Abstract
Background:
Certain cancer types and subsequent treatment can cause or worsen pain and emotional distress, leading to functional limitation, particularly among a growing population of older adults with cancer..
Methods:
We constructed a national sample of older adult Medicare beneficiaries with cancer using the 2007– 2012 Surveillance, Epidemiology and End Results (SEER)-Medicare Health Outcomes Survey (MHOS) database linked to Medicare Part D enrollment and prescription claims data. MHOS survey responses described functional limitations due to pain and emotional distress. Using multivariable logistic regression, we estimated the association between participant characteristics and patient-reported functional limitation due to pain and emotional distress and subsequent prescription medication use.
Results:
Among 9,105 older adults with cancer, aged 66–102 years (y), 68.6% reported moderate to severe functional limitation due to pain, and 48.3% reported moderate to severe functional limitation due to emotional distress. Nearly 10% reported severe functional limitation due to co-occurring symptoms of pain and emotional distress. Significant predictors of severe functional limitation due to co-occurring symptoms included age ≥80y (ref: 66–69y, adjusted relative risk (aRR): 1.74; 95% confidence interval (CI) 1.39–2.18, p<.001), stage IV disease at diagnosis (ref: stage I, aRR: 2.08; CI 1.52–2.86, p<.001), and lung cancer (ref: breast cancer, aRR: 1.84; CI 1.30–2.61, p<.001). Among 892 participants reporting co-occurring symptoms, 32.5% received neither pain nor emotional distress prescription medication.
Conclusions:
Functional limitation due to pain and emotional distress persist among older adults with cancer, particularly octogenarians. Efforts to identify and target unmet supportive care needs to maintain functional independence are needed.
Keywords: Functioning, cancer pain, patient reported outcomes, Medicare
Introduction
Loss of functional independence, termed functional limitation, is a major concern among older adults with multiple chronic diseases including cancer.1 Cancer and cancer treatment can lead to functional limitation among older adults with up to 40% of older adults experiencing functional decline after a new cancer diagnosis.2 Functional limitation among older adults with cancer is increasingly important as older adults are the fastest growing demographic in the United States and worldwide.3,4 In addition, by 2030, nearly two-thirds of all cancer diagnoses will be among older adults,5,6 rendering the problem of functional limitation an important area of study in a growing geriatric oncology population.
Certain cancer types and subsequent treatment can cause or worsen pain and emotional distress, leading to functional limitation, which may or may not improve with cancer treatment. Among patients of all ages with cancer, studies have highlighted the high prevalence of pain and emotional distress. A recent meta-analysis found that 38% of patients with cancer report moderate to severe pain, with the number rising to 66.4% for patients with advanced, metastatic, or terminal cancer.7 Moreover, cancer-associated pain is significantly associated with higher levels of emotional distress.8,9 Previous studies using the 36-item Short Form Health Survey (SF-36) to examine patterns of pain and emotional distress among older adults have shown that pain and emotional distress were notable concerns for this population.10 Despite its importance, patterns of functional limitation due to pain or emotional distress and the co-occurrence of pain and emotional distress is underreported in older adults with cancer. Prior studies focused on older adults and the development of functional limitation due to pain but were not cancer-specific. 11,12 There is a lack of data describing the prevalence, disease, and patient-related factors associated with downstream functional limitation related to pain and emotional distress specifically among older adults with cancer. Furthermore, most studies focus on either pain or emotional distress separately rather than the co-occurrence of both pain and emotional distress. The co-occurrence of symptoms is common among adults both with13,14 and without cancer15; and can great affect quality-of-life16 even more so than a single symptom. To better target symptom management, we need to understand the extent to which the co-occurrence of pain and emotional distress limit function among older adults with cancer and to identify the predictors of severe functional limitation.
With ongoing efforts to improve the quality of cancer care, it is also important to examine older adults’ use of prescription medication targeted at the management of pain and emotional distress. A number of studies have reported on receipt of supportive care medications, including opioids, non-opioids, antidepressants, and sleep aids by older adults with cancer.17–24 However, relatively few studies have linked medication use to patient-reported symptoms (i.e. pain)25, and to our knowledge, no published study has linked prescription medications with self-reported functional limitation due to co-occurring symptoms. Patient-reported symptoms and prescription medication claims data are usually analyzed separately. Characterization of patient-reported symptoms with subsequent prescription medication claims data can highlight which older adults are receiving pharmacologic intervention for this understudied and important patient-reported outcome: functional limitation. In addition, determining which characteristics are associated with severe functional limitation could help clinicians determine which older adults are likely to be at risk for severe symptomatology and hence, provide clinicians with the opportunity to intervene.
In this study, we describe both the prevalence and predictors of functional limitations due to pain, due to emotional distress, and co-occurring pain and emotional distress among older adults with cancer. In addition, we use linked Medicare Part D claims data to describe prescription medication use associated with patient-reported functional limitation.
Methods
Data
We used data from the linked Surveillance, Epidemiology and End Results (SEER)-Medicare Health Outcomes Survey (MHOS)26, with a novel linkage to Medicare Part D claims. SEER provides detailed cancer registry data from 19 regions, including cancer type(s), stage, histology, date of diagnosis, and initial treatment modalities (surgery or radiation only). The MHOS includes self-reported information on sociodemographic characteristics, and responses to questions in the Veteran’s RAND 12-item survey27,28, in addition to selected questions related to health status. It is administered annually to a random sample of Medicare beneficiaries enrolled in each Medicare Advantage plan with a minimum of 500 enrollees. The SEER-MHOS represents a linkage for beneficiaries with a SEER-reported cancer who responded to an MHOS survey. Medicare Part D prescription drug claims for the period from 2007 through 2012 were linked for Part D enrolled MHOS respondents in the database. Part D claims provide detailed information on medications prescribed and filled, fill dates, route, strength, quantity, and days supplied for all oral prescription drugs covered by Medicare. Part D claims do not include medications obtained over-the-counter. Part D enrollees are not permitted to use supplemental coverage, patient assistance provided by industry or coupons, hence, these sources should not affect reliability of claims data.
