Abstract
The coxsackievirus–adenovirus receptor (CAR) is the described primary receptor for adenovirus serotype 5 (Ad5), a common human pathogen that has been exploited as a viral vector for gene therapy and vaccination. This study showed that monocytes and dendritic cells (DCs), such as freshly isolated human blood myeloid DCs, plasmacytoid DCs and monocyte-derived DCs, are susceptible to recombinant Ad5 (rAd5) infection despite their lack of CAR expression. Langerhans cells and dermal DCs from skin expressed CAR, but blocking CAR only partly decreased rAd5 infection, together suggesting that other receptor pathways mediate viral entry of these cells. Lactoferrin (Lf), an abundant protein in many bodily fluids known for its antiviral and antibacterial properties, promoted rAd5 infection in all cell populations except plasmacytoid DCs using a CAR-independent process. Lf caused phenotypic differentiation of the DCs, but cell activation played only a minor role in the increase in infection frequencies. The C-type lectin receptor DC-SIGN facilitated viral entry of rAd5–Lf complexes and this was dependent on high-mannose-type N-linked glycans on Lf. These results suggest that Lf present at high levels at mucosal sites can facilitate rAd5 attachment and enhance infection of DCs. A better understanding of the tropism and receptor mechanisms of Ad5 may help explain Ad5 pathogenesis and guide the engineering of improved rAd vectors.
INTRODUCTION
Adenoviruses are frequent causes of acute upper respiratory tract infections and can be responsible for ocular and gastrointestinal illnesses in humans. They have also been exploited for the development of replication-incompetent viral vectors because their genomes allow large inserts of expression cassettes, thereby offering efficient gene delivery. Recombinant adenoviruses (rAds), serotype 5 (rAd5) in particular, are commonly used vectors for gene therapy and vaccination trials (Barouch & Nabel, 2005; McConnell & Imperiale, 2004; Tatsis & Ertl, 2004). The primary receptor described for infection of most Ad species (A, C, D, E and F) is the coxsackievirus and adenovirus receptor (CAR) (Bergelson et al., 1997; Roelvink et al., 1998; Tomko et al., 1997). As currently understood, infection of Ad5 (species C) involves binding of the viral fiber knob to CAR on the target cells (Roelvink et al., 1996), followed by an interaction between the viral penton base with α v integrins on the cell surface (Wickham et al., 1993). This allows virus–receptor complexes to enter clathrin-coated pits via endocytosis (Li et al., 1998; Wang et al., 1998; Wickham et al., 1993). Efficient infection of Ad5 via CAR has mainly been demonstrated in vitro using immortalized cell lines with well-exposed CAR. However, CAR is naturally expressed in tight junctions between cells and therefore has limited accessibility on the apical cell surface (Cohen et al., 2001). As a likely consequence of this, CAR has been shown to play a minor role in Ad5 infection of epithelial cells (Johansson et al., 2007), hepatocytes (Smith et al., 2003), fibroblasts (Hidaka et al., 1999; Leopold et al., 2006) and antigen-presenting cells (APCs) such as dendritic cells (DCs) (Cheng et al., 2007; Lore et al., 2007; Rozis et al., 2005; Tillman et al., 1999). Thus, CAR-independent infection pathways used by Ad5 have been proposed. For example, heparan sulfate glycosaminoglycans have been demonstrated to mediate Ad5 infection (Cheng et al., 2007; Dechecchi et al., 2001, 2000; Xie et al., 2006). As opposed to the fiber knob, the hexon of Ad5 was shown to be able to directly bind the coagulation factor X and mediate Ad5 infection of the liver (Waddington et al., 2008). Moreover, lactoferrin (Lf), an iron-binding protein present at mucosal sites and in many bodily fluids, was shown to facilitate binding of Ad5 to human epithelial cells and enhance infection via a CAR-independent manner using a yet undefined receptor (Johansson et al., 2007). Interestingly, Lf is mainly known for its immunomodulatory properties, which enhance anti-microbial and anti-inflammatory responses (Legrand et al., 2005). High levels of Lf present during inflammation were recently shown to directly recruit and activate DCs (de la Rosa et al., 2008; Spadaro et al., 2008).
The receptor pathways used for Ad5 entry into DCs are poorly understood. We have previously demonstrated that primary human CD11c+ myeloid DCs (MDCs) and CD123+ plasmacytoid DCs (PDCs) are susceptible to rAd5 and are able to present rAd5-encoded antigen to activate specific T-cell responses (Lore et al., 2007). In this study, we investigated the involvement of CAR and the influence of Lf in rAd5 infection in relevant primary human APC populations from both blood and skin.
METHODS
Isolation of blood DCs.
This study was approved by the ethical committee at the Karolinska Institutet, Stockholm, Sweden, and the NIH, Bethesda, MD, USA. Our sorting procedures for direct isolation of DCs from blood have been described previously (Lore et al., 2003, 2005, 2007; Smed-Sorensen et al., 2005). DCs were cultured in complete medium composed of RPMI 1640 and 10 % fetal calf serum (FCS; Sigma-Aldrich). To maintain viability, the medium for PDCs and MDCs was supplemented with interleukin (IL)-3 (1 ng ml−1; R&D Systems) or granulocyte–monocyte colony-stimulating factor (GM-CSF, 2 ng ml−1; PeproTech), respectively.
In vitro differentiation of DCs from monocytes.
Monocyte-derived dendritic cells (MDDCs) were prepared as described previously (Lore et al., 1998) with some modifications. Adherent monocytes were cultured for 6 days in complete medium supplemented with IL-4 (150 ng ml−1; R&D Systems) and GM-CSF (300 ng ml−1) to obtain >90 % CD1a+ CD14− immature DCs.
Human monocyte enrichment.
Adherent monocytes were collected by cell scraper and cultured in complete medium to obtain enriched monocyte cultures (>90 % CD14+).
Isolation of skin DC subsets.
Skin was collected from patients undergoing breast-reconstruction surgery (Karolinska University Hospital, Stockholm, Sweden). Skin DCs were isolated as described previously with some modifications (Lore et al., 1998). Following surgery, skin was processed with a skin graft mesher (Zimmer). Meshed skin was incubated with dispase (3.4 U ml−1 in PBS; Gibco) for 90 min at 37 °C before the dermis and epidermis were separated with forceps. The epidermis and dermis were incubated separately in complete medium (with 40 ng GM-CSF ml−1) for 48 h at 37 °C to induce the migration of epidermal Langerhans cells (LCs; CD1a+, HLA-DR+) and dermal DCs (dDCs; CD1a+/−, HLA-DR+), respectively. All experiments were carried out on freshly harvested DCs.
Phenotypic characterization of DCs.
Cells were harvested, washed in PBS supplemented with 2 % FCS and surface stained with different combinations of anti-CD1a, anti-CD11c, anti-CD123, anti-CD14, anti-CD80, anti-CD86, anti-HLA-DR (BD Biosciences), anti-CAR (Upstate), anti-DC-SIGN (R&D Systems), and anti-langerin (Beckman-Coulter) monoclonal antibodies (mAbs) for 15 min at 4 °C and then washed. The cells were collected on a FACSCalibur flow cytometer (BD Biosciences) and all data were analysed using FlowJo software (version 8.5; Treestar).
Production of Ad construct.
E1/E3-deleted, replication-incompetent rAd5 or Ad35 vectors were generated in PER.C6/55K cells as described previously (Barouch et al., 2004; Havenga et al., 2001). Viral titres were determined by high performance liquid chromatography and infectivity was assessed by plaque assays using PER.C6/55K cells.
rAd5 and rAd35 infection of cells.
Cell populations were cultured at 1×106 cells ml−1 (with ≥3×105 cells per tube) at 37 °C in polystyrene round-bottomed tubes (BD Labware) and exposed to either rAd5 or rAd35 encoding green fluorescent protein (rAd35–GFP) at the indicated inoculums of infectious particles (i.p.) per cell for 24 h. Infections were evaluated using flow cytometry.
Lf and transferrin treatment of rAd.
rAd5 or rAd35 was incubated with human Lf from milk or recombinant human Lf produced in rice (HLf), bovine Lf from milk (BLf) or human transferrin (HTf) (Sigma-Aldrich) at the indicated concentrations for 3 h at 25 °C before exposure to cells. In the indicated experiments, the various Lfs or HTf were added simultaneously with rAd to the cells.
Stimulation of MDDCs.
MDDCs were stimulated with Lf or lipopolysaccharide (LPS) from Escherichia coli 055 : B5 (Sigma-Aldrich) for 24 h and evaluated for CD86 expression using flow cytometry.
Endotoxin removal of Lf stocks.
