Presentation of Case
Dr. Aditya Bardia: A 62-year-old woman was evaluated at this hospital after she had identified a mass in her left breast, confirmed by her physician on physical examination, during the pandemic of coronavirus disease 2019 (Covid-19), the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The patient, who was of Ashkenazi Jewish ancestry, had no known family history of breast or ovarian cancer. Medical history included asthma and a fibroadenoma in the left breast, for which she had undergone excisional biopsy 30 years earlier. Menarche had occurred at 12 years of age and menopause at 54 years of age; she had not received hormone-replacement therapy.
Physical examination revealed a mass, measuring 3 cm in greatest dimension, in the left breast. No other masses or axillary lymph nodes were palpable. The patient underwent imaging studies in accordance with the American College of Radiology guidelines.1 Both breasts were imaged, since the patient’s last mammogram had been obtained 7 years earlier.
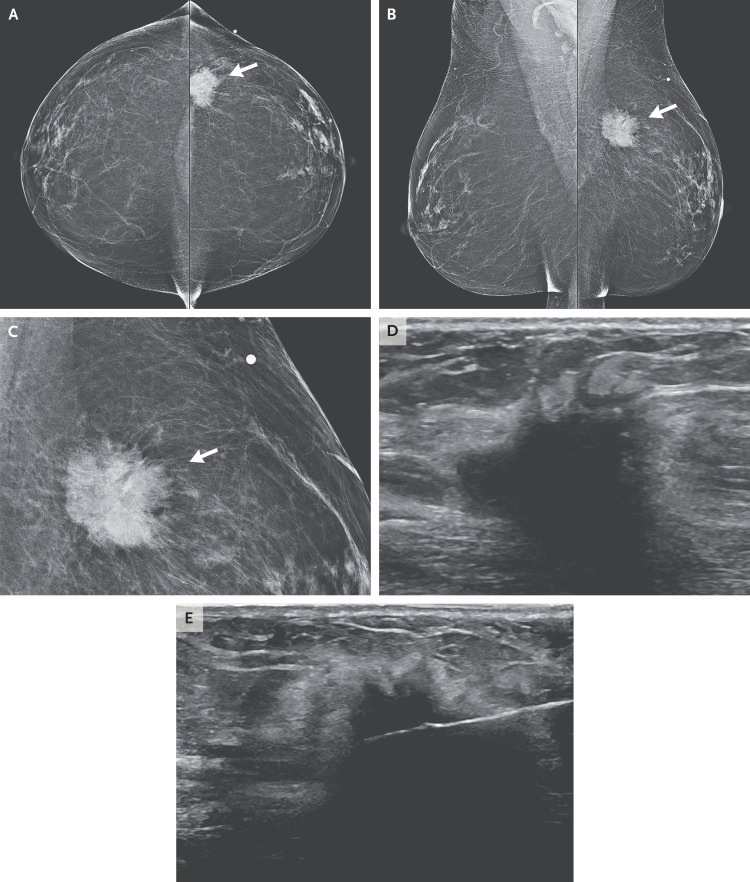
Dr. Gary X. Wang: Mammography revealed an irregular mass with spiculated margins underlying the skin marker in the left breast, with imaging characteristics highly suggestive of cancer (Figure 1A, 1B, and 1C).2 Subsequent ultrasound examination revealed a solid, irregular mass in the left breast that measured 3.1 cm by 1.5 cm by 1.2 cm (Figure 1D) and normal left axillary lymph nodes. Tissue sampling with core-needle biopsy under ultrasonographic guidance was performed (Figure 1E).
Figure 1. Imaging Studies of the Breast.
Bilateral mammograms obtained from the craniocaudal and mediolateral oblique views (Panels A and B, respectively) show a mass in the left breast underlying the skin marker (arrows). At higher magnification (Panel C), the mass appears irregular and spiculated (arrow). An ultrasound image (Panel D) shows a solid, irregular mass, measuring 3.1 cm by 1.5 cm by 1.2 cm. An image obtained during core-needle biopsy under ultrasonographic guidance (Panel E) shows the needle positioned within the mass.
Pathological Discussion
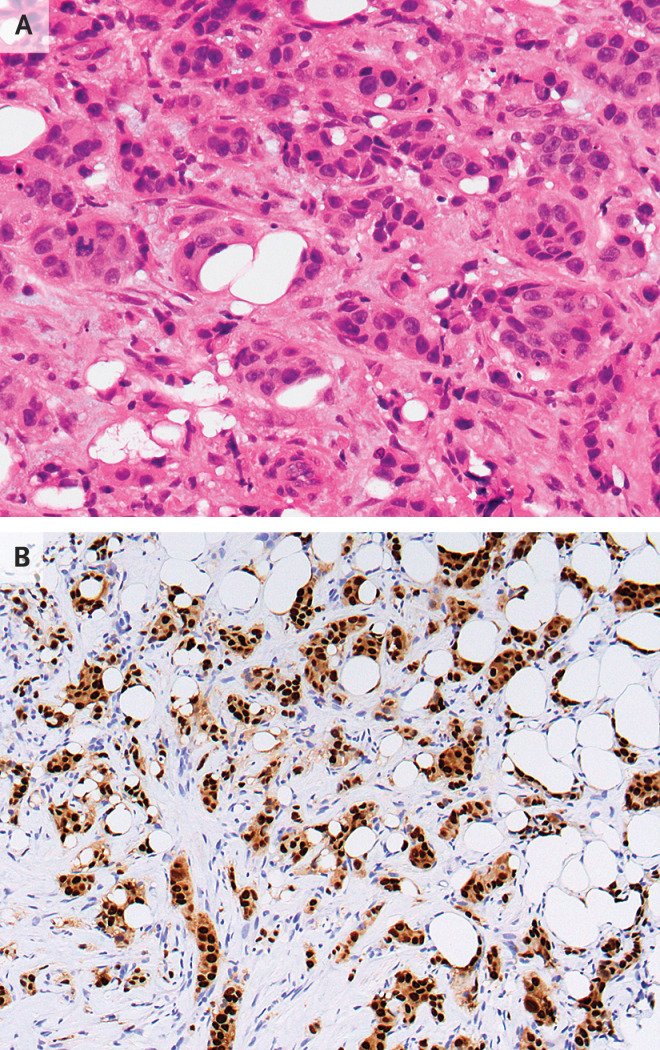
Dr. Amy Ly: Histologic evaluation of the biopsy specimen revealed invasive ductal carcinoma, grade 2, spanning at least 1.6 cm in greatest dimension. No definitive lymphovascular invasion or carcinoma in situ was identified (Figure 2A). Immunohistochemical staining showed tumor cells that were strongly and diffusely positive for estrogen receptor (ER) and progesterone receptor (PR) (Figure 2B). Human epidermal growth factor receptor 2 (HER2) overexpression was equivocal on immunohistochemical staining. Subsequent fluorescence in situ hybridization for HER2 did not reveal amplification.
