Abstract
Chronic pruritus of unknown origin (CPUO) is a refractory condition. The expression of Interleukin-31 (IL-31), a major pruritogenic cytokine, in CPUO patients has not been investigated. This study aimed to investigate the potential association of IL-31 with CPUO. This was a cross-sectional, analytical study. Patients diagnosed with CPUO and healthy subjects were included at a ratio of 1:2. Serum IL-31 levels were measured in both groups and compared. There were 10 CPUO and 20 healthy subjects who participated in this study. The median IL-31 level in the CPUO group was significantly higher than in the healthy group (127.3 vs 34.4 pg/mL; P < .001). The presence of CPUO was independently associated with IL-31 levels with a coefficient of 89.678 (P < .001). The serum IL-31 cutoff point for CPUO was 56.8 pg/mL, with an area under the receiver operating characteristic curve (ROC) of 100%. Chronic pruritus of unknown origin was significantly and independently associated with higher IL-31 levels. Further clinical trials of IL-31-related treatment may be justified in CPUO patients.
Keywords: Pruritus, treatment, interleukin
Introduction
Chronic pruritus, defined by itching persisting longer than 6 weeks, is one of the most common problems encountered in dermatology clinics and significantly impacts patients’ quality of life.1 It can be caused by primary cutaneous diseases, such as atopic dermatitis, psoriasis, lichen planus, and prurigo nodularis, or systemic conditions including chronic kidney disease, liver disease, thyroid dysfunction, hematologic disorders, and neuropsychiatric diseases.1,2 However, some patients are classified as having chronic pruritus of unknown origin (CPUO) after all known causes of chronic itch have been excluded.1 Chronic pruritus of unknown origin patients usually have generalized pruritus without skin lesions or with nonspecific skin findings such as excoriations. This condition is difficult to treat and refractory to conventional treatment. The pathogenesis of chronic pruritus is complex and not completely understood but involves cross-talk among exogenous and endogenous pruritogens, their receptors, itch-selective unmyelinated C fibers in the nonhistaminergic nerve, immune cells, and cytokines.3
Interleukin (IL)-31 is a major pruritogenic cytokine mainly produced by activated Th2 cells, although mast cells, macrophages, dendritic cells, eosinophils, and basophils also express IL-31.4 Interleukin-31 signals through a heterodimeric receptor complex composed of IL-31 receptor alpha (IL-31RA) and oncostatin M receptor beta (OSMRβ), which are expressed on activated macrophages, dendritic cells, eosinophils, basophils, and keratinocytes cells. It subsequently activates signaling pathways, including the JAK/STAT (Janus-activated kinase/signal transducer and activator of transcription), PI3K/AKT (phosphatidylinositol 3-kinase/protein kinase), and MAPK (mitogen-activated protein kinase) pathways, resulting in the release of chemokines and proinflammatory cytokines.5,6 Recently, several studies have shown that IL-31 plays a key role in inducing pruritus in chronic inflammatory skin conditions and autoimmune skin diseases including atopic dermatitis, prurigo nodularis, and contact dermatitis.7-9 To our knowledge, the expression of IL-31 in CPUO patients has not yet been investigated. We, therefore, conducted this study to investigate the potential association of IL-31 with CPUO.
Methods
Participants
This was a cross-sectional, analytical study conducted at Khon Kaen University’s Srinagarind Hospital in Thailand. Patients diagnosed with CPUO between October 2017 and September 2019 were included. Chronic pruritus of unknown origin was defined as generalized itching lasting longer than 6 weeks without skin rash or systemic causes of pruritus including hematologic disorders such as iron deficiency anemia, polycythemia vera, chronic kidney disease, liver disease, thyroid disease, malignancy, and neuropsychiatric disorders. Exclusion criteria included use of any systemic steroids, immunosuppressive drugs, or phototherapy within 3 months prior to participating in the study. The healthy group consisted of healthy adults undergoing plastic surgical reconstructive or excisional procedures at Srinagarind Hospital with no pruritic symptoms or co-morbid diseases including malignancy. The CPUO to healthy patient ratio was 1:2 with a simple random sampling of the healthy subjects.
