] [PubMed] [Google Scholar]
- Countryman J., Miller G. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc Natl Acad Sci U S A. 1985;82:4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. H., Ando I. EB virus induction is associated with B-cell maturation. Immunology. 1986;59:405–409. [PMC free article] [PubMed] [Google Scholar]
- Dojcinov S. D., Venkataraman G., Pittaluga S., Wlodarska I., Schrager J. A., Raffeld M., Hills R. K., Jaffe E. S. Age-related EBV-associated lymphoproliferative disorders in the Western population: a spectrum of reactive lymphoid hyperplasia and lymphoma. Blood. 2011;117:4726–4735. doi: 10.1182/blood-2010-12-323238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton A., Reynolds G. M., Dawson C. W., Young L. S., Murray P. G. Constitutive activation of phosphatidyl-inositide 3 kinase contributes to the survival of Hodgkin’s lymphoma cells through a mechanism involving Akt kinase and mTOR. J Pathol. 2005;205:498–506. doi: 10.1002/path.1725. [DOI] [PubMed] [Google Scholar]
- Engels N., Yigit G., Emmerich C. H., Czesnik D., Schild D., Wienands J. Epstein-Barr virus LMP2A signaling in statu nascendi mimics a B-cell antigen receptor-like activation signal. Cell Commun Signal. 2012;10:9. doi: 10.1186/1478-811X-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell K. J., Biddulph J. P., Powell J. E., Parkes S. E., Redfern D., Weinreb M., Nelson P., Mann J. R., Young L. S., Murray P. G. South Asian ethnicity and material deprivation increase the risk of Epstein-Barr virus infection in childhood Hodgkin’s disease. Br J Cancer. 2001;85:350–356. doi: 10.1054/bjoc.2001.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gires O., Zimber-Strobl U., Gonnella R., Ueffing M., Marschall G., Zeidler R., Pich D., Hammerschmidt W. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J. 1997;16:6131–6140. doi: 10.1093/emboj/16.20.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser S. L., Lin R. J., Stewart S. L., Ambinder R. F., Jarrett R. F., Brousset P., Pallesen G., Gulley M. L., Khan G., other authors Epstein-Barr virus-associated Hodgkin’s disease: epidemiologic characteristics in international data. Int J Cancer. 1997;70:375–382. doi: 10.1002/(SICI)1097-0215(19970207)70:4<375::AID-IJC1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- He B., Raab-Traub N., Casali P., Cerutti A. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching. J Immunol. 2003;171:5215–5224. doi: 10.4049/jimmunol.171.10.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg D., Middeldorp J. M., Catalina M., Sullivan J. L., Luzuriaga K., Thorley-Lawson D. A. Demonstration of the Burkitt’s lymphoma Epstein-Barr virus phenotype in dividing latently infected memory cells in vivo. Proc Natl Acad Sci U S A. 2004;101:239–244. doi: 10.1073/pnas.2237267100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudnall S. D., Ge Y., Wei L., Yang N. P., Wang H. Q., Chen T. Distribution and phenotype of Epstein-Barr virus-infected cells in human pharyngeal tonsils. Mod Pathol. 2005;18:519–527. doi: 10.1038/modpathol.3800369. [DOI] [PubMed] [Google Scholar]
- Jarrett R. F., Gallagher A., Jones D. B., Alexander F. E., Krajewski A. S., Kelsey A., Adams J., Angus B., Gledhill S., Wright D. H. Detection of Epstein-Barr virus genomes in Hodgkin’s disease: relation to age. J Clin Pathol. 1991;44:844–848. doi: 10.1136/jcp.44.10.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E., Rickinson A. B. Epstein–Barr virus and its replication. In: Knipe D. M., Howley P. M., editors. Fields Virology. 4th edn. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2511–2573. Edited by. [Google Scholar]
- Klein U., Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- Kube D., Holtick U., Vockerodt M., Ahmadi T., Haier B., Behrmann I., Heinrich P. C., Diehl V., Tesch H. STAT3 is constitutively activated in Hodgkin cell lines. Blood. 2001;98:762–770. doi: 10.1182/blood.V98.3.762. [DOI] [PubMed] [Google Scholar]
- Küppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer. 2005;5:251–262. doi: 10.1038/nrc1589. [DOI] [PubMed] [Google Scholar]
- Kurth J., Spieker T., Wustrow J., Strickler G. J., Hansmann L. M., Rajewsky K., Küppers R. EBV-infected B-cells in infectious mononucleosis: viral strategies for spreading in the B-cell compartment and establishing latency. Immunity. 2000;13:485–495. doi: 10.1016/S1074-7613(00)00048-0. [DOI] [PubMed] [Google Scholar]
