Abstract
Background
In population-based studies, the genetic variability of the APOE E alleles have been associated with health outcomes. Health problems are common in subjects with obesity. This study explored associations between the APOE E alleles and comorbidity in subjects with morbid obesity.
Methods
The study included consecutive subjects referred for evaluation of bariatric surgery with morbid obesity (defined as BMI > 40 or > 35 kg/m2 with complications related to obesity). The subjects followed a conservative weight loss program for 6 months before surgery and had a follow-up visit 12 months after surgery. Demographic data and a set psychosomatic scores (musculoskeletal pain, WHO-5 Well-Being Index, Rosenberg Self-Esteem Scale, Hopkins Symptom Check-list 10; Epworth Sleepiness Scale, and Fatigue Severity Scale) were collected, and blood samples were analysed for haematological and biochemical parameters and APOE alleles.
Results
One hundred and forty subjects (men/women: 32 (23%)/108 (77%) with mean age 43.0 (SD 8.7) years and BMI 42.1 (SD 3.8) kg/m2 were included. One hundred and eight and 92 subjects had data after conservative treatment and 12 months after surgery, respectively. The prevalence of the APOE alleles were: E2E2: 1 (0.7%), E2E3: 13 (9.3%), E2E4: 4 (2.9%), E3E3: 71 (50.7%), E3E4: 47 (33.6%), and E4E4: 4 (2.9%). The prevalence rates were as anticipated in a Norwegian population. The weight loss during conservative treatment and after bariatric surgery was independent of E allele variability. E2 was associated with a significant or clear trend toward improvement of all psychosomatic disorders. There was a significant fall in CRP during the two treatment periods with weight loss. E2 and E4 were significantly associated with high and low CRP, respectively, but no associations were seen between CRP and comorbidity.
Conclusions
The most marked finding was the association between E2 and improvement of all psychosomatic disorders. The positive and negative associations between CRP and E2 and E4, respectively, could indicate effects on inflammation and immunological reactions.
Keywords: APOE, Comorbidity, CRP, Inflammation, Obesity, Psychosomatic disorders
Background
The human APOE gene has three common allelic variants E2, E3, and E4 encoded on chromosome 19. Depending on the genetic variability, the gene has been ascribed significant positive and negative health effects such as longevity and shortened lifespan, neurological and psychosomatic disorders, cognitive decline and Alzheimer disease, altered lipoprotein profile, atherosclerosis and cardiovascular disease, type II diabetes, changes in the immune response, oxidative stress, quality of life, physical activity, and obesity [1–4]. At large, E4 has been ascribed unfavourable health outcomes and E2 health-promoting effects [1, 5–11].
Obesity is a global health challenge due to the high prevalence of serious comorbidity [12–14]. The comorbidity of obesity (metabolic syndrome and type II diabetes, cardiovascular disease, musculoskeletal disorders and cancer) coincide in part with disorders associated with variability of the APOE isoforms. The associations between biomarkers and disease in subjects with obesity are, however, not always as expected, as exemplified by the protective effect of obesity on the outcome of cardiovascular disease (the obesity paradox) [15]. Except for a possible association between E4 and obesity, little is known about associations between APOE variability and morbidity in subjects with obesity [2, 3].
The paramount aim of this study was to explore associations between the APOE E alleles and psychosomatic disorders and inflammatory markers in subjects with morbid obesity. The study aimed primarily at exploring the prevalence of APOE E4 in subjects with morbid obesity and the effect of E4 on conservative and surgically induced weight reduction. Secondary aims were to study the effects of all APOE alleles on weight and weight reduction, comorbidity and biochemical biomarkers. The associations between comorbidity and inflammatory markers and the APOE alleles were performed as post hoc analyses.
Methods
Study design
The data is a subset of the prospective cohort study in subjects with morbid obesity, the MO-BiPS study, (Morbid Obesity – Bio-Psycho-Social disorders) referred for evaluation of bariatric surgery at Innlandet Hospital Trust, Gjøvik, Norway [16–18]. After inclusion and before surgery, the participants followed a conservative weight-loss program for 6 months with dietary advice and increased physical activity (the first treatment period). A follow-up visit was scheduled 12 months after surgery (the second treatment period).
Subjects
Consecutive subjects aged 18–65 years with morbid obesity (defined as BMI ≥ 40 kg/m2 or ≥ 35 kg/m2 with complications related to obesity) were from December 2012 to September 2014 included in the study. The doctors were responsible for the medical history and the physical examination, and decided necessary supplementary examinations. Blood samples were collected. The patints filled in paper-based questionnaires. Subjects with a somatic disease or psychiatric disorders in need of treatment and judged as unrelated to obesity, were excluded. A study nurse was responsible for the care of the patients and most of the practical work.
Variables
The following variables were collected at inclusion:
Gender, age (years), body mass index (BMI, kg/m2), smoking habits (never / previously / daily smokers), all regular use of drugs and present or previous somatic disorders including hypertension and diabetes (yes / no).
Blood samples were analysed for standard haematological and biochemical variables, inflammatory markers [C-reactive protein (CRP), Interleukin-6 (Il-6) and Tumor Necrosis Factor (TNF)] and APOE genotype.
Two questions assessed physical activity: Easy activity (not sweaty/breathless): none; < 1 h; 1–2 h; ≥3 h/week (score 0–3). Strenuous activity (sweaty/breathless): none; < 1 h; 1–2 h; ≥3 h/week (score 0, 3, 4, 5). Sum score physical activity 0–8.
Musculoskeletal pain from six parts of the body (score 0–12).
WHO-5 Well-Being Index (scores 0–100; scores ≤50 indicate low mood and scores ≤28 indicate likely depression) [19]
Hopkins Symptom Checklist 10 measuring psychological distress (score 1–4; scores ≥1.85 indicate mental distress) [20].
Fatigue Severity Scale. A Norwegian translation of the Fatigue Severity Scale was used (score 9–63; scores ≥36 indicate further evaluation) [21].
Rosenberg Self-Esteem Scale. A validated Norwegian translation of the international questionnaire was used (score 10–40; values < 25 indicate low self-esteem) [22, 23].
