Abstract
Hendra virus (HeV) is lethal to humans and horses, and little is known about its epidemiology. Biosecurity restrictions impede advances, particularly on understanding pathways of transmission. Quantifying the environmental survival of HeV can be used for making decisions and to infer transmission pathways. We estimated HeV survival with a Weibull distribution and calculated parameters from data generated in laboratory experiments. HeV survival rates based on air temperatures 24 h after excretion ranged from 2 to 10 % in summer and from 12 to 33 % in winter. Simulated survival across the distribution of the black flying fox (Pteropus alecto), a key reservoir host, did not predict spillover events. Based on our analyses we concluded that the most likely pathways of transmission did not require long periods of virus survival and were likely to involve relatively direct contact with flying fox excreta shortly after excretion.
Introduction
Hendra virus (HeV) is a recently discovered paramyxovirus in the genus Henipavirus. The natural reservoir hosts are the four Australian fruit bat species known as flying foxes (Pteropus spp.) (Halpin et al., 2000). HeV was discovered during an outbreak of a respiratory and neurological disease in 20 horses and one human in 1994 (Murray et al., 1995), and was classified as a Biosecurity Level (BSL) 4 pathogen. Understanding of the mechanisms driving its emergence and transmission to spillover hosts has been slow to develop. Despite the recent availability of a commercial vaccine for horses, spillover events continue to occur. Many at-risk horses remain unvaccinated due to the cost of the vaccine, risk perceptions of horse owners and other factors (Middleton et al., 2014). Undertaking more cost-effective alternative mitigation strategies is constrained by a lack of implementation of the management recommendations. Thus, improving the understanding of transmission routes of HeV from flying foxes to horses will result in reducing necessary costs and efforts to implement effective prevention. The most likely route is thought to be exposure of horses to infectious bat excretions (Field et al., 2011; Marsh et al., 2011).
According to the most plausible scenario, once HeV is excreted by bats, the time it remains infectious determines the transmission window within which horses must be exposed to become infected with HeV (Plowright et al., 2015). If the virus survival is very short, rapid and direct exposure to bat excretions (faeces, urine, saliva or birth/foetal fluids) may be necessary. Longer survival provides more diverse opportunities for exposure. For example, infectious excreta may be able to accumulate in feed. Clearly, improving our understanding of virus survival in the environment will be critical for inferring likely transmission mechanisms and mitigating risk.
Inferences about HeV survival within the environment have thus far been based on a study of the effects of pH, desiccation, urine and fruit juice at two different temperatures. This study also examined Nipah virus (NiV) survival using the same conditions and substrates (Fogarty et al., 2008). HeV and NiV were resistant to a wide pH range (3–11) but sensitive to desiccation, surviving for <15 min without moisture at 37 °C. Whilst these experiments indicated that the decay of infectious HeV occurs quickly, the effect of the magnitude of the environmental variables (and their change through time) on virus survival are still unknown. For this reason, we cannot estimate HeV survival in eastern Australia where spillover events occur (Fig. S1, available in the online Supplementary Material). Moreover, we cannot use HeV survival to predict spillover events or infer the most likely transmission routes.
To estimate and understand the contribution of virus survival in the environment to spillover risk we developed a model from experimental data of HeV survival at three different temperatures. With our model we quantified the relationship between changes in temperature and survival of HeV. The model accounted for the effect of temperature on the decay rate of the virus and was based on the Weibull cumulative density function (CDF). The model was used to perform simulations under the temperature conditions of the current geographical distribution of HeV spillover events. The simulations estimated how much virus would survive 12 and 24 h after excretion at 3 : 00 a.m. across the distribution of the black flying fox (Pteropus alecto), which encompasses the current distribution of spillover events. In addition, we showed that previous calculations that determined longer survival (Scanlan et al., 2014) are explained through recording errors, different model-fitting procedures and the nature of environmental data used in simulations (air versus soil temperatures). The differences in results have implications for understanding and managing HeV transmission.
Results
Observed survival in laboratory and calculated half-lives
The experimental data consisted of measurements of the concentration of viral particles in TCID50. The measurements and the length of intervals between measurements are shown in Table S1. Our estimates of virus survival differed substantially from previously published analyses of the data (Scanlan et al., 2014). For instance, our estimations of virus half-life at 56 and 22 °C were ~1900 and 17.3 times less, respectively, than reported by Scanlan et al. (2014).