Figure 2. Core-Needle Biopsy Specimens of the Breast.
Hematoxylin and eosin staining of a tissue core (Panel A) shows invasive ductal carcinoma. Immunohistochemical staining (Panel B) shows invasive carcinoma cells that are strongly and diffusely positive for estrogen receptor and progesterone receptor.
The patient was referred to a breast surgeon for further evaluation. To carefully consider various therapeutic options, the breast surgeon saw the patient in a multidisciplinary clinic that included consultants from the radiation oncology and medical oncology services.
Discussion of Management
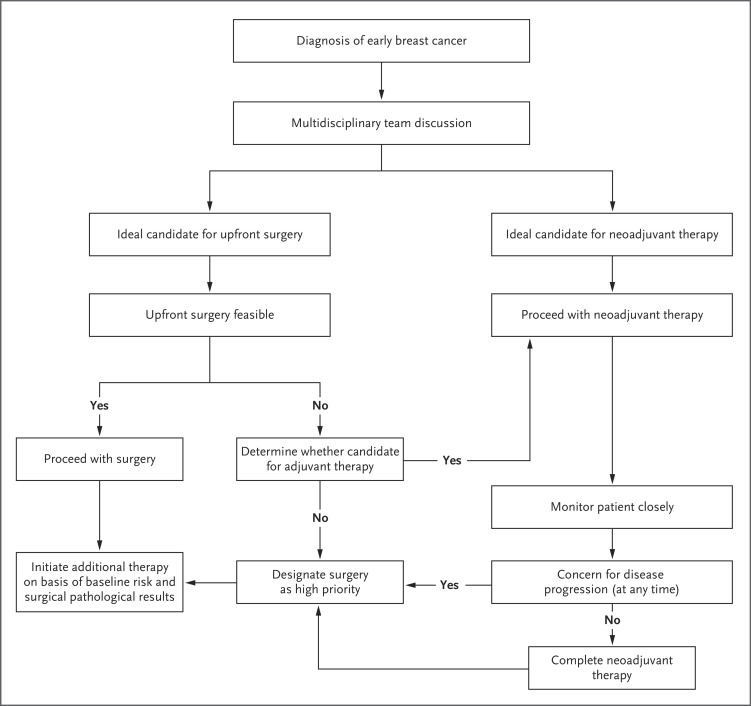
Dr. Bardia: The Covid-19 pandemic poses a major challenge to the health care system, and several organizations have released consensus recommendations for management of breast cancer during this unprecedented situation.3-5 Major differences in management before and during the pandemic are outlined in Table 1, and key principles are reviewed here. First, during this pandemic, although the choice and sequence of method of treatment may be affected, the overall goal of management remains cure. Second, the benefit of treatment needs to be carefully weighed against the known risks associated with treatment and against the potential risk of transmission of SARS-CoV-2 to patients and health care providers. Third, the treatment regimen may need to be modified on the basis of the individual clinical and pathological scenario. Finally, it is important to organize a multidisciplinary plan of alternative options that can be implemented in a resource-constrained environment (Figure 3).
Table 1. General Management of Select Early Breast Cancer Scenarios before and during the Covid-19 Pandemic.*.
| Clinical Scenario | Typical Management, before Covid-19 Pandemic | Modified Management, during Covid-19 Pandemic† |
|---|---|---|
| Newly diagnosed postmenopausal early HR-positive, HER2-negative breast cancer |
|
|
| Newly diagnosed premenopausal early HR-positive, HER2-negative breast cancer |
|
|
| Newly diagnosed localized HER2-amplified breast cancer |
|
|
| Newly diagnosed localized triple-negative breast cancer |
|
|
Of note, these are broad treatment principles, and there could be exceptions. Ultimately, management of breast cancer needs to be individualized. HER2 denotes human epidermal growth factor receptor 2, and HR hormone receptor.