Epworth Sleepiness Scale. A validated Norwegian translation was used (score 0–24; normal 0–10; Mild 11–14; Moderate 15–18; Severe 19–25) [24].
Sense of humour. A Norwegian version of the short form SHQ-6: “Attitudes toward humour” was used (score 6–24) [25].
Body weight and blood tests were available at inclusion, and before and 12 months after surgery for subjects following the scheduled plan.
Statistics
The results have been reported as mean (with SD or 95% CI), and number with proportion (in percentage). Between groups compaisons were analysed with chi-square tests, t-test, and Pearson’s correlation analyses depending on the type of data. Independent predictors of demographics, comorbidity and biochemical biomarkers and APOE status were assessed with linear or logistic regression analyses and reported as B-value or Odds Ratio (OR) with 95% confidence interval (CI) and p-values. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp was used for the analyses. P-values < 0.05 were judged as statistically significant. The genetic analyses were performed in previously collected blood samples [16–18]. The analyses were explorative, and no formal power calculation was performed.
Results
Three hundred and fifty Caucasian subjects with morbid obesity were referred to the obesity unit during the inclusion period. One hundred and eleven were not included because study nurse was unavailable, 80 refused participation, 17 were excluded after erroneous inclusion, and two had had to be excluded because they did not fill in the questionnaires. The study included 140 subjects: men/women 32 (23%)/108 (77%) with mean age 43.0 (SD 8.7) years and BMI 42.1 (SD 3.8) kg/m2. One hundred and eight and 92 subjects had data on body weight and inflammatory markers immediately before bariatric surgery and 12 months after surgery, respectively.
The prevalence rates of the APOE alleles were: E2E2: 1 (0.7%), E2E3: 13 (9.3%), E2E4: 4 (2.9%), E3E3: 71 (50.7%), E3E4: 47 (33.6%), and E4E4: 4 (2.9%) giving prevalence rates of the E2, E3, and E4 alleles of 6.8, 72.1 and 21.1% respectively. The E3 allele was not significantly associated with any of the variables in the study (data not shown).
Demographics and APOE E2 and E4 alleles
There was a non-significant trend towards an increased weight reduction in subjects with the E4 allele after adjusting for age and gender. No other differences in the demographic variables were related to the E2 and E4 alleles. Table 1 gives the details.
Table 1.
Associations between the subjects’ demographics and APOE E4 and E2 variants
| Subject demographics (no is given if less than 140) |
APOE-E4 Present (no 55) |
APOE-E4 Absent (no 85) |
Statistics p-value |
Adjusted* p-value |
APOE-E2 Present (no 18) |
APOE-E2 Absent (no 122) |
Statistics p-value |
Adjusted* p-value |
|---|---|---|---|---|---|---|---|---|
| Gender (female) | 43/55 (78%) | 65/85 (76%) | p = 0.84 | 13/18 (72%) | 95/122 (78%) | 0.76 | ||
| Age (years) | 42.6 (9.5) | 43.3 (8.1) | P = 0.68 | 41.8 (8.4) | 43.2 (8.7) | 0.53 | ||
| Body weight (kg) | 124.5 (19.3) | 123.3 (18.4) | P = 0.72 | 126.2 (20.7) | 123.4 (18.4) | 0.56 | ||
| Height (cm) | 171.6 (9.6) | 170.7 (8.5) | P = 0.59 | 172.2 (9.2) | 170.9 (8.9) | 0.58 | ||
| BMI (kg / m2) | 42.1 (4.0) | 42.1 (3.7) | P = 0.99 |
−0.07 (−1.26;1.13) p = 0.91 ** |
42.4 (4.9) | 42.1 (3.6) | 0.73 |
0.00 (− 1.74; 1.74) p = 1.00 ** |
| BMI reduction during the first treatment period (no 95) | 3.2 (1.6) | 2.9 (1.5) | P = 0.28 |
0.34 (−0.21; 0.98) p = 0.30 |
2.7 (1.4) | 3.1 (1.6) | 0.48 |
−0.28 (−1.22;0.66) P = 0.56 |
| BMI reduction during the last treatment period (no 92) | 11.0 (3.7) | 10.5 (3.2) | P = 0.46 |
0.94 (−0.37;2.25) p = 0.16 |
10.0 (4.5) | 10.8 (3.2) | 0.44 |
−0.41 (−2.30;1.48) p = 0.67 |
| BMI reduction total (no 92) | 14.3 (3.8) | 13.4 (3.5) | P = 0.21 |
1.36 (−0.04;2.75) p = 0.057 |
12.7 (5.1) | 13.9 (3.3) | 0.45 |
−0.72 (−2.76;1.32) p = 0.49 |
The results are given as mean (SD) or number (proportion). Chi-square and T-test were used for the comparisons. *Adjusted associations between the subject demographics (dependent variable) and APOE E4 and E2 variants were analysed with linear regression analyses adjusted for age, gender and BMI and reported as unstandardized coefficients (B-value) with 95% confidence intervals and p-values. ** Adjusted for age and gender
Comorbidity and APOE E2 and E4 alleles
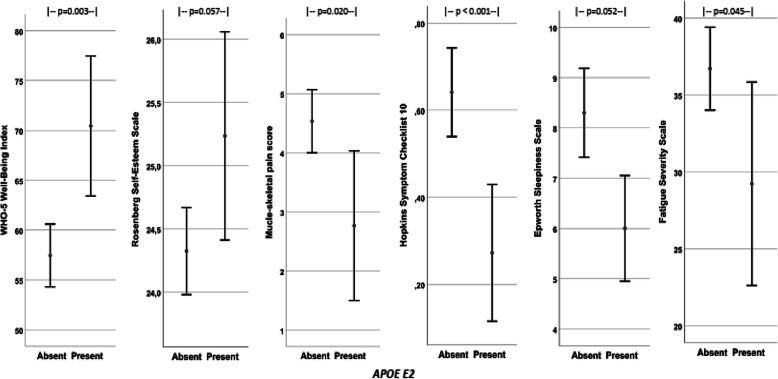
Presence of the E2 allele was favourably associated with all the psychosomatic variables (i.e. increased well-being and self-esteem, and reduced musculoskeletal pain, psychological distress, fatigue and sleepiness). Table 2 gives the details, and Fig. 1 shows the scores for psychosomatic comorbidity in subjects with and without E2 with comparisons between the groups. Presence of E2 was furthermore associated with hypertension in the adjusted analyses. No differences in comorbidity were seen between subjects with and without E4.