HeV survival was relatively short and highly dependent on temperature. A small proportion of HeV particles were capable of surviving for several hours in Earle’s minimum essential medium (EMEM) at 22 °C, whilst survival increased at 4 °C and decreased significantly at 56 °C (Figs 1 and S2). The half-life of virus decreased exponentially with increasing temperature; therefore, small increases in temperature resulted in large increases in decay rates. The relationship between ln S (where S is the proportion of virus surviving at time t) and time was increasingly non-log-linear at higher temperatures. Consequently, half-life decreased not only exponentially with temperature, but followed a power law dependent on temperature. This resulted in higher decay rates immediately after virus excretion, because the shape parameter of the Weibull model was <1 at all temperatures and decreased even more at higher temperatures (Fig. 1, Table S1).
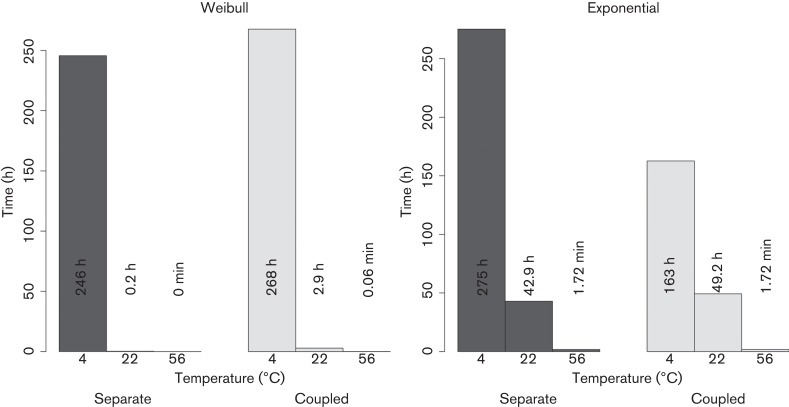
Fig. 1.
Half-lives calculated for each temperature: Weibull model versus exponential model. The estimations between the separate models for each temperature differ less with respect to the time–temperature coupled model in the Weibull model than in the exponential model.
As a result of the non-log-linearity of the data (Fig. S2), the Weibull model achieved the best fit as shown by the Akaike weights test between models (P<0.05). The half-life estimations between models differed substantially, especially at high temperatures. Weibull half-life estimations were 268 h, 2.9 h and 3.5 s at 4, 22 and 56 °C, respectively. In contrast, half-lives we calculated with the exponential model were 275 h, 42.9 h and 1.72 min, respectively (Fig. 1). Given the significantly lower Akaike information criterion values, we only show detailed results for the Weibull model. Parameters of all fitted models are given in Tables S2 and S3.
Survival rates calculated with air temperature
The amount of virus surviving 24 h ranged from 2 % (0.04–6.7 % with se of estimated parameters) in the northernmost location (Cairns) to 10 % (1.6–18 % se) in the southernmost location (Kempsey) during summer. In the coldest month of winter, the amount of virus surviving was 12 % (2.4–21.2 % se) in Cairns and 33.1 % (20.4–40.4 % se) in Kempsey (Fig. 2).
Fig. 2.
Simulated virus decay curves for 96 h after excretion at 3 : 00 a.m. Summer conditions are represented by the mean temperature of the hottest month and winter conditions are represented by the mean temperature of the coldest month. Autumn and spring are represented by the mean temperature of the first month of the season. The dashed lines represent the simulations with upper and lower standard errors of estimated parameters. The grey shaded area on the left side of each graph highlights the survival curve over the first 24 h.
Survival rates at 24 h were highly variable amongst years; however, variability within months could be greater than pooled variability between years as evidenced by the smaller 95% confidence intervals (95% CIs) of air temperature variability (Fig. 3a). Regarding the two major spillover clusters to date, 2011 [southern Queensland and northern New South Wales (NSW)] and 2012 (northern Queensland; Fig S1), the events in 2011 had a mean survival slightly higher than the 1993–2012 mean temperatures, but within normal variability, whereas events from the 2012 cluster had survival rates very close to the mean for each location. The mean survival of 2012 locations was lower than the mean survival of 2011 locations, suggesting that clustering of spillover events was not driven by conditions that promoted survival (Fig. 2).
Fig. 3.
(a) Comparison of the proportion of HeV surviving 24 h after excretion at the mean temperature conditions between 1993 and 2012 (coloured bars) at each of the spillover locations and the mean temperature of the month where spillover events occurred (black points). Thin continuous error bars show 95 % CIs for temperature between 1993 and 2012. Dashed error bars show 95 % CIs of HeV survival for the month of spillover. Location of the point with respect to the thin error bars indicates how different the month of spillover was from the most common conditions (coloured bars). The grey shaded areas indicate the events that occurred in summer. Results are only shown for locations where reliable temperature data were available from a meteorological station. (b) Bimodal distribution of HeV spillover events with respect to potential virus survival.