Endotoxin contents were reduced in all protein stocks using 1 % Triton X-114 extraction (Calbiochem). HLf and BLf stocks at 1 mg ml−1 contained ≤1.25 and ≤0.125 ng endotoxin ml−1, respectively, levels that were reduced 10-fold by this procedure. The final concentrations of endotoxin in the Lf/rAd5 mixes added to DC cultures were 23-fold more diluted. Endotoxin was measured using a Limulus amoebocyte lysate assay.
rAd blocking assays.
Cells were pre-treated with anti-CAR (clone RmcB, 20 μg ml−1; Upstate), anti-CD46 (clone 13/42, 5 μg ml−1; BMA Biomedicals), anti-DC-SIGN (clone 120507, 10 μg ml−1; R&D Systems) or anti-langerin (clone DCMG4, 20 μg ml−1, Beckman-Coulter) mAbs, mannan (200 μg ml−1; Sigma-Aldrich) or heparin sodium salt (200 μg ml−1; Sigma-Aldrich) for 1 h at 37 °C before exposure to rAd for 24 h.
Enzymic digestion of N-linked carbohydrates.
Native or denatured HLf or BLf (5 μg) was incubated with 15 mU endoglycosidase H (Endo H; Roche Applied Science) ml−1 in 50 mM sodium acetate buffer (pH 5.3) for 20 h at 37 °C. Enzyme-treated and untreated proteins were then separated by 7.5 % Tris/HCl SDS-PAGE (Bio-Rad) and the protein bands were visualized with Coomassie blue staining. A high-range rainbow molecular mass marker was run simultaneously to identify the 78 kDa lactoferrin band (Amersham Biosciences).
Statistical analyses.
Statistical analyses were performed using Student's paired t-test (Fig. 1 and Supplementary Fig. S1) or Wilcoxon signed rank test (Figs 2–7) with Graph Pad Prism software.
Fig. 1.
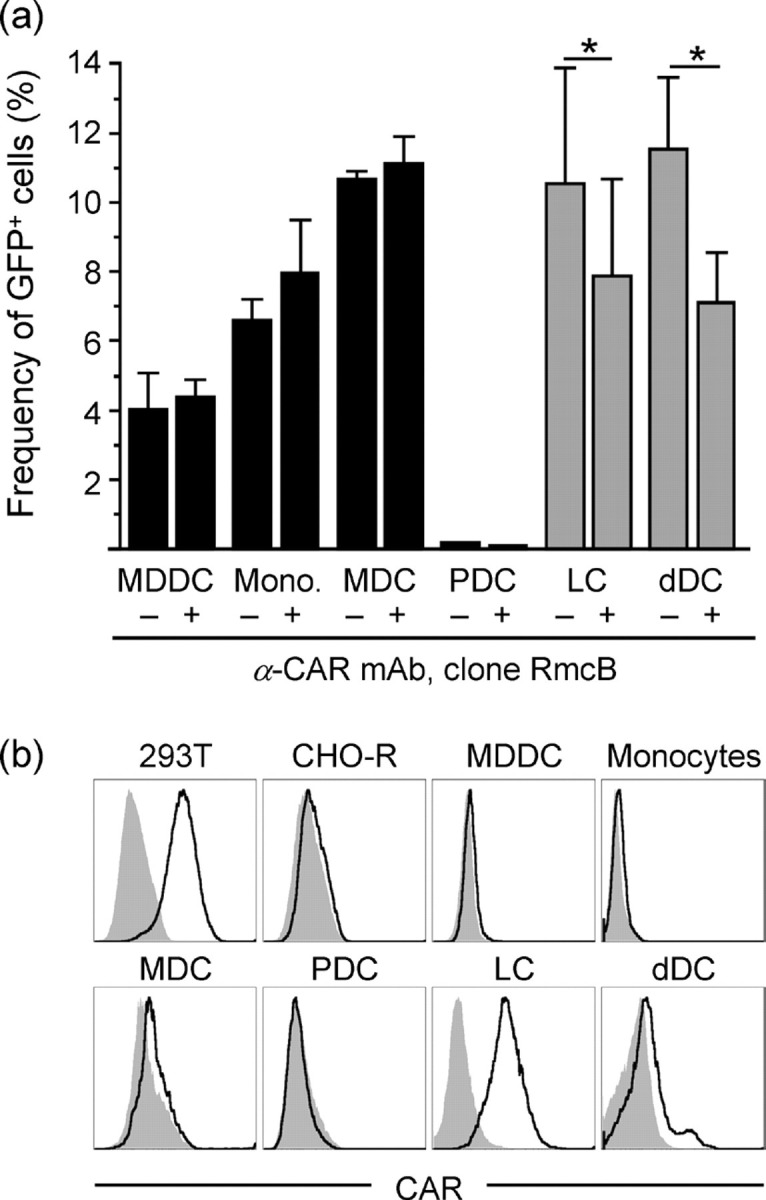
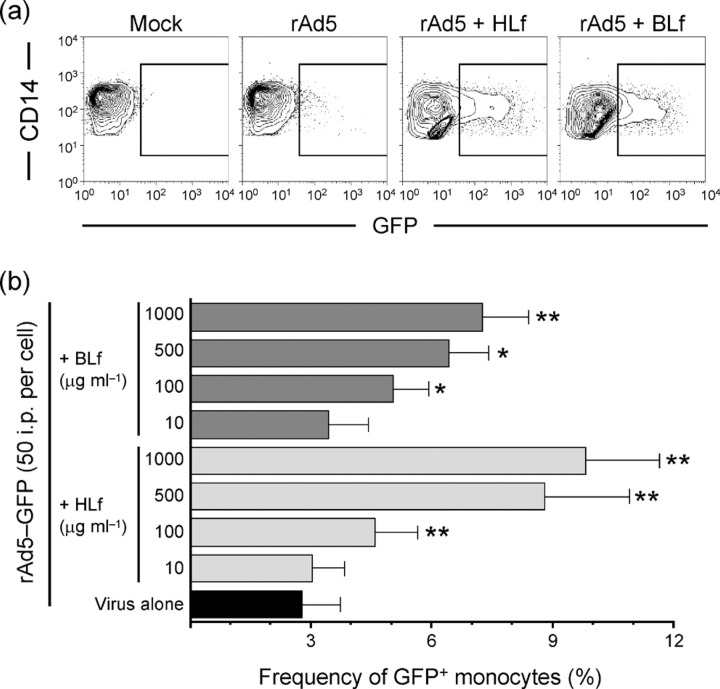
Differential CAR expression and CAR-dependent rAd5 infection of DC subsets. (a) MDDCs, monocytes (Mono.), MDCs, PDCs, LCs and dDCs were exposed to rAd5–GFP for 24 h in the presence or absence of anti-CAR mAb. The frequencies of GFP+ cells were assessed by flow cytometry. Results are shown as means±sem; *P<0.05. (b) Histograms show CAR expression on various cell populations where black lines indicate stained cells and grey-filled areas indicate unstained cells. 293T and CHO-R cell lines were used as positive and negative CAR staining controls, respectively.
Fig. 2.