Table 2.
Associations between comorbidity and APOE E4 and E2 variants
| Subjects’ psychosomatic disorders (no is given if less than 140) |
APOE-E4 Present (no 55) |
APOE-E4 Absent (no 85) |
Statistics p-value |
Adjusted* p-value |
APOE-E2 Present (no 18) |
APOE-E2 Absent (no 122) |
Statistics p-value |
Adjusted* p-value |
|---|---|---|---|---|---|---|---|---|
| Diabetes (no 135) | 12/54 (22%) | 14/81 (17%) | p = 0.51 |
OR† 0.30 (0.55;3.33) p = 0.51 |
6/18 (33%) | 20/117 (17%) | 0.12 |
OR† 0.92 (0.77;8.1) p = 0.12 |
| Hypertension (no 135) | 19/54 (35%) | 27/81 (33%) | p = 0.85 |
OR† 0.11 (0.051;2.48) p = 0.78 |
10/18 (56%) | 36/117 (31%) | 0.06 |
OR† 1.34 (1.21;12.1) p = 0.022 |
| Physical activity (no 134) | 4.5 (2.3) | 4.5 (2.2) | p = 0.84 | - 0.076 (−0.868;0.715) p = 0.85 | 5.1 (1.6) | 4.4 (2.3) | 0.15 | 0.666 (−0.465;1.798) p = 0.25 |
| Musculoskeletal pain (no 137) | 4.5 (3.0) | 4.2 (2.9) | p = 0.66 |
−0.21 (− 0.81;1.23) p = 0.68 |
2.8 (2.5) | 4.5 (3.0) | 0.020 | −1.74 (−3.23;-0.26) p = 0.022 |
| WHO-5 Well-Being Index (no139) | 58.2 (18.5) | 59.8 (17.1) | p = 0.61 |
−1.60 (−7.73;4.52) p = 0.61 |
70.4 (14.1) | 57.5 (17.5) | 0.003 | 12.89 (4.22;21.56) p = 0.004 |
| Hopkins Symptom Checklist 10 (no 135) | 0.62 (0.57) | 0.57 (0.53) | p = 0.58 |
0.06 (−0.13;0.25) p = 0.54 |
0.27 (0.32) | 0.64 (0.56) | < 0.001 | −0.36 (− 0.62;-0.09) p = 0.009 |
| Rosenberg Self-Esteem (no 131) | 24.7 (1.9) | 24.3 (1.8) | p = 0.24 |
0.37 (−0.29;1.02) p = 0.27 |
25.2 (1.6) | 24.3 (1.9) | 0.057 | 0..87 (−0.08;1.81) p = 0.07 |
| Fatigue Severity Scale (no 137) | 36.6 (15.2) | 35.2 (14.6) | p = 0.61 |
1.34 (−3.75;6.43) p = 0.52 |
29.2 (13.3) | 36.7 (14.8) | 0.045 | −6.95 (− 14.22;0.32) p = 0.06 |
| Epworth Sleepiness Scale (no138) | 8.9 (5.1) | 7.4 (4.3) | p = 0.06 |
1.52 (−0.09;3.12) p = 0.06 |
6.0 (2.1) | 8.3 (4.9) | 0.052 | −2.22 (− 4.54;0.11) p = 0.06 |
| Sense of humour (no 138) | 19.6 (2.5) | 18.9 (2.8) | p = 0.12 |
−0.71 (− 0.19;1.60) p = 0.12 |
18.9 (2.5) | 19.2 (2.7) | 0.70 | −0.36 (−1.67;0.96) p = 0.60 |
The results are given as mean (SD) or number (proportion). Chi-square and t-test were used for the comparisons. *Adjusted associations between the subjects’ psychosomatic disorders (dependent variable) and APOE E4 and E2 were analysed with linear and logistic regression analyses adjusted for age, gender and BMI. The results are reported as unstandardized coefficients (B-value) or †Odds Ratio (OR) with 95% confidence intervals and p-values
Fig. 1.
Shows the scores for the psychosomatic disorders in subjects with and without the APOE E2 allele. The results are given as mean with 95% CI of the mean
Biochemical biomarkers and APOE E2 and E4 alleles
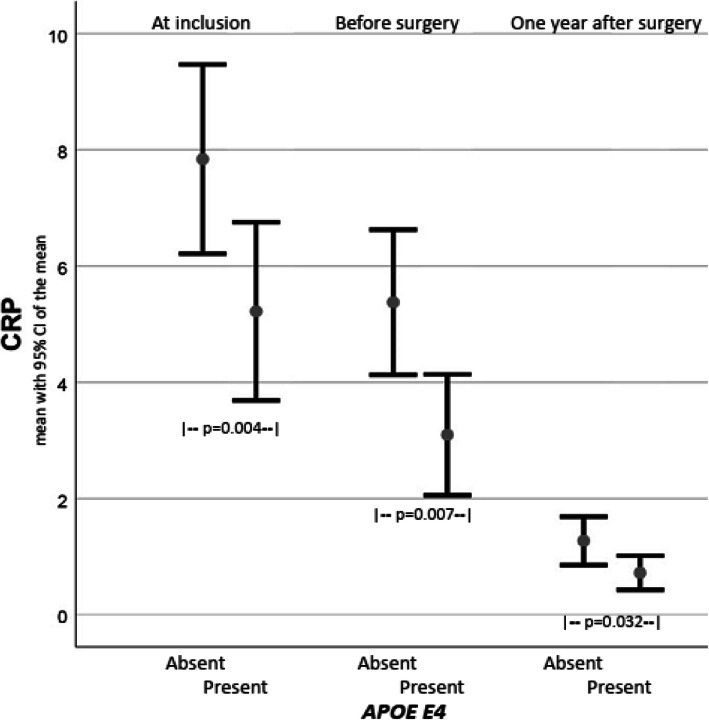
CRP was elevated at inclusion, was reduced after conservative induced weight loss, and continued to normalise after bariatric surgery. Presence of the E4 allele was associated with lower CRP at all points of time. The presence of E2 was associated with high CRP and HbA1c. Table 3 gives the details, and Fig. 2 shows CRP values in subjects with and without E4 at inclusion, after conservative treatment and 12 months after surgery.