Predictive ability of survival rates in space and time
The survival model was projected to three sets of layers: a set of layers of interpolated air temperature, and two sets of soil temperature at zero depth under 0 and 50 % shade (microclim sets). Soil temperature simulations were only performed 12 h after excretion. The difference between air and soil temperature was very large, which resulted in much lower survival estimates for both series of simulations at 0 and 50 % shade at soil level than with air temperature. For instance, the highest survival rate 12 h after excretion at spillover locations under 50 % shade in winter was 10 % compared with ~54 % at 24 h with air temperature. Furthermore, surviving fractions at soil temperature implied that in most of the cases there was no virus left at 12 h, even under 50 % shade in winter conditions. With soil temperature, more events occurred where 12 h survival was almost zero (Figs S3 and S4). The results described below correspond to simulations under air temperature, which likely represented the best case scenario for virus survival.
According to air temperature, most spillover events occurred where 24 h survival was ~40 % (Fig. 3b). Summer and spring spillover events occurred when and where virus survival was lowest across the current geographical distribution of spillover (Fig. 4). The mean 24 h survival of these events was 3.5 % [n = 6, 95% CI 1.5–5.2 %] and the mean survival in buffer zones of 80 km (background survival) was 4.1 % (95% CI 0.2–7.8 %). For autumn events, mean survival was 6.8 % (n = 2, 95% CI 2.0–11.0 %) and background survival was 10 % (95% CI 4.1–16 %). In winter, mean survival at location of spillover was 34.7 % (n = 32, 95% CI 19.8–49.7 %) and background survival was 38.8 % (95% CI 15.0–62.5 %). Finally, in spring, mean survival was 9.6 % (n = 8, 95% CI 0.0–20.0 %) and background survival was 12.5 % (95% CI 0.6–24.4 %).
Fig. 4.
Maps showing the amount of HeV surviving 24 h after being excreted whilst temperature is the mean minimum for each season. Crosses show the locations of spillover events and circles correspond to the buffers used in the statistical analyses. Events were divided into season of occurrence. WA, Western Australia; NT, Northern Territory; SA, South Australia; QLD, Queensland; NSW, New South Wales. The x- and y-axes show longitude and latitude, respectively.
Predicted viral survival rates during spillover events were close to the mean survival for those areas both within and between years (Fig. 3a). Therefore, spillover events mostly occurred at times of intermediate virus survival. However, the distribution of virus survival amongst locations of spillover was bimodal (Fig. 3b). This means that when temperature conditions were cold, spillover tended to occurr in cold areas; when temperatures were higher, spillover occurred in warm areas. Similarly, spillover occurred close to the coastal line, where temperatures were higher than immediately farther inland (Fig. 4).
The distribution and timing of spillover events was not significantly influenced by virus survival rates 12 and 24 h after excretion. The survivorship models did not perform significantly better than random predictions of spillover [area under the curve (AUC) ratio not significantly >1] in space and time. Furthermore, Kolmogorov–Smirnov tests suggested no significant difference between viral survivorship at the location of spillover events with randomly selected background (non-event) survivorship samples in summer. In winter, however, the empirical CDF of the events was significantly smaller (P = 0.001). This association of higher mean survivorship at spillover locations in winter was caused by the positive correlation of temperature with latitude in the southern hemisphere. The majority of the detected spillover events in winter/autumn occurred in the southernmost part of the black flying fox (spillover) geographical range (Fig. 4). When we restricted sampling of non-events to a buffer zone of 40 km around each spillover event, the difference between survival at events versus non-events was not statistically significant (P>0.05).
Discussion
HeV transmission may need to occur shortly after the virus is excreted by bats and probably requires that horses directly contact fresh bat excreta. The spatial simulations with the microclim dataset (Kearney et al. 2014) suggest that HeV potential survival is much shorter than previously estimated with air temperatures. We conclude this based on the higher rate of HeV mortality immediately after excretion, the very low survival rates 12 h after excretion in soil microclimates and the lack of explanatory power of the pattern of spillover by virus survival, with both air and microclim temperatures.