Sample selection and characterization
We selected Medicare beneficiaries in the SEER-MHOS dataset who had a first and only primary cancer, invasive disease (Stages I-IV, with the addition of Stage 0 breast cancer), diagnosed between January 2003 and December 2012 (up to 5 years prior to first MHOS survey). Beneficiaries had to complete at least one MHOS survey between January 2008 and December 2012 within 5 years of their cancer diagnosis. We excluded observations with an unknown month of cancer diagnosis, cancer diagnosis first reported on death certificate or on autopsy, age 65 years or younger at MHOS survey date, and without continuous enrollment in Medicare Parts A, B, & D or Medicare Advantage & Part D during the 12 months pre- and post- the MHOS month or until death.
Participant sociodemographics were measured based on MHOS responses (age, race/ethnicity, sex, marital status, educational attainment) or linked at the zipcode level (percent of the population living in poverty, 4 regions as defined by the U.S. Census Bureau). Chronic health conditions were self-reported on a condition checklist included in the MHOS. Characteristics of the cancer (type, stage at diagnosis, and initial therapy) were reported as part of the SEER registry data. Current receipt of cancer therapy was self-reported on the MHOS for only breast, prostate, lung, and colorectal cancers. We captured initial treatment with radiation or surgery available through SEER; systemic treatment information is not available. Medicare/Medicaid dual enrollment was reported as part of the Medicare enrollment data.
Outcome Measures
The main outcome measures were self-reported activity limitation due to pain (pain interference) or emotional distress (emotional interference). Pain interference was measured based on the bodily pain subscale of the VR-12, based on responses to the MHOS question, “During the past 4 weeks, how much did pain interfere with your normal work (include both work outside the home and housework)?” This question is part of The Veterans RAND 12 Item Health Survey (VR-12)29, a patient-reported global health measure. We grouped the responses into three levels: Little/None (Not at all; a little bit); Moderate (some of the time); and Severe (quite a bit, extremely). Emotional interference was measured using the Role-Emotional subscale29 based on responses to two MHOS questions, “During the past 4 weeks, have you had any of the following problems with your work or other regular daily activities as a result of any emotional problems (such as feeling depressed or anxious): (1) accomplished less than you would like; or .... (2) didn’t do work or other activities as carefully as usual.” For each item, we grouped the responses into three levels: Little/None (none of the time; a little of the time); Moderate (some of the time) and Severe (most of the time, or all of the time). Patients’ functional limitation due to emotional distress was categorized based on a hierarchy: they were classified as having severe functional limitation if they answered either of these emotional distress MHOS questions with the “severe” answer; they were assigned to the moderate category if they answered either question with the “moderate” answer; and they were assigned to the little/none category if they did not meet either of the above criteria. For regression analyses, responses were grouped into two levels of activity limitation for pain and emotional distress, respectively: severe or not severe (moderate/little/none). We also constructed an indicator for respondents who reported severe activity limitation from co-occurring pain and emotional distress dichotomized as “severe” limitation in both pain and emotional distress compared with not “severe” for both types of functional limitation.
We also assessed the receipt of prescription medications for treatment of pain or emotional interference as of the MHOS survey date and the following three months. We categorized pain medications by: opioids, non-steroidal anti-inflammatory drugs (NSAIDs), local anesthetics, antiepileptic and selected tricyclic antidepressants commonly used in pain management. We categorized emotional symptom medications by antidepressants (excluding those already assigned to pain management), anxiolytics/sedatives, and antipsychotics. We identified relevant individual medications for pain and emotional distress based on input from the literature,23,30 clinical guidelines,31 and input from palliative care clinicians on the research team (Table S1). Once we identified the medications, we searched Part D claims for the study sample to identify the relevant generic drug names, including combinations identified by our clinical team. Indicators for individual drugs were combined to construct indicators for any pain or emotional distress medication, by category, and category combinations.
Analysis
We used bivariate analysis with chi-squared statistics to describe the unadjusted associations between type and degree of functional limitation and beneficiary characteristics. We used multivariable logistic regression analysis to test for associations between severe functional limitation and covariates of interest in three distinct models. Our first model evaluated severe functional limitation due to pain, a second model estimated severe limitation due to emotional distress, and a third model estimated severe limitation due to both pain and emotional distress. Covariates of interest included age, stage of cancer, and cancer type. For each model, we included covariates associated with hypothesized associations or that were significant in the bivariate analysis; we assessed the significance of the relative risk and used predicted margins to report on levels of functional limitation adjusted for covariates. As a subset analysis, to determine the effect of current treatment on function, we limited our sample to patients with the four cancer types that included information on receipt of current cancer therapy and added that covariate to the multivariable regression models. Hosmer-Lemeshow32 and C-statistics were used to determine model goodness-of-fit. Lastly, we determined whether any medication was used to manage pain or emotional distress, and the types of medications used by type and degree of functional limitation.
All statistical tests were two-sided with alpha = .05. All analyses were performed using SAS version 9.4 and STATA version 15.
As per federal regulation 45 CFR 46.101(b)(4), the Yale Human Investigation Committee determined that this research was exempt from further review..
Results
We identified 9,105 Medicare beneficiaries with prostate (30.7%), breast (19.2%), colorectal (10.5%), lung (6.4%) or other (33.2%) cancer between 2007 and 2012. The mean age was 75 years (range: 66–102 years); 23.9% of the sample was ≥80 years, 44.4% were female, 24.9% had less than a high school education, and the majority were non-Hispanic white race (Table 1; Additional patient characteristics available in Table S2).
Table 1.