Table 3.
Associations between biochemical biomarkers and APOE E4 and E2 variants
| Biochemical biomarkers |
APOE-E4 Present (no 55) |
APOE-E4 Absent (no 85) |
Statistics p-value |
Adjusted* B-value (CI) p-value |
APOE-E2 Present (no 18) |
APOE-E2 Absent (no 122) |
Statistics p-value |
Adjusted* B-value (CI) p-value |
|---|---|---|---|---|---|---|---|---|
| Hb (no 139) | 14.6 (1.1) | 14.4 (1.1) | P = 0.23 |
0.24 (−0.06;0.54) P = 0.12 |
14.6 (1.2) | 14.5 (1.1) | p = 0.57 |
0.09 (−0.36;0.53) p = 0.70 |
| CRP at inclusion (no 139) | 5.3 (4.6) | 8.2 (7.1) | p = 0.004 |
−2.98 (−5.06;-0.91) p = 0.005 |
9.6 (7.7) | 6.7 (6.1) | p = 0.07 |
2.80 (−0.27;5.87) p = 0.07 |
| CRP before surgery (no 108) | 3.1 (2.8) | 5.2 (4.4) | p = 0.007 |
−1.65 (−3.12;-0.19) p = 0.028 |
7.1 (7.1) | 4.0 (3.0) | p = 0.112 |
3.46 (1.48;5.42) p = 0.001 |
| CRP 1 year after surgery (no 88) | 0.7 (0.8) | 1.3 (1.6) | p = 0.032 |
−0.53 (−1.13;0.07) p = 0.08 |
2.3 (2.4) | 0.9 (1.0) | p = 0.062 |
1.55 (0.77;2.33) p < 0.001 |
| HbA1C (no 139) | 5.9 (1.4) | 5.8 (1.3) | p = 0.62 |
0.14 (−0.32;0.60) p = 0.54 |
6.5 (2.0) | 5.8 (1.2) | P = 0.133 |
0.79 (0.14;1.44) p = 0.018 |
| HDL cholesterol (no 139) | 1.2 (0.4) | 1.2 (0.3) | p = 0.69 |
0.03 (−0.08;0.13) p = 0.64 |
1.1 (0.3) | 1.2 (0.3) | p = 0.25 |
−0.07 (− 0.22;0.09) p = 0.38 |
| LDL cholesterol (no 139) | 3.5 (0.8) | 3.3 (0.8) | p = 0.08 |
0.25 (−0.04;0.55) p = 0.09 |
3.0 (1.0) | 3.4 (0.8) | p = 0.06 |
−0.41 (− 0.83;0.02) p = 0.06 |
| Il-6 (no 134) | 2.5 (1.5) | 3.0 (3.5) | p = 0.32 |
−0.52 (−1.55;0.51) p = 0.32 |
2.8 (1.4) | 2.8 (3.1) | p = 0.95 |
−0.02 (−1.50;1.49) p = 0.98 |
| TNF (no 135) | 11.4 (28.8) | 12.9 (49.5) | p = 0.84 |
−1.25 (− 16.3;13.8) p = 0.87 |
6.6 (2.5) | 13.2 (45.7) | p = 0.54 |
−5.7 (−27.2;15.9) p = 0.60 |
The results are given as mean (SD). T-test was used for the comparisons. * Adjusted associations between the biochemical biomarkers (dependent variable) and APOE E4 and E2 were analysed with linear regression analyses adjusted for age, gender and BMI. The results are reported as unstandardized coefficients (B-value) with 95% confidence intervals and p-values
Fig. 2.
Shows the CRP values in subjects with and without the APOE E4 allele at inclusion, before bariatric surgery (i.e. after 6 months with conservative weight loss) and 12 months after bariatric surgery
Comorbidity and inflammation
E2 and E4 were associated with high and low CRP values, respectively. No associations were seen between APOE genotype and Il-6 and TNF. Table 4 gives the associations between the comorbidities and CRP, E2 and E4 adjusted for age, gender and BMI. The psychosomatic comorbidities and hypertension were associated with E2 but not with CRP and E4. Diabetes was associated with CRP.
Table 4.