Additional supporting evidence that HeV survival is likely lower than that predicted by Scanlan et al. (2014) is the non-log-linear relationship between ln S and time. This kind of relationship can result from heterogeneous susceptibilities of individual viruses or heterogeneous conditions in the experimental substrates (Peleg, 1996; van Boekel, 2002). It is likely that, in a natural setting, the resistance of free particles and the environmental conditions are more heterogeneous than under laboratory conditions. Therefore, the resulting decay rates might be greater, resulting in shorter lifespans. Low survival will be exacerbated by additional stresses, such as desiccation (Fogarty et al., 2008) and UV radiation. UV radiation is very effective at killing other viruses (Murray & Jackson, 1993; Weber & Stilianakis, 2008), but has not been tested for HeV. HeV is an enveloped virus (Paterson et al., 1998) and therefore could be highly sensitive to UV radiation, which will additionally reduce survival after sunrise.
Heterogeneous conditions might also be caused by microclimates. A key difference with the term microclimate used previously is the variability of the macroclimate with respect to the conditions formed by vegetation or topography at smaller scales. Microclimates in this sense can differ from the macroclimate, decreasing maximum temperatures, increasing moisture, decreasing desiccation rates and creating a refuge from UV radiation (Chen et al., 1996). The effect of microclimates on temperature may be applicable to this study. A downscaling of interpolated air temperature from the macroclimate will not be representative of microclimates because soil characteristics are important determinants of temperature at ground level (Kearney et al., 2014). For instance, Scanlan et al. (2014) assumed that a microclimate is a colder version of the macroclimate by up to 5 °C, whilst a common effect of microhabitats is an increase in minimuma (Chen et al., 1996), which may result in decreasing survival. We have shown here that soil level conditions dramatically decrease the survival of HeV even under 50 % shade compared with air temperature (Kearney et al., 2014). Therefore, a homogeneous reduction in temperatures is not representative of microclimates. Microclimatic effects should be represented by direct measurements, and would benefit from consideration of the joint effects of moisture, temperature and desiccation on virus survival.
We have shown that HeV survival in the environment is likely to be short. The ability of a pathogen to survive outside its hosts is indicative of the possible transmission pathways between hosts. For instance, influenza viruses may survive up to 2 months in water, and therefore can be transmitted by direct and indirect routes (Domanska-Blicharz et al., 2010). Less resistant, non-vector-transmitted pathogens such as human immunodeficiency virus (survival up to 1 week on smooth surfaces) are usually transmitted directly, whilst indirectly transmitted pathogens such as poliovirus survive for up to 2 months (Kramer et al., 2006). The ability of a pathogen to survive in the environment can be an evolutionary consequence of virulence/transmission trade-offs (Walther & Ewald, 2004). Consequently, the environmental susceptibility of HeV might be a consequence of evolutionary trade-offs that occurred within flying fox populations over long periods of time. For example, the clustering of bats in tree canopies, and subsequent exposure of many individuals to a mist of aerosolized urine, might favour transmission routes that do not require long environmental survival (Markwell & Shortridge, 1982; Plowright et al., 2015). The sit-and-wait strategy of highly resistant pathogens such as the causative agents of polio, smallpox and tuberculosis in humans (Walther & Ewald, 2004) may not be necessary for HeV transmission amongst flying foxes. Therefore Transmission routes to horses may be similar to some of the transmission routes between flying foxes.
The precise route of viral entry and infectious dose of HeV in flying foxes and horses are unknown. Some other animal viruses enter through nasal and corneal routes at lower minimum infectious doses than required for oral routes of infection (Ward et al., 1984). For example, experimental nasal inoculation of NiV in pigs and hamsters leads to central nervous system disease (Munster et al., 2012; Weingartl et al., 2005). Experimental infections with HeV in horses successfully reproduced disease with oral and nasal inoculation; however, doses were relatively large (2×106 and 5×104 TCID50, respectively) and were therefore unlikely to represent natural conditions (Marsh et al., 2011). Other experimental studies failed to reproduce transmission from flying foxes to horses by ingestion of contaminated feed (Williamson et al., 1998). Whilst oral transmission of HeV would require contaminated feed or water, nasal transmission may occur through inhalation of aerosolized fluids, droplets or loose contaminated particles in the air (Weber & Stilianakis, 2008). As HeV survival in feed might resemble survival under desiccation, transmission would have to occur very shortly after excretion. For example, time to death of 90 % of HeV (1 log reduction) changed from 24 h in urine to 1 min under desiccation at 22 °C (Fogarty et al., 2008). Similarly, the low survival under desiccation makes inhalation of contaminated dust unlikely to be a route of transmission. Generally, non-smooth, dusty and dry environments further reduce the survival of pathogens (Wagenvoort & Penders, 1997). Given the above, and that urine is probably the most important route of HeV excretion (Williamson et al., 1998, 2000), we suggest that direct exposure to infectious flying fox urine may be an important transmission pathway. However, we cannot completely rule out indirect exposure through contaminated feed during some of the spillover events, especially where predicted 24 h virus survival and moisture are high. A special circumstance is present when water troughs are placed under trees, which eliminates death of viruses by desiccation.