If chemotherapy is deemed absolutely necessary during the Covid-19 pandemic, it is important to consider alterations to chemotherapy regimens, including minimizing glucocorticoid use, to decrease the extent of myelosuppression. Once-weekly paclitaxel could be substituted for paclitaxel given every 2 weeks, with the trade-off of the need for a greater number of visits. Alternatively, docetaxel given every 3 weeks can be used, with growth factor support. For HER2-positive tumors with homogeneous HER2 expression, trastuzumab emtansine (with or without pertuzumab) could be considered instead of chemotherapy, particularly for smaller tumors. For stage I triple-negative breast cancer, docetaxel plus cyclophosphamide may be considered, although for patients for whom chemotherapy is not otherwise recommended, surgery should remain a high priority.
Figure 3. Multidisciplinary Approach for Management of Early Breast Cancer during the Covid-19 Pandemic.
Ideally, the multidisciplinary team would include a surgical oncologist, a radiation oncologist, a medical oncologist, a genetics expert (if needed), a social worker (if needed), and a person with expertise in symptom management, palliative medicine, or both (if needed). If a patient does not undergo upfront surgery, the chemotherapy regimen that would have been selected for adjuvant therapy can be administered as neoadjuvant therapy to allow for deferred surgery.
Management of Breast Cancer before the Covid-19 Pandemic
Dr. Michelle C. Specht: The new diagnosis of breast cancer in this patient gives us an opportunity to reflect on the ways in which patients were typically cared for before the widespread onset of Covid-19. We can then consider appropriate management strategies that can be implemented during the pandemic.
Upfront Surgical Options
Before the emergence of Covid-19, a patient with clinical stage T2N0 (according to the tumor–node–metastasis classification system), hormone receptor (HR)–positive, HER2-negative breast cancer, such as this patient, would be a candidate for upfront surgery. Surgical options would include mastectomy or lumpectomy with radiation; these approaches are associated with equivalent disease-free survival.6 Given the size of this patient’s breast, the tumor would be resectable; therefore, most surgeons would recommend lumpectomy to preserve body image and sexual sensation.7,8 Sentinel-node biopsy of the axilla would be performed at the time of lumpectomy. The patient would be informed of the risks associated with the procedure, including a 17 to 59% risk of reoperation if the surgical margin is positive, a 3 to 23% risk of lymphedema, and a less than 10% risk of postoperative infection, seroma, or hematoma.9,10 Genetic counseling and testing would be recommended, because this patient is of Ashkenazi Jewish ancestry. Although overall survival among patients who have a BRCA1 or BRCA2 mutation is similar after either breast-conserving therapy or mastectomy, the risk of a second, contralateral breast cancer after breast-conserving therapy is 26 to 40% at 20 years.11 Therefore, some women choose bilateral mastectomy instead of lumpectomy in order to prevent a second primary cancer.
Options for Systemic Treatment
Dr. Laura M. Spring: After upfront surgery is performed, the final pathological interpretation of the surgical specimen would determine adjuvant systemic treatment. The mainstay of adjuvant treatment for an HR-positive, HER2-negative tumor is endocrine therapy, and some patients also receive chemotherapy. Because this patient is postmenopausal, daily treatment with an aromatase inhibitor for 5 to 10 years12,13 or upfront treatment with tamoxifen, followed by an aromatase inhibitor, would be the most likely treatment choice.14
Standard clinicopathological features are used to determine whether a patient with HR-positive, HER2-negative breast cancer should receive adjuvant chemotherapy before endocrine therapy to further reduce the risk of recurrence. Additional genomic tests such as an RNA-based risk-score assay can be performed to estimate both the risk of recurrence and the potential for risk reduction with chemotherapy.12,15-18 For example, the 21-gene recurrence-score assay Oncotype DX (Genomic Health) is a gene-expression assay that evaluates 16 cancer-related genes involved in tumor-cell proliferation and hormonal response, along with 5 reference genes; scores range from 0 to 100.15 A high recurrence score (defined as either ≥31 or ≥26, depending on the specific trial) is associated with a greater risk of distant recurrence and is predictive of chemotherapy benefit, whereas a low score (<11) indicates a low risk of recurrence and limited benefit with chemotherapy.15 Chemotherapy can also be safely omitted in patients who have an intermediate score (11 to 25), on the basis of the Trial Assigning Individualized Options for Treatment (TAILORx) study, which showed that efficacy outcomes associated with adjuvant endocrine therapy and with chemotherapy plus endocrine therapy were similar, although a subgroup analysis suggested some benefit with the addition of chemotherapy among women who were 50 years of age or younger and had lower recurrence scores.19
If we assume that this patient’s pathological results are consistent with the clinical stage T2N0, grade 2 tumor, performing a genomic assay is recommended, given that she has certain features that support the use of chemotherapy (e.g., large tumor size) and other features that do not support the use of chemotherapy (e.g., strongly HR-positive tumor). If her recurrence score indicates a chemotherapy benefit, a regimen such as docetaxel and cyclophosphamide would be a reasonable choice.20,21
Options for Radiation Therapy
Dr. Rachel B. Jimenez: Before the Covid-19 pandemic, radiation therapy would typically be considered after lumpectomy to reduce the risk of a recurrence in the same breast and to increase survival.22 Treatment with radiation usually begins 4 to 8 weeks after surgery unless chemotherapy is given, in which case radiation therapy starts once chemotherapy is complete, since delaying chemotherapy may reduce its effectiveness.23 In the United States, radiation treatment has historically been administered daily to the entire breast over the course of approximately 4.5 to 5 weeks, often followed by a 1-to-1.5-week radiation “boost” focused on the area within a few centimeters of the lumpectomy cavity. However, randomized trials have shown that the efficacy and safety of “hypofractionated” regimens lasting 3 to 4 weeks are similar to those of “conventional” fractionation, and therefore, such regimens are preferred over conventional fractionation for most patients.24 Accelerated partial breast irradiation, which is used to treat only the area immediately around the lumpectomy cavity over the course of 1 to 2 weeks, is an option for select patients.25-27
Management of Breast Cancer during the Covid-19 Pandemic
Dr. Specht: On March 15, 2020, the Massachusetts Department of Public Health issued an order to suspend nonessential elective invasive procedures to protect patients and health care workers and to conserve hospital resources during the Covid-19 pandemic. With this mandate, the multidisciplinary treatment of this patient with newly diagnosed breast cancer changed. The risks associated with surgery during the pandemic — including patient and staff exposure to SARS-CoV-2 and the need for personal protective equipment, ventilators, and medical staff who could otherwise be deployed to care for patients with Covid-19 — were weighed against the risk of tumor progression while the patient was receiving systemic therapy. Alternative therapeutic options were discussed in the multidisciplinary clinic.