Associations between comorbidity and CRP
| Comorbidity | CRP* | CRP Adjusted† |
E2 Adjusted† |
E4 Adjusted† |
|---|---|---|---|---|
| Diabetes (no 135) |
r = 0.096 p = 0.27 |
OR = 1.08 (1.004;1.16) p = 0.039 | OR = 2.42 (0.71;8.21) p = 0.157 | OR = 0.20 (0.72;5.08) p = 0.20 |
| Hypertension (no 135) |
r = − 0.096 p = 0.27 |
OR = 0.98 (0.92;1.06) p = 0.66 | OR = 4.50 (1.34;15.11) p = 0.015 | OR = 1.15 (0.49;2.73) p = 0.76 |
| Musculoskeletal pain score (no 137) |
r = −0.134 P = 0.12 |
B = -0.07 (−0.15;0.02) p = 0.12 | B = -1.55 (−3.05;-0.05) p = 0.043 | B = -0.025 (−1.29;0.78) p = 0.63 |
| WHO-5 Well-Being Index (no139) |
r = −0.034 P = 0.69 |
B = -0.21 (−0.70;0.29) p = 0.41 | B = 13.1 (4.3;22.0) p = 0.004 | B = -0.53 (−6.7;5.7) p = 0.87 |
| Hopkins Symptom Checklist 10 (no 135) |
r = 0.049 P = 0.57 |
B = 0.01 (−0.01;0.02) p = 0.35 | B = -0.37 (−0.64;-0.10) p = 0.009 | B = 0.05 (− 0.15;0.24) p = 0.64 |
| Rosenberg Self-Esteem (131) |
r = 0.005 P = 0.96 |
B = -0.01 (−0.06;0.05) p = 0.77 | B = 1.01 (0.05;1.98) p = 0.040 | B = 0.44 (−0.24;1.12) p = 0.20 |
| Fatigue Severity Scale (no 137) |
r = 0.021 p = 0.81 |
B = 0.08 (−0.34;0.50) p = 0.71 | B = -6.9 (−14.4;0.5) p = 0.069 | B = 0.70 (−4.57;5.97) p = 0.79 |
| Epworth Sleepiness Scale (no138) |
r = 0.019 P = 0.83 |
B = 0.07 (−0.07;0.20) p = 0.33 | B = -2.10 (− 4.45;0.25) p = 0.080 | B = 1.52 (− 0.13;3.17) p = 0.071 |
| Sense of humour (no 138) |
r = −0.028 p = 0.75 |
B = -0.03 (− 0.11;0.05) p = 0.42 | B = -0.31 (−1.47;1.20) p = 0.85 | B = 0.55 (− 0.39;1.49) p = 0.25 |
*The results are given as Pearson’s correlation coefficient (r). † Adjusted associations between the comorbidity (dependent variable) and CRP, E2 and E4, age, gender and BMI were analysed with logistic and linear regression analyses. The results are reported as Odds Ratio (OR) and unstandardized coefficients (B-value) with 95% confidence intervals and p-values
Discussion
The prevalence rates of the APOE alleles were consistent with those reported in both clinical and population-based studies from Scandinavia [1, 26–28].. The prevalence of E4 might seem high, but considering the increasing south-north gradient and comparisons with studies from the same region, the prevalence rates were as expected [28, 29]. The observed distribution of the different genotypes was in complete agreement with the predicted genotypes according to Hardy-Weinberg equilibrium. In a case-control study with 198 obese and normal-weight subjects, the prevalence of E4 was high in subjects with obesity [3]. In a review of pooled data from seven studies with 27,863 Caucasians, E4 was associated with reduced BMI in individuals above 60 years of age but not in younger [2]. This study gives no support for an abnormal distribution of the E alleles in subjects with morbid obesity but is too small for a valid conclusion. Neither was the presence of any of the E alleles significantly associated with weight reduction after conservative treatment or bariatric surgery.
The main finding related to the E4 allele was the significant association with low CRP. Similar findings have been reported in previous reports; the relative reduction of CRP has been estimated to 34% compared to subjects with zero E4 alleles [30–33]. There was a marked reduction of CRP during both treatments periods. The significant differences in CRP between subjects with and without the E4 allele persisted at all points of time. The reduction in CRP after weight loss was expected because CRP is strongly related to total fat mass, particularly in women, and most subjects in this study were women [34]. In the general population, high CRP is an independent predictor of cancer-, cardiovascular-, and overall mortality and ischemic stroke [35, 36]. Therefore, it seems contradictory that E4, which is associated with low CRP, is a predictor of cardiovascular disease and mortality, and reduced longevity [1, 37–40]. The same contradiction applies to cognitive functions and Alzheimer disease. CRP has been associated with subsequent cognitive impairment and age-related cognitive decline, whereas the E4 allele, which was associated with low CRP, increases the risk of cognitive reduction and Alzheimer disease [5, 6, 41, 42]. The interactions between CRP, the APOE genotype, cardiovascular diseases and cognitive functions and dementia are confusing and might depend on gender, age, and disease such as obesity [43–46]. The association between CRP and atherosclerosis might be diminished in subjects with obesity [44]. In this study, after adjusting for age, gender and BMI, the E2 allele was associated with high CRP-values after the two treatment periods and with hypertension. This was unexpected since E2 has shown favourable health outcomes such as reduced low-density lipoprotein and increased survival [7]. Consequently, using CRP to estimate overall disease risk or prognosis may be misleading in subjects with morbid obesity.
Obesity with comorbidity (hypertension, metabolic syndrome, diabetes) leads to cardiovascular disease but protects against the clinical outcome such as short and long term mortality rates in subjects with cardiovascular disease [15, 47, 48]. The phenomenon has been referred to as the “obesity paradox”. Compared with normal-weight subjects, subjects with obesity have an unexplained preservation of vascular function with higher flow-mediated dilatation and reduced intima-media thickness [49]. The significance of inflammation on the obesity paradox is inconclusive. In one study, body fat protected against mortality in a subgroup with high CRP [50]. The association between APOE genotype and coronary disease was described as a puzzling paradox in 2006; the paradox is still not solved [51]. There is emerging evidence for plasma APOE as a risk factor for dementia, and to a lesser extent for ischemic heart disease, but the associations seem to be independent of APOE genotype [52, 53]. A recently published study suggests that other genetic factors might contribute to the obesity paradox [54].
In this study, the E2 allele was associated with significant or clear trends toward the improvement of all the psychosomatic variables: Musculoskeletal pain, WHO-5 Well-Being Index, Rosenberg Self-Esteem Scale, Hopkin Symptoms Check-list 10; Epworth Sleepiness Scale, and Fatigue. Protection of E2 against depression and reduced vulnerability to psychiatric and somatic diseases has also been reported in other studies [8–10]. In all, the E2 allele seems to have a range of health-promoting effects. In addition to the psychosomatic effects, E2 has been associated with longevity, delayed onset of cognitive decline, reduced LDL-cholesterol and reduced overall risk of cardiovascular diseases [1].
High and low CRP were associated with E2 and E4, respectively. Inflammation has been linked to psychosomatic disorders [55–57]. Therefore, post hoc analyses were performed to rule out if inflammation could confound the associations between the psychosomatic disorders and the APOE alleles. E2 was the only independent predictor of the psychosomatic disorders after adjusting for E4, CRP, gender, age and BMI (Table 4). CRP was associated with diabetes. These results support the reduced importance of CRP as a disease modifier in subjects with obesity [44].
Strengths and limitation
Inclusion of consecutive subjects with morbid obesity referred for evaluation of bariatric surgery improves the validity of the results. However, since this group is only a minority of all subjects with obesity, the transferability of the results to all subjects with obesity is uncertain, and the results are not transferrable to subjects with normal weight. The study was too small for valid comparisons of the prevalence of the E alleles in subjects with morbid obesity and the general population, and for evaluation of the effects of the E alleles on weight loss, but no striking differences were observed. The association between low CRP and E4 observed in this study is in accordance with other studies in subjects with and without obesity [30–33]. The lack of associations between CRP and comorbidity is known from other studies in subjects with obesity, which contrasts to findings in the general population [44]. Favourable effects of E2 have also been reported in subjects with other disorders [1, 8–10]. Four subjects with the combination E2:E4 were included in two groups, in the group with E4 and the group with E2, and might have weakened the analyses. In all, the consistent findings indicate high external validity of the study.