Given that virus survival is not a key driver of the pattern of spillover, in seasons and areas with higher viral survival but no reported cases there must be other factors limiting transmission. These factors must occur with periodicity in southern Queensland and northern NSW in order to explain the seasonal clustering of events in winter. These factors may be less variable in the north where events are more evenly spread throughout the year. For instance, flying foxes may spend more time feeding in agricultural landscapes (e.g. horse paddocks) in winter in the subtropics due to loss of winter feeding habitat, increasing the opportunities for horse exposure; this seasonal dietary shift may not be as apparent in the northern tropics (Plowright et al., 2015).
Scanlan et al. (2014) developed a viral survival model based on similar experimental data to that used here. The overall structure of their model and methods were similar; however, their results and conclusions differ from ours. They stated that HeV survival is a major driver of transmission and seasonality, and that the absence of detected events in the south during summer is driven by chance. In contrast, we found that virus survival did not influence the pattern of spillovers. We identified three main reasons for our different results. First, Scanlan et al. (2014) include recording error. The interval between virus titrations during the survival experiment at 56 °C was 10 min. Scanlan et al. (2014) reported this to be 10 h, and based their half-life estimates and subsequent regression analysis on this unit of measurement. The second reason is the use of different models; Scanlan et al. (2014) used an exponential model compared with a Weibull model used here. As mortality rates are usually not constant over time, there is little support for the use of the exponential model in survival studies (McCallum, 2000; Peleg & Cole, 1998). To account for such an effect, other models and distributions (e.g. Gompertz, Gamma or Weibull) are usually more appropriate (Gil et al., 2011; van Boekel, 2002). The Weibull is simple and flexible enough to account for constant, accelerating or decelerating rates. The third reason is the use of additional data points taken from experiments not designed to measure the effect of temperature (Fogarty et al., 2008). These data points alter the relationship between half-life and temperature. Another contributing factor is that the model used to estimate half-life in Fogarty et al. (2008) (one-phase exponential decay) was different from that used by Scanlan et al. (2014) (linear regression between ln TCID50 and time). Consequently, half-lives used do not correspond to measurements of the same processes with equivalent models. Rather, they are a mixture of half-lives of survival experiments under urine interpolated with a one-phase decay model and survival experiments in EMEM interpolated with the classic exponential model.
Although we do not find a substantial effect of temperature on the epidemiology of HeV through virus survival, there are other ecological processes involved that are influenced by temperature. For instance, present and past temperatures affect the flowering status of many native Eucalyptus species (Hudson et al., 2010), which then influences the distribution and abundance of flying foxes, the reservoir hosts. Temperature also influences pasture quality and the feeding behaviour of horses (Plowright et al., 2015). We also have to consider that absolute abundance of HeV is not only a function of its mortality, but of the excretion rate and the number of infected flying foxes (Field et al., 2011). For example, with more infected flying foxes, more fruiting or flowering trees attracting more flying foxes can increase environmental loads of HeV. It has been shown recently that black and spectacled flying fox densities are higher in areas where spillovers occur (Smith et al., 2014). On the horses side, conditions that increase the time horses spend under trees, basic management practices such as feeding horses and placing water troughs under trees may increase opportunities for horse exposure to the virus (Plowright et al., 2015).
Despite the advantage of the wide range of temperatures in our experiments, there is no information to validate our simulations because the response of HeV is probably different under changing temperatures. Another limitation is that the interval between measurements is too wide to precisely estimate half-lives. Thus, we recommend that future survival experiments be performed over shorter time periods that allow more confidence for interpolation. Experiments should also include the effect of other potential processes that affect HeV survival, such as desiccation and UV radiation (Zhao et al., 2012).