Sample Characteristics*
| Total (N=9105) | N | % |
|---|---|---|
| Age | ||
| 66–69 years (y) | 2323 | 25.5 |
| 70–74 y | 2556 | 28.1 |
| 75–79 y | 2048 | 22.5 |
| 80+ y | 2178 | 23.9 |
| Sex | ||
| Male | 5059 | 55.6 |
| Female | 4046 | 44.4 |
| Race/Ethnicity | ||
| White, non-Hispanic | 6142 | 67.5 |
| Black, non-Hispanic | 911 | 10.0 |
| Hispanic | 878 | 9.6 |
| Asian, Other | 1174 | 12.9 |
| Marital Status | ||
| Married | 5321 | 58.4 |
| Never/formerly married | 3784 | 41.6 |
| Education | ||
| < High school degree | 2266 | 24.9 |
| High school degree | 2791 | 30.7 |
| > High school degree | 4048 | 44.5 |
| Comorbidity | ||
| Coronary Artery Disease | 1331 | 14.6 |
| Stroke | 827 | 9.1 |
| Pulmonary Disease | 1538 | 16.9 |
| Diabetes | 2428 | 26.7 |
| Depression | 1399 | 15.4 |
| Cancer type | ||
| Breast | 1746 | 19.2 |
| Prostate | 2798 | 30.7 |
| Colorectal | 958 | 10.5 |
| Lung | 582 | 6.4 |
| Other** | 3021 | 33.2 |
| Stage at diagnosis*** | ||
| I | 2324 | 25.5 |
| II | 3387 | 37.2 |
| III | 888 | 9.8 |
| IV | 516 | 5.7 |
| Unknown | 1990 | 21.9 |
| Initial therapy | ||
| Surgery | 5693 | 62.5 |
| Radiation | 2923 | 32.1 |
| MHOS survey year | ||
| 2008 | 1771 | 19.5 |
| 2009 | 2338 | 25.7 |
| 2010 | 1882 | 20.7 |
| 2011 | 1680 | 18.5 |
| 2012 | 1434 | 15.8 |
| Diagnosis to survey (months) | ||
| 0–12 | 2205 | 24.2 |
| 13–24 | 1958 | 21.5 |
| 25–36 | 1666 | 18.3 |
| 37–48 | 1587 | 17.4 |
| 49–60 | 1689 | 18.55 |
Datasource: SEER-MHOS
Stage I included in situ carcinoma for breast cancer only
Other cancers included cancers of: oral cavity and pharynx, digestive system excluding colon and rectum, liver and intrahepatic bile duct, respiratory system excluding lung and bronchus, bones and joints, soft tissue, skin excluding basal and squamous, female genital system, male genital system excluding prostate, urinary system, eye and orbit, brain and nervous system, endocrine system, lymphoma, myeloma, leukemia and miscellaneous.
Functional limitation due to pain
Nearly 70% of beneficiaries (68.6%) reported functional limitation due to pain, with 46.0% reporting moderate functional limitation and 22.6% reporting severe functional limitation due to pain. Overall, the highest reported rates of severe limitation due to pain were among individuals ≥80 years of age (28.9%), female (25.9%), with lung cancer (37.1%), stage IV (32.8%) at diagnosis, comorbid depression diagnosis (53.0%) and less than a high school education (33.3%) (P<.01, Table 2).
Table 2:
Unadjusted associations between type and degree of functional limitation and SEER-MHOS participant characteristicsa
| Pain N=6244 | Emotional Distress N=4396 | Severe Pain and Emotional Distress | ||||
|---|---|---|---|---|---|---|
| Severe n=2054 | Moderate n=4190 | Severe n=1339 | Moderate n=3057 | n=893 | ||
| Row % | Row % | Row % | Row % | Row % | ||
| Total Sample | ||||||
| N=9105 | 22.6 | 46.0 | 14.7 | 33.6 | 9.8 | |
| Age category | ||||||
| 66–69 | 20.2 | 44.8 | 11.7 | 30.1 | 8.0 | |
| 70–74 | 19.5 | 47.1 | 13.3 | 32.2 | 8.9 | |
| 75–79 | 22.4 | 46.3 | 13.7 | 34.9 | 8.8 | |
| 80+ | 28.9 | 45.7 | 20.4 | 37.7 | 13.7 | |
| Sex | ||||||
| Male | 19.9 | 46.1 | 13.9 | 31.8 | 9.1 | |
| Female | 25.9 | 46.0 | 15.7 | 35.7 | 10.7 | |
| Race/Ethnicity | ||||||
| White, non-Hispanic | 20.6 | 46.7 | 12.4 | 32.1 | 8.3 | |
| Black, non-Hispanic | 29.9 | 41.6 | 18.3 | 36.7 | 12.6 | |
| Hispanic | 28.3 | 45.0 | 22.4 | 36.5 | 14.9 | |
| Asian, non-Hispanic, | ||||||
| Other | 22.7 | 46.8 | 18.1 | 36.8 | 11.8 | |
| Marital Status | ||||||
| Currently married | 19.5 | 47.1 | 12.3 | 31.7 | 8.3 | |
| Never/formerly married | 26.9 | 44.6 | 18.1 | 36.2 | 12.0 | |
| Education | ||||||
| < High school degree | 33.3 | 43.7 | 25.1 | 35.8 | 17.4 | |
| High school degree | 22.7 | 46.9 | 13.8 | 34.6 | 9.4 | |
| > High school degree | 16.5 | 46.7 | 9.6 | 31.6 | 5.8 | |
| Comorbidity | ||||||
| Coronary Artery | ||||||
| Disease | 35.5 | 45.8 | 23.4 | 36.9 | 18.1 | |
| Stroke | 37.9 | 40.5 | 28.8 | 35.3 | 20.6 | |
| Pulmonary Disease | 36.1 | 44.2 | 25.1 | 35.2 | 18.3 | |
| Diabetes | 28.7 | 47.7 | 20.0 | 35.2 | 14.0 | |
| Depression | 53.0 | 37.3 | 49.8 | 40.4 | 37.3 | |
| Poverty rates | ||||||
| Low (<5%) | 17.5 | 46.7 | 10.2 | 30.7 | 6.5 | |
| 5% to <10% | 19.7 | 47.6 | 11.9 | 33.7 | 8.0 | |
| 10% to <20% | 24.5 | 45.4 | 17.1 | 34.5 | 11.5 | |
| High (20% +) | 30.3 | 43.9 | 20.9 | 35.8 | 14.3 | |
| Cancer type | ||||||
| Breast | 23.5 | 47.4 | 13.2 | 35.2 | 8.9 | |