Conclusions
The most important finding was the marked improvement of all psychosomatic disorders related to the presence of the E2 allele in subjects with morbid obesity. The E2 allele has been ascribed health promoting effects, but the effects seemed more pronounced than in studies in non-obese groups. High and low CRP were associated with the E2 and E4 alleles respectively, but not associated with obesity-related disorders. The uncertain associations between CRP and comorbidity observed in other studies in subjects with obesity seem to imply also to those with morbid obesity. No notable effect of the E alleles was observed on BMI or reduction of BMI after conservative treatment or bariatric surgery.
Acknowledgements
The authors want to thank senior nursing officer Janne Dahlby Rostad at the obesity unit for tailoring the practical work to the research activities and study nurse Anja Byfuglien at Innlandet Hospital Trust for conscientious help with the practical work, and the Medical Laboratory Scientists at the Unit for Special Analyses, Department of Medical Biochemistry at Oslo University Hospital for APOE genotyping.
Abbreviations
- BMI
Body Mass Index
- CI
Confidence interval
- CRP
C-reactive protein
- Il-6
Interleukin-6
- OR
Odds Ratio
- SD
Standard Deviation
- TNF
Tumor Necrosis Factor
- WHO
World Health Organization
Authors’ contributions
PGF is the guarantor of the project. He designed the main study, and has been responsible for the practical implementation, performed the statistical analyses, wrote the manuscript and is responsible for the integrity of the work. HR has been responsible for the APOE analyses and interpretation of these results. KH has had shared responsibility for the main study and has been responsible for the evaluation of the psychosomatic results. All authors have contributed to the interpretation of the results, given valuable comments on the manuscript and approved the last version.
Authors’ information
Not applicable.
Funding
The work was funded by an unrestricted grant from Innlandet Hospital Trust, Brumunddal, Norway; and Oslo University Hospital, Oslo, Norway.
Availability of data and materials
The raw datasets generated and analysed during the current study are not publicly available in order to protect participant confidentiality. Case report forms (CRFs) on paper are safely stored. The data were transferred to SPSS for statistical analyses and the data files are stored by Innlandet Hospital Trust, Brumunddal, Norway, on a server dedicated to research. The security follows to the rules given by The Norwegian Data Protection Authority, P.O. Box 8177 Dep. NO-0034 Oslo, Norway. The data are available on request to the authors.
Ethics approval and consent to participate
The study was approved by the Norwegian Regional Committees for Medical and Health Research Ethics, PB 1130, Blindern, 0318 Oslo, Norway (reference number 2012/966 with an amendment of June 28, 2018). The study was performed in accordance with the Declaration of Helsinki including written informed consent from all participants before inclusion.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Per G. Farup, Email: Per.Farup@ntnu.no, Email: per.farup@ntnu.no
Helge Rootwelt, Email: hrootwel@ous-hf.no.
Knut Hestad, Email: Knut.Hestad@inn.no.
References
- 1.Abondio P, Sazzini M, Garagnani P, Boattini A, Monti D, Franceschi C, et al. The genetic variability of APOE in different human populations and its implications for longevity. Genes (Basel) 2019;10:222. doi: 10.3390/genes10030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulminski AM, Loika Y, Culminskaya I, Huang J, Arbeev KG, Bagley O, et al. Independent associations of TOMM40 and APOE variants with body mass index. Aging Cell. 2019;18:e12869. doi: 10.1111/acel.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alharbi KK, Syed R, Alharbi FK, Khan IA. Association of Apolipoprotein E Polymorphism with impact on Overweight University pupils. Genet Test Mol Biomarkers. 2017;21:53–57. doi: 10.1089/gtmb.2016.0190. [DOI] [PubMed] [Google Scholar]
- 4.Klimentidis YC, Raichlen DA, Bea J, Garcia DO, Wineinger NE, Mandarino LJ, et al. Genome-wide association study of habitual physical activity in over 377,000 UK biobank participants identifies multiple variants including CADM2 and APOE. Int J Obes. 2018;42:1161–1176. doi: 10.1038/s41366-018-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spinney L. Alzheimer's disease: the forgetting gene. Nature. 2014;510:26–28. doi: 10.1038/510026a. [DOI] [PubMed] [Google Scholar]
- 6.Farlow MR, He Y, Tekin S, Xu J, Lane R, Charles HC. Impact of APOE in mild cognitive impairment. Neurology. 2004;63:1898–1901. doi: 10.1212/01.WNL.0000144279.21502.B7. [DOI] [PubMed] [Google Scholar]
- 7.Kulminski AM, Raghavachari N, Arbeev KG, Culminskaya I, Arbeeva L, Wu D, et al. Protective role of the apolipoprotein E2 allele in age-related disease traits and survival: evidence from the long life family study. Biogerontology. 2016;17:893–905. doi: 10.1007/s10522-016-9659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou KL. Moderating effect of apolipoprotein genotype on loneliness leading to depressive symptoms in Chinese older adults. Am J Geriatr Psychiatry. 2010;18:313–322. doi: 10.1097/JGP.0b013e3181c37b2a. [DOI] [PubMed] [Google Scholar]
- 9.Fan PL, Chen CD, Kao WT, Shu BC, Lung FW. Protective effect of the apo epsilon2 allele in major depressive disorder in Taiwanese. Acta Psychiatr Scand. 2006;113:48–53. doi: 10.1111/j.1600-0447.2005.00686.x. [DOI] [PubMed] [Google Scholar]
- 10.Julian LJ, Vella L, Frankel D, Minden SL, Oksenberg JR, Mohr DC. ApoE alleles, depression and positive affect in multiple sclerosis. Mult Scler. 2009;15:311–315. doi: 10.1177/1352458508099478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suri S, Heise V, Trachtenberg AJ, Mackay CE. The forgotten APOE allele: a review of the evidence and suggested mechanisms for the protective effect of APOE varepsilon2. Neurosci Biobehav Rev. 2013;37:2878–2886. doi: 10.1016/j.neubiorev.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Obesity and overweight [https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight] (accessed on 27 June 2020).