Approaches to Radiation Therapy
Dr. Jimenez: During the Covid-19 pandemic, a few treatment options can be considered regarding the administration of radiation therapy after surgery. First, the initiation of radiation therapy after breast-conserving surgery can be delayed in order to limit a patient’s exposure to health care facilities. Several retrospective studies showed that, among patients who were not receiving chemotherapy, the efficacy of radiation therapy was not affected by delaying the start of radiation up to 20 weeks after breast-conserving surgery.28-30 Second, radiation therapy courses can be shortened with the use of hypofractionated regimens or accelerated partial-breast irradiation for certain patients and by omitting a boost in some patients.24,27,31 Administering the radiation boost simultaneously with the delivery of whole-breast radiation can also shorten the duration of radiation therapy, although results of randomized trials comparing such an approach with sequential boosts are not yet available.32 So-called “ultrahypofractionated” regimens, in which the entire breast is treated once weekly for 5 weeks or daily over the course of 5 consecutive days, have shown acceptable short-term tumor control and rates of toxic effects, although the long-term efficacy and safety of these regimens are incompletely established; therefore, such a regimen should be used selectively, even during the Covid-19 pandemic.33-36
Options for Neoadjuvant Endocrine Therapy in Lieu of Surgery
Dr. Spring: In lieu of upfront surgery, an alternative treatment option for this patient would be preoperative (neoadjuvant) therapy.12,37 Neoadjuvant endocrine therapy has been shown to improve surgical outcomes by increasing rates of eligibility to undergo breast-conserving therapy and by increasing response rates.38-40 Such therapy would be an option for this patient even in the pre–Covid-19 era, although it is vastly underused in the United States.41
It is well established that aromatase inhibitors are more effective than tamoxifen when used as neoadjuvant therapy in postmenopausal women.40,42-46 Initiation of treatment with an aromatase inhibitor before lumpectomy could lead to a partial or complete response, thereby increasing the likelihood of negative surgical margins and an improved cosmetic outcome. In one study, 69.8% of the patients had a partial or complete response after receiving an aromatase inhibitor for 3 months.47 The risk of disease progression while a patient is receiving neoadjuvant endocrine therapy is low. Furthermore, during the period when neoadjuvant therapy is administered, results from genetic testing would typically become available, allowing the patient to make a more informed surgical decision regarding breast-conserving therapy or mastectomy.
This approach also allows identification of endocrine-sensitive disease, thereby enabling some patients to avoid chemotherapy.37,48 A small number of randomized clinical trials have shown that the response rates and rates of breast-conserving therapy associated with neoadjuvant endocrine therapy are similar to those associated with neoadjuvant chemotherapy in the appropriate patient population, while neoadjuvant endocrine therapy confers fewer adverse effects.38,39,49 The duration of neoadjuvant endocrine therapy is typically 3 to 6 months, although treatment can be extended if the tumor continues to respond; response rates are generally higher when the duration of treatment is longer.50
One potential concern associated with neoadjuvant endocrine therapy is the risk of disease progression. Therefore, timely follow-up examination and imaging, if indicated, are important to monitor for progression. Furthermore, it is reasonable to perform a genomic analysis of the diagnostic core-needle biopsy specimen to assess whether neoadjuvant chemotherapy should be considered instead of neoadjuvant endocrine therapy when the decision is not clear. Although the validation of genomic assays has occurred mostly for adjuvant treatment, emerging clinical response data from studies evaluating the use of neoadjuvant therapy have become available. In the TransNEOS study, among patients who received neoadjuvant letrozole, the incidence of disease progression was low if the score on the 21-gene recurrence-score assay was below 31 (<1% among patients with a score of <18 and 4% among those with a score of 18 to 30); in contrast, the incidence of progression was considerably higher among patients with a score of 31 or higher (17%).51 If this patient’s recurrence score is found to be 31 or higher, a multidisciplinary discussion and consideration of resources would be needed to determine whether the patient should proceed to surgery or begin neoadjuvant chemotherapy.