| Prostate | 17.2 | 46.0 | 10.9 | 29.8 | 7.0 | |
| Colorectal | 19.9 | 47.1 | 14.9 | 36.6 | 9.6 | |
| Lung | 37.1 | 40.6 | 27.2 | 34.2 | 20.8 | |
| Other | 25.0 | 46.0 | 16.6 | 35.0 | 10.9 | |
| Stage at diagnosis | ||||||
| I | 22.0 | 47.3 | 14.1 | 34.2 | 9.6 | |
| II | 19.3 | 46.4 | 11.6 | 32.3 | 7.4 | |
| III | 24.3 | 46.1 | 18.5 | 30.9 | 13.0 | |
| IV | 32.8 | 39.2 | 25.2 | 35.3 | 18.2 | |
| Unknown | 25.4 | 45.7 | 16.3 | 35.9 | 10.7 | |
| Initial therapy | ||||||
| Surgery | 21.3 | 47.1 | 13.3 | 33.6 | 9.0 | |
| Radiation | 20.7 | 46.5 | 13.1 | 33.3 | 8.7 | |
| Current treatmentb | ||||||
| Breast Cancer | 25.8 | 48.6 | 14.8 | 37.2 | 10.0 | |
| Prostate Cancer | 18.4 | 48.2 | 12.3 | 32.8 | 7.8 | |
| Colorectal Cancer | 22.1 | 49.0 | 18.0 | 42.6 | 12.1 | |
| Lung Cancer | 41.8 | 42.1 | 31.9 | 34.6 | 24.9 | |
| Region | ||||||
| Northeast | 20.2 | 45.1 | 11.8 | 36.1 | 8.0 | |
| South | 19.1 | 49.3 | 10.7 | 31.8 | 5.9 | |
| Central | 25.9 | 44.8 | 17.9 | 32.1 | 12.7 | |
| West | 22.3 | 46.2 | 14.8 | 33.9 | 9.7 | |
| Medicaid dual enrolled | ||||||
| No | 20.1 | 46.3 | 12.6 | 32.4 | 8.3 | |
| Yes | 36.5 | 44.6 | 26.5 | 39.9 | 18.1 | |
| MHOS survey year | ||||||
| 2008 | 22.8 | 46.0 | 14.9 | 36.4 | 9.3 | |
| 2009 | 21.7 | 45.6 | 13.8 | 32.8 | 9.0 | |
| 2010 | 22.5 | 48.2 | 15.4 | 35.3 | 10.4 | |
| 2011 | 22.8 | 45.2 | 15.2 | 31.0 | 10.9 | |
| 2012 | 23.5 | 44.8 | 14.5 | 32.2 | 9.6 | |
| Diagnosis to survey (months) | ||||||
| 0–12 | 24.9 | 44.9 | 16.1 | 35.3 | 11.1 | 24.9 |
| 13–24 | 22.8 | 45.9 | 14.5 | 33.8 | 9.8 | 22.8 |
| 25–36 | 20.2 | 47.3 | 14.5 | 32.2 | 9.1 | 20.2 |
| 37–48 | 22.9 | 45.5 | 13.7 | 33.2 | 9.5 | 22.9 |
| 49–60 | 21.1 | 47.0 | 14.3 | 32.9 | 9.2 | 21.1 |
bold values indicate bivariate analysis between each column and covariate Χ2 p<.01 gray values indicate bivariate analysis between each column and covariate Χ2 p<.05
percentages were taken from total N for each cancer type. Treatment with surgery or radiation from SEER data
Functional limitation due to emotional distress
Almost half of the sample (48.3%) reported functional limitation due to emotional distress, with 33.6% of those cases reporting moderate functional limitation and 14.7% reporting severe functional limitation. The highest rates of severe limitation due to emotional distress were among adults ≥80 years of age (20.4%), with lung cancer (27.2%), stage IV at diagnosis (25.2%), depression (49.8%), and less than a high school education (25.1%) (unadjusted, P<.01, Table 2).
Functional limitation due to co-occurrence of pain and emotional distress
Nearly 10% of the sample reported severe limitation due to both pain and emotional distress. The characteristics significantly associated with patient-reported functional limitation due to severe pain co-occurring with severe emotional distress were most similar to those associated with emotional distress only (≥80 years of age, lung cancer, stage IV at diagnosis, depression, and less than a high school education; unadjusted, P<.01, Table 2).
Factors associated with functional limitation
In multivariable regression models, significant predictors of severe functional limitation due to co-occurring symptoms included age ≥80y (ref: 66–69y, adjusted relative risk (aRR): 1.74; 95% confidence interval (CI) 1.39–2.18, p<.001), stage IV disease at diagnosis (ref: stage I, aRR: 2.08; CI 1.52–2.86, p<.001), and lung cancer (ref: breast cancer, aRR: 1.84; CI 1.30–2.61, p<.001; Figure 1). Other significant predictors include Hispanic ethnicity, less than a high school education, Medicare/Medicaid dual enrollment, initial treatment with surgery, unmarried status, and central or western region of the United States (aRR in Table S3). Significant predictors of severe functional limitation due to either pain or emotional distress modeled separately include age ≥80 years (y), lung cancer type, stage IV at diagnosis, initial therapy with surgery, pulmonary disease, stroke, less than a high school education, and Medicare/Medicaid dual enrollment (Figures S1 &S2, aRR reported in Table S3). Self-reported depression (aRR: 4.1, 95% CI 3.5–4.8; Table S4) was significantly associated with severe functional limitation due to pain and emotional distress, while female sex, marital status, and Hispanic ethnicity was a significant predictor of severe functional limitation due to emotional distress (aRR: 1.4, 95% CI 1.1–1.8; Table S4). Model C-statistics suggested good predictive ability (0.76–0.79).