- Kurth J., Hansmann M. L., Rajewsky K., Küppers R. Epstein-Barr virus-infected B-cells expanding in germinal centers of infectious mononucleosis patients do not participate in the germinal center reaction. Proc Natl Acad Sci U S A. 2003;100:4730–4735. doi: 10.1073/pnas.2627966100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laichalk L. L., Thorley-Lawson D. A. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J Virol. 2005;79:1296–1307. doi: 10.1128/JVI.79.2.1296-1307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K. I., Lin Y., Calame K. Repression of c-myc is necessary but not sufficient for terminal differentiation of B lymphocytes in vitro. Mol Cell Biol. 2000;20:8684–8695. doi: 10.1128/MCB.20.23.8684-8695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan I. C. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Mancao C., Altmann M., Jungnickel B., Hammerschmidt W. Rescue of “crippled” germinal center B-cells from apoptosis by Epstein-Barr virus. Blood. 2005;106:4339–4344. doi: 10.1182/blood-2005-06-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín P., Gomez-Lozano N., Montes S., Salas C., Provencio M., Bellas C. Epstein-Barr virus in the germinal centres of adenopathies affected by classic Hodgkin lymphoma. Histopathology. 2011;59:349–352. doi: 10.1111/j.1365-2559.2011.03904.x. [DOI] [PubMed] [Google Scholar]
- Martín P., Coronado M. J., Bellas C. Evidence of the intersection of Epstein-Barr virus with germinal center. APMIS. 2012;120:253–254. doi: 10.1111/j.1600-0463.2011.02837.x. [DOI] [PubMed] [Google Scholar]
- Maruo S., Zhao B., Johannsen E., Kieff E., Zou J., Takada K. Epstein-Barr virus nuclear antigens 3C and 3A maintain lymphoblastoid cell growth by repressing p16INK4A and p14ARF expression. Proc Natl Acad Sci U S A. 2011;108:1919–1924. doi: 10.1073/pnas.1019599108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. L., Lee J. H., Kieff E., Longnecker R. An integral membrane protein (LMP2) blocks reactivation of Epstein-Barr virus from latency following surface immunoglobulin crosslinking. Proc Natl Acad Sci U S A. 1994;91:772–776. doi: 10.1073/pnas.91.2.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedobitek G., Herbst H., Young L. S., Brooks L., Masucci M. G., Crocker J., Rickinson A. B., Stein H. Patterns of Epstein-Barr virus infection in non-neoplastic lymphoid tissue. Blood. 1992;79:2520–2526. [PubMed] [Google Scholar]
- Niedobitek G., Agathanggelou A., Herbst H., Whitehead L., Wright D. H., Young L. S. Epstein-Barr virus (EBV) infection in infectious mononucleosis: virus latency, replication and phenotype of EBV-infected cells. J Pathol. 1997;182:151–159. doi: 10.1002/(SICI)1096-9896(199706)182:2<151::AID-PATH824>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Niedobitek G., Agathanggelou A., Steven N., Young L. S. Epstein-Barr virus (EBV) in infectious mononucleosis: detection of the virus in tonsillar B lymphocytes but not in desquamated oropharyngeal epithelial cells. Mol Pathol. 2000;53:37–42. doi: 10.1136/mp.53.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama T., Ichimura K., Suzuki R., Suzumiya J., Ohshima K., Yatabe Y., Yokoi T., Kojima M., Kamiya Y., other authors Senile EBV+ B-cell lymphoproliferative disorders: a clinicopathologic study of 22 patients. Am J Surg Pathol. 2003;27:16–26. doi: 10.1097/00000478-200301000-00003. [DOI] [PubMed] [Google Scholar]
- Oyama T., Yamamoto K., Asano N., Oshiro A., Suzuki R., Kagami Y., Morishima Y., Takeuchi K., Izumo T., other authors Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: a study of 96 patients. Clin Cancer Res. 2007;13:5124–5132. doi: 10.1158/1078-0432.CCR-06-2823. [DOI] [PubMed] [Google Scholar]
- Panagopoulos D., Victoratos P., Alexiou M., Kollias G., Mosialos G. Comparative analysis of signal transduction by CD40 and the Epstein-Barr virus oncoprotein LMP1 in vivo. J Virol. 2004;78:13253–13261. doi: 10.1128/JVI.78.23.13253-13261.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince S., Keating S., Fielding C., Brennan P., Floettmann E., Rowe M. Latent membrane protein 1 inhibits Epstein-Barr virus lytic cycle induction and progress via different mechanisms. J Virol. 2003;77:5000–5007. doi: 10.1128/JVI.77.8.5000-5007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickinson A., Kieff E. Epstein–Barr virus. In: Knipe D. M., Howley P. M., editors. Fields Virology. 4th edn. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2575–2627. Edited by. [Google Scholar]