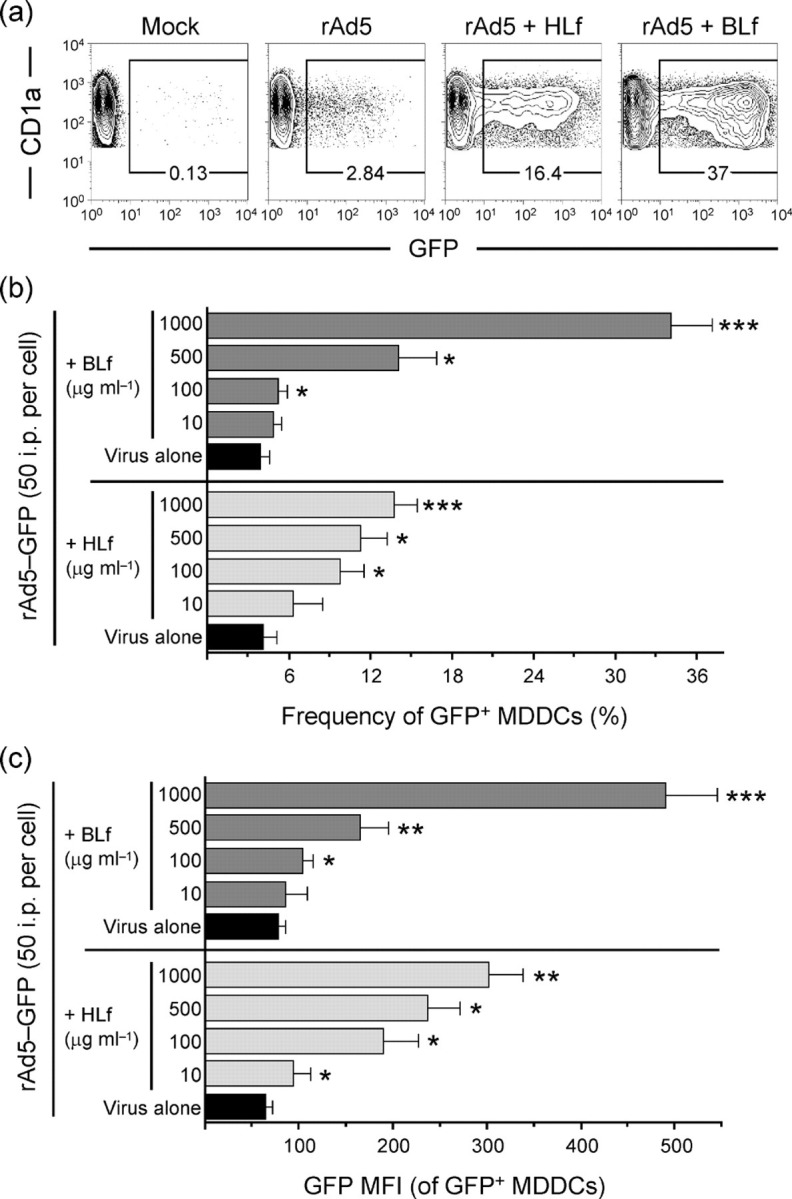
Lf facilitates rAd5 infection of MDDCs. (a) Representative dot plots of infection frequencies in MDDCs exposed for 24 h to rAd5–GFP pre-incubated with HLf or BLf. (b, c) Dose-dependent increases in HLf- and BLf-enhanced rAd5 infection frequencies (b) and the GFP MFI of infected cells (c). Results are shown as means±sem; *P<0.05; **P<0.005; ***P<0.0005.
Fig. 7.
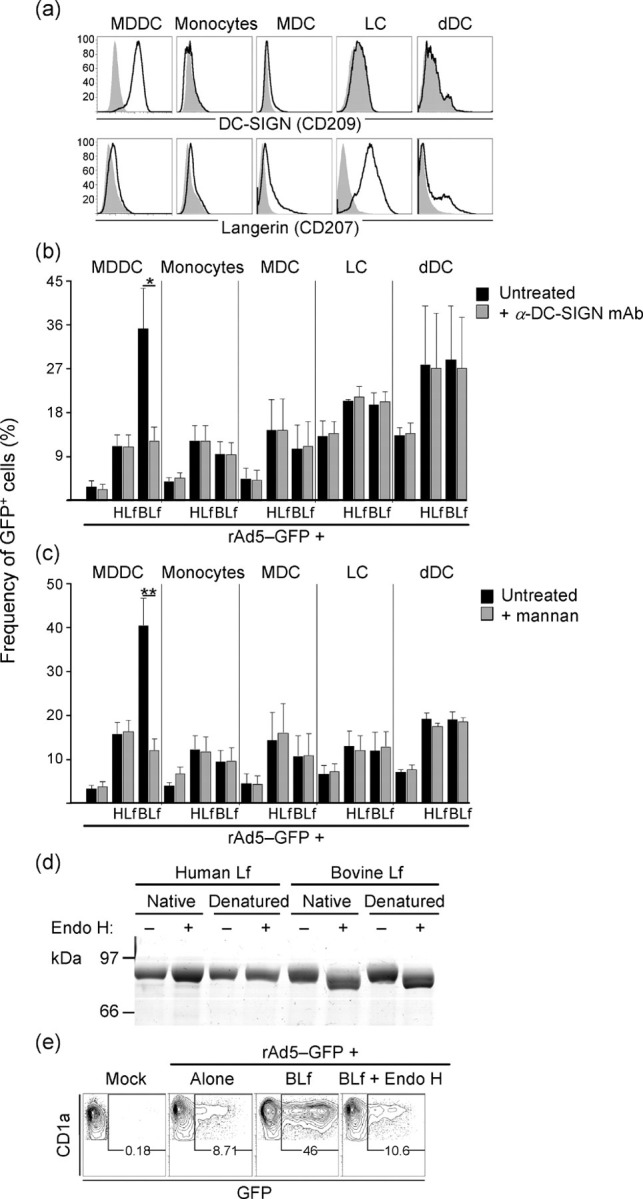
BLf can mediate rAd5 infection via DC-SIGN. (a) The histograms show DC-SIGN and langerin expression where the black lines depict stained cells and grey-filled areas depict unstained cells. (b, c) Cells were pre-treated with anti-DC-SIGN mAb or mannan before exposure to rAd5. Results are shown as means±sem; *P<0.05; **P<0.005. (d) HLf and BLf were treated with Endo H and analysed by an SDS-PAGE mobility shift assay. (e) rAd5 pre-treated with deglycosylated BLf was exposed to MDDCs and showed no appreciable increase in infection compared with rAd5 alone.
RESULTS
CAR-independent rAd5 infection of human DCs
We initiated these studies by performing a side-by-side comparison of various APC subsets for their Ad5 susceptibility and the involvement of CAR in this process. We exposed in vitro-differentiated MDDCs and freshly sorted monocytes, MDCs and PDCs from blood, and LCs and dDCs from skin, to replication-incompetent rAd5 encoding GFP. Thus, infected cells expressed GFP but did not produce new viral particles. We exposed the cell populations to an inoculum of 100 i.p. rAd5–GFP per cell and analysed the frequency of infected cells after 24 h by flow cytometry as described previously (Lore et al., 2007). Among the APCs purified from blood, MDDCs, monocytes and MDCs were susceptible to rAd5, whilst PDCs showed markedly lower infection frequencies (Fig. 1a) as defined by our criteria of monitoring GFP expression, which does not rule out virus entry (Rea et al., 2001). No reduction in rAd5 infection was observed in any of the blood APC populations when CAR was blocked using neutralizing anti-CAR mAbs (clone RmcB; Hsu et al., 1988) during virus exposure (Fig. 1a). This mAb partially reduced Ad5 infection (by ≤40 %) in CAR-expressing 293T cell lines (data not shown). An alternative anti-CAR mAb (clone E1-1; Ebbinghaus et al., 2001) also did not show any reduction in rAd5 infection in blood APCs (data not shown). LCs isolated from epidermis and DCs isolated from dermis were also susceptible to rAd5 infection (Fig. 1a). However, in contrast to the blood APCs, CAR blocking resulted in a partial and significant reduction in rAd5 infection in LCs (mean 25 % reduction, P=0.02) and dDCs (mean 38 % reduction, P=0.01; n=3) (Fig. 1a).
We next analysed the cell-surface expression of CAR on all of these APC populations. The cell lines 293T (expressing CAR) and CHO-R (not expressing CAR) were used as CAR staining controls. The APC populations from blood were found to exhibit no or very low levels of CAR expression (Fig. 1b). In contrast, epidermal LCs had a high and uniform expression of CAR, whilst most dDCs expressed low or undetectable levels except for a small subpopulation that exhibited clear CAR expression (Fig. 1b). Because the various APC subsets were susceptible to rAd5 infection, showed variable surface expression of CAR and blocking of CAR did not completely eliminate susceptibility to rAd5, we hypothesized that CAR-independent pathways can be used by rAd5 for infection of these cells.
Lf enhances rAd5 infection of MDDCs
The short fibers present on Ad5 used for binding to cells may indicate that the virus needs a well-exposed receptor or alternative soluble components promoting viral attachment to cells and infection. As mentioned earlier, it was reported recently that the iron-binding protein Lf can promote infection of Ad5 in epithelial cells where CAR is not accessible (Johansson et al., 2007). We therefore investigated whether Lf influenced and/or promoted infection of rAd5 in human APCs. We tested two commercial sources of Lf by pre-incubating rAd5 with various doses (10–1000 μg ml−1) of either HLf or BLf, representing physiological doses in vivo. The Lf–rAd5 mix was added to MDDCs at a fixed inoculum of 50 i.p. per cell for 24 h. Both HLf and BLf significantly enhanced the in vitro transduction efficiency of rAd5 in MDDCs in a dose-dependent manner (Fig. 2a, b). At a concentration of 1 mg ml−1, BLf and HLf increased rAd5 infection in MDDCs 8.7- and 3.4-fold, respectively (P=0.0001; n=18 and 13, respectively; Fig. 2b). Lowering the concentration of Lf to 100 μg ml−1 still increased rAd5 infection of MDDCs (P≤0.039). Lf promoted rAd5 infection when pre-incubated with the virus, suggesting that Lf first binds to rAd5 and that the complex then interacts with the cells. Pre-treating MDDCs with Lf prior to rAd5 exposure also enhanced the rAd5 infection frequency, although this effect was less pronounced than when rAd5 was pre-treated with Lf (data not shown). Also, pre-treating rAd5 with similar doses of Lf but using lower or higher concentrations of rAd5 showed a similar Lf-mediated augmentation of MDDC infection (data not shown).