Figure 1.
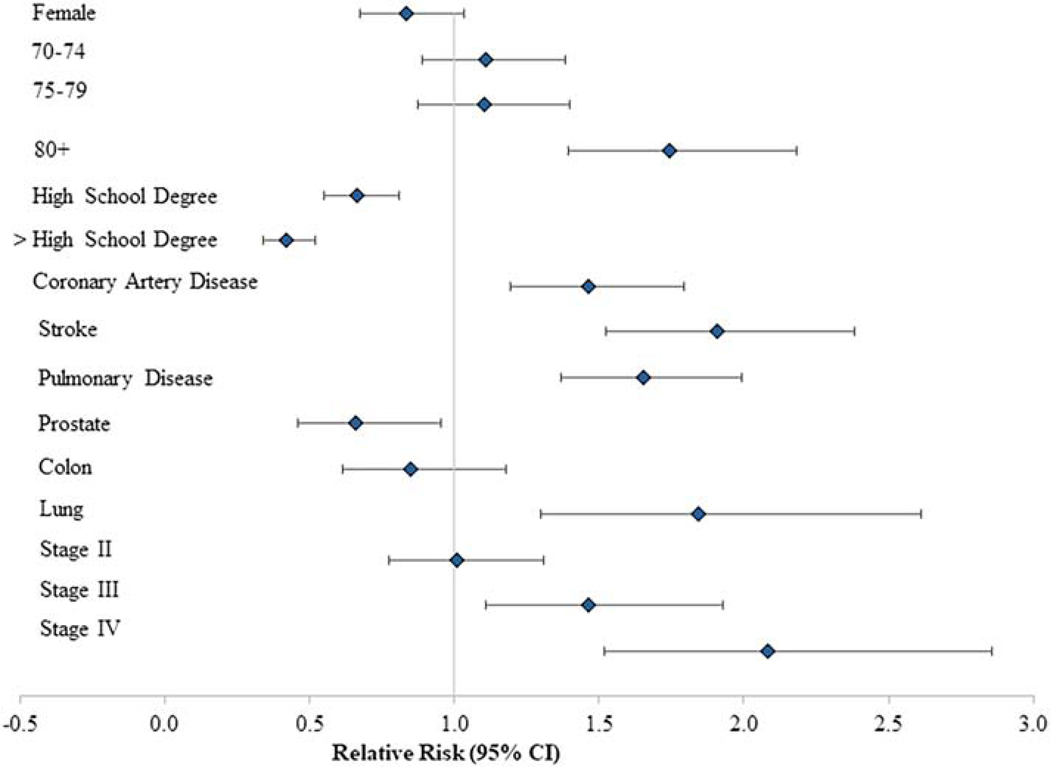
Adjusted association of covariates with both pain and emotional distress: Severe pain and emotional distress vs. no severe pain and emotional distress (moderate + none). Models controlled for all variables described under Methods. Reference categories for reported characteristics were age 66–69 years, no High School diploma, breast cancer, Stage I disease at diagnosis.
Prescription medication use
Pain:
Overall, among 6,244 individuals reporting moderate to severe functional limitation due to pain, 38.1% received at least one prescription for pain medication. Among individuals reporting severe functional limitation, 55.2% received at least one prescription pain medication, compared to 29.7% of individuals with moderate functional limitation. The majority of individuals reporting severe functional limitation due to pain were prescribed an opioid or NSAID (Figure 2).
Figure 2:
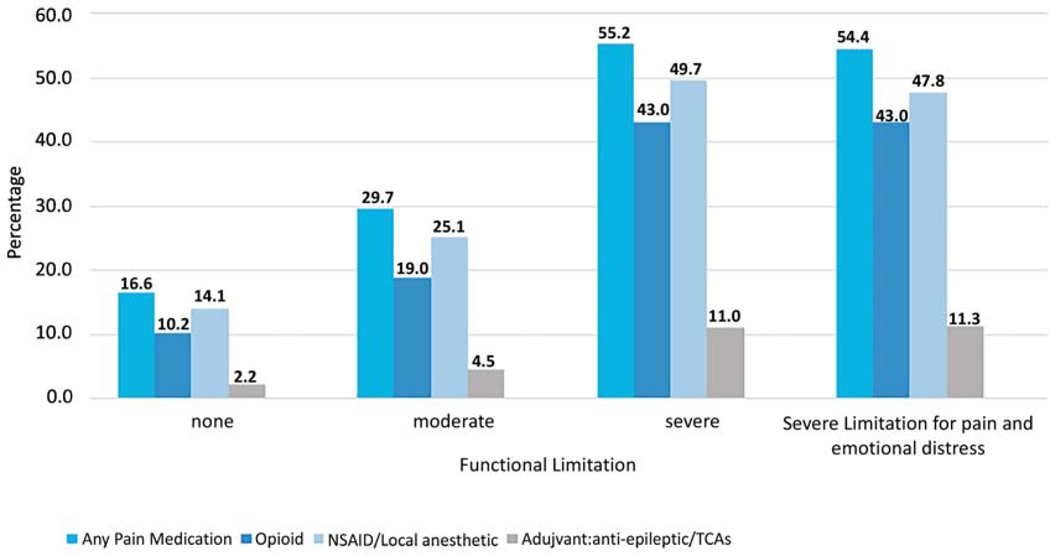
Receipt of pain medication for self-reported functional limitation among SEER-MHOS respondents with cancer. All P-values are <0.01 across functional limitation categories for receipt of any pain medication and each type of pain medication. Note: Participants could be taking more than one medication class. Abbreviations: NSAID: non-steroidal anti-inflammatory drug; TCA: tricyclic antidepressant.