We conclude that the timing and geographical distribution of HeV spillover events cannot be explained by virus survival in the environment. Spillover events occurred when the suitability of temperatures for survival was intermediate to very low. Transmission routes requiring short periods of virus survival, such as direct contact with fresh bat excreta, are likely to be important, whilst routes of transmission requiring longer survival, such as consumption of contaminated feed, should be less important. The winter-dominant seasonal pattern of HeV transmission to horses in southern Queensland and northern NSW is likely driven by an additional seasonal factor (not virus survival), such as the relative availability of food for flying foxes in horse paddocks. In summary, virus survival in the environment is unlikely to be the driver of the pattern of spillover events. We must explore other seasonal risk factors that occur periodically at southern latitudes but are relatively constant in the tropics. Behavioural studies of the interactions between flying foxes and horses are needed to determine the route of HeV transmission.
Methods
We developed two models based on the Weibull and exponential CDFs. The effect of temperature on survival was included when transforming parameters of each distribution into functions of temperature. To run simulations, we generated temperature profiles to represent temperature changes between day and night. The profiles accounted for the variability and extremes observed at meteorological stations over the previous 20 years in order to emulate real-world conditions. Simulations represented the survival of an excreted viral population over a period of at least 24 h. This method allowed us to assess the likelihood of virus accumulation in the environment on successive days. The model was constructed from experimental data at three constant temperatures.
Measurement of HeV survival.
Survival experiments were performed by P. S. in the Australian Animal Health Laboratory (Commonwealth Scientific and Industrial Research Organisation, Geelong, Victoria, Australia) under BSL4 conditions with the isolated HeV strain in the index case. Survival of HeV was measured at three constant temperatures: 4, 22 and 56 °C. Virus cultures were titrated at intervals of 1 week for the experiments at 4 and 22 °C, and 10 min at 56 °C. Virus concentration was reported as TCID50. The detailed experimental design and methods are described in Scanlan et al. (2014).
Model parameterization and simulations.
Optimization was performed with the Gauss–Newton algorithm for non-linear regression implemented in the ‘nls’ routine of the statistical computing language r 3.1.0 (R Core Team, 2014). For a detailed description of the model fitting and simulation procedure, see the Supplementary Information.
Environmental data used in simulations was downloaded from the Bureau of Meteorology (http://www.bom.gov.au), and consisted of minimum and maximum daily air temperatures averaged from 1993 to 2012 from four locations where spillover events were recorded. All simulations were run with the optimum parameter values and their ses with the r package deSolve (Soetaert et al., 2010).
We also generated a series of maps of the potential survival of HeV across the geographical distribution of the black flying fox – the only flying fox species with a distribution covering all spillover sites and consistently found closer to spillover events (Field et al., 2011). These maps represented the proportion of HeV surviving 12 h after excretion at 3 : 00 a.m. Simulations were used to test whether survival was a good predictor of the spatial location of spillover in each season. Data for these simulations were obtained from the microclim dataset (Kearney et al., 2014) for soil temperatures and the Bureau of Meteorology for air temperatures. These simulations were run with the raster 2.2-31 and dismo 0.9-3 r packages (Hijmans, 2013; Hijmans et al., 2013).
Measuring the predictive ability of survival.
Assuming that inter-annual variation in temperature would affect virus survival over the entire geographical region, we performed a Partial ROC (receiver operating characteristic) analysis to assess the performance of virus survival as predictor of spillover (Peterson et al., 2008). The Partial ROC test measures the area under curve (AUC) formed by the predicted extent versus the proportion of predicted events and compares it to a random predictor (Peterson et al., 2008). Tests were run with the Partial ROC tool (Barve, 2008). This is because our survival maps can represent the fundamental niche of spillover events. (Kearney & Porter, 2009). Detailed methods are provided in the Supplementary Information. Under the same assumption, we compared survival in the location of spillover to 100 random samples of survival rates in the background (no-event areas) with a Kolmogorov–Smirnov test. Significance was assessed by calculating the probability that the measured P values were >0.05 if they were drawn from a normal distribution of P values.
Supplementary Data
Acknowledgements
The School of Public Health, Tropical Medicine and Rehabilitation Sciences, James Cook University was contracted by the Rural Industries Research and Development Corporation to undertake this research project. This research was funded by the Commonwealth of Australia, the State of New South Wales and the State of Queensland under the National Hendra Virus Research Program. HeV incident locations are by courtesy of the State of Queensland, through the Department of Agriculture, Fisheries and Forestry, Biosecurity Queensland. We would also like to thank Deborah Middleton (Australian Animal Health Laboratory) and Craig Smith (Queensland Centre for Emerging Infectious Diseases) for collaboration and data sharing, and reviewers whose feedback helped improve the quality of this paper.
Footnotes
Supplementary material is available with the online Supplementary Material.