Pruritus assessment
The average intensity of pruritus was assessed by a 11-point numeric rating scale (NRS-11) ranging from 0 (no itch) to 10 (worst imaginable itch) and itching severity scale (ISS) score ranging from 0 to 3: 0 = no pruritus, 1 = mild, 2 = moderate (troublesome but does not disturb normal daily activity or sleep), and 3 = severe (disturbs normal daily activity or sleep).
Baseline characteristics and laboratory parameters
Baseline characteristics, including age, sex, co-morbid diseases and concurrent medications, duration and severity of pruritus, laboratory test results, and serum IL-31 levels, of all participants were recorded. Laboratory tests included a complete blood count and measurements of serum blood urea nitrogen, serum creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), serum albumin, serum globulin, and total bilirubin. All laboratory tests were performed at the hospital’s central laboratory.
Analysis of IL-31 serum levels
Serum specimens were collected and stored at −80° C. Concentrations of IL-31 in serum were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) with standard kits according to the manufacturer’s instructions (Human IL-31 ELISA Kit; Abcam, Cambridge, MA, USA).
Statistical analysis
Descriptive statistics were used to calculate median (range) and proportions of the studied variables. Numerical variables were compared between the pruritus group and healthy group using a Wilcoxon rank sum test, while categorical variables between both groups were compared using Fisher exact test. The associations between IL-31 levels and the studied variables were determined using univariate and multivariate linear regression analyses. Those factors with a P value of less than .20 by univariate analysis were subjected to subsequent multivariate analysis. Factors with collinearity were checked. Results were reported as coefficient (standard error) with a P value. The IL-31 cutoff point to indicate CPUO was calculated using logistic regression analysis. A receiver operating characteristic curve (ROC) was also created. Statistical analyses were computed using STATA version 10.1 (College Station, TX, USA).
Results
There were 10 CPUO patients and 20 healthy subjects who participated in this study. The CPUO patients had a median score of 8.5 of 10 on the pruritus NRS-11 score (range: 8–10). Scores of 2 and 3 on the ISS were reported in 4 and 6 patients, respectively. The median duration of pruritus was 12 months. Five (50.0%) of the 10 CPUO patients had at least one co-morbid disease, the most common of which was hypertension (2 patients). Other co-morbid diseases included diabetes (1 patient), tumid LE (1 patient), dyslipidemia (1 patient), migraine (1 patient), and gastritis (1 patient). Median (range) thyroid-stimulating hormone (TSH) in the CPUO group was 2.05 (1.66-2.08). The pruritus group differed from the healthy group in terms of age and serum blood urea nitrogen (Table 1), which were significantly higher in the former. Moreover, serum IL-31 levels in the pruritus group were significantly higher than in the healthy group (Figure 1).
Table 1.
Baseline characteristics and laboratory results of CPUO patients and healthy subjects.
| Factors | Healthy subjects n = 20 |
CPUO patients n = 10 |
P value |
|---|---|---|---|
| Age, years | 50 (20-70) | 64 (47-80) | .041 |
| Male sex, n (%) | 9 (45.00) | 4 (40.00) | .999 |
| Hemoglobin, g/dL | 13.3 (11.7-15.3) | 12.1 (10.8-15.3) | .123 |
| White blood cell, cells/mm3 | 7180 (4350-10 550) | 6100 (4540-15 400) | .094 |
| PMN, % | 58.4 (41.4-77.1) | 52.2 (41.9-70.7) | .252 |
| Lymphocyte, % | 29.6 (17.6-46.3) | 34.1 (12.0-42.6) | .234 |
| Monocyte, % | 7.3 (4.4-11.6) | 7.1 (5.9-9.8) | .894 |
| Eosinophils, % | 4.5 (0-16.5) | 3.4 (0.4-10.1) | .270 |
| Blood urea nitrogen, mg/dL | 10.6 (7.0-19.1) | 14.7 (10.6-18.4) | .029 |
| Serum creatinine, mg/dL | 0.75 (0.48-1.31) | 0.84 (0.69-1.41) | .074 |
| Albumin, g/dL | 4.4 (2.7-4.7) | 4.5 (4.2-5.1) | .224 |
| Globulin, g/dL | 3.1 (2.5-4.0) | 3.1 (2.7-3.5) | .839 |
| Total bilirubin, mg/dL | 0.4 (0.1-1.3) | 0.5 (0.3-0.8) | .475 |
| ALT, U/L | 15.0 (9.0-26.0) | 23.0 (6.0-41.0) | .340 |
| AST, U/L | 20.0 (14.0-34.0) | 26.5 (13.0-54.0) | .548 |
| Alkaline phosphatase, U/L | 73.0 (41.0-120.0) | 74.0 (35.0-103.0) | .698 |
| Interleukin-31, pg/mL | 34.4 (20.4-56.8) | 127.3 (100.5-154.2) | <.001 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPUO, chronic pruritus of unknown origin; PMN, polymorphonuclear leukocytes.