Emotional distress:
In contrast, only 26.4% of the 4,396 individuals reporting moderate to severe functional limitation due to emotional distress, received any emotional distress prescription medication. The most commonly prescribed medication types for individuals reporting severe functional limitation were antidepressants (26.2%) followed by anxiolytics/sedatives (14.3%) and antipsychotics (3.7%) (Figure 3).
Figure 3:
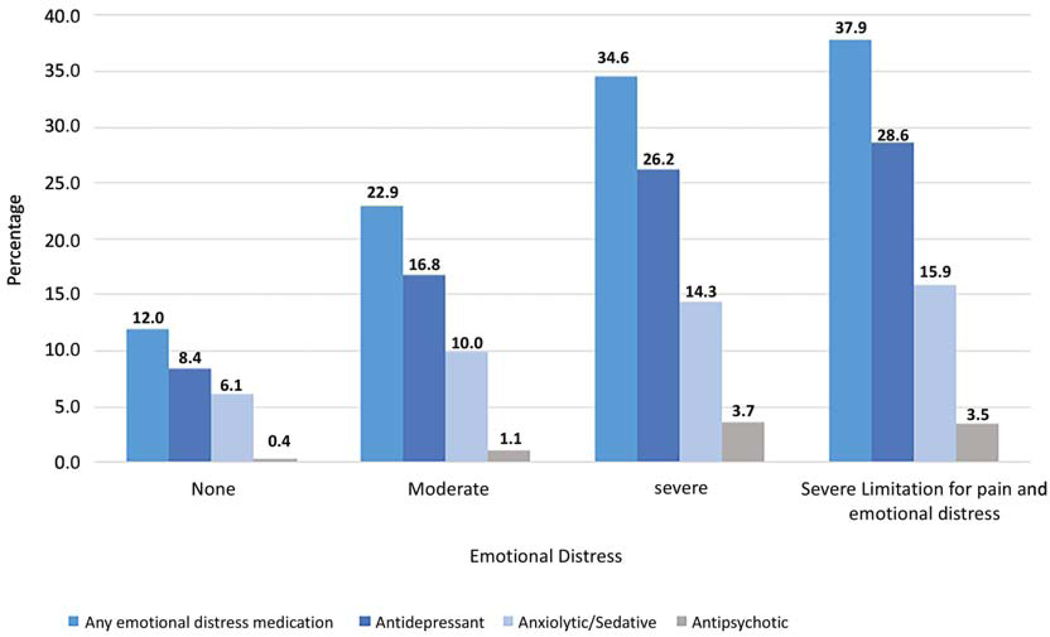
Receipt of emotional distress medication for self-report functional limitation among SEER-MHOS respondents with cancer. All P-values are <0.01 across functional limitation categories for receipt of any emotional distress medication and each type of emotional distress medication. Note: Participants could be taking more than one medication class.
Co-occurring symptoms:
Among the 893 participants who reported co-occurring severe pain and emotional distress, 13% received only emotional distress medication, 29.6% received only pain medication, 24.9 % received medication for both symptoms, and 32.5% received no medication for either symptom.
Discussion
Pain is a highly reported health problem for older adults and continues to increase in prevalence and severity as compared to younger adults.12,33 Using a novel linkage of SEER-MHOS data to Part D Medicare prescription medication claims, the present study found that nearly 70% of Medicare beneficiaries with cancer aged 66–102 years reported moderate to severe functional limitation due to pain, while 50% reported moderate to severe functional limitations due to emotional distress. These findings are consistent with prior work linking pain with functional limitation and disability among older adults.11,33,34 Importantly, this study focused on older adults with cancer and identified characteristics significantly associated with severe functional limitation due to pain or emotional distress, including age ≥80 years, stage IV disease at diagnosis, and lung cancer. Many patients, despite reporting severe functional limitation, did not receive any prescription medication for reported pain or emotional distress. This may be a result of lack of Medicare cover for benzodiazepines, a medication class often used for emotional distress, until 2013.
This study documents significant functional limitation among cancer patients aged 80 years and older, due to the symptoms of pain and emotional distress. Octogenarians are the fastest growing age demographic worldwide, but are underrepresented in cancer clinical trials and understudied in cancer outcomes research.35 Notably, this disconnect creates a large evidence gap in how best to diagnose and intervene upon functional limitations and related disabling symptoms such as pain and emotional distress. Monitoring functional status as a key patient-reported outcome could inform future interventions on how to prevent functional decline and improve resiliency, particularly among cancer patients aged 80 years and older. Given previous research highlighting that only one-third of patients with cancer would choose to undergo a treatment regimen if it resulted in functional limitation,1 functional status is a particularly important outcome to consider when caring for these patients.
In addition to age, lung cancer and stage IV disease were also significant predictors of functional limitation, likely due to the presence of disabling symptoms. Lung cancer is commonly diagnosed at an advanced stage with metastasis to other organs or fluid accumulation in the lungs, causing symptoms such as pain or emotional distress due to shortness of breath.36 The early integration of palliative care specifically in advanced lung cancer can result in improved quality-of-life, fewer depressive symptoms, and improved overall survival.37 Given the overall large societal burden of lung cancer as the leading cause of cancer-related mortality in the United States,38 additional research targeting functional status evaluation39,40 and supportive care during treatment such as early palliative care integration, specifically designed for older adults with lung cancer, could improve symptom-related disability.