References
- Barve N. Tool for Partial-ROC. Lawrence, KS: Biodiversity Institute; 2008. [Google Scholar]
- Chen J., Saunders S. C., Crow T. R., Naiman R. J., Brosofske K. D., Mroz G. D., Brookshire B. L., Franklin J. F. Microclimate in forest ecosystem and landscape ecology: variations in local climate can be used to monitor and compare the effects of different management regimes. Bioscience. 1996;49:288–297. doi: 10.2307/1313612. [DOI] [Google Scholar]
- Domanska-Blicharz K., Minta Z., Smietanka K., Marché S., van den Berg T. H5N1 high pathogenicity avian influenza virus survival in different types of water. Avian Dis. 2010;54(Suppl):734–737. doi: 10.1637/8786-040109-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Field H. E., de Jong C., Melville D., Smith C., Smith I., Broos A., Kung Y. H. N., McLaughlin A., Zeddeman A. Hendra virus infection dynamics in Australian fruit bats. PLoS One. 2011;6:e28678. doi: 10.1371/journal.pone.0028678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty R., Halpin K., Hyatt A. D., Daszak P., Mungall B. A. Henipavirus susceptibility to environmental variables. Virus Res. 2008;132:140–144. doi: 10.1016/j.virusres.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil M. M., Miller F. A., Brandão T. R. S., Silva C. L. M. On the use of the Gompertz model to predict microbial thermal inactivation under isothermal and non-isothermal conditions. Food Eng Rev. 2011;3:17–25. doi: 10.1007/s12393-010-9032-2. [DOI] [Google Scholar]
- Halpin K., Young P. L., Field H. E., Mackenzie J. S. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J Gen Virol. 2000;81:1927–1932. doi: 10.1099/0022-1317-81-8-1927. [DOI] [PubMed] [Google Scholar]
- http://cran.r-project.org/web/packages/raster/index.html Hijmans, R. J. (2013). raster: Geographic data analysis and modeling.
- http://cran.r-project.org/web/packages/dismo/index.html Hijmans, R. J., Leathwick, L. & Elith, J. (2013). dismo: Species distribution modelling.
- Hudson I. L., Kim S. W., Keatley M. R. Climatic influences on the flowering phenology of four eucalypts: a GAMLSS approach. In: Hudson I. L., Keatley M. R., editors. Phenological Research. London: Springer; 2010. pp. 209–228. Edited by. [DOI] [Google Scholar]
- Kearney M., Porter W. Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecol Lett. 2009;12:334–350. doi: 10.1111/j.1461-0248.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- Kearney M. R., Isaac A. P., Porter W. P. microclim: Global estimates of hourly microclimate based on long-term monthly climate averages. Sci Data. 2014;1:140006. doi: 10.1038/sdata.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A., Schwebke I., Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell D. D., Shortridge K. F. Possible waterborne transmission and maintenance of influenza viruses in domestic ducks. Appl Environ Microbiol. 1982;43:110–115. doi: 10.1128/aem.43.1.110-115.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh G. A., Haining J., Hancock T. J., Robinson R., Foord A. J., Barr J. A., Riddell S., Heine H. G., White J. R., et al. Experimental infection of horses with Hendra virus/Australia/horse/2008/Redlands. Emerg Infect Dis. 2011;17:2232–2238. doi: 10.3201/eid1712.111162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum H. Population Parameters?: Estimation for Ecological Models. Brisbane: Blackwell Science; 2000. [Google Scholar]
- Middleton D., Pallister J., Klein R., Feng Y.-R., Haining J., Arkinstall R., Frazer L., Huang J.-A., Edwards N., et al. Hendra virus vaccine, a one health approach to protecting horse, human, and environmental health. Emerg Infect Dis. 2014;20:372–379. doi: 10.3201/eid2003.131159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster V. J., Prescott J. B., Bushmaker T., Long D., Rosenke R., Thomas T., Scott D., Fischer E. R., Feldmann H., de Wit E. Rapid Nipah virus entry into the central nervous system of hamsters via the olfactory route. Sci Rep. 2012;2:736. doi: 10.1038/srep00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. G., Jackson G. A. Viral dynamics: a model of the interaction of ultraviolet light and mixing processes on virus survival in seawater. Mar Ecol Prog Ser. 1993;102:105–114. doi: 10.3354/meps102105. [DOI] [Google Scholar]