The clinical scenario is more complicated among premenopausal women, given the paucity of data with neoadjuvant endocrine therapy; most phase 3 randomized trials evaluating this treatment have focused on postmenopausal women. Although the randomized phase 2 GEICAM/2006-03 study showed a significant benefit of neoadjuvant chemotherapy over neoadjuvant endocrine therapy among premenopausal women, with higher response rates seen among those who had a high Ki-67 proliferation index (a marker of cellular proliferation), the use of neoadjuvant endocrine therapy remains a valid approach in the appropriate clinical situation.12,37,38 With regard to choice of endocrine therapy, the STAGE study showed that neoadjuvant treatment with an aromatase inhibitor plus a luteinizing hormone–releasing hormone (LHRH) agonist resulted in significantly greater response rates than an LHRH agonist plus tamoxifen.45 However, the length of time it takes the combination of an aromatase inhibitor and an LHRH agonist to suppress estrogen — with maximal suppression typically achieved by week 4 — is a potential concern.45 During the Covid-19 pandemic, the administration of an LHRH agonist every 3 months is preferred over a monthly dose to minimize clinic visits. As noted previously, performing a genomic analysis of the core-needle biopsy specimen is a reasonable approach to determine whether chemotherapy would be appropriate. Although the threshold for recommending chemotherapy is lower for women 50 years or age or younger, on the basis of the results of the TAILORx study,19 it is important to balance the potential benefit of chemotherapy with the risk of immunosuppression during the Covid-19 pandemic; moreover, the potential role of suppression of ovarian function in lieu of chemotherapy among patients who have a lower clinical risk should also be carefully considered.52 With regard to this patient, the core-needle biopsy specimen was sent for genomic analysis, which revealed an intermediate recurrence score of 24 on the 21-gene assay.
Alternative Scenarios during the Covid-19 Pandemic
Dr. Beverly Moy: Although this patient has clinical stage T2N0, HR-positive, HER2-negative breast cancer, we also want to consider appropriate treatment strategies for women who present with other subtypes of breast cancer during the pandemic.
Patients with HER2-Amplified Breast Cancer
A widely accepted evidence-based treatment approach used in patients with early HER2-positive breast cancer is surgery, followed by adjuvant therapy, for patients with clinical stage T1N0 disease and neoadjuvant systemic therapy, followed by surgery, for patients with clinical stage T2–4N0 or node-positive disease.53,54 On the basis of this approach, the preferred initial treatment for a patient with clinical stage T2N0 HER2-positive breast cancer — even in the absence of the Covid-19 pandemic — would be neoadjuvant therapy. This approach is based in part on the KATHERINE trial, which showed improved outcomes with the use of adjuvant trastuzumab emtansine (T-DM1) among women with early HER2-positive breast cancer who had had residual invasive disease after receiving multiagent, HER2-targeted neoadjuvant therapy.54 However, the Covid-19 pandemic has led oncologists to more carefully weigh the risks and the benefits of standard neoadjuvant HER2-targeted regimens.
Commonly used regimens that have been evaluated extensively in clinical trials include doxorubicin and cyclophosphamide, followed by paclitaxel, trastuzumab, and pertuzumab, as well as combination therapy with docetaxel, carboplatin, trastuzumab, and pertuzumab.55,56 Although these evidence-based regimens are extremely efficacious, they are also associated with a high degree of immunosuppression, and their use could lead to further consequences if a patient becomes infected with SARS-CoV-2. The KRISTINE trial evaluated the effects of replacing standard neoadjuvant HER2-targeted regimens with a less immunosuppressive regimen, T-DM1 plus pertuzumab.57 In that trial, despite being associated with fewer toxic effects, neoadjuvant treatment with T-DM1 plus pertuzumab led to a lower pathological complete response rate than combination therapy with docetaxel, carboplatin, trastuzumab, and pertuzumab (44.4% vs. 55.7%), possibly because a strictly HER2-targeted therapy approach may be less active among patients whose cancer has heterogeneous HER2 expression. Another alternative neoadjuvant regimen is paclitaxel administered with trastuzumab and pertuzumab, which will be more carefully studied in the upcoming COMPASS trial (ClinicalTrials.gov number, NCT04266249). The downside of treatment with paclitaxel, trastuzumab, and pertuzumab is that the weekly dose of paclitaxel requires frequent trips to a clinic. Adjustment of the chemotherapy regimen, whereby paclitaxel or docetaxel could be administered every 3 weeks, could be performed, but the use of granulocyte colony-stimulating factors to reduce immunosuppression during the pandemic should be considered.
If a patient has a small clinical stage T1N0 HER2-positive tumor, the surgical restrictions resulting from the Covid-19 pandemic could prevent a standard upfront surgical approach. Therefore, neoadjuvant systemic therapy is needed, but there is no clear standard regimen. In the absence of clear pathological confirmation of lymph-node status, it may not be advisable to eliminate the use of pertuzumab, since it is associated with modest benefit regarding disease-free survival in early HER2-positive breast cancer.58 Therefore, reasonable neoadjuvant regimens in this context include paclitaxel, trastuzumab, and pertuzumab or T-DM1 plus pertuzumab.
Patients with Triple-Negative Breast Cancer
Dr. Steven J. Isakoff: Chemotherapy remains the cornerstone of systemic treatment for early ER-negative, PR-negative, HER2-negative (triple-negative) breast cancer. For this patient with clinical stage T2N0 cancer, the standard approach for triple-negative breast cancer before the Covid-19 pandemic would have involved neoadjuvant chemotherapy with deferred surgery, and this remains the preferred approach during the pandemic. In addition to facilitating successful surgical resection, the neoadjuvant approach allows for the response to neoadjuvant therapy to inform adjuvant therapy decisions; in the Capecitabine for Residual Cancer as Adjuvant Therapy (CREATE-X) trial, treatment with adjuvant capecitabine showed a survival benefit among patients with HER2-negative breast cancer who had had residual disease after receiving anthracycline-based neoadjuvant therapy.59 The neoadjuvant regimens used for triple-negative breast cancer tumors that are larger than 2 cm in diameter or that are node-positive typically include an anthracycline and taxane.12 The addition of neoadjuvant carboplatin remains controversial; although pathological complete response rates are higher with the addition of carboplatin,60,61 the effect on long-term outcomes remains uncertain, and, especially during the Covid-19 pandemic, the added risks of hematologic toxic effects and immunosuppression must be carefully considered.