In addition to the increased frequencies of rAd5-infected MDDCs mediated by Lf, the infected cells were found to produce more GFP on a per-cell basis, as measured by the elevated median fluorescence intensity (MFI) of GFP (Fig. 2c). Treatment with 1 mg BLf or HLf ml−1 increased the GFP MFI of rAd5-infected cells 6.4- and 4.7-fold, respectively, compared with rAd5 alone (P=0.0002; n=16 and 9, respectively). Lowering the concentration of Lf to 100 μg ml−1 still increased the GFP MFI of rAd5-exposed MDDCs (P=0.016 and P=0.027).
Lf enhances rAd5 infection in primary human APCs
We next turned to primary APCs. Lf also significantly enhanced rAd5 infection of monocytes in a dose-dependent manner (Fig. 3a, b). As was observed in MDDCs, Lf treatment of rAd5 was accompanied by a significantly higher GFP MFI within the infected monocytes (Fig. 3a and data not shown). Lf also increased rAd5 infection of freshly isolated MDCs from blood 2-fold (1 mg ml−1, P=0.008, n=7; Fig. 4a, b). Lf-mediated infection was again associated with an increase in the GFP MFI (Fig. 4c). In contrast, rAd5 infection of PDCs was found to be low and Lf did not increase infection of these cells (Fig. 4a, b). Increasing the dose of either rAd5 or Lf did not lead to an appreciable Lf-mediated increase in rAd5 infection in PDCs (data not shown). In contrast, Lf increased the frequencies of rAd5 infection in dDCs. Infection of LCs increased 1.6-fold when pre-incubated with 1 mg ml−1 of either Lf (P=0.016, n=6; Fig. 4d, e). Similarly, Lf increased infection in dDCs ≥1.9-fold (P=0.016, n=6; Fig. 4d, e). An increased GFP MFI of the infected dDCs accompanied the increased infection frequencies (Fig. 4d).
Fig. 3.
Lf facilitates rAd5 infection of monocytes. (a) Representative dot plots of infection frequencies in monocytes exposed for 24 h to rAd5–GFP pre-incubated with HLf or BLf. (b) Graph showing combined data of infection frequencies in monocytes exposed to rAd5 pre-incubated with various doses of HLf or BLf. Results are shown as means±sem; *P<0.05; **P<0.005.
Fig. 4.
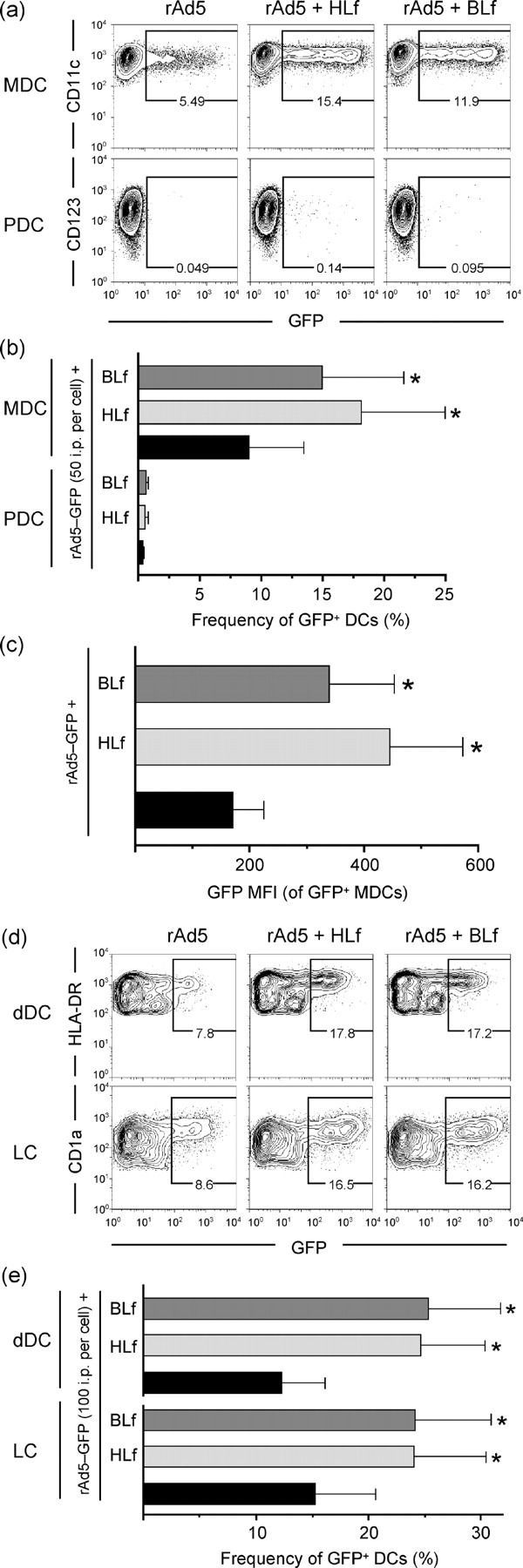
Lf facilitates rAd5 infection of primary human DC subsets. rAd5–GFP was pre-incubated with HLf or BLf and exposed to MDCs, PDCs, LCs and dDCs at the indicated doses for 24 h. Representative dot plots for MDCs and PDCs, and for LCs and dDCs are shown in (a) and (d), respectively. The bar graphs show the combined data of infection frequencies in MDCs, LCs, and dDCs (b, e), and the GFP MFI of infected MDCs (c). Results in (b), (c) and (e) are shown as means±sem; *P<0.05.
Lf induces phenotypic maturation of DCs
We examined the extent by which Lf induces activation of DCs by assessing phenotypic maturation and how this affected rAd5 infection. MDDCs were exposed for 24 h to Lf. Exposure to doses ≥100 μg ml−1 led to full maturation of DCs visualized by complete upregulation of CD86 (Fig. 5a) and CD80 and CD83 (data not shown). As the immunostimulatory effects of Lf may involve its capacity to form complexes with endotoxin (i.e. LPS; Miyazawa et al., 1991), we compared DC maturation using preparations of Lf with and without LPS removed. Although the maturation was reduced, it was still induced by Lf with undetectable LPS levels (<4×10−5 ng ml−1; Fig. 5a). Stimulation by LPS alone at concentrations between 0.0005 and 0.043 ng ml−1, mimicking the range of detected LPS contamination in untreated Lf, showed maturation with the highest dose and a gradual decrease showing no or weak maturation using the lower dose (Fig. 5a). Therefore, it is likely that LPS in untreated Lf preparations contributes to induction of DC maturation, but Lf itself is also able to cause differentiation of DCs. Furthermore, we examined whether maturation per se led to increased susceptibility to infection or increased gene expression from rAd5. Importantly, we found that CAR expression was still undetectable after maturation (data not shown). MDDCs were exposed to rAd5 pre-incubated with either untreated Lf or Lf with undetectable LPS levels. Lf-mediated augmentation of rAd5 infection was slightly lower when LPS was removed from the Lf preparations (Fig. 5b). Simultaneous LPS stimulation and rAd5 exposure excluding Lf also led to a minor but non-significant increase in infection (Fig. 5b). Thus, whilst it is possible that DC maturation induced by Lf may contribute to the enhancement of Ad5 entry and/or gene expression, DC maturation does not account for the majority of the Lf-mediated enhancement of rAd5 infection observed.
Fig. 5.