- Murray K., Selleck P., Hooper P., Hyatt A., Gould A., Gleeson L., Westbury H., Hiley L., Selvey L., et al. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268:94–97. doi: 10.1126/science.7701348. [DOI] [PubMed] [Google Scholar]
- Paterson D. L., Murray P. K., McCormack J. G. Zoonotic disease in Australia caused by a novel member of the paramyxoviridae. Clin Infect Dis. 1998;27:112–118. doi: 10.1086/514614. [DOI] [PubMed] [Google Scholar]
- Peleg M. Evaluation of the Fermi equation as a model of dose-response curves. Appl Microbiol Biotechnol. 1996;46:303–306. doi: 10.1007/s002530050821. [DOI] [Google Scholar]
- Peleg M., Cole M. B. Reinterpretation of microbial survival curves. Crit Rev Food Sci Nutr. 1998;38:353–380. doi: 10.1080/10408699891274246. [DOI] [PubMed] [Google Scholar]
- Peterson A. T., Papes M., Soberon J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol Modell. 2008;213:63–72. doi: 10.1016/j.ecolmodel.2007.11.008. [DOI] [Google Scholar]
- Plowright R. K., Eby P., Hudson P. J., Smith I. L., Westcott D., Bryden W. L., Middleton D., Reid P. A., McFarlane R. A., et al. Ecological dynamics of emerging bat virus spillover. Proc Biol Sci. 2015;282:20142124. doi: 10.1016/j.ecolmodel.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . r: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2014. [Google Scholar]
- Scanlan J. C., Kung N. Y., Selleck P. W., Field H. E. Survival of Hendra virus in the environment: modelling the effect of temperature. EcoHealth. 2014 doi: 10.1007/s10393-014-0920-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C., Skelly C., Kung N., Roberts B., Field H. Flying-fox species density – a spatial risk factor for Hendra virus infection in horses in eastern Australia. PLoS One. 2014;9:e99965. doi: 10.1371/journal.pone.0099965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- http://journal.r-project.org/archive/2010-2/RJournal_2010-2_Soetaert~et~al.pdf Soetaert, K., Petzoldt, T. & Woodrow, R. (2010). Solving differential equations in r.
- van Boekel M. A. On the use of the Weibull model to describe thermal inactivation of microbial vegetative cells. Int J Food Microbiol. 2002;74:139–159. doi: 10.1016/S0168-1605(01)00742-5. [DOI] [PubMed] [Google Scholar]
- Wagenvoort J. H., Penders R. J. Long-term in-vitro survival of an epidemic MRSA phage-group III-29 strain. J Hosp Infect. 1997;35:322–325. doi: 10.1016/S0195-6701(97)90229-2. [DOI] [PubMed] [Google Scholar]
- Walther B. A., Ewald P. W. Pathogen survival in the external environment and the evolution of virulence. Biol Rev Camb Philos Soc. 2004;79:849–869. doi: 10.1017/S1464793104006475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. L., Akin E. W., D’Alessio D. J. Minimum infective dose of animal viruses. Crit Rev Environ Control. 1984;14:297–310. doi: 10.1080/10643388409381721. [DOI] [Google Scholar]
- Weber T. P., Stilianakis N. I. Inactivation of influenza A viruses in the environment and modes of transmission: a critical review. J Infect. 2008;57:361–373. doi: 10.1016/j.jinf.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartl H., Czub S., Copps J., Berhane Y., Middleton D., Marszal P., Gren J., Smith G., Ganske S., et al. Invasion of the central nervous system in a porcine host by Nipah virus. J Virol. 2005;79:7528–7534. doi: 10.1128/JVI.79.12.7528-7534.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson M. M., Hooper P. T., Selleck P. W., Gleeson L. J., Daniels P. W., Westbury H. A., Murray P. K. Transmission studies of Hendra virus (equine morbillivirus) in fruit bats, horses and cats. Aust Vet J. 1998;76:813–818. doi: 10.1111/j.1751-0813.1998.tb12335.x. [DOI] [PubMed] [Google Scholar]
- Williamson M. M., Hooper P. T., Selleck P. W., Westbury H. A., Slocombe R. F. Experimental Hendra virus infection in pregnant guinea-pigs and fruit bats (Pteropus poliocephalus) J Comp Pathol. 2000;122:201–207. doi: 10.1053/jcpa.1999.0364. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Aarnink A. J., Dijkman R., Fabri T., de Jong M. C., Groot Koerkamp P. W. Effects of temperature, relative humidity, absolute humidity, and evaporation potential on survival of airborne Gumboro vaccine virus. Appl Environ Microbiol. 2012;78:1048–1054. doi: 10.1128/AEM.06477-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.