Among patients with triple-negative breast cancer tumors that measure 2 cm or less in diameter and are node-negative, administration of adjuvant chemotherapy is standard practice for patients with tumors larger than 1 cm in diameter (T1c) and is often considered for patients with tumors larger than 0.5 cm in diameter (T1b).12 However, if access to surgery is limited because of the Covid-19 pandemic, the chemotherapy regimen that would have been selected for adjuvant therapy can be administered as neoadjuvant therapy to allow for deferred surgery. For example, docetaxel and cyclophosphamide, a regimen commonly used for adjuvant therapy,21 may be administered as neoadjuvant therapy.62 Surgery should remain a high priority for patients whose triple-negative breast cancer tumors are small and for whom chemotherapy is not otherwise recommended3; alternative systemic approaches are not suitable for smaller tumors, and the biologic characteristics of triple-negative breast cancer arouse concern that a sustained delay in surgery could result in tumor growth and upstaging, which in turn may increase the risk of recurrence.
Patients Who Have Completed Neoadjuvant Therapy and Need Surgery
Dr. Isakoff: Patients completing neoadjuvant chemotherapy regimens during the Covid-19 pandemic who are unable to proceed to surgery because of limitations in hospital resources require special attention to ensure that long-term outcomes are not compromised. As a general principle, surgery for patients who have progression of disease during neoadjuvant therapy or who have no alternative systemic therapy options is given high priority.3 Patients with HR-positive breast cancer who are receiving neoadjuvant endocrine therapy without clinical progression may safely continue endocrine therapy for 6 months or more, with the timing of surgery depending largely on factors relating to hospital resources. For patients with HER2-positive disease, several options are available, depending on the response to neoadjuvant therapy. For patients who have a clinical complete response or a substantial partial response, continuation of trastuzumab and pertuzumab as maintenance treatment may be reasonable. In addition, taxane-based HER2-targeted therapy may be safely continued if not limited by the development of adverse effects.63 For patients with HER2-positive disease who have minimal clinical response or no response, surgery is preferred, but such patients may transition to neoadjuvant treatment with T-DM1 until surgery is feasible, with plans to continue adjuvant therapy.54 It is recommended that surgery for patients who have triple-negative breast cancer and are completing neoadjuvant therapy be given high priority.3 If surgery is not feasible within a reasonable time frame after completion of a standard regimen of neoadjuvant therapy, patients may receive additional cycles of nonanthracycline chemotherapy, such as weekly paclitaxel, capecitabine, or other agents, to provide a bridge to surgery; however, surgery should be completed as soon as feasible.
Communication with Patients during the Covid-19 Pandemic
Dr. Jennifer A. Shin: During the Covid-19 pandemic, providers are faced with the challenge of communicating with patients about their cancer diagnosis and treatment plan, while also addressing concerns about Covid-19 and how it might affect cancer care. An additional challenge is that these conversations may be occurring virtually, rather than in person. Patients may express feeling overwhelmed by a breast cancer diagnosis and the associated therapies. In the Covid-19 era, patients may have additional anxiety and fear about coming to the hospital or about the possibility that administration of cancer therapies may increase their risk of Covid-19 and death.64,65 Patients may also be concerned about a treatment plan that deviates from routine standard of care and whether this alternative approach could affect their clinical outcome.
Communication is at the core of the medical profession, and effective and empathic communication can have a positive effect on a patient’s quality of life, satisfaction with care, and medical outcomes.66-68 During a clinical visit, identifying and addressing concerns and emotions are a key first step before proceeding to the other parts of the visit.68,69 It is difficult for a patient to absorb medical information if the provider does not acknowledge any worry, anxiety, and distress about the diagnosis and the pandemic. At the start of the visit, checking in with the patient is important. This patient may express worry about the delay in her breast surgery and may ask whether this might affect her cancer outcome.
Follow-up
Dr. Bardia: After discussing the care of this patient during a virtual multidisciplinary tumor board conference, we determined that upfront surgery was not an option because of Covid-19 restrictions; consequently, neoadjuvant endocrine therapy with an aromatase inhibitor was initiated. The patient is currently doing well and has a 2-month follow-up visit scheduled with the multidisciplinary team.
Final Diagnosis
Invasive ductal carcinoma of the left breast, clinical prognostic stage IB (T2N0), estrogen receptor–positive, progesterone receptor–positive, human epidermal growth factor receptor 2–negative, grade 2, with an intermediate recurrence score on the 21-gene assay.