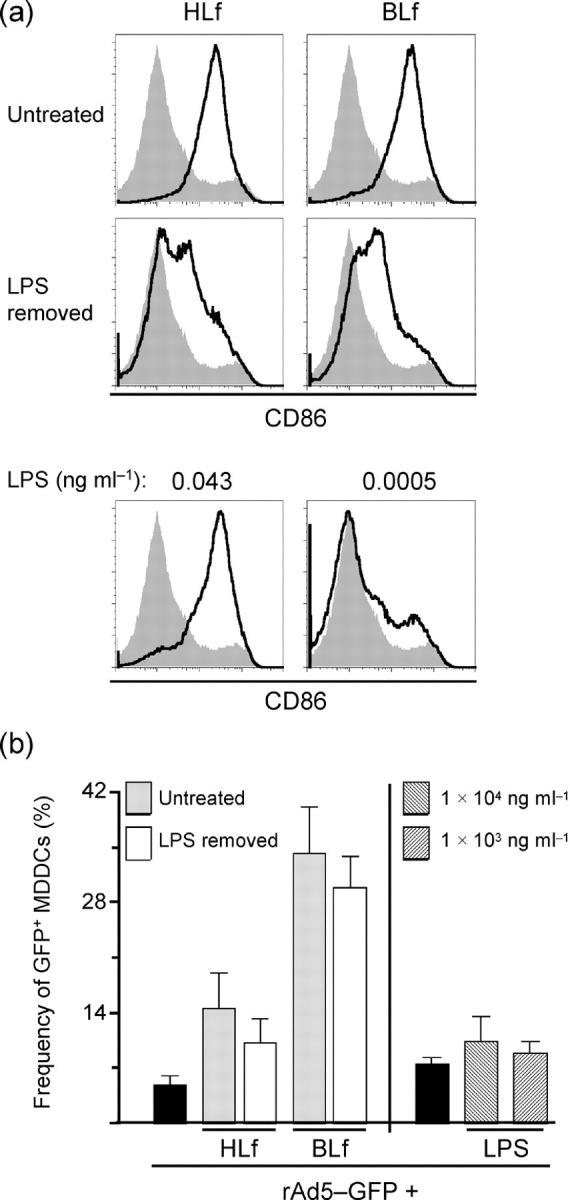
Lf causes the differentiation of MDDCs. (a) MDDCs were exposed to untreated Lf, Lf with LPS removed or LPS alone for 24 h. CD86 upregulation, a marker for DC maturation, is presented as histograms where black lines indicate stimulated MDDCs and grey-filled areas are unstimulated MDDCs. (b) Infection frequencies are shown for MDDCs exposed to rAd5 pre-incubated with untreated Lf or Lf with LPS removed. DC maturation per se did not lead to enhanced rAd5 infection. Results are shown as means±sem.
Lf-mediated rAd5 infection does not involve CAR binding
To examine the specificity of the rAd5–Lf interaction and subsequent enhancement of rAd5 infection, a related iron-binding protein, HTf (Wally & Buchanan, 2007), was studied for its influence on Ad5 infection. Pre-treating rAd5 with HTf at various concentrations ranging from 40 to 1000 μg ml−1 had a slight but non-significant effect on the ability of rAd5 to infect MDDCs (P=0.07, n=5; Fig. 6a).
Fig. 6.
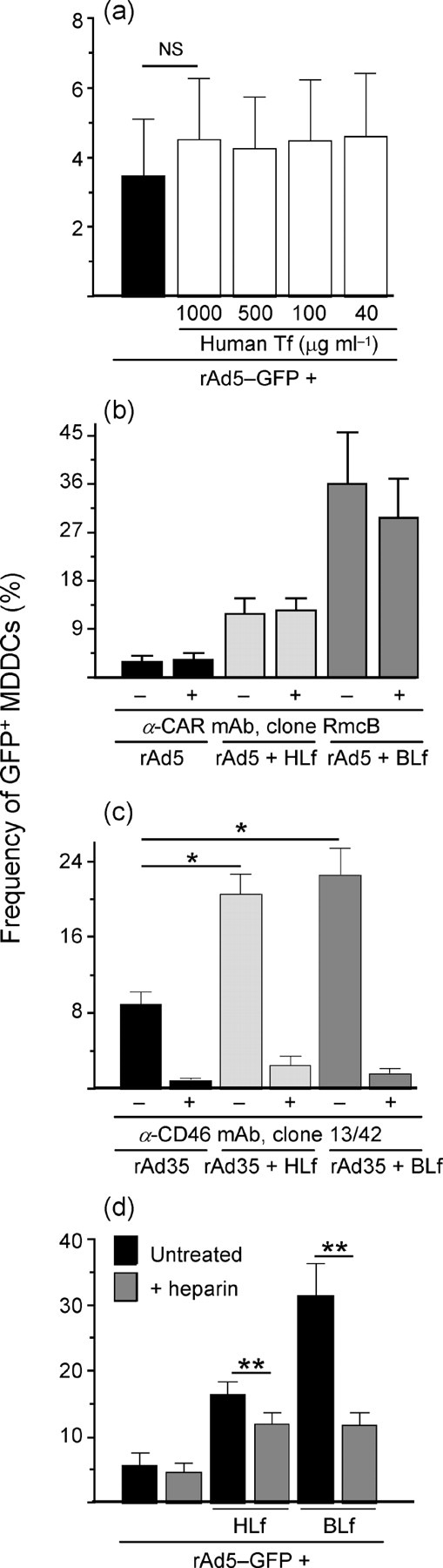
Characterization of Lf specificity. (a) rAd5–GFP was pre-incubated with various doses of HTf and exposed to MDDCs. (b) Infection frequencies are shown for MDDCs exposed to Lf–rAd5 complexes in the presence of anti-CAR mAb. (c) rAd35 was pre-treated with HLf or BLf and exposed to MDDCs in the presence or absence of anti-CD46 mAb. (d) Infection frequencies are shown for MDDCs exposed to Lf–rAd5 complexes in the presence of heparin. Results are shown as means±sem; ns, not significant; *P<0.05; **P<0.005.
Blocking CAR did not reduce the Lf-enhanced infection frequencies of rAd5, suggesting that Lf promotes rAd5 infection using a CAR-independent pathway (Fig. 6b). To test whether the entry-promoting effect of Lf was specific to rAd5, we extended our studies to include a species B Ad (rAd35), which utilizes CD46 for entry (de Gruijl et al., 2006; Lore et al., 2007). rAd35 encoding GFP was pre-treated with Lf and then exposed to MDDCs for 24 h at 10 i.p. per cell. At a concentration of 1 mg ml−1, Lf significantly increased rAd35 infection in MDDCs by ≥2.3-fold (P=0.03, n=5; Fig. 6c). In contrast to rAd5 infection where we found that Lf operates independently of CAR, Lf-mediated enhancement of rAd35 infection was dependent on the primary receptor CD46, as the presence of neutralizing anti-CD46 mAbs (clone 13/42) almost abolished infection (Fig. 6c).
Treatment of MDDCs with heparin sodium salt significantly reduced the BLf- and HLf-mediated rAd5 infection rates by means of 62.5 and 17 %, respectively, compared with the heparin-untreated cells (P=0.004, n=8), but had no inhibitory effect on rAd5 infection alone (Fig. 6d). A heparin-sensitive rAd5 infection pathway as reported for DCs (Cheng et al., 2007) may therefore be present in these cells if a bridging molecule such as Lf is involved.
Lf-binding receptors expressed on DCs and monocytes
As Lf-mediated rAd5 infection apparently occurred independently of CAR, we searched for APC-related receptors with potential to interact with Lf. There is no known exclusive receptor for Lf (Suzuki & Lonnerdal, 2002; Suzuki et al., 2005). HLf and BLf share similar amino acid sequences and structures (Pierce et al., 1991). However, BLf has four potential N-linked glycosylation sites, of which two to three have been shown to be occupied by high-mannose-type oligosaccharides, whereas natural HLf has two N-acetyl-lactosaminic-type oligosaccharides (Coddeville et al., 1992; Spik et al., 1988; van Berkel et al., 1996). Based on these different glycosylation patterns, BLf and HLf may interact with different carbohydrate-binding receptors. A number of receptors for mannose-containing proteins have been described including C-type lectin receptors such as the mannose receptor (CD206) and the DC-specific intercellular adhesion molecule-3 (ICAM-3)-grabbing nonintegrin (DC-SIGN or CD209; Groot et al., 2005; Naarding et al., 2005). Another C-type lectin receptor known to bind both mannose and N-acetylglucosamine is langerin (CD207).
We found that MDDCs expressed high levels of DC-SIGN but expressed no langerin (Fig. 7a). Monocytes expressed no DC-SIGN or langerin. Although MDCs occasionally showed a small proportion of DC-SIGN+ cells, most cells showed no expression. Some MDCs expressed low levels of langerin. LCs isolated from epidermis had uniform and high expression of langerin but did not express DC-SIGN. dDCs contained a subpopulation that resembled and could potentially be contaminating epidermal LCs, but the majority of the cells did not express langerin or DC-SIGN. As our prior studies showed that PDCs had poor susceptibility to Lf-mediated rAd5 infection, we excluded them from this analysis.