Only half of older adults reporting severe functional limitation due to pain in this study received a prescription pain medication and only one third reporting severe functional limitation due to emotional distress received a prescription medication to target these symptoms. The stigma associated with mental health issues and the mandatory reporting of pain as the “5th vital sign” may explain the differences in prescribing patterns found in this study.41 In addition, depression was significantly associated with severe functional limitation due to pain, highlighting the need for intensified co-management of emotional distress with physical symptoms such as pain. Efforts to improve both evaluation i.e. geriatric assessement39 and treatment of functional limitation due to disabling symptoms should be a priority for older adults with cancer.
Limitations
This study has limitations typically associated with use of survey and administrative claims . While the SEER-MHOS is population-based, it is limited to Medicare beneficiaries enrolled in Medicare Advantage plans. Their experience with symptom assessment and treatment may differ from that of beneficiaries enrolled in the traditional Medicare benefit. However, over 20% of the sample comprised octogenarians with a cancer diagnosis (over 2,000 participants), which is comparable to other large cancer cohort studies.42 The study bases symptom report on the VR-12 which is incorporated into the MHOS. The VR-12 patient-reported outcome measures are generic, and may not distinguish aspects of symptom burden specific to cancer such as neuropathic pain from chemotherapy agents versus nociceptive pain. In addition, it doesn’t capture side effects of symptom management medications such as falls with benzodiazepines or constipation with opioids. Further, they are a snapshot in time, and do not capture information on symptom burden that may have preceded the cancer diagnosis or treatment. Our analysis included self-report of chronic conditions that may have been alternate contributors to pain or emotional distress. The study used Part D claims data to identify prescription medications used for management of pain and emotional distress. We acknowledge that some medications may be used for both pain and emotional distress i.e. duloxetine; our clinical team assigned drugs to the symptom category where the drug was most commonly prescribed. We note that Part D claims reflect prescriptions that are filled, but do not capture medications recommended but not prescribed, or prescribed but not filled. Further, claims do not indicate whether medications were ingested as prescribed. Part D claims do not capture medications filled over-the counter or excluded from coverage, as in the case of benzodiazepines during the observation period. We also note that our analysis only includes pharmacologic symptom management, and only for oral prescription medications . Model fit could be stronger, but since we were hypothesis testing, we did not want to include interactions or other modifications that may improve fit but obscure interpretation. Lastly, this study was only able to document cancer-directed treatment among four major cancer types (subset analysis). Regardless of the source or onset of symptoms, clinicians need to be cognizant of unmanaged symptoms, as they interfere with cancer treatment, functional status, and quality of life.
Conclusion
Despite an increased focus on symptom management in recent years, functional limitation due to pain and emotional distress remain major issues among older adults with cancer, particularly those individuals 80 years and older. Over one-third of older adults reporting severe functional limitation did not receive any prescription medications for pain or emotional distress. Efforts to identify and target unmet supportive care needs to maintain functional independence in this population are greatly needed.
Supplementary Material
Acknowledgments
Presented at ASCO Annual Meeting Chicago, IL 2018
Work was funded in part by Contract# HHSN261201700690P.
Conflict of Interest:
Carolyn J. Presley: Dr. Presley reports grants from the Ohio State University (K12 CA133250), the Yale Lung Spore Career Development Award (P50CA196530), and the Robert Wood Johnson Veterans Affairs Clinical Scholar Program during the conduct of the study. She also serves on the clinical advisory board for Potentia Metrics outside the submitted work
Maureen Canavan: Nothing to disclose.
Shi-Yi Wang: Dr. Wang receives funding from Genentech for his research.
Shelli L. Feder: Nothing to disclose.
Jennifer Kapo: Nothing to disclose.
Maureen L. Saphire: Dr. Saphire was formerly employed by Janssen Pharmaceuticals.
Ella Sheinfeld: Nothing to disclose.
Erin E. Kent: Nothing to disclose.
Amy J. Davidoff: Dr. Davidoff’s institution receives funding for her research from Celgene, and she has a family member who receives advisory board and consulting income from Celgene, Abbvie, Jazz Pharmaceuticals, Daiichi Sankyo, and Kyowa Hakko Kirin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fried TR, Bradley EH, Towle VR, et al. : Understanding the Treatment Preferences of Seriously Ill Patients. New England Journal of Medicine 346:1061–1066, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Presley CJ, Han L, Leo-Summers L, et al. : Functional trajectories before and after a new cancer diagnosis among community-dwelling older adults. Journal of Geriatric Oncology, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith BD, Smith GL, Hurria A, et al. : Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 27:2758–65, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Mitka M: Iom report: Aging us population, rising costs, and complexity of cases add up to crisis in cancer care. JAMA 310:1549–1550, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Smith BD, Smith GL, Hurria A, et al. : Future of Cancer Incidence in the United States: Burdens Upon an Aging, Changing Nation. Journal of Clinical Oncology 27:2758–2765, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Hurria A, Naylor M, Cohen H: improving the quality of cancer care in an aging population: Recommendations from an iom report. JAMA 310:1795–1796, 2013 [DOI] [PubMed] [Google Scholar]
- 7.van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, et al. : Update on Prevalence of Pain in Patients With Cancer: Systematic Review and Meta-Analysis. J Pain Symptom Manage 51:1070–1090 e9, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Spiegel D, Sands S, Koopman C: Pain and depression in patients with cancer. Cancer 74:2570–2578, 1994 [DOI] [PubMed] [Google Scholar]