- Roughan J. E., Thorley-Lawson D. A. The intersection of Epstein-Barr virus with the germinal center. J Virol. 2009;83:3968–3976. doi: 10.1128/JVI.02609-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan J. E., Torgbor C., Thorley-Lawson D. A. Germinal center B-cells latently infected with Epstein-Barr virus proliferate extensively but do not increase in number. J Virol. 2010;84:1158–1168. doi: 10.1128/JVI.01780-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaadt E., Baier B., Mautner J., Bornkamm G. W., Adler B. Epstein-Barr virus latent membrane protein 2A mimics B-cell receptor-dependent virus reactivation. J Gen Virol. 2005;86:551–559. doi: 10.1099/vir.0.80440-0. [DOI] [PubMed] [Google Scholar]
- Skalska L., White R. E., Franz M., Ruhmann M., Allday M. J. Epigenetic repression of p16(INK4A) by latent Epstein-Barr virus requires the interaction of EBNA3A and EBNA3C with CtBP. PLoS Pathog. 2010;6:e1000951. doi: 10.1371/journal.ppat.1000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson-Mungerson M. A., Caldwell R. G., Bultema R., Longnecker R. Epstein-Barr virus LMP2A alters in vivo and in vitro models of B-cell anergy, but not deletion, in response to autoantigen. J Virol. 2005;79:7355–7362. doi: 10.1128/JVI.79.12.7355-7362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K., Shimizu N., Sakuma S., Ono Y. Trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J Virol. 1986;57:1016–1022. doi: 10.1128/jvi.57.3.1016-1022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328–1337. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Duca K. A., Shapiro M. Epstein-Barr virus: a paradigm for persistent infection – for real and in virtual reality. Trends Immunol. 2008;29:195–201. doi: 10.1016/j.it.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Vockerodt M., Morgan S. L., Kuo M., Wei W., Chukwuma M. B., Arrand J. R., Kube D., Gordon J., Young L. S., other authors The Epstein–Barr virus oncoprotein, latent membrane protein-1, reprograms germinal centre B-cells towards a Hodgkin’s Reed–Sternberg-like phenotype. J Pathol. 2008;216:83–92. doi: 10.1002/path.2384. [DOI] [PubMed] [Google Scholar]
- Vockerodt M., Wei W., Nagy E., Prouzova Z., Schrader A., Kube D., Rowe M., Woodman C. B., Murray P. G. Suppression of the LMP2A target gene, EGR-1, protects Hodgkin’s lymphoma cells from entry to the EBV lytic cycle. J Pathol. 2013;230:399–409. doi: 10.1002/path.4198. [DOI] [PubMed] [Google Scholar]
- Vrzalikova K., Vockerodt M., Leonard S., Bell A., Wei W., Schrader A., Wright K. L., Kube D., Rowe M., other authors Down-regulation of BLIMP1 by the EBV oncogene, LMP-1, disrupts the plasma cell differentiation program and prevents viral replication in B-cells: implications for the pathogenesis of EBV-associated B-cell lymphomas. Blood. 2011;117:5907–5917. doi: 10.1182/blood-2010-09-307710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L. M., Movahed L. A., Warnke R. A., Sklar J. Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin’s disease. N Engl J Med. 1989;320:502–506. doi: 10.1056/NEJM198902233200806. [DOI] [PubMed] [Google Scholar]
- White R. E., Rämer P. C., Naresh K. N., Meixlsperger S., Pinaud L., Rooney C., Savoldo B., Coutinho R., Bödör C., other authors EBNA3B-deficient EBV promotes B-cell lymphomagenesis in humanized mice and is found in human tumors. J Clin Invest. 2012;122:1487–1502. doi: 10.1172/JCI58092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. C., Mann R. B., Charache P., Hayward S. D., Staal S., Lambe B. C., Ambinder R. F. Detection of EBV gene expression in Reed-Sternberg cells of Hodgkin’s disease. Int J Cancer. 1990;46:801–804. doi: 10.1002/ijc.2910460509. [DOI] [PubMed] [Google Scholar]
- Young L. S., Murray P. G. Epstein-Barr virus and oncogenesis: from latent genes to tumours. Oncogene. 2003;22:5108–5121. doi: 10.1038/sj.onc.1206556. [DOI] [PubMed] [Google Scholar]
- Young L. S., Rickinson A. B. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]