DC-SIGN expressed on MDDCs acts as a receptor for BLf-mediated rAd5 infection
To test whether these receptors are functionally involved in Lf-mediated rAd5 infection, we employed two known blocking agents: a neutralizing mAb against DC-SIGN (clone 120507; Wu et al., 2002) and mannan (Naarding et al., 2005). The anti-DC-SIGN antibody specifically blocks DC-SIGN, whereas mannan can reduce both DC-SIGN and langerin function (Turville et al., 2002). We found that if the cells were incubated with anti-DC-SIGN prior to virus exposure, the BLf-enhanced rAd5 infection in MDDCs was reduced by a mean of 65 %, but HLf-enhanced rAd5 infection or rAd5 alone infection was not blocked (Fig. 7b). The effect of mannan treatment was similar to that of the anti-DC-SIGN antibody and only blocked infection of BLf-treated rAd5 by a mean of 70 % (Fig. 7c). Neither anti-DC-SIGN nor mannan decreased rAd5 infection in other APCs, regardless of Lf treatment (Fig. 7b, c). These findings suggest that DC-SIGN binds to BLf–rAd5 complexes to facilitate entry of rAd5 into DC-SIGN+ cells but is not involved in HLf-mediated rAd5 or rAd5 alone infection. As monocytes, MDCs, LCs and dDCs express very little or no DC-SIGN, blocking DC-SIGN had no effect on rAd5 infectivity in these cells (Fig. 7b). Mannan also did not appear to block any infection of MDCs, LCs or dDCs, which would indicate that langerin's binding capacity to mannose is insufficient to partake in BLf-mediated rAd5 entry. However, langerin's binding capacity to N-acetylglucosamine would theoretically include interaction with HLf. Both HLf- and BLf-mediated infection of langerin+ LCs was reduced when an anti-langerin antibody (clone DCGM4) was present during virus exposure, which suggests that langerin may have an involvement in this process (see Supplementary Fig. S1, available in JGV Online).
To investigate whether the involvement of DC-SIGN in the Lf-mediated enhancement depended on the respective glycosylation patterns of BLf and HLf, we treated the Lf proteins with Endo H to remove high-mannose-type oligosaccharides enzymically. Only BLf was sensitive to Endo H digestion, consistent with the presence of two to three high-mannose-type oligosaccharides on this protein (Fig. 7d) (Coddeville et al., 1992). Pre-treating rAd5 with deglycosylated native BLf did not lead to any increase in rAd5 infection of MDDCs compared with rAd5 exposure alone (Fig. 7e). In contrast, the HLf-mediated enhancement of rAd5 infection in MDDCs was not reduced when Endo H-treated HLf was used (data not shown). In conclusion, DC-SIGN probably interacts with BLf through its high-mannose residues to mediate rAd5 infection. The receptor(s) involved in HLf-mediated infection remains elusive.
DISCUSSION
Although CAR is defined as the primary receptor for Ad5, several CAR-deficient cells are susceptible to Ad5 infection. Our results demonstrated that CAR is not expressed on the surface of circulating DCs (MDCs, PDCs and MDDCs) or on monocytes from the blood but only on DCs that reside in intact tissue, represented by the skin LCs and dDCs. Consistent with these findings, previous reports have demonstrated that tissue DCs express numerous tight-junction proteins, including ZO-1, which associates with CAR (Cohen et al., 2001; Rescigno et al., 2001; Sung et al., 2006). Blocking CAR on skin DCs significantly, although not completely, reduced their susceptibility to rAd5 at a similar magnitude as documented for CAR-expressing cell lines. In contrast, rAd5 infection of APCs from blood was not affected by blocking CAR. Based on these initial observations, we concluded that CAR has only limited involvement in rAd5 infection of DCs. This led us to hypothesize that other receptor pathways are likely to be utilized by rAd5 to infect these cells.
The structure of rAd5 including the relatively short fibers suggests that attachment to cells via a receptor that is poorly exposed such as CAR would make the infection process very inefficient. Therefore, it seems likely that other soluble components and/or cell-surface receptors are involved in promoting Ad5 binding and infection. As Lf was recently shown to facilitate attachment of Ad5 to epithelial cells by serving as a bridge between the virus and the target cell surface (Johansson et al., 2007), we investigated the effect of Lf on infection of APCs. Lf is present in mucosal tissues and in most bodily fluids, such as milk, tear fluid, saliva, nasal secretions and vaginal mucus, and at low concentration in serum (Masson et al., 1966; Weinberg, 2001). Lf is of interest with regard to DCs as it can interact directly with these cells and modulate important functions such as migration, cell activation and induction of T-cell responses (de la Rosa et al., 2008; Puddu et al., 2009; Spadaro et al., 2008). It is therefore likely that Lf is present at high concentration at anatomical sites where peripheral DCs encounter pathogens such as Ad5 in vivo. Lf has been demonstrated to interfere with cellular entry by viruses such as human immunodeficiency virus type 1 (Groot et al., 2005), Semliki Forest virus (Waarts et al., 2005), human papillomavirus (Drobni et al., 2004) and Ad2 (Di Biase et al., 2003; Pietrantoni et al., 2003), indicating that Lf often acts to inhibit, not promote, viral entry (van der Strate et al., 2001)
We found that Lf of both human and bovine origin enhanced rAd5 infection in a dose-dependent manner in all APC populations tested with the exception of PDCs. The mechanism by which this enhancement of infection occurs is probably initiated by Lf binding to rAd5, as pre-treating the cells with Lf had less effect than pre-treating the virus with Lf. Subsequently, a number of mechanisms by which rAd5–Lf complexes attach to the cell surface may be envisioned. These include increased accessibility of rAd5 to appropriate cell-surface receptors, reduced steric hindrance allowing for favourable electron charges between the virus and the cell, and perhaps the possibility of using alternative receptors for viral entry that are not available to rAd5 in the absence of Lf. We did not find any evidence that the Lf-mediated increased infection of rAd5 involved CAR. Instead, the C-type lectin receptor DC-SIGN facilitated infection by Lf–rAd5 complexes. DC-SIGN was only shown to be involved in the infection of Lf–rAd5 complexes on MDDCs, the only cells examined in our studies that express DC-SIGN. Whilst not studied here, there are distinct DC-SIGN+ DC subsets residing in submucosal and dermal tissues in vivo. However, DC-SIGN is known to be downregulated during migration and isolation of such cells, which is probably why we were unable to demonstrate DC-SIGN expression on the skin DCs isolated using our protocol (Turville et al., 2002). rAd5 variants engineered to target DC-SIGN or its natural ligand ICAM-3 have been reported to enter DC-SIGN+ cells more efficiently (Korokhov et al., 2005; Maguire et al., 2006). As our data indicated that DC-SIGN interacts with BLf via its high-mannose oligosaccharides, it was only involved in the BLf- and not in HLf-mediated enhancement of rAd5. The relevance for DC-SIGN–Lf-mediated enhancement of Ad5 infection in humans therefore remains unknown, but other soluble proteins equipped with high-mannose N-linked glycans may play a role in promoting rAd5 infection of APCs expressing lectin receptors such as DC-SIGN. The previously reported modest effects of BLf compared with HLf for enhancing Ad5 infection of epithelial cells may be explained by a lack of or low expression of receptors with binding affinity to the high-mannose carbohydrate residues on BLf (Johansson et al., 2007). Due to the wide tropism of Ad5, the virus probably utilizes various cell-surface proteins depending on the target cell type.
In addition to Lf-mediated rAd5 infection in DCs, we found that rAd35 pre-treated with Lf showed enhanced infection. However, whereas the receptor usage for Lf-mediated rAd5 infection was independent of CAR, the primary receptor CD46 was essential for Lf-mediated rAd35 infection, at least in the studied population of MDDCs (Fig. 6d). As CD46 is not a C-type lectin receptor and has no reported binding affinity to carbohydrates, it is likely that Lf mediates rAd5 and rAd35 infection through different mechanisms. Therefore, Lf may enhance infection by various Ad species in DCs, but the receptors used to enhance viral entry are different. It is possible that the net positive charge of Lf may act to shield negatively charged residues on Ad capsids, thereby reducing repulsive forces from the negatively charged plasma membrane and allowing more efficient binding to cellular receptors.
It remains to be elucidated whether Lf-mediated enhancement of Ad5 infection is primarily a consequence of binding and uptake mechanisms being enhanced so that more virions enter the cells or whether Lf stimulates the DCs and thereby supports viral replication, or in this case transcription of GFP. In regard to the latter, the elevated GFP MFI values of infected cells with Lf can be indicative of increased GFP transcription or translation. However, the strong maturation induced by LPS stimulation alone did not lead to significantly more GFP expression, suggesting that DC activation per se does not play a major role in enhancing Ad5 infection.
In conclusion, we have identified possible mechanisms that rAd5 can utilize to infect APCs independently of CAR. The differential CAR expression on various APCs and their respective ability to utilize alternative receptors may play a role in their susceptibility to natural infection by Ad5 in vivo, as well as having consequences for the administration of rAd5 vaccine or gene therapy vectors. The infection efficiency of DCs by wild-type Ad or Ad-based viral vectors, and the subsequent levels of antigen expression, antigen presentation and phenotypic differentiation of such cells, may greatly impact on the magnitude of the adaptive immune responses that are generated. Thus, a better understanding of the receptor pathways used by Ad5 to infect APCs may help guide the development of rAds with greater immunogenicity or target-cell specificity. The findings presented here may also lead to more efficient methods for in vitro transduction of DCs, thereby facilitating basic studies of the Ad5 infectious life cycle.