Disclosure Forms
This article was published on July 1, 2020, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Expert Panel on Breast Imaging. ACR Appropriateness Criteria® palpable breast masses. J Am Coll Radiol 2017;14:S203-S224. [DOI] [PubMed] [Google Scholar]
- 2.D’Orsi C. 2013 ACR BI-RADS atlas: breast imaging reporting and data system. Reston, VA: American College of Radiology, 2014. [Google Scholar]
- 3.Dietz JR, Moran MS, Isakoff SJ, et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. Breast Cancer Res Treat 2020;181:487-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marron JM, Joffe S, Jagsi R, Spence RA, Hlubocky FJ. Ethics and resource scarcity: ASCO recommendations for the oncology community during the COVID-19 pandemic. J Clin Oncol 2020. April 28 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 5.Mauri D, Kamposioras K, Tolia M, Alongi F, Tzachanis D. Summary of international recommendations in 23 languages for patients with cancer during the COVID-19 pandemic. Lancet Oncol 2020;21:759-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher B, Jeong J-H, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 2002;347:567-575. [DOI] [PubMed] [Google Scholar]
- 7.Wellisch DK, DiMatteo R, Silverstein M, et al. Psychosocial outcomes of breast cancer therapies: lumpectomy versus mastectomy. Psychosomatics 1989;30:365-373. [DOI] [PubMed] [Google Scholar]
- 8.Pozo C, Carver CS, Noriega V, et al. Effects of mastectomy versus lumpectomy on emotional adjustment to breast cancer: a prospective study of the first year postsurgery. J Clin Oncol 1992;10:1292-1298. [DOI] [PubMed] [Google Scholar]
- 9.Coopey S, Smith BL, Hanson S, et al. The safety of multiple re-excisions after lumpectomy for breast cancer. Ann Surg Oncol 2011;18:3797-3801. [DOI] [PubMed] [Google Scholar]
- 10.Shah C, Arthur D, Riutta J, Whitworth P, Vicini FA. Breast-cancer related lymphedema: a review of procedure-specific incidence rates, clinical assessment AIDS, treatment paradigms, and risk reduction. Breast J 2012;18:357-361. [DOI] [PubMed] [Google Scholar]
- 11.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017;317:2402-2416. [DOI] [PubMed] [Google Scholar]
- 12.Gradishar WJ, Anderson BO, Abraham J, et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2020;18:452-478. [DOI] [PubMed] [Google Scholar]
- 13.Pan H, Gray R, Braybrooke J, et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 2017;377:1836-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386:1341-1352. [DOI] [PubMed] [Google Scholar]
- 15.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351:2817-2826. [DOI] [PubMed] [Google Scholar]
- 16.Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 2015;373:2005-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 2016;375:717-729. [DOI] [PubMed] [Google Scholar]
- 18.Dowsett M, Sestak I, Lopez-Knowles E, et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol 2013;31:2783-2790. [DOI] [PubMed] [Google Scholar]
- 19.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 2018;379:111-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blum JL, Flynn PJ, Yothers G, et al. Anthracyclines in early breast cancer: the ABC Trials — USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol 2017;35:2647-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones S, Holmes FA, O’Shaughnessy J, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol 2009;27:1177-1183. [DOI] [PubMed] [Google Scholar]
- 22.Early Breast Cancer Trialists’ Collaborative Group. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellon JR, Come SE, Gelman RS, et al. Sequencing of chemotherapy and radiation therapy in early-stage breast cancer: updated results of a prospective randomized trial. J Clin Oncol 2005;23:1934-1940. [DOI] [PubMed] [Google Scholar]
- 24.Smith BD, Bellon JR, Blitzblau R, et al. Radiation therapy for the whole breast: executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol 2018;8:145-152. [DOI] [PubMed] [Google Scholar]
- 25.Vicini FA, Cecchini RS, White JR, et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet 2019;394:2155-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whelan TJ, Julian JA, Berrang TS, et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): a randomised controlled trial. Lancet 2019;394:2165-2172. [DOI] [PubMed] [Google Scholar]
- 27.Correa C, Harris EE, Leonardi MC, et al. Accelerated partial breast irradiation: executive summary for the update of an ASTRO evidence-based consensus statement. Pract Radiat Oncol 2017;7:73-79. [DOI] [PubMed] [Google Scholar]
- 28.Olivotto IA, Lesperance ML, Truong PT, et al. Intervals longer than 20 weeks from breast-conserving surgery to radiation therapy are associated with inferior outcome for women with early-stage breast cancer who are not receiving chemotherapy. J Clin Oncol 2009;27:16-23. [DOI] [PubMed] [Google Scholar]
- 29.Karlsson P, Cole BF, Colleoni M, et al. Timing of radiotherapy and outcome in patients receiving adjuvant endocrine therapy. Int J Radiat Oncol Biol Phys 2011;80:398-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vujovic O, Yu E, Cherian A, Dar AR, Stitt L, Perera F. Time interval from breast-conserving surgery to breast irradiation in early stage node-negative breast cancer: 17-year follow-up results and patterns of recurrence. Int J Radiat Oncol Biol Phys 2015;91:319-324. [DOI] [PubMed] [Google Scholar]
- 31.Bartelink H, Maingon P, Poortmans P, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol 2015;16:47-56. [DOI] [PubMed] [Google Scholar]
- 32.Freedman GM, White JR, Arthur DW, Allen Li X, Vicini FA. Accelerated fractionation with a concurrent boost for early stage breast cancer. Radiother Oncol 2013;106:15-20. [DOI] [PubMed] [Google Scholar]
- 33.The FAST Trialists Group. First results of the randomised UK FAST Trial of radiotherapy hypofractionation for treatment of early breast cancer (CRUKE/04/015). Radiother Oncol 2011;100:93-100. [DOI] [PubMed] [Google Scholar]
- 34.Brunt AM, Wheatley D, Yarnold J, et al. Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the UK FAST-Forward Trial. Radiother Oncol 2016;120:114-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunt AM, Haviland JS, Wheatley DA, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020;395:1613-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dragun AE, Ajkay NJ, Riley EC, et al. First results of a phase 2 trial of once-weekly hypofractionated breast irradiation (WHBI) for early-stage breast cancer. Int J Radiat Oncol Biol Phys 2017;98:595-602. [DOI] [PubMed] [Google Scholar]