- 9.O’Connor M, Weir J, Butcher I, et al. : Pain in patients attending a specialist cancer service: prevalence and association with emotional distress. J Pain Symptom Manage 43:29–38, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Kent EE, Ambs A, Mitchell SA, et al. : Health-related quality of life in older adult survivors of selected cancers: data from the SEER-MHOS linkage. Cancer 121:758–65, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covinsky KE, Lindquist K, Dunlop DD, et al. : Pain, functional limitations, and aging. Journal of the American Geriatrics Society 57:1556–1561, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmer Z, Zajacova A: Persistent, Consistent, and Extensive: The Trend of Increasing Pain Prevalence in Older Americans. The Journals of Gerontology: Series B, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Cheng KK, Lee DT: Effects of pain, fatigue, insomnia, and mood disturbance on functional status and quality of life of elderly patients with cancer. Crit Rev Oncol Hematol 78:127–37, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Lin S, Chen Y, Yang L, et al. : Pain, fatigue, disturbed sleep and distress comprised a symptom cluster that related to quality of life and functional status of lung cancer surgery patients. J Clin Nurs 22:1281–90, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Loyland B: The co-occurrence of chronic pain and psychological distress and its associations with salient socio-demographic characteristics among long-term social assistance recipients in Norway. Scand J Pain 11:65–72, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Roiland RA, Heidrich SM: Symptom clusters and quality of life in older adult breast cancer survivors. Oncol Nurs Forum 38:672–80, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Maio M, Perrone F, Gallo C, et al. : Supportive care in patients with advanced non-small-cell lung cancer. Br J Cancer 89:1013–21, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maggiore RJ, Gross CP, Togawa K, et al. : Use of complementary medications among older adults with cancer. Cancer 118:4815–23, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbera L, Seow H, Husain A, et al. : Opioid prescription after pain assessment: a population-based cohort of elderly patients with cancer. J Clin Oncol 30:1095–9, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Syrowatka A, Chang S-L, Tamblyn R, et al. : Psychotropic and Opioid Medication Use in Older Patients With Breast Cancer Across the Care Trajectory: A Population-Based Cohort Study. Journal of the National Comprehensive Cancer Network 14:1412–1416, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Zuckerman IH, Davidoff AJ, Erten MZ, et al. : Use of and spending on supportive care medications among Medicare beneficiaries with cancer. Support Care Cancer 22:2185–95, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Ashbury FD, Madlensky L, Raich P, et al. : Antidepressant prescribing in community cancer care. Support Care Cancer 11:278–85, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Check DK, Rosenstein DL, Dusetzina SB: Early supportive medication use and end-of-life care among Medicare beneficiaries with advanced breast cancer. Support Care Cancer 24:3463–72, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Check DK, Samuel CA, Rosenstein DL, et al. : Investigation of Racial Disparities in Early Supportive Medication Use and End-of-Life Care Among Medicare Beneficiaries With Stage IV Breast Cancer. J Clin Oncol 34:2265–70, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidoff AJ, Canavan ME, Feder S, et al. : Patterns of pain medication use associated with reported pain interference in older adults with and without cancer. Support Care Cancer, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kent EE, Malinoff R, Rozjabek HM, et al. : Revisiting the Surveillance Epidemiology and End Results Cancer Registry and Medicare Health Outcomes Survey (SEER-MHOS) Linked Data Resource for Patient-Reported Outcomes Research in Older Adults with Cancer. J Am Geriatr Soc 64:186–92, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Selim AJ, Rogers W, Fleishman JA, et al. : Updated U.S. population standard for the Veterans RAND 12-item Health Survey (VR-12). Qual Life Res 18:43–52, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Usman Iqbal S, Rogers W, Selim A, et al. : The Veterans Rand 12 Item Health Survey (VR-12): What it is and how it is used, Technical report., 2016 [Google Scholar]
- 29.Kazis L E, Selim A, Rogers W, et al. : Veterans RAND 12 Item Health Survey (VR12): A White Paper Summary, 2019 [Google Scholar]
- 30.Barbera L, Sutradhar R, Chu A, et al. : Opioid Prescribing Among Cancer and Non-cancer Patients: Time Trend Analysis in the Elderly Using Administrative Data. J Pain Symptom Manage 54:484–492 e1, 2017 [DOI] [PubMed] [Google Scholar]
- 31.Denlinger CS, Ligibel JA, Are M, et al. : Survivorship: pain version 1.2014. J Natl Compr Canc Netw 12:488–500, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosmer DW, Hosmer T, Le Cessie S, et al. : A comparison of goodness‐of‐fit tests for the logistic regression model. Statistics in medicine 16:965–980, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Leveille SG, Fried L, Guralnik JM: Disabling symptoms: what do older women report? J Gen Intern Med 17:766–73, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mercadante S, Casuccio A, Fulfaro F: The course of symptom frequency and intensity in advanced cancer patients followed at home. J Pain Symptom Manage 20:104–12, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Hesketh PJ, Lilenbaum RC, Chansky K, et al. : Chemotherapy in patients >= 80 with advanced non-small cell lung cancer: Combined results from SWOG 0027 and LUN 6. Journal of Thoracic Oncology 2:494–498, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Collins LG, Haines C, Perkel R, et al. : Lung cancer: Diagnosis and management. American Family Physician 75:56–63, 2007 [PubMed] [Google Scholar]
- 37.Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363:733–42, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2019. CA: A Cancer Journal for Clinicians [DOI] [PubMed] [Google Scholar]
- 39.Decoster L, Kenis C, Schallier D, et al. : Geriatric Assessment and Functional Decline in Older Patients with Lung Cancer. Lung 195:619–626, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Kenis C, Decoster L, Bastin J, et al. : Functional decline in older patients with cancer receiving chemotherapy: A multicenter prospective study. J Geriatr Oncol 8:196–205, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Mularski RA, White-Chu F, Overbay D, et al. : Measuring pain as the 5th vital sign does not improve quality of pain management. J Gen Intern Med 21:607–12, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paskett ED, Caan BJ, Johnson L, et al. : The Women’s Health Initiative (WHI) Life and Longevity After Cancer (LILAC) Study: Description and Baseline Characteristics of Participants. Cancer Epidemiology Biomarkers & Prevention 27:125–137, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