Acknowledgments
This study was supported by the Swedish International Development Cooperation Agency (Sida), the Swedish Council for Research (Vetenskapsrådet), Åke Wiberg's Research Foundation, the Jeansson's Foundation, Clas Groschinsky's Foundation and the Swedish Society for Medicine (K. L. funding recipient). The authors would also like to thank Dr Leif Perbeck, Karolinska University Hospital, for providing skin specimens, as well as Kjell-Olof Hedlund, Swedish Institute for Infectious Disease Control, and Pia Dosenovic and Christopher Sundling, Karolinska Institutet, for technical assistance and advice.
Footnotes
A supplementary figure showing that Lf can mediate rAd5 infection via langerin is available with the online version of this paper.
References
- Barouch D. H., Nabel G. J. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum Gene Ther. 2005;16:149–156. doi: 10.1089/hum.2005.16.149. [DOI] [PubMed] [Google Scholar]
- Barouch D. H., Pau M. G., Custers J. H., Koudstaal W., Kostense S., Havenga M. J., Truitt D. M., Sumida S. M., Kishko M. G., other authors Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- Bergelson J. M., Cunningham J. A., Droguett G., Kurt-Jones E. A., Krithivas A., Hong J. S., Horwitz M. S., Crowell R. L., Finberg R. W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Cheng C., Gall J. G., Kong W. P., Sheets R. L., Gomez P. L., King C. R., Nabel G. J. Mechanism of Ad5 vaccine immunity and toxicity: fiber shaft targeting of dendritic cells. PLoS Pathog. 2007;3:e25. doi: 10.1371/journal.ppat.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddeville B., Strecker G., Wieruszeski J. M., Vliegenthart J. F., van Halbeek H., Peter-Katalinic J., Egge H., Spik G. Heterogeneity of bovine lactotransferrin glycans. Characterization of α -d-Galp-(1→3)- β -d-Gal- and α -NeuAc-(2→6)- β -d-GalpNAc-(1→4)- β -d-GlcNAc-substituted N -linked glycans. Carbohydr Res. 1992;236:145–164. doi: 10.1016/0008-6215(92)85013-P. [DOI] [PubMed] [Google Scholar]
- Cohen C. J., Shieh J. T., Pickles R. J., Okegawa T., Hsieh J. T., Bergelson J. M. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci U S A. 2001;98:15191–15196. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechecchi M. C., Tamanini A., Bonizzato A., Cabrini G. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology. 2000;268:382–390. doi: 10.1006/viro.1999.0171. [DOI] [PubMed] [Google Scholar]
- Dechecchi M. C., Melotti P., Bonizzato A., Santacatterina M., Chilosi M., Cabrini G. Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus types 2 and 5. J Virol. 2001;75:8772–8780. doi: 10.1128/JVI.75.18.8772-8780.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gruijl T. D., Ophorst O. J., Goudsmit J., Verhaagh S., Lougheed S. M., Radosevic K., Havenga M. J., Scheper R. J. Intradermal delivery of adenoviral type-35 vectors leads to high efficiency transduction of mature, CD8+ T cell-stimulating skin-emigrated dendritic cells. J Immunol. 2006;177:2208–2215. doi: 10.4049/jimmunol.177.4.2208. [DOI] [PubMed] [Google Scholar]
- de la Rosa G., Yang D., Tewary P., Varadhachary A., Oppenheim J. J. Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. J Immunol. 2008;180:6868–6876. doi: 10.4049/jimmunol.180.10.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Biase A. M., Pietrantoni A., Tinari A., Siciliano R., Valenti P., Antonini G., Seganti L., Superti F. Heparin-interacting sites of bovine lactoferrin are involved in anti-adenovirus activity. J Med Virol. 2003;69:495–502. doi: 10.1002/jmv.10337. [DOI] [PubMed] [Google Scholar]
- Drobni P., Naslund J., Evander M. Lactoferrin inhibits human papillomavirus binding and uptake in vitro. Antiviral Res. 2004;64:63–68. doi: 10.1016/S0166-3542(04)00123-8. [DOI] [PubMed] [Google Scholar]
- Ebbinghaus C., Al-Jaibaji A., Operschall E., Schoffel A., Peter I., Greber U. F., Hemmi S. Functional and selective targeting of adenovirus to high-affinity Fc γ receptor I-positive cells by using a bispecific hybrid adapter. J Virol. 2001;75:480–489. doi: 10.1128/JVI.75.1.480-489.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot F., Geijtenbeek T. B., Sanders R. W., Baldwin C. E., Sanchez-Hernandez M., Floris R., van Kooyk Y., de Jong E. C., Berkhout B. Lactoferrin prevents dendritic cell-mediated human immunodeficiency virus type 1 transmission by blocking the DC-SIGN–gp120 interaction. J Virol. 2005;79:3009–3015. doi: 10.1128/JVI.79.5.3009-3015.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenga M. J., Lemckert A. A., Grimbergen J. M., Vogels R., Huisman L. G., Valerio D., Bout A., Quax P. H. Improved adenovirus vectors for infection of cardiovascular tissues. J Virol. 2001;75:3335–3342. doi: 10.1128/JVI.75.7.3335-3342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka C., Milano E., Leopold P. L., Bergelson J. M., Hackett N. R., Finberg R. W., Wickham T. J., Kovesdi I., Roelvink P., Crystal R. G. CAR-dependent and CAR-independent pathways of adenovirus vector-mediated gene transfer and expression in human fibroblasts. J Clin Invest. 1999;103:579–587. doi: 10.1172/JCI5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu K. H., Lonberg-Holm K., Alstein B., Crowell R. L. A monoclonal antibody specific for the cellular receptor for the group B coxsackieviruses. J Virol. 1988;62:1647–1652. doi: 10.1128/jvi.62.5.1647-1652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C., Jonsson M., Marttila M., Persson D., Fan X. L., Skog J., Frangsmyr L., Wadell G., Arnberg N. Adenoviruses use lactoferrin as a bridge for CAR-independent binding to and infection of epithelial cells. J Virol. 2007;81:954–963. doi: 10.1128/JVI.01995-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korokhov N., de Gruijl T. D., Aldrich W. A., Triozzi P. L., Banerjee P. T., Gillies S. D., Curiel T. J., Douglas J. T., Scheper R. J., Curiel D. T. High efficiency transduction of dendritic cells by adenoviral vectors targeted to DC-SIGN. Cancer Biol Ther. 2005;4:289–294. doi: 10.4161/cbt.4.3.1499. [DOI] [PubMed] [Google Scholar]
- Legrand D., Elass E., Carpentier M., Mazurier J. Lactoferrin: a modulator of immune and inflammatory responses. Cell Mol Life Sci. 2005;62:2549–2559. doi: 10.1007/s00018-005-5370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold P. L., Wendland R. L., Vincent T., Crystal R. G. Neutralized adenovirus-immune complexes can mediate effective gene transfer via an Fc receptor-dependent infection pathway. J Virol. 2006;80:10237–10247. doi: 10.1128/JVI.00512-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E., Stupack D., Klemke R., Cheresh D. A., Nemerow G. R. Adenovirus endocytosis via α v integrins requires phosphoinositide-3-OH kinase. J Virol. 1998;72:2055–2061. doi: 10.1128/jvi.72.3.2055-2061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lore K., Sonnerborg A., Spetz A. L., Andersson U., Andersson J. Immunocytochemical detection of cytokines and chemokines in Langerhans cells and in vitro derived dendritic cells. J Immunol Methods. 1998;214:97–111. doi: 10.1016/S0022-1759(98)00040-4. [DOI] [PubMed] [Google Scholar]
- Lore K., Betts M. R., Brenchley J. M., Kuruppu J., Khojasteh S., Perfetto S., Roederer M., Seder R. A., Koup R. A. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J Immunol. 2003;171:4320–4328. doi: 10.4049/jimmunol.171.8.4320. [DOI] [PubMed] [Google Scholar]
- Lore K., Smed-Sorensen A., Vasudevan J., Mascola J. R., Koup R. A. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J Exp Med. 2005;201:2023–2033. doi: 10.1084/jem.20042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lore K., Adams W. C., Havenga M., Precopio M. L., Holterman L., Goudsmit J., Koup R. A. Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. J Immunol. 2007;179:1721–1729. doi: 10.4049/jimmunol.179.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire C. A., Sapinoro R., Girgis N., Rodriguez-Colon S. M., Ramirez S. H., Williams J., Dewhurst S. Recombinant adenovirus type 5 vectors that target DC-SIGN, ChemR23 and α v β 3 integrin efficiently transduce human dendritic cells and enhance presentation of vectored antigens. Vaccine. 2006;24:671–682. doi: 10.1016/j.vaccine.2005.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Dive C. H. An iron-binding protein common to many external secretions. Clin Chim Acta. 1966;14:735–739. doi: 10.1016/0009-8981(66)90004-0. [DOI] [Google Scholar]