- 37.Spring LM, Gupta A, Reynolds KL, et al. Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: a systematic review and meta-analysis. JAMA Oncol 2016;2:1477-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alba E, Calvo L, Albanell J, et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol 2012;23:3069-3074. [DOI] [PubMed] [Google Scholar]
- 39.Semiglazov VF, Semiglazov VV, Dashyan GA, et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer 2007;110:244-254. [DOI] [PubMed] [Google Scholar]
- 40.Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol 2001;12:1527-1532. [DOI] [PubMed] [Google Scholar]
- 41.Chiba A, Hoskin TL, Heins CN, Hunt KK, Habermann EB, Boughey JC. Trends in neoadjuvant endocrine therapy use and impact on rates of breast conservation in hormone receptor-positive breast cancer: a National Cancer Data Base study. Ann Surg Oncol 2017;24:418-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cataliotti L, Buzdar AU, Noguchi S, et al. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the Pre-Operative “Arimidex” Compared to Tamoxifen (PROACT) trial. Cancer 2006;106:2095-2103. [DOI] [PubMed] [Google Scholar]
- 43.Ellis MJ, Coop A, Singh B, et al. Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol 2001;19:3808-3816. [DOI] [PubMed] [Google Scholar]
- 44.Harper-Wynne CL, Sacks NPM, Shenton K, et al. Comparison of the systemic and intratumoral effects of tamoxifen and the aromatase inhibitor vorozole in postmenopausal patients with primary breast cancer. J Clin Oncol 2002;20:1026-1035. [DOI] [PubMed] [Google Scholar]
- 45.Masuda N, Sagara Y, Kinoshita T, et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): a double-blind, randomised phase 3 trial. Lancet Oncol 2012;13:345-352. [DOI] [PubMed] [Google Scholar]
- 46.Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 2005;23:5108-5116. [DOI] [PubMed] [Google Scholar]
- 47.Dixon JM, Renshaw L, Macaskill EJ, et al. Increase in response rate by prolonged treatment with neoadjuvant letrozole. Breast Cancer Res Treat 2009;113:145-151. [DOI] [PubMed] [Google Scholar]
- 48.Ellis MJ, Suman VJ, Hoog J, et al. Ki67 proliferation index as a tool for chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of breast cancer: results from the American College of Surgeons Oncology Group Z1031 Trial (Alliance). J Clin Oncol 2017;35:1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmieri C, Cleator S, Kilburn LS, et al. NEOCENT: a randomised feasibility and translational study comparing neoadjuvant endocrine therapy with chemotherapy in ER-rich postmenopausal primary breast cancer. Breast Cancer Res Treat 2014;148:581-590. [DOI] [PubMed] [Google Scholar]
- 50.Allevi G, Strina C, Andreis D, et al. Increased pathological complete response rate after a long-term neoadjuvant letrozole treatment in postmenopausal oestrogen and/or progesterone receptor-positive breast cancer. Br J Cancer 2013;108:1587-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iwata H, Masuda N, Yamamoto Y, et al. Validation of the 21-gene test as a predictor of clinical response to neoadjuvant hormonal therapy for ER+, HER2-negative breast cancer: the TransNEOS study. Breast Cancer Res Treat 2019;173:123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and economic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med 2019;380:2395-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tolaney SM, Guo H, Pernas S, et al. Seven-year follow-up analysis of adjuvant paclitaxel and trastuzumab trial for node-negative, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2019;37:1868-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Minckwitz G, Huang C-S, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 2019;380:617-628. [DOI] [PubMed] [Google Scholar]
- 55.Gianni L, Pienkowski T, Im YH, et al. 5-Year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol 2016;17:791-800. [DOI] [PubMed] [Google Scholar]
- 56.Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278-2284. [DOI] [PubMed] [Google Scholar]
- 57.Hurvitz SA, Martin M, Jung KH, et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2-positive breast cancer: three-year outcomes from the phase III KRISTINE study. J Clin Oncol 2019;37:2206-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 2017;377:122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017;376:2147-2159. [DOI] [PubMed] [Google Scholar]
- 60.Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 2015;33:13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loibl S, O’Shaughnessy J, Untch M, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol 2018;19:497-509. [DOI] [PubMed] [Google Scholar]
- 62.Nakatsukasa K, Koyama H, Oouchi Y, et al. Docetaxel and cyclophosphamide as neoadjuvant chemotherapy in HER2-negative primary breast cancer. Breast Cancer 2017;24:63-68. [DOI] [PubMed] [Google Scholar]
- 63.Carey LA, Berry DA, Cirrincione CT, et al. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol 2016;34:542-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov 2020. May 1 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qian Y, Wu K, Xu H, et al. A survey on physical and mental distress among cancer patients during the COVID-19 epidemic in Wuhan, China. J Palliat Med 2020. May 14 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 66.Baile WF, Aaron J. Patient-physician communication in oncology: past, present, and future. Curr Opin Oncol 2005;17:331-335. [DOI] [PubMed] [Google Scholar]
- 67.Rai A, Han X, Zheng Z, Yabroff KR, Jemal A. Determinants and outcomes of satisfaction with healthcare provider communication among cancer survivors. J Natl Compr Canc Netw 2018;16:975-984. [DOI] [PubMed] [Google Scholar]
- 68.Back A, Tulsky JA, Arnold RM. Communication skills in the age of COVID-19. Ann Intern Med 2020;172:759-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Back AL, Arnold RM, Baile WF, Tulsky JA, Fryer-Edwards K. Approaching difficult communication tasks in oncology. CA Cancer J Clin 2005;55:164-177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.