- 13.Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med. 2017;5:161. doi: 10.21037/atm.2017.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghoorah K, Campbell P, Kent A, Maznyczka A, Kunadian V. Obesity and cardiovascular outcomes: a review. Eur Heart J Acute Cardiovasc Care. 2016;5:77–85. doi: 10.1177/2048872614523349. [DOI] [PubMed] [Google Scholar]
- 16.Aasbrenn M, Lydersen S, Farup PG. A conservative weight loss intervention relieves bowel symptoms in morbidly obese subjects with irritable bowel syndrome: a prospective cohort study. J Obes. 2018;2018:3732753. doi: 10.1155/2018/3732753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farup PG, Valeur J. Changes in Faecal short-chain fatty acids after weight-loss interventions in subjects with morbid obesity. Nutrients. 2020;12:802. doi: 10.3390/nu12030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blom-Hogestol IK, Aasbrenn M, Chahal-Kummen M, Brunborg C, Eribe I, Kristinsson J, et al. Irritable bowel syndrome-like symptoms and health related quality of life two years after Roux-en-Y gastric bypass - a prospective cohort study. BMC Gastroenterol. 2019;19:204. doi: 10.1186/s12876-019-1103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topp CW, Ostergaard SD, Sondergaard S, Bech P. The WHO-5 well-being index: a systematic review of the literature. Psychother Psychosom. 2015;84:167–176. doi: 10.1159/000376585. [DOI] [PubMed] [Google Scholar]
- 20.Søgaard AJ, Bjelland I, Tell GS, Røysamb E. A comparison of the CONOR mental health index to the HSCL-10 and HADS. Norsk Epidemiologi. 2003;13:279–284. [Google Scholar]
- 21.Lerdal A, Wahl A, Rustoen T, Hanestad BR, Moum T. Fatigue in the general population: a translation and test of the psychometric properties of the Norwegian version of the fatigue severity scale. Scand J Public Health. 2005;33:123–130. doi: 10.1080/14034940410028406. [DOI] [PubMed] [Google Scholar]
- 22.Alessandri G, Vecchione M, Eisenberg N, Laguna M. On the factor structure of the Rosenberg (1965) general self-esteem scale. Psychol Assess. 2015;27:621–635. doi: 10.1037/pas0000073. [DOI] [PubMed] [Google Scholar]
- 23.von Soest T. Rosenbergs selfølelsesskala: Validering av en norsk oversettelse. Tidsskr Nor Psykologforen. 2005;42:226–228. [Google Scholar]
- 24.Beiske KK, Kjelsberg FN, Ruud EA, Stavem K. Reliability and validity of a Norwegian version of the Epworth sleepiness scale. Sleep Breath. 2009;13:65–72. doi: 10.1007/s11325-008-0202-x. [DOI] [PubMed] [Google Scholar]
- 25.Svebak S. The development of the sense of humor questionnaire: from SHQ to SHQ-6. Humor Int J Humor Res. 1996;9:341–362. doi: 10.1515/humr.1996.9.3-4.341. [DOI] [Google Scholar]
- 26.Hestad KA, Engedal K, Whist JE, Farup PG. The effect of ApoE e4 on blood pressure in patients with and without depression. Neuropsychiatr Dis Treat. 2016;12:1365–1370. doi: 10.2147/NDT.S106933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hestad K, Kveberg B, Engedal K. Low blood pressure is a better predictor of cognitive deficits than the apolipoprotein e4 allele in the oldest old. Acta Neurol Scand. 2005;111:323–328. doi: 10.1111/j.1600-0404.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- 28.Singh PP, Singh M, Mastana SS. APOE distribution in world populations with new data from India and the UK. Ann Hum Biol. 2006;33:279–308. doi: 10.1080/03014460600594513. [DOI] [PubMed] [Google Scholar]
- 29.Adler G, Adler MA, Urbanska A, Skonieczna-Zydecka K, Kiseljakovic E, Valjevac A, et al. Bosnian study of APOE distribution (BOSAD): a comparison with other European populations. Ann Hum Biol. 2017;44:568–573. doi: 10.1080/03014460.2017.1346708. [DOI] [PubMed] [Google Scholar]
- 30.Judson R, Brain C, Dain B, Windemuth A, Ruano G, Reed C. New and confirmatory evidence of an association between APOE genotype and baseline C-reactive protein in dyslipidemic individuals. Atherosclerosis. 2004;177:345–351. doi: 10.1016/j.atherosclerosis.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Chasman DI, Kozlowski P, Zee RY, Kwiatkowski DJ, Ridker PM. Qualitative and quantitative effects of APOE genetic variation on plasma C-reactive protein, LDL-cholesterol, and apoE protein. Genes Immun. 2006;7:211–219. doi: 10.1038/sj.gene.6364289. [DOI] [PubMed] [Google Scholar]
- 32.Hubacek JA, Peasey A, Pikhart H, Stavek P, Kubinova R, Marmot M, et al. APOE polymorphism and its effect on plasma C-reactive protein levels in a large general population sample. Hum Immunol. 2010;71:304–308. doi: 10.1016/j.humimm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martiskainen H, Takalo M, Solomon A, Stancakova A, Marttinen M, Natunen T, et al. Decreased plasma C-reactive protein levels in APOE epsilon4 allele carriers. Ann Clin Transl Neurol. 2018;5:1229–1240. doi: 10.1002/acn3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khera A, Vega GL, Das SR, Ayers C, McGuire DK, Grundy SM, et al. Sex differences in the relationship between C-reactive protein and body fat. J Clin Endocrinol Metab. 2009;94:3251–3258. doi: 10.1210/jc.2008-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Han W, Gong D, Man C, Fan Y. Hs-CRP in stroke: a meta-analysis. Clin Chim Acta. 2016;453:21–27. doi: 10.1016/j.cca.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Zhong X, Cheng G, Zhao C, Zhang L, Hong Y, et al. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: a meta-analysis. Atherosclerosis. 2017;259:75–82. doi: 10.1016/j.atherosclerosis.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Stengard JH, Weiss KM, Sing CF. An ecological study of association between coronary heart disease mortality rates in men and the relative frequencies of common allelic variations in the gene coding for apolipoprotein E. Hum Genet. 1998;103:234–241. doi: 10.1007/s004390050811. [DOI] [PubMed] [Google Scholar]