Data presented as median (range) unless indicated otherwise.
Figure 1.
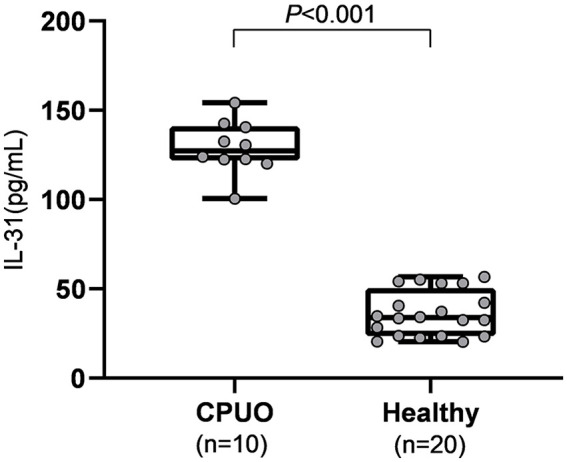
Comparison of serum IL-31 levels between CPUO and healthy subjects. Serum IL-31 levels were significantly higher in CPUO patients than in healthy subjects. CPUO indicates chronic pruritus of unknown origin; IL-31, interleukin-31.
Median serum IL-31 in the CPUO group was about 3.7 times higher than that of the healthy group (127.3 vs 34.4 pg/mL). Age, serum blood urea nitrogen, and CPUO status were significantly associated with serum IL-31 levels according to univariate linear regression analysis. However, only CPUO was independently associated, with a coefficient of 89.678 (P < .001), as shown in Table 2. The serum IL-31 cutoff point for CPUO was 56.8 pg/mL with an area under the ROC of 100%, as the highest serum IL-31 levels in the control group were lower than the lowest serum IL-31 levels in the CPUO group (Table 1).
Table 2.
Factors associated with interleukin-31 levels in patients with chronic pruritus of unknown origin and healthy subjects by multivariate linear regression analysis.
| Factors | Univariate coefficient (SE) | P value | Multivariate coefficient | P value |
|---|---|---|---|---|
| Age | 1.045 (0.469) | 0.034 | 0.006 (0.166) | .970 |
| White blood cell | –0.004 (0.003) | 0.147 | –0.001 (0.001) | .205 |
| Blood urea nitrogen | 5.008 (2.275) | 0.036 | 0.480 (0.803) | .555 |
| Pruritus | 92.605 (5.134) | <0.001 | 89.678 (6.018) | <.001 |
Abbreviation: SE, standard error.
Discussion
This study showed that patients with CPUO had significantly higher serum IL-31 levels than healthy subjects and that CPUO was an independent predictor of IL-31. This is similar to findings in patients with other chronic pruritic inflammatory skin diseases such as atopic dermatitis, prurigo nodularis, and contact dermatitis, meaning that the higher IL-31 levels in these conditions may be caused by a similar mechanism. Previous studies have found IL-31, a mediator in the gp130/IL6 family, to be overexpressed in NC/Nga mice.10,11 It has also been found to be high in serum of humans in chronic pruritic skin conditions with absence or subtle skin inflammation such as uremic pruritus and cholestasis of pregnancy.12,13 Although these skin diseases are associated with higher serum IL-31 levels, the amount varies by disease. This might be due to commercial ELISA kits for IL-31 having different reference levels. However, previous studies have found mean serum IL-31 levels in adult patients with atopic dermatitis to be around 1.7 times higher than in controls (1060.4 vs 617.6 pg/mL),7 and those of allergic contact dermatitis patients to be 1.6 times higher (8.27 vs 5.18 pg/mL).9 In one study, median serum IL-31 levels in patients with cholestasis of pregnancy were 1.2 times higher than in the control group (620.8 vs 515.9 pg/mL).13 Our study found this difference to be even higher, with IL-31 levels in the CPUO group at 3.7 times those of the healthy group (127.3 vs 34.4 pg/mL). These findings imply that IL-31 levels differ depending on the cause of disease.
Previous reports have also found differences in serum uric acid, ALT, and AST between pruritus patients and controls.12,13 We found several factors that differed significantly between the CPUO and healthy subjects, including IL-31 levels, age, and serum blood urea nitrogen (Tables 1 and 2), of which only IL-31 was positively and independently associated with CPUO. This implies that CPUO was associated with changes in IL-31 levels after adjustment for other factors, an association that has not been shown in any previous studies. The suggested IL-31 cutoff point for CPUO is 56.8 pg/mL, which yielded perfect prediction (100% area under ROC). These results imply that treatment related to IL-31 may be beneficial and justify future clinical trial.
Chronic pruritus of unknown origin usually only occurs in a small segment of the population (especially in older adults) but has a highly significant impact on patients’ quality of life due to severe itching and that it is difficult to treat. One retrospective study showed that only 11% (63 of 597) of patients with chronic pruritus had CPUO.14 Although the pathogenesis of the condition is not yet fully understood, 3 main factors are involved in its development: keratinocytes and skin barrier dysfunction, immune system–related conditions, and nonhistaminergic sensory neuropathy.3,15 Elderly patients often suffer from immunosenescence, a defect in Th1 function skewing toward increasing Th2 response, and the resulting Th2 polarization can cause itching.15 Our study showed that CPUO tended to disproportionately affect elderly people. This might be because of immunosenescence-related Th2 activation. Significantly higher IL-31 levels (which is one of the Th2 cytokines in CPUO patients) might play a crucial role in the pathogenesis of the disease.
IL-31 acts through the IL-31 receptor complex: IL-31RA and OSMRβ. After IL-31 binds to its heterodimeric receptor, the JAK/STAT, PI3K/AKT, and MAPK signaling pathways are activated, resulting in induction of chemokines and proinflammatory cytokines, regulation of cell proliferation, and stimulation of dorsal root ganglia sensory neurons.5,6 In 2 recent studies, a total of 10 patients with severe CPUO who were refractory to conventional treatment, including systemic steroids and potent immunosuppressive therapy, had significant itch improvement after taking tofacitinib, an oral JAK inhibitor.16,17 This supports the hypothesis that interaction of IL-31 and the JAK/STAT pathway is involved in the pathogenesis of CPUO. Further studies may show drugs targeting IL-31 to be effective in relieving pruritic symptoms in CPUO patients.
There were some limitations to this study. First, only IL-31 was measured. However, previous reports have found IL-31 to be a potent pruritogenic cytokine associated with itching. Second, there were several factors that differed between groups due to the non-randomized study design. However, the presence of CPUO was still significantly related with IL-31 levels after controlling for confounding factors using multivariate linear regression analysis. Third, due to the small sample size and the fact that all of the patients had moderate to severe pruritus, comparative analysis between those experiencing mild versus severe itching could not be conducted. Fourth, the group of healthy individuals was not age- or sex-matched. Finally, family history of atopy was not evaluated in either group.
In summary, this was the first study to show CPUO to be significantly and independently associated with higher IL-31 levels.
Acknowledgments
The authors would like to thank Dr. Dylan Southard (USA) for editing this manuscript. The authors also would like to thank the Sleep Apnea Research Group and Khon Kaen University (Khon Kaen, Thailand).
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the Khon Kaen University Faculty of Medicine (Thailand; IN61211).
Declaration of Conflicting Interest:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: SC and CC designed the study. SC, KS, KW, and CC collected samples, performed the laboratory assays. SC and KS performed the statistical analyses. SC, KS, and KS wrote the manuscript. The final version of the manuscript was approved by all authors.
ORCID iD: Kittisak Sawanyawisuth  https://orcid.org/0000-0003-3570-8474
https://orcid.org/0000-0003-3570-8474
References
- 1. Kim BS, Berger TG, Yosipovitch G. Chronic pruritus of unknown origin (CPUO): uniform nomenclature and diagnosis as a pathway to standardized understanding and treatment. J Am Acad Dermatol. 2019;81:1223-1234. [DOI] [PubMed] [Google Scholar]
- 2. Hashimoto T, Yosipovitch G. Itching as a systemic disease. J Allergy Clin Immunol. 2019;144:375-380. [DOI] [PubMed] [Google Scholar]
- 3. Yosipovitch G, Rosen JD, Hashimoto T. Itch: from mechanism to (novel) therapeutic approaches. J Allergy Clin Immunol. 2018;142:1375-1390. [DOI] [PubMed] [Google Scholar]
- 4. Gibbs BF, Patsinakidis N, Raap U. Role of the pruritic cytokine IL-31 in autoimmune skin diseases. Front Immunol. 2019;10:1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Salvo E, Ventura-Spagnolo E, Casciaro M, Navarra M, Gangemi S. IL-33/IL-31 axis: a potential inflammatory pathway. Mediators Inflamm. 2018;2018:3858032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakashima C, Otsuka A, Kabashima K. Interleukin-31 and interleukin-31 receptor: new therapeutic targets for atopic dermatitis. Exp Dermatol. 2018;27:327-331. [DOI] [PubMed] [Google Scholar]
- 7. Nygaard U, Hvid M, Johansen C, et al. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J Eur Acad Dermatol Venereol. 2016;30:1930-1938. [DOI] [PubMed] [Google Scholar]
- 8. Raap U, Weissmantel S, Gehring M, Eisenberg AM, Kapp A, Folster-Holst R. IL-31 significantly correlates with disease activity and Th2 cytokine levels in children with atopic dermatitis. Pediatr Allergy Immunol. 2012;23:285-288. [DOI] [PubMed] [Google Scholar]
- 9. Guarneri F, Minciullo PL, Mannucci C, et al. IL-31 and IL-33 circulating levels in allergic contact dermatitis. Eur Ann Allergy Clin Immunol. 2015;47:156-158. [PubMed] [Google Scholar]
- 10. Dillon SR, Sprecher C, Hammond A, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752-760. [DOI] [PubMed] [Google Scholar]
- 11. Takaoka A, Arai I, Sugimoto M, Yamaguchi A, Tanaka M, Nakaike S. Expression of IL-31 gene transcripts in NC/Nga mice with atopic dermatitis. Eur J Pharmacol. 2005;516:180-181. [DOI] [PubMed] [Google Scholar]
- 12. Ko MJ, Peng YS, Chen HY, et al. Interleukin-31 is associated with uremic pruritus in patients receiving hemodialysis. J Am Acad Dermatol. 2014;71:1151.e1-1159e1. [DOI] [PubMed] [Google Scholar]
- 13. Basile F, Santamaria A, Mannucci C, et al. Interleukin 31 is involved in intrahepatic cholestasis of pregnancy. J Matern Fetal Neonatal Med. 2017;30:1124-1127. [DOI] [PubMed] [Google Scholar]
- 14. Mollanazar NK, Sethi M, Rodriguez RV, et al. Retrospective analysis of data from an itch center: integrating validated tools in the electronic health record. J Am Acad Dermatol. 2016;75:842-844. [DOI] [PubMed] [Google Scholar]
- 15. Berger TG, Steinhoff M. Pruritus in elderly patients—eruptions of senescence. Semin Cutan Med Surg. 2011;30:113-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217.e13-228.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang F, Morris C, Bodet ND, Kim BS. Treatment of refractory chronic pruritus of unknown origin with tofacitinib in patients with rheumatoid arthritis [published online ahead of print September 18, 2019]. JAMA Dermatol. doi: 10.1001/jamadermatol.2019.2804. [DOI] [PubMed] [Google Scholar]