- McConnell M. J., Imperiale M. J. Biology of adenovirus and its use as a vector for gene therapy. Hum Gene Ther. 2004;15:1022–1033. doi: 10.1089/hum.2004.15.1022. [DOI] [PubMed] [Google Scholar]
- Miyazawa K., Mantel C., Lu L., Morrison D. C., Broxmeyer H. E. Lactoferrin–lipopolysaccharide interactions. Effect on lactoferrin binding to monocyte/macrophage-differentiated HL-60 cells. J Immunol. 1991;146:723–729. [PubMed] [Google Scholar]
- Naarding M. A., Ludwig I. S., Groot F., Berkhout B., Geijtenbeek T. B., Pollakis G., Paxton W. A. Lewis X component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes. J Clin Invest. 2005;115:3256–3264. doi: 10.1172/JCI25105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce A., Colavizza D., Benaissa M., Maes P., Tartar A., Montreuil J., Spik G. Molecular cloning and sequence analysis of bovine lactotransferrin. Eur J Biochem. 1991;196:177–184. doi: 10.1111/j.1432-1033.1991.tb15801.x. [DOI] [PubMed] [Google Scholar]
- Pietrantoni A., Di Biase A. M., Tinari A., Marchetti M., Valenti P., Seganti L., Superti F. Bovine lactoferrin inhibits adenovirus infection by interacting with viral structural polypeptides. Antimicrob Agents Chemother. 2003;47:2688–2691. doi: 10.1128/AAC.47.8.2688-2691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddu P., Valenti P., Gessani S. Immunomodulatory effects of lactoferrin on antigen presenting cells. Biochimie. 2009;91:11–18. doi: 10.1016/j.biochi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Rea D., Havenga M. J., van Den Assem M., Sutmuller R. P., Lemckert A., Hoeben R. C., Bout A., Melief C. J., Offringa R. Highly efficient transduction of human monocyte-derived dendritic cells with subgroup B fiber-modified adenovirus vectors enhances transgene-encoded antigen presentation to cytotoxic T cells. J Immunol. 2001;166:5236–5244. doi: 10.4049/jimmunol.166.8.5236. [DOI] [PubMed] [Google Scholar]
- Rescigno M., Urbano M., Valzasina B., Francolini M., Rotta G., Bonasio R., Granucci F., Kraehenbuhl J. P., Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- Roelvink P. W., Kovesdi I., Wickham T. J. Comparative analysis of adenovirus fiber–cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J Virol. 1996;70:7614–7621. doi: 10.1128/jvi.70.11.7614-7621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelvink P. W., Lizonova A., Lee J. G., Li Y., Bergelson J. M., Finberg R. W., Brough D. E., Kovesdi I., Wickham T. J. The coxsackievirus–adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozis G., de Silva S., Benlahrech A., Papagatsias T., Harris J., Gotch F., Dickson G., Patterson S. Langerhans cells are more efficiently transduced than dermal dendritic cells by adenovirus vectors expressing either group C or group B fibre protein: implications for mucosal vaccines. Eur J Immunol. 2005;35:2617–2626. doi: 10.1002/eji.200425939. [DOI] [PubMed] [Google Scholar]
- Smed-Sorensen A., Lore K., Vasudevan J., Louder M. K., Andersson J., Mascola J. R., Spetz A. L., Koup R. A. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J Virol. 2005;79:8861–8869. doi: 10.1128/JVI.79.14.8861-8869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. A., Idamakanti N., Marshall-Neff J., Rollence M. L., Wright P., Kaloss M., King L., Mech C., Dinges L., other authors Receptor interactions involved in adenoviral-mediated gene delivery after systemic administration in non-human primates. Hum Gene Ther. 2003;14:1595–1604. doi: 10.1089/104303403322542248. [DOI] [PubMed] [Google Scholar]
- Spadaro M., Caorsi C., Ceruti P., Varadhachary A., Forni G., Pericle F., Giovarelli M. Lactoferrin, a major defense protein of innate immunity, is a novel maturation factor for human dendritic cells. FASEB J. 2008;22:2747–2757. doi: 10.1096/fj.07-098038. [DOI] [PubMed] [Google Scholar]
- Spik G., Coddeville B., Montreuil J. Comparative study of the primary structures of sero-, lacto- and ovotransferrin glycans from different species. Biochimie. 1988;70:1459–1469. doi: 10.1016/0300-9084(88)90283-0. [DOI] [PubMed] [Google Scholar]
- Sung S. S., Fu S. M., Rose C. E., Jr, Gaskin F., Ju S. T., Beaty S. R. A major lung CD103 ( α E)- β 7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176:2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. A., Lonnerdal B. Characterization of mammalian receptors for lactoferrin. Biochem Cell Biol. 2002;80:75–80. doi: 10.1139/o01-228. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. A., Lopez V., Lonnerdal B. Mammalian lactoferrin receptors: structure and function. Cell Mol Life Sci. 2005;62:2560–2575. doi: 10.1007/s00018-005-5371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsis N., Ertl H. C. Adenoviruses as vaccine vectors. Mol Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillman B. W., de Gruijl T. D., Luykx-de Bakker S. A., Scheper R. J., Pinedo H. M., Curiel T. J., Gerritsen W. R., Curiel D. T. Maturation of dendritic cells accompanies high-efficiency gene transfer by a CD40-targeted adenoviral vector. J Immunol. 1999;162:6378–6383. [PubMed] [Google Scholar]
- Tomko R. P., Xu R., Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci U S A. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turville S. G., Cameron P. U., Handley A., Lin G., Pohlmann S., Doms R. W., Cunningham A. L. Diversity of receptors binding HIV on dendritic cell subsets. Nat Immunol. 2002;3:975–983. doi: 10.1038/ni841. [DOI] [PubMed] [Google Scholar]
- van Berkel P. H., van Veen H. A., Geerts M. E., de Boer H. A., Nuijens J. H. Heterogeneity in utilization of N -glycosylation sites Asn624 and Asn138 in human lactoferrin: a study with glycosylation-site mutants. Biochem J. 1996;319:117–122. doi: 10.1042/bj3190117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Strate B. W., Beljaars L., Molema G., Harmsen M. C., Meijer D. K. Antiviral activities of lactoferrin. Antiviral Res. 2001;52:225–239. doi: 10.1016/S0166-3542(01)00195-4. [DOI] [PubMed] [Google Scholar]
- Waarts B. L., Aneke O. J., Smit J. M., Kimata K., Bittman R., Meijer D. K., Wilschut J. Antiviral activity of human lactoferrin: inhibition of alphavirus interaction with heparan sulfate. Virology. 2005;333:284–292. doi: 10.1016/j.virol.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Waddington S. N., McVey J. H., Bhella D., Parker A. L., Barker K., Atoda H., Pink R., Buckley S. M., Greig J. A., other authors Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Wally J., Buchanan S. K. A structural comparison of human serum transferrin and human lactoferrin. Biometals. 2007;20:249–262. doi: 10.1007/s10534-006-9062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Huang S., Kapoor-Munshi A., Nemerow G. Adenovirus internalization and infection require dynamin. J Virol. 1998;72:3455–3458. doi: 10.1128/jvi.72.4.3455-3458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Human lactoferrin: a novel therapeutic with broad spectrum potential. J Pharm Pharmacol. 2001;53:1303–1310. doi: 10.1211/0022357011777792. [DOI] [PubMed] [Google Scholar]
- Wickham T. J., Mathias P., Cheresh D. A., Nemerow G. R. Integrins α v β 3 and α v β 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-E. [DOI] [PubMed] [Google Scholar]
- Wu L., Martin T. D., Vazeux R., Unutmaz D., KewalRamani V. N. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J Virol. 2002;76:5905–5914. doi: 10.1128/JVI.76.12.5905-5914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Chiang L., Contreras J., Wu K., Garner J. A., Medina-Kauwe L., Hamm-Alvarez S. F. Novel fiber-dependent entry mechanism for adenovirus serotype 5 in lacrimal acini. J Virol. 2006;80:11833–11851. doi: 10.1128/JVI.00857-06. [DOI] [PMC free article] [PubMed] [Google Scholar]