- 38.Gerdes LU, Gerdes C, Kervinen K, Savolainen M, Klausen IC, Hansen PS, et al. The apolipoprotein epsilon4 allele determines prognosis and the effect on prognosis of simvastatin in survivors of myocardial infarction : a substudy of the Scandinavian simvastatin survival study. Circulation. 2000;101:1366–1371. doi: 10.1161/01.CIR.101.12.1366. [DOI] [PubMed] [Google Scholar]
- 39.Kumar NT, Liestol K, Loberg EM, Reims HM, Brorson SH, Maehlen J. The apolipoprotein E polymorphism and cardiovascular diseases--an autopsy study. Cardiovasc Pathol. 2012;21:461–469. doi: 10.1016/j.carpath.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Garatachea N, Emanuele E, Calero M, Fuku N, Arai Y, Abe Y, et al. ApoE gene and exceptional longevity: insights from three independent cohorts. Exp Gerontol. 2014;53:16–23. doi: 10.1016/j.exger.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Marioni RE, Stewart MC, Murray GD, Deary IJ, Fowkes FG, Lowe GD, et al. Peripheral levels of fibrinogen, C-reactive protein, and plasma viscosity predict future cognitive decline in individuals without dementia. Psychosom Med. 2009;71:901–906. doi: 10.1097/PSY.0b013e3181b1e538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gong C, Wei D, Wang Y, Ma J, Yuan C, Zhang W, et al. A meta-analysis of C-reactive protein in patients with Alzheimer’s disease. Am J Alzheimers Dis Other Dement. 2016;31:194–200. doi: 10.1177/1533317515602087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bojar I, Gujski M, Pinkas J, Raczkiewicz D, Owoc A, Humeniuk E. Interaction between C-reactive protein and cognitive functions according to APOE gene polymorphism in post-menopausal women. Arch Med Sci. 2016;12:1247–1255. doi: 10.5114/aoms.2016.62868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta NK, de Lemos JA, Ayers CR, Abdullah SM, McGuire DK, Khera A. The relationship between C-reactive protein and atherosclerosis differs on the basis of body mass index: the Dallas heart study. J Am Coll Cardiol. 2012;60:1148–1155. doi: 10.1016/j.jacc.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 45.Lima TA, Adler AL, Minett T, Matthews FE, Brayne C, Marioni RE. Medical Research Council cognitive F, ageing S: C-reactive protein, APOE genotype and longitudinal cognitive change in an older population. Age Ageing. 2014;43:289–292. doi: 10.1093/ageing/aft193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones NS, Rebeck GW. The synergistic effects of APOE genotype and obesity on Alzheimer's disease risk. Int J Mol Sci. 2018;20:63. doi: 10.3390/ijms20010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Csige I, Ujvarosy D, Szabo Z, Lorincz I, Paragh G, Harangi M, et al. The impact of obesity on the cardiovascular system. J Diabetes Res. 2018;2018:3407306. doi: 10.1155/2018/3407306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oreopoulos A, Padwal R, Norris CM, Mullen JC, Pretorius V, Kalantar-Zadeh K. Effect of obesity on short- and long-term mortality postcoronary revascularization: a meta-analysis. Obesity (Silver Spring) 2008;16:442–450. doi: 10.1038/oby.2007.36. [DOI] [PubMed] [Google Scholar]
- 49.Biasucci LM, Graziani F, Rizzello V, Liuzzo G, Guidone C, De Caterina AR, et al. Paradoxical preservation of vascular function in severe obesity. Am J Med. 2010;123:727–734. doi: 10.1016/j.amjmed.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 50.De Schutter A, Kachur S, Lavie CJ, Boddepalli RS, Patel DA, Milani RV. The impact of inflammation on the obesity paradox in coronary heart disease. Int J Obes. 2016;40:1730–1735. doi: 10.1038/ijo.2016.125. [DOI] [PubMed] [Google Scholar]
- 51.Reilly M, Rader DJ. Apolipoprotein E and coronary disease: a puzzling paradox. PLoS Med. 2006;3:e258. doi: 10.1371/journal.pmed.0030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rasmussen KL, Tybjaerg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. Plasma levels of apolipoprotein E and risk of ischemic heart disease in the general population. Atherosclerosis. 2016;246:63–70. doi: 10.1016/j.atherosclerosis.2015.12.038. [DOI] [PubMed] [Google Scholar]
- 53.Rasmussen KL. Plasma levels of apolipoprotein E, APOE genotype and risk of dementia and ischemic heart disease: a review. Atherosclerosis. 2016;255:145–155. doi: 10.1016/j.atherosclerosis.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 54.Brodsky SV, Ganju R, Mishra S, Ivanov I, Fadda P, Wang H, et al. Genomic analysis of an obesity paradox: a microarray study of the aortas of morbidly obese decedents with mild and severe atherosclerosis. Crit Pathw Cardiol. 2019;18:57–60. doi: 10.1097/HPC.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naude PJW, Roest AM, Stein DJ, de Jonge P, Doornbos B. Anxiety disorders and CRP in a population cohort study with 54,326 participants: the LifeLines study. World J Biol Psychiatry. 2018;19:461–470. doi: 10.1080/15622975.2018.1433325. [DOI] [PubMed] [Google Scholar]
- 57.Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med. 2014;76:181–189. doi: 10.1097/PSY.0000000000000049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw datasets generated and analysed during the current study are not publicly available in order to protect participant confidentiality. Case report forms (CRFs) on paper are safely stored. The data were transferred to SPSS for statistical analyses and the data files are stored by Innlandet Hospital Trust, Brumunddal, Norway, on a server dedicated to research. The security follows to the rules given by The Norwegian Data Protection Authority, P.O. Box 8177 Dep. NO-0034 Oslo, Norway. The data are available on request to